Abstract
Chimeric antigen receptor (CAR) T-cell therapy has transformed the treatment of relapsed/refractory B-cell acute lymphoblastic leukemia (ALL). However, this new paradigm has introduced unique considerations specific to the patients receiving CAR T-cell therapy, including prognostic uncertainty, symptom management, and psychosocial support. With increasing availability, there is a growing need for evidence-based recommendations that address the specific psychosocial needs of the children who receive CAR T-cell therapy and their families. To guide and standardize the psychosocial care offered for patients receiving CAR T-cell therapy, we propose the following recommendations for addressing psychosocial support.
Keywords: chimeric antigen receptor t-cell, acute lymphoblastic leukemia, psychosocial, interdisciplinary care, communication, symptom management, palliative care, pediatric oncology
Introduction
Chimeric antigen receptor (CAR) T-cell therapy is an immunotherapy which uses modified patient-derived T-cells to treat cancer. In patients with relapsed or refractory acute lymphoblastic leukemia (ALL), high rates of remission have been acheived.1–4 Although CAR T-cell therapy provides promise for patients with B-cell ALL whose disease has been resistant to traditional chemotherapies, many patients experience severe acute toxicity and most patients continue to carry a poor or uncertain prognosis.1,3,4 Moreover, most patients continue to receive this type of therapy at specialized referral centers, away from the primary oncology team with which they are most familiar.5 Altogether, these factors result in a complex situation for both the family and the medical team, emphasizing the need for interdisciplinary comprehensive patient-centered care. As the availability of CAR T-cell therapy grows, there is an increasing need for standardization of care and recommendations specific to the unique challenges and opportunities accompanied by this novel therapeutic paradigm. We hope to use this hypothetical case presentation to provide an initial roadmap for navigating clinical and psychosocial practice scenarios for children and adolescents undergoing CAR T-cell therapies, supplementing existing consensus statements with expert opinion specifically addressing interdisciplinary psychosocial support.6,7
Case Presentation
Penny was 8 years old when she was diagnosed with high risk B-cell ALL at a community-based pediatric hospital. Although her initial treatment course was uncomplicated, she experienced an early isolated medullary relapse. Her disease was refractory to the standard relapse chemotherapy regimen and she has been referred to the nearest regional academic pediatric hospital to receive CAR T-cell therapy. In communication with Penny’s primary oncology team, your team learns that the family is from a rural community. The town recently hosted a fundraising event to help support the family during their time away. Since it is summer break from school, Penny’s mother, grandmother, and two younger siblings will stay locally with her during her treatment, while Penny’s father and older brother will stay home to take care of the family business.
Domain 1: Psychosocial Evaluation and Support
As outlined in the Institute of Medicine’s Cancer Care for the Whole Patient: Meeting Psychosocial Needs, “health and disease are determined by dynamic interactions among biologic, psychological, behavioral, and social factors,” and the incorporation, acknowledgement, and management of psychosocial needs is an essential component of quality care.8,9 Indeed, providing psychosocial support has a direct effect on wellbeing and may minimize downstream negative health outcomes.10,11 As a key component of its first recommendation, the Institute of Medicine calls out the importance of “identifying each patient’s psychosocial health needs” and “systematically following up on, reevaluating, and adjusting plans.”8 This is echoed in the Standards for Psychosocial Care for Children with Cancer and their Families.12
Comprehensive evaluation considering the medical, psychological, and social contexts of an individual’s experience is most effective upfront and continued along the treatment trajectory.13 For patients referred from another institution, evaluation should begin at initial consultation, prior to transfer of care. Components of the comprehensive care of young patients undergoing CAR T-cell therapy include assessment of past and present psychosocial needs, knowledge and expectations about treatment, mutual understanding of goals of care, and communication with the primary oncology team (Figure 1). A comprehensive psychosocial evaluation of both the patient and the family should include screening for potential risk factors for distress and poor health outcomes, including prior psychiatric history, poor social support, and socioeconomic status.12,14
FIGURE 1.
