Abstract
Hydrogen production from renewable resources and its reconversion into electricity are two important pillars toward a more sustainable energy use. The efficiency and viability of these technologies heavily rely on active and stable electrocatalysts. Basic research to develop superior electrocatalysts is commonly performed in conventional electrochemical setups such as a rotating disk electrode (RDE) configuration or H-type electrochemical cells. These experiments are easy to set up; however, there is a large gap to real electrochemical conversion devices such as fuel cells or electrolyzers. To close this gap, gas diffusion electrode (GDE) setups were recently presented as a straightforward technique for testing fuel cell catalysts under more realistic conditions. Here, we demonstrate for the first time a GDE setup for measuring the oxygen evolution reaction (OER) of catalysts for proton exchange membrane water electrolyzers (PEMWEs). Using a commercially available benchmark IrO2 catalyst deposited on a carbon gas diffusion layer (GDL), it is shown that key parameters such as the OER mass activity, the activation energy, and even reasonable estimates of the exchange current density can be extracted in a realistic range of catalyst loadings for PEMWEs. It is furthermore shown that the carbon-based GDL is not only suitable for activity determination but also short-term stability testing. Alternatively, the GDL can be replaced by Ti-based porous transport layers (PTLs) typically used in commercial PEMWEs. Here a simple preparation is shown involving the hot-pressing of a Nafion membrane onto a drop-cast glycerol-based ink on a Ti-PTL.
Keywords: oxygen evolution reaction, gas diffusion electrode setup, water electrolyzer, iridium-based catalysts, Ti porous transport layer, performance screening
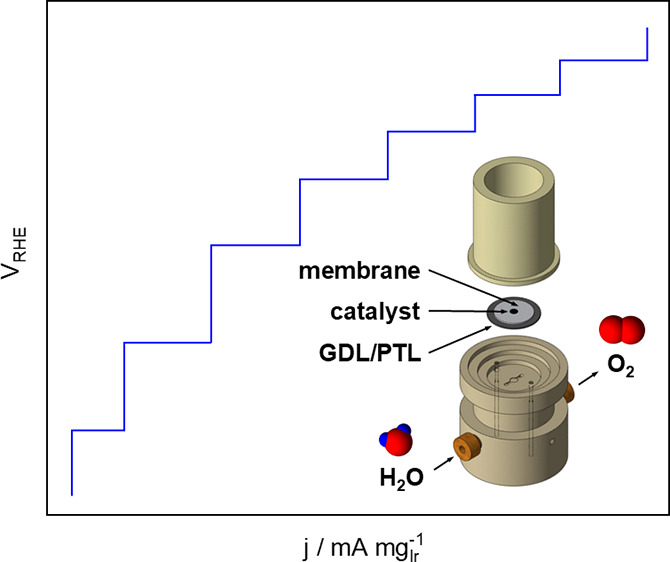
PEMWEs coupled to renewable energy sources such as wind and solar are a promising technology for energy conversion and long-term storage because of their higher current density as compared with alkaline electrolysis cells (AECs).1−3 For PEMWEs, the preferred anode materials are Ir and Ir alloys because of the combination of high activity and stability of IrO2.4−6 As Ir is one of the rarest precious metals,7 an efficient testing platform requiring only small amounts of Ir to screen PEMWE catalysts is necessary to optimize the anode performance. In this work, an in-house developed GDE setup8−11 previously used for oxygen reduction reaction (ORR) studies is adapted to enable OER measurements. In this modified setup, the stainless-steel body is replaced by polychlorotrifluoroethylene for enhanced chemical stability at high voltages (see Figure 1 for the scheme of the developed GDE setup for straightforward OER measurements and Figures S1–S2).
Figure 1.
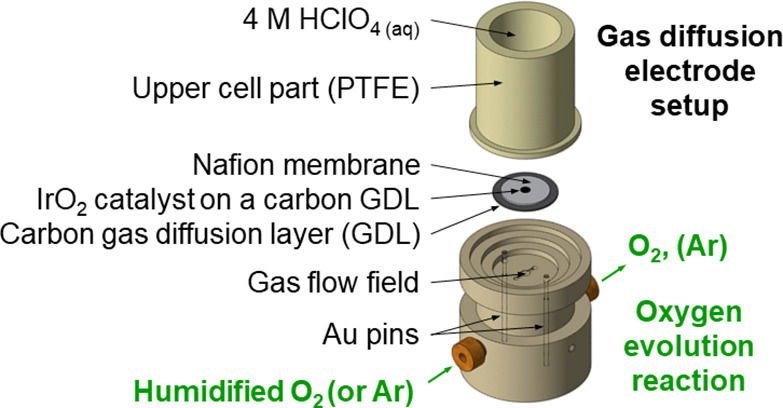
Scheme of the developed GDE setup for straightforward OER measurements.
