Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2, was first reported in December 2019 in Wuhan, Hubei province, China. It is now known as a pandemic and a global crisis due to rapid human-to-human transmission with the vast expansion that has affected almost all countries. The primary source of the disease is still unknown, but it is possible that the virus was transmitted through bat to an intermediate host and then to humans. The main and early symptoms of COVID-19 infection are fatigue, fever, dry cough, myalgia, and dyspnea. The incubation period of the disease is about 2–14 days, which is one of the important parameters for planning to prevent disease outbreak. PT-polymerase chain reaction test is used to diagnose the disease; chest computed tomography scan, chest X-ray, blood tests, and symptoms are also very helpful in diagnosing the disease. There is a strong emphasis on controlling infections and hand hygiene to prevent the transmission of the disease. There is not enough knowledge about this disease yet, and there are no specific vaccines or medications available to prevent and treat this disease. The current review study uses articles indexed on databases of Embase, Elsevier, PubMed, and World Health Organization and Centers for Disease Control and Prevention, and keywords of coronavirus, COVID-19, acute respiratory distress syndrome and China.
Keywords: COVID-19, epidemic, novel coronavirus, pandemic, Infectious disease, SARS-CoV-2, health emergency, review
Introduction
On December 31, 2019, China presented cluster reports of cases of pneumonia with an unknown cause, later known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease was often associated with fever, cough, and dyspnea for 2–14 days after exposure.[1] The disease resulted in a syndrome that in some cases led to the need for intensive care.[2] Coronaviruses were first identified in the 1960s and are large RNA viruses that infect many animals.[3] Genome sequencing revealed that an emerging disease has developed in Wuhan, China, due to a novel coronavirus, and like SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), the newly emerged SARS-CoV-2 virus belongs to Beta coronaviruses.[4,5] Although the primary source of the disease is still unknown, the initial cases of infection were in the Huanan Seafood Market in Wuhan, but in addition to seafood, it has been reported on social media that some wild animals are also sold in this market and region. Some studies reported that bats may be potential hosts for SARS-CoV-2.[6,7,8,9,10,11] Thirty-three of the 585 environmental samples in the Huanan Seafood Market were positive for the novel coronavirus. Some of these include a variety of live animals, such as hedgehogs, badgers, snakes, turtles, birds and pangolins. Therefore, the bats may not have had direct contact with humans, and direct transmission of the virus from bat to human seems unlikely. In the past, SARS-CoV-2 and MERS-CoV have also been transmitted from bat to human, through intermediate hosts of civet and camel, respectively. The SARS-CoV-2 was isolated from pangolins; it has been found that strains isolated from infected humans are similar to COVID-19 by 99%. It has been suggested that the route of transmission and completion was from bat to pangolins (intermediate hosts), and then to humans.[12,13,14,15] Understanding the source and host of SARS-CoV-2 can play an important role in preventing the onset and outbreak of the disease.[4] Coronaviruses in humans cause mild respiratory illness with flu-like symptoms, but they have also been proven to develop severe and fatal respiratory diseases, such as SARS epidemic in 2002-2003 and MERS disease in 2012-2013.[3,16] Since 2003, there have been no reports of human infection with SARS to the World Health Organization (WHO). The virus infected 8098 people in 2002–2003 and killed 744 people due to acute respiratory failure (about 11%).[17,18,19] Most patients with MERS were reported from Saudi Arabia. The virus responsible for the disease was first isolated in December 2012 by an Egyptian virologist Dr. Ali Mohamed Zaki from the patient's lung. The result of this experiment was the detection of an emerging viral disease. In May 2013, the International Committee on Taxonomy of Viruses proposed the name MERS-CoV.[3] Rapid human-to-human transmission of COVID-19 disease has led to the creation of steep curves in many areas.[20,21] In many cases, the transmission through asymptomatic carriers has been reported.[3] With the massive global spread of the novel coronavirus, on March 11, 2020, the WHO approved the 2019 novel coronavirus (COVID-19) as a pandemic description of Figure 1.[1,22,23]
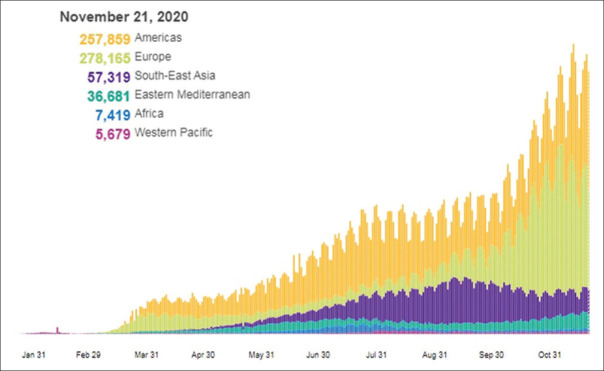
Figure 1.
