Abstract
In recent years, immune checkpoint inhibitors (ICPI) have become widely used for multiple solid malignancies. Reliable predictive biomarkers for selection of patients who would benefit most are lacking. Several tumor types with somatic or germline alterations in genes involved in the DNA damage response (DDR) pathway harbor a higher tumor mutational burden, possibly associated with an increased tumoral neoantigen load. These neoantigens are thought to lead to stronger immune activation and enhanced response to ICPIs. We present a series of seven patients with different malignancies with germline disease-associated variants in DDR genes (BRCA1, BRCA2, CHEK2) responding favorably to ICPIs.
Keywords: DNA damage response, immune checkpoint inhibitors, BRCA1, BRCA2, CHEK2
1. Introduction
Since the approval of the cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) inhibitor ipilimumab for the treatment of metastatic melanoma in 2011, immune checkpoint inhibitors (ICPI) have become a cornerstone in the treatment of solid malignancies [1]. These monoclonal antibodies target immune checkpoint molecules such as CTLA-4, programmed death (PD)-1 or programmed death ligand (PD-L)1, through which they counteract the inhibition of T cells and promote the antitumor immune response. They are able to induce profound and durable responses in a subset of patients; however, selecting these patients remains a challenge that has given rise to substantial research for predictive biomarkers.
Tumor mutational burden (TMB) is a potential predictive biomarker that has shown promising results in malignant melanoma (MM) [2] and non-small cell lung carcinoma [3,4]. A higher TMB leads to a higher load of neoantigens that are recognized by cytotoxic T-lymphocytes, inducing an exhausted antitumoral immune reaction [5,6]. Upon treatment with ICPIs, the immune response is activated, leading to the antitumor immune response [7,8,9,10]. Previous pan-cancer research has demonstrated a response rate of 58% in patients with high TMB (>20 mut/Mb), whereas in patients with intermediate to low TMB the response rate is still around 20% [11].
Tumors with impaired DNA damage response (DDR) pathways, through bi-allelic mutations or copy number changes, usually harbor a higher TMB [12,13], leading to increased tumoral neoantigen load [14]. Additionally, increasing evidence has emerged of a direct link between DDR pathways and the innate immune system which can enhance the antitumor immune response independent of neoantigen burden [15,16]. Tumors arising in patients with germline disease-associated DDR variants could, therefore, display more favorable responses to ICPIs. Of the main pathways of DNA repair mechanisms (base excision repair, nucleotide excision repair, mismatch repair (MMR), homologous recombination (including Fanconi anemia) and nonhomologous end joining), remarkable responses to ICPI have been extensively demonstrated in MMR deficient tumors [17,18]. These groundbreaking trials led to FDA approval for the PD-1 antibody pembrolizumab for MMR deficient solid tumors regardless of primary tumor site [19].
In this study, we present a multitumor case series of patients carrying a germline DDR alteration displaying favorable outcomes on ICPIs.
2. Materials and Methods
2.1. Patient Selection
We retrospectively included patients treated with ICPIs who were screened for germline DNA alterations across two institutions (University Hospitals Leuven, Leuven, Belgium; AZ Groeninge Hospital, Kortrijk, Belgium). The patients were divided into two groups: with and without germline DDR alterations. As the predictive effect of MMR impairment on ICPIs has been well demonstrated [17,18], we did not include germline MMR alterations in the current study.
Tumor type was not a selection criterion, as far as there was an approved indication for ICPIs in monotherapy. Patients treated with ICPIs in combination with chemotherapy or targeted therapies were not included. Patients were treated according to institutional standards, following good clinical practice, with the PD-1-antibody nivolumab or pembrolizumab, the PD-L1-antibody atezolizumab, or the combination of an PD1-antibody with the CTLA-4-antibody ipilimumab.
We extracted baseline clinical characteristics from the patient files, including age, sex, tumor type, presence of other tumors, number of metastatic disease sites at the start of ICPI treatment, Eastern Cooperative Oncology Group Performance Status (ECOG PS), type of ICPI, best response, progression-free survival (PFS) and overall survival (OS). Response evaluation was based on Immune-related Response Evaluation Criteria In Solid Tumors (irRECIST) [20]. We extracted biochemical data with known prognostic impact at the start of ICPI treatment, including C-reactive protein (CRP), neutrophil/lymphocyte ratio (NLR) [21,22,23], albumin and lactate dehydrogenase (LDH).
2.2. Genetic Testing
DNA testing was performed as part of clinical routine (A) to detect familial cancer syndromes in patients with multiple malignancies and/or young age at diagnosis, or (B) in patients with a family member carrying a known disease-associated germline variant. DNA was extracted from peripheral white blood cells by magnetic separation on a Chemagic 360 instrument (Perkin Elmer, Waltham, MA, USA) using the Chemagic DNA 4 k blood kit (Perkin Elmer, Waltham, MA, USA) according to the manufacturer’s instructions. Patients with a family member carrying a known disease-associated germline variant were tested specifically for that variant with Sanger sequencing. Patients without a known familial variant were tested with next generation sequencing (NGS) gene panels for hereditary cancer syndromes, with the panel composition expanded over the years as more genes for family cancer syndromes became known (time range 2016–2020). Initially, the BRCA hereditary cancer MASTR plus kit (Multiplicom/Agilent, Santa Clara, CA, USA) was used to detect small variants. Duplications and/or (multi) exonic deletions were investigated via MLPA (MRC Holland, Amsterdam, The Netherlands). From 2019 onwards, the HaloPlex panel (Agilent, custom design v2) was used to investigate disease-associated small variants and copy number variants. One patient was tested at another clinical center, where FamCanc panel and additional genetic testing for Precision-2 trial was performed. All panels included BRCA1, BRCA2, PALB2, CHEK2 and TP53. The following reference sequences were used for the detected disease-associated variants: LRG_292t1 (BRCA1), LRG_293t1 (BRCA2), NM_007194.4 (CHEK2). The American College of Medical Genetics and Genomics guidelines were used to classify the variants [24]. In general, only class 4 (i.e., likely pathogenic) and class 5 (i.e., pathogenic) variants were reported.
