Abstract
BACKGROUND:
Transfusion of healthy red blood cells (RBCs) after storage is important. One of the storage lesions on blood bags is oxidative stress. One way to prevent increased oxidative stress is to use antioxidant nanoparticles (NPs). Superoxide dismutase (SOD) and catalase (CAT) play an important role in antioxidant defense on RBC. poly lactic-co-glycolic acid (PLGA) is a nontoxic biodegradable polymer that is approved by the Food and Drug Administration for drug delivery. This study aimed to assess dose-dependent efficacy of SOD-CAT-polyethylene glycol -PLGA on RBCs storage.
MATERIALS AND METHODS:
Using a descriptive study, during 1 month, twenty donors from Bojnourd Blood Donation Center were selected. NPs with different concentrations were injected into the satellite bags after directing blood to them. On target days, experiments were performed on the samples taken. Electrospray was employed to prepare SOD-CAT-PLGA NPs. Twenty packed RBCs were isolated from the whole blood bags by the mechanical method, and certain amount of product was transferred to the satellite bags. On days 1, 7, 14, 21, 28, and 35, bags were sampled. Malondialdehyde (MDA), prooxidant-antioxidant balance (PAB), and Annexin V were performed on the samples taken. The repeated measures analysis with the help of SPSS software version 20 was performed on samples.
RESULTS:
MDA increased in both groups. The maximum increase in test group was seen in concentration 12 mg (MDA Day 14, test [1.93 ± 0.3], [P MDA < 0.001]). Maximum increase in PAB was seen in concentration 12 mg (from 444 ± 1.7 to 563 ± 2.5) (P PAB = 0.000). Furthermore, PS expression increased in the concentration of 12 mg greater than other concentration in consecutive (from 5.00 ± 0.8 to 22.26 ± 1.7, [P < 0.001]).
CONCLUSION:
Evaluation of dose dependency showed that different concentrations of antioxidant NPs affect RBC. This effect can be changed oxidative stress and apoptosis. Using both changes to evaluate functional and toxicity can be helpful.
Keywords: Antioxidant effect, blood bank, eryptosis, nanoparticles, oxidative stress
Introduction
Transfusion of the stored red blood cells (RBCs) is an ideal technique for improving oxygen delivery to tissue when other treatments are no longer suitable, especially in fetal medicine and neonatal intensive care, trauma, surgery, and cancer.[1] Depending on the blood bag preservative solution, the shelf life of RBCs can be estimated for up to 35 or 42 days.[2] While millions of whole blood and red blood cellular products are transfused annually, the red blood packed cells are still the most commonly transfused component.[3] Blood bags stored under blood bank conditions undergo structural and functional deterioration, collectively referred to as “RBC storage lesions.”[4] RBCs play an important role in antioxidant defense as oxygen carriers.[5] In normal RBCs, a balance is established between oxidants and antioxidants. So that the produced superoxide ions would be converted into hydrogen peroxide by the superoxide dismutase (SOD) enzyme; thus, its toxicity would be decreased and again the produced hydrogen peroxide is converted into water by the glutathione peroxidase (GPX) enzyme. GPX needs other enzymes including glutathione reductase and glucose-6-phosphate dehydrogenase, to function properly. Catalase (CAT), however, does not require the same enzymes with the same function.[6] Under the condition of blood storage, this balance can be disrupted, and oxidative stress occurs resulting in the oxidation of proteins and lipids of the RBC membrane. Oxidative stress is now recognized as one of the pathological factors in various diseases including chronic diseases, depression, liver diseases, and venous thrombosis.[7,8,9,10] Transfusion of healthy RBCs after storage, today, is key issues faced by researchers in the field of blood transfusion.[11,12]
One way to prevent increased oxidative stress is to use antioxidant nanoparticles (NPs).[13,14] Polylactic-co-glycolic acid (PLGA) is a nontoxic biodegradable polymer that is currently approved by the Food and Drug Administration (FDA) for several applications in drug delivery, diagnosis, and other clinical applications.[15] Polyethylene glycol (PEG) is a polymer of choice in drug delivery systems. This USFDA-approved polymer is popular due to its tunable properties and well-established safety profile.[16] It also increases the solubility of the compound. Antioxidant NPs containing SOD and CAT enzymes can prevent the increase of oxidative stress in blood storage that finally improves transfusion of healthy RBC storage. Despite the widespread use of NPs, they can also have negative effects including on RBCs.[17,18,19] One of the effects of antioxidant NPs can be eryptosis.[20] In addition to their beneficial roles, NPs can also have negative effects. These adverse effects on the use of NPs that can be injected into humans should be given more attention. One of the negative effects of NPs can be oxidative stress.[21] In addition to the ineffectiveness of antioxidant NPs, oxidative stress can cause adverse effects on RBCs. Therefore, using a suitable concentration to prevent these negative effects seems useful. The dose of NPs is one of the important factors in the efficiency of NPs.[22]
The purpose of this study is to identify the extent of reduction in antioxidant enzymes and the dose-dependent effect of antioxidant NPs in RBCs storage. We used PLGA NPs containing of SOD-CAT antioxidant enzymes (SOD-CAT-PEG-PLGA) to neutralize oxidative stress. Finding the optimum concentration to prevent toxicity in RBCs can be effective in preventing storage lesion.
