Abstract
Topically applied vasoconstrictor is a new strategy to prevent oral mucositis and alopecia, two complications of chemotherapy and stem-cell transplant. We sought to determine whether mice treated with topical vasoconstrictor minutes before chemotherapy to suppress L1210 leukemia would develop a vasoconstrictor-induced L1210 cell sanctuary, and with it, significantly worse survival outcomes. B6D2F1 mice received 104 mouse L1210 leukemia cells via retro-orbital intravenous injection and were then divided into treatment groups, which included: i) no further treatment, ii) a single, sub-curative, intraperitoneal dose of cyclophosphamide (90 μg/gm bw) 24 hr after L1210 cell inoculation, iii) topical epinephrine (25-400 mM) to clipped dorsal backs 20 min before cyclophosphamide, or iv) orotopical phenylephrine (16-130 mM), epinephrine (10 mM) or norepinephrine (25 mM) 20 min before cyclophosphamide. All mice were then followed until day of death. Differences in median survival time and percent survival between mice receiving cyclophosphamide alone and mice treated with either orotopical phenylephrine, epinephrine or norepinephrine; or topical epinephrine before cyclophosphamide were not significantly different. A discernible leukemia sanctuary was not created by topical vasoconstrictor treatment prior to chemotherapy; there was no significant difference in leukemia progression between untreated mice and those treated with either orotopical or topical vasoconstrictor before chemotherapy. We have opened a Phase I/IIa dose escalation trial to evaluate the safety and efficacy of orotopical phenylephrine in preventing oral mucositis in subjects undergoing hematopoietic stem cell transplant conditioning with cyclophosphamide plus total body irradiation. This could provide a cost-effective and convenient method to prevent oral mucositis.
Keywords: epinephrine, phenylephrine, Cytoxan, cyclophosphamide, L1210
Introduction
Cancer therapy, while life-saving in the majority of cases, has a myriad of side effects. Oral mucositis and alopecia are two common complications of cancer therapy; they can be both costly1 and a strong disincentive to participating in chemotherapy2. The Palifermin and Dignicap cold cap alternatives are both costly and limited in their efficacy. Though tissue chilling (cryotherapy) has been used to prevent chemotherapy adverse events, this approach has been limited in duration and cumbersome, and it is uncomfortable for patients.
Oral mucositis causes significant morbidity, including pain, susceptibility to infection, poor nutrition, and rarely, upper airway compromise, as well as significant increase in cost of therapy1. Oral mucositis occurs in: i) up to 80% of patients who receive high-dose chemotherapy in preparation for hematopoietic stem cell transplant (HSCT), ii) ~95% of HSCT patients who receive the cyclophosphamide + total body irradiation conditioning regimen, and iii) leads to prolonged hospital stays and increased cost of therapy due to frequent need of narcotics and parenteral nutrition1,3. An existing vasoconstriction approach (i.e. cryotherapy) has shown modest success in decreasing oral mucositis in a limited number of chemotherapy regimens, but the use of oral cryotherapy is limited due to a number of practical considerations4.
Alopecia, while not directly leading to mortality, influences patients’ cancer therapy compliance and affects their psychological well-being5. Of 72 scored side effects of cancer therapy6, alopecia was ranked the second most distressing by patients. One study found that 8% of women are at risk of forgoing chemotherapy because of the alopecia risk7. An existing vasoconstriction approach (i.e., cold caps) has been used since the 1970s, but its efficacy: i) is very modest, ii) is limited to short half-life chemotherapy drugs, and iii) can be very uncomfortable for patients8. Use of scalp cooling devices are also either labor intensive for nursing staff (due to need to replace caps frequently) or are highly restrictive for patients (due to need to be connected to a scalp cooling machine9.
A new strategy to suppress skin and mucosal cancer therapy side effects10 has shown that neonate rats treated with topical epinephrine or norepinephrine prior to radiation or chemotherapy had complete prevention of alopecia in the topical treatment area and were denuded elsewhere. In addition, mice and hamsters that received orotopical phenylephrine, norepinephrine or epinephrine minutes prior to radiation had up to 100% suppression of oral mucositis, including complete restoration of weight gain and substantially decreased inflammation of the oral mucosa11. A strategy to use topically applied vasoconstrictor to constrict those vessels located about 1 mm beneath the skin or mucosa (i.e., the first encountered below the surface) would have no cold discomfort to the patient’s mouth or scalp, and vasoconstriction could be maintained for extended periods through reapplication, allowing use with chemotherapeutic drugs with longer half-lives.
One concern expressed about the cryotherapy/vasoconstriction approach is that by suppressing delivery of chemotherapy to vasoconstricted tissue (i.e., that anatomically undefined tissue that is chilled), one may provide a sanctuary of reduced chemotherapy concentration, and by so doing, lead to increased risk of cancer relapse from scalp or mucosal metastases, particularly with hematopoietic malignancies12-14.
In this report, we describe one step in the process of determining whether any discernible sanctuary for blood-borne leukemia cells is created in mice treated with topical vasoconstrictor by using the L1210 leukemia model15. We quantitatively assess whether cyclophosphamide-conferred increased survival is affected by prior topical treatment of skin or oral cavity with the same doses and vasoconstrictor currently being used in a human clinical trial of this technology16.
