Abstract
The past 20 years have seen dramatic changes in the delivery of radiation therapy, but the impact of radiobiology on the clinic has been far less substantial. A major consideration in the use of radiotherapy has been on how best to exploit differences between the tumour and host tissue characteristics, which in the past has been achieved empirically by radiation-dose fractionation. New advances are uncovering some of the mechanistic processes that underlie this success story. In this Review, we focus on how these processes might be targeted to improve the outcome of radiotherapy at the individual patient level. This approach would seem a more productive avenue of treatment than simply trying to increase the radiation dose delivered to the tumour.
Introduction
Radiation therapy is the most-effective cytotoxic therapy available for the treatment of localized solid cancers. The success of this approach is exemplified by the fact that about 60% of patients with cancer in the USA continue to receive curative radiation therapy—a century after its invention and despite advances in many other treatment modalities. In the past 20 years, there has been a dramatic increase in physical dose delivery options in clinical radiation therapy.1 Major improvements in computer-aided, 3D treatment-planning systems with high-precision techno logy that include tracking organ motion during delivery have been noted. These delivery improvements have been aligned with advances in imaging that support strategies based on intensity-modulated radiation therapy (IMRT), whereby specified doses can be targeted to avoid critical structures and to falloff sharply outside the cancer volume, thereby minimizing dose and toxicity to neighbouring normal tissue. The armamentarium available to clinicians to achieve the same aim has been expanded by novel accelerators for the delivery of proton and heavy-ion charged-particle therapy (CPT) that enable a sharp increase in dose at a very defined depth (Bragg peak),2 albeit at greatly increased monetary cost. In some situations, these new approaches enable higher fraction sizes to be delivered, with desirable decreases in treatment times, as in stereotactic (body) radiation therapy (SBRT).3 Demonstrating that the advances in physical delivery of ionizing radiation have translated into therapeutic benefit, however, has been challenging. The implementation of these new technologies has been largely empirical and driven by the belief that increasing dose will increase cure, rather than being guided by solid clinical or radiobiological data. We present the view that ionizing radiation is rather different from other cancer therapies, and that biological and chemical targeting should take account of these differences and be optimally integrated with new radiation-delivery techniques. This approach is more likely to advance radiation therapy than strategies aimed at increasing the radiation dose delivered to the tumour.
Classic radiobiology phenomena
For the past 40 years, radiation oncologists have often been guided by the classic radiobiological phenomena that underline fractionated radiotherapy and that are enshrined by Withers as the ‘4Rs of radiotherapy’—namely, repair, redistribution/reassortment, repopulation, and reoxygenation.4 These four phenomena are often extended by a fifth ‘R’, that of intrinsic radiosensitivity, defined as the initial DNA damage caused by radiation.5 These phenomena help explain how conventional daily low-dose fractions of around 2 Gy, given five times per week, can exploit differences between normal tissues and tumours, and yield outcomes that are often superior to radiotherapy given in fewer single large doses.4 These principles have stood the test of time; however, they come from an era before advances such as IMRT and molecular profiling were available, and their adaptation to new clinical realities has been slow.6 Even treatment failure in the form of radioresistance, which is known to be associated with certain histologies (such as melanoma and glioblastoma) or oncogenic mutations (for example, KRAS and EGFR), or tumour-associated hypoxia and their relationship with radiotherapy dose delivery requires further elucidation. Nevertheless, the magnitude of these biological influences on outcome argues that the widely held belief of increasing the radiation dose, even if practically achievable with new technologies, is unlikely to significantly increase the cure rate in many cancers. Rather, a paradigm shift towards biological interventions that are tailored specifically to radiation-related parameters is needed.7,8
Recent discoveries in cancer research have given some hope for new avenues of radiation therapy. First, mutations in DNA-damage-response (DDR) pathways have been found to occur very frequently in cancer. These mutations can promote radioresistance, genomic instability, and increase tumour heterogeneity, but they might also represent a potential Achilles’ heel for intervention in cancer radiation therapy and in immunotherapy.7,9 Second, novel biologically targeted agents have been introduced, which, although not necessarily designed for interaction with radiation, might radiosensitize tumours. Even if their synergy with radiation is limited, such agents often have little cytotoxic action when used alone; thus, radiotherapy might be needed to achieve a sufficient cell kill. Furthermore, many examples exist in which a subset of cells escapes the attention of such agents by virtue of losing the targeted molecule or as a result of bypass (escape) pathways. In such scenarios, targeting radiation therapy to the residual tumour deposits would seem appropriate. Third, radiation therapy can alter the tumour microenvironment, which argues for its role as part of a combination therapy; for example, to engage the immune system or improve drug penetration. Hypofractionated SBRT protocols might be superior in this regard.10 Fourth, the ability of IMRT and CPT to deliver radiation dose more precisely to tumour sites will help to localize and amplify any drug–radiotherapy synergy, which would be important for drugs that target radiation-related processes such as DNA repair.7
Of note, radiation therapy is unique in that the normal tissue adjacent to the tumour will often receive doses that are close to the maximum doses that can be tolerated. Addition of even a minimally cytotoxic drug could, therefore, be sufficient to precipitate a crisis. Ironically, radiation dose de-escalation might, on occasion, be the only way to increase the therapeutic benefit of some combined therapies,11 which is a clinically challenging concept. We believe that many potential biological approaches can increase the radiotherapeutic benefit, but we focus herein on only a few that we consider the most promising.
Targeting DNA damage and repair
Ionizing radiation is unique as an anticancer modality in its ability to generate lethal lesions. Clusters of ionization events are generated ubiquitously in cells and tissues, which in turn cause clusters of diverse molecular lesions. In DNA, simple forms of damage can be repaired with relative ease, but dense lesions formed within one to two loops of the helix are more problematic, with complex DNA double-strand breaks (DSBs) being formed that are often lethal to cells. DSBs can be formed as a direct result of clusters of base changes and strand breaks or indirectly during lesion processing and repair, or by conversion at replication forks through homologous recombination (HR).12 In any event, this proclivity of ionizing radiation to form large, complex DSBs explains its efficiency as a cytotoxic agent. DNA-repair pathways are, therefore, valid targets for radiotherapeutic interventions in cancer therapy.7 What is perhaps surprising is the growing evidence for interconnectivity between the diverse mechanisms underlying the ‘5Rs of radiotherapy’. For instance, radiosensitization resulting from ‘targeting’ one pathway could in fact be a result of unexpected effects on DNA repair.
This crosstalk between pathways in response to radiation exposure has been obvious since the DDR was discovered.13 This evolutionarily conserved signalling cascade senses and responds to DNA DSBs to regulate cell-cycle progression and cell-fate decisions, such as apoptosis and senescence, with the main aim of maintaining genomic integrity. Investigations into the crosstalk were greatly facilitated by an improved assay for DSBs.14 The protein product of the gene mutated in ataxia telangiectasia (ATM), along with ATR (ATM-related and RAD3-related) and DNA-PKcs (DNA-dependent protein kinase catalytic subunit) form a phalanx of kinases triggered by DNA damage. One result is that ATM phosphorylates serine139 of a histone variant, H2AX, in the surrounding chromatin to produce γH2AX, which marks the DSB site. The number of γH2AX radiation-induced foci (RIF) in the nucleus is now used routinely to assess the amount of DNA damage and its repair kinetics. This RIF assay has been extended to interrogate other proteins involved in the dynamic orchestration of chromatin-directed DNA-repair programmes.15–17 The nature of the molecules within the RIF reflects the repair mechanism involved. Each repair mechanism is associated with an exclusive set of recruited RIF proteins, but many molecules that include H2AX, ATM, MRN, BRCA1, PARP-1, and DNA-PKcs, are involved in more than one mechanism, illustrating the considerable crosstalk between DNA-repair systems.
Non-homologous end joining (NHEJ) is the main DNA DSB-repair mechanism evoked after ionizing radiation exposure. This pathway normally requires DNA-PK activation, but can also proceed by a slower, alternative DNA-PK-independent pathway (error-prone microhomology-mediated end joining). NHEJ catalyses a simple rejoining of two DNA DSB ends irrespective of their origin18,19 without or with only minimal (for the alternative pathway) guidance from a template and, as a result, is an error-prone process. By contrast, the other major DSB-repair system, HR, faithfully restores the DNA sequence using the sister chromatid as a template,20 and is therefore active only in late S and G2 phases of the cell cycle (Figure 1).21 ATR responds to a wider array of DNA damage than ATM, and is activated through DSB resection and the ssDNA-replication protein A (RPA) complex, and responds to stalled or collapsed replication forks.22 Defects in HR precipitate increases in mutational load, tumour heterogeneity and cancer progression, indicating the critical role for HR in maintenance of genomic integrity as well as DNA repair.
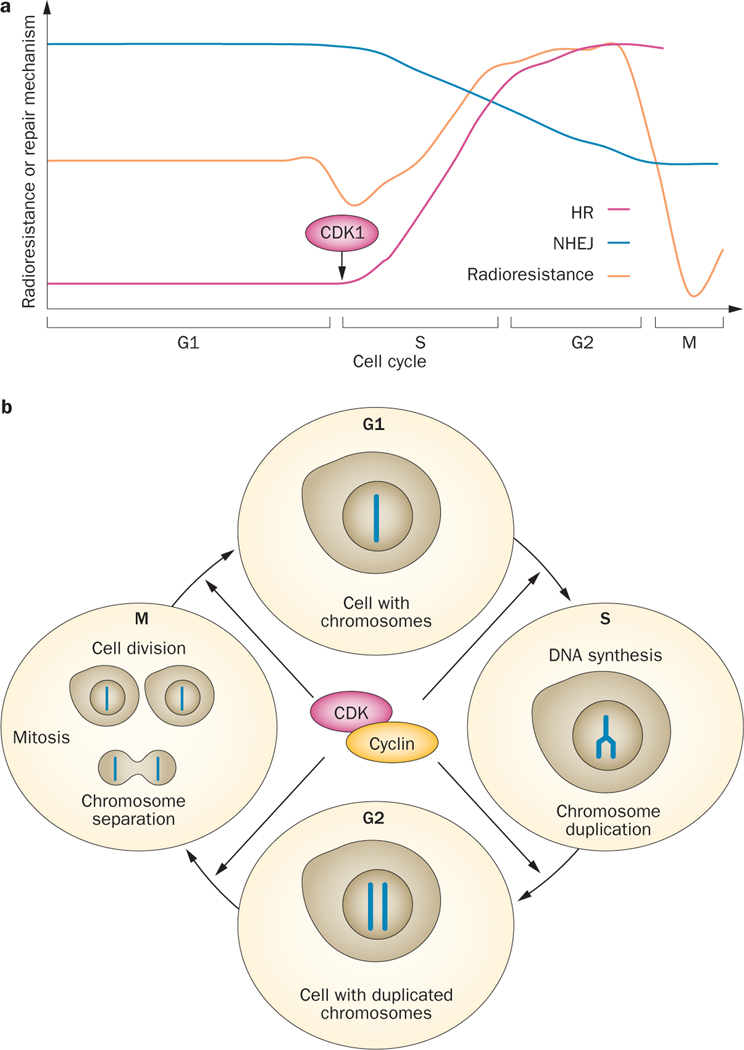
Figure 1 |.