Materials and Methods
PEG-PLGA with a lactide to glycolide ratio of 50:50 (MW = 5–50 kDa) was purchased from Iran Polymer and Petrochemical Institute. The SOD (S7571) and CAT (C40) were purchased from Sigma-Aldrich Co. SOD and CAT assay kits were purchased from ZellBio GmbH Co. Sampling Site Coupler sold as 96/cs by Fenwal. CPD-A1 blood bag (JMS Blood Transfer Bag-Singapore). Phosphatidylserine detection kit (IQ-Product-Netherlands) and all other chemicals were obtained from Merck chemicals (Germany).
Preparation of nanoparticles
Electrospray was employed to prepare SOD, CAT-loaded PLGA NPs (SOD-CAT-PLGA NPs). Initially, required amount of soft the PEG-PLGA polymer were dissolved in deionized water (0.5%–2% w/w). Then, SOD and CAT were added to PLGA solution at polymer/enzyme ratio of 40–50:1. The final solution was stirred for 30 min at ambient temperature to allow a complete dissolution of polymer and enzymes. The solution was then loaded into a 1 ml plastic syringe with blunt-ended 21G stainless steel needle. The polymeric solution was electrosprayed through the nozzle at a flow rate of 1 ml/h using a programmable syringe pump (SP1000HOM, Fannavaran Nano-Meghyas [FNM] Ltd., Tehran, Iran) and an applied voltage of 8.8 kV by high voltage generators (HV35POC, FNM Ltd., Tehran, Iran). The positive electrode was connected to the needle with alligator clips. The distance between needle tip and collector was set to 15 cm. Particles were collected on a sheet of aluminum foil for 1 h (at a temperature of 20°C and the relative humidity 50%).
Particle size and zeta potential measurement
Particle size, polydispersity index (PDI), and zeta potential of SOD and CAT-loaded polymeric NPs were measured using dynamic light scattering (DLS, Zetasizer Nano-ZS, Malvern Instruments Ltd., UK).
Evaluation of efficacy and toxicity of nanoparticles
To evaluate the performance of NPs after dissolution in phosphate-buffered saline (PBS), the activity of SOD and CAT enzymes was measured in supernatant with SOD and CAT assay kits. Furthermore, to study the release of enzymes, activity was measured at specified intervals ultracentrifugation at 30.000 rpm for 30 min at 4°C[23] in the supernatant (Beckman L8-70M Ultracentrifuge: Beckman Instruments, Palo Alto, CA, USA).
Subjects
Out of all the donors referring for donations to blood donation center, twenty donors were selected. Participants were apparently healthy, and an informed consent form was completed by them. After initial screening of donors by doctor, the blood bags were collected. All blood bags were made of PVC with three satellite bags and CPDA1 preservative solution.
Blood bags
After performing viral tests such as HIV-Ab, HBS-Ag, HCV-Ab, and HTLV-Ab, packed RBCs were isolated from the whole blood bags by the mechanical method. For each blood bag, a certain amount of product was transferred to three satellite bags. Then, specific concentrations of NPs entered to satellite bags by sampling coupler (test group). The packed red cell concentrates were kept for 35 days from the time of donation (5 weeks) under the standard conditions, and on days 1, 7, 14, 21, 28, and 35, bags were sampled. Experiments were performed on the samples taken. Satellite bags containing NPs were compared with matched control bags.
Oxidative stress in blood bags
Malondialdehyde (MDA) test was performed in the target days on samples.
