Abstract
Genetic screens in Drosophila have identified p50cdc37 to be an essential component of the sevenless receptor/mitogen-activated kinase protein (MAPK) signaling pathway, but neither the function nor the target of p50cdc37 in this pathway has been defined. In this study, we examined the role of p50cdc37 and its Hsp90 chaperone partner in Raf/Mek/MAPK signaling biochemically. We found that coexpression of wild-type p50cdc37 with Raf-1 resulted in robust and dose-dependent activation of Raf-1 in Sf9 cells. In addition, p50cdc37 greatly potentiated v-Src-mediated Raf-1 activation. Moreover, we found that p50cdc37 is the primary determinant of Hsp90 recruitment to Raf-1. Overexpression of a p50cdc37 mutant which is unable to recruit Hsp90 into the Raf-1 complex inhibited Raf-1 and MAPK activation by growth factors. Similarly, pretreatment with geldanamycin (GA), an Hsp90-specific inhibitor, prevented both the association of Raf-1 with the p50cdc37-Hsp90 heterodimer and Raf-1 kinase activation by serum. Activation of Raf-1 via baculovirus coexpression with oncogenic Src or Ras in Sf9 cells was also strongly inhibited by dominant negative p50cdc37 or by GA. Thus, formation of a ternary Raf-1–p50cdc37–Hsp90 complex is crucial for Raf-1 activity and MAPK pathway signaling. These results provide the first biochemical evidence for the requirement of the p50cdc37-Hsp90 complex in protein kinase regulation and for Raf-1 function in particular.
The mitogen-activated protein kinase (MAPK) phosphorylation cascade, composed of Raf kinase, Mek (MAPK kinase), and Erk (MAPK) itself, relays proliferative and differentiative signals from the plasma membrane to the transcriptional and cell cycle progression machinery (38). Although it is established that Ras-GTP is required to tether Raf-1 to the plasma membrane (reviewed in reference 1), the subsequent events that lead to Raf-1 activation are poorly understood. The major reasons for this are (i) only a small fraction (∼3%) of the total Raf-1 cytoplasmic pool needs to become activated for effective signaling (23) and (ii) the entire process of Raf-1 plasma membrane recruitment and activation is rapid and transient (for reviews, see references 37 and 45). Thus, identification of both crucial intermediates and the causative relationships in Raf-1 activation has been difficult. However, it is clear that the N-terminal domain of Raf-1 acts to repress the activity of the C-terminal kinase domain and that its deletion results in constitutive activation of the kinase (25, 68). Phosphorylation of Raf-1 and association with other proteins in response to receptor activation most likely leads to a conformational change in Raf-1 that relieves this repression (37, 45).
Raf-1 fractionated from various cell types exists in large (300- to 500-kDa) multiprotein complexes (78). Known Raf-1-associated proteins include 14-3-3, Hsp90, and pp50, a 50-kDa Hsp90-associated protein (45, 78). 14-3-3 is required for Raf-1 function but probably is not directly involved in the Raf-1 activation process (37, 42, 44). The function of the pp50-Hsp90 complex in Raf-1 activation has yet to be addressed. pp50 had previously been widely found in Hsp90-containing kinase complexes, notably involving v-Src (reviewed in reference 4), and with both cytoplasmic and membrane localized Raf-1 (66, 78). Hsp90-associated pp50 has recently been identified immunologically and by peptide mapping to be the 50-kDa gene product of the mammalian Cdc37 homologue p50cdc37 (51).
Cdc37 was originally identified in yeast as a cell cycle mutant that gives a G1 cell cycle arrest phenotype (56). Cutforth and Rubin (8) subsequently isolated an allele of Drosophila Cdc37 (Dcdc37) that functioned as a dominant enhancer of the sevenless phenotype in the Drosophila eye. However, these genetic experiments have not identified where and how Dcdc37 functions in the sevenless mitogen-activated protein kinase (MAPK) pathway. Vertebrate Cdc37 was cloned first from chicks (21, 27) and subsequently from mammals (20, 33, 50, 51, 69). The structure of Cdc37 reveals no significant homologies to proteins of known function. The yeast protein is homologous to mammalian and Dcdc37 through only the first 30 amino acids and diverges significantly thereafter. Despite this limited homology, Dcdc37 will complement the yeast gene (8). The cell cycle phenotype of cdc37 appears to be due to a diminished capacity of G1 cyclins and the cyclin-dependent kinase Cdc28 to associate (19). Subsequent work by ourselves and others has found that mammalian p50cdc37 interacts with Cdk4 and accumulates Hsp90 to it (9, 20, 33, 69). Though p50cdc37 has been found to interact with diverse kinase families, its interactions are selective in that, for instance, among cyclin-dependent kinases, it interacts with Cdk4 and the closely related Cdk6 but not with Cdk2 (9, 28, 69). Thus, from genetic studies, Cdc37 appears to operate in both the cell cycle and the Ras/Raf/MAPK pathway in close cooperation with its Hsp90 chaperone partner (28).
Hsp90 is an abundant and highly conserved protein (54) that is essential in yeast and Drosophila (2, 8). Unlike the more general Hsp70 and Hsp60 chaperones, Hsp90 appears to have substrate-specific folding activity (30, 47, 54). It has been best characterized for its essential role in steroid hormone receptor signaling, where it interacts with and modulates receptor function through a dynamic and regulated series of interactions with a defined set of chaperone cofactors (54, 65). Hsp90’s conformation and activity have been proposed to be regulated by nucleotide binding, and its associations and activity can be inhibited by geldanamycin (GA) an Hsp90-specific antibiotic which competes for ATP binding to Hsp90 (22, 55). It has been further proposed that p50cdc37 may serve to target Hsp90 to a subset of protein kinases and thereby help them achieve an active conformation (28, 53). However, the distantly related yeast Cdc37p by itself has been shown to have chaperone activity in vitro (32).
The available mammalian association data (63, 66, 78), although not informative about the functional significance of Raf-1 association with Hsp90 and p50cdc37, nevertheless are complemented by genetic evidence from Drosophila. Cutforth and Rubin (8) found that Hsp90 mutations enhance the sevenless phenotype in the Drosophila eye as does Dcdc37 and thus also functions in the MAPK pathway. Subsequently, van der Straten et al. (76) identified Hsp90 alleles that suppress the multiple R7 phenotype caused by the constitutive high-level activation of a membrane-targeted D-Raf kinase domain (RaftorY9). In fact, the two Hsp90 point mutations recovered in this screen were the strongest dominant suppressors of the multiple R7 photoreceptor cell phenotype caused by the Ras-independent, activated Torso RTK-Raf chimeric protein. Importantly, the mutant Hsp90 proteins identified in these genetic screens exhibited reduced binding to D-Raf-1 and correlated with diminished Raf kinase activity (76). Thus, neither deletion of the N-terminal suppression domain nor membrane anchoring bypasses the requirement of D-Raf-1 for Hsp90 association.
Here, we have addressed directly the biochemical role of p50cdc37 and its partner, Hsp90, during Raf-1 activation and signaling to Mek and Erk. We found that p50cdc37 and Hsp90 each interact directly with Raf-1 but that p50cdc37 is the main determinant of the assembly of heterotrimeric complex. Disruption of the Raf-1–p50cdc37–Hsp90 ternary complex with the Hsp90 inhibitor GA or with a dominant negative p50cdc37 inhibits Raf-1 activity. Serum stimulation promotes Raf-1–p50cdc37–Hsp90 complex formation and coexpression of p50cdc37 with Raf-1 in insect cells is sufficient to activate Raf-1. Moreover, p50cdc37 synergizes with Src for Raf-1 activation. Our data, coupled with the aforementioned genetic studies, indicate that p50cdc37 and Hsp90 are critical components of the MAPK cascade and of the Raf-1 activation complex in particular.
MATERIALS AND METHODS
Cell culture and transfections.
Cos-1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 0.1 μg of penicillin and streptomycin per ml. Freshly plated cells were transfected at 70 to 80% confluence with a total of 7.5 μg of DNAs per 100-mm-diameter dish, using Lipofectamine (Life Technologies) or Targefect (Targetting Systems, San Diego, Calif.). In experiments requiring replicate transfected cultures, cells were split 24 h after the start of transfection into appropriate smaller dishes so that 20 to 24 h later cultures would have achieved confluence. At this point, cells were serum starved for an additional 16 to 18 h. For stimulations, serum (at 20%) or epidermal growth factor (EGF; 100 ng/ml) was directly added for 5 more min before cells were lysed. A 2-mg/ml stock solution of geldanamycin GA in dimethyl sulfoxide (DMSO) or DMSO alone was diluted 1:1,000 in the culture media for the times indicated before cells were either lysed directly or serum stimulated. Solubilized cell extracts were then quantitated for protein content by the Bradford assay and analyzed by direct Western blotting or by protein purification using antibodies or, for overexpressed glutathione S-transferase (GST) fusion proteins, by glutathione (GSH)-Sepharose chromatography, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. Baculovirus infection and culture of Spodoptera frugiperda Sf9 cells was performed essentially as described by Morrison (43). Unless otherwise indicated, all baculoviruses were infected at comparable levels of multiplicity of infection (MOI).
Antibody reagents.
The anti-p50cdc37 antibodies were raised in rabbits against the chick (pNG13 clone [21]) or human GST-p50cdc37 protein. Anti-epitope tag antibodies obtained from Boehringer (antihemagglutinin [anti-HA] 12CA5 and anti-Myc 9E10) or from Kodak (anti-FLAG M5). Santa Cruz Biotechnology was the supplier for additional antibodies, including ones against Raf-1 (C-12) and GST (Z-5). Monoclonal antibodies against Raf-1 and p50cdc37, used in the experiment described in Fig. 1B, were purchased from Transduction Laboratories. Anti-active MAPK polyclonal antibody V6671 was obtained from Promega, and antibodies directed against Hsp90 (SPA-830 and SPA-771) and recombinant human Hsp90 purified from Escherichia coli (SPP-771) were obtained from Stressgen.
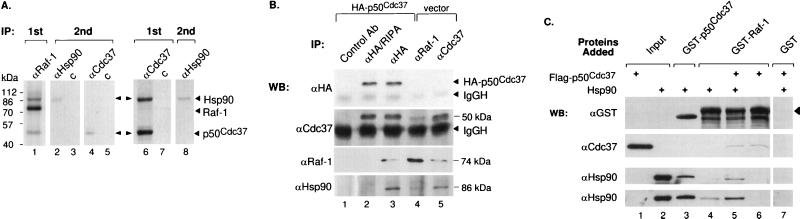
FIG. 1.