The effect of the cell-cycle phase on radiosensitivity and on the DNA-repair pathway that is utilized. a | The schema shows how CDK1 engages HR in the S and G2 phase of the cell cycle, which coincides with an increase in radioresistance, at the expense of NHEJ. b | Progression through the cell cycle is under the control of a network of CDKs that rely on oscillating cyclin expression. Once a cell moves forward, out of G1 and into S phase, its DNA gets duplicated and therefore increasingly allowing for DNA damage repair to follow the more accurate route of HR. Abbreviations: CDK1, cyclin-dependent kinase 1; HR, homologous recombination; NHEJ, non-homologous end joining.
Initial DNA damage is dependent on the nature (or quality) of the ionizing radiation, which defines the spatial density of the ionization events. For example, densely ionizing heavy-ion CPT has a high relative biological effectiveness. That the quality of ionizing radiation can also affect the balance between repair pathways has now become clear,23,24 with heavy ions causing a shift towards greater use of DSB resection and alternative-end joining in G1, as well as HR in late S/G2, when compared with sparsely ionizing photon irradiation.25 Clearly, the time that tumour cells spend in G0/G1, overall, will impact on the balance between repair mechanisms,26 although much remains to be learnt about this aspect of the response. For example, the exact molecular mechanisms defining this balance and the default pathway chosen require clarification; however, findings that low-dose pre-exposure chromatin structure,27 and cell-cycle phase can influence the balance between the repair pathways suggest considerable complexity in the DNA-repair process.
Variation in radiosensitivity with cell-cycle phase (Figure 1), with cells in late S phase being markedly more resistant than those in G1, is a presumed result of the high efficiency of HR and changes in chromatin organization. This phenomenon is seen in another radiobiological tenet, namely reassortment, which enables fractionation to preferentially kill proliferating cells within radiosensitive cell-cycle phases. The link between HR and the S/G2 cell-cycle phase28,29 has been attributed largely to a requirement for cyclin-dependent kinase 1 (CDK1) for DNA resection (Figure 1).30 CDK1 activates HR by phosphorylating key recombination factors, and phosphorylates the XRCC4-like factor (XLF; also known as Cernunnos) to downregulate NHEJ, at least in yeast.31 Repair is also linked to the cell cycle by Chk1, an effector kinase of ATR that promotes checkpoint arrest in S and G2/M, and DNA repair through RAD51 and HR.32 Chk1 inhibition leads to DNA damage and cell death,33 and is a potential target for improving the outcome of radiation therapy.
Of note, many chemotherapeutic drugs that are used in conjunction with radiotherapy target or interfere with DNA repair. The nucleoside analogues 5-fluorouracil (5-FU) and gemcitabine, and the topoisomerase I inhibitors primarily target HR, whereas cisplatin plus radiation synergize by impairing the NHEJ pathway.7,34 Despite clear treatment success, the reality of chemoradiation in many clinical contexts is, however, that it often comes with increased toxicity and only a modest improvement in outcome,11 which emphasizes the need for more in-depth consideration of how best to translate what we know about DNA repair into an increased therapeutic benefit.
Most cancers have defects in DNA repair, but little is known about the impact of such mutations on outcomes in radiation therapy. The current ‘poster child’ for targeting DNA repair in the clinic is poly(ADP-ribose) polymerase-1 (PARP-1). PARP-1 and its 17 related family members have central roles in many cellular processes. This protein is a sensor of SSBs in DNA, but has a plurality of functions in DNA replication, transcription, DSB repair, histone/chromatin modification, and cell death, as well as inflammation.35 The central concept driving the use of PARP inhibitors is that they block SSB repair, and increase the number and complexity of lesions that have to be dealt with by HR (Figure 2). This concept that radiation- induced, non-DSB, clustered DNA-damage repair impacts on HR, is further supported by the S-phase specificity of killing by PARP inhibitors.36 Defects in HR repair, which might be caused by a BRCA mutation, result in double jeopardy—analogous to ‘synthetic lethality’ (Figure 2). Initially, this explanation was proposed for the single-agent efficacy of PARP inhibitors in BRCA-mutated breast cancers.37,38 However, many DNA-repair mutations other than BRCA1 might sensitize tumours to PARP inhibitors, which could potentially explain the relatively high response rate in patients with non-BRCA-mutated ovarian cancer.37 Because radiation therapy itself exploits differences in repair capacity between tissues, that the combination of radiation with PARP inhibitors is effective against cancers that have BRCA, MRE11, and other DNA-repair-protein mutations is no surprise.39–44 However, PARP-1 is also activated by oxidative and nitrative stress, and is a known cofactor for nuclear factor κB (NF-κB)-driven inflammatory gene expression;45 radiosensitization by PARP inhibitors might also be the result of inhibition of this crucial survival pathway (Figure 2).46 Not surprisingly, many clinical trials of PARP inhibitors in combination with radiation therapy are ongoing.41,47 In evaluating their outcomes, it must be remembered that patients with germline mutations in DNA-repair genes will be also at increased risk of radiation-induced normal tissue toxicity in the presence of PARP inhibitors, and that different PARP inhibitors are available with differing specificities for what is a functionally very-complex family of molecules. A salutary lesson comes from the failure of the putative PARP inhibitor iniparib, in combination with gemcitabine/carboplatin, in a phase III clinical trial in women with triple-negative breast cancer:48 iniparib was later shown to have little PARP-specific activity even in cells in vitro.49
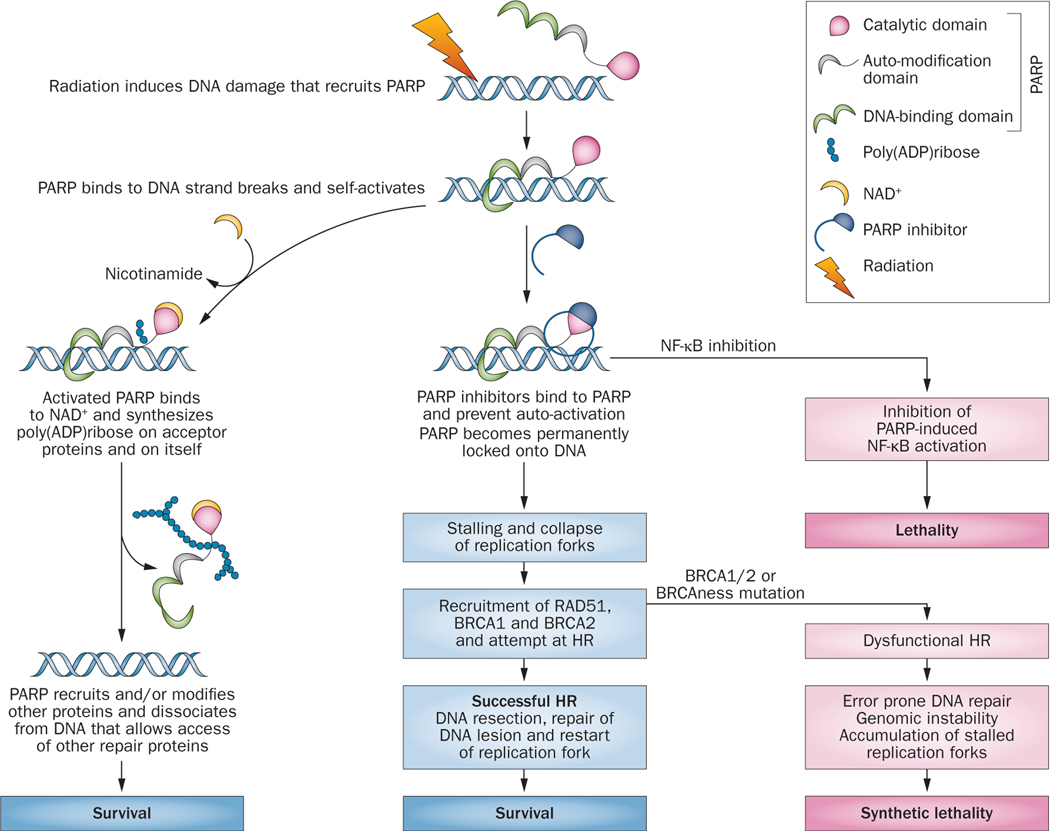
Figure 2 |.
Possible mechanisms by which PARP-1 inhibitors might interact with radiation-induced DNA damage for therapeutic benefit. PARP inhibitors cause synthetic lethality in cells that have a compromised HR apparatus or can block cell survival pathways activated through NF-κB. On the left of the diagram, once PARP disassociates from the DNA complex, recruitment of repair proteins XRCC1 and DNA ligase III for repair of SSBs by BER commences; MRE11 and ATM facilitate DSB repair through HR. MRE11 also facilitates restart of stalled replication forks. The repair proteins help regulate chromatin structure, DNA methylation, histone H1 binding to chromatin, and transcriptional regulation of survival genes, such as NF-kB. Abbreviations: BER, base-excision repair; CD, C-terminal catalytic domain; DSB, double-strand break; HR, homologous recombination; NF-κB, nuclear factor κB; SSBs, single-strand breaks; PARP, poly(ADPribose) polymerase.
Many new drugs have been developed that target molecules involved in DNA repair and the DDR, including ATM, ATR, Chk1/2, DNA-PKcs, and WEE1, and other targets specific to HR.50 Some of these are in phase I clinical trials. There is hope for further rational approaches to combined drug–radiotherapy treatment, although their optimal implementation will probably require prospective molecular profiling to define the tumour-related DNA-repair and DDR processes.
Chromatin structure and targeting
One incidental consequence of the discovery of RIF as markers for DNA DSBs was that it illuminated the spatiotemporal organization of repair processes within functional and structural chromatin domains. Increasing evidence indicates that chromatin architecture imposes important constraints on DNA damage and repair, and that the chromatin arrangement changes on formation of DSBs. Chromatin structure is an e merging potential target for radiation therapy.