Total number of infected cases in the United States on November 21, 2020; Source: World Health Organization website[42]
Materials and Methods
The current review study uses articles indexed on databases of Embase, Elsevier, PubMed, and WHO and Centers for Disease Control and Prevention (CDC), and keywords of coronavirus, COVID-19, acute respiratory distress syndrome (ARDS) and China. In general, the articles were read and reviewed.
Epidemiology
On December 31, 2019, China submitted reports of cases of pneumonia of unknown cause, later known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).[1] The rapid human-to-human transmission of the disease involved many countries.[20] Most cases in Wuhan were initially in adults,[24] and the mortality rate from the disease was reported to be about 3%, and most of the deaths were elated to elderly people with underlying diseases. Due to the potential asymptomatic transmission of the virus by carriers, it was expected to have a very rapid and high prevalence. On February 11, 2020, the WHO reported a total of 43103 infected patients. The disease was widespread in China and abroad, and then the trend of new cases of infection in China declined but continued to rise outside China. As of February 11, 2020, 28 countries were affected by the novel coronavirus, including mainland China, Japan, Singapore, Hong Kong, Thailand, South Korea, Taiwan, Australia, Malaysia, Germany, Vietnam, the United States, Macao, the United Arab Emirates, Canada, France, Philippines, the United Kingdom, Italy, India, Russia, Finland, Sweden, Sri Lanka, Cambodia, Nepal, Spain, and Belgium.[25,26,27] On February 21, 2020, the WHO reported a total of 76,769 infected patients, with 32 countries or regions reporting positive cases. In addition to the countries mentioned above, Iran, Egypt, Israel and Lebanon were added to the infection cases. The WHO reports:[28,29]
Routes of transmission
The novel coronavirus can be transmitted from human to human through respiratory droplets, but the respiratory tract is probably not the only route of transmission. The virus is also transmitted through direct and indirect contact with mucous membranes such as the eyes, mouth and nose.[12,30,31] However, there is also the possibility of transmission through airborne particles in a closed environment with constant exposure to high aerosol concentrations. These patients also have some gastrointestinal symptoms and it is likely that the virus will be transmitted through fecal-oral route.[4,12,32,33] Angiotensin-converting enzyme 2 (ACE2) has been suggested as a receptor for the virus. The ubiquitous expression of this enzyme in various cells such as lung, esophageal, epithelial, and absorptive enterocytes of ileum and colon may play a role in the multi-tissue infection of novel coronavirus.[12,34] Further studies are needed to examine the exact routes of transmission. Accurate information is not available on whether SARS-CoV-2 can be transmitted through breast milk or vertically from mother to fetus.[4]
Incubation period
Incubation period is the time between exposure to the source of transmission and the onset of symptoms.[35] The incubation period is a key parameter in epidemiological discussions, and the range of incubation period of the disease is essential to explain epidemiological episodes and to determine quarantine time, which is one of the main factors in preventing further transmission of the disease.[36] The length of the incubation period helps to estimate the epidemic rate.[6,36] Li et al. initially used the information of 10 patients positive for SARS-CoV-2 in Wuhan to estimate the incubation period of the disease, which was estimated to be 5.2 days. Backer et al. tried to estimate the incubation period of the disease by considering the length of stay in Wuhan and the time of onset of symptoms in 88 patients who had traveled to Wuhan, and they estimated that this time averaged 6.4 days (approximately between 2 and 11 days).[36,37] In a study of Guan et al., the mean incubation period of the disease was estimated to be 3 days, which can last up to 24 days.[35] In a study of Lauer et al., the mean incubation period of the disease was estimated to be 5 days (4.4–5.6 days) with a range of 2–14 days. It has been reported that the SARS-CoV-2 asymptomatic patients (carriers) living during the incubation period can transmit the disease to healthy individuals.[4,38,39] Estimates of the incubation period of SARS-CoV-2 are roughly the same as the incubation period of other known coronaviruses in humans, for example, in SARS (mean 5 days with a range of 2–14 days) and MERS (mean 5–7 days with a range of 2–14 days).[40] Summing up most studies, the incubation period of the disease is estimated to be about 2–14 days.