Association of p50cdc37, Hsp90, and Raf-1 in vivo and in vitro. (A) Lane 1, anti-Raf-1 IP from [35S]methionine-labeled Cos-1 cells. Lanes 2 to 5, after the primary anti-Raf IP was boiled for 2 min in the presence of 0.5% SDS, a second IP was carried out with anti-Hsp90 or control (c) antibody (lanes 2 and 3) or with polyclonal anti-p50cdc37 or nonimmune rabbit (c) antibody (lanes 4 and 5, respectively). Lanes 6 and 7, anti-p50cdc37 primary IPs and nonimmune rabbit serum IPs, respectively, from [35S]methionine-labeled Cos-1 cells. A second IP with anti-Hsp90 antibody (lane 8) was performed with a fraction of the anti-p50cdc37 primary immunoprecipitate identical to that run in lane 6. The relative migration of molecular weight marker proteins is indicated. (B) Plasmids pMT3-HA-p50cdc37 and pMT3-HA were transiently transfected into Cos-1 cells, and extracts were immunoprecipitated with anti-FLAG antibody (Ab) M5 as a control (lane 1) or anti-HA monoclonal antibody 12CA5 under either denaturing or mild conditions (RIPA or NP-40 LB buffer; lanes 2 and 3, respectively) or, to purify endogenous Raf-1 and p50cdc37 proteins, with anti-Raf-1 (lane 4) and anti-p50cdc37 (lane 5) monoclonal antibodies. Immunoprecipitated proteins were examined by Western blotting (WB) and ECL for the presence of transfected HA-p50cdc37 with anti-HA antibody or for the presence of both transfected and endogenous p50cdc37 with anti-p50cdc37 rabbit antisera. Endogenous Raf-1 and Hsp90 proteins were detected with rabbit-anti-Raf-1 antibodies and rat-anti-Hsp90, respectively (top to bottom panels). IgGH, precipitating IgG antibody heavy chains. (C) FLAG-p50cdc37 (immunoaffinity purified from baculovirus-infected Sf9 cells) and Hsp90 (recombinant E. coli; Stressgen) were assayed in vitro for binding to bacterially produced GST-Raf-1, GST-p50cdc37, or GST alone as indicated by GSH-Sepharose pull-down assays and Western blotting (WB) with the indicated antibodies as described in Materials and Methods. Anti-Hsp90 immunoblotting performed with two distinct Hsp90-specific antibodies (SPA-830 and SPA-771) is shown (bottom two panels). The first two lanes indicate the input amounts of purified proteins added. The arrowhead denotes the position of the full-length GST-Raf-1 above the breakdown products.
Cloning and constructs.
For eukaryotic expression, the complete open reading frame for the human p50cdc37 cDNA was subcloned by PCR into the EcoRI sites of pMT3 and pSG5 vectors and in frame with N-terminal HA and FLAG, respectively, peptide epitopes. Similarly, GST-p50cdc37 constructs were placed by PCR into the BamHI-NotI sites of the pEBG eukaryotic (57) and pGEX2T (Pharmacia) prokaryotic expression vectors. For expression in insect (S. frugiperda Sf9) cells, the entire open reading frame for the FLAG-p50cdc37 fusion protein was subcloned from the pSG5 constructs into the EcoRI/NotI sites of the pFASTBAC1 (Life Technologies) baculovirus vector. Deleted versions of the FLAG-p50cdc37 fusion protein were produced by using appropriate enzyme digestion of the full-length inserts in pSG5, followed by agarose gel electrophoresis and DNA religation and further subcloned into pFASTBAC1 by the same approach. Cloned inserts were verified by DNA sequencing. Expression plasmids for Raf-1, Ras, and v-Src used in this study have been described previously (14, 35, 46, 63).
In vitro synthesis of radiolabeled p50cdc37.
Different full-length and deletion forms of p50cdc37 were transcribed and translated in vitro from the pSG5 expression constructs in the presence of 20 μCi of [35S]methionine (EXPRESS protein labeling mix; NEN), using the coupled rabbit reticulocyte lysate and T7 RNA polymerase system (Promega).
Metabolic labeling.
Nontransfected or transfected cells 48 to 60 h posttransfection were initially incubated for 2 h in methionine-free medium containing 2% dialyzed fetal serum and then labeled for 4 h with [35S]methionine (NEN) in fresh medium. Cells were then lysed, and equal amounts (counts per minute) of labeled lysate were immunoprecipitated, as described below for nonlabeled lysates, and analyzed by SDS-PAGE and fluorography.
Immunoprecipitation and immunoblotting.
Cells were harvested 48 to 60 h after transfection and extracted in Nonidet P-40 lysis buffer (NP-40 LB; 0.5% NP-40, 20 mM HEPES [pH 7.5], 0.1 M NaCl, 2 mM EGTA, 10% glycerol, 50 mM glycerophosphate, 2 mM dithiothreitol [DTT]) containing protease and phosphatase inhibitors (2 mM sodium vanadate, 1 mM NaF, 0.2 mM phenylmethylsulfonyl fluoride, 10 μg each of leupeptin and aprotinin per ml). For measuring Raf-1 kinase activity in Sf9 cells in the experiments represented in Fig. 4, 5B, and C, and 6A, NP-40 LB was substituted with radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris [pH 8.0], 137 mM NaCl, 10% [vol/vol] glycerol, 1% [vol/vol] NP-40, 0.1% [wt/wt] SDS, 0.5% sodium deoxycholate, 2 mM EDTA). Cell lysates were cleared by centrifugation at 4°C for 15 min. The protein concentration was measured with a kit from Bio-Rad and normalized for all samples in each individual total Western or immunoprecipitation (IP) experiment. Equivalent aliquots of cleared supernatants were mixed with Laemmli SDS-loading buffer (25 mm Tris [pH 6.8], 1% SDS, 2.5% β-mercaptoethanol, 0.5 mg of bromophenol blue per ml, 5% glycerol), separated by SDS-PAGE, and transferred to a Hybond-ECL membrane (Amersham). Following preclearing, IP was performed for 2 h at 4°C, using 0.5 μg of purified anti-FLAG, anti-c-Myc, anti-HA monoclonal antibody or indicated purified rabbit polyclonal antisera. Immune complexes were then recovered by binding to GammaBind-Plus Sepharose (Pharmacia). Alternatively, GST fusion proteins were purified using preequilibrated GSH-Sepharose (Pharmacia) as described elsewhere (64). After three washes with 50 volumes lysis buffer, GSH-Sepharose-bound proteins and immunocomplexes were processed for electrophoresis as described above. The entire protein purification procedure was done at 4°C. Immunoblot detection was performed with specified antibodies in 5% dried milk in phosphate-buffered saline and developed as described by the manufacturer of the enhanced chemiluminescence (ECL) system (Amersham). For reblotting, membranes were incubated in 20 mM DTT–1% SDS in phosphate-buffered saline for 10 min at ambient temperature.
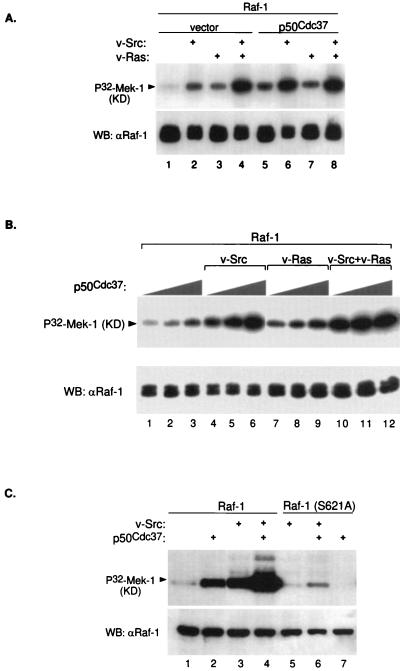
FIG. 4.
Sf9 cell coinfection with p50cdc37 results in Raf-1 activation. (A) Baculoviruses encoding Raf-1, v-Src, v-Ras, or p50cdc37 were infected in Sf9 cells in the combinations indicated; 48 h postinfection, Raf-1 was immunoprecipitated with anti-Raf-1 polyclonal antibody C-12 in RIPA buffer and tested for its ability to phosphorylate recombinant kinase-defective (KD) Mek-1 as described in Materials and Methods (top). As controls, kinase assay reactions were also Western blotted (WB) with the same anti-Raf-1 antibody (bottom). (B) Baculovirus coinfection followed by Raf-1 kinase assay (top) and Western blot (bottom) were performed as for panel A. In each set, increasing amounts of p50cdc37 baculovirus (at 1, 3, and 9×) were added as indicated. (C) Wild-type Raf-1 and Raf-1(S621A) were either expressed alone or coexpressed with indicated v-Src or p50cdc37 baculovirus constructs, immunoprecipitated, and assayed for in vitro kinase activity as for panel A.
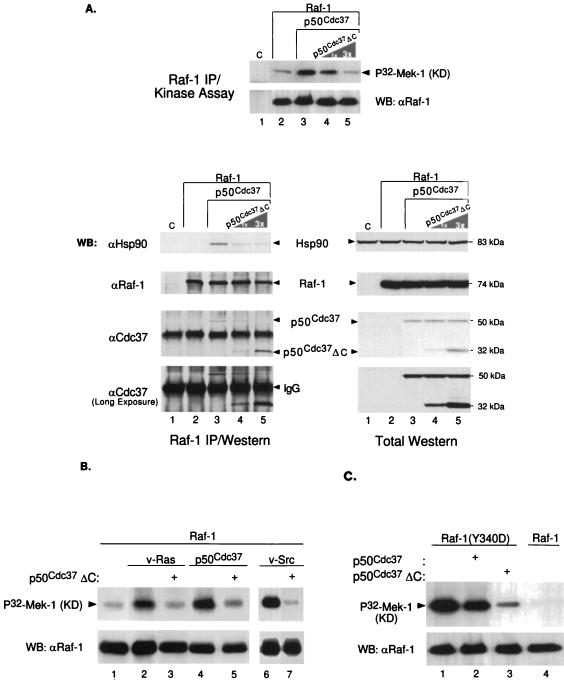
FIG. 5.
p50cdc37ΔC disrupts Raf-1–p50cdc37–Hsp90 complex formation and abrogates p50cdc37-mediated Raf-1 activation. (A) A baculovirus encoding p50cdc37ΔC mutant was coinfected at the same MOI or a threefold greater excess MOI with p50cdc37 (lanes 4 and 5) and Raf-1. Control Sf9 cultures included an empty-vector baculovirus infection (C; lane 1) and cultures infected with Raf-1 alone or in combination with p50cdc37 (lanes 2 and 3, respectively); 48 h postinfection, cells were solubilized in NP-40 LB, and a portion of each of the five extracted cultures was harvested, subjected to anti-Raf-1 IPs under nondenaturing conditions using NP-40 LB (see Materials and Methods), and analyzed either for Raf-1 kinase activity toward kinase-defective (KD) recombinant Mek-1 (top) or for p50cdc37- and Hsp90-associated proteins. For assessment of protein expression, control Western blots (WB) of total cellular extracts are shown on the right. (B and C) p50cdc37ΔC inhibits v-Src and v-Ras activation of Raf-1. (B) Raf-1 was immunoprecipitated and analyzed for its activity toward recombinant inactive Mek-1 from Sf9 cells coinfected with the indicated baculoviruses as described for Fig. 4A. The effect of v-Src (lanes 6 and 7) was examined in a separate experiment involving a shorter kinase assay exposure. (C) The effect of p50cdc37ΔC on the constitutively active Raf-1(Y340D) mutant was examined as described above. For comparison, wild-type Raf-1 was subjected to similar analysis and is shown in lane 4.