Much of the damage from photon ionizing radiation is indirect, through the generation of free radicals, especially reactive oxygen species (ROS) that are generated by radiolysis of water. Radiation with densely ionizing tracks, such as heavy-ion CPT, is less dependent on this pathway. At the molecular level, little is known about how the tightness of the binding of chromatin-associated water, histones, antioxidants, and other molecules influences the lesions formed by ionizing radiation, but some evidence from isolated nuclei suggests that ROS cannot easily penetrate condensed chromatin.51 A role for chromatin in determining radiosensitivity was suggested more than 25 years ago,52 but RIF assays have enabled more detailed investigations of differences in DNA damage and repair between condensed heterochromatin and looser, more transcriptionally active euchromatin. RIF are not readily detected in the centre of hetero chromatic regions following ionizing radiation, but damaged DNA seems to relocate to the periphery of these regions where ATM-dependent repair occurs,53–55 which slows the repair process compared with DNA damage that occurs in euchromatin.56–58 These differences have been found with the use of both sparse and dense ionizing radiation, and have led to the conclusion that chromatin and nuclear architecture influences the dynamics and extent of DNA damage and repair even within one cell-cycle phase. Extrapolating these data to cell survival needs caution, but several reports suggest that radioresistant cells have higher heterochromatin levels than radiosensitive cells.54,59 Also, chromatin structure varies with cell activity and status, and this variation is a suggested explanation for observed differences in radiosensitivity between T-cell subsets.60 In the longer term, greater understanding of the dynamics of RIF viz-a-viz chromatin structure should give rise to new biomarkers of radiosensitivity and identify novel therapeutic targets.
Little is known about how the ionizing-radiation-induced local and global chromatin unwinding processes are regulated following lesion formation, and during ongoing DNA repair and restructuring, so as to maintain genomic integrity.61 Radiation-induced local decondensation of DNA in the vicinity of DSBs could potentially enhance the mobility of damaged chromatin domains and increase repair fidelity. Remarkably, additional roles for ATM and its effector kinase Chk2 have been identified in relaxing the heterochromatin structure to allow access of the DNA-repair m achinery.62–64 This involves phosphorylation of transcription intermediary factor 1-β (TIF1-β; also known as TRIM28 and KAP1), a master repressor that maintains local heterochromatin architecture.65,66 A similar chromatin- modifying role has been suggested for tumour suppressor p53-binding protein 1 (53BP1).67 The forces that operate to compact or relax chromatin and those that tether it to nuclear structures must be carefully controlled to promote different aspects of repair in heterochromatin and euchromatin, and the slow ATM-dependent DSB repair of densely packed chromatin domains at the periphery referred to earlier is probably just one manifestation of these forces at work. Radiation therapy clearly disrupts local and distant chromatin architecture, its epigenetic landscape, and gene function. How radiotherapy impacts on the complex social interactions between multiple chromatin regulatory factors remains unclear, but the evidence is growing that higher-order chromatin structure can dramatically affect the radiation responses, and provide targets for improving the benefit of radiation therapy.
The primary mechanisms that dictate chromatin dynamics are methylation and histone acetylation. Both potentially alter radiation responses, with agents targeting the latter process being more advanced in clinical testing.68 Several inhibitors of histone deacetylases (HDACs) have entered clinical trials.69 Vorinostat (SAHA) and romidepsin (FK228) were shown to improve outcomes for patients with cutaneous T-cell lymphoma, and others HDAC inhibitors are being tested in a variety of cancers.70 Combining radiation with HDAC inhibitors was demonstrated to be a promising approach by findings that these drugs radiosensitize various cancer cell lines in culture and in vivo.71–77 This combination approach was first tested in the clinic in 2009,78 and since then several trials have been initiated and have produced some promising early data.79 The main rationale presented for the use of these inhibitors is that they prevent HDACs from maintaining tightly packed chromatin that might promote radioresistance.59,80 However, numerous non-histone proteins are deacetylated by HDACs,68,80 which presents many other possible mechanistic interpretations for the anticancer action of HDAC inhibitors. Similarly, the existence of four classes of 18 different HDACs, all with multifaceted roles in coordinating intracellular signalling pathways with genetic and epigenetic functions, supports alternative mechanisms. Not surprisingly, multiple end points for HDAC-inhibitor activity have been reported, including gene expression, cell-cycle arrest, cell differentiation, antiangiogenesis, cell death, and autophagy.81 In addition, HDAC inhibitors have been reported to generate ROS and modulate redox levels in cells,82–87 resulting in DNA damage and activation of the DDR.88,89 Others have shown that key DNA-repair molecules including Ku70/Ku80, DNA-PK, RAD50, RAD51, BRCA1/2, and MRE11 are downregulated by vorinostat and other HDAC inhibitors in combination with radiotherapy, leading to RIF persistence.75,77,90–92 Furthermore, DNA-repair-defective cancers are more sensitive to HDAC inhibition.69 For many reasons, these drugs might make good candidates for combination with radiotherapy, but the mechanisms might be obscure. Importantly, reports suggest that HDAC inhibitors do not radiosensitize normal cells in the same way as they sensitize cancer cells.74 In fact, HDAC inhibitors have even been shown to protect against the lethality of whole-body irradiation in mice,75,93 indicating a possible radiotherapeutic differential. This radioprotection might be due to their anti-inflammatory effects, evidence of which is growing.94–96 A critical issue for further advancement of HDAC inhibitors is their specificity and validation. Currently, two opposite design strategies are underway: highly selective and/or multitargeted. It will be interesting to see which of these approaches is most effective in a radiation-therapy setting.
Growth factors and radiotherapy
Recently, one of the most interesting convergences in radiobiology has taken place between growth factor signalling and DNA repair. This relationship is exemplified by EGFR, which has become a paradigm for growth-factor-driven radioresistance.97–99 EGFR is overexpressed or mutated in intestinal, lung, brain, and head and neck cancers, among other tumours, and this pathway has been targeted using monoclonal antibodies, such as cetuximab, and small-molecule inhibitors, including gefitinib and erlotinib.100 Monotherapy with these agents is not particularly effective, in part because of bypass mutations or pathways, which spurred efforts to optimize the combination of these agents with radiotherapy and chemotherapy. Building on earlier promising results, a phase III clinical trial with radiotherapy in locoregional advanced-stage head and neck cancer showed that cetuximab markedly increased survival from 29 months with radiotherapy alone to 49 months, with a 9.2% overall long-term survival.101,102 Many other phase II/III trials that are combining radiation with cetuximab are currently underway in various treatment settings.97 Of note, cetuximab failed to improve outcomes when added to a radiotherapy–cisplatin regimen, 103 suggesting convergent pathways. Also, in rectal cancer combining cetuximab with chemoradiation produced disappointing results, possibly because the antiproliferative effect of cetuximab compromised the activity of the chemotherapeutics.104 One potential mechanism of radiosensitization by EGFR inhibition that has been studied is through p53-dependent G1 arrest, but this pathway does not necessarily lead to improved tumour control.105 These findings provide a salutary lesson that unless the mechanistic basis for radiosensitization is known and biomarkers are available, success in one system might not be easily translated to another.
The initial rationale for combining EGFR inhibition with radiotherapy was that ionizing radiation activates multiple tyrosine-kinase receptors and signal-transduction pathways,106 including PI3K/AKT and RAS/RAF/MEK/ERK, and would therefore drive cells to favour increased survival and proliferation.98,107 Radiation-driven activation of EGFR is a rapid, ROS-dependent process involving phosphatase inactivation.108,109 An essential feature of growth-factor signalling, also involving ROS, is the shuttling of activated protein kinases between the cytoplasm and nucleus.110–112 In fact, nuclear EGFR in tumours has been linked to a worse prognosis.113 The Rodemann group114,115 were first to present another explanation for EGFR-driven radioresistance by linking ionizing radiation- induced EGFR nuclear translocation to superior DNA repair, and demonstrating that nuclear EGFR enhanced DNA-PKcs activity. EGFR has also been reported to bind to excision repair cross complementation group 1 (ERCC1) protein116 and EGFR-stimulated PI3K/AKT to interact with DNA-PKcs.117 Furthermore, somatic activating mutations in EGFR that have been linked to gefitinib and erlotinib responsiveness in patients with non-small-cell lung cancer, have been reported to make cells more radiosensitive than those with non-mutated EGFR.117 These effects extend to chromatin structure, as EGFR can be found in RIF, where it associates with histone acetyltransferase KAT5 (Tip60) to regulate ATM phosphorylation of TIF1-β with resultant heterochromatin relaxation.118–120 One message from these studies is that cells can integrate cues from the microenvironment into DNA repair and chromatin dynamics to ultimately influence cell death and survival. This level of integration will probably also exist for other signalling pathways, which might expand the possibilities for targeted radiotherapeutic intervention. Unfortunately, the complexity of the interactive network governed by growth factors and/or their receptors, such as EGFR, makes it difficult to develop biomarkers that could reliably predict outcome, and clearly many factors need to be accounted for and optimized before this approach can be reliably used in the clinic.
Cancer stem cells and radiotherapy
Major concerns in cancer therapy relate to the role of cancer stem cells (CSCs), and whether these cells express the same targets as the cancer as a whole and have the same sensitivity to cytotoxic agents. The CSC hypothesis posits a relatively small number of stem cells as a self-renewing force that drives tumour growth and metastasis, and that CSCs contribute disproportionately to tumour recurrence.121,122 CSCs from several human solid cancers seem to be particularly resistant to radio therapy,121,123–128 although reports to the contrary exist.129,130 Radioresistance has been associated with a metabolically quiescent state,131 increased levels of free-radical scavengers, lower ROS levels, increased DNA repair, cell-cycle checkpoints,121,124,132 and survival.133,134 The finding that CSCs are not resistant to heavy-ion CPT, which causes more direct clustered and complex DNA damage, also suggests that indirect free radical, ROS-directed pathways are involved in their resistance to photon radiation.135 Chromatin in CSCs has been reported to be more condensed than in non-CSCs,59,136 and this factor might also have a role in radio resistance, although this might oversimplify these complex dynamic systems.
Under conditions of pathological stress, including radiation,137,138 hypoxia,139 and oncogene expression,140 checkpoints that restrict developmental cellular outputs can be lifted, allowing cells to reprogramme for ‘stemness’.141 Such reprogramming could be an essential part of normal healing, but during fractionated courses of radiation it could generate a nidus for recurrence. Reprogramming can be likened to induction of pluripotency in somatic-cell populations via the four Yamanaka factors (OCT3/4, SOX2, KLF4, c-MYC) that are highly expressed in embryonic stem cells, which demonstrates the plasticity inherent in many cell types.137 This process also highlights the substanstial phenotypic and functional heterogeneity within CSCs that manifests as a continuum of stemness-related gene expression.137,142 This heterogeneity might explain why definitive CSC markers are lacking, although major characteristic pheno types are recognized. One could argue that radioresistance and reprogramming of CSCs add to the classic 4Rs and 5Rs that impact the outcome of a course of fractionated radiotherapy, making a case for 6Rs.