Prooxidant-antioxidant balance
The oxidant-antioxidant balance can be estimated using tetramethylbenzidine powder and two enzymatic and chemical reactions. In the enzymatic reaction, tetramethylbenzidine chromogen is oxidized by hydrogen peroxidase to cationic tetramethylbenzidine, and in the chemical reaction, cationic tetramethylbenzidine is reduced by the uric acid (antioxidant).[24]
Flow cytometric assay
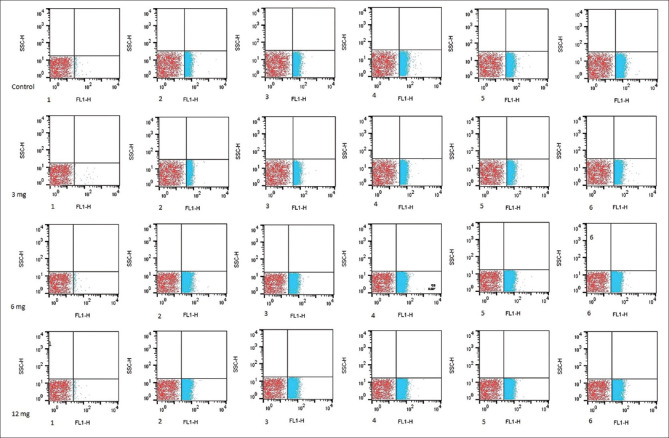
Phosphatidylserine (PS) expression on the surface of erythrocytes was evaluated on the three satellite bag samples by Annexin V-FITC assay kit, and it was done according to the principles stated in the IQ-Product company kit. In summary, RBCs were washed once in (PBS, pH 7.4) and adjusted at 1.0 × 106 cells/mL with manufacturer's buffer. 100 μL of cell suspension incubated with 10 μL Annexin V-FITC, at room temperature in the dark for 30 min. Then, samples of at least 1 × 105 cells were subjected to fluorescence-activated cell sorting (FACS) analysis (BD FACSCalibur Flow Cytometry System: BD Biosciences-USA). The results were analyzed by FlowJo software version 10 (Becton, Dickinson and Company, ashland, oregon USA).
Statistical analysis
Results are expressed as mean values and standard error of the mean. Differences between means were evaluated using the repeated Measurement test when appropriate. Data were managed with the use of SPSS software package (IBM company, New York, USA) 26. P < 0.05 was considered to be statistically significant.
Results
Characterization of nanoparticles
The mean size of the NPs measured using DLS was 291 nm with PDI = 0.08 and zeta potentials = 28.6 ± 1.5 mv. Concentrations of 3, 6, and 12 mg NPs entered the test blood bags.
Subject
The participants were twenty donors referring to the blood transfusion organization of Bojnurd, Iran. All donors were men with O blood group.
Oxidative stress changes parameters
The amount of MDA increased in both control and test groups [Table 1]. This increase was greater in the control group than in the test. The maximum increase in the test group were seen in concentration 12 mg (MDA Day14, Test [1.93 ± 0.3], [p MDA < 0.001]).
Table 1.
Comparison of malondialdehyde and prooxidant-antioxidant balance parameters of control and concentration of 3, 6, and 12 mg test groups in target weeks
| Parameters | MDA | PAB | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Group | Control | Test | Control | Test | ||||
|
|
|
|||||||
| Concentration (mg) | - | 3 | 6 | 12 | - | 3 | 6 | 12 |
| Day 1 | 0.93±0.1 | 0.90±0.5 | 0.91±0.2 | 0.92±0.1 | 448±2.7 | 438±2.2 | 442±1.9 | 444±1.7 |
| Day 7 | 0.90±0.0 | 0.88±0.3 | 0.90±0.1 | 0.92±0.4 | 470±2.8 | 455±2.5 | 461±2.4 | 466±1.0 |
| Day 14 | 2.03±0.3 | 1.87±0.1 | 1.89±0.3 | 1.93±0.3 | 497±3.1 | 481±2.0 | 487±2.6 | 490±1.3 |
| Day 21 | 2.57±0.2 | 2.40±0.3 | 2.46±0.2 | 2.51±0.2 | 528±1.3 | 5.22±1.4 | 524±2.5 | 526±2.4 |
| Day 28 | 3.03±0.4 | 2.57±0.2 | 2.69±0.6 | 2.92±0.5 | 549±1.0 | 536±2.8 | 540±1.8 | 540±2.8 |
| Day 35 | 3.69±0.7 | 3.18±0.5 | 3.36±0.4 | 3.46±0.5 | 567±1.7 | 550±2.5 | 558±2.1 | 563±2.5 |
Values are expressed as mean±SEM. MDA=Malondialdehyde, PAB=Prooxidant-antioxidant balance, SEM=Standard error of mean
Furthermore, PAB changed in blood bags in the target weeks after donation (Control from 448 ± 2.7 to 567 ± 1.7) [Table 1]. The maximum increase in the test group was seen in concentration 12 mg (from 444 ± 1.7 to 563 ± 2.5) (P PAB < 0.001).