Symptoms
The main manifestations of the disease at the onset of the disease are fatigue, fever, dry cough, myalgia and dyspnea, and less common symptoms include nasal congestion, headache, rhinorrhea, sore throat, vomiting, and diarrhea.[4,41] In severe patients, dyspnea or hypoxemia often develops about a week after the onset of symptoms, followed by septic shock, ARDS, disorder in correction of metabolic acidosis, and coagulation dysfunction. However, critically ill patients may have a low fever and even no obvious fever, or mild patients may have a low fever and mild fatigue and may not have pneumonia. The asymptomatic patients can also transmit the disease to others.[4] The clinical features of SARS-CoV-2 are similar to those of SARS-CoV, fever and cough are common symptoms of the disease, and gastrointestinal symptoms are rare. Fever is initially seen in only 43.8% of patients and increases in 87.9% of hospitalized patients. The absence of fever in SARS-CoV-2 is higher than in SARS-CoV (1%) and MERS-CoV (2%), so over-focusing on the diagnosis of fever may cause the patient to be undiagnosed.[35,42] Despite the high similarity between flu and COVID-19 symptoms, it is requisite to develop high sensitivity and specificity laboratory diagnostic kits with enough rapid and accurate detection.[43]
Diagnosis
The number of cases of the disease is increasing sharply. Therefore, early diagnosis and isolation of patients is of great importance to eradicate the source of infection and break the transmission chain.[12] The main and only confirmed method of laboratory diagnosis is currently the detection of nucleic acid. Real-time polymerase chain reaction (PCR), also called quantitative PCR, is a molecular biological technique based on the identification of nucleic acid sequence. The SARS-CoV-2 genome sequence exists in the GeneBank. Therefore, the SARS-CoV-2 nucleic acid can be detected by quantitative reverse transcription PCR (RT-qPCR) or viral gene sequencing using nasopharyngeal and oropharyngeal swabs, as well as stool, sputum, and blood samples. Saliva samples are less invasive and are more commonly used.[4,44] There is also the possibility of false negative results. In the RT-qPCR measurements for nasopharyngeal and oropharyngeal samples, only 65% and 70% were positive, respectively. In a study on the analysis of computed tomography (CT) scan findings among suspected SARS-CoV-2 cases, 48% of the cases that were considered negative using nasopharyngeal and oropharyngeal samples were estimated to be very likely for SARS-CoV-2 infection, and 33% were reported as possible cases. In fact, a negative sample of an upper airway swab cannot rule out the SARS-CoV-2 infection, and collecting the samples from other airways, such as the lower airway, can help diagnose the disease. In addition, the detection of a pathogen other than SARS-CoV-2, especially in suspected patients, may not indicate that SARS-CoV-2 is not present, and diagnostic tests should be performed for the disease. A positive swab sample confirms the disease and is sufficient to initiate treatment and control the infection.[45] According to reports, the SARS-CoV-2 uses ACE2 as a human cell receptor, which initially causes lung damage and subsequent parenchymal changes in lung tissue. The chest CT scan can show different patterns depending on the severity and duration of the disease. Findings observed in the chest CT scan can include:
Ground glass opacity: Is similar to hazy or opaque glass by increasing the concentration without blurring of the bronchi and peripheral arteries, which may be caused by minor air movement. The patients with SARS-CoV-2 suffer from unilateral or bilateral involvement with peripheral or subpleural ground glass opacity
Consolidation: In fact, the alveolar space is filled with fluids, cells, or tissues instead of air, and this increases the density of the lung parenchyma and causes diffuse or multifocal opacities with indistinct boundaries
Reticular pattern: defined as thickened pulmonary interstitial structures as a set of very fine lines in the CT scan. The formation of these patterns is associated with interstitial lymphocytic infiltration, which causes interlobular septal thickening
Crazy paving pattern: is a paving pattern due to the thickening of the interlobular and interlobular septa.
Figure 2 show are some images from the various CT scans taken by Ye et al.:[46]
Figure 2.
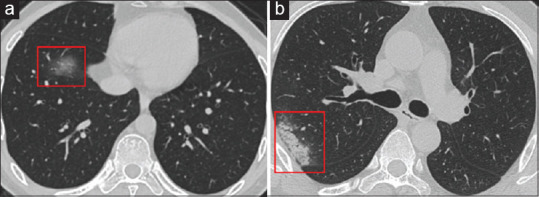
Active lesion in the lung of patient on admission on day 20 of the disease
However, the CT scan findings also depend on the timing of the CT scan, which may be normal in the early days of the illness and do not rule out a normal CT scan, especially at the onset of COVID-19.