Heterogeneity within CSC populations also makes a ful understanding of the effects of therapies challenging. Reports indicate that PARP1 is overexpressed in certain CSC subsets and that these cell populations are more sensitive to PARP inhibitors.143,144 HDAC inhibitors have been reported to radiosensitize CSCs.59 By contrast, EGFR-targeting agents might be less efficacious when CSCs lack expression of EGFR, as has been suggested for some CSCs from head and neck cancers.145 Exclusive targeting of CSCs to reduce radioresistance and to block stress-induced reprogramming remains in its infancy, but therapeutic avenues for elimination of CSCs have been identified within the developmental pathways driven by the four key stem-cell factors that include fibroblast growth factor (FGF)/MAPK, Notch, WNT, Hedgehog (HH), JAK/STAT, and transforming growth factor β (TGF-β) pathways.146,147 Not surprisingly, these signalling cascades are often dysregulated in cancer. Some of the first stem-cell-targeting agents tested were γ-secretase inhibitors (GSIs) that disrupt Notch signalling, and were found to radiosensitize tumours in preclinical studies.148–150 Some GSIs are in clinical trials for cancer treatment,151 although global inhibition of γ-secretases is associated with toxicity.152 Interestingly, chloroquine, which can radiosensitize tumours153–155 and unmask radiation-induced antitumour immunity,156 showed some specificity for CSCs that was mediated by inhibition of CXCL12/CXCR4 and the HH pathways, rather than by blocking autophagy—the activity generally attributed to this agent.157
A commonly expressed CSC marker is CD44, in particular, the splice variant CD44v. That CD44v is associated with radioresistance in prostate cancer cells might not be coincidental.158 An intriguing aspect of CD44v-positive CSCs is that they regulate their ROS levels through the activity of the cysteine transporter subunit xCT, a subunit of the cysteine–glutamate antiporter system that promotes glutathione synthesis.145,159 xCT is upregulated in about 30% of triple-negative breast cancer cell lines160,161 and might represent a good target for radiosensitization.162 Interestingly, the HDAC inhibitor vorinostat has been shown to normalize xCT-containing transporter levels in gliomas with an accompanying increase in ROS levels. 86 The cysteine transport system maintains intracellular cysteine and glutathione pools in many cells to counter oxidative stress. This system is under control of nuclear factor-erythroid 2 p45-related factor 2 (Nrf2), which provides a potential link for CSC radioresistance through expression of free -radical scavengers.
The transcriptional factor Nrf2 is the master regulator of cellular redox homeostasis and cytoprotection, acting by upregulating a plethora of antioxidant response element (ARE)-bearing gene products, including γ-glutamylcysteine synthetase (GCS), the limiting enzyme of glutathione synthesis (Figure 3). This battery of antioxidative and cytoprotective gene products generally promotes cell survival and cellular radioresistance.163 As oxidative stress is a hallmark of most cancers, not surprisingly, Nrf2 or its inhibitor Keap 1 is frequently mutated, for example, in lung cancer.164,165 Intriguing evidence indicates that oncogenic KRAS confers tumour chemoresistance by upregulating Nrf2.166 By regulating redox, the Nrf2 network also guides anti-inflammatory responses and counterbalances proinflammatory transcription factors, such as NF-κB, setting the T-helper cell type 1/2 (TH1/TH2) immune balance.167 Moreover, the Nrf2 network has metabolic influences, facilitating flux through the pentose phosphate pathway, increasing NADPH regeneration and purine biosynthesis, and seems to direct metabolic reprogramming during cellular stress.168
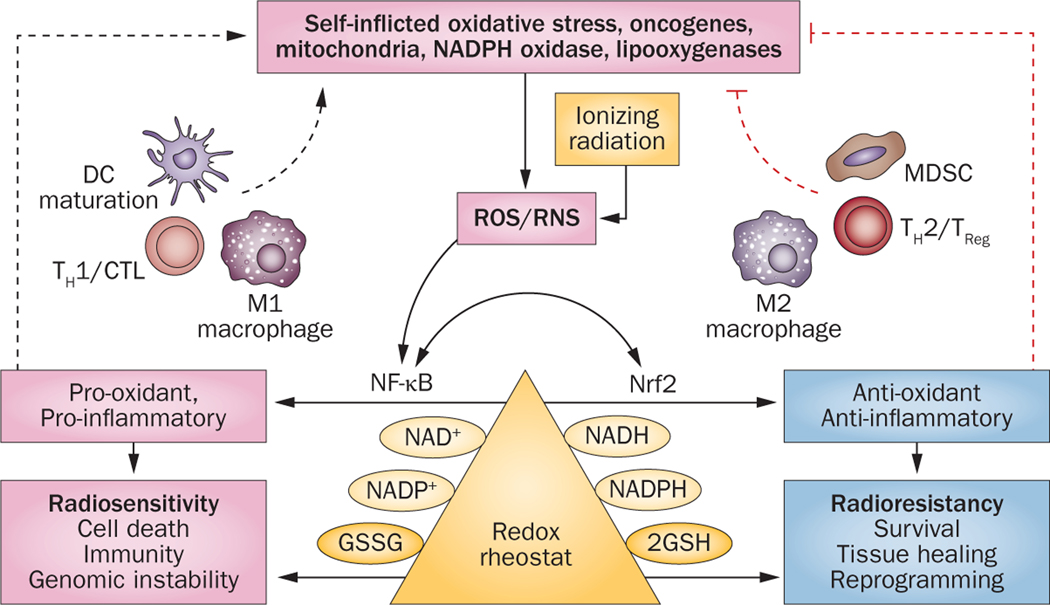
Figure 3 |.
Redox and how it might influence both radiation responses and the immune system. The concept presented is that radiation-induced ROS/RNS drives the generation of a pro-oxidant state that results in acute inflammation, proinflammatory cytokines, more ROS/RNS production, and self-inflicted oxidative damage. At the same time, ROS has the ability to promote antigen presentation by DCs, TH1 cells, CTL and M1 macrophage responses, with further production of proinflammatory cytokines—all of which affirm the pro-oxidative state (left-hand side). The redox imbalance that is created eventually drives an antioxidant response (right-hand side) with increased glutathione synthesis, cell survival, and radioresistance. Under the same influences, immune-control mechanisms, including TREG cells, MDSC, M2 macrophages, and TH2 responses, as well as antioxidants and anti-inflammatory cytokines, will be favoured. Abbreviations: 2GSH, 2 monomeric glutathione molecules; CTL, cytotoxic T-cell lymphocyte; DC, dendritic cell; GSSG, glutathione disulphide; MDSC, myeloid-derived suppressor cells; Nrf2, nuclear factor (erythroid-derived 2)-like 2; ROS, reactive oxygen species; RNS, reactive nitrogen species; TH1, T-helper type 1 (cell); TH2, T-helper type 2 (cell); TREG, regulatory T (cell).
Redox processes are central to the basic functioning of cells and tissues, as well as to radiation damage.169 They are key to regulating biochemical pathways and networks operating to affect signal transduction, DNA and RNA synthesis, protein synthesis, enzyme activation, metabolism, and regulation of the cell cycle. The level of ROS generated and the initial redox state are important elements in deciding responses. Quantitative assessment of these factors is difficult, especially because of intracellular compartmentalization, although new and better probes are becoming available.169,170 The redoxome is large and many important transcriptional programmes, including those driven by Nrf2, NF-κB, and AP-1, can be triggered by redox changes, and by ionizing radiation. ATM can be directly activated by oxidation by a mechanism distinct from the DDR,171,172 as can AMP-activated protein kinase,173 and many phosphatases. The largely proinflammatory effects of ionizing radiation are through the generation of free radicals and activation of NF-κB.174 Redox is, therefore, a natural, well-recognized target for radiation response modification. Importantly, marked differences in redox status exist between normal and cancer cells, although exploiting these variations remains in its infancy. Metformin, a drug widely used to treat type 2 diabetes, regulates redox, has direct anti-CSC activity,175 and is a radiosensitizer.176 Clinical trials of metformin combined with radiation show promising results,177–179 but the increased acute locoregional toxicity of radio therapy observed in diabetic patients receiving metformin urges caution and detailed consideration of the therapeutic ratio.180
Persistent damage, ROS, and senescence
Classic radiobiology concepts posit that ROS are generated very rapidly DNA damage and repair that is measured in minutes to hours, whereas time to expression of tissue damage is dictated by its turnover time. These contentions are only partly true. In fact, ionizing radiation can trigger waves of self-inflicted oxidative stress with DNA and tissue damage that can persist for weeks and months after exposure.134,163,181–183 These effects are associated with inflammatory cytokine production.174 Senescent cells can be detected in vitro by a hallmark increase in p16INK4a, p21CIP1, and β-galactosidase expression.184 In vivo, irradiation of mice and human cells results in similar p16INK4a and DNA-damage foci that persist for many months.183 Senescent cells remain metabolically active and undergo a plethora of changes, including the development of a senescence-associated secretory phenotype (SASP) with persistent nuclear foci and elevated Chk2 and p53 levels as part of a delayed DDR.185 DNA lesions that persist have been called ‘DNA segments with chromatin alterations reinforcing senescence’ (DNA-SCARS) and shown to functionally regulate multiple aspects of the senescent phenotype.185 A major feature of SASP is secretion of inflammatory cytokines, especially IL-6, IL-8, growth factors, and proteases in response to DNA damage.186,187 Chronic proinflammatory cytokine responses involving recurring spikes of tumour necrosis factor α (TNF-α) and IL-1 have also been observed in tissues long after irradiation.174 The relationship between these late manifestations of cytokine expression following radiation exposure requires further study, but they almost certainly contribute to the pathogenesis of radiation damage and might be instrumental in forming reprogramming niches. Juxtacrine, paracrine, and endocrine effects are an inevitable consequence of these responses that are often placed under the general rubric of radiation-induced, non-targeted, bystander effects.
The general concept that emerges is that after radiation exposure, acute tissue inflammation, which is largely pro-oxidant, can cause further DNA DSBs, and cell and tissue damage (Figure 3).188 Under normal inflammatory circumstances, the danger of acute inflammation, which is required for pathogen removal, passes and in time a shift occurs to antioxidant processes with anti-inflammatory and growth-stimulatory cytokines that focus on tissue repair.189 In this scenario, both cancer causation and prevention are possible outcomes of inflammation.190,191 High doses of ionizing radiation disrupt this normal progression of events, generating chronic tissue damage that fails to heal appropriately; a concept supported by the recent development of mitigators of radiation damage that seem to target acute inflammation192 and enhance stem-cell recovery after irradiation.
Local and systemic responses
As discussed, evidence indicates that radiotherapy can trigger a pro-oxidant, proinflammatory ‘dangerous’ microenvironment and that this microenvironment can serve as an immunological niche for the generation of adaptive immune responses.174,189,193 Indeed, radiotherapy can generate tumour-specific immune responses both in mouse models and in humans.194–196 Markers of activation include the upregulation of major histocompatibility complex and cell-adhesion molecules, expression of proinflammatory cytokine family members and their receptors, and molecules with damage-associated molecular patterns (DAMPS) for immune stimulation.174 Antigen-presenting dendritic cells mature in this environment and gain in their ability to cross-present tumour antigens.197 Optimal stimulation of adaptive immunity in radiation therapy might require threefold higher fraction sizes than the conventional 2Gy, which can be achieved by SBRT,195,198 and perhaps by CPT. These higher doses may be needed to release sufficient neoantigens, DAMPS, and immunostimulatory molecules.195,198 It is of interest that high individual doses might be suboptimal at stimulation.195,198
Success in immune-mediated tumour regression and in immunotherapy requires the tumour to express an appropriate neoantigen target landscape,199 and investigations are ongoing to determine if radiotherapy can expand this landscape by promoting epitope spreading.200 Release of highly charged histone and DNA fragments as well as oxidized molecules after radiotherapy might broaden immune responses, and genomic instability might, ironically, assist in doing the same. This possibility comes from evidence that microsatellite instability defines the immune microenvironment in some colorectal cancers,201 that the presence of a high mutation rate improves immunotherapy responses,9 and that suicide-gene therapy and photodynamic therapy increase tumour immunogenicity.202,203 HDAC and PARP inhibitors as well as other chemo therapeutic agents could potentially enhance radiation-induced tumour immunity by similar mechanisms.