The GDE consists of a catalyst layer deposited on top of a carbon GDL by vacuum filtration as introduced by Yarlagadda et al.12 The vacuum filtration enables a reproducible film quality with catalyst loadings comparable to PEMWEs.7,13 Element mapping by energy-dispersive X-ray spectroscopy (EDS) reveals a clearly separated IrO2 layer on top of the microporous carbon layer (MPL) of the GDL (see Figure 2). To establish a measurement protocol for the new GDE setup, the influence of several experimental parameters such as the gas flow rate, gas atmosphere (Ar, O2), substrate, and temperature on the measured reaction rate were investigated (see Table S1 for a summary). In addition, the results were compared to conventional RDE measurements.14−17
Figure 2.
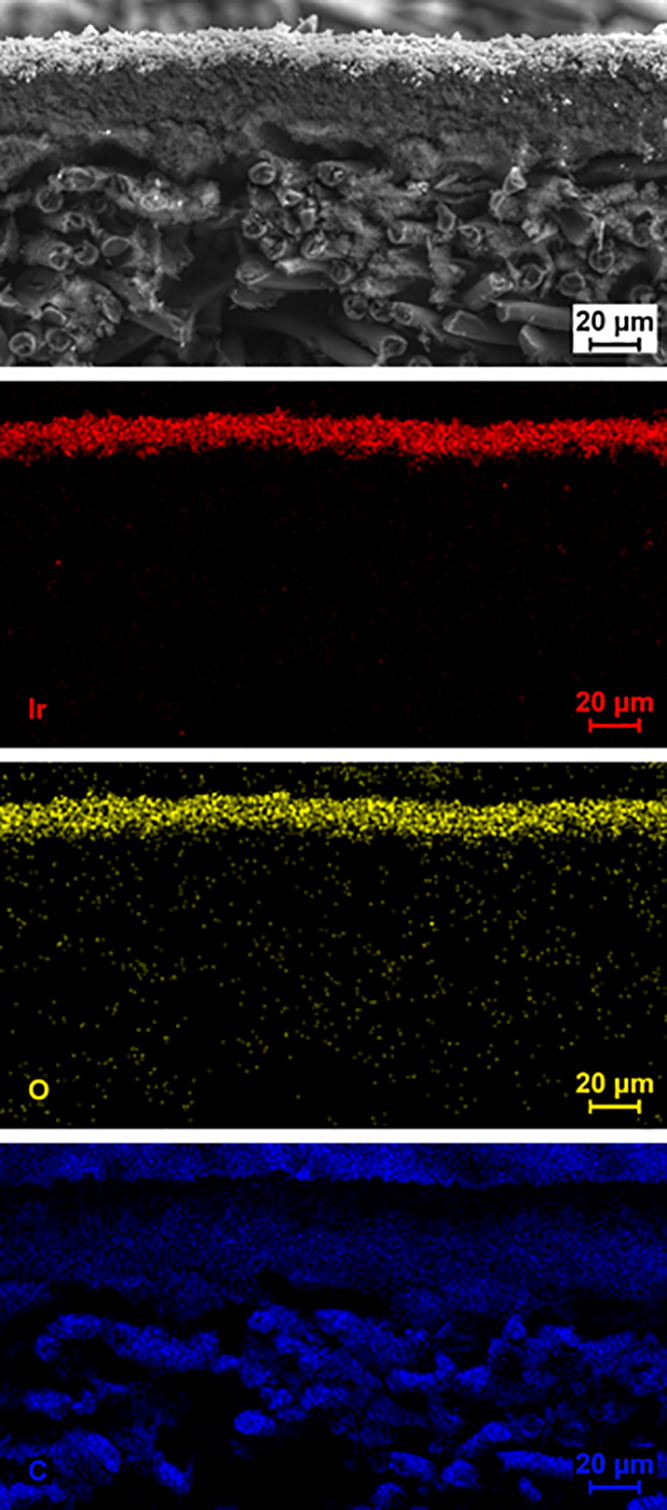
SEM cross-section of an activated IrO2 catalyst film deposited on a GDL in combination with EDS mapping of iridium (Ir, red), oxygen (O, yellow), and carbon (C, blue). A clear separation of the catalyst film, the carbon containing MPL, and the carbon fibers of the GDL is apparent.
The influence of the reactant gas flow (humidified Ar) on the observed OER activity is shown in Figure S4. Based on the measurements, possible mass transport limitations can be identified. The results demonstrate that the apparent OER activities and Tafel slopes are relatively constant (41.4 ± 0.9 mV dec–1) over a wide range of the reactant gas flow (40–190 mL min–1). Only for low flow rates (10 mL min–1), the observed OER rates are inhibited and the Tafel slope shows nonlinear behavior. These observations indicate mass transport limitations at too low flow rates because of a lack of water transported to the catalyst or the difficulty in removing produced O2 from the surface. Hence, in the following, a flow rate of 40 mL min–1 was used to ensure sufficient reactant mass transport.