Protein purification and in vitro association assays.
GST fusion proteins were produced and purified by GSH-Sepharose affinity chromatography in NETN buffer (20 mM Tris, [pH 8.0], 0.1 M NaCl, 1 mM EDTA, 0.5% NP-40) supplemented with proteinase and phosphatase inhibitors as previously described (64). Kinase-defective bacterial His6-Mek-1 (K97M) was similarly prepared, using a kit from Qiagen. FLAG-p50cdc37 was immunoaffinity purified by agarose-cross-linked anti-FLAG monoclonal antibody M2 (Kodak) according to the supplier’s instructions. For studying in vitro associations, GSH-Sepharose-bound GST fusion proteins were then directly incubated with either purified or in vitro-translated proteins in NETN buffer for 2 h at 4°C. Bound complexes were subsequently washed three times in 50 volumes of prechilled NETN buffer, and after SDS-PAGE they were either immunoblotted or, for [35S]methionine-labeled proteins, directly analyzed by fluorography.
Protein kinase assays.
For kinase reactions, GSH-Sepharose-bound GST fusion proteins or immunocomplexes, prepared as described above, were additionally washed in 50 volumes of kinase buffer (25 mM HEPES [pH 7.5], 10 mM MgCl2, 10 mM MnCl2, 1 mM DTT), drained, and incubated for 15 min at 30°C in 30 μl of fresh kinase buffer containing 20 μM ATP, 5 μCi of [γ-32P]ATP (6,000 Ci/mmol; NEN), and 0.5 μg of recombinant kinase-defective His6-Mek-1(K97M). Assays were terminated by the addition of Laemmli SDS-loading buffer, the boiled samples were resolved by SDS-PAGE, and phosphorylated substrate proteins were quantitated by phosphorimager analysis and autoradiography.
RESULTS
p50cdc37 bridges Hsp90 to Raf-1.
Previously Hsp90 and p50cdc37 were detected by immunological methods in a complex with Raf-1 (51, 66, 78). Here we have used cloned p50cdc37 and Raf-1 proteins to reconstitute and further characterize the precise interactions among p50cdc37, Hsp90, and Raf-1. Cos-1 cells express Raf-1, which is the principal Raf isoform (16), and both Hsp90 and p50cdc37. In accordance with previous findings for other tissues (11, 12, 34, 78), two proteins of approximately 90 and 50 kDa coprecipitate with endogenous Raf-1 in Cos-1 cells (Fig. 1A). Subsequent disruption of the complex and a second round of IP with anti-Hsp90 and anti-p50cdc37 antisera indicates that these two coprecipitating proteins are immunologically related to Hsp90 and p50cdc37, respectively (Fig. 1A, lanes 1 to 5). The converse experiment precipitating first with anti-p50cdc37 antibodies shows stoichiometric coimmunoprecipitation with Hsp90 but reveals only a faint Raf-1 band at the expected 74-kDa range (lanes 6 to 8). This is probably due to the fact that although a significant proportion of Raf-1 protein is bound to p50cdc37 and Hsp90 (19a, 34, 60, 78), only a fraction of p50cdc37, which is present in excess over Raf-1 (not shown) and Hsp90 (1 to 2% of total cytosolic protein), is in a complex with the kinase. Our findings with [35S]methionine-labeled proteins (Fig. 1A, lanes 6 to 8) and by silver staining (not shown) indicate that Hsp90 copurifies in approximately equimolar quantities with p50cdc37 and that the p50cdc37-Hsp90 interaction also occurs in vitro in the absence of other proteins (63).
That the cloned p50cdc37 protein indeed associates with Raf-1 is further supported by the experiments presented in Fig. 1B. HA-p50cdc37 or vector plasmids were transiently transfected into Cos-1 cells, and extracts were immunoprecipitated with anti-FLAG antibody M5 as a control (lane 1) or anti-HA monoclonal antibody 12CA5 under either denaturing or mild conditions (RIPA or NP-40 LB buffer; lanes 2 and 3, respectively) or, to purify endogenous Raf-1 and p50cdc37 proteins, with anti-Raf-1 (lane 4) or anti-p50cdc37 (lane 5) monoclonal antibodies. Immunoprecipitated proteins were then examined by Western blotting and ECL for the presence of transfected HA-p50cdc37 or endogenous p50cdc37 with anti-HA antibody and anti-p50cdc37 rabbit antisera, respectively. Endogenous Hsp90 or Raf-1 proteins were detected with rat-anti-Hsp90 and rabbit-anti-Raf-1 antibodies. In both situations, 50-kDa proteins were found in complex with endogenous Raf-1 and Hsp90. p50cdc37’s associations were sensitive to RIPA buffer (lane 2) and were specific, in that no Hsp90 or Raf-1 could be observed in control antibody IPs (lane 1). Conversely, anti-Raf-1 IPs, followed by Western blotting analysis, identified both p50cdc37 and Hsp90 at lower levels, but in a reproducible manner, to copurify with endogenous Raf-1. Thus, by its size and characteristics of its interaction with Raf-1 and Hsp90, cloned p50cdc37 is most likely pp50, the previously described 50-kDa Hsp90 partner present in the Raf-1 IPs along with Hsp90.
Similar conclusions were reached in vitro, using combinations of purified Hsp90 and p50cdc37 proteins to reconstitute these associations (Fig. 1C). To test whether posttranslationally unmodified Raf-1 can bind to Hsp90 and p50cdc37, GSH-Sepharose-bound GST–Raf-1 that had been produced in E. coli was allowed to associate either with p50cdc37 or Hsp90 alone or with a mixture of the two proteins. Both p50cdc37 and Hsp90 (purified to apparent homogeneity, as judged by silver staining) were found to interact directly and independently with recombinant Raf-1 in vitro (Fig. 1C, bottom panel). Notably, Hsp90’s association with Raf-1 greatly increased when p50cdc37 was present. This result suggests that Hsp90’s association with Raf-1 is induced by a p50cdc37-mediated Raf-1 conformational change or that, more likely, the enhanced association between Raf-1 and Hsp90 (lane 5) is mediated by p50cdc37 acting directly to recruit Hsp90 to Raf-1. In the latter scenario, the existence of two distinct sites on Hsp90, one for associating with the Raf-1 bound p50cdc37 and a second for directly binding to Raf-1, can be envisioned (Fig. 2D). These experiments demonstrate that recombinant p50cdc37 and Hsp90 associate directly and stably with Raf-1, confirming earlier conclusions reached by immunological means (51, 60, 66, 78). Notably, relative to the in vivo situation, Raf-1 association with p50cdc37 Raf-1 association with p50cdc37 is rather modest, suggesting that modifications such as phosphorylation or association with other proteins may regulate the Raf-1 interaction with p50cdc37 and Hsp90 as is the case for its association with 14-3-3 (42).
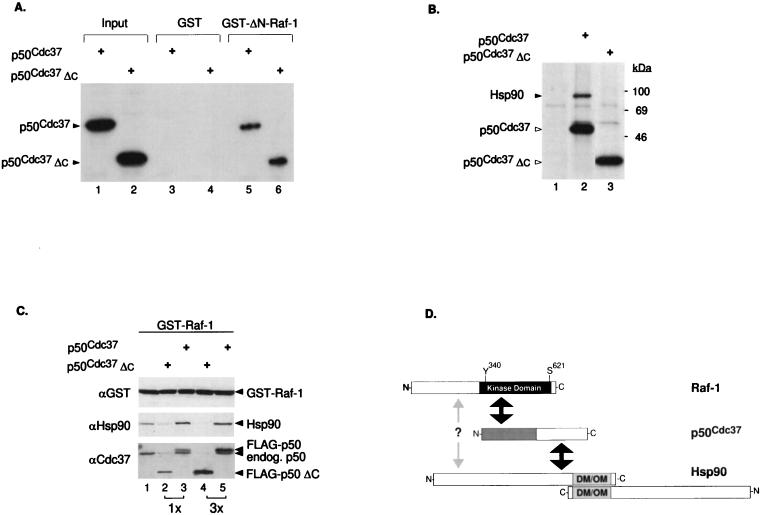
FIG. 2.
The N-terminal half of p50cdc37 mediates association with the catalytic domain of Raf-1 but is impaired for Hsp90 interaction and accumulation to Raf-1. (A) Plasmids pSG5-p50cdc37 and pSG5-p50cdc37ΔC were transcribed and translated in vitro, using T7 RNA polymerase and a reticulocyte lysate system (Promega); 5 μl of each reaction mixture was either analyzed directly (input lanes) or assayed in vitro for binding to either GST or bacterially purified GST–ΔN-Raf-1(Δ26-309) and visualized by SDS-PAGE and fluorography. Comparable results were obtained with full-length GST–Raf-1 (not shown). (B) Cos-1 cells transfected with pSG5-FLAG vector, pSG5-FLAG-p50cdc37, and pSG5-FLAG-p50cdc37ΔC were [35S]methionine labeled, and anti-FLAG IPs in NP-40 LB of each transfected sample were analyzed by SDS-PAGE and fluorography (lanes 1 to 3, respectively). Proteins at the sizes predicted for overexpressed FLAG-p50cdc37 proteins or associated endogenous Hsp90 are also indicated. (C) Two micrograms of pEBG-GST-Raf-1 was cotransfected with 5 μg of pSG5-FLAG vector (lane 1), pSG5-FLAG-p50cdc37 (lanes 2 and 3), or pSG5-FLAG-p50cdc37ΔC (lanes 4 and 5) at 5 or 15 μg as indicated. After 48 h in DMEM-FBS, all five cultures were harvested and lysed in NP-40 LB, and GST–Raf-1 was GSH-Sepharose purified and tested for associated p50cdc37 or Hsp90 proteins with rabbit anti-p50cdc37 or rat anti-Hsp90 antibody. A control anti-GST immunoblot was also included to detect overexpressed GST–Raf-1 (top panel). (D) Diagram indicating regions of interaction between p50cdc37, Raf-1, and Hsp90. The N-terminal half of p50cdc37 (gray area) which corresponds to p50cdc37ΔC is sufficient for interacting with the C-terminal kinase domain of Raf-1, while its C-terminal half mediates Hsp90 interaction (indicated by black arrows). A distinct weak interaction of Raf-1 directly with Hsp90 through as yet unidentified domains is also proposed and is indicated by the gray arrow. Relative positions of the Y340 and S621 phosphorylation sites present on Raf-1 are also indicated. Since Hsp90 can both homodimerize and form oligomers through its C terminus (DM/OM) (41, 48, 49), higher-order complexes of p50cdc37–Raf-1–Hsp90 can also be envisioned.