Obviously, radiotherapy does not always induce clinically relevant antitumour immunity, even in mouse models.204 The tumour–host relationship is well established before therapy starts, often with an immunosuppressive tumour microenvironment to protect from excessive damage to self, although this varies between tumours.205,206 Historically, the negative impact of the tumour microenvironment on radiotherapy outcomes has been ascribed to the presence of hypoxia, but this concept might be expanded considering the antioxidant effects of regulatory T (TREG) cells, myeloid-derived suppressor cells (MDSC), M2 macrophages and anti-inflammatory cytokines, such as TGF-β, that can be associated with the tumour mass (Figure 3). An oversimplified view is that infiltrates are defined by tumour properties and fall between two polar extremes, best exemplified by plasticity within the myeloid lineage. For example, some tumours have functional M1 macro phages and are genuinely capable of generating T-cell-mediated immunity, even if this is often downregulated by suppressor cells, while others are polarized to an M2 phenotype early during growth and are generally ignored by the adaptive immune system.205,207 A reductionist view is that these extremes most probably reflect the counterbalance between proinflammatory, pro-oxidant pathways that are regulated by factors such as NF-κB,208,209 versus antioxidant, anti-inflammatory pathways that are regulated by factors such as Nrf2 (Figure 3). Whether tumours with a proinflammatory immune profile have a better response to radiotherapy, which could be harnessed through the generation of adaptive immunity or through regulation of redox status, remains to be established.
Radiotherapy without additional intervention is unlikely to reproducibly overcome a well-established negative immune microenvironment present within many tumours, and might even enhance the immune suppressive environment. Thus, although radiation can act as an immune adjuvant, it can also enhance TREG-cell representation, perhaps as a response to radiation-induced ‘danger’ signals,196,210 and can also stimulate M2 macrophage infiltration.211 The response probably varies from tumour to tumour but, at least in some preclinical models, radiation-induced vascular damage, which might be greater after SBRT or CPT,10 can increase the extent of chronic, at the expense of acute, hypoxia.211 Many tumours experience an influx of CD11b+ myeloid cells that evolve into M2 growth-stimulatory macro phages in chronic hypoxic regions.204,212–217 The radiation-induced influx of myeloid cells into these tumours can be prevented through the blockade of the HIF1α/SDF-1/CXCR4214,218 or CSF1/CSF1R213,216 axes resulting in tumour radiosensitization. Such approaches are now entering clinical trials.219 In addition, angiogenesis is inhibited by such treatments, forcing irradiated tissues and tumours to become more reliant on vasculogenesis, which is a less effective process.211,215,220
Unmasking radiation-induced antitumour immunity by targeting negative immune forces has generated considerable enthusiasm. Not least because of the success of antibody-mediated inhibition of immune checkpoints that control antigen-specific T-cell responses against tumours. Triggering of the T-cell-receptor complex not only requires the antigen to be recognized on the surface of an antigen-presenting cell, but also needs a second signal to be sent in a coordinated fashion through a co-stimulatory receptor. The long-standing co-stimulatory proteins CD28 and B7, along with B7 and other protein families, are relevant targets for immune therapies. Some co-stimulatory protein are co-inhibitory (PD-1, PD-L1, CTLA-4, BTLA) rather than co-stimulatory (CD28, ICOS, 4–1BB, CD40, OX40, CD27) and operate to switch off responses.221 These co-inhibitory molecules integrate with TREG cells, MDSC, and M2 macrophages to downregulate immune responses. Tumours often thrive by expressing co-inhibitory molecules, but the remarkable efficacy of anti-CTLA4 and anti-PD-1/PD-L1 strategies in rebalancing antitumour immunity in favour of the host has excited the oncology community.221
Blockade of immune checkpoints enhances radiotherapy-induced immunity in preclinical models,222–225 and clinical observations of out-of-field (abscopal) responses in patients receiving similar treatment have been noted,226–228 leading to multiple ongoing clinical trials combining immune-checkpoint inhibitors with radiation therapy. In the longer term, full exploitation of this approach might be best achieved through a combination of radiation sensitizers and/or myeloid-cell inhibitors plus immune-checkpoint inhibitors, which raises the question as to how to interrogate the immune status of patients with a view to optimizing the choice of treatments. The duality within the host–cancer relationship is thought to arise from having to deal with pathogens without causing dangerous autoimmune responses, while healing tissues. As redox is a nexus for so many of the pathways that are intricately linked to cancer (oxidative stress, genomic instability, mutations, altered metabolism, the tumour microenvironment, inflammation, and host immune responses), it will be interesting to investigate how redox-related or immune biomarkers reflect this status. This knowledge will be critical for evaluating how best to individually tailor therapies for combination with radiotherapy. Indeed, it would not be surprising if these markers also served as an index of response to classic radiation therapy.
Conclusions
In spite of the complexity of radiation responses, a unifying concept that has its roots in the polarizing effects of redox regulation in multiple pathways can integrate our understanding tumour radiosensitization and radioprotection of normal tissue. Multiple antioxidant and pro-oxidant pathways can be expressed, but a major direction for future advances in radiotherapy will be to examine how these pathways are balanced in patients with cancer, and how to tip this balance in favour of the host by therapeutic approaches, be they targeted to DNA repair, growth-factor inhibition, the tumour microenvironment, immune-checkpoint inhibition, CSCs, or mitigation and protection of normal tissue radiation damage.
Key points
Radiotherapy needs a paradigm shift to include biological interventions that are tailored to radiation-related phenomena
DNA-repair mechanisms are obvious targets for interventions aimed at improving the radiotherapeutic benefit
Chromatin structure and nuclear architecture critically influence the dynamics and extent of DNA damage and repair, and thus the response to radiation
Cells exist along a wide spectrum of radiation responsiveness, with cancer stem cells generally being radioresistant
Radiation therapy can be an antitumour immune adjuvant and new approaches to immunotherapy will offer the opportunity to exploit this interaction
Biomarkers that are redox-related or immune-related might help evaluate the status of patients with cancer and provide insight into how best to combine radiotherapy with biological treatments in each individual
Review criteria
The PubMed database was searched for published, full-text articles in English. The same searches were also used to search Google Scholar, Highwire, JSTOR, and the Web of Science, and through reading articles. Main search terms were “DNA repair”, “chromatin structure”, “PARP”, “PARP inhibitors”, “non-homologous end joining”, “homologous recombination”, “senescence”, “H2AX”, “ATM”, “BRCA1”, “chromatin dynamics”, “histone deacetylation”, “HDAC Inhibitors”, “Nrf2”, “redox”, “immunity”, “macrophage subsets”, and “immune activation”. No restriction was placed on the year of publication. Broad searches were performed first before adding limiting search terms, such as “ionizing radiation”, “charged-particle therapy”, and “SBRT”.
Acknowledgements
The authors would like to dedicate this article to H. Rodney Withers, past Chair of Radiation Oncology at the University of California, Los Angeles (UCLA), who died on 25 February 2015. ‘Rod’ was an intellectual giant who shaped the discipline of radiobiology as it related to radiotherapy. He is greatly missed. The authors would also like to thank Dr Ekaterini Angelis for editorial assistance. Financial support for the authors came from the US Army MTA W81XWH-11-1-0531 (W.H.M.) and NIAID 2U19 AI067769 (W.H.M.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Thariat J, Hannoun-Levi JM, Sun Myint A, Vuong T & Gerard JP Past, present, and future of radiotherapy for the benefit of patients. Nat. Rev. Clin. Oncol. 10, 52–60 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Loeffler JS & Durante M. Charged particle therapy--optimization, challenges and future directions. Nat. Rev. Clin. Oncol. 10, 411–424 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Lo SS et al. Stereotactic body radiation therapy: a novel treatment modality. Nat. Rev. Clin. Oncol. 7, 44–54 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Withers HR The 4Rs of radiotherapy in Advances in Radiation Biology Vol. 5 (eds Lett JT & Alder H) 241–249 (New York: Academic Press, 1975). [Google Scholar]
- 5.Steel GG, McMillan TJ & Peacock JH The 5Rs of radiobiology. Int. J. Radiat. Biol. 56, 1045–1048 (1989). [DOI] [PubMed] [Google Scholar]
- 6.Good JS & Harrington KJ The hallmarks of cancer and the radiation oncologist: updating the 5Rs of radiobiology. Clin. Oncol. (R. Coll. Radiol.) 25, 569–577 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Helleday T, Petermann E, Lundin C, Hodgson B & Sharma RA DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 8, 193–204 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Chinnaiyan P, Allen GW & Harari PM Radiation and new molecular agents, part II: targeting HDAC, HSP90, IGF-1R, PI3K, and Ras. Semin. Radiat. Oncol. 16, 59–64 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Rizvi NA et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuks Z & Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell 8, 89–91 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Masterson L. et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cochrane Database of Systematic Reviews, Issue 2. Art. No.: CD010271 10.1002/14651858.CD010271.pub2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomax ME, Folkes LK & O’Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 25, 578–585 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B & Craig RW Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51, 6304–6311 (1991). [PubMed] [Google Scholar]
- 14.Rogakou EP, Pilch DR, Orr AH, Ivanova VS & Bonner WM DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Jackson SP & Bartek J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X. et al. Chk1-mediated phosphorylation of FANCE is required for the Fanconi anemia/BRCA pathway. Mol. Cell Biol. 27, 3098–3108 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bekker-Jensen S & Mailand N. Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair (Amst.) 9, 1219–1228 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Lieber MR NHEJ and its backup pathways in chromosomal translocations. Nat. Struct. Mol. Biol. 17, 393–395 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieber MR & Wilson TE SnapShot: nonhomologous DNA end joining (NHEJ). Cell 142, 496–496.e1 (2010). [DOI] [PubMed] [Google Scholar]