Materials and Methods
Materials
(±)-epinephrine HCl, (R)-phenylephrine HCl, Nile Red dye, cyclophosphamide, erioglaucine blue dye and solvents for drug delivery were all obtained from Sigma (St. Louis, MO). L-norepinephrine-HCl was obtained from ChemPacific (Baltimore, MD). Female B6D2F1 mice (20-21 g) were obtained from Jackson Labs (Bar Harbor, Maine), and female ICR mice (20-22g) were obtained from Harlan (Indianapolis, IN). Mice were maintained on 12 hr light/dark cycle and provided ad lib water and lab chow. All animal procedures were conducted according to a protocol (#M02632) approved by the University of Wisconsin Institutional Animal Care and Use Committee.
L1210 cell line
Tumorigenic L1210 cells were propagated in B6D2F1 mice. Cells were injected intravenously (IV) and L1210 cell-laden spleens were harvested and used for spleen brei preparation. For brei preparation, spleens from moribund mice, typically 7-8 days following IV injection of 105 L1210 cells, were sterilely removed, weighed, minced with scissors, and then briefly homogenized with five up and down passes with a Teflon Potter-Elvehjem homogenizer. The homogenate was passed through a 20 g needle eight times, and a diluted aliquot was counted using a hemocytometer. Because control spleens weighed 26% of L1210 spleens, L1210 cells were assumed to represent 74% of the total spleen brei cell counts. Spleen brei cells were stored frozen in liquid nitrogen in 10% DMSO media. Thawed cells were washed and diluted in phosphate-buffered saline prior to mouse injections.
L1210 Leukemia Mouse Survival Assay
Anesthetized B6D2F1 mice were positioned in a nose-cone that supplied 2-3% isoflurane, the eyeball was manually displaced to the side, and a 200 μl bolus injection of phosphate-buffered saline containing L1210 cells was administered using a 28 g needle and 0.5 ml syringe to the retro-orbital sinus. Mice were observed twice a day (AM and PM) and dead mice were recorded and removed from cages.
For irradiation, 24 hr after L1210 injections, mice in plastic cages were positioned within an x-ray irradiator, and a single 5.5 Gy dose of x-rays was administered.
For Cytoxan injections, 24 hr after L1210 injections, mice were individually weighed, and volumes of a 12 mg/ml Cytoxan stock solution in water were administered by intraperitoneal injection with a 28 g needle and 0.5 ml syringe.
Topical Vasoconstrictor Impact on L1210 Leukemia Therapy
Skin:
Twenty four hr after the L1210 cell inoculation (per above), mice were anaesthetized with 2-3% isoflurane, their dorsal backs were clipped with a clipper, and 45 μl of a 70:30 (ethanol:water) formulation containing epinephrine was applied to an ~1.5 cm2 area of dorsal back skin. Twenty min later, each mouse was weighed and an aliquot of a 12 mg/ml Cytoxan solution in water was administered by intraperitoneal injection.
Oral Cavity:
Twenty four hr after the L1210 cell inoculation, mice were anesthetized with 2-3% isoflurane and 40 ul of an optimized oral delivery vehicle17 containing phenylephrine, norepinephrine or epinephrine was applied to the tongue and oral cavity of anesthetized mice with a Pipetman with the mouse head tilted forward; 20 min later, mice were weighed and received a single intraperitoneal Cytoxan injection.
Topical Vasoconstrictor Exclusion of Blue Dye from Skin
Dorsal back coats of female ICR mice, which received 2-3% isoflurane anesthesia, were first clipped and then Nair was applied to the skin. After 5-6 min, the Nair and residual coat were washed off under a warm water stream. Skin was briefly scrubbed with soap and warm water, and sleeping mice were positioned under a lamp to dry off. One day later, 20 ul of epinephrine in a topical 70:30 (ethanol:water) delivery vehicle was carefully applied with a P20 Pipetman to an ~1 cm2 area of skin. Skin blanch was photographed in some mice. At 20 min – 3 hr after topical epinephrine application, a 200 ul bolus injection of erioglaucine blue dye (2% in phosphate-buffered saline) was injected IV into the retro-orbital sinus. Five min later, backs of sleeping mice were photographed, and exclusion (0-100%) of the systemic dye from the patch of vasoconstrictor-treated skin was scored visually.
Statistical Analysis
Graphpad Prizm software, was used for plotting survival and for analysis of statistical differences between treatment groups.