On the basis of the investigations, we established a measurement protocol, where a set current (normalized to the mass of the precious metal catalyst) is applied and the electrode potential is recorded as a function of time (see Figure S4). Given the controlled current, the average of the iR-corrected potential of the last 60 s (when not stated differently) of every step is defined as the OER.
Thus, conducted OER measurements with a commercial IrO2 benchmark catalyst are presented in Figure 3. In Figure 3a, we compare GDE measurements to conventional RDE measurements at 30 °C in an O2 atmosphere. It is seen that the determined mass activities and Tafel slopes are similar in both setups. At 1.48 VRHE, mass activities of 4.3 and 4.9 mA mgIr–1 are determined for the galvanostatic GDE and potentiostatic RDE measurements, respectively, which is comparable to the values reported by Alia et al.18,19 The determined Tafel slopes are 45.4 ± 1.0 and 39.2 ± 0.4 mV dec–1, respectively.
Figure 3.
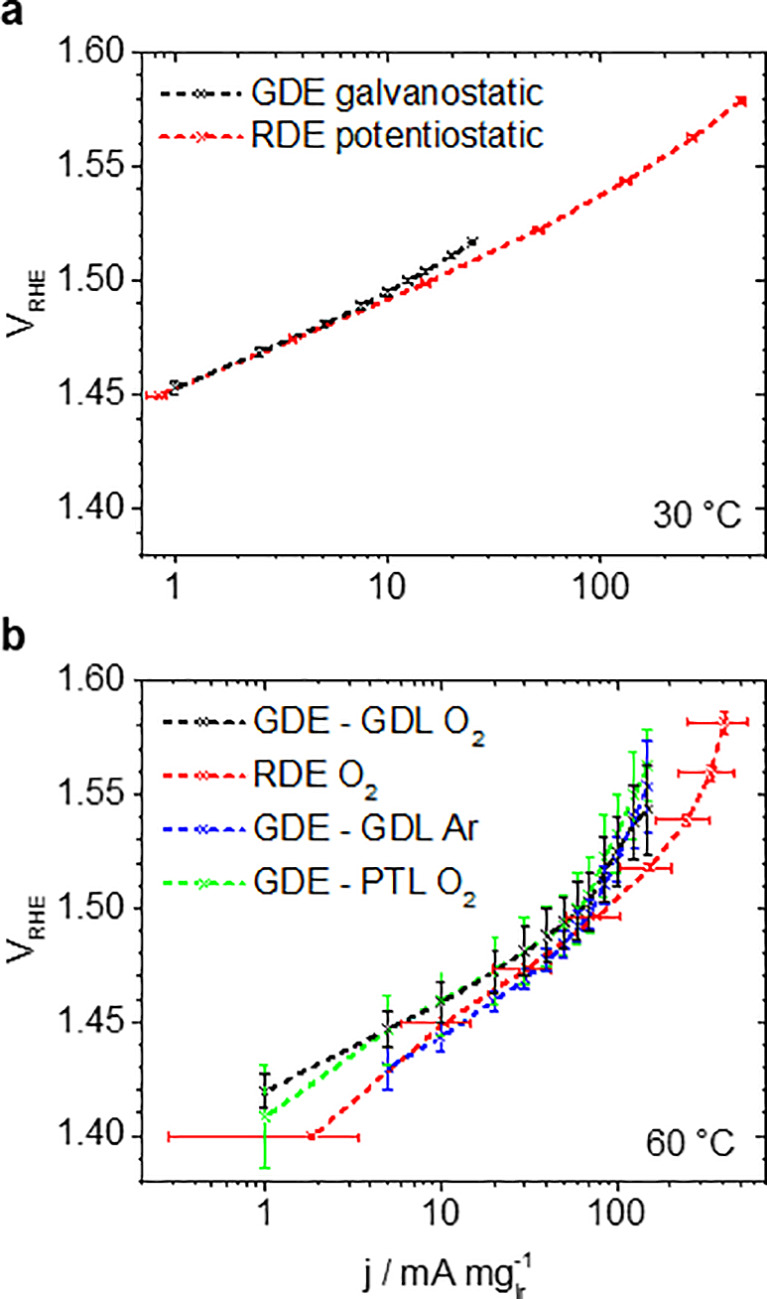
Tafel plots of OER mass activity j of the IrO2 catalyst. Comparison of GDE (galvanostatic, 4 M HClO4, 1 mgIr cm–2geo) and RDE (potentiostatic in O2 atmosphere, 0.1 M HClO4, 50 μgIr cm–2geo) measurements (a) at 30 °C in O2 and (b) 60 °C using a GDL in O2 and Ar or Ti-PTL in O2 atmosphere in the GDE setup. The error bars show the standard deviation of three independent measurements; the data points are connected by dashed lines as guide for the eye.