Since the catalytic C-terminal half of Raf-1 has been reported to be sufficient for interaction with pp50 (66), we tested whether recombinant p50cdc37 binds to the same Raf-1 region. In vitro-translated p50cdc37 bound efficiently to immobilized GST–ΔN-Raf-1, a viral Raf form-like construct (3, 63), but not to GST alone (Fig. 2A) or to the N-terminal Raf-1 regulatory domain alone (not shown). This interaction of p50cdc37 with Raf-1 occurs via the N-terminal half of p50cdc37, as a deletion mutant (p50cdc37ΔC) truncated at Met164 to half the original size is sufficient to interact strongly with GST–ΔN-Raf-1. Interestingly, p50cdc37ΔC is severely compromised in its ability to associate with Hsp90 in transfected Cos-1 cells (Fig. 2B) compared with full-length p50cdc37 which readily associates with its chaperone partner.
We then sought to determine whether this mutant could disrupt the Hsp90–Raf-1 association in a dominant fashion. When p50cdc37ΔC was further coexpressed in Cos-1 cells with GST-tagged Raf-1, endogenous Hsp90 association to Raf-1 was strongly inhibited in a dose-dependent manner, with increasing amounts of p50cdc37ΔC binding to the kinase (Fig. 2C). In contrast, overexpressed wild-type p50cdc37 not only binds to Raf-1 but also recruits Hsp90 to the complex, in agreement with results of the in vitro experiment shown in Fig. 1C. A likely interpretation of this observation is that overexpressed p50cdc37ΔC competes with endogenous p50cdc37 for binding to Raf-1 and that the subsequent Hsp90 association with GST–Raf-1, which largely depends on intact p50cdc37, is prevented (Fig. 2C; compare lanes 1, 3, and 5). Thus, although some direct Hsp90 binding to Raf-1 cannot be ruled out (Fig. 1C, lane 4), we conclude that the p50cdc37 greatly potentiates Hsp90 accumulation into the Raf-1 complex (Fig. 2D) most likely by bridging Hsp90 to Raf-1. This result also suggests that p50cdc37ΔC might interfere with the function of Hsp90 in the Raf-1 complex and potentially acts as a dominant negative allele of p50cdc37 in functional assays (described below).
Inability of Raf-1 to respond to serum activation correlates with its inability to complex with p50cdc37-Hsp90 heterodimers.
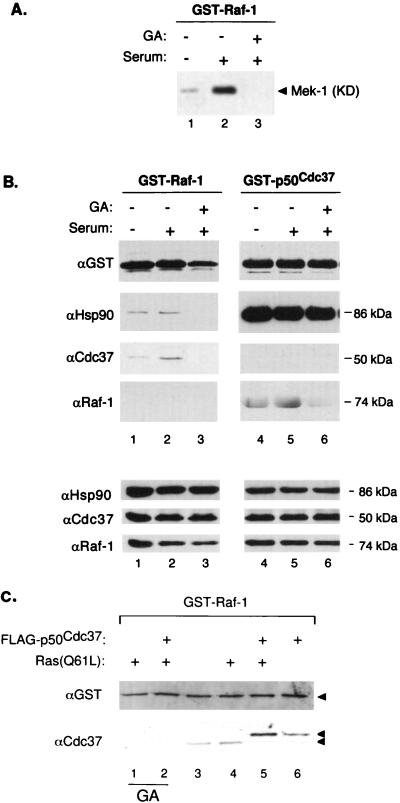
GA, a benzoquinone ansamycin (10), was originally described as a protein kinase inhibitor (74). However, subsequent examination has shown that its effects on kinases are indirect and that it specifically binds to and inhibits the action of Hsp90 (80, 81). GA has been established to be a specific reagent for assessing Hsp90’s role in various signaling systems, including v-Src (80), Raf-1 (60, 61), Lck (24), heme-regulated eukaryotic initiation factor 2α kinase (75), and steroid nuclear receptors (31, 65) (reviewed in references 52 and 58). GA competitively displaces ATP and locks Hsp90 into its ADP-specific inactive conformation, disrupting a dynamic equilibrium in which unliganded steroid receptor complexes alternate among various chaperone heterocomplex intermediates (22, 31, 55, 65). GA-bound Hsp90 is then unable to form productive complexes with its steroid receptor and kinase targets, which subsequently results in their degradation upon prolonged in vivo GA treatment (59, 60, 62, 80). In an attempt to define the roles of p50cdc37 and Hsp90 in Raf-1 kinase heterocomplex formation and activity, we used GA to abrogate Hsp90–Raf-1 association and Raf-1 activation as has been shown by Schulte et al. (60, 61). However, to directly correlate Raf-1’s ability to interact with p50cdc37 and Hsp90 with its kinase activity, we have designed our experiments to assess the effects of GA on Raf-1 at a stage prior to the time when Raf-1 is depleted from the cells due to prolonged GA treatment. In addition, to improve the detection of associated proteins, we have alternatively used GST fusion cDNAs of Raf-1 or p50cdc37 transiently transfected in mammalian cells. GSH-Sepharose-purified GST–Raf-1 and GST-p50cdc37 were then analyzed both for associated proteins and for kinase activity (57, 64).
Cos-1 cells were transfected with either GST–Raf-1 or GST-p50cdc37 and replated into three identical cultures. After these cultures were serum starved overnight, two of the replicate transfections were stimulated with 20% serum with or without a 6-h preincubation with GA, as indicated, while the third plate was left untreated. The resulting cellular extracts were analyzed for overall protein expression and protein association with each purified GST-protein. Further, the purified GST–Raf-1 complexes were examined for in vitro kinase activity, using a recombinant kinase-inactive form of Mek-1 as a substrate (Fig. 3A). Western blotting of total cell extracts revealed that expression of the transfected GST-fusion proteins was approximately three times the level of the corresponding endogenous p50cdc37 and Raf-1 proteins (not shown) and that under these conditions GA treatment slightly reduced the levels of Raf-1 expression but had no apparent effect on p50cdc37 and Hsp90 steady-state levels. From this experiment, the following observations can be made. Consistent with the existing literature, transfected GST–Raf-1 kinase activities was induced by serum but not after GA pretreatment (Fig. 3A). Accordingly, serum stimulation results in small but reproducible enhancement of associations of endogenous p50cdc37 and Hsp90 with GST–Raf-1 (Fig. 3B lanes 1 and 2). In contrast, GA pretreatment abolished activation of Raf-1 by serum and almost entirely eliminated this association (Fig. 3B, lanes 3 and 6). Importantly, Raf-1’s association with p50cdc37-Hsp90 correlates closely with its activity (Fig. 3B, lanes 1 to 3). Previously, GA was shown to decrease Raf-1 activity and expression in NIH 3T3 cells by destabilizing the protein (60, 61). Note that in this experiment, by assaying Raf-1 levels after a much shorter treatment of Cos-1 cells with GA, GST–Raf-1 expression is only modestly reduced at this time (Fig. 3B, lanes 3), but both Hsp90 and p50cdc37 associations with GST–Raf-1 are nearly abolished. Thus, disruption of the Raf-1–p50cdc37–Hsp90 complex by GA occurs prior to Raf-1 degradation and correlates with the inability of Raf-1 to be activated by serum growth factors even though it remains present in the cell at substantial concentrations. Our results with the p50cdc37ΔC further confirm the requirement for Hsp90 association with Raf-1 independently of effects on Raf-1 protein degradation (see below).
FIG. 3.
(A and B) Association of p50cdc37 and Hsp90 with Raf-1 correlates closely with Raf-1 kinase activity. Two micrograms each of pEBG-GST-Raf and pEBG-p50cdc37 were transfected into subconfluent Cos-1 cells, and next day each of the transfected 150-mm-diameter plates was further split into three 100-mm-diameter plates; 16 h later, cultures were fed with serum-free medium for an additional 16 h. GA or only DMSO diluent was then added, followed by serum stimulation as indicated, and the three replicate cultures of each transfection were harvested and solubilized in NP-40 LB. (B, top panels) GST fusion proteins were then purified by GSH affinity chromatography as described in Materials and Methods and analyzed for associated proteins by SDS-PAGE and immunoblotting with the indicated antibodies; (A) 0.2-volume extract portions were similarly processed and tested for GST–Raf-1 kinase activity toward recombinant kinase-defective (KD) Mek-1. (B, bottom panels) Control immunoblots of total cell extracts. Control transfections with empty pEBG vector, followed by GSH pull-down assays and Western blotting, showed that no p50cdc37, Hsp90, or Raf-1 associated with the GST propeptide alone (not shown). (C) pEBG-GST-Raf-1 was transfected into Cos-1 cells alone or with pMT2-Ras(Q61L) and pSG5-FLAG-p50cdc37 as indicated; 48 h later, GST–Raf-1 was isolated from NP-40 LB-solubilized cell extracts and tested by Western blotting and ECL for associated endogenous and overexpressed p50cdc37, using anti-Cdc37 antiserum (bottom). Anti-GST blotting was performed to verify levels of GST–Raf-1 expression and recovery. For lanes 1 and 2, GA (2 μg/ml) was included in the growth medium for 6 h before harvest.
Interestingly, overexpressed GST-p50cdc37 remained sequestered with endogenous Hsp90, and no changes in the association of Hsp90 with GST-p50cdc37 were observed under all experimental conditions, including GA pretreatment. Thus, the locking of Hsp90 into the ADP-bound conformation by GA effects the ability of the Hsp90-p50cdc37 complex to remain associated with Raf-1. Since p50cdc37ΔC does not bind Hsp90 but can nevertheless still bind to Raf-1, this finding implies that the GA-bound conformation of Hsp90 inhibits the ability of bound p50cdc37 to associate with Raf-1 through either steric hindrance, allosteric regulation, or an indirect mechanism. p50cdc37 and Hsp90’s respective associations with endogenous Raf-1 also showed small but reproducible serum-mediated enhancement and almost complete elimination by GA (Fig. 3B lanes 4 to 6). Thus, during serum activation of Raf-1, there is a stabilization of p50cdc37–Hsp90–Raf-1 complex formation. A weak associated MAPKKK activity could be detected in GST-p50cdc37 pull-down-in vitro kinase assays from cells coexpressing exogenous Raf-1 (not shown), consistent with both our observation that the bulk of p50cdc37 is not Raf-1 associated (Fig. 1A) and the fact that only a small fraction of Raf-1 kinase actually becomes activated during signaling (23, 37, 45). A previous related study (78) using standard antibody-based Raf-1 purification found no changes in endogenous p50cdc37 and Hsp90 coprecipitating with active and inactive transfected Raf-1. The availability of cloned p50cdc37, including a new array of Cdc37-specific antibodies, enabled us to perform reciprocal GST-p50cdc37 and GST–Raf-1 pull-down assays. Further, the antibody-free method of isolation allowed us to use higher-stringency GST-protein purification for more accurate assessment of changes in endogenous Raf-1 and p50cdc37 complexed with GST-p50cdc37 and GST–Raf-1, respectively. This, especially in the case of p50cdc37, which on SDS-PAGE migrates closely with immunoprecipitating antibodies, is, as we also find, technically difficult. We have also observed that coexpression of one GST-tagged protein with a non-GST-tagged version of the other improves further the detection of an increase in Raf-1–p50cdc37 association during serum Raf-1 activation (not shown; see Fig. 3C).