Results
Propagation of the L1210 leukemia cell line
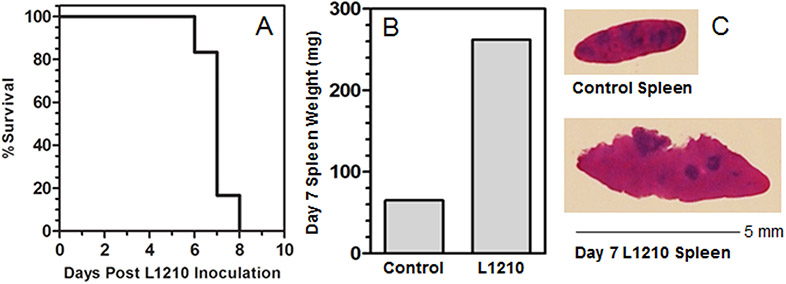
Skipper and colleagues15,18 established the mouse L1210 leukemia as a core tumorigenesis model that continues to be widely used for studies of tumor growth and drug development. Propagation of the L1210 cells within mice, and the harvest of enlarged L1210 cell-laden spleens (68 mg Control versus 275 mg L1210 spleen weights; Fig. 1B, 1C) for tumorigenic seed stock, yielded a leukemogenic cell stock suitable for L1210 growth and mouse death studies (Fig. 1A) that closely replicated Skipper’s results (15) in which IV inoculation with 105 L1210 cells resulted in 50% mouse survival on Day 7. Mouse death was reported to represent a tumor load of 109 L1210 cells per mouse15.
Figure 1.
(A) Survival of mice after IV bolus inoculation of 105 L1210 cells. Eight mice received a 200 μl IV inoculation to the retro-orbital sinus on Day 0 and were observed daily. (B) On day 7-8 post-inoculation, moribund mice were euthanized and spleens were removed by sterile dissection and weighed. (C) Portions of control and L1210 spleens were processed for histology and spleen brei preparation.
Screening of radiation- or chemotherapy-induced increased survival
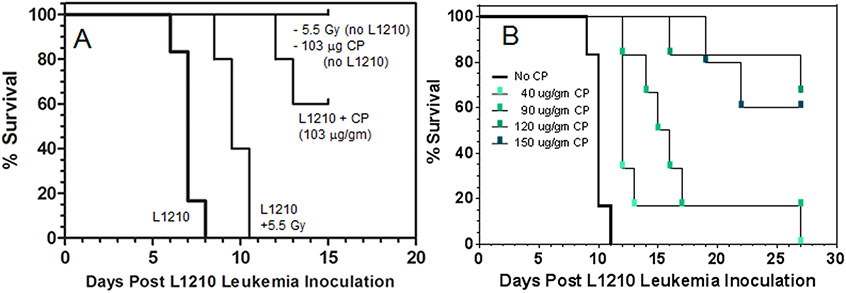
To determine whether topically applied vasoconstrictor would negatively impact the therapeutic efficacy of either radiation- or chemotherapy-induced L1210 cell kill, we performed an initial screen to identify radiation or cyclophosphamide doses that demonstrably affected survival of mice following L1210 inoculation. B6D2F1 mice were inoculated IV with 105 L1210 cells, and 24 hr later, the mice were exposed to either: i) nothing, ii) 5.5 Gy of whole-body x-rays, or iii) 103 μg/gm bw of cyclophosphamide (Fig. 2A). Mouse deaths were logged twice per day (AM, PM) to maximize resolution between treatment groups of “% Survival.” The 5.5 Gy x-ray dose was reported19 to be the “LD0” dose above which mice would begin dying from radiation-induced toxicities. The 5.5 Gy dose conferred a 2.5 day increase in survival time. Though there was a 2.5 day prolongation in survival time, this window is operationally small, and a change in survival time due to topically applied vasoconstrictor would be difficult to establish with statistical significance. Doses of whole-body radiation greater than 5.5 Gy become lethal in mice19 so the irradiation model was abandoned.
Figure 2.
(A) Preliminary screen of the impact of whole-body irradiation (5.5 Gy) or intraperitoneal cyclophosphamide (CP) chemotherapy (103 μg/gm bw) upon % survival and survival time following IV inoculation of mice with bolus of 105 L1210 cells. Mice received either a 5.5 Gy x-ray dose or intraperitoneal cyclophosphamide 24 hr following L1210 inoculation. (B) Cyclophosphamide dose-dependent improvement in survival in mice inoculated with 105 L1210 leukemia cells 24 hr before the indicated IP cyclophosphamide doses were administered. There were 8 mice per treatment group.
The 103 ug/gm bw cyclophosphamide dose led to a sizable increase in both survival time (>6 days) and percent survival (60%) in the L1210 mice. Previous reports using the L1210 mouse model showed that increasing doses of cyclophosphamide led to dose-dependent increases in survival times. To identify an optimum, cyclophosphamide-induced, increase in survival time at the 50% survival point, mice were injected with 105 L1210 cells, and 24 hr later they received low – high doses of cyclophosphamide by intraperitoneal injection (Fig. 2B). Cyclophosphamide at 90 ug/gm bw induced a 5.0 day increase in survival, still with <50% eventual survival. This would enable comparison of curves at 50% survival, therefore this dose was chosen for our model.
Topical Vasoconstrictor: Skin Blanch and Dye Exclusion
To determine whether a topical vasoconstrictor, here epinephrine, applied to skin created a transient, reduced chemotherapy sanctuary for L1210 leukemia cells in mice, we first needed to determine what range of topical epinephrine doses were pharmacologically active when applied to mouse skin, i.e., the range of doses that conferred a pertinent phenotype to mouse skin.