In Figure 3b, the results at an increased temperature of 60 °C are shown. In a RDE setup, potentiodynamic measurements reveal performance degradation during continuous cycling (see Figure S5), and potentiostatic measurements show a large standard deviation for the measured current density. By comparison, in the GDE setup, three independent measurements confirm a good reproducibility of the obtained OER activities under steady-state conditions (see also Table S1). The fast performance degradation, as seen in the RDE, is most likely inhibited in the GDE setup because of the use of a Nafion membrane avoiding direct contact of the catalyst to the liquid electrolyte leading to a more realistic setup in the GDE comparable to a PEMWE. Furthermore, it is demonstrated that the OER activity can be conveniently studied in O2 or Ar gas atmosphere. The latter leads to apparent activity improvements, which can be understood by the influence of the O2 partial pressure in the Nernst equation. The same effect influences the Tafel behavior. In O2 atmosphere and 60 °C, a Tafel slope of 41.1 ± 4.8 mV dec–1 is observed at low current densities, which at higher current densities increases significantly. This observation is linked to oxygen bubble formation, which leads to inhibited OER rates.20,21 As a consequence, with increasing oxygen formation rates (i.e., higher current densities), the apparent OER activity in Ar atmosphere decreases and approaches that in O2. Interestingly, nonlinearities in the Tafel slope at low current densities, which are often observed in potentiodynamic RDE measurements,22 are not apparent in the GDE setup.
Most importantly, no fundamental difference is observed if these OER activity measurements are performed with a catalyst film deposited onto a carbon GDL or Ti-PTL, justifying the substantially less complex GDL approach for catalyst screening; see more details about the film preparation in the SI. To further corroborate that carbon corrosion of the GDL does not pose a problem during the activity measurement, the current density was increased stepwise from 1 to 10 mA mgIr–1 and held at 10 mA mgIr–1 for 1 h. Figure 4 shows that even with the carbon-based GDL, it is possible to perform such suggested stability measurements,23 although the potential increase in repeat 2 (of three independent samples) can probably be traced back to bubble formation or carbon degradation. Hence the GDL is a suitable substrate for determining the activity of OER catalysts in screening-type studies.
Figure 4.
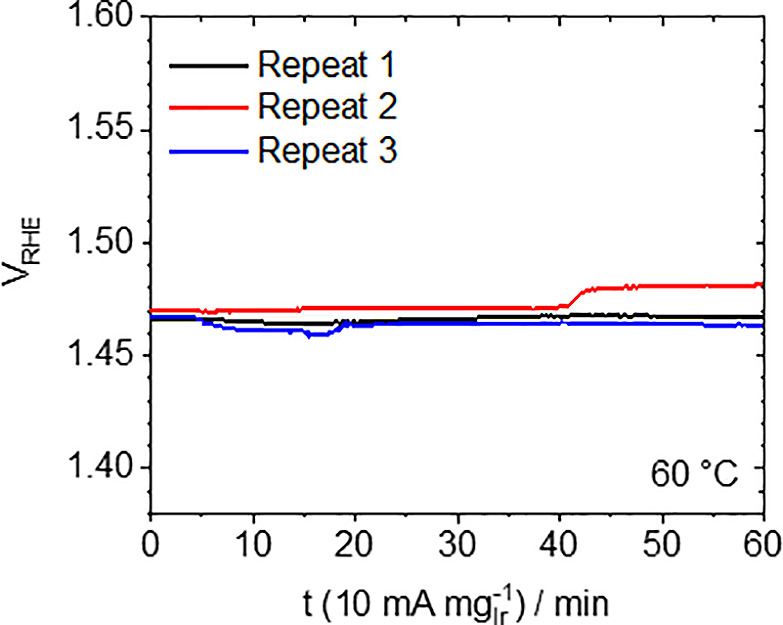
Stability measurements of IrO2/GDL applying 10 mA mgIr–1 for 1 h.
For long-term stability measurements under harsher conditions, catalyst-coated membranes (CCMs) pressed to Ti-PTLs might be used.24,25 However, CCM preparation is time-consuming and needs special equipment; hence, it is not available in all laboratories for a fast screening test protocol. Because of the high porosity of the PTL, vacuum filtration or drop-casting using an ink of water and IPA as for the GDLs preparation is not possible. A simple alternative to CCM preparation is to use a glycerol-based catalyst ink. To completely avoid the ink permeation into the PTL, Teflon was sprayed on top of the PTL before drop-casting (note that the MPL on top of the carbon fiber structure of the GDL also contains Teflon). Furthermore, as compared with the GDL preparation, the temperature during pressing needs to be increased; otherwise, a poor electric connection between Nafion membrane and PTL can lead to high resistances of several hundred ohms. However, a homemade hot-press was sufficient to enable the use of a PTL and achieve results comparable to the GDL approach (see Figure 5b). Therefore, the use of a PTL is possible in the presented GDE setup even without specialized equipment. However, because of the simple hot-pressing, the membrane can peel off with time at high O2 formation rates. Additionally, because of the high costs of the PTL, we believe that the GDL approach is more suitable for a first activity screening.