In addition to its effects on serum activation of Raf-1, in experiments similar to the one shown in Fig. 3A, we found that GA also inhibits Raf-1 activity driven by cotransfected Ras(Q61L), a constitutively active Ras mutant (not shown). This result indicates that inhibition of Raf-1 by GA occurs downstream of Ras, in agreement with the original observations of Schulte et al. (60, 61), who found that GA had no effect on Ras levels and on Raf-1–Ras-GTP interaction. We have further observed that as with serum induction, activated Ras potentiates Raf-1 association with the p50cdc37 complex (Fig. 3C; compare lanes 3 and 4 and lanes 5 and 6), but in the presence of GA, this association is entirely abolished (lanes 1 and 2) although the p50cdc37-Hsp90 association again remained unaffected (not shown). Altogether, the above results suggest that Raf-1’s ability to respond to upstream activating stimuli correlates with its ability to form heterotrimeric complexes with p50cdc37 and Hsp90.
Activation of Raf-1 by p50cdc37 overexpression.
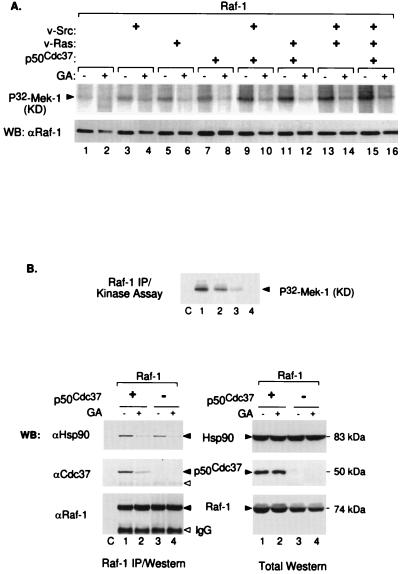
The Sf9 insect cell-baculovirus expression system is currently the most widely used in vivo system for evaluating potential Raf-1 activators (reviewed in references 43 and 44). Therefore, we used this system to further analyze the possible involvement of p50cdc37 in the Raf-1 activation process. Baculoviruses expressing full-length p50cdc37 and Raf-1, together or in triple combinations with v-Src- or v-Ras-expressing baculoviruses (Fig. 4A), were used to coinfect Sf9 cells. At 48 h postinfection, Raf-1 was immunoprecipitated from Sf9 cells in RIPA buffer and subsequently assayed for its ability to phosphorylate inactive recombinant Mek-1. Consistent with previous reports (reviewed in reference 44), v-Src and, to a lesser extent, v-Ras both activate Raf-1, an effect most prominent when the two oncoproteins are coexpressed (Fig. 4A, lanes 1 to 4). Surprisingly, p50cdc37, a unique protein with no apparent kinase or other recognizable enzymatic domain, by itself strongly activated Raf-1 to an even greater extent than v-Ras and almost as well as, although never better than, v-Src. In coinfected combinations, the p50cdc37–v-Src effect was synergistic (compare lanes 2, 5, and 6), but only modest cooperation was observed between p50cdc37 and v-Ras (lanes 3 and 7). The cooperation of p50cdc37 with v-Src and its dose-dependent activation of Raf-1 are shown even more clearly in the dose-response experiment shown in Fig. 4B.
Ser621 of Raf-1 is an indispensable major phosphorylation site whose deletion (25) or substitution by either alanine or even negatively charged aspartate inactivates the protein (17, 46), possibly by compromising the activation-competent conformation of the Raf-1 catalytic domain (44). Neither v-Src nor p50cdc37 could substantially induce Raf-1(S621A) activation compared with the strong positive effect of each on wild-type Raf-1 (Fig. 4C, lanes 5 to 7). Interestingly, however, p50cdc37 also enhanced the weak effect of v-Src on the Raf-1 mutant as it did for wild-type Raf-1 (lanes 4 and 6). This result suggests that p50cdc37, in conjunction with its more abundant partner Hsp90, may be rate limiting in insect cells under these conditions and act as a chaperone by increasing the proportion of Raf-1 which is in the active conformation.
Inhibition of Raf-1 activation by dominant negative p50cdc37 and GA.
Since the deletion mutant p50cdc37ΔC fails to bind to both mammalian and insect Hsp90, we sought to determine whether this mutant might interfere with Raf-1 activity by displacing the wild-type insect p50cdc37-Hsp90 complex from Raf-1 since it retains the ability to bind to Raf-1 (Fig. 2). In the experiment shown in Fig. 5A, we attempted to correlate the effects of p50cdc37ΔC on Raf-1 activity with its aforementioned ability to displace the full-length p50cdc37 protein upon overexpression (Fig. 2C). Previously it has been found that endogenous insect Hsp90 and p50cdc37 associate with overexpressed mammalian Raf-1 in Sf9 cells (11, 12). However, since our p50cdc37 antibodies fail to recognize p50cdc37 from insect cells, Sf9 cells were coinfected with baculoviruses expressing mammalian p50cdc37 and Raf-1 alone or with increasing amounts of a baculovirus expressing p50cdc37ΔC. Extracts of infected cells were then immunoprecipitated with anti-Raf-1 and analyzed for associated mammalian p50cdc37 proteins and Hsp83, the endogenous insect homologue of Hsp90 (8), as well as for Raf-1 kinase activity. Figure 5A shows that, as we had previously observed in mammalian cells (Fig. 2C), p50cdc37ΔC efficiently and in a dose-dependent manner displaced its full-length counterpart from Raf-1 in coinfected Sf9 cells and strongly reduced the association of insect Hsp90 with Raf-1. The dissociation of p50cdc37 and Hsp90 from Raf-1 correlated closely with the reduction of Raf-1 activation to basal levels (Fig. 5A, top). A control Western blot of total cellular extracts from this experiment indicates that this effect was not due to decreased expression of wild-type p50cdc37, endogenous Hsp90, or Raf-1 kinase (Fig. 5A). We conclude that p50cdc37ΔC functions as a dominant negative for the p50cdc37-mediated Raf-1–p50cdc37–Hsp90 complex formation and subsequent Raf-1 kinase activation.
We also examined whether p50cdc37ΔC could inhibit Raf-1 activation by Ras and v-Src and again found that overexpression of p50cdc37ΔC in insect cells abrogated Raf-1 activation by oncogenic Src and Ras (Fig. 5B). Thus, activation of Raf-1 by both v-Src and v-Ras in Sf9 cells is dependent on the ability of p50cdc37 and Hsp90 to form a productive complex with Raf-1 kinase. To gain more insight into the mechanism of p50cdc37-dependent Raf-1 activation, we assessed the effects of wild-type and dominant negative p50cdc37 on the activity of Raf-1 catalytic domain site mutants by coinfection of Sf9 cells. As expected, Raf-1(K375M), which is kinase inactive (14), could not be stimulated by p50cdc37 or Src (not shown). Tyr340 and to a lesser extent Tyr341 have previously shown to be important regulatory sites, whose phosphorylation by tyrosine kinases presumably activates Raf-1 by interfering with negative regulation of the catalytic domain by the amino terminus of the protein (14). Since, as shown above, p50cdc37 binds both in vivo and in vitro to the catalytic half of the Raf-1 protein and interacts also both physically and functionally with Src kinases (references 4 and 13 and data not shown), we reasoned that p50cdc37’s role might be auxiliary to tyrosine kinase function, i.e., by facilitating or promoting Raf-1 tyrosine phosphorylation or by preserving the active Raf-1 conformation. To test this, we coexpressed in Sf9 cells p50cdc37 along with Raf-1(Y340D), a constitutively active mutant (14). Indeed, p50cdc37’s coexpression with Raf-1(Y340D) (Fig. 5C), even at the highest possible amounts (not shown), failed to further superinduce the already high basal activity of this mutant, consistent with the above-hypothesized role for p50cdc37. However, when we also tested the effect of p50cdc37ΔC on Raf-1(Y340D), we found again the previously noted strong inhibition of Raf-1 activity (Fig. 5C). Consistent with this, we have found that both p50cdc37 and p50cdc37ΔC associate with Raf-1(Y340D), as judged by examination of the coexpressed proteins (not shown). The above results argue strongly for a potential dual role of p50cdc37 and its Hsp90 chaperone cofactor in the Raf-1 activation process: one where p50cdc37-Hsp90 might be involved both in the efficient activation of Raf-1 and a second involving maintenance of the active kinase conformation, once relief from repression by the N-terminal domain is achieved either through tyrosine phosphorylation by v-Src (Fig. 4) or by activation of amino acid mutations (Fig. 5C).
Using a complementary experimental approach, we then tested whether GA-mediated inhibition of insect cell Hsp90 would abrogate baculovirus Raf-1 activation as we had observed in Cos-1 cells. Indeed, GA treatment of Sf9 cells coinfected with Raf-1 and viruses expressing v-Src, v-Ras, or p50cdc37 resulted in dramatic decreases in Raf-1 activity (Fig. 6A) that correlated with a substantial loss of endogenous Hsp90 binding to Raf-1 in all tested combinations (Fig. 6B and data not shown). It is of note that under the conditions used, GA resulted in only slight depletion in Raf-1 protein, which, interestingly, exhibited a noticeable mobility up-shift during SDS-PAGE. Thus, the dramatic reduction in Raf-1 kinase activity cannot be accounted for by changes in levels of Raf-1 protein expression (control anti-Raf-1 immunoblot in Fig. 6A). As we have additionally observed, coexpression of Raf-1 with Hsp90 deletion constructs also abrogate Raf-1 activation without causing Raf-1 protein degradation (data not shown). Thus, Raf-1 activation by coexpression with p50cdc37, v-Src, or v-Ras is dependent in each case on functional endogenous insect Hsp90.
FIG. 6.
GA inhibits Raf-1 activation in Sf9 cell by disrupting Raf-1–Hsp90–p50cdc37 complex formation. (A) Raf-1 alone or in combination with v-Src, v-Ras, or p50cdc37 was expressed in Sf9 cells, incubated for 48 h, immunoprecipitated with anti-Raf-1 polyclonal antisera in RIPA buffer, and tested for in vitro kinase activity. Even-numbered lanes represent parallel cultures treated with GA (2 μg/ml) for 4 h before being harvested and analyzed similarly. Blotted kinase reactions (top panel) were tested for immunoprecipitated Raf-1 protein levels, using rabbit anti-Raf-1 Western blotting (WB) (bottom). Note that GA-treated Raf-1 migrates slower than nontreated samples (bottom) and is severely deficient in phosphorylating recombinant kinase-defective (KD) Mek-1 (top panel). (B) Sf9 cell cultures coinfected with Raf-1 and p50cdc37 or empty-vector baculovirus were each split into two replicate cultures 24 h postinfection; 24 h later, one replicate culture was treated with GA (2 μg/ml) for 2 h while the other was similarly treated with only DMSO diluent as indicated. Cell extracts in NP-40 LB were subjected to Raf-1 IP followed by Raf-1 kinase assay (top panel) or Western blot analysis (bottom left) or, additionally, directly analyzed for respective Raf-1, p50cdc37, or Hsp90 protein expression (lane C is like lane 3 except that immunoprecipitating Raf-1 antibody was omitted.) Open arrowheads denote positions of immunoprecipitating anti-Raf-1 antibodies.