In a study by Fang et al. on 51 COVID-19 patients with RT-PCR and chest CT tests, it was shown that CT scan sensitivity was 98% for diagnosing COVID-19 infection, while RT-PCR sensitivity was 71%. Reasons for less sensitivity of RT-PCR may include the following: (1) low development of this diagnostic technology, (2) differences in the level of diagnostic sensitivity in different manufacturers, (3) low viral load, and (4) incorrect clinical sampling. The use of chest CT is recommended especially in patients with clinical symptoms of the disease and with negative RT-PCR.[47]
In a study by Farnoosh et al. on 167 COVID-19 patients with RT-PCR and chest CT tests, the CT scan evidence of viral infection has been shown to be positive in some patients with negative RT-PCR.[12] In fact, in the patients with negative RT-PCR who have clinical symptoms, it is best to not only test, but also use CT scan and other diagnostic methods.
Most patients on radiography also have bilateral opacity.[48,49] Various radiological patterns are observed during the course of the disease.[50] At the beginning of the disease, patchy shadows and interstitial changes are seen at the margins of the lungs in the chest X-ray. As the disease progresses in more severe cases, the bronchi and even the entire lung may become involved, and pleural thickening and pleural effusion may occur.[4]
No active lesion in the lung of patient A on admission on day 20 of the disease (a1) is seen, but in the same patient the upper right lung interstitial infiltration can be observed six days after admission (a2). In patient B, interstitial infiltration is seen on admission in the left lower lobe on day 15 of the disease (b1) and remains for five days after admission (b2). The Figure 3 was adapted from a study by Hsih et al.[51]
Figure 3.
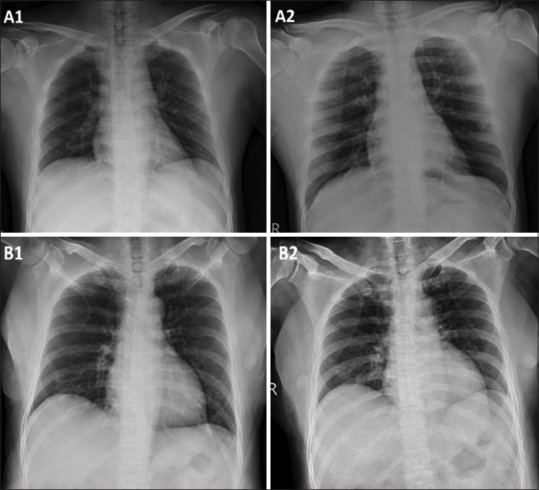
No active lesion in the lung of patient A on admission on day 20 of the disease (a1) is seen, but in the same patient the upper right lung interstitial infiltration can be observed six days after admission (a2). In patient B, interstitial infiltration is seen on admission in the left lower lobe on day 15 of the disease (b1) and remains for five days after admission (b2). Source: Images adapted from Hsih et al.[51]
Another finding that helps diagnose SARS-CoV-2 in patients is laboratory serological findings. Jingliu et al. examined the immunological properties of peripheral blood in patients with COVID-19, and showed that the severity of the disease was associated with neutrophil count (increased number of neutrophils may indicate the severity of inflammatory responses), T cell count, especially CD8+ T cells (lymphopenia indicate the impairment of immune system by the viral infection) and the level of inflammatory cytokines in the peripheral blood. In this study, they stated that N8R (the neutrophil-to-CD8+ T cell ratio) and neutrophil-to-lymphocyte ratio may serve as a useful factor reflecting the severity of the imbalance of inflammation and immune responses in patients with COVID-19, and can be used as a powerful predictor of early detection of severe COVID-19 cases, and may help to gain a better understanding of immune system dysfunction, and may help physicians to provide timely intervention to patients with COVID-19. The study also evaluated 40 positive patients, 13 of which had severe symptoms and the rest mild disease; increased white blood cell, elevated neutrophils, lymphopenia, increased alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, creatine kinase, and ferritin, were observed especially in severe cases.[25] Guan et al. examined 1099 patients with confirmed positive SARS-CoV-2, 926 of which had mild symptoms and 173 had severe symptoms. Lymphopenia, leukopenia, and decreased platelet count, and total bilirubin were seen, especially in more severe patients.[52] Alhazzani et al. showed that 25% of patients had leukopenia and 63% had lymphocytopenia, as well as aspartate aminotransferase levels were elevated in 37% of patients.[45] In a study of Guan et al., 82.1% of patients had lymphopenia.[35] In a study of Rodriguez-Morales et al., the leukopenia was mentioned as one of the important factors in the diagnosis of SARS-CoV-2.[2] Zhang et al. reported a decrease in the count of leukocytes, lymphocytopenia, an increase in inflammatory parameters such as C reactive protein, and anti-inflammatory cytokines (interleukin [IL]-6, tumor necrosis factor-α, IL-8, etc.).