Expression of phosphatidylserine
PS expression increased in the concentration of 12 mg greater than other test groups in consecutive weeks [Table 2] (concentration 12 mg of PS from 5.00 ± 0.8 to 22.26 ± 1.7, [P < 0.001]).
Table 2.
Comparison of phosphatidylserine parameters of control and concentration of 3, 6, and 12 mg test groups in target weeks
| Parameters | PS | |||
|---|---|---|---|---|
|
| ||||
| Group | Control | Test | ||
|
| ||||
| Concentration (mg) of NPs | - | 3 | 6 | 12 |
| Day 1 | 3.99±0.5 | 3.89±0.5 | 4.00±0.6 | 5.00±0.8 |
| Day 7 | 7.25±1.2 | 6.42±0.8 | 19.60±0.4 | 20.20±0.2 |
| Day 14 | 16.20±1.4 | 14.43±1.2 | 16.03±0.7 | 18.70±0.4 |
| Day 21 | 13.13±0.6 | 11.93±0.5 | 21.30±0.5 | 21.70±0.5 |
| Day 28 | 15.43±0.4 | 14.60±1.1 | 21.80±1.1 | 21.70±0.7 |
| Day 35 | 10.78±0.69 | 9.04±0.8 | 23.10±0.9 | 22.26±0.7 |
Values are expressed as mean±SEM. PS=Phosphatidylserine, SEM=Standard error of mean
Discussion
Based on the results, the performance of antioxidant nanoparticles containing SOD and CAT in blood storage is dose dependent [Figure 1]. Due to their very small size, nanoparticles can enter tissues. Therefore, it is important to pay attention to the different dimensions of their impact in vivo. Physicochemical factors can affect the cytotoxicity of nanoparticles including size, surface, shape, aggregation, and dose dependent.[25] There are several methods for investigating the toxicity of nanoparticles in RBC including oxidative stress and PS expression (eryptosis).
Figure 1.
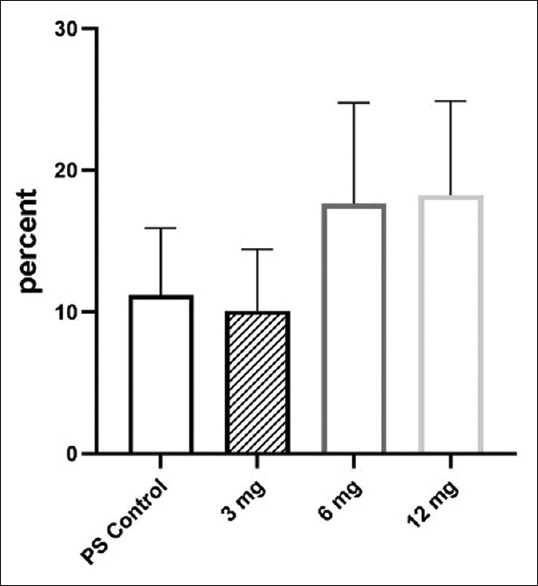
Comparison of PS expression of control and different concentration of antioxidant nanoparticle in target weeks. Values are expressed as mean ± standard error of the mean. PS: Phosphatidylserine
Nanoparticle aggregation plays a vital role in creating intracellular response.[26] In the study of Santiago Martinez Legaspi and Laura Segatori, one of the effective factors in cell autophagy is the aggregation of nanoparticles.[25] With increasing storage time, Changes in pH, electrolyte, or salt in the blood storage can nanoparticles aggregation. In a study of M Vippola et al.,[27] it was shown that one of the reasons for the aggregation of nanoparticles is the change in pH. In our study, changes in blood bag pH could be the cause of aggregation and ultimately changes in cellular response to cell death so that the amount of PS increased at the cell surface. Further more, an increase in nanoparticle concentration can intensify these aggregations. Lankoff et al. found that the concentration of silver nanoparticles plays a role in its accumulation and toxicity, and the lower the concentration, the lower the toxicity.[28]
In our study, the toxicity increased with increasing nanoparticle concentration. Antioxidant nanoparticles prevent oxidative stress. However, aggregation in nanoparticles can affect the binding and functional regions of nanoparticles and protein enzymes (SOD and CAT).[29] Sun et al. showed in a study One of the causes of eryptosis is oxidative stress.[30] In our study, with increasing the nanoparticle concentration, PS expression increased [Figure 2], which could be the cause of damage in RBCs [Table 2]. Increases in MDA and PAB can indicate a defect in the function of antioxidant nanoparticles [Table 1]. Wadhwa et al. Showed that uptake and phagocytosis of NPs by RBCs lead to membrane lipid oxidation and increased antioxidant enzyme activity.[31] Therefore, the optimum dose of nanoparticles can affect its ability to prevent oxidative stress.