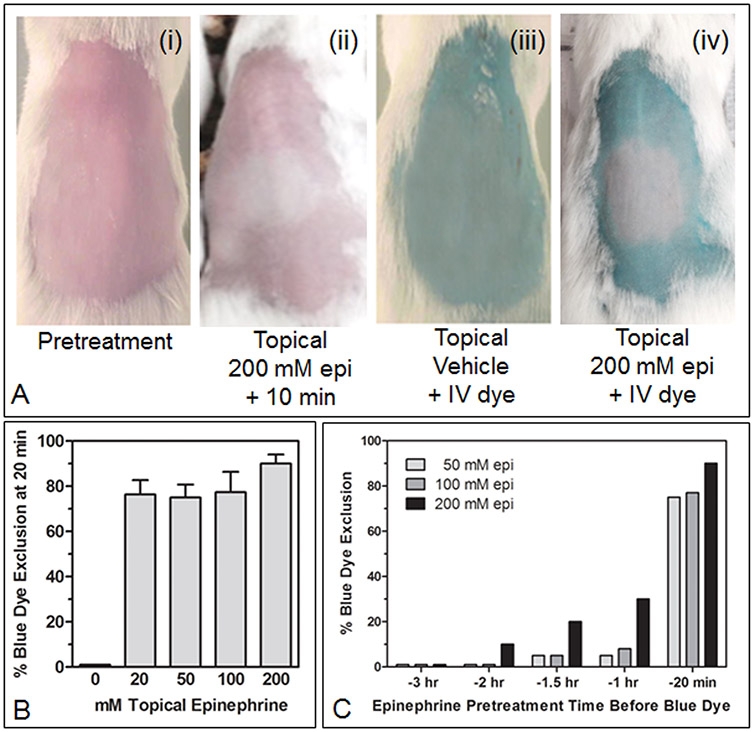
Fig. 3A shows that topical application of 200 mM epinephrine in an ethanol:water (70:30) vehicle resulted in the exclusion of both red blood (Fig. 3A, ii) as well as IV-administered blue dye (Fig. 3A, iv). Initial skin blanch was visible within one min of topical epinephrine application and was profound by 10 min. IV 2% erioglaucine dye was administered 20 min after topical epinephrine application, and percent dye exclusion from the treated skin patch was scored five min later (Fig. 3A, iv). A range of epinephrine doses (i.e., 20 μl of 20 – 200 mM) and application times (20 min – 3 hr) prior to scoring dye exclusion were tested. At 20 min following topical application, there was no significant difference in percent dye exclusion between 20 and 200 mM epinephrine (Fig. 3B); all doses were highly effective, excluding >75% of the dye relative to the surrounding, topically untreated skin. If applied an hr or more before scoring dye exclusion, dye exclusion was significantly decreased at all epinephrine concentrations, and after two hours, only the 200mM dose level of epinephrine still showed discernible dye exclusion (Fig. 3C).
Figure 3.
(A, i-iv) Dorsal backs of female ICR mice that were clipped, denuded with a topical depilatory agent, and washed with detergent and water 24 hr before topical epinephrine (epi) or vehicle treatments. (ii-iv) Twenty μl of epinephrine (200 mM) in ethanol:water (70:30) or 20 μl of vehicle alone were carefully applied over ~1 cm2 of skin using a Pipetman (over a ~30 second period). (ii) skin blanch was recorded 10 min after epi application. (iii-iv) 20 min following topical epinephrine or vehicle application, a 200 μl bolus injection of 2% erioglaucine blue dye in saline was injected IV into the retro-orbital sinus; five min later digital photos of mice were recorded. (B) Twenty min after topical epinephrine application (and five min after IV erioglaucine), backs of mice were photographed, and (C) the extent of blue dye exclusion ((i) = 100% dye exclusion, (iii) = 0% dye exclusion) in each treatment group (3 mice/group) was visually estimated. (C) Topical epinephrine formulations, at the indicated concentrations, were applied to mouse backs (3 mice/group) at the indicated times prior to photographing mice to visually estimate blue dye exclusion from topically treated skin.
The range of topical 20- 200 mM epinephrine doses that showed a discernible vasoconstriction phenotype in mouse skin, i.e., the physical exclusion of red blood or a dye molecule systemically borne in red blood, provided us with a range that could then be tested in L1210-inoculated mice to see if a significant drug-reduced sanctuary for L1210 occurred in transiently vasoconstricted mouse skin.