Treatment
There is currently no antiviral treatment or vaccine for SARS-CoV-2. Vaccine production is a big challenge, and research and development of new drugs is a long process. In the event of a sudden outbreak of such epidemics, where scientists cannot produce a new drug with traditional principles, a regular screening will usually be performed on the effect of existing drugs.[12] Common treatments for these patients include paracetamol, nonsteroidal anti-inflammatory drugs, nasal irrigation, chloroquine, and mucolytics.[20]
Wang et al. found a satisfactory effect of remdesivir and chloroquine on 2019-nCoV control. Previous experience with the use of systemic steroids in the treatment of coronavirus-related infections such as SARS and MERS has not yielded acceptable outcomes, so the use of systemic steroids in the treatment of SARS-CoV-2 is not recommended. There are intrinsic differences in the epidemiology of COVID-19 and seasonal influenza, though symptoms overlap. Molecular diagnostic testing for SARS-CoV-2 is critical for all patients with acute respiratory illness. If the COVID-19 and influenza epidemics overlap and peak around the same time, tightening mitigation strategies are helpful to control virus spread. Social distancing and stay-at-home are proper measurements and can reduce demand on hospitals and protect vulnerable populations [Table 1].
Table 1.
Comparison between coronavirus disease 2019 and flu-like diseases symptoms[43]
| Symptoms | Coronavirus | Influenza | Cold |
|---|---|---|---|
| Speed of onset | Rarely | Suddenly | Rarely |
| Fever | Common (99%), <40°C | Common (99%), >40°C | Common (99%), <40°C |
| Tremor | Mild, rarely | Common, severe | Moderate, rarely |
| Headache | Rarely (7%) | More common | Rarely |
| Cough | Common (60%), dry | Common (60%), wet | Rarely, dry |
| Sore throat | Rarely (20%) | Common | Common |
| Myalgia | Relatively rare (35%) | More common | Rarely |
| Fatigue feeling | Moderately common (70%) | Severely common | Rarely, weak |
| Rhinorrhea (runny nose) | Very rare | Rare | Very common |
| Nasal congestion | Moderately common | Rare | More common |
| Anorexia | Relatively common (40%) | Common | Relatively common |
| Abdominal cramp | Rarely (3%) | Rarely | Highly rare |
| Sneezing | Severely rare | Severely rare | Severely common |
| Vomiting and nausea | Rarely (≥10%) | Relatively common (25%) | Severely rare |
| Diarrhea | Rarely (10%) | Relatively common (25%) | - |
| Impacts of predisposing factor | Completely effective | Completely effective | Not so effective |
Traditional Chinese medicine has also been used to improve the treatment process. A few notable points:
These antiviral drugs may be promising therapeutic options for the treatment of COVID-19
Interaction of antiviral drugs with other therapeutic drugs should be considered
Lopinavir/ritonavir side effects, such as diarrhea, nausea, vomiting, and liver damage, should also be considered
The use of three or more antiviral drugs at the same time is not recommended and the use of drugs associated with severe side effects in the patient is not recommended
Further studies are needed to determine the effectiveness of these drugs in the treatment of SARS-CoV-2 Table 2.[4,25]
Table 2.
Recommended drugs[43]
| One of the candidate drugs in the treatment of SARS-CoV-2, which prevents viral replication through direct intervention and increases the body immune response | Interferon-alpha (IFN α) |
| it was first known as a protease inhibitor in the synthesis and replication of human immunodeficiency virus (HIV), which causes the virus to produce immature and non-infectious particles. Evidence has proven that lopinavir/ritonavir alone or with other viral drugs has been shown to be effective in the treatment of SARS or MERS | Lopinavir/ritonavir (Kaletra) |
| it is a nucleoside analogue with extensive antiviral activity that can prevent RNA and DNA replication in the virus. It has been used to treat patients with SARS alone or in combination with steroids. It can be considered as a candidate in the treatment of SARS-CoV-2 | Ribavirin |
| It is a low-cost, low-risk anti-malarial drug that has been also used as a drug against autoimmune diseases for more than 70 years. It effectively suppresses SARS-CoV-2, in vitro[43] | Chloroquine |
| it is an antiviral drug against influenza infection, and has an acceptable in vitro inhibitory effect on reducing SARS-CoV replication. A retrospective cohort study found that Arbidol alone or in combination with other antiviral drugs had favorable outcomes in the treatment of COVID-19 pneumonia. | Arbidol (umifenovir) |
| there are reports of SARS-CoV and MERS-CoV inhibition in the body. An in vitro study showed that the drug blocked SARSCoV-2 at low micromolecular concentrations | Remdesivir |
In patients who are unable to provide adequate immune response against disease, passive immunization is a suitable method for treating some diseases.[54]
Plasma therapy is one of the treatments that have been achieved so far with the desired results. Mortality was reportedly decreased in a number of patients with SARS-CoV in Taiwan and Hong Kong who received plasma therapy at the onset of the disease, resulting in significant benefits such as reduced plasma viral load to an unmeasurable level within 24 h after plasma injection. Thus, in theory, the plasma therapy can have suitable outcomes in the treatment of SARS-CoV-2, although more studies are still needed.[4,55] Studies have shown that the use of Live Plasma in the treatment of SARS-CoV-1 and MERS-CoV has had very good outcomes and has reduced the viral load without side effects and reduced mortality in patients.[52] The US Food and Drug Administration approved the use of convalescent plasma to treat severe and life-threatening COVID-19 patients. Currently, there are contradictory opinions about the use of convalescent plasma. Some guidelines oppose the use of convalescent plasma in critically ill patients by stating that level of neutralizing antibodies against SARS-CoV-2 is still unknown.