2.3. Statistical Analysis
The main objective was to report cases of patients carrying DDR germline alterations and their favorable outcome on treatment with ICPIs. This study was not conceived for a formal comparison between subgroups. PFS and OS were estimated with Kaplan Meier survival analysis and compared with the log-rank test. Best responses were compared using Pearson’s Chi Squared test, and objective response rate (ORR) was analyzed using Fisher’s Exact test. Analyses were performed using R (version 4.0.03) (R Core Team, Vienna, Austria) software.
2.4. Ethical Approval
The study was approved by the Ethics Committee Research UZ/KULeuven (registration number S53479/S63833).
3. Results
3.1. Included Patients
We collected seven patient cases with germline DDR alteration carriers (BRCA1 (n = 3), BRCA2 (n = 3) and CHEK2 (n = 1)). These patients were treated for MM, transitional cell carcinoma (TCC), renal cell carcinoma (RCC) or squamous cell carcinoma (SCC), with nivolumab (n = 3), pembrolizumab (n = 2), atezolizumab (n = 1) or ipilimumab/nivolumab (n = 1). We collected 13 patient cases for whom no class 4 or 5 variants were found. These patients were diagnosed with MM or RCC and treated with nivolumab (n = 8), pembrolizumab (n = 1) or ipilimumab/nivolumab (n = 4). Full patient details are reported in Table S1. Baseline prognostic parameters are reported in Table 1. Baseline albumin levels and ECOG PS were similar for both subgroups. The mean number of metastatic sites, LDH and NLR were higher in patients with germline alteration, but baseline CRP levels were higher in patients without. No statistical comparison was done due to small patient numbers.
Table 1.
Baseline characteristics.
| Germline DDR Variant Present (n = 7) | Germline DDR Variant Absent (n = 13) | |
|---|---|---|
| Age at diagnosis (median, range) | 59 (31–73) | 57 (43–71) |
| Gender | 5 males—2 females | 7 males—6 females |
| Tumor Type (n) | ||
| Malignant melanoma | 3 | 2 |
| Squamous cell carcinoma of unknown origin | 1 | 0 |
| Renal cell carcinoma | 1 | 11 |
| Transitional cell carcinoma | 2 | 0 |
| Second Tumor (n) | ||
| Prostate adenocarcinoma | 2 | 2 |
| Colorectal adenocarcinoma | 1 | 2 |
| Transitional cell carcinoma | 0 | 1 |
| Breast cancer | 0 | 5 |
| Malignant melanoma | 0 | 2 |
| Basocellular carcinoma (skin) | 0 | 2 |
| Squamous cell carcinoma (skin) | 0 | 1 |
| Endometrial cancer | 0 | 1 |
| Clinical and Biochemical Characteristics | ||
| Number of metastatic sites (mean, SD) | 3.57 ± 1.8 | 2.23 ± 1.0 |
| CRP (mg/L) (mean, SD) | 28.5 ± 63.0 | 51.2 ± 77.9 |
| Albumin (g/L) (mean, SD) | 40.9 ± 5.34 | 39.3 ± 3.9 |
| NLR (mean, SD) | 5.85 ± 7.1 | 3.54 ± 2.6 |
| LDH (U/l) (mean, SD) | 237.9 ± 87.2 | 207.2 ± 65.2 |
| ECOG Performance Status | ||
| ECOG 0 (n, %) | 5 (71.4%) | 8 (61.5%) |
| ECOG 1 (n, %) | 2 (28.6%) | 5 (38.5%) |
| Type of ICPI | ||
| Nivolumab | 3 (42.9%) | 8 (61.5%) |
| Ipilimumab and nivolumab | 1 (14.3%) | 4 (30.8%) |
| Pembrolizumab | 2 (28.6%) | 1 (7.7%) |
| Atezolizumab | 1 (14.3%) | 0 (0%) |
Abbreviations: CRP = C-reactive protein; SD = standard deviation; NLR = neutrophil to lymphocyte ratio; LDH = lactate dehydrogenase; ECOG PS = Eastern Cooperative Oncology Group performance status; ICPI = immune checkpoint inhibitor.
3.2. Genetic Testing and Outcome on ICPI Treatment
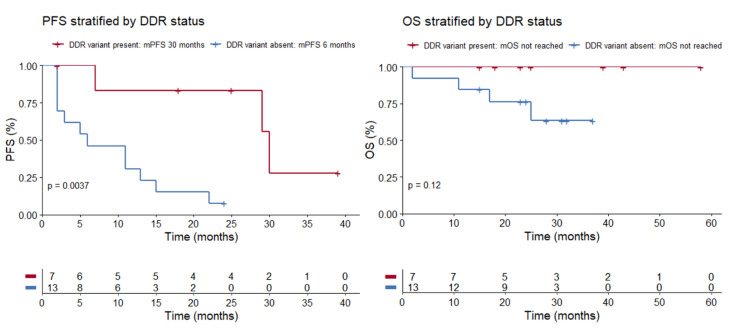
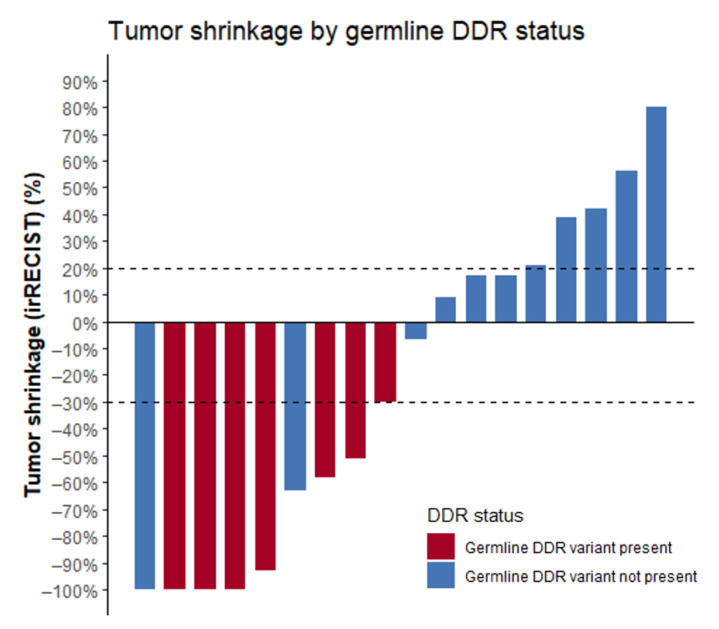
The seven patients with detected germline alterations displayed very favorable outcomes on ICPIs. ORR was 86%, median PFS (mPFS) was 30 months (range 2 to 39 months), and OS was not reached (Figure 1; Table 2). At the time of analysis, response was ongoing in three patients and progression reached in three patients. One patient switched to second-line therapy because of severe toxicity on ICPI. Complete response (CR) was achieved in three patients. Best response on ICPI according to irRECIST is shown in Figure 2.