We then examined whether, as previously found for Cos-1 cells, the GA inhibitory effect in Sf9 cells could be due to disruption of complex formation between Raf-1 and p50cdc37-Hsp90. In agreement with both in vitro (Fig. 1C) and in vivo reconstitution data for Cos-1 cells (Fig. 2C), the results in Fig. 6B show that coexpression of mammalian p50cdc37 with Raf-1 in Sf9 cells results in strong p50cdc37–Raf-1 complex formation and enhanced recruitment of endogenous Hsp90 into the kinase complex (compare lanes 1 and 3). This correlates well with p50cdc37-mediated Raf-1 activation as evidenced by the in vitro kinase activity of immunoprecipitated Raf-1 in a parallel assay (Fig. 6B, top panel). However, in GA-treated replicate cultures, both of these effects were almost entirely eliminated. We conclude, therefore, that under all conditions tested in both mammalian and insect cells, Raf-1 must be able to efficiently complex with both p50cdc37 and Hsp90 in order to achieve and/or maintain its activated state.
p50cdc37 contributes to the transduction of EGF signals that activate the MAPK cascade via Raf-1.
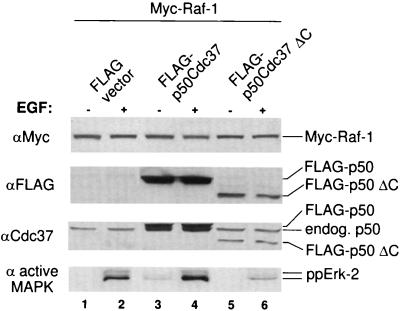
Activated Raf-1 transduces signals to multiple pathways. The best-studied of these is the MAPK pathway. If, therefore, the association of the p50cdc37-Hsp90 complex with Raf-1 contributes to the activation of Raf-1, the dominant negative mutant p50cdc37ΔC, which disrupts this complex, would be expected to interfere with the transduction of physiological signals from Raf-1 to the MAPK cascade. To test this hypothesis, we overexpressed p50cdc37ΔC or its full-length p50cdc37 counterpart in combination with Raf-1 in Cos-1 cells, using the Targefect high-efficiency transfection system. Duplicate serum-starved cultures were harvested with or without EGF stimulation, and solubilized cell extracts were then examined by Western blotting with an antibody against activated phospho-MAPK or with control antibodies against transfected Raf-1 or p50cdc37 (Fig. 7). The results revealed that in contrast to the wild-type protein (Fig. 7, lanes 3 and 4), transfected p50cdc37ΔC inhibited EGF-stimulated Raf-1 activation as judged by Raf-1 kinase assay (not shown) and subsequent MAPK activation as determined by detection of dually phosphorylated endogenous Erk-2 with anti-phospho-Erk antibodies (lanes 5 and 6). Thus, not only is the p50cdc37 C-terminal mutant unable to support Raf-1 activation, but it also prevents Raf-1-mediated downstream signaling through the MAPK pathway. Thus, GA and p50cdc37ΔC, which target the Hsp90 and p50cdc37 components of the Raf-1 activation complex, respectively, produce similar adverse effects: disruption of the native Raf-1 heterocomplex, inhibition of Raf-1 activation, and interruption of signaling to downstream Raf-1 effectors. These findings show that the p50cdc37-Hsp90 complex contributes to the activation of Raf-1 by growth factors and plays a critical role in the transduction of growth factor-generated Raf-1 signals to the MAPK pathway.
FIG. 7.
Dominant negative p50cdc37 inhibits MAPK activation. Cos-1 cells transiently transfected by using Targefect with pMT2-Raf-1 and p50cdc37 or p50cdc37ΔC, or with vector alone, were split; one set of duplicates was serum starved, while the other was stimulated with EGF. Solubilized extracts were then analyzed either with anti-active-Erk rabbit antiserum (bottom) or for levels of expression with the indicated antibodies (top three panels).
DISCUSSION
Based on observations that both Hsp90 and p50cdc37 copurify with various protein kinases, it has been proposed that these two proteins comprise a complex that regulates kinase conformation and activity (4, 28, 53). However, this hypothesis has yet to be examined biochemically. The recent cloning of p50cdc37 has allowed us to directly investigate the role of the p50cdc37-Hsp90 complex in the regulation of the Raf-1. We have found that coexpression of p50cdc37 with Raf-1 leads to Raf-1 activation and that disruption of the p50cdc37-Hsp90 heterodimer interaction with Raf-1 by either p50cdc37ΔC or GA inhibits Raf-1 activation and signaling through Erk. These results indicate that the concerted action of p50cdc37 and Hsp90 on Raf-1 plays a critical role in cell signaling via the Raf-1/Mek/Erk pathway.
Although it has previously been hypothesized that Hsp90 brings p50cdc37 into a complex with Raf and Src (29, 67, 79), our analysis indicates that Raf-1–Hsp90 association is for the most part p50cdc37 dependent and that p50cdc37 is the factor which primarily mediates the Raf-1–p50cdc37–Hsp90 complex formation. More specifically, p50cdc37 binds to the catalytic domain of Raf-1 through its N terminus and tethers Hsp90 to Raf-1 through a second domain located at its C-terminal half (Fig. 2D). This finding is consistent with the observation of Stepanova et al. (69) that p50cdc37 accumulates Hsp90 to Cdk4, although in this case there were no clear effects on kinase activity. Further support for our conclusion stated above is given by the observation that p50cdc37ΔC associates with Raf-1 even though it cannot bind to Hsp90. Moreover, this mutant prevents the accumulation of Hsp90 into the complex by displacing its endogenous full-length counterpart from Raf-1.
Surprisingly, however, GA disrupts the association of the Hsp90-p50cdc37 complex with Raf-1 even though it is known to bind only Hsp90 and fails to dissociate the Hsp90-p50cdc37 complex itself. This could be explained in several ways. GA is known to competitively displace ATP and, by binding tightly to Hsp90, to lock the chaperone into its ADP-specific inactive conformation (7, 22, 55, 72). This conformation may prevent complex binding by steric hindrance, since most of p50cdc37 is bound to Hsp90. p50cdc37ΔC, in contrast, being unable to bind Hsp90, would be free to associate with Raf-1. Alternatively, p50cdc37 may bind to the GA-Hsp90 complex in such a way that it is no longer able to bind to Raf-1. Thus, both Hsp90 and p50cdc37 must be in a functional complex in order to form a productive heterotrimeric complex with Raf-1. In general, however, these results validate experimentally the earlier proposal that Hsp90’s specific associations might be mediated through Hsp90-associated cofactors and that pp50, in particular, might function in targeting Hsp90 to v-Src and Raf-1 kinases (6, 52, 53). It is notable that Hsp90 and p50cdc37 can sometimes function independently of each other. p50cdc37 has not been detected in steroid receptor complexes (54), and we have found that Mek-1 forms a tight complex with p50cdc37 that is characteristically devoid of Hsp90 (19a).
Several lines of evidence indicate that p50cdc37-Hsp90 association with Raf-1 is necessary for the Raf-1 kinase activity. First, overexpressed p50cdc37ΔC reduces both Hsp90 association with Raf-1 and Raf-1 kinase activity by competitively displacing wild-type p50cdc37 from the Raf-1 complex. Second, GA, an Hsp90-specific inhibitor, blunted Raf-1 activation by serum (Fig. 3), and this inhibition correlated with a dramatic loss of p50cdc37-Hsp90 heterodimers from the kinase. That occupation of the ATP/ADP binding pocket of Hsp90 by GA results in dissociation of the protein from Raf-1 is consistent with the notion that alternating cycles of ATP and ADP binding regulate Hsp90 conformation and, in turn, its ability to mediate the formation of productive signaling heterocomplexes (7, 22, 55, 72). The inhibition by GA was also observed with BXB-Raf-1, a constitutively active N-terminal Raf-1 deletion mutant (3), which consistently binds to p50cdc37 and Hsp90 even more strongly than its full-length counterpart (19a). Coupled with our findings that the vast majority of cytoplasmic p50cdc37 is sequestered in heterodimeric complexes by Hsp90 and that it is primarily responsible for bringing Hsp90 into the Raf-1 complex, these results suggest that the interface of p50cdc37–Raf-1 interaction is a target of GA action and that GA-induced conformational alteration of the Hsp90-p50cdc37 heterodimer either leads to the release of the heterodimer as a whole from Raf-1 or prevents it from rebinding to Raf-1. Freed Raf-1 then becomes subject to accelerated degradation as previously observed by Schulte et al. (60). Interestingly, p50cdc37ΔC binding to Raf-1 excludes Hsp90 from the complex but does not lead, as GA treatment does, to Raf-1 degradation. p50cdc37ΔC further inhibits Raf-1 activation, which also suggests that Hsp90 and p50cdc37 play an active and positive role in Raf-1 signaling rather than merely serving to stabilize the kinase.
Strikingly, we have found that p50cdc37 itself, upon coinfection in insect cells with Raf-1, results in strong dose-dependent Raf-1 catalytic activity. This activation is even stronger than that observed with v-Ras and only slightly weaker than v-Src-mediated Raf-1 activation. Moreover, p50cdc37 was able to enhance the weak v-Src-mediated activation of Raf-1(S621A), a well-characterized conformation-compromised, and thus inactive, Raf-1 mutant. Given that Hsp90, p50cdc37’s partner, is a highly abundant protein, these results suggest that p50cdc37 may be a rate-limiting component under conditions of Raf-1 overexpression and may contribute to the formation or stabilization of the active Raf-1 conformational state. As with v-Src and v-Ras, this effect requires phosphorylatable Ser621 for function (46). In contrast, p50cdc37 failed to induce further the already high constitutive activity of Raf-1(Y340D), an N-terminal repression-relieved activated Raf-1 mutant (14). One possible interpretation of this result is that p50cdc37 enhances Src-mediated phosphorylation and activation of Raf-1, a notion supported by the observed physical and functional interactions between Src kinases and p50cdc37 (reference 4 and unpublished results), including their strong synergistic effect on activating Raf-1 activation (Fig. 4). However, our finding that the dominant negative p50cdc37 deletion also down-regulates Raf-1(Y340D) (Fig. 5) in a dose-dependent fashion (not shown) indicates that some of the effects of p50cdc37-Hsp90 complex are independent of tyrosine phosphorylation as well. Thus, it is likely that the p50cdc37-Hsp90 complex is further required to maintain the activated Raf-1 kinase in its active conformation. This latter interpretation would be consistent as well with the findings that activated Ras-independent Drosophila Raf alleles still require Hsp90 association for constitutive function at the membrane (76). It is not yet known whether the Drosophila cdc37 mutation can also suppress this activated Raf allele. This genetic result also indicates that Hsp90 affects Raf-1 activity independently of Raf-1 translocation to the plasma membrane.