- 20.San Filippo J, Sung P & Klein H. Mechanism of eukaryotic homologous recombination. Annu. Rev. Biochem. 77, 229–257 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Onn I, Heidinger-Pauli JM, Guacci V, Unal E & Koshland DE Sister chromatid cohesion: a simple concept with a complex reality. Annu. Rev. Cell Dev. Biol. 24, 105–129 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Maréchal A & Zou L. RPA-coated single-stranded DNA as a platform for post-translational modifications in the DNA damage response. Cell Res. 25, 9–23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore S, Stanley FK & Goodarzi AA The repair of environmentally relevant DNA double strand breaks caused by high linear energy transfer irradiation--no simple task. DNA Repair (Amst.) 17, 64–73 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Takahashi A. et al. Nonhomologous end-joining repair plays a more important role than homologous recombination repair in defining radiosensitivity after exposure to high-LET radiation. Radiat. Res. 182, 338–344 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Averbeck NB et al. DNA end resection is needed for the repair of complex lesions in G1-phase human cells. Cell Cycle 13, 2509–2516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durante M. New challenges in high-energy particle radiobiology. Br. J. Radiol. 87, 20130626 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima NI et al. Pre-exposure to ionizing radiation stimulates DNA double strand break end resection, promoting the use of homologous recombination repair. PLoS ONE 10, e0122582 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shrivastav M, De Haro LP & Nickoloff JA Regulation of DNA double-strand break repair pathway choice. Cell Res. 18, 134–147 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Hufnagl A. et al. The link between cell-cycle dependent radiosensitivity and repair pathways: A model based on the local, sister-chromatid conformation dependent switch between NHEJ and HR. DNA Repair (Amst.) 27, 28–39 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Aylon Y, Liefshitz B & Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 23, 4868–4875 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hentges P, Waller H, Reis CC, Ferreira MG & Doherty AJ Cdk1 restrains NHEJ through phosphorylation of XRCC4-like factor Xlf1. Cell Rep. 9, 2011–2017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorensen CS et al. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat. Cell Biol. 7, 195–201 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Bryant C, Scriven K & Massey AJ Inhibition of the checkpoint kinase Chk1 induces DNA damage and cell death in human leukemia and lymphoma cells. Mol. Cancer 13, 147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sears CR & Turchi JJ Complex cisplatin-double strand break (DSB) lesions directly impair cellular non-homologous end-joining (NHEJ) independent of downstream damage response (DDR) pathways. J. Biol. Chem. 287, 24263–24272 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson BA & Kraus WL New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 13, 411–424 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Noel G. et al. Radiosensitization by the poly(ADP-ribose) polymerase inhibitor 4-amino-1,8-naphthalimide is specific of the S phase of the cell cycle and involves arrest of DNA synthesis. Mol. Cancer Ther. 5, 564–574 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Audeh MW et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376, 245–251 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Fong PC et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Koppensteiner R. et al. Effect of MRE11 loss on PARP-inhibitor sensitivity in endometrial cancer in vitro. PLoS ONE 9, e100041 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L. et al. MK-4827, a PARP-1/-2 inhibitor, strongly enhances response of human lung and breast cancer xenografts to radiation. Invest. New Drugs 30, 2113–2120 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Reiss KA et al. A phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy in patients with advanced solid malignancies and peritoneal carcinomatosis. Clin. Cancer Res. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chow JP et al. PARP1 is overexpressed in nasopharyngeal carcinoma and its inhibition enhances radiotherapy. Mol. Cancer Ther. 12, 2517–2528 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Nowsheen S, Bonner JA & Yang ES The poly(ADP-Ribose) polymerase inhibitor ABT-888 reduces radiation-induced nuclear EGFR and augments head and neck tumor response to radiotherapy. Radiother. Oncol. 99, 331–338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatterjee P. et al. PARP inhibition sensitizes to low dose-rate radiation TMPRSS2–ERG fusion gene-expressing and PTEN-deficient prostate cancer cells. PLoS ONE 8, e60408 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castri P. et al. Poly(ADP-ribose) polymerase-1 and its cleavage products differentially modulate cellular protection through NF-κB-dependent signaling. Biochim. Biophys. Acta 1843, 640–651 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunter JE et al. NF-κB mediates radio-sensitization by the PARP-1 inhibitor, AG-014699. Oncogene 31, 251–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng FY et al. Targeted radiosensitization with PARP1 inhibition: optimization of therapy and identification of biomarkers of response in breast cancer. Breast Cancer Res. Treat. 147, 81–94 (2014). [DOI] [PubMed] [Google Scholar]
- 48.O’Shaughnessy J. et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N. Engl. J. Med. 364, 205–214 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Patel AG, De Lorenzo SB, Flatten KS, Poirier GG & Kaufmann SH Failure of iniparib to inhibit poly(ADP-ribose) polymerase in vitro. Clin. Cancer Res. 18, 1655–1662 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan MA, Parsels LA, Maybaum J & Lawrence TS Improving the efficacy of chemoradiation with targeted agents. Cancer Discov. 4, 280–291 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takata H. et al. Chromatin compaction protects genomic DNA from radiation damage. PLoS ONE 8, e75622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapman JD et al. Condensed chromatin and cell inactivation by single-hit kinetics. Radiat. Res. 151, 433–441 (1999). [PubMed] [Google Scholar]
- 53.Chiolo I. et al. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 144, 732–744 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Storch K. et al. Three-dimensional cell growth confers radioresistance by chromatin density modification. Cancer Res. 70, 3925–3934 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Jakob B. et al. DNA double-strand breaks in heterochromatin elicit fast repair protein recruitment, histone H2AX phosphorylation and relocation to euchromatin. Nucleic Acids Res. 39, 6489–6499 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiolo I, Tang J, Georgescu W & Costes SV Nuclear dynamics of radiation-induced foci in euchromatin and heterochromatin. Mutat. Res. 750, 56–66 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jezkova L. et al. Function of chromatin structure and dynamics in DNA damage, repair and misrepair: gamma-rays and protons in action. Appl. Radiat. Isot. 83 (Pt B), 128–136 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Falk M, Lukasova E & Kozubek S. Higherorder chromatin structure in DSB induction, repair and misrepair. Mutat. Res. 704, 88–100 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Frame FM et al. HDAC inhibitor confers radiosensitivity to prostate stem-like cells. Br. J. Cancer 109, 3023–3033 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pugh JL et al. Histone deacetylation critically determines T cell subset radiosensitivity. J. Immunol. 193, 1451–1458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kruhlak MJ, Celeste A & Nussenzweig A. Monitoring DNA breaks in optically highlighted chromatin in living cells by laser scanning confocal microscopy. Methods Mol. Biol. 523, 125–140 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Becker A, Durante M, Taucher-Scholz G & Jakob B. ATM alters the otherwise robust chromatin mobility at sites of DNA double-strand breaks (DSBs) in human cells. PLoS ONE 9, e92640 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodarzi AA, Jeggo P & Lobrich M. The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax. DNA Repair (Amst.) 9, 1273–1282 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Goodarzi AA & Jeggo PA The heterochromatic barrier to DNA double strand break repair: how to get the entry visa. Int. J. Mol. Sci. 13, 11844–11860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iyengar S & Farnham PJ KAP1 protein: an enigmatic master regulator of the genome. J. Biol. Chem. 286, 26267–26276 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee DH et al. Phosphoproteomic analysis reveals that PP4 dephosphorylates KAP-1 impacting the DNA damage response. EMBO J. 31, 2403–2415 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dimitrova N, Chen YC, Spector DL & de Lange T. 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature 456, 524–528 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Averbeck NB & Durante M. Protein acetylation within the cellular response to radiation. J. Cell Physiol. 226, 962–967 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Rosato RR & Grant S. Histone deacetylase inhibitors in cancer therapy. Cancer Biol. Ther. 2, 30–37 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Zhang L. et al. Recent progress in the development of histone deacetylase inhibitors as anti-cancer agents. Mini Rev. Med. Chem. 13, 1999–2013 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Camphausen K. et al. Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int. J. Cancer 114, 380–386 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Cerna D, Camphausen K & Tofilon PJ Histone deacetylation as a target for radiosensitization. Curr. Top. Dev. Biol. 73, 173–204 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Shabason JE, Tofilon PJ & Camphausen K. HDAC inhibitors in cancer care. Oncology (Williston Park) 24, 180–185 (2010). [PMC free article] [PubMed] [Google Scholar]
- 74.Jung M. et al. Novel HDAC inhibitors with radiosensitizing properties. Radiat. Res. 163, 488–493 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Konsoula Z, Velena A, Lee R, Dritschilo A & Jung M. Histone deacetylase inhibitor: antineoplastic agent and radiation modulator. Adv. Exp. Med. Biol. 720, 171–179 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chinnaiyan P. et al. Postradiation sensitization of the histone deacetylase inhibitor valproic acid. Clin. Cancer Res. 14, 5410–5415 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karagiannis TC, Kn H & El-Osta A. The epigenetic modifier, valproic acid, enhances radiation sensitivity. Epigenetics 1, 131–137 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Noguchi H. et al. Successful treatment of anaplastic thyroid carcinoma with a combination of oral valproic acid, chemotherapy, radiation and surgery. Endocr. J. 56, 245–249 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Ree AH et al. Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: the Pelvic Radiation and Vorinostat (PRAVO) phase 1 study. Lancet Oncol. 11, 459–464 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Chen HP, Zhao YT & Zhao TC Histone deacetylases and mechanisms of regulation of gene expression. Crit. Rev. Oncog. 20, 35–47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ren J. et al. HDAC as a therapeutic target for treatment of endometrial cancers. Curr. Pharm. Des. 20, 1847–1856 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Nakajima S & Kitamura M. Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response. Free Radic. Biol. Med. 65, 162–174 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Yoon JY, Ishdorj G, Graham BA, Johnston JB & Gibson SB Valproic acid enhances fludarabine-induced apoptosis mediated by ROS and involving decreased AKT and ATM activation in B-cell-lymphoid neoplastic cells. Apoptosis 19, 191–200 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Jeong SG & Cho GW Trichostatin a modulates intracellular reactive oxygen species through SOD2 and FOXO1 in human bone marrow-mesenchymal stem cells. Cell Biochem. Funct. 33, 37–43 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Park S. et al. Suberoylanilide hydroxamic acid induces ROS-mediated cleavage of HSP90 in leukemia cells. Cell Stress Chaperones 20, 149–157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolf IM et al. Histone deacetylases inhibition by SAHA/vorinostat normalizes the glioma microenvironment via xCT equilibration. Sci. Rep. 4, 6226 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sholler GS et al. PCI-24781 (abexinostat), a novel histone deacetylase inhibitor, induces reactive oxygen species-dependent apoptosis and is synergistic with bortezomib in neuroblastoma. J. Cancer Ther. Res. 2, 21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bakkenist CJ & Kastan MB DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 (2003). [DOI] [PubMed] [Google Scholar]