Figure 1.
mPFS was 30 months in the patient group where a germline DDR variant was present, compared to 6 months in the patient group where a germline variant was absent (p = 0.0037, log-rank test). mOS was not reached, both in the patient group where a germline DDR variant was present and where it was absent (p = 0.12, log-rank test). Abbreviations: PFS = progression free survival, DDR = DNA damage response, mPFS = median progression free survival, OS = overall survival, mOS = median overall survival.
Table 2.
Best response according to irRECIST in the patient groups with or without germline DDR variant.
| Germline DDR Status | Partial Response | Stable Disease | Progressive Disease | Clinical Benefit | p-Value (Chi-Square Test) |
|---|---|---|---|---|---|
| Germline DDR variant present | 6/7 (86%) | 1/7 (14%) | 0/7 (0%) | 0/7 (0%) | 0.02 |
| Germline DDR variant absent | 2/13 (15.4%) | 4/13 (30.8%) | 5/13 (38.5%) | 2/13 (15.4%) |
Abbreviations: DDR = DNA damage response.
Figure 2.
Waterfall plot displaying the best response to ICPIs according to irRECIST criteria. Note: two patients without DDR alterations were not included in this figure as precise tumor shrinkage was not available. Best response was defined as “clinical benefit”. Abbreviations: ICPIs = immune checkpoint inhibitors, DDR = DNA damage response.
The 13 patients without detected alterations displayed modest outcomes. mPFS was rather short (6 months; range 2 to 39 months) and ORR low (15.4%), with one patient achieving a CR. At time of analysis, response was ongoing in one patient and progression reached in 12 patients. Median OS (mOS) was not reached. Best response on ICPI according to irRECIST is shown in Figure 2. The ORRs in these patients are in line with the expected outcomes in nonselected RCC and MM patients treated with nivolumab or ipilimumab/nivolumab.
Table 3 summarizes results of the genetic testing in each included patient. In six out of seven patients with a germline alteration, only the known familial disease-associated variant was tested without further germline testing. In one of these seven patients, germline testing for BRCA1 was performed after somatic NGS revealed a BRCA1 mutation in 50% of the alleles. In the 13 patients without germline alteration, several gene panels for hereditary cancer syndromes were used, all including BRCA1, BRCA2, CHEK2, Partner And Localizer Of BRCA2 (PALB2) and Tumor Protein P53 (TP53).
Table 3.
Genetic testing performed in each patient.
| Patient | Genetic Variant Identified | Genetic Screening Performed |
| 1 | BRCA1 (c.212+3A>G, p.?) | Analysis of known family variant |
| 2 | BRCA2 (c.6644_6647del, p.Ty2215Serfs*13) | Analysis of known family variant |
| 3 | CHEK2 (c.1100del, p.Thr367Metfs*15) | Analysis of known family variant |
| 4 | BRCA2 (c.516+1G>A, p.?) | Analysis of known family variant |
| 5 | BRCA1 (c.5186T>A, p.Leu1729Gln) |
ATM, BRIP1, CDH1, CHEK2, MLH1, MSH2, MSH6, NBN (only c.657_661del), PALB2, PTEN, RAD51C, RAD51D, TP53
Additional testing: NGS sequencing of tumor tissue |
| 6 | BRCA2 (c.4936_4939del, p.Glu1646Glnfs*23) | Analysis of known family variant |
| 7 | BRCA1 (c.212+3A>G, p.?) | Analysis of known family variant |
| 8 | No class 4 or 5 variant found | HaloPlex panel |
| 9 | No class 4 or 5 variant found | HaloPlex panel |
| 10 | No class 4 or 5 variant found | HaloPlex panel |
| 11 | No class 4 or 5 variant found | HaloPlex panel |
| 12 | No class 4 or 5 variant found | HaloPlex panel |
| 13 | No class 4 or 5 variant found | HaloPlex panel |
| 14 | No class 4 or 5 variant found | HaloPlex panel |
| 15 | No class 4 or 5 variant found | HaloPlex panel |
| 16 | No class 4 or 5 variant found | BRCA hereditary cancer MASTR plus kit |
| 17 | No class 4 or 5 variant found | BRCA hereditary cancer MASTR plus kit |
| 18 | No class 4 or 5 variant found | BRCA hereditary cancer MASTR plus kit |
| 19 | No class 4 or 5 variant found | FamCanc + Precision 2 trial (BMSO) |
| 20 | No class 4 or 5 variant found | HaloPlex panel |
Abbreviations: ATM = Ataxia Telangiectasia Mutated; BRCA1 = BRCA1 DNA Repair Associated; BRCA2 = BRCA2 DNA Repair Associated; BRIP1 = BRCA1 interacting protein C-terminal helicase 1; CDH1 = cadherin 1; CHEK2 = checkpoint kinase 2; MLH1 = MutL Homolog 1; MSH2 = MutS Homolog 2; MSH6 = MutS Homolog 6; NBN = Nibrin; PALB2 = Partner And Localizer Of BRCA2; PTEN = Phosphatase And Tensin Homolog; RAD51C = RAD51 paralog C; RAD51D = RAD51 paralog D; TP53 = Tumor Protein P53; NGS = next generation sequencing.