Mere addition of purified p50cdc37 and Hsp90 to Raf-1 does not activate the kinase in vitro (unpublished observation). Furthermore, it is worth noting that under commonly used kinase assay conditions, Raf-1, precipitated in RIPA buffer and thus presumably stripped of bound p50cdc37 and Hsp90, remains active. This finding suggests that p50cdc37 and Hsp90 exert their activation role in vivo in conjunction with additional Raf-1 activation factors and do not need to stay associated with Raf-1 in vitro in order for the kinase to remain active; it also argues against a strictly structural role for the p50cdc37-Hsp90 complex in maintaining Raf-1 activity. This observation may also explain why we can detect only a relatively weak associated MAPKKK activity in p50cdc37 immunoprecipitates. As with other chaperone proteins, the p50cdc37-Hsp90 complex may interact with Raf-1 in a transient manner and release after catalyzing conformational changes in Raf-1.
Previous work in Raf-1 overexpression systems has suggested that there may be a limiting cytosolic factor which is required for maximal Raf-1 activation (5, 26, 36, 70, 78). Our results suggest that p50cdc37 could well be a component of this activity. However, since p50cdc37 is more abundant than Raf-1, the ability of p50cdc37 overexpression alone to activate endogenous Raf-1 is modest relative to its marked ability to activate coexpressed Raf-1. This finding suggests that in unstimulated cells there may be a stoichiometric inhibitor of Raf-1 signaling whose effects are partially overcome by overexpression of Raf-1. Conceivably, under these conditions, the p50cdc37-Hsp90 complex becomes limiting and overexpressed p50cdc37 complexes with the already abundant Hsp90 to reconstitute the Raf-1–p50cdc37–Hsp90 complex and allow activation of the kinase. That the Hsp90-p50cdc37 complex would be limiting in these experiments would also be consistent with a model in which the complex serves as a scaffold for Raf-1 oligomerization. There is evidence both that oligomerization can lead to Raf-1 activation (15, 35) and that Hsp90 forms homodimers and oligomers (40, 41, 48, 49). In further support, most of native Raf-1 is found in large (300- to 500-kDa) complexes with p50cdc37 and Hsp90, and it is this form of Raf-1 that becomes membrane activated (78).
An important remaining question is whether the associations or the activity of the p50cdc37-Hsp90 complex are subject to regulation. First, we have found increased formation of the Raf-1–p50cdc37–Hsp90 ternary complex after serum stimulation and in response to activated Ras. It is possible that this contributes to the activation of the small fraction of Raf-1 that is reportedly sufficient for effective signaling. This would be consistent as well with our finding that coexpression of p50cdc37 with Raf-1 accumulates Hsp90 and activates Raf-1 in a dose-dependent manner. Analogously, Garcia-Cardena et al. (18) have recently found that extracellular regulators of endothelial nitric oxide synthase induce the rapid recruitment of Hsp90 to the enzyme, resulting in its membrane activation. It is also possible that changes in protein association or modifications of preexisting Raf-1–Hsp90–p50cdc37 trimeric complexes are sufficient to cause Raf-1 activation or derepression during cell stimulation. Since both p50cdc37 and Hsp90 are phosphoproteins (4, 34, 78, 79), their protein associations within the Raf-1 signalsome could in turn be modulated by phosphorylation. Indeed, phosphorylation-dependent interactions appear to be involved in the regulatory interaction of other kinases with Hsp90, including v-Src (39), Lck (24), and HRI (73, 75). In addition, serum regulation of the phosphorylation state of the Hsp90-p50cdc37 complex could play an important role in Raf-1 activation. Alternatively, serum might regulate the nucleotide binding state and conformation of Hsp90 (22, 55, 72) that is associated with p50cdc37 and Raf-1 and thereby allosterically regulate its effects on Raf-1. This may occur either through assisting Raf-1 in the conformational transition to the activated state or by allowing it to achieve a configuration where it is competent to respond to upstream activators.
In summary, our findings complement and extend genetic data for Drosophila and indicate that the p50cdc37-Hsp90 chaperone complex is essential for signaling through the MAPK pathway at the level of Raf-1. Interestingly, the fact that Raf-1 (71, 77), Hsp90 (54), and, as verified by both mRNA and protein analyses (8, 19a), p50cdc37 all involve ubiquitously expressed proteins points to a potentially universal Raf-1–Hsp90–p50cdc37 signaling complex. Future experiments will address both the exact nature of Raf-1 regulation by the p50cdc37-Hsp90 heterodimer and whether additional kinases are similarly modulated.
ACKNOWLEDGMENTS
GA was provided by the Developmental Therapeutics Program of the NCI. We gratefully acknowledge J. Kyriakis, Z. Luo, J. Avruch, D. Morrison, and T. Roberts for supplying reagents, L. Feig, G. Mosialos, P. Dice and J. Kyriakis for reviewing the manuscript and for useful discussions, D.-W. Kim for assisting with p50cdc37 antibody preparation, and J. Lee for help with graphics.
This work was support by DOD Breast Cancer Research Program grants DAMD17-97-1-7990 and NIH grant GM51551 to B.H.C.
Footnotes
N.G. dedicates this paper to John, George, and Bill.
REFERENCES
- 1.Avruch J, Zhang X F, Kyriakis J M. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 2.Borkovich K A, Farrelly F W, Finkelstein D B, Taulien J, Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989;9:3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruder J, Heidecker G, Rapp U. Serum-, TPA-, and ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992;6:545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- 4.Brugge J S. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. doi: 10.1007/978-3-642-70810-7_1. [DOI] [PubMed] [Google Scholar]
- 5.Chow Y H, Pumiglia K, Jun T H, Dent P, Sturgill T W, Jove R. Functional mapping of the N-terminal regulatory domain in the human Raf-1 protein kinase. J Biol Chem. 1995;270:14100–14106. doi: 10.1074/jbc.270.23.14100. [DOI] [PubMed] [Google Scholar]
- 6.Courtneidge S A, Bishop J M. Transit of pp60v-src to the plasma membrane. Proc Natl Acad Sci USA. 1982;79:7117–7121. doi: 10.1073/pnas.79.23.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csermely P, Kahn C R. The 90-kDa heat shock protein (hsp-90) possesses an ATP binding site and autophosphorylating activity. J Biol Chem. 1991;266:4943–50. [PubMed] [Google Scholar]
- 8.Cutforth T, Rubin G M. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994;77:1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 9.Dai K, Kobayashi R, Beach D. Physical interaction of mammalian CDC37 with CDK4. J Biol Chem. 1996;271:22030–22034. doi: 10.1074/jbc.271.36.22030. [DOI] [PubMed] [Google Scholar]
- 10.DeBoer C, Meulman P A, Wnuk R J, Peterson D H. Geldanamycin, a new antibiotic. J Antibiot (Tokyo) 1970;23:442–447. doi: 10.7164/antibiotics.23.442. [DOI] [PubMed] [Google Scholar]
- 11.Dent P, Jelinek T, Morrison D K, Weber M J, Sturgill T W. Reversal of Raf-1 activation by purified and membrane-associated protein phosphatases. Science. 1995;268:1902–1906. doi: 10.1126/science.7604263. [DOI] [PubMed] [Google Scholar]
- 12.Dent P, Reardon D B, Morrison D K, Sturgill T W. Regulation of Raf-1 and Raf-1 mutants by Ras-dependent and Ras-independent mechanisms in vitro. Mol Cell Biol. 1995;15:4125–4135. doi: 10.1128/mcb.15.8.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dey B, Lightbody J J, Boschelli F. CDC37 is required for p60v-src activity in yeast. Mol Biol Cell. 1996;7:1405–1417. doi: 10.1091/mbc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabian J R, Daar I O, Morrison D K. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol Cell Biol. 1993;13:7170–7179. doi: 10.1128/mcb.13.11.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrar M A, Alberol I, Perlmutter R M. Activation of the Raf-1 kinase cascade by coumermycin-induced dimerization. Nature. 1996;383:178–181. doi: 10.1038/383178a0. [DOI] [PubMed] [Google Scholar]
- 16.Faure M, Bourne H R. Differential effects on cAMP on the MAP kinase cascade: evidence for a cAMP-insensitive step that can bypass Raf-1. Mol Biol Cell. 1995;6:1025–1035. doi: 10.1091/mbc.6.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrier A F, Lee M, Anderson W B, Benvenuto G, Morrison D K, Lowy D R, DeClue J E. Sequential modification of serines 621 and 624 in the Raf-1 carboxyl terminus produces alterations in its electrophoretic mobility. J Biol Chem. 1997;272:2136–2142. doi: 10.1074/jbc.272.4.2136. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa W C. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 19.Gerber M R, Farrell A, Deshaies R J, Herskowitz I, Morgan D O. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc Natl Acad Sci USA. 1995;92:4651–4655. doi: 10.1073/pnas.92.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Grammatikakis, N. Unpublished data.