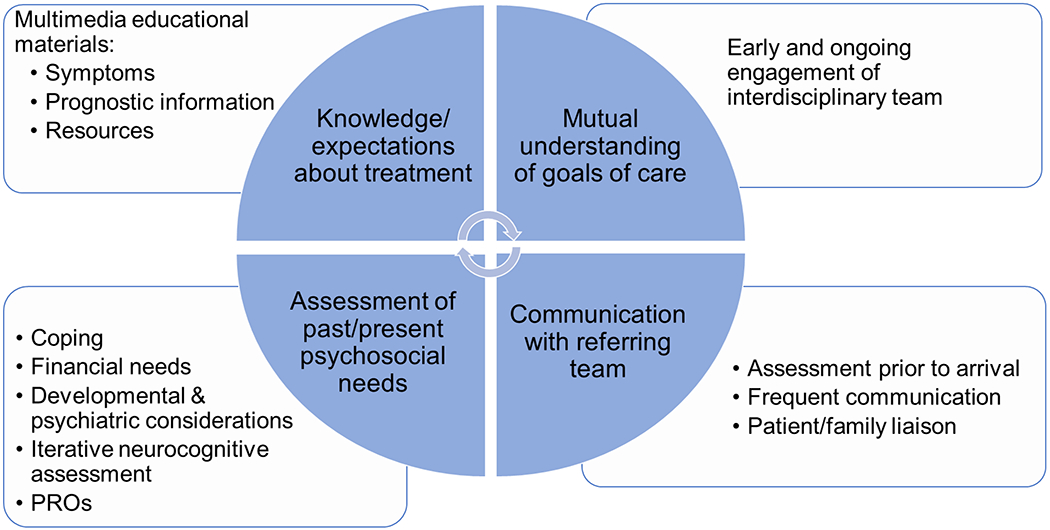
Model of Comprehensive Care. Domains of comprehensive psychosocial care, represented by wedges of the circle, include: knowledge and expectations about treatment, mutual understanding of goals of care, assessment of past and present psychosocial needs, and communication with the referring oncology team. Actionable objectives of assessment are included in adjacent boxes.
An interdisciplinary team approach is considered the standard of oncology care 8,15,16 and provides the framework for effective comprehensive evaluation.12 Such a team should include pediatric-trained representatives from the medical team, interdisciplinary psychosocial services (including, but not limited to, psychology, palliative care, and social work), allied health professionals, and ancillary support staff (Table 1).16–18 This approach is particularly important with patients undergoing CAR T-cell therapy due to the complexity of disease and variability of patient and family needs.13,15,19 Interdisciplinary teams are associated with improved medical team satisfaction and patient quality of life.13,20–22 The diversity of expertise provided by each discipline allows for a continual multi-faceted evaluation of the patient’s needs and enables the application of a variety of strategies to ease tension in difficult scenarios.23
TABLE 1.
Key Stakeholders of the CAR T-cell Therapy Interdisciplinary Team
| Stakeholder Group | Members | Primary Role in Psychosocial Care |
|---|---|---|
| Patient and Family | Patient Parents Siblings Other caregivers Extended social network |
Advocate for the needs of the patient |
| Medical Team | Primary Oncology team | Serve as patient/family liaison Share history of prior experiences, preferences, and needs of child and family Offer long-term therapeutic relationship |
| CAR T-cell Oncology team | Educate patient/family on symptoms, prognosis Facilitate baseline and ongoing discussion |
|
| Psychosocial Services | Psychologist Psychiatrist |
Explore psychosocial needs and provide relevant interventions |
| Social work Chaplain |
Above, plus: Champion family-centered communication Provide support in transitions of care Provide bereavement support and care |
|
| Palliative Care Team | Above, plus: Assess and help manage symptoms Explore goals of care |
|
| Allied Health Professionals | Physical therapist Occupational therapist Pharmacist Nutritionist Health educators Child Life Specialists, Art Therapists, Music Therapists |
Provide comprehensive education and symptom support |
| Ancillary Support Staff | Translators (in-person whenever possible) Clinical research team members (nurses, research assistants) |
Support navigation of medical system |
| Additional Important Stakeholders | ICU medical teams Emergency Medicine teams Hospital Administration |
Help identify challenges and opportunities for streamlining care |
The patient and the patient’s family should be considered key stakeholders in the interdisciplinary model.13 A patient’s prior experience plays a large role in their understanding of and reaction to subsequent events. For example, in children undergoing hematopoietic stem cell therapy, higher parental report of both parental and child distress with prior medical treatment is predictive of subsequent parental distress.24 Understanding families’ perceptions of prior experiences and their specific needs can help minimize or prevent future conflict, especially in times of stress. Specifically, understanding the family’s experience with times of crisis, attitudes toward the intensive care unit, or previous need for support from other consulting services (i.e. pain team, nutrition, physical therapy) will help determine current and future social support needs.