Figure 2.
Dose-dependent effect of different concentration (3, 6, and 12 mg) of antioxidant nanoparticle on phosphatidylserine expression in target groups in consecutive weeks (1–6). Red dot blot areas indicate negative control and blue dot blot indicates red blood cells attached to Annexin V-FITC
The effect of dose on cytotoxicity is important. Generally, nanoparticles increase apoptosis in a dose-dependent effect.[32] In our study, a similar result was observed due to the increased expression of PS. Finding the minimum dose of cytotoxicity, in addition, to reduce the potential risks of nanoparticles in vivo can help the health of stored blood. Therefore, it is important to determine the optimum dose to prevent the effects of oxidative stress. Dose dependence causes oxidative stress and eryptosis by increasing the entry of nanoparticles into cells.[33,34] Furthermore, Libi et al. showed that the association between PLGA-containing nanoparticles and erythrocyte membranes is concentration dependent. As the concentration increases, this bond increases.[35] In our study, this increase in binding can be seen in the amount of PS. Binding of nanoparticles to the surface of RBCs can also cause changes in its surface properties which causes deformation and eventually hemolysis.[31]
Conclusion
For proper performance of antioxidant nanoparticles, using the optimum dose is helpful. Otherwise, it will cause storage lesion in blood storage. The use of injectable nanoparticles in blood storage can be a way to prevent the loss of insufficient sources of stored blood. This can be very important for RBCs, which are most commonly used in blood transfusions. There are several methods for investigating toxicity and dose dependency in different nanoparticles. Some of these methods are based on changes in oxidative stress. Methods based on apoptosis but help more. Using both methods to evaluate functional and toxicity can be helpful. It is suggested to consider the dose dependence of different polymers before presenting more useful methods for the effect of antioxidant nanoparticles on RBCs. Furthermore, the effects of nanoparticles in whole blood also need further investigation. The use of a method to remove nanoparticles from blood bag before transfusion can also be considered. One of the limitations using of antioxidant nanoparticles RBC storage is the antigenic effects on other cells.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgment
The authors would like to thank all the individuals involved in preparing and supporting this work, especially the faculty members of Iran University of Medical Sciences and staff of the Blood Transfusion Organization.
This article is the result of an approved research project No. 98-1-5-14647 with ethics number IR. IUMS. REC.1398.314 in Iran University of Medical Sciences, School of Allied Medical Sciences.