Risk Factors
Although most patients are middle-aged adults, children are also affected. The SARSCoV-2 is more common in men than in women, health workers are also one of the groups at risk for the disease.[24] According to reports from China[52,53,56] and Italy, older people, especially those with underlying diseases, are at higher risk for serious illness and death. Some of the risk factors for more serious illness and hospitalization in the intensive care units include over the age of 60 years, male gender, underlying conditions such as diabetes, malignancies, cardiovascular disease, chronic respiratory disease, chronic kidney disease, hypertension, and weakened immune system.[4,45]
Prevention
Health measures and infection control are essential to reduce the damage caused by SARSCoV-2; reducing and controlling human-to-human transmission is vital to prevent the secondary infections. Based on previous experience in managing SARS and MERS infections, the WHO recommends that infection control interventions reduce the risk of transmitting acute respiratory infections. Some of the infection control interventions include preventing close contact with people suffering from acute respiratory infections, frequent hand washing, especially after direct contact with infected people or their surroundings, and preventing contact with domestic or wild animals. In addition, people with symptoms of an acute respiratory infection should observe the etiquette of coughing and sneezing (keeping distance, covering the mouth and nose when coughing and sneezing, and washing hands). Standards for infection prevention and control, especially in emergency departments, are recommended for the health-care providers.[25,56,57] The healthcare workers should follow the procedures and policies of infection control of healthcare institutions to prevent the transmission of the infection. The employees exposed to patient aerosols are advised to use well-equipped respirators (N95, FFP2, or similar) in addition to surgical/medical masks and other protective equipment (gloves, clothing, goggles, and face shield). The aerosols can be produced during endotracheal intubation, bronchoscopy, open suction, nebulizer therapy, manual ventilation before intubation, physical contact with the patient, weaning the patient from mechanical ventilation, noninvasive positive-pressure ventilation, tracheostomy, and cardiopulmonary resuscitation.[45,58]
Conclusion
One of the most dangerous pandemics the world has ever faced is COVID-19, which is caused by the SARS-CoV-2 and is a major threat to the general public and healthcare workers. There is not enough knowledge about this disease yet, and there is no specific vaccine or drug to prevent and treat this disease, and it has affected the countries with the most advanced healthcare systems. Currently, the most effective strategy for disease management is to follow the recommendations for infection control, especially regular hand washing and appropriate social distancing policies. It is not clear when people will be able to return to normal life. It seems that people need to get used to this way of life until research leads to the development of an effective vaccine and drug to manage the disease. In fact, the viral epidemics require an integrated international strategy to control and prevent the outbreak of the disease. It is very important for all people to follow the rules and strategies to help control the disease, and these laws must be enforced and people must obey them. Cooperation between countries is also essential for effective policies to control the disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We appreciate all the medical staff working at the frontline against COVID-19 and the citizens' contributing to the society during this outbreak.