4. Case Descriptions
4.1. Patient 1: BRCA1 (c.212+3A>G, p.?): Renal Cell Carcinoma
At the age of 65, this patient underwent radical prostatectomy for a localized prostate carcinoma (PC). Three years later, he underwent a nephrectomy for localized clear cell RCC. At the age of 70, he was diagnosed with localized rectal adenocarcinoma, treated with neoadjuvant radiotherapy, surgery and adjuvant chemotherapy. At the age of 81, local relapse with metastatic spread of the RCC was diagnosed with several liver lesions, confirmed by biopsy. Upon progression on sunitinib and axitinib, third-line treatment with nivolumab was initiated, leading to confirmed deep partial response (PR) (−93% irRECIST). Nivolumab was paused after 22 months because of sustained response but was reinitiated after 13 months due to progressive disease (PD). Upon further disease progression after 6 months, talazoparib was started in compassionate use. He currently has stable disease. OS was censored at 43 months. The corresponding phase 3 trial comparing nivolumab with everolimus in pretreated advanced RCC (Checkmate 025) demonstrated a mPFS of 4.6 months, an ORR of 25% and a mOS of 25 months in nivolumab-treated patients [25]. This case was previously published by Beulque et al. [26].
4.2. Patient 2: BRCA2 (c.6644_6647del, p.Ty2215Serfs*13): Squamous Cell Carcinoma of Unknown Origin
At the age of 68, this patient underwent a radical prostatectomy for a localized PC. Subsequently, a local relapse was treated with radiotherapy and a biochemical recurrence by androgen deprivation therapy. Three years later, he presented with a symptomatic brain metastasis. After complete resection, pathologic evaluation showed it to be a SCC metastasis. Additional staging demonstrated diffuse lymph node and bone metastases, with a low prostate specific antigen (PSA) level. First-line treatment with cisplatin-fluorouracil proved inefficient. Nivolumab in second line led to a PR (−58% irRECIST), which is ongoing after 25 months. Meanwhile, docetaxel was associated because of rapidly rising PSA levels, leading to a biochemical response and discontinuation of docetaxel after 7 months. This patient was censored for OS at 25 months. In a corresponding phase 3 trial in patients with head and neck SCC with recurrent disease on platinum-based chemotherapy (CheckMate 141), nivolumab-treated patients had a mPFS of 2 months, an ORR of 13.3% and a mOS of 7.5 months [27].
4.3. Patient 3: CHEK2 (c. 1100del, p.Thr367Metfs*15): Malignant Melanoma
Patient 3 was diagnosed with a localized BRAF-wild type MM at the age of 31 and treated with wide excision. One year later, staging showed lung, lymph node, liver and spleen metastases. First-line systemic therapy with pembrolizumab was initiated. After two months, staging showed a slight increase of liver and lymph node metastases but complete response (CR) of lung and spleen metastases. Surgical resection of the liver metastasis and axillary lymph nodes showed pathological confirmed CR. Pembrolizumab is still ongoing after 39 months with sustained CR. In the KEYNOTE-006 trial investigating pembrolizumab vs. ipilimumab in advanced melanoma, pembrolizumab-treated patients had a mPFS of 4.1 months and an ORR of 32.9% [28].
4.4. Patient 4: BRCA2 (c.516+1G>A, p.?): Malignant Melanoma
Patient 4 was diagnosed with a BRAF-mutated MM at the age of 57, with lymph node, lung and brain metastases. Shortly after the start of ipilimumab/nivolumab in first-line, he developed severe auto-immune meningitis and pneumonitis. Staging showed a decrease of the largest brain metastasis and adenopathies (−29% irRECIST); however two subcentrimetric brain metastasis had slightly increased. Due to severe toxicity, ICPI was switched to dabrafenib-trametinib with an ongoing PR after 13 months of therapy. The patient was censored for PFS at two months and for OS at 23 months. The corresponding phase 3 trial in patients with previously untreated advanced melanoma (CheckMate-067) demonstrated a mPFS of 11.5 months, an ORR of 57.6% and a mOS of more than 60 months in the patient group treated with ipilimumab-nivolumab [29].
4.5. Patient 5: BRCA1 (c.5186T>A, p.Leu1729Gln): Malignant Melanoma
Patient 5 discovered a breast nodule at age 38, which was confirmed to be a BRAF-mutated MM metastasis with additional liver and lymph node metastases. First-line therapy with dabrafenib-trametinib led to a PR. Tumor tissue NGS showed a BRCA1 class 3 variant in 55% of the reads. Subsequent testing confirmed it to be a germline variant. This patient had a second degree female relative diagnosed with breast cancer at the age of 48 (no germline testing available for this relative). Therefore, we included this patient in this study even though the variant was not of class 4 or 5. After 27 months, for multifocal progressive disease, ipilimumab/nivolumab was started, leading to CR. After 30 months, staging showed a new breast nodule with axillary lymph nodes. Pathological examination couldn’t differentiate between local MM relapse, clinically the more likely alternative, or triple negative breast cancer. However, on a multitumor board it was advised to consider this new tumor as breast carcinoma and to offer a potentially curative treatment. Immunotherapy was discontinued. After mastectomy and adjuvant chemo-radiotherapy, she has been without evidence of disease for 21 months. To avoid bias through a more favorable interpretation of results, we considered the new tumor in the breast as a MM relapse after 30 months of ICPI treatment for this study, and not as a new breast carcinoma. The patient was censored for OS at 58 months. As mentioned above, in the CheckMate-067 trial in advanced melanoma patients, ipilimumab-nivolumab treated patients had a mPFS of 11.5 months, an ORR of 57.6% and a mOS of more than 60 months [29].