References
- 1.Rudrappan RB, Promotion H. Evaluating the knowledge and practices of nurses and paramedics in blood transfusion services – A survey in the states of Tamil Nadu and Pondicherry, India. 2019;8:48. doi: 10.4103/jehp.jehp_293_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meledeo MA, Peltier GC, McIntosh CS, Bynum JA, Cap AP. Optimizing whole blood storage: Hemostatic function of 35-day stored product in CPD, CP2D, and CPDA-1 anticoagulants. Transfusion. 2019;59:1549–59. doi: 10.1111/trf.15164. [DOI] [PubMed] [Google Scholar]
- 3.Adams F, Bellairs G, Bird AR, Oguntibeju OO. Biochemical storage lesions occurring in nonirradiated and irradiated red blood cells: a brief review. BioMed research international. 2015 Jan;29:2015. doi: 10.1155/2015/968302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida T, Prudent M, D'Alessandro AJ. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 2019;17:27–52. doi: 10.2450/2019.0217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Öztaş Y, Boşgelmez İİ. Pathology. Academic Press; 2020. Jan 1, Oxidative stress in sickle cell disease and emerging roles for antioxidants in treatment strategies; pp. 65–75. [Google Scholar]
- 6.Ghafouri-Khosrowshahi A, Ranjbar A, Mousavi L, Nili-Ahmadabadi H, Ghaffari F, Zeinvand-Lorestani H, et al. Chronic exposure to organophosphate pesticides as an important challenge in promoting reproductive health: A comparative study. J Educ Health Promot. 2019;8:149. doi: 10.4103/jehp.jehp_148_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. 2020;25:1270–6. doi: 10.1016/j.drudis.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radical Biology and Medicine. 2020 Mar 8; doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 9.García-Sánchez A, Miranda-Díaz AG, Cardona-Muñoz EG. The role of oxidative stress in physiopathology and pharmacological treatment with pro-and antioxidant properties in chronic diseases. Oxid Med Cell Longev. 2020;2020:2082145. doi: 10.1155/2020/2082145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Zennadi R. Oxidative stress and thrombosis during aging: The roles of oxidative stress in RBCs in venous thrombosis. Int J Mol Sci. 2020;21:4259. doi: 10.3390/ijms21124259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nalbant D, Cancelas JA, Mock DM, Kyosseva SV, Schmidt RL, Cress GA, et al. In premature infants there is no decrease in 24-hour posttransfusion allogeneic red blood cell recovery after 42 days of storage. Transfusion. 2018;58:352–8. doi: 10.1111/trf.14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Alessandro A, Reisz JA, Zhang Y, Gehrke S, Alexander K, Kanias T, et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 2019;3:884–96. doi: 10.1182/bloodadvances.2018029629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrabi SS, Yang J, Gao Y, Kuang Y, Labhasetwar V. Nanoparticles with antioxidant enzymes protect injured spinal cord from neuronal cell apoptosis by attenuating mitochondrial dysfunction. J Control Release. 2020;317:300–11. doi: 10.1016/j.jconrel.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.dos Santos Tramontin N, da Silva S, Arruda R, Ugioni KS, Canteiro PB, de Bem Silveira G, et al. Gold nanoparticles treatment reverses brain damage in Alzheimer's disease model. Mol Neurobiol. 2020;57:926–36. doi: 10.1007/s12035-019-01780-w. [DOI] [PubMed] [Google Scholar]
- 15.Cappellano G, Comi C, Chiocchetti A, Dianzani U. Exploiting PLGA-based biocompatible nanoparticles for next-generation tolerogenic vaccines against Autoimmune Disease. Int J Mol Sci. 2019;20:204. doi: 10.3390/ijms20010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’souza AA, Shegokar R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13:1257–75. doi: 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- 17.Fang W, Chi Z, Li W, Zhang X, Zhang Q. Comparative study on the toxic mechanisms of medical nanosilver and silver ions on the antioxidant system of erythrocytes: From the aspects of antioxidant enzyme activities and molecular interaction mechanisms. J Nanobiotechnology. 2019;17:66. doi: 10.1186/s12951-019-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian Y, Kim K, Ngo T, Kim I, Bae ON, Lim KM, et al. Silver nanoparticles promote procoagulant activity of red blood cells: A potential risk of thrombosis in susceptible population. Part Fibre Toxicol. 2019;16:9. doi: 10.1186/s12989-019-0292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-Rodríguez NL, Tavárez S, González-Sánchez ZI. In vitro toxicity assessment of zinc and nickel ferrite nanoparticles in human erythrocytes and peripheral blood mononuclear cell. Elsevier. 2019;57:54–61. doi: 10.1016/j.tiv.2019.02.011. doi: 10.1016/j.tiv. 2019.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Ferdous Z, Beegam S, Tariq S, Ali BH, Nemmar A. The in vitro effect of polyvinylpyrrolidone and citrate coated silver nanoparticles on erythrocytic oxidative damage and eryptosis. Cell Physiol Biochem. 2018;49:1577–88. doi: 10.1159/000493460. [DOI] [PubMed] [Google Scholar]