- 20.Grammatikakis N, Grammatikakis A, Piwnica-Worms H, Toole B P, Cochran B H. The cell cycle. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1996. p. 72. [Google Scholar]
- 21.Grammatikakis N, Grammatikakis A, Yoneda M, Yu Q, Banerjee S D, Toole B P. A novel glycosaminoglycan-binding protein is the vertebrate homologue of the cell cycle control protein, Cdc37. J Biol Chem. 1995;270:16198–16205. doi: 10.1074/jbc.270.27.16198. [DOI] [PubMed] [Google Scholar]
- 22.Grenert J P, Sullivan W P, Fadden P, Haystead T A J, Clark J, Mimnaugh E, Krutzsch H, Ochel H J, Schulte T W, Sausville E, Neckers L M, Toft D O. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–2350. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 23.Hallberg B, Rayter S I, Downward J. Interaction of Ras and Raf in intact mammalian cells upon extracellular stimulation. J Biol Chem. 1994;269:3913–3916. [PubMed] [Google Scholar]
- 24.Hartson S D, Ottinger E A, Huang W, Barany G, Burn P, Matts R L. Modular folding and evidence for phosphorylation-induced stabilization of an hsp90-dependent kinase. J Biol Chem. 1998;273:8475–8482. doi: 10.1074/jbc.273.14.8475. [DOI] [PubMed] [Google Scholar]
- 25.Heidecker G, Huleihel M, Cleveland J L, Kolch W, Beck T W, Lloyd P, Pawson T, Rapp U R. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol Cell Biol. 1990;10:2503–2512. doi: 10.1128/mcb.10.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howe L R, Leevers S J, Gomez N, Nakielny S, Cohen P, Marshall C J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992;71:335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- 27.Huang L, Grammatikakis N, Toole B P. Organization of the chick CDC37 gene. J Biol Chem. 1998;273:3598–3603. doi: 10.1074/jbc.273.6.3598. [DOI] [PubMed] [Google Scholar]
- 28.Hunter T, Poon R Y C. Cdc37: a protein kinase chaperone? Trends Cell Biol. 1997;7:157–161. doi: 10.1016/S0962-8924(97)01027-1. [DOI] [PubMed] [Google Scholar]
- 29.Hutchison K A, Brott B K, De Leon J H, Perdew G H, Jove R, Pratt W B. Reconstitution of the multiprotein complex of pp60src, hsp90, and p50 in a cell-free system. J Biol Chem. 1992;267:2902–2908. [PubMed] [Google Scholar]
- 30.Jakob U, Buchner J. Assisting spontaneity: the role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem Sci. 1994;19:205–211. doi: 10.1016/0968-0004(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 31.Johnson J L, Toft D O. Binding of p23 and hsp90 during assembly with the progesterone receptor. Mol Endocrinol. 1995;9:670–678. doi: 10.1210/mend.9.6.8592513. [DOI] [PubMed] [Google Scholar]
- 32.Kimura Y, Rutherford S L, Miyata Y, Yahara I, Freeman B C, Yue L, Morimoto R I, Lindquist S. Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 1997;11:1775–1785. doi: 10.1101/gad.11.14.1775. [DOI] [PubMed] [Google Scholar]
- 33.Lamphere L, Fiore F, Xu X, Brizuela L, Keezer S, Sardet C, Draetta G F, Gyuris J. Interaction between Cdc37 and Cdk4 in human cells. Oncogene. 1997;14:1999–2004. doi: 10.1038/sj.onc.1201036. [DOI] [PubMed] [Google Scholar]
- 34.Lovric J, Bischof O, Moelling K. Cell cycle-dependent association of Gag-Mil and hsp90. FEBS Lett. 1994;343:15–21. doi: 10.1016/0014-5793(94)80598-9. [DOI] [PubMed] [Google Scholar]
- 35.Luo Z, Tzivion G, Belshaw P J, Vavvas D, Marshall M, Avruch J. Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature. 1996;383:181–185. doi: 10.1038/383181a0. [DOI] [PubMed] [Google Scholar]
- 36.Marais R, Light Y, Paterson H F, Mason C S, Marshall C J. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272:4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 37.Marais R, Marshall C J. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- 38.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 39.Mimnaugh E G, Worland P J, Whitesell L, Neckers L M. Possible role for serine/threonine phosphorylation in the regulation of the heteroprotein complex between the hsp90 stress protein and the pp60v-src tyrosine kinase. J Biol Chem. 1995;270:28654–28659. doi: 10.1074/jbc.270.48.28654. [DOI] [PubMed] [Google Scholar]
- 40.Minami Y, Kawasaki H, Miyata Y, Suzuki K, Yahara I. Analysis of native forms and isoform compositions of the mouse 90-kDa heat shock protein, HSP90. J Biol Chem. 1991;266:10099–10103. [PubMed] [Google Scholar]
- 41.Minami Y, Kimura Y, Kawasaki H, Suzuki K, Yahara I. The carboxy-terminal region of the mammalian HSP90 is required for its dimerization and function in vivo. Mol Cell Biol. 1994;14:1459–1464. doi: 10.1128/mcb.14.2.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison D. 14-3-3: modulators of signaling proteins? Science. 1994;266:56–57. doi: 10.1126/science.7939645. [DOI] [PubMed] [Google Scholar]
- 43.Morrison D K. Activation of Raf-1 by Ras in intact cells. Methods Enzymol. 1995;255:301–310. doi: 10.1016/s0076-6879(95)55033-x. [DOI] [PubMed] [Google Scholar]
- 44.Morrison D K. Mechanisms regulating Raf-1 activity in signal transduction pathways. Mol Reprod Dev. 1995;42:507–514. doi: 10.1002/mrd.1080420420. [DOI] [PubMed] [Google Scholar]
- 45.Morrison D K, Cutler R E. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 46.Morrison D K, Heidecker G, Rapp U R, Copeland T D. Identification of the major phosphorylation sites of the Raf-1 kinase. J Biol Chem. 1993;268:17309–17316. [PubMed] [Google Scholar]
- 47.Nathan D F, Vos M H, Lindquist S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci USA. 1997;94:12949–12956. doi: 10.1073/pnas.94.24.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nemoto T, Ohara-Nemoto Y, Ota M, Takagi T, Yokoyama K. Mechanism of dimer formation of the 90-kDa heat-shock protein. Eur J Biochem. 1995;233:1–8. doi: 10.1111/j.1432-1033.1995.001_1.x. [DOI] [PubMed] [Google Scholar]
- 49.Nemoto T, Sato N. Oligomeric forms of the 90-kDa heat shock protein. Biochem J. 1998;330:989–995. doi: 10.1042/bj3300989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozaki T, Irie K, Sakiyama S. Molecular cloning and cell cycle-dependent expression of a novel gene that is homologous to cdc37. DNA Cell Biol. 1995;14:1017–1023. doi: 10.1089/dna.1995.14.1017. [DOI] [PubMed] [Google Scholar]
- 51.Perdew G H, Wiegand H, Vanden Heuvel J P, Mitchell C, Singh S S. A 50 kilodalton protein associated with raf and pp60(v-src) protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry. 1997;36:3600–3607. doi: 10.1021/bi9612529. [DOI] [PubMed] [Google Scholar]
- 52.Pratt W B. The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;217:420–434. doi: 10.3181/00379727-217-44252. [DOI] [PubMed] [Google Scholar]
- 53.Pratt W B. The role of heat-shock proteins in regulating the function, folding and trafficking of the glucocorticoid receptor. J Biol Chem. 1993;268:21455–21458. [PubMed] [Google Scholar]
- 54.Pratt W B, Toft D O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocrine Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 55.Prodromou C, Roe S M, O’Brien R, Ladbury J E, Piper P W, Pearl L H. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 56.Reed S I. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics. 1980;95:561–577. doi: 10.1093/genetics/95.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 58.Scheibel T, Buchner J. The Hsp90 complex—a super-chaperone machine as a novel drug target. Biochem Pharmacol. 1998;56:675–682. doi: 10.1016/s0006-2952(98)00120-8. [DOI] [PubMed] [Google Scholar]
- 59.Schneider C, Sepp-Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, Hartl F U. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci USA. 1996;93:14536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schulte T W, Blagosklonny M V, Ingui C, Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- 61.Schulte T W, Blagosklonny M V, Romanova L, Mushinski J F, Monia B P, Johnston J F, Nguyen P, Trepel J, Neckers L M. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1–MEK–mitogen-activated protein kinase signalling pathway. Mol Cell Biol. 1996;16:5839–5845. doi: 10.1128/mcb.16.10.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Segnitz B, Gehring U. The function of steroid hormone receptors is inhibited by the hsp90-specific compound geldanamycin. J Biol Chem. 1997;272:18694–18701. doi: 10.1074/jbc.272.30.18694. [DOI] [PubMed] [Google Scholar]
- 63.Silverstein A M, Grammatikakis N, Cochran B H, Chinkers M, Pratt W B. p50(cdc37) binds directly to the catalytic domain of Raf as well as to a site on hsp90 that is topologically adjacent to the tetratricopeptide repeat binding site. J Biol Chem. 1998;273:20090–20095. doi: 10.1074/jbc.273.32.20090. [DOI] [PubMed] [Google Scholar]
- 64.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 65.Smith D F, Whitesell L, Nair S C, Chen S, Prapapanich V, Rimerman R A. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stancato L F, Chow Y H, Hutchison K A, Perdew G H, Jove R, Pratt W B. Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J Biol Chem. 1993;268:21711–21716. [PubMed] [Google Scholar]
- 67.Stancato L F, Chow Y H, Owens-Grillo J K, Yem A W, Deibel M R, Jr, Jove R, Pratt W B. The native v-Raf.hsp90.p50 heterocomplex contains a novel immunophilin of the FK506 binding class. J Biol Chem. 1994;269:22157–22161. [PubMed] [Google Scholar]
- 68.Stanton V P, Jr, Nichols D W, Laudano A P, Cooper G M. Definition of the human Raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989;9:639–47. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stepanova L, Leng X, Parker S, Harper J. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- 70.Stokoe D, McCormick F. Activation of c-Raf-1 by Ras and Src through different mechanisms: activation in vivo and in vitro. EMBO J. 1997;16:2384–2396. doi: 10.1093/emboj/16.9.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Storm S M, Cleveland J L, Rapp U R. Expression of raf family proto-oncogenes in normal mouse tissues. Oncogene. 1990;5:345–351. [PubMed] [Google Scholar]
- 72.Sullivan W, Stensgard B, Caucutt G, Bartha B, McMahon N, Alnemri E S, Litwack G, Toft D. Nucleotides and two functional states of hsp90. J Biol Chem. 1997;272:8007–8012. doi: 10.1074/jbc.272.12.8007. [DOI] [PubMed] [Google Scholar]
- 73.Szyszka R, Kramer G, Hardesty B. The phosphorylation state of the reticulocyte 90-kDa heat shock protein affects its ability to increase phosphorylation of peptide initiation factor 2 alpha subunit by the heme-sensitive kinase. Biochemistry. 1989;28:1435–1438. doi: 10.1021/bi00430a001. [DOI] [PubMed] [Google Scholar]
- 74.Uehara Y, Murakami Y, Suzukake-Tsuchiya K, Moriya Y, Sano H, Shibata K, Omura S. Effects of herbimycin derivatives on src oncogene function in relation to antitumor activity. J Antibiot (Tokyo) 1988;41:831–834. doi: 10.7164/antibiotics.41.831. [DOI] [PubMed] [Google Scholar]
- 75.Uma S, Hartson S D, Chen J J, Matts R L. Hsp90 is obligatory for the heme-regulated eIF-2alpha kinase to acquire and maintain an activable conformation. J Biol Chem. 1997;272:11648–11656. doi: 10.1074/jbc.272.17.11648. [DOI] [PubMed] [Google Scholar]
- 76.van der Straten A, Rommel C, Dickson B, Hafen E. The heat shock protein 83 (Hsp83) is required for Raf-mediated signalling in Drosophila. EMBO J. 1997;16:1961–1969. doi: 10.1093/emboj/16.8.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wadewitz A G, Winer M A, Wolgemuth D J. Developmental and cell lineage specificity of raf family gene expression in mouse testis. Oncogene. 1993;8:1055–1062. [PubMed] [Google Scholar]
- 78.Wartmann M, Davis R J. The native structure of the activated Raf protein kinase is a membrane-bound multi-subunit complex. J Biol Chem. 1994;269:6695–6701. [PubMed] [Google Scholar]
- 79.Whitelaw M L, Hutchison K, Perdew G H. A 50-kDa cytosolic protein complexed with the 90-kDa heat shock protein (hsp90) is the same protein complexed with pp60v-src hsp90 in cells transformed by the Rous sarcoma virus. J Biol Chem. 1991;266:16436–16440. [PubMed] [Google Scholar]
- 80.Whitesell L, Mimnaugh E G, De Costa B, Myers C E, Neckers L M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whitesell L, Shifrin S D, Schwab G, Neckers L M. Benzoquinonoid ansamycins possess selective tumoricidal activity unrelated to src kinase inhibition. Cancer Res. 1992;52:1721–1728. [PubMed] [Google Scholar]