4.6. Patient 6: BRCA2 (c.4936_4939del, p.Glu1646Glnfs*23): Transitional Cell Carcinoma
At the age of 63, patient 6 was diagnosed with stage IV TCC. First-line therapy with cisplatin-gemcitabine led to PR. At disease progression, he received the PARP-inhibitor olaparib. This treatment was discontinued after two months because of PD. Atezolizumab was initiated and is currently, after 10 months, ongoing with a PR (−51% irRECIST). At 7 months of therapy, his disease progressed. He was censored for OS at 15 months. In the IMvigor 211 trial comparing atezolizumab with chemotherapy in platinum-pretreated patients, patients in the ICPI arm had a mPFS of 2 months, an ORR of 13.4% and a mOS of 8.6 months [30,31].
4.7. Patient 7: BRCA1 (c.212+3A>G, p.?): Transitional Cell Carcinoma
At the age of 57, patient 7 underwent neoadjuvant chemotherapy and radical cystoprostatectomy for a muscle invasive TCC. Several months later pembrolizumab was initiated for diffuse metastatic spread (PD-L1 combined positive score > 10), leading to a CR. Therapy is currently ongoing, 18 months after start. In the corresponding phase 3 trial, previously untreated patients with advanced urothelial carcinoma who were treated with pembrolizumab in monotherapy had an ORR of 30.3% and a mOS 15.6 months (KEYNOTE-361) [32].
5. Discussion
We report on 7 patients with a germline BRCA1, BRCA2 or CHEK2 alteration with distinct metastatic malignancies displaying favorable responses on ICPIs in terms of RR, number of CRs and mPFS. In a group of 13 patients for whom genetic testing did not show evidence of germline alterations, outcomes were more modest. Additionally, the outcomes of patients with DDR alterations are generally more favorable than outcomes demonstrated in the phase 3 trials investigating the respective ICPI agent in each cancer type.
Our findings further support the concept that pre-existing germline alterations in DNA repair systems could enhance response to ICPIs. Impaired DDR pathways lead to higher levels of intratumor genomic instability, more potential for neoantigens and higher immunogenicity [3,8]. Additionally, accumulating damaged DNA fragments in the cytosol of cells with impaired DDR pathways activate the type I interferon response [33]. The resulting stimulation of the innate antitumor immune response is correlated with durable responses to ICPIs [34]. Of the main pathways of DNA repair mechanisms (base excision repair, nucleotide excision repair, mismatch repair, homologous recombination (including Fanconi anemia) and nonhomologous end joining), remarkable responses to ICPI have been demonstrated in MMR deficient tumors [17,18]. In 2017, pembrolizumab received FDA approval for MMR deficient solid tumors, regardless of primary tumor site [19]. Furthermore, pembrolizumab recently received FDA approval for tumors with high TMB (≥10 mut/Mb) [35]. A noncomprehensive list of reports showing similar findings in other DDR pathways is shown in Table 4. Evidence appears strongest and most concordant in bladder carcinoma, followed by RCC, and is more conflicting in prostate carcinoma and melanoma. However, most publications report a positive correlation between the presence of somatic DDR alterations and clinical outcome in a variety of different cancers. Despite the consistency of this association, one needs to take into account that the level of evidence is fairly low, since most publications encompass case reports or small case series.
Table 4.
Literature evidence regarding the effect of DDR disease-associated variants on the response to ICPIs.
| Authors | n | Somatic or Germline | ICPI | Concordant with DDR Hypothesis | Findings |
|---|---|---|---|---|---|
| Pan-cancer | |||||
| Zhou et al. [36] | 141 | Somatic | ICPI in monotherapy or combination | Yes | Patients with somatic BRCA2 alterations had improved OS (median OS 31 vs. 18 months, p = 0.02). Patients with BRCA2 altered tumors with low TMB had comparable OS with patients with high TMB tumors (median OS 44 vs. 41 months; p < 0.001). |
| Metastatic urothelial carcinoma | |||||
| Teo et al. [37] | 60 | Somatic | Anti-PD(L)1 antibodies in monotherapy | Yes | Response rate of 80% in patients with deleterious DDR alteration (n = 18), 54% in patients with DDR VUS (n = 15) and 19% in wild type tumors (n = 17) (p < 0.001). Median PFS not reached, 15.8 and 2.9 months, respectively, and median OS not reached, 23.0 and 9.3 months, respectively. |
| Joshi et al. [38] | 53 | Somatic | Anti-PD(L)1 antibodies | Yes | DDR alterations, somatic or germline, were associated with trend towards longer OS. Increased number of DDR alterations were associated with trend for higher ORR. |
| Powles et al. [39] | 559 | Somatic | Avelumab versus BSC | Yes | DDR alterations were associated with improved OS when treated with ICPI (HR 0.65; 95%CI 0.504–0.847) compared to BSC. Association was not observed in DDR wild type tumors (HR 0.89; 95%CI 0.489–1.612). |
| Metastatic renal cell carcinoma | |||||
| Labriola et al. [40] | 34 | Somatic | Nivolumab, ipilimumab—nivolumab or pembrolizumab | Yes | 68.8% of patients with disease control (n = 16) had enrichment in somatic DDR alterations vs. 38.9% of patients with PD (n = 18) (p = 0.03). |
| Ged et al. [41] | 107 | Germline (27%) Somatic (73%) |
Anti-PD1 monotherapy (68%) and combination ICPIs (32%) | Yes | 19 patients had deleterious DDR alterations and 88 patients wild type/VUS DDR. Deleterious DDR was associated with improved OS on ICPI (p = 0.049). This effect was not seen in control group of 118 patients treated with angiogenesis inhibitors. |