The integration of interdisciplinary palliative care is recommended for all patients with advanced cancer, including children and adolescents with hematologic malignancies.18,25,26 Interdisciplinary palliative care teams can include physicians, advance practice providers, nurses, social workers, psychologists, spiritual care providers, case managers, bereavement specialists, and child life specialists, ideally with additional subspecialty training in pediatric palliative medicine.27 Possible models for the integration of interdisciplinary palliative care have been proposed and consider resources unique to the institution.28,29 Consultation with palliative care clinicians is recommended early in the disease course and should continue concurrently with treatment. Early palliative care intervention has been associated with better symptom management, higher quality of life, greater patient satisfaction, lower distress, and less aggressive end of life care (i.e. less chemotherapy in the last month of life, fewer deaths in the hospital, more patients enrolled on hospice).22,30–36 Transitions in the disease trajectory, including diagnosis of relapse and changes in therapy, have been proposed as highly appropriate times to consider involvement of subspecialty palliative care clinicians.25,26,28 This makes early conversations considering CAR T-cell therapy (i.e. therapeutic consent conferences, transfer to CAR T-cell therapy center) a natural time to introduce interdisciplinary palliative care. Symptom burden assessment and intervention and effective communication have been identified as high priority areas of patient care likely to benefit from subspecialty palliative care;18 additional considerations of subspecialty palliative care involvement specific to these subjects are detailed in the sections below.
For patients referred from other institutions, the primary oncology team is an integral component of the interdisciplinary team. The expertise of the primary oncology team should be leveraged throughout the treatment course, beginning prior to transfer and continuing until transition back to the primary institution. Routine communication with the primary team at frequent intervals (i.e. once a week) can help to lessen conflict during challenging times that may arise. The primary oncology team (including the medical team, social worker, psychologists) can provide context to the patient’s prior experience and aid in identification of unique care needs.
Psychosocial evaluation prior to arrival can serve to explore the family’s health literacy, family-specific concerns, and their understanding of the disease and expected treatment course.37 This is particularly relevant for international patients. There is little published guidance on the care of international patients. However, suggestions for improving care of international patients include: pre-appointment orientation to the medical system, establishing collaborative expectations, understanding cultural norms and any associated specific needs, identifying ways that support and other resources may differ from the patient’s home country, and early and deliberate transition of care back to the primary medical team.37–40 Identification of a primary contact within the home institution at the time of initial consultation and regular communication during the treatment course is necessary to facilitate transfer of care back to the primary team.
Social determinants of health are well known to impact many patient outcomes.9 Nearly 40% of families at a single, large pediatric cancer center reported material hardship while their child received a stem cell transplant.41 Moreover, transplant-related income losses are disproportionately reported by low income families.41 The prevalence of financial hardship is likely similarly under-identified among patients undergoing CAR T-cell therapies. Although a recent report evaluated the cost-effectiveness of CAR T-cell therapies, potential patient-specific financial toxicities were not examined.42 However, long-term financial toxicity is a well-known complication of surviving childhood cancer. 43–45 Thus, evaluation of risk for financial hardship should be considered for all patients undergoing CAR T-cell therapy. Since most CAR T-cell treatment is provided away from the family’s usual social and financial supports, this evaluation should be completed prior to arrival in order for timely identification of financial stressors and housing needs.37 Ongoing risk assessment is also important; the transition to outpatient or home care has been identified as a particularly high-risk time for distress.46 This is disproportionately true for families from lower socioeconomic status, reflecting disparate availability of financial, instrumental, and social support resources.46
Your team is meeting the family for the first time. Penny is accompanied by her mother and father in the exam room. Penny’s parents have many questions about CAR T-cell therapy. How long will they have to stay locally? Is she going to have to stay in the hospital? What is the likelihood of this therapy working? Will she need a bone marrow transplant afterward? What happens if it isn’t successful? Penny’s mother mentions they are close family friends with another patient who was recently treated at your institution for CAR T-cell therapy and she has been avidly reading about CAR T-cell therapy on an online parent forum.