- 21.Ferraro SA, Domingo MG, Etcheverrito A, Olmedo DG, Tasat DR. Neurotoxicity mediated by oxidative stress caused by titanium dioxide nanoparticles in human neuroblastoma (SH-SY5Y) cells. J Trace Elem Med Biol. 2020;57:126. doi: 10.1016/j.jtemb.2019.126413. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2020;10:1–24. doi: 10.1038/s41573-020-0090-8. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singhal A, Morris VB, Labhasetwar V, Ghorpade A. Nanoparticle-mediated catalase delivery protects human neurons from oxidative stress. Cell Death Dis. 2013;4:e903. doi: 10.1038/cddis.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghayour-Mobarhan M, Alamdari DH, Moohebati M, Sahebkar A, Nematy M, Safarian M, et al. Determination of prooxidant – Antioxidant balance after acute coronary syndrome using a rapid assay: A pilot study. Angiology. 2009;60:657–62. doi: 10.1177/0003319709333868. [DOI] [PubMed] [Google Scholar]
- 25.Martinez Legaspi S, Segatori L. Aggregation behavior of nanoparticle-peptide systems affects autophagy. Bioconjug Chem. 2019;30:1986–97. doi: 10.1021/acs.bioconjchem.9b00266. [DOI] [PubMed] [Google Scholar]
- 26.Budama-Kilinc Y, Cakir-Koc R, Zorlu T, Ozdemir B, Karavelioglu Z, Egil AC, Kecel-Gunduz S. Assessment of Nano-toxicity and Safety Profiles of Silver Nanoparticles. In: Khan M, editor. Silver nanoparticles-fabrication, characterization and applications. London: Intechopen; 2018. Jul 18, p. 185. doi: 10.5772.intechopen. 75645. [Google Scholar]
- 27.Vippola M, Falck GC, Lindberg HK, Suhonen S, Vanhala E, Norppa H, et al. Preparation of nanoparticle dispersions for in-vitro toxicity testing. Hum Exp Toxicol. 2009;28:377–85. doi: 10.1177/0960327109105158. [DOI] [PubMed] [Google Scholar]
- 28.Lankoff A, Sandberg WJ, Wegierek-Ciuk A, Lisowska H, Refsnes M, Sartowska B, et al. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol Lett. 2012;208:197–213. doi: 10.1016/j.toxlet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Kittler S, Greulich C, Gebauer JS, Diendorf J, Treuel L, Ruiz L, Gonzalez-Calbet JM, Vallet-Regi M, Zellner R, Köller M, Epple M. The influence of proteins on the dispersability and cell-biological activity of silver nanoparticles. Journal of Materials Chemistry. 2010;20(3):512–8. doi: 10.5772.intechopen. 75645. [Google Scholar]
- 30.Sun Y, Liu G, Jiang Y, Wang H, Xiao H, Guan G. Erythropoietin protects erythrocytes against oxidative stress-induced eryptosis in vitro. Clin Lab. 2018;64:365–9. doi: 10.7754/Clin.Lab.2017.170924. [DOI] [PubMed] [Google Scholar]
- 31.Wadhwa R, Aggarwal T, Thapliyal N, Kumar A, Priya, Yadav P, et al. Red blood cells as an efficient in vitro model for evaluating the efficacy of metallic nanoparticles. 3 Biotech. 2019;9:279. doi: 10.1007/s13205-019-1807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar V, Sharma N, Maitra SS. In vitro and in vivo toxicity assessment of nanoparticles. International Nano Letters. 2017 Dec 1;7(4):243–56. doi: 10.5772.59381. [Google Scholar]
- 33.Kong B, Seog JH, Graham LM, Lee SB. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine (Lond) 2011;6:929–41. doi: 10.2217/nnm.11.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthuraman P, Ramkumar K, Kim DH. Analysis of dose-dependent effect of zinc oxide nanoparticles on the oxidative stress and antioxidant enzyme activity in adipocytes. Appl Biochem Biotechnol. 2014;174:2851–63. doi: 10.1007/s12010-014-1231-5. [DOI] [PubMed] [Google Scholar]
- 35.Libi S, Calenic B, Astete CE, Kumar C, Sabliov CM. Investigation on hemolytic effect of poly (lactic co-glycolic) acid nanoparticles synthesized using continuous flow and batch processes. Nanotechnology Reviews. 2017 Apr 1;6(2):209–20. doi: 10.1515/ntrev-2016-0045. [Google Scholar]