| Metastatic malignant melanoma | |||||
| Hugo et al. [42] | 469 | Somatic | Nivolumab and pembrolizumab | Yes | 28 patients had somatic BRCA2 mutation. BRCA2 mutations were significantly more frequent in responders compared to nonresponders (OR 6.2, p = 0.002). |
| Amaral et al. [43] | 4 | Germline | Combination ICPIs | No | None of the patients (two with BRCA2, one with BAP1 and one with PALB2 germline alteration) responded well. |
| Metastatic ovarian carcinoma | |||||
| Matsuo et al. [44] | 6 | Germline | Nivolumab | Yes | In these six heavily pretreated patients with germline BRCA1/2 mutations, ORR was 67%. |
| Liu et al. [45] | 134 | Somatic of germline | ICPIs | No | 31 patients had deleterious somatic or germline BRCA1/2 mutations. No association was found between mutation status and response to ICPIs. |
| Metastatic castration resistant prostate carcinoma | |||||
| Boudadi et al. [46] | 15 | Somatic | Ipilimumab—nivolumab | Yes | Six out of these 15 patients with an aggressive subtype of AR-V7 expressing prostate carcinoma carried a somatic DDR mutation (three BRCA2, two in ATM and one in ERCC4) and showed improved PFS (HR 0.31, p = 0.01) compared to the nine patients without DDR mutations. |
| Markowski et al. [47] | 3 | One germline, two somatic | PD-1 inhibitors | Yes | Three patients with inactivating BRCA2 or ATM mutations showed profound and durable response to ICPI. |
| Antonarakis et al. [48] | 153 | Somatic | Pembrolizumab | No | 29 patients with somatic DDR mutations, response to ICPI was not associated with mutational status. |
| Case reports in other tumors | |||||
| Pang et al. [49] | 1 | Germline | Pembrolizumab | Yes | Patient with metastatic PDAC with germline and somatic PALB2 mutation, had a durable PR. |
| Boeck et al. [50] | 1 | Germline | Pembrolizumab | No | Patient with metastatic PDAC, with a germline PALB2 mutation, had PD as best response. |
| Dizon et al. [51] | 1 | Germline and somatic | Pembrolizumab | Yes | Patient with high grade Mullerian adenocarcinoma, with germline BRCA1 and somatic BRCA2 mutation, had a CR. |
| Santin et al. [52] | 1 | Somatic | Nivolumab | Yes | Patient with hypermutated endometrial tumor with a POLE mutation had a profound PR. |
| Momen et al. [53] | 1 | Germline | Pembrolizumab | Yes | Patient with xeroderma pigmentosum (germline XPC mutation) and metastatic angiosarcoma (somatic POLE mutation and high TMB) achieved PR. |
| Johanns et al. [54] | 1 | Germline | Pembrolizumab | Yes | Patient with a hypermutated glioblastoma and germline POLE mutation showed evidence of clinical and immunological response to ICPI. |
Note: for this literature overview, we did not consider studies on the impact of MMR because this has already been studied intensively and ICPI have been approved by the FDA in a tumor-agnostic way.
Limitations of this study are the retrospective nature of the data and small patient number, subject to recall bias. Relapses were not routinely confirmed through biopsy. Molecular data about TMB or somatic DDR alterations were not available and therefore extrapolated from germline data. However, it is unlikely that this is a source of inconsistency.
6. Conclusions
Taken together, our data further support the existing evidence for a potential role of germline DDR disease-associated variants as predictive biomarkers for ICPI response, which is worthwhile to be further studied prospectively in several tumor types, particularly in bladder carcinoma and RCC. Our data also suggest that patients with metastatic cancers harboring germline DDR mutations should be offered ICPIs, and that larger randomized clinical trials comparing standard of care with ICPI in first line should be conducted in this rare population of cancer patients.
Abbreviations
mRCC = metastatic renal cell carcinoma; ICPI = immune checkpoint inhibitor; OS = overall survival; TMB = tumor mutational burden; PD-1 = programmed death receptor 1; PD-L1 = programmed death receptor ligand 1; VUS = variant of unknown significance; DDR = DNA damage response; mUC = metastatic urothelial carcinoma; ORR = objective response rate; BSC = best supportive care; ERCC4 = Excision Repair Cross-Complementation Group 4; ATM = Ataxia Telangiectasia Mutated; PDAC = pancreatic ductal adenocarcinoma; PALB2 = Partner and Localizer of Breast Cancer 2; PR = partial response; PD = progressive disease; CR = complete remission; HRR = homologous recombinational repair; MSI = microsatellite instability; PFS = progression-free survival; mCRPC = metastatic castration resistant prostate cancer; POLE = DNA Polymerase Epsilon; MSH6 = mutS homolog 6.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/curroncol28050280/s1, Table S1: Characteristics of included patients.
Author Contributions
Conceptualization, L.K. and B.B.; data curation, L.K. and B.B.; formal analysis, L.K. and B.B.; funding acquisition, B.B.; methodology, L.K., H.B. and B.B.; project administration, B.B.; resources, B.B.; supervision, B.B.; visualization, L.K.; writing—original draft, L.K. and B.B.; writing—review and editing, L.K., O.B., K.P., P.R.D., H.B., P.C., E.R., Y.V.H., M.A., M.B., P.S. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
L.K. received a grant from “Kom op tegen Kanker” (Stand up to Cancer), the Flemish cancer society. E.R. received an unrestricted research grant from Pfizer and Ipsen. B.B. has received grants from Research Foundation-Flanders (FWO).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by Ethics Committee Research UZ/KU Leuven (registration number S53479/S63833).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in Table S1.