Topical Epinephrine Application to Skin in L1210-Inoculated Mice
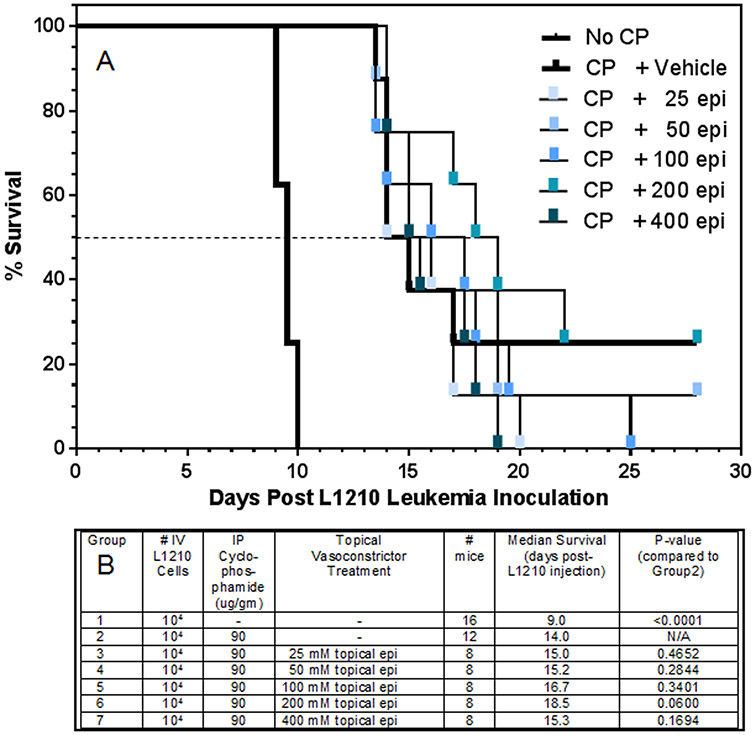
To rigorously test the vasoconstrictor-conferred sanctuary hypothesis, an expanded range of epinephrine doses (25 - 400 mM epinephrine) was applied to the clipped skin of B6D2F1 mice 20 min before systemic cyclophosphamide was administered. Each mouse had been inoculated with 104 L1210 cells 24 hr before. Mice that received only L1210 cells had a 50% survival time of 9.5 days (Fig. 4A). In the mice that received a therapeutic dose of cyclophosphamide 24 hr later, i) the cyclophosphamide dose (90 μg/gm bw) in topical vehicle-treated controls conferred a 5.5 day increase in survival time (15 days), ii) the 50% survival times in topical epinephrine + cyclophosphamide groups varied by 0 to +4 days longer, and iii) none of the differences between treatment groups in 50% survival time achieved a P value of < 0.05 (Groups 1 to 7; Fig. 4B).
Figure 4.
The impact of topically applied epinephrine to skin upon the survival of mice after IV inoculation of L1210 cells. Twenty four hr after IV L1210 inoculation (104 cells), backs of mice were clipped with a clippers, 45 μl of the indicated epinephrine formulations in an ethanol:water (70:30) vehicle was applied to an ~1.5 cm2 area of clipped skin, and 20 min later, mice were weighed and received a single IP injection of cyclophosphamide (CP, 90 μg/gm bw). Mice were observed every 12 hrs to record deaths per half day. There were 8 mice per treatment group. A dashed line showing the 50% survival point, where survival times in each group were statistically compared, is indicated.
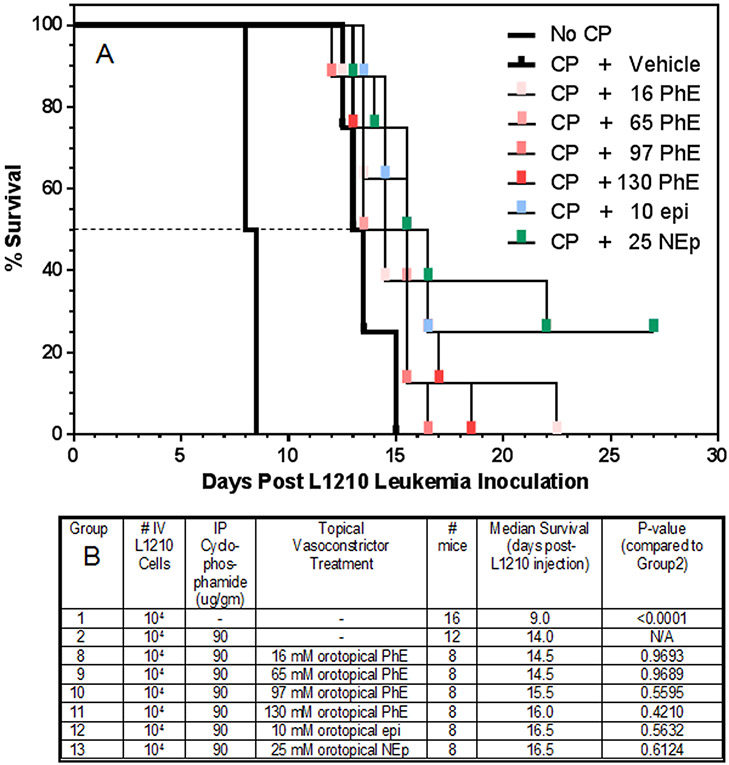
Orotopical Phenylephrine, Norepinephrine or Epinephrine in L1210-Inoculated Mice
The doses of phenylephrine (14-136 mM), norepinephrine (27 mM) or epinephrine (12 mM) that conferred 100% prevention of oral mucositis in both a hamster and a mouse model of radiation-induced oral mucositis were previously determined in work from our lab11. For these L1210 experiments, increasing doses (40 μl of 16-130 mM phenylephrine, 10 mM epinephrine or 25 mM norepinephrine) of each vasoconstrictor were applied topically to the oral cavities of anesthetized mice in an optimized oral delivery vehicle17, and after 20 min, systemic cyclophosphamide (90 μg/gm bw) was administered. Each mouse had been inoculated with 104 L1210 cells 24 hr before. Mice that received only L1210 cells had a 50% survival time of 8.5 days (Fig. 5A). In the mice that received a therapeutic dose of cyclophosphamide 24 hr later, i) the cyclophosphamide dose (90 μg/gm bw) in topical vehicle-treated controls conferred a 5.0 day increase in survival time (13.5 days), ii) the 50% survival times in all of the topical vasoconstrictor + cyclophosphamide groups varied by 0 to +1.5 days longer, and iii) none of the differences between treatment groups in 50% survival time achieved a P value of < 0.05 (Groups 8 to 13; Fig. 5B).