- 89.Wang H. et al. The HDAC inhibitor depsipeptide transactivates the p53/p21 pathway by inducing DNA damage. DNA Repair (Amst.) 11, 146–156 (2012). [DOI] [PubMed] [Google Scholar]
- 90.Blattmann C. et al. Enhancement of radiation response in osteosarcoma and rhabdomyosarcoma cell lines by histone deacetylase inhibition. Int. J. Radiat. Oncol. Biol. Phys. 78, 237–245 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Kachhap SK et al. Downregulation of homologous recombination DNA repair genes by HDAC inhibition in prostate cancer is mediated through the E2F1 transcription factor. PLoS ONE 5, e11208 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ren J. et al. Epigenetic interventions increase the radiation sensitivity of cancer cells. Curr. Pharm. Des. 20, 1857–1865 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Brown SL, Kolozsvary A, Liu J, Ryu S & Kim JH Histone deacetylase inhibitors protect against and mitigate the lethality of total-body irradiation in mice. Radiat. Res. 169, 474–478 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grabiec AM, Tak PP & Reedquist KA Function of histone deacetylase inhibitors in inflammation. Crit. Rev. Immunol. 31, 233–263 (2011). [DOI] [PubMed] [Google Scholar]
- 95.Kim HJ & Chuang DM HDAC inhibitors mitigate ischemia-induced oligodendrocyte damage: potential roles of oligodendrogenesis, VEGF, and anti-inflammation. Am. J. Transl. Res. 6, 206–223 (2014). [PMC free article] [PubMed] [Google Scholar]
- 96.Felice C, Lewis A, Armuzzi A, Lindsay JO & Silver A. Review article: selective histone deacetylase isoforms as potential therapeutic targets in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 41, 26–38 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Morris ZS & Harari PM Interaction of radiation therapy with molecular targeted agents. J. Clin. Oncol. 32, 2886–2893 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen DJ & Nirodi CS The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin. Cancer Res. 13, 6555–6560 (2007). [DOI] [PubMed] [Google Scholar]
- 99.Kim K. et al. Epidermal growth factor receptor vIII expression in U87 glioblastoma cells alters their proteasome composition, function, and response to irradiation. Mol. Cancer Res. 6, 426–434 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baumann M. et al. EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother. Oncol. 83, 238–248 (2007). [DOI] [PubMed] [Google Scholar]
- 101.Bonner JA et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006). [DOI] [PubMed] [Google Scholar]
- 102.Bonner JA et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 11, 21–28 (2010). [DOI] [PubMed] [Google Scholar]
- 103.Ang KK et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J. Clin. Oncol. 32, 2940–2950 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Debucquoy A, Machiels JP, McBride WH & Haustermans K. Integration of epidermal growth factor receptor inhibitors with preoperative chemoradiation. Clin. Cancer Res. 16, 2709–2714 (2010). [DOI] [PubMed] [Google Scholar]
- 105.Kriegs M. et al. Radiosensitization of NSCLC cells by EGFR inhibition is the result of an enhanced p53-dependent G1 arrest. Radiother. Oncol. 10.1016/j.radonc.2015.02.018 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Dent P. et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat. Res. 159, 283–300 (2003). [DOI] [PubMed] [Google Scholar]
- 107.Liccardi G, Hartley JA & Hochhauser D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 71, 1103–1114 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Knebel A, Rahmsdorf HJ, Ullrich A & Herrlich P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 15, 5314–5325 (1996). [PMC free article] [PubMed] [Google Scholar]
- 109.Schmidt-Ullrich RK et al. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene 15, 1191–1197 (1997). [DOI] [PubMed] [Google Scholar]
- 110.Kim J, Adam RM & Freeman MR Trafficking of nuclear heparin-binding epidermal growth factor-like growth factor into an epidermal growth factor receptor-dependent autocrine loop in response to oxidative stress. Cancer Res. 65, 8242–8249 (2005). [DOI] [PubMed] [Google Scholar]
- 111.Kefaloyianni E, Gaitanaki C & Beis I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-κB transactivation during oxidative stress in skeletal myoblasts. Cell Signal. 18, 2238–2251 (2006). [DOI] [PubMed] [Google Scholar]
- 112.Papaiahgari S, Zhang Q, Kleeberger SR, Cho HY & Reddy SP Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS–EGFR–PI3K–Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid. Redox Signal. 8, 43–52 (2006). [DOI] [PubMed] [Google Scholar]
- 113.Han W & Lo HW Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 318, 124–134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dittmann K. et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J. Biol. Chem. 280, 31182–31189 (2005). [DOI] [PubMed] [Google Scholar]
- 115.Dittmann K, Mayer C & Rodemann HP Nuclear EGFR as novel therapeutic target: insights into nuclear translocation and function. Strahlenther. Onkol. 186, 1–6 (2010). [DOI] [PubMed] [Google Scholar]
- 116.Liccardi G, Hartley JA & Hochhauser D. Importance of EGFR/ERCC1 interaction following radiation-induced DNA damage. Clin. Cancer Res. 20, 3496–3506 (2014). [DOI] [PubMed] [Google Scholar]
- 117.Javvadi P. et al. Threonine 2609 phosphorylation of the DNA-dependent protein kinase is a critical prerequisite for epidermal growth factor receptor-mediated radiation resistance. Mol. Cancer Res. 10, 1359–1368 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim IA. et al. Epigenetic modulation of radiation response in human cancer cells with activated EGFR or HER-2 signaling: potential role of histone deacetylase 6. Radiother. Oncol. 92, 125–132 (2009). [DOI] [PubMed] [Google Scholar]
- 119.Dittmann K, Mayer C, Rodemann HP & Huber SM EGFR cooperates with glucose transporter SGLT1 to enable chromatin remodeling in response to ionizing radiation. Radiother. Oncol. 107, 247–251 (2013). [DOI] [PubMed] [Google Scholar]
- 120.Dittmann K. et al. Nuclear epidermal growth factor receptor modulates cellular radiosensitivity by regulation of chromatin access. Radiother. Oncol. 99, 317–322 (2011). [DOI] [PubMed] [Google Scholar]
- 121.Vlashi E, McBride WH & Pajonk F. Radiation responses of cancer stem cells. J. Cell Biochem. 108, 339–342 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vlashi E & Pajonk F. Targeted cancer stem cell therapies start with proper identification of the target. Mol. Cancer Res. 8, 291 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Phillips TM, McBride WH & Pajonk F. The response of CD24-/low/CD44+ breast cancer-initiating cells to radiation. J. Natl Cancer Inst. 98, 1777–1785 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Bao S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Woodward WA et al. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc. Natl Acad. Sci. USA 104, 618–623 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim Y, Joo KM, Jin J & Nam DH Cancer stem cells and their mechanism of chemo-radiation resistance. Int. J. Stem Cells 2, 109–114 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rich JN Cancer stem cells in radiation resistance. Cancer Res. 67, 8980–8984 (2007). [DOI] [PubMed] [Google Scholar]
- 128.Bourguignon LY, Shiina M & Li JJ Hyaluronan–CD44 interaction promotes oncogenic signaling, microRNA functions, chemoresistance, and radiation resistance in cancer stem cells leading to tumor progression. Adv. Cancer Res. 123, 255–275 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McCord AM, Jamal M, Williams ES, Camphausen K & Tofilon PJ CD133+ glioblastoma stem-like cells are radiosensitive with a defective DNA damage response compared with established cell lines. Clin. Cancer Res. 15, 5145–5153 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zielske SP, Spalding AC, Wicha MS & Lawrence TS Ablation of breast cancer stem cells with radiation. Transl. Oncol. 4, 227–233 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vlashi E. et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proc. Natl Acad. Sci. USA 108, 16062–16067 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Diehn M. et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458, 780–783 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zafarana G & Bristow RG Tumor senescence and radioresistant tumor-initiating cells (TICs): let sleeping dogs lie! Breast Cancer Res. 12, 111 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dong Q. et al. Radioprotective effects of BMI-1 involve epigenetic silencing of oxidase genes and enhanced DNA repair in normal human keratinocytes. J. Invest. Dermatol. 131, 1216–1225 (2011). [DOI] [PubMed] [Google Scholar]
- 135.Pignalosa D & Durante M. Overcoming resistance of cancer stem cells. Lancet Oncol. 13, e187–e188 (2012). [DOI] [PubMed] [Google Scholar]
- 136.Chen T. et al. Effects of heterochromatin in colorectal cancer stem cells on radiosensitivity. Chin. J Cancer 29, 270–276 (2010). [DOI] [PubMed] [Google Scholar]
- 137.Vlashi E & Pajonk F. Cancer stem cells, cancer cell plasticity and radiation therapy. Semin. Cancer Biol. 31, 28–35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lagadec C, Vlashi E, Della Donna L, Dekmezian C & Pajonk F. Radiation-induced reprogramming of breast cancer cells. Stem Cells 30, 833–844 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shoshani O & Zipori D. Stress as a fundamental theme in cell plasticity. Biochim. Biophys. Acta 1849, 371–377 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Chlon TM et al. High-risk human papillomavirus E6 protein promotes reprogramming of Fanconi anemia patient cells through repression of p53 but does not allow for sustained growth of induced pluripotent stem cells. J. Virol. 88, 11315–11326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gomez-Casal R. et al. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol. Cancer 12, 94 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Patel SS, Shah KA, Shah MJ, Kothari KC & Rawal RM Cancer stem cells and stemness markers in oral squamous cell carcinomas. Asian Pac. J. Cancer Prev. 15, 8549–8556 (2014). [DOI] [PubMed] [Google Scholar]
- 143.Venere M. et al. Therapeutic targeting of constitutive PARP activation compromises stem cell phenotype and survival of glioblastoma-initiating cells. Cell Death Differ. 21, 258–269 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gilabert M. et al. Poly(ADP-ribose) polymerase 1 (PARP1) overexpression in human breast cancer stem cells and resistance to olaparib. PLoS ONE 9, e104302 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yoshikawa M. et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 73, 1855–1866 (2013). [DOI] [PubMed] [Google Scholar]
- 146.Greenow K & Clarke AR Controlling the stem cell compartment and regeneration in vivo: the role of pluripotency pathways. Physiol. Rev. 92, 75–99 (2012). [DOI] [PubMed] [Google Scholar]
- 147.Takebe N. et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat. Rev. Clin. Oncol. 10.1038/nrclinonc.2015.61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mizugaki H. et al. γ-secretase inhibitor enhances antitumour effect of radiation in Notch-expressing lung cancer. Br. J. Cancer 106, 1953–1959 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin J, Zhang XM, Yang JC, Ye YB & Luo SQ γ-secretase inhibitor-I enhances radiosensitivity of glioblastoma cell lines by depleting CD133+ tumor cells. Arch. Med. Res. 41, 519–529 (2010). [DOI] [PubMed] [Google Scholar]
- 150.Lagadec C. et al. Radiation-induced Notch signaling in breast cancer stem cells. Int. J. Radiat. Oncol. Biol. Phys. 87, 609–618 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Diaz-Padilla I. et al. A phase Ib combination study of RO4929097, a γ-secretase inhibitor, and temsirolimus in patients with advanced solid tumors. Invest. New Drugs 31, 1182–1191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.De Strooper B & Chavez Gutierrez L. Learning by failing: ideas and concepts to tackle γ-secretases in Alzheimer disease and beyond. Annu. Rev. Pharmacol. Toxicol. 55, 419–437 (2015). [DOI] [PubMed] [Google Scholar]
- 153.Kim SH, Kim JH & Fried J. Enhancement of the radiation response of cultured tumor cells by chloroquine. Cancer 32, 536–540 (1973). [DOI] [PubMed] [Google Scholar]