Figure 5.
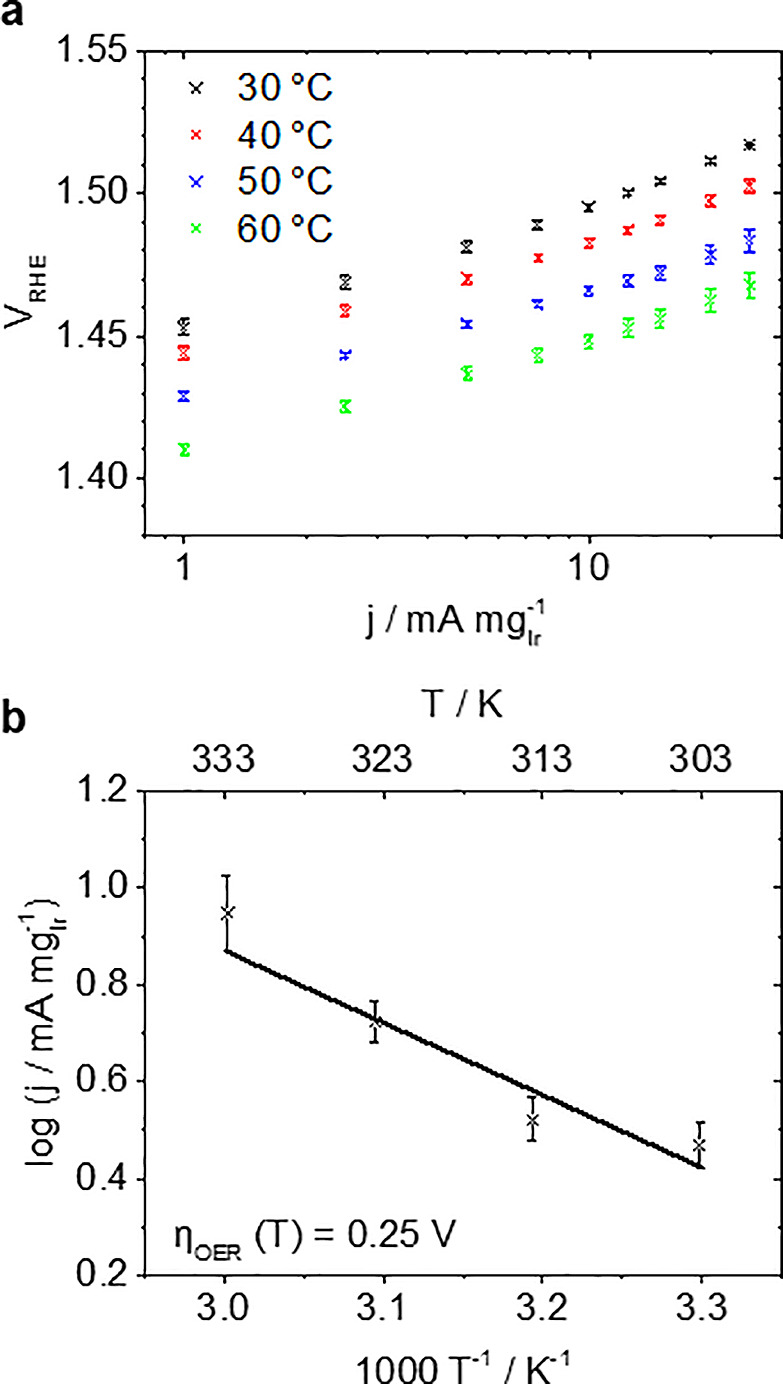
(a) Temperature-dependent OER mass activity j plotted as Tafel plots obtained in a GDE setup applying current steps versus VRHE. (b) The corresponding Arrhenius plot at an overpotential ηOER of 0.25 V.