Figure 5.
The impact of topically applied vasoconstrictor (epinephrine, phenylephrine, or norepinephrine) to the oral cavity upon the survival of mice after IV inoculation of L1210 cells. Twenty four hr after IV L1210 inoculation (104 cells), 40 μl of the indicated vasoconstrictor formulations in an optimized orotopical delivery vehicle was applied to the tongue and oral cavity of anesthetized mice with a Pipetman, and 20 min later, mice were weighed and received a single IP injection of cyclophosphamide (CP, 90 μg/gm bw). Mice were observed every 12 hrs to record deaths per half day. There were 8 mice per treatment group. A dashed line showing the 50% survival point, where survival times in each group were statistically compared, is indicated.
Discussion
The results of our study indicate that a discernible sanctuary site for L1210 leukemia cells was not created by the topical application of a vasoconstrictor. The data show no significant difference in leukemia progression between mice treated with low-very high doses of vasoconstrictors prior to chemotherapy, either orotopically with phenylephrine, epinephrine or norepinephrine, or topically with epinephrine. If any of the topical vasoconstrictor treatments had created a significant sanctuary site for L1210 cells, we would have expected the 50% survival times of mice treated with topical vasoconstrictor prior to cyclophosphamide to be significantly lower than those treated with cyclophosphamide alone. However, in Figs. 4 and 5, median survival times of mice treated with any vasoconstrictor, even at concentrations well beyond those envisioned clinically, showed no statistical difference when compared to the median survival times of mice treated with cyclophosphamide alone.
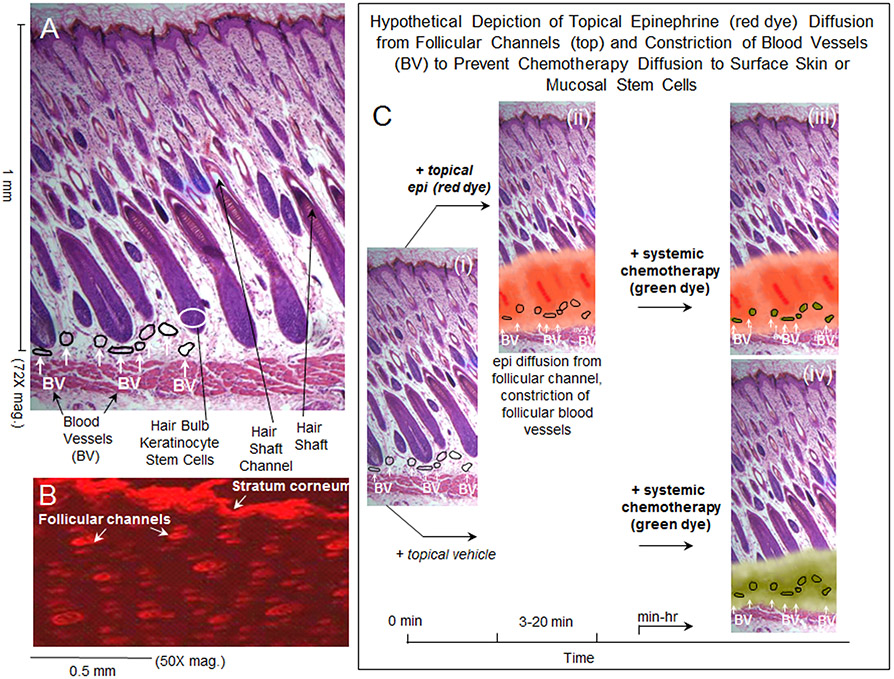
The mechanism by which topically applied vasoconstrictor is presumed to suppress toxicity to skin and mucosal stem cells is depicted in Fig. 6. Hair follicle stem cells, as well as stem cells of oral epithelium11, are located about 1 mm beneath the surface, and they are enmeshed in small blood vessels (Fig. 6A). Consistent with published studies20,21 our previous experiments10 showed rapid delivery of a topically applied red dye down hair shaft channels and diffusion of the small molecule dye around the hair bulb stem cells (Fig. 6B). This delivery and diffusion of red dye, seen as a surrogate for epinephrine delivery, is depicted in Fig. 6C, i -. iii). The retention of systemic chemotherapy within the epinephrine-vasoconstricted subcutaneous blood vessels is hypothetically depicted in Fig. 6C, iii. The delivery, and free diffusion of systemic chemotherapy to hair bulb stem cells, in skin untreated with epinephrine, is hypothetically depicted in Fig. 6C, iv. Killing of blood-borne leukemia cells should be unabated in panel 6C, iii. How the scenario in panel 6C, iii would impact killing of non-blood-borne leukemia or solid tumor cells in the 1 mm of surface tissue above the transiently vasoconstricted vessels will ultimately only be assessable by clinical trials of tumor recurrence with long term follow ups. As discussed below, studies to date using oral or scalp cryotherapy in cancer chemotherapy patients have shown no difference in tumor recurrence in patients treated with oral ice or scalp cooling.