References
- 1.McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, et al. Epidemiology of Covid-19 in a long-term care facility in king county, washington. N Engl J Med. 2020;382:205–11. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Morales AJ, Cardona-Ospina JA, Ocampo GE, Peña VR, Rivera HY, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Diseas. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jalilian N, Ziapour A, Mokari Z, Kianipour N. A study of the relationship between the components of spiritual health and happiness of students at Kermanshah University of Medical Sciences in 2016. Ann Trop Med Public Health. 2017;10:1010–4. [Google Scholar]
- 4.Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID-19): Current status and future perspective. Int J Antimicrob Agents. 2020;55:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoosefi Lebni Y, Abbas J, Moradi F, Salahshoor MR, Chaboksavar F, Irandoost SF, et al. How the COVID-19 pandemic effected economic, social, political, and cultural factors: A lesson from Iran. Int J Soc Psychiatry. 2020. pp. 1–3. In Press. https://journals.sagepub.com/doi/full/100.1177/0020764020939984 . [DOI] [PMC free article] [PubMed]
- 6.Yoosefi lebni J, Irandoost S, Mehedi N, Sedighi S, Ziapour A. The Role of Celebrities during the COVID-19 Pandemic in Iran: Opportunity or Threat? Disaster Med Public Health Preparedness. 2021:1–7. doi: 10.1017/dmp.2021.297. In Press. doi: 10.1017/dmp.2020.498. [DOI] [PubMed] [Google Scholar]
- 7.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbas J, Wang D, Su Z, Ziapour A. The Role of Social Media in the Advent of COVID-19 Pandemic: Crisis Management, Mental Health Challenges and Implications. Risk Manag Healthcare Policy. 2021;14:1917–32. doi: 10.2147/RMHP.S284313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NeJhaddadgar N, Ziapour A, Zakkipour G, Abbas J, Abolfathi M, Shabani M. Effectiveness of telephone-based screening and triage during COVID-19 outbreak in the promoted primary healthcare system: a case study in Ardabil province, Iran. J Public Health. 2020. pp. 1–6. In Press. https://link.springer.com/article/10.1007/s10389-020-01407-8 . [DOI] [PMC free article] [PubMed]
- 10.Ziapour A, Zokaei A, Kahrizy F. A Theoretical study of the standing of social investment in the health sector. Soc Sci. 2016;11:3682–7. [Google Scholar]
- 11.Abbasi P, Kianipour N, Ziapour A. A study of the status of students' social health at kermanshah university of medical sciences and the role of demographic variables. J Clin Diagn Res. 2018;12:VC10–4. doi: 10.7860/JCDR/2017/25114.10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farnoosh G, Alishiri G, Hosseini Zijoud SR, Dorostkar R, Farahani JA. Understanding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease (COVID-19) Based on available evidence-a narrative review. J Mil Med. 2020;22:1–11. [Google Scholar]
- 13.Mohammadi M, Esfandnia A, Rezaei S, Ziapour A. Performance evaluation of hospitals under supervision of kermanshah medical sciences using pabonlasoty diagram of a five-year period (2008-2012) Life Sci J. 2014;11(1):77–81. [Google Scholar]
- 14.Montazeri N, Kianipour N, Nazari B, Ziapour A, Bakhshi S. Health promoting behaviors among university students: a case-sectional study of Kermanshah University of Medical Sciences. Int J Pediatr. 2017;5:5091–9. [Google Scholar]
- 15.Abbasi P, Kianipour N, Demir Özdenk G, Ziapour A. Dataset of leisure time among students at Kermanshah University of Medical Sciences and its relationship with health-related quality of life (HRQOL) Data Brief. 2018;21:122–7. doi: 10.1016/j.dib.2018.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azizi MR, Atlasi R, Ziapour A, Abbas J, Naemi R. Innovative human resource management strategies during the COVID-19 pandemic: A systematic narrative review approach. Heliyon. 2021:e07233. doi: 10.1016/j.heliyon.2021.e07233. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet. 2003;361:1767–72. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbas J, Ling J, Ziapour A, Hamza Shuja K. The Role of interventions to manage reduce covid-19 mortality rate of the COVID-19 patients worldwide. Foundation Univ J Psychol. 2020;4:33–6. [Google Scholar]
- 19.Bhattacharya S, Singh A, Hossain MM. Health system strengthening through Massive Open Online Courses (MOOCs) during the COVID-19 pandemic: An analysis from the available evidence. J Educ Health Promot. 2020;9:195. doi: 10.4103/jehp.jehp_377_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter european study. Europ Arch Oto-Rhino-Laryngol. 2020;277:2251–61. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbasi P, Kianipour N, Ziapour A. Correlation of the components of student's lifestyles and their health promotion. J Clin Diagn Res. 2018;12:LC01–4. [Google Scholar]
- 22.Karyani KA, Faramani SR, Amini S, Doroh RV, Berenjian F, Dizaj M, et al. World one-hundred days after COVID-19 outbreak: Incidence, case fatality rate, and trend. J Educ Health Promot. 2020;9:199. doi: 10.4103/jehp.jehp_483_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kootesh BR, Raisi M, Ziapour A. Investigation of relationship internet addict with mental health and quality sleep in students. Acta Med Mediterran. 2016;32(5):1921–5. [Google Scholar]