To complete the IrO2 catalyst characterization, the temperature dependency was investigated between 30 and 60 °C. In Figure 5a, it is shown that, as expected, the OER rate increases with temperature.26 Furthermore, comparable Tafel slopes (38.5–45.4 mV dec–1) are observed, indicating that the OER reaction mechanism is the same in the investigated temperature range. Hence, the electrochemical activation energy EA can be estimated assuming Arrhenius behavior. For a rough estimation, the formal EA determined with respect to the reference potential of the used reversible hydrogen reference electrode (RHE) can be used (see SI). For a more precise determination, the temperature-dependent shift of the reversible oxygen potential versus RHE needs to be considered. This correction is also required when determining the exchange current density j0. The precise Tafel slope allows for an extrapolation of the linear fits to the reversible potential to determine j0 for the OER. In conventional, potentiodynamic RDE measurements, determining j0 for the OER is daring and usually not pursued because an extrapolation of the measured current densities over several orders of magnitude is required. Error margins in the Tafel slope therefore can lead to substantial uncertainties of several orders of magnitude. For the determination of the temperature-dependent reversible potential Erev,T different equations can be found (e.g., from Parthasarathy et al.27 or from Bratsch).28 The latter contains an approximation of the exact dependence. Determining Erev,T using the equation of Parthasarathy et al.27j0 of 10.6 ± 6.0, 3.5 ± 1.8, 2.1 ± 1.2, and 7.2 ± 3.8 × 10–9 A mgIr–1 are obtained for 30, 40, 50, and 60 °C, respectively. It should be noted that three independent samples were measured while consecutively applying the four temperatures. Determining Erev,T following Bratsch28 leads to comparable but slightly higher values of j0 (see Tables S2–S3). The obtained j0 are comparable to 7.3 × 10–9 A mgIr–2 calculated from the data reported by Lu et al.22 The limitation of the extrapolation, however, is apparent from the increase in j0 going from 50 to 60 °C. For the determination of EA at constant overpotential, Erev,T has been calculated according to Parthasarathy et al.27 (see Figure S6 for a Arrhenius plot according to the correction by Bratsch28). The linear fit of the Arrhenius plot at an overpotential ηOER of 0.25 V (Figure 5b) leads to EA of 28.5 ± 6.6 kJ mol–1 being a bit lower than 47 kJ mol–1 reported by Suermann et al.29 in a PEMWE, which is mainly related to the point at 30 °C. Using kinetic data of four temperatures certainly only allows a rough estimation. Nevertheless, this example shows the potential of the presented GDE approach to provide high quality data.
To sum up, we demonstrate a new strategy to test OER catalysts in a GDE setup. The GDE setup is a more straightforward technique than RDE10,11 and the GDE cell can be built in any research workshop (see technical drawings in SI). Depositing the catalyst onto a carbon GDL is suitable for OER steady-state activity screening and allows the determination of key kinetic data. In contrast to conventional potentiodynamic RDE measurements, the catalyst layers contain Nafion and exhibit realistic loadings comparable to PEMWEs. Accurate Tafel slopes can be obtained and extrapolated to determine the exchange current density. Even short-term stability tests are feasible with carbon GDL, which are cost-efficient substrates for catalyst screening. Furthermore, the GDE setup can be used for more elaborate stability screenings using Ti-PTLs as substrate and performing a simple hot-pressing procedure.
Acknowledgments
This Project has received funding from the European Union’s Horizon 2020 Research and Innovation program under grant agreement N. 861960 (“Recycalyse” project) and the Swiss National Science Foundation (SNSF) via the project No. 200021_184742. J.Q acknowledges the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 840523 (CoSolCat). J.Q. and M.A. thank Dr. L. Theil Kuhn and Dr. S. B. Simonsen, Technical University of Denmark (DTU) for access to TEM facilities. H.A.E.-S. and M.F.T. gratefully acknowledge the German Ministry of Education and Research for financial support of this work within the innoKA project (BMWi, 03ET6096A). Dr. Nedjeljko Seselj of Danish Power Systems (DPS) is acknowledged for his advice concerning the catalyst film formation on the PTL.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.1c00015.
Experimental (chemicals, materials, and gases; gas diffusion electrode setup; rotating disk electrode setup; ink formation; catalyst film preparation; electrochemical measurements; characterization), OER activity measurements (GDE and RDE data; Tafel plot determination; exchange current density and activation energy), XPS and TEM measurements (PDF)
Author Present Address
¶ F.B.: Empa, Ueberlandstrasse 129, 8600 Dübendorf, Switzerland.