Figure 6.
(A) Normal skin architecture showing hair follicle and blood vessel organization; some perifollicular blood vessels have been outlined. (B) Topical application of Nile Red dye in an ethanol:water vehicle to the skin surface, followed 30 min later by frozen section and fluorescence microscopy of skin sections, shows that delivery of the small molecule dye into the skin occurs almost entirely via hair follicle channels. (C) (i) normal skin architecture with some perifollicular blood vessels outlined; (ii) hypothetical depiction of red dye diffusion, as an example for epinephrine diffusion, from the hair follicle base into surrounding tissue that contains perifollicular blood vessels; (iii) hypothetical depiction showing that systemically administered chemotherapy is retained within the lumen of the constricted, perifollicular blood vessels with little or no diffusion to the surrounding hair follicle stem cells; (iv) hypothetical depiction showing that systemically administered chemotherapy readily diffuses to surrounding structures, including hair follicle stem cells, from non-constricted perifollicular blood vessels.
Three significant advantages of the scalp vasoconstrictor strategy shown in Fig. 6C include: i) it’s not cold and uncomfortable, ii) by increasing the topical vasoconstrictor dose or potentially by applying multiple applications over time, it could be used for extended time periods for long half-life chemotherapy regimens, and iii) it’s portable; patients aren’t tied to either chilling machines or ready ice supplies and nursing staff for extended periods, they can go home wearing a simple head covering before inactivating the epinephrine and washing their hair.
Oral and scalp cryotherapy is: i) technically challenging for staff to maintain, ii) uncomfortable to use for patients, iii) generally ineffective against all but a few short half-life chemotherapy agents, iv) not suitable for protection during radiotherapy, and v) not portable because scalp chilling machines or even ready supplies of ice are not portable for patient use outside the clinic setting.
Several studies of either oral or scalp cryotherapy during chemotherapy have reported, for example, no increase in scalp metastases at 5.8 years after chemotherapy with or without scalp cooling22,23, or no increase in 5 year tumor recurrence rates in patients with cancer and bone marrow transplant who received bolus 5-FU or high-dose melphalan ± total-body irradiation24 with or without oral cryotherapy. Also, topical delivery of vasoconstrictor to vessels ~1 mm beneath the skin or mucosal surface would be expected to induce vasoconstriction in a much more anatomically-confined tissue space than topical application of ice.
We have opened a Phase I/IIa dose escalation trial to evaluate the safety and efficacy of orotopical phenylephrine in the prevention of oral mucositis in subjects undergoing hematopoietic stem cell transplant conditioning with cyclophosphamide plus total body irradiation16. Thus far, patient enrollment has focused on the lowest of five phenylephrine dose levels; initial data indicate: i) efficacy in suppressing oral mucositis, and ii) application of orotopical phenylephrine prior to cyclophosphamide or twice-daily total body irradiation is both feasible and well-tolerated. This strategy could provide a cost-effective and convenient method of preventing oral mucositis. If ongoing patient phenylephrine pharmacokinetic studies demonstrate minimal phenylephrine systemic absorption after orotopical application, future treatment groups within this study will include enrollment of pediatric patients.
Novelty and Impact.
A new strategy of topically applied vasoconstrictor has shown complete suppression of chemotherapy- or radiotherapy-induced oral mucositis and alopecia. Our studies here showed that no detectable sanctuary of L1210 leukemia cells was formed in mice treated with orotopical or topical vasoconstrictor before systemic cyclophosphamide was administered, even at topical vasoconstrictor doses well above those imagined for clinical use. In a recently opened Phase I/IIa trial of orotopical phenylephrine in cyclophosphamide + total body irradiation stem cell transplant patients, i) orotopical phenylephrine is well tolerated, and ii) oral mucositis is suppressed. The topical vasoconstrictor strategy should provide a convenient method of preventing oral mucositis and alopecia.
Grant Sponsor
This work was supported in part by research funds from the Division of Hematology, Oncology and Bone Marrow Transplant, Pediatrics Department, University of Wisconsin.