- 24.Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARSCoV-2): Facts and myths. J Microbiol Immunol Infect. 2020;53:404–12. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrobial Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panahi S, Ashrafi-rizi H, Panahi M. Exposure to coronavirus (COVID-19) using narrative and simulated experience approaches: A commentary. J Educ Health Promot. 2020;9:135. doi: 10.4103/jehp.jehp_267_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoosefi Lebni J, Ziapour A, Mehedi N, Irandoost SF. The Role of Clerics in Confronting the COVID-19 Crisis in Iran. J Relig Health. 2021:1–8. doi: 10.1007/s10943-021-01295-6. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santacroce L, Charitos IA, Del Prete R. COVID-19 in Italy: An overview from the first case to date. Electronic J General Med. 2020;17:Em235. [Google Scholar]
- 29.Shanbehzadeh M, Kazemi-Arpanahi H, Mazhab-Jafari K, Haghiri H. Coronavirus disease 2019 (COVID-19) surveillance system: Development of COVID-19 minimum data set and interoperable reporting framework. J Educ Health Promot. 2020;9:203. doi: 10.4103/jehp.jehp_456_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Cw, Liu Xf, Jia Zf. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet (London, England) 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–94. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalhori RP, Ziapour A, Kianipour N, Foroughinia A. A study of the relationship between lifestyle and happiness of students at Kermanshah University of Medical Sciences over 2015–2016. Ann Trop Med Public Health. 2017;10:1004. [Google Scholar]
- 34.Zokaei A, Ziapour A, Kianipour N. Evaluation of relationship between organizational culture and job satisfaction among employee of Kermanshah University of Medical Sciences. Soc Sci. 2016;11:4005–12. [Google Scholar]
- 35.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv. 2020;26:1–6. [Google Scholar]
- 36.Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill. 2020;25(5):1–5. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James SL, Lucchesi LR, Bisignano C, Castle CD, Dingels ZV, Fox JT, et al. Morbidity and mortality from road injuries: results from the Global Burden of Disease Study 2017. Injury prev. 2020;26(Supp 1):46–56. doi: 10.1136/injuryprev-2019-043302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quilty BJ, Clifford S, Flasche S, Eggo RM CMMID nCoV working group. Effectiveness of airport screening at detecting travellers infected with novel coronavirus (2019-nCoV) Euro Surveill. 2020;25(5):1–5. doi: 10.2807/1560-7917.ES.2020.25.5.2000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nazari B, Bakhshi S, Kaboudi M, Dehghan F, Ziapour A, Montazeri N. A Comparison of quality of life, anxiety and depression in children with cancer and healthy children, kermanshah-iran. Int J Pediatr. 2017;5:5305–14. [Google Scholar]
- 40.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann Intern Med. 2020;172:577–82. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–36. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. World Health Organization; 2020. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV)Infection is Suspected: Interim Guidance, 25 January 2020. [Google Scholar]
- 43.Rezabakhsh A, Ala A, Khodaei SH. Novel coronavirus (COVID-19): A new emerging pandemic threat. J Res Clin Med. 2020;8:1–6. [Google Scholar]
- 44.Djalante R, Lassa J, Setiamarga D, Mahfud C, Sudjatma A, Indrawan M, et al. Review and analysis of current responses to COVID-19 in Indonesia: Period of January to March 2020. Progress Disaster Sci. 2020;6:100091. doi: 10.1016/j.pdisas.2020.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–87. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur Radiol. 2020;30:4381–9. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;296:E115–E117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir Med. 2020;8:506–17. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbasi P, Timareh M, Ziapour A, Kianipour N. A study of the components of happiness and the role of demographic variables among the students at kermanshah university of medical sciences. J Postgrad Med Instit. 2018;32:173–8. [Google Scholar]
- 50.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis. 2020;20:425–34. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsih WH, Cheng MY, Ho MW, Chou CH, Lin PC, Chi CY, et al. Featuring COVID-19 cases via screening symptomatic patients with epidemiologic link during flu season in a medical center of central Taiwan. J Microbiol Immunol Infect. 2020;53:459–66. doi: 10.1016/j.jmii.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeh KM, Chiueh TS, Siu LK, Lin JC, Chan PK, Peng MY, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–22. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: Old tricks for new challenges. Crit Care. 2020;24:91. doi: 10.1186/s13054-020-2818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaboudi M, Dehghan F, Ziapour A. The effect of acceptance and commitment therapy on the mental health of women patients with type II diabetes. Ann Trop Med Public Health. 2017;10:1709–13. [Google Scholar]
- 56.Mehta N, Mazer-Amirshahi M, Alkindi N, Pourmand A. Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am J Emerg Med. 2020;38:1488–93. doi: 10.1016/j.ajem.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaboudi M, Kianipour N, Ziapour A, Dehghan F. A study of health literacy components and their relationships with health-promoting behaviors in students at kermanshah university of medical sciences. Int J Pediatr. 2017;5:6721–29. [Google Scholar]
- 58.Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;296:E115–7. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]