Conflicts of Interest
P.R.D. received honoraria from Astra-Zeneca, Sanofi, Bayer, MSD, Roche, BMS, and Merck KGaA and institutional research funding from Pfizer. The others declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forschner A., Battke F., Hadaschik D., Schulze M., Weißgraeber S., Han C.T., Kopp M., Frick M., Klumpp B., Tietze N., et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma—Results of a prospective biomarker study. J. Immunother. Cancer. 2019;7:180. doi: 10.1186/s40425-019-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S., et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizvi H., Sanchez-Vega F., La K., Chatila W., Jonsson P., Halpenny D., Plodkowski A., Long N., Sauter J.L., Rekhtman N., et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J. Clin. Oncol. 2018;36:633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strickland K.C., Howitt B.E., Shukla S.A., Rodig S., Ritterhouse L.L., Liu J.F., Garber J.E., Chowdhury D., Wu C.J., D’Andrea A.D., et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587–13598. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J., Lu L.Y., Yu X. The role of BRCA1 in DNA damage response. Protein Cell. 2010;1:117–123. doi: 10.1007/s13238-010-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P., Ho T.S., et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 9.Yarchoan M., Hopkins A., Jaffee E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z., Zhao J., Wang G., Zhang F., Zhang Z., Zhang F., Zhang Y., Dong H., Zhao X., Duan J., et al. Comutations in DNA damage response pathways serve as potential biomarkers for immune checkpoint blockade. Cancer Res. 2018;78:6486–6496. doi: 10.1158/0008-5472.CAN-18-1814. [DOI] [PubMed] [Google Scholar]
- 11.Goodman A.M., Kato S., Bazhenova L., Patel S.P., Frampton G.M., Miller V., Stephens P.J., Daniels G.A., Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mei P., Freitag C.E., Wei L., Zhang Y., Parwani A.V., Li Z. High tumor mutation burden is associated with DNA damage repair gene mutation in breast carcinomas. Diagn. Pathol. 2020;15:50. doi: 10.1186/s13000-020-00971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh A.R., He Y., Hong T.S., Corcoran R.B., Clark J.W., Ryan D.P., Zou L., Ting D.T., Catenacci D.V., Chao J., et al. Analysis of DNA Damage Response Gene Alterations and Tumor Mutational Burden Across 17,486 Tubular Gastrointestinal Carcinomas: Implications for Therapy. Oncologist. 2019;24:1340–1347. doi: 10.1634/theoncologist.2019-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chae Y.K., Anker J.F., Bais P., Namburi S., Giles F.J., Chuang J.H. Mutations in DNA repair genes are associated with increased neo-antigen load and activated T cell infiltration in lung adenocarcinoma. Oncotarget. 2018;9:7949–7960. doi: 10.18632/oncotarget.23742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatzinikolaou G., Karakasilioti I., Garinis G.A. DNA damage and innate immunity: Links and trade-offs. Trends Immunol. 2014;35:429–435. doi: 10.1016/j.it.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Barber G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015;15:760–770. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marabelle A., Le D.T., Ascierto P.A., Di Giacomo A.M., de Jesus-Acosta A., Delord J.P., Geva R., Gottfried M., Penel N., Hansen A.R., et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/ mismatch repair–deficient cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus L., Lemery S.J., Keegan P., Pazdur R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 2019;25:3753–3758. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 20.Wolchok J.D., Hoos A., O’Day S., Weber J.S., Hamid O., Lebbé C., Maio M., Binder M., Bohnsack O., Nichol G., et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 21.Cupp M.A., Cariolou M., Tzoulaki I., Aune D., Evangelou E., Berlanga-Taylor A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18 doi: 10.1186/s12916-020-01817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roussel E., Kinget L., Verbiest A., Debruyne P.R., Baldewijns M., Van Poppel H., Albersen M., Beuselinck B. C-reactive protein and neutrophil-lymphocyte ratio are prognostic in metastatic clear-cell renal cell carcinoma patients treated with nivolumab. Urol. Oncol. Semin. Orig. Investig. 2021;39:239.e17–239.e25. doi: 10.1016/j.urolonc.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J.T., Miner T.J., Vezeridis M.P. Is the neutrophil-to-lymphocyte ratio a useful prognostic indicator in melanoma patients? Melanoma Manag. 2020;7:MMT47. doi: 10.2217/mmt-2020-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beulque Y., Deleu A.L., Punie K., De Wever L., Baldewijns M., Caruso S., Couchy G., Zucman-Rossi J., Beuselinck B. Immunogenomics of Metastatic Clear-Cell Renal Cell Carcinoma: Remarkable Response to Nivolumab in a Patient With a Pathogenic Germ Line BRCA1 Mutation. Clin. Genitourin. Cancer. 2019;17:e909–e912. doi: 10.1016/j.clgc.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Ferris R.L., Blumenschein G., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K., Kasper S., Vokes E.E., Even C., et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 29.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.-J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 30.Powles T., Durán I., van der Heijden M.S., Loriot Y., Vogelzang N.J., De Giorgi U., Oudard S., Retz M.M., Castellano D., Bamias A., et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 31.Van der Heijden M.S., Loriot Y., Durán I., Ravaud A., Retz M., Vogelzang N.J., Nelson B., Wang J., Shen X., Powles T. Atezolizumab Versus Chemotherapy in Patients with Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: A Long-term Overall Survival and Safety Update from the Phase 3 IMvigor211 Clinical Trial. Eur. Urol. 2021;80:7–11. doi: 10.1016/j.eururo.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Powles T., Csőszi T., Özgüroğlu M., Matsubara N., Géczi L., Cheng S.Y.S., Fradet Y., Oudard S., Vulsteke C., Morales Barrera R., et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:931–945. doi: 10.1016/S1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 33.Corrales L., Gajewski T.F. Molecular pathways: Targeting the Stimulator of Interferon Genes (STING) in the immunotherapy of cancer. Clin. Cancer Res. 2015;21:4774–4779. doi: 10.1158/1078-0432.CCR-15-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun W., Zhang Q., Wang R., Li Y., Sun Y., Yang L. Targeting DNA Damage Repair for Immune Checkpoint Inhibition: Mechanisms and Potential Clinical Applications. Front. Oncol. 