Footnotes
Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, Hayden V, Eilers J, Epstein JB, LeVeque FG, Miller C, Peterson DE, Schubert MM, Spijkervet FK, Horowitz M. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 2001;19:2201–5 [DOI] [PubMed] [Google Scholar]
- 2.Tierney AJ, Taylor J, Closs SJ. Knowledge, expectations and experiences of patients receiving chemotherapy for breast cancer. Scand J Caring Sci 1992;6:75–80. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhry HM, Bruce AJ, Wolf RC, Litzow MR, Hogan WJ, Patnaik MS, Kremers WK, Phillips GL, Hashmi SK. The incidence and severity of oral mucositis among allogeneic hematopoietic stem cell transplantation patients: A systematic review. Biol Blood Marrow Transplant 2015;15:639–4. [DOI] [PubMed] [Google Scholar]
- 4.Peterson DE, Ohrn K, Bowen J, Fliedner M, Lees J, Loprinzi C, Mori T, Osaguona A, Weikel DS, Elad S, Lalla RV. Systematic review of oral cryotherapy for management of oral mucositis caused by cancer therapy. Support Care Cancer 2013;21:327–32. [DOI] [PubMed] [Google Scholar]
- 5.Belum VR, Marulanda K, Ensslin C, Gorcey L, Busam KJ, Gerber PA, Lacouture ME. Alopecia in patients treated with molecularly targeted anticancer therapies. Ann Oncol 2015; epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carelle N, Piotto E, Bellanger A. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer 2002;95:155–163. [DOI] [PubMed] [Google Scholar]
- 7.McGarvey EL, Baum LD, Pinkerton RC, Rogers LM. Psychological sequelae and alopecia among women with cancer. Cancer Pract 2001;6:283–9. [DOI] [PubMed] [Google Scholar]
- 8.Protière C, Evans K, Camerlo J, d'Ingrado MP, Macquart-Moulin G, Viens P, Maraninchi D, Genre D. Efficacy and tolerance of a scalp-cooling system for prevention of hair loss and the experience of breast cancer patients treated by adjuvant chemotherapy. Support Care Cancer 2002;10:529–37. [DOI] [PubMed] [Google Scholar]
- 9. http://www.breastcancer.org/tips/hair_skin_nails/cold-caps.
- 10.Soref CM, Fahl WE. A new strategy to prevent chemotherapy and radiotherapy-Induced alopecia using topically applied vasoconstrictor. Int J Cancer 2015;136:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soref CM, Fahl WE. A new topical vasoconstrictor-based strategy for prevention of oral mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;117:454–461. [DOI] [PubMed] [Google Scholar]
- 12.Christodoulou C, Tsakalos G, Galani E, Skarlos DV. Scalp metastases and scalp cooling for chemotherapy-induced alopecia prevention. Ann Oncol 2006;17:350. [DOI] [PubMed] [Google Scholar]
- 13.Grevelman EG, Breed WP. Prevention of chemotherapy-induced hair loss by scalp cooling. Ann Oncol 2005;16:352–358. [DOI] [PubMed] [Google Scholar]
- 14.Witman G, Cadman E, Chen M. Misuse of scalp hypothermia. Cancer Treat Rep 1981;65:507–8. [PubMed] [Google Scholar]
- 15.Skipper HE. The effects of chemotherapy on the kinetics of leukemic cell behavior. Cancer Res 1965;25:1544–1550. [PubMed] [Google Scholar]
- 16.www.clinicaltrials.gov (identifier: NCT02434146)
- 17.Soref CM, Fahl WE. Optimum topical delivery of adrenergic agonists to oral mucosa vasculature. Pharm Res 2015;32:492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skipper HE, Schabel FM, Wilcox WS. Experimental evaluation of potential anticancer agents. XIII. On the criteria and kinetics associated with “curability” of experimental leukemia. Ca Chemoth Rep 1964;35:1–111. [PubMed] [Google Scholar]
- 19.Storer JB, Serrano LJ, Darden EB, Jernigan MC, Ullrich RL, Mitchell TJ. Life Shortening in RFM and BALB/c Mice as a Function of Radiation Quality, Dose, and Dose Rate. Rad Res 1979;78:122–161. [PubMed] [Google Scholar]
- 20.Grice JE, Ciotti S, Weiner N, Lockwood P, Cross SE, Roberts MS. Relative uptake of minoxidil into appendages and stratum corneum and permeation through human skin in vitro. J Pharm Sci 2010;99:712–718. [DOI] [PubMed] [Google Scholar]
- 21.Ciotti SN, Weiner N. Follicular liposomal delivery systems. J Liposome Res 2002;12:143–148. [DOI] [PubMed] [Google Scholar]
- 22.Lemieux J, Amireault C, Provencher L, Maunsell E. Incidence of scalp metastases in breast cancer: a retrospective cohort study in women who were offered scalp cooling. Breast Cancer Res Treat 2009;118:547–52. [DOI] [PubMed] [Google Scholar]
- 23.Lemieux J, Provencher L, Perron L, Brisson J, Amireault C, Blanchette C, Maunsell E. No effect of scalp cooling on survival among women with breast cancer. Breast Cancer Res Treat 2015;149:263–8. [DOI] [PubMed] [Google Scholar]
- 24.Svanberg A, Ohrn K, Birgegard G. Five-year follow-up of survival and relapse in patients who received cryotherapy during high-dose chemotherapy for stem cell transplantation shows no safety concerns. Eur J Cancer Care 2012;21:822–8. [DOI] [PubMed] [Google Scholar]