Author Contributions
G.K.H.W. designed the GDE setup for the OER measurements. J.S., M.A., H.A.E.-S. designed the electrochemical experiments in the GDE setup, prepared and performed by J.S. V.A.M. performed the RDE measurements. J.S. prepared the samples for TEM and XPS measurements. J.Q. performed TEM and M.F.T. XPS measurements and analysis. E.B. did the SEM and EDS measurements. A.B. did initial tests on the hot pressing. F.B. did initial OER tests in the GDE setup. H.A.E.-S. and M.A. supervised the research. J.S. and M.A. wrote the first draft of the paper, read, and commented by all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Buttler A.; Spliethoff H. Current Status of Water Electrolysis for Energy Storage, Grid Balancing and Sector Coupling via Power-to-Gas and Power-to-Liquids: A Review. Renewable and Sustainable Energy Reviews 2018, 82, 2440–2454. 10.1016/j.rser.2017.09.003. [DOI] [Google Scholar]
- Babic U.; Suermann M.; Büchi F. N.; Gubler L.; Schmidt T. J. Critical Review—Identifying Critical Gaps for Polymer Electrolyte Water Electrolysis Development. J. Electrochem. Soc. 2017, 164 (4), F387–F399. 10.1149/2.1441704jes. [DOI] [Google Scholar]
- Schmidt O.; Gambhir A.; Staffell I.; Hawkes A.; Nelson J.; Few S. Future Cost and Performance of Water Electrolysis: An Expert Elicitation Study. Int. J. Hydrogen Energy 2017, 42 (52), 30470–30492. 10.1016/j.ijhydene.2017.10.045. [DOI] [Google Scholar]
- Fabbri E.; Habereder A.; Waltar K.; Kötz R.; Schmidt T. J. Developments and Perspectives of Oxide-Based Catalysts for the Oxygen Evolution Reaction. Catal. Sci. Technol. 2014, 4 (11), 3800–3821. 10.1039/C4CY00669K. [DOI] [Google Scholar]
- Antolini E. Structural Parameters of Supported Fuel Cell Catalysts: The Effect of Particle Size, Inter-Particle Distance and Metal Loading on Catalytic Activity and Fuel Cell Performance. Applied Catalysis B: Environmental 2016, 181, 298–313. 10.1016/j.apcatb.2015.08.007. [DOI] [Google Scholar]
- McCrory C. C. L.; Jung S.; Peters J. C.; Jaramillo T. F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135 (45), 16977–16987. 10.1021/ja407115p. [DOI] [PubMed] [Google Scholar]
- Carmo M.; Fritz D. L.; Mergel J.; Stolten D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrogen Energy 2013, 38 (12), 4901–4934. 10.1016/j.ijhydene.2013.01.151. [DOI] [Google Scholar]
- Wiberg G. K. H.; Fleige M.; Arenz M. Gas Diffusion Electrode Setup for Catalyst Testing in Concentrated Phosphoric Acid at Elevated Temperatures. Rev. Sci. Instrum. 2015, 86 (2), 024102. 10.1063/1.4908169. [DOI] [PubMed] [Google Scholar]
- Inaba M.; Jensen A. W.; Sievers G. W.; Escudero-Escribano M.; Zana A.; Arenz M. Benchmarking High Surface Area Electrocatalysts in a Gas Diffusion Electrode: Measurement of Oxygen Reduction Activities under Realistic Conditions. Energy Environ. Sci. 2018, 11 (4), 988–994. 10.1039/C8EE00019K. [DOI] [Google Scholar]
- Alinejad S.; Inaba M.; Schröder J.; Du J.; Quinson J.; Zana A.; Arenz M. Testing Fuel Cell Catalysts under More Realistic Reaction Conditions: Accelerated Stress Tests in a Gas Diffusion Electrode Setup. J. Phys. Energy 2020, 2 (2), 024003. 10.1088/2515-7655/ab67e2. [DOI] [Google Scholar]
- Schröder J.; Quinson J.; Mathiesen J. K.; Kirkensgaard J. J. K.; Alinejad S.; Mints V. A.; Jensen K. M. Ø.; Arenz M. A New Approach to Probe the Degradation of Fuel Cell Catalysts under Realistic Conditions: Combining Tests in a Gas Diffusion Electrode Setup with Small Angle X-Ray Scattering. J. Electrochem. Soc. 2020, 167 (13), 134515. 10.1149/1945-7111/abbdd2. [DOI] [Google Scholar]
- Yarlagadda V.; McKinney S. E.; Keary C. L.; Thompson L.; Zulevi B.; Kongkanand A. Preparation of PEMFC Electrodes from Milligram-Amounts of Catalyst Powder. J. Electrochem. Soc. 2017, 164 (7), F845–F849. 10.1149/2.1461707jes. [DOI] [Google Scholar]
- Pan L.; Ott S.; Dionigi F.; Strasser P. Current Challenges Related to the Deployment of Shape-Controlled Pt Alloy Oxygen Reduction Reaction Nanocatalysts into Low Pt-Loaded Cathode Layers of Proton Exchange Membrane Fuel Cells. Curr. Opin. Electrochem. 2019, 18, 61–71. 10.1016/j.coelec.2019.10.011. [DOI] [Google Scholar]