2021;11:648687. doi: 10.3389/fonc.2021.648687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samstein R.M., Lee C.H., Shoushtari A.N., Hellmann M.D., Shen R., Janjigian Y.Y., Barron D.A., Zehir A., Jordan E.J., Omuro A., et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019;51:202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Z., Li M. Evaluation of BRCA1 and BRCA2 as Indicators of Response to Immune Checkpoint Inhibitors. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teo M.Y., Seier K., Ostrovnaya I., Regazzi A.M., Kania B.E., Moran M.M., Cipolla C.K., Bluth M.J., Chaim J., Al-Ahmadie H., et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. J. Clin. Oncol. 2018;36:1685–1694. doi: 10.1200/JCO.2017.75.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi M., Grivas P., Mortazavi A., Monk P., Clinton S.K., Sue-Ann Woo M., Holder S.L., Drabick J.J., Yin M. Alterations of DNA damage response genes correlate with response and overall survival in anti-PD-1/PD-L1-treated advanced urothelial cancer. Cancer Med. 2020;9:9365–9372. doi: 10.1002/cam4.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powles T.B., Loriot Y., Bellmunt J., Sternberg C.N., Sridhar S., Petrylak D.P., Tambaro R., Dourthe L.M., Alvarez-Fernandez C., Aarts M., et al. 699O Avelumab first-line (1L) maintenance + best supportive care (BSC) vs BSC alone for advanced urothelial carcinoma (UC): Association between clinical outcomes and exploratory biomarkers. Ann. Oncol. 2020;31:S552–S553. doi: 10.1016/j.annonc.2020.08.771. [DOI] [Google Scholar]
- 40.Labriola M.K., Zhu J., Gupta R., McCall S., Jackson J., Kong E.F., White J.R., Cerqueira G., Gerding K., Simmons J.K., et al. Characterization of tumor mutation burden, PD-L1 and DNA repair genes to assess relationship to immune checkpoint inhibitors response in metastatic renal cell carcinoma. J. Immunother. Cancer. 2020;8:319. doi: 10.1136/jitc-2019-000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ged Y., Chaim J.L., DInatale R.G., Knezevic A., Kotecha R.R., Carlo M.I., Lee C.H., Foster A., Feldman D.R., Teo M.Y., et al. DNA damage repair pathway alterations in metastatic clear cell renal cell carcinoma and implications on systemic therapy. J. Immunother. Cancer. 2020;8:230. doi: 10.1136/jitc-2019-000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hugo W., Zaretsky J.M., Sun L., Song C., Moreno B.H., Hu-Lieskovan S., Berent-Maoz B., Pang J., Chmielowski B., Cherry G., et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amaral T., Schulze M., Sinnberg T., Nieser M., Martus P., Battke F., Garbe C., Biskup S., Forschner A. Are pathogenic germline variants in metastatic melanoma associated with resistance to combined immunotherapy? Cancers. 2020;12:1101. doi: 10.3390/cancers12051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuo K., Spragg S.E., Ciccone M.A., Blake E.A., Ricker C., Pham H.Q., Roman L.D. Nivolumab use for BRCA gene mutation carriers with recurrent epithelial ovarian cancer: A case series. Gynecol. Oncol. Rep. 2018;25:98–101. doi: 10.1016/j.gore.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y.L., Selenica P., Zhou Q., Iasonos A., Callahan M., Feit N.Z., Boland J., Vazquez-Garcia I., Mandelker D., Zehir A., et al. BRCA Mutations, Homologous DNA Repair Deficiency, Tumor Mutational Burden, and Response to Immune Checkpoint Inhibition in Recurrent Ovarian Cancer. JCO Precis. Oncol. 2020;4:665–679. doi: 10.1200/PO.20.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boudadi K., Suzman D.L., Anagnostou V., Fu W., Luber B., Wang H., Niknafs N., White J.R., Silberstein J.L., Sullivan R., et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget. 2018;9:28561–28571. doi: 10.18632/oncotarget.25564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markowski M.C., Shenderov E., Eisenberger M.A., Kachhap S., Pardoll D.M., Denmeade S.R., Antonarakis E.S. Extreme responses to immune checkpoint blockade following bipolar androgen therapy and enzalutamide in patients with metastatic castration resistant prostate cancer. Prostate. 2020;80:407–411. doi: 10.1002/pros.23955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antonarakis E.S., Piulats J.M., Gross-Goupil M., Goh J., Ojamaa K., Hoimes C.J., Vaishampayan U., Berger R., Sezer A., Alanko T., et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: Multicohort, open-label phase II KEYNOTE-199 study. J. Clin. Oncol. 2020;38:395–405. doi: 10.1200/JCO.19.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pang X., Qian J., Jin H., Zhang L., Lin L., Wang Y., Lei Y., Zhou Z., Li M., Zhang H. Durable benefit from immunotherapy and accompanied lupus erythematosus in pancreatic adenocarcinoma with DNA repair deficiency. J. Immunother. Cancer. 2020;8:e000463. doi: 10.1136/jitc-2019-000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boeck S., Mehraein Y., Ormanns S., Kruger S., Westphalen C.B., Haas M., Jung A., Kirchner T., Heinemann V. Mismatch-repair-deficient metastatic pancreatic ductal adenocarcinoma with a germline PALB2 mutation: Unusual genetics, unusual clinical course. Ann. Oncol. 2017;28:438–439. doi: 10.1093/annonc/mdw564. [DOI] [PubMed] [Google Scholar]
- 51.Dizon D.S., Dias-Santagata D., Bregar A., Sullivan L., Filipi J., DiTavi E., Miller L., Ellisen L., Birrer M., DelCarmen M. Complete Remission Following Pembrolizumab in a Woman with Mismatch Repair-Deficient Endometrial Cancer and a Germline BRCA1 Mutation. Oncologist. 2018;23:650–653. doi: 10.1634/theoncologist.2017-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santin A.D., Bellone S., Buza N., Choi J., Schwartz P.E., Schlessinger J., Lifton R.P. Regression of chemotherapy-resistant polymerase ϵ (POLE) ultra-mutated and MSH6 hyper-mutated endometrial tumors with nivolumab. Clin. Cancer Res. 2016;22:5682–5687. doi: 10.1158/1078-0432.CCR-16-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Momen S., Fassihi H., Davies H.R., Nikolaou C., Degasperi A., Stefanato C.M., Dias J.M.L., Dasgupta D., Craythorne E., Sarkany R., et al. Dramatic response of metastatic cutaneous angiosarcoma to an immune checkpoint inhibitor in a patient with xeroderma pigmentosum: Whole-genome sequencing aids treatment decision in end-stage disease. Cold Spring Harb. Mol. Case Stud. 2019;5:a004408. doi: 10.1101/mcs.a004408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johanns T.M., Miller C.A., Dorward I.G., Tsien C., Chang E., Perry A., Uppaluri R., Ferguson C., Schmidt R.E., Dahiya S., et al. Immunogenomics of hypermutated glioblastoma: A patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. 2016;6:1230–1236. doi: 10.1158/2159-8290.CD-16-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in Table S1.