- 154.Firat E, Weyerbrock A, Gaedicke S, Grosu AL & Niedermann G. Chloroquine or chloroquine–PI3K/Akt pathway inhibitor combinations strongly promote gamma-irradiation-induced cell death in primary stem-like glioma cells. PLoS ONE 7, e47357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maycotte P & Thorburn A. Targeting autophagy in breast cancer. World J. Clin. Oncol. 5, 224–240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ratikan JA, Sayre JW & Schaue D. Chloroquine engages the immune system to eradicate irradiated breast tumors in mice. Int. J. Radiat. Oncol. Biol. Phys. 87, 761–768 (2013). [DOI] [PubMed] [Google Scholar]
- 157.Balic A. et al. Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol. Cancer Ther. 13, 1758–1771 (2014). [DOI] [PubMed] [Google Scholar]
- 158.Xiao W. et al. CD44 is a biomarker associated with human prostate cancer radiation sensitivity. Clin. Exp. Metastasis 29, 1–9 (2012). [DOI] [PubMed] [Google Scholar]
- 159.Ni J. et al. CD44 variant 6 is associated with prostate cancer metastasis and chemo-/radioresistance. Prostate 74, 602–617 (2014). [DOI] [PubMed] [Google Scholar]
- 160.Timmerman LA et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 24, 450–465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang F & Yang Y. Suppression of the xCT–CD44v antiporter system sensitizes triple-negative breast cancer cells to doxorubicin. Breast Cancer Res. Treat. 147, 203–210 (2014). [DOI] [PubMed] [Google Scholar]
- 162.Takeuchi S. et al. Sulfasalazine and temozolomide with radiation therapy for newly diagnosed glioblastoma. Neurol. India 62, 42–47 (2014). [DOI] [PubMed] [Google Scholar]
- 163.McDonald JT et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 70, 8886–8895 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bauer AK, Hill T 3rd & Alexander CM The involvement of NRF2 in lung cancer. Oxid. Med. Cell Longev. 2013, 746432 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hast BE et al. Cancer-derived mutations in KEAP1 impair NRF2 degradation but not ubiquitination. Cancer Res. 74, 808–817 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tao S. et al. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res. 74, 7430–7441 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Reuter S, Gupta SC, Chaturvedi MM & Aggarwal BB Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49, 1603–1616 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hayes JD & Dinkova-Kostova AT The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 39, 199–218 (2014). [DOI] [PubMed] [Google Scholar]
- 169.Buettner GR, Wagner BA & Rodgers VG Quantitative redox biology: an approach to understand the role of reactive species in defining the cellular redox environment. Cell Biochem. Biophys. 67, 477–483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma T. et al. Dual-functional probes for sequential thiol and redox homeostasis sensing in live cells. Analyst 140, 322–329 (2015). [DOI] [PubMed] [Google Scholar]
- 171.Kruger A & Ralser M. ATM is a redox sensor linking genome stability and carbon metabolism. Sci. Signal. 4, pe17 (2011). [DOI] [PubMed] [Google Scholar]
- 172.Perry JJ & Tainer JA All stressed out without ATM kinase. Sci. Signal. 4, pe18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shirwany NA & Zou MH AMPK: a cellular metabolic and redox sensor. A minireview. Front. Biosci. (Landmark Ed.) 19, 447–474 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McBride WH et al. A sense of danger from radiation. Radiat. Res. 162, 1–19 (2004). [DOI] [PubMed] [Google Scholar]
- 175.Vazquez-Martin A. et al. Repositioning chloroquine and metformin to eliminate cancer stem cell traits in pre-malignant lesions. Drug Resist. Updat. 14, 212–223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Najbauer J, Kraljik N & Nemeth P. Glioma stem cells: markers, hallmarks and therapeutic targeting by metformin. Pathol. Oncol. Res. 20, 789–797 (2014). [DOI] [PubMed] [Google Scholar]
- 177.Skinner HD et al. Metformin use and improved response to therapy in rectal cancer. Cancer Med. 2, 99–107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Spratt DE et al. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur. Urol. 63, 709–716 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Skinner HD et al. Metformin use and improved response to therapy in esophageal adenocarcinoma. Acta Oncol. 52, 1002–1009 (2013). [DOI] [PubMed] [Google Scholar]
- 180.Ferro A. et al. Evaluation of diabetic patients with breast cancer treated with metformin during adjuvant radiotherapy. Int. J. Breast Cancer 2013, 659723 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eckers JC, Kalen AL, Xiao W, Sarsour EH & Goswami PC Selenoprotein P inhibits radiation-induced late reactive oxygen species accumulation and normal cell injury. Int. J. Radiat. Oncol. Biol. Phys. 87, 619–625 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gao Z. et al. Late ROS accumulation and radiosensitivity in SOD1-overexpressing human glioma cells. Free Radic. Biol. Med. 45, 1501–1509 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Le ON et al. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell 9, 398–409 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sabin RJ & Anderson RM Cellular Senescence—its role in cancer and the response to ionizing radiation. Genome Integr. 2, 7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rodier F. et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J. Cell Sci. 124, 68–81 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Durante M, Reppingen N & Held KD Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol. Med. 19, 565–582 (2013). [DOI] [PubMed] [Google Scholar]
- 187.Coppe JP et al. Tumor suppressor and aging biomarker p16INK4a induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 286, 36396–36403 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Westbrook AM et al. The role of tumour necrosis factor-alpha and tumour necrosis factor receptor signalling in inflammation-associated systemic genotoxicity. Mutagenesis 27, 77–86 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schaue D & McBride WH Links between innate immunity and normal tissue radiobiology. Radiation Res. 173, 406–417 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kansara M. et al. Immune response to RB1-regulated senescence limits radiation-induced osteosarcoma formation. J. Clin. Invest. 123, 5351–5360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Klammer H, Mladenov E, Li F & Iliakis G. Bystander effects as manifestation of intercellular communication of DNA damage and of the cellular oxidative status. Cancer Lett. 356, 58–71 (2015). [DOI] [PubMed] [Google Scholar]
- 192.Kim K. et al. High throughput screening of small molecule libraries for modifiers of radiation responses. Int. J. Radiat. Biol. 87, 839–845 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Demaria S, Bhardwaj N, McBride WH & Formenti SC Combining radiotherapy and immunotherapy: a revived partnership. Int. J. Radiat. Oncol. Biol. Phys. 63, 655–666 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Formenti SC & Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int. J. Radiat. Oncol. Biol. Phys. 84, 879–880 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schaue D, Ratikan JA, Iwamoto KS & McBride WH Maximizing tumor immunity with fractionated radiation. Int. J. Radiat. Oncol. Biol. Phys. 83, 1306–1310 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schaue D. et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin. Cancer Res. 14, 4883–4890 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liao Y-P, Meng WS & McBride WH Antigen presentation by dendritic cells is affected after irradiation [abstract]. Proc. Am. Assoc. Cancer Res. 43, 480–481 (2002). [Google Scholar]
- 198.Dewan MZ et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Snyder A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Butterfield LH et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin. Cancer Res. 9, 998–1008 (2003). [PubMed] [Google Scholar]
- 201.Llosa NJ et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 5, 43–51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mullen CA, Coale MM, Lowe R & Blaese RM Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res. 54, 1503–1506 (1994). [PubMed] [Google Scholar]
- 203.Pizova K. et al. Photodynamic therapy for enhancing antitumour immunity. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 156, 93–102 (2012). [DOI] [PubMed] [Google Scholar]
- 204.Chen FH et al. Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin. Cancer Res. 15, 1721–1729 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.McBride WH Phenotype and functions of intratumoral macrophages. Biochim. Biophys. Acta 865, 27–41 (1986). [DOI] [PubMed] [Google Scholar]
- 206.Gajewski TF et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr. Opin. Immunol. 25, 268–276 (2013). [DOI] [PubMed] [Google Scholar]
- 207.Dougherty GJ & McBride WH Immunoregulating activity of tumor-associated macrophages. Cancer Immunol. Immunother. 23, 67–72 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kikuchi N. et al. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and TH1/TH2 balance. Respir. Res. 11, 31 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rockwell CE, Zhang M, Fields PE & Klaassen CD TH2 skewing by activation of Nrf2 in CD4+ T cells. J. Immunol. 188, 1630–1637 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schaue D, Xie MW, Ratikan JA & McBride WH Regulatory T cells in radiotherapeutic responses. Front. Oncol. 2, 90 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tsai CS et al. Macrophages from irradiated tumors express higher levels of iNOS, arginase-1 and COX-2, and promote tumor growth. Int. J. Radiat. Oncol. Biol. Phys. 68, 499–507 (2007). [DOI] [PubMed] [Google Scholar]
- 212.Kioi M. et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Invest. 120, 694–705 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tseng D, Vasquez-Medrano DA & Brown JM Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas. Br. J. Cancer 104, 1805–1809 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen FH et al. Combination of vessel-targeting agents and fractionated radiation therapy: the role of the SDF-1/CXCR4 pathway. Int. J. Radiat. Oncol. Biol. Phys. 86, 777–784 (2013). [DOI] [PubMed] [Google Scholar]
- 215.Brown JM Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy. Br. J. Radiol. 87, 20130686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Xu J. et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 73, 2782–2794 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chiang CS et al. Irradiation promotes an M2 macrophage phenotype in tumor hypoxia. Front. Oncol. 2, 89 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ahn GO & Brown JM Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives. Cell Cycle 8, 970–976 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mantovani A & Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 212, 435–445 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tsai JH et al. Ionizing radiation inhibits tumor neovascularization by inducing ineffective angiogenesis. Cancer Biol. Ther. 4, 1395–1400 (2005). [DOI] [PubMed] [Google Scholar]
- 221.Pardoll DM The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Johnson DB, Rioth MJ & Horn L. Immune checkpoint inhibitors in NSCLC. Curr. Treat. Options Oncol. 15, 658–669 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Demaria S. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005). [PubMed] [Google Scholar]
- 224.Pilones KA, Vanpouille-Box C & Demaria S. Combination of radiotherapy and immune checkpoint inhibitors. Semin. Radiat. Oncol. 25, 28–33 (2015). [DOI] [PubMed] [Google Scholar]
- 225.Deng L. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124, 687–695 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stamell EF, Wolchok JD, Gnjatic S, Lee NY & Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int. J. Radiat. Oncol. Biol. Phys. 85, 293–295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hiniker SM et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl. Oncol. 5, 404–407 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Postow MA, Harding J & Wolchok JD Targeting immune checkpoints: releasing the restraints on anti-tumor immunity for patients with melanoma. Cancer J. 18, 153–159 (2012). [PMC free article] [PubMed] [Google Scholar]