- Bernt M.; Hartig-Weiß A.; Tovini M. F.; El-Sayed H. A.; Schramm C.; Schröter J.; Gebauer C.; Gasteiger H. A. Current Challenges in Catalyst Development for PEM Water Electrolyzers. Chem. Ing. Tech. 2020, 92 (1–2), 31–39. 10.1002/cite.201900101. [DOI] [Google Scholar]
- Oakton E.; Lebedev D.; Povia M.; Abbott D. F.; Fabbri E.; Fedorov A.; Nachtegaal M.; Copéret C.; Schmidt T. J. IrO2-TiO2: A High-Surface-Area, Active, and Stable Electrocatalyst for the Oxygen Evolution Reaction. ACS Catal. 2017, 7 (4), 2346–2352. 10.1021/acscatal.6b03246. [DOI] [Google Scholar]
- Reier T.; Oezaslan M.; Strasser P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2 (8), 1765–1772. 10.1021/cs3003098. [DOI] [Google Scholar]
- Oh H. S.; Nong H. N.; Reier T.; Bergmann A.; Gliech M.; Ferreira De Araújo J.; Willinger E.; Schlögl R.; Teschner D.; Strasser P. Electrochemical Catalyst-Support Effects and Their Stabilizing Role for IrOx Nanoparticle Catalysts during the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2016, 138 (38), 12552–12563. 10.1021/jacs.6b07199. [DOI] [PubMed] [Google Scholar]
- Alia S. M.; Rasimick B.; Ngo C.; Neyerlin K. C.; Kocha S. S.; Pylypenko S.; Xu H.; Pivovar B. S. Activity and Durability of Iridium Nanoparticles in the Oxygen Evolution Reaction. J. Electrochem. Soc. 2016, 163 (11), F3105–F3112. 10.1149/2.0151611jes. [DOI] [Google Scholar]
- Alia S. M.; Shulda S.; Ngo C.; Pylypenko S.; Pivovar B. S. Iridium-Based Nanowires as Highly Active, Oxygen Evolution Reaction Electrocatalysts. ACS Catal. 2018, 8 (3), 2111–2120. 10.1021/acscatal.7b03787. [DOI] [Google Scholar]
- El-Sayed H. A.; Weiß A.; Olbrich L. F.; Putro G. P.; Gasteiger H. A. OER Catalyst Stability Investigation Using RDE Technique: A Stability Measure or an Artifact?. J. Electrochem. Soc. 2019, 166 (8), F458–F464. 10.1149/2.0301908jes. [DOI] [Google Scholar]
- Hartig-Weiss A.; Tovini M. F.; Gasteiger H. A.; El-Sayed H. A. OER Catalyst Durability Tests Using the Rotating Disk Electrode Technique: The Reason Why This Leads to Erroneous Conclusions. ACS Appl. Energy Mater. 2020, 3, 10323. 10.1021/acsaem.0c01944. [DOI] [Google Scholar]
- Lu Y.; Wang W.; Xie F. Investigation of Oxygen Evolution Reaction Kinetic Process and Kinetic Parameters on Iridium Electrode by Electrochemistry Impedance Spectroscopy Analysis. J. Electroanal. Chem. 2020, 871, 114281. 10.1016/j.jelechem.2020.114281. [DOI] [Google Scholar]
- Spöri C.; Kwan J. T. H.; Bonakdarpour A.; Wilkinson D. P.; Strasser P. The Stability Challenges of Oxygen Evolving Catalysts: Towards a Common Fundamental Understanding and Mitigation of Catalyst Degradation. Angewandte Chemie - International ed. 2017, 56, 5994–6021. 10.1002/anie.201608601. [DOI] [PubMed] [Google Scholar]
- Rakousky C.; Reimer U.; Wippermann K.; Carmo M.; Lueke W.; Stolten D. An Analysis of Degradation Phenomena in Polymer Electrolyte Membrane Water Electrolysis. J. Power Sources 2016, 326, 120–128. 10.1016/j.jpowsour.2016.06.082. [DOI] [Google Scholar]
- Bühler M.; Holzapfel P.; McLaughlin D.; Thiele S. From Catalyst Coated Membranes to Porous Transport Electrode Based Configurations in PEM Water Electrolyzers. J. Electrochem. Soc. 2019, 166 (14), F1070–F1078. 10.1149/2.0581914jes. [DOI] [Google Scholar]
- Zhang G.; Wang H.; Yang J.; Zhao Q.; Yang L.; Tang H.; Liu C.; Chen H.; Lin Y.; Pan F. Temperature Effect on Co-Based Catalysts in Oxygen Evolution Reaction. Inorg. Chem. 2018, 57 (5), 2766–2772. 10.1021/acs.inorgchem.7b03168. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A.; Srinivasan S.; Appleby A. J.; Martin C. R. Temperature Dependence of the Electrode Kinetics of Oxygen Reduction at the Platinum/Nafion® Interface—A Microelectrode Investigation. J. Electrochem. Soc. 1992, 139 (9), 2530–2537. 10.1149/1.2221258. [DOI] [Google Scholar]
- Bratsch S. G. Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18 (1), 1–21. 10.1063/1.555839. [DOI] [Google Scholar]
- Suermann M.; Schmidt T. J.; Büchi F. N. Comparing the Kinetic Activation Energy of the Oxygen Evolution and Reduction Reactions. Electrochim. Acta 2018, 281, 466–471. 10.1016/j.electacta.2018.05.150. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