Domain 2: Communication
A strong therapeutic alliance between the health care team, patient, and family is a cornerstone of high quality care.47 This alliance begins with the first interaction between families and the health care team and is highly dependent on effective communication.47 Here, we outline some of the common themes in effective communication, underscoring components that are especially relevant in the care of young patients receiving CAR T-cell therapies. For those interested in additional guidance on communicating difficult issues, conversation guides and checklists are available to aid in difficult conversations.48–53 Moreover, interdisciplinary psychosocial care providers, including palliative care clinicians, psychologists, and social workers, are skilled in navigating difficult conversations; integration of their services should be considered along the care trajectory.16,18
From the beginning, engage patients and families by encouraging their participation in the decision-making process.13 As described above, the family and primary oncology team can help provide context from previous experiences even before transfer to the CAR T-cell therapy team takes place. This can provide insight into what the family may expect with the therapy, help to identify potential areas for difficulty that may arise during the upcoming treatment course, and allow for early intervention and prevention.13 Explore families’ preference for learning (e.g. written, visual) and extent of knowledge desired.54 Understand information sources the family has identified as valuable and integral to their decision-making process. Many families look to their extended social network for information, including families of other patients and online support groups; this is especially true during times of distress or uncertainty.13,54 Understanding where families find information is necessary to identify sources of possible misinformation and to clarify expectations. Including care team members from various disciplines can augment the process of risk factor identification and aid in the process of intervention implementation. Additionally, understanding the support resources that were available in prior phases of treatment, what worked well and what didn’t, can save time and strengthen the therapeutic alliance between the family and the care team.
Exploring expectations, hopes, and worries in early interactions with a family can emphasize what the medical team needs to learn from the family to optimize care.54 Both families and health care professionals identify times of stability as the best time to have important conversations.55 Using simple “what if” questions as well as exploring “other hopes” can provide a great deal of insight, enrich the therapeutic alliance, and can be done without substantial probing.56–58 Topics that may be specifically relevant to families of children and adolescents receiving CAR T-cell therapy may include (Table 2): What does the family and the patient already know about this treatment? What communication strategies worked well with them and their primary team and how may that be incorporated into their current care? What is their understanding of how likely this treatment is to cure their child’s cancer diagnosis? Beyond hope for cure, what else is the family hoping for?
TABLE 2.
The Three “E’s” of Effective Communication.
| Engage | •Champion patient and family as key care team members ○“You know your child better than anyone else. We value your insight and want to know when you are worried.” •Leverage primary oncology team in times of transition ○“If we need to talk about something serious, who do you want to make sure is part of the conversation?” ○“Who from your team at home is important to include in important conversations?” |
| Explore | •Ask about preference and information needs of the family •Ask about prior experiences and how this shapes expectations ○“When your child first went through treatment, what did you find most challenging?” ○“At your hospital near home, were there people or other services that helped you when you were struggling?” ○“What worked well in your relationship with your team at home?” ○“What do you already know about CAR T-cell therapy?” •Ask about hopes and goals of therapy ○“What is your understanding of the goal of CAR T-cell therapy for your child?” ○“Beyond hoping for a cure, what else do you hope for?” ○“Would it be helpful to talk about the chances of cure (or of meeting other goals)?” •Ask about worries and sources of distress ○“What worries you most about the treatment course ahead?” |
| Edit | •Revisit conversations often •Schedule next conversation ○“Some families find it helpful to talk about (chance of cure/symptoms/what the future may look like) when things are otherwise going well. How would you feel about talking about this at our next appointment?” •Schedule “check-in” visits with family, CAR T-cell therapy team, and primary oncology team to re-evaluate new concerns and needs. |
Approach conversations directly and with honesty, supporting experience with evidence.54,59 Honest disclosure about prognosis supports hope, even when outcomes are anticipated to be poor.60 Withholding information has the potential to promote false hope and may lead to feelings of betrayal and mistrust.61,62 CAR T-cell therapy is fast-paced and changes may occur rapidly. Moreover, prognostic conversations may vary substantially and may include discussion about chances of cure and what the child’s life may be like during treatment and beyond.47 Revisit conversations often; families appreciate an iterative approach, allowing for ample opportunity to engage in these conversations with the medical team.13,54
Uncertainty can be a source of unease for both families and care teams.63 In an effort to cope with uncertainty, some families employ hypervigilance in information-seeking.63 Prolonged periods of uncertainty may result in poorer psychosocial outcomes in the long-term.10 For care teams, chronically managing uncertainty can lead to burnout, especially in the absence of necessary support systems.64 Uncertainty is pervasive with CAR T-cell therapies: in who develops toxicity, how severe toxicity will be, whether or not remission will be achieved, and if it is, for how long, and what life will look life after CAR T-cells with regard to lasting adverse effects. Although uncertainty is inherent to any life-threatening illness, there are strategies that are shown to help patients and their families manage uncertainty. These include integration of clinicians trained to facilitate optimal patient-provider communication (such as palliative care providers and skilled oncology nurses);65–67 interventions aiming to improve cancer knowledge and access to information resources;68–71 mindfulness-based practices;72 and Cognitive-Behavioral Therapy.73,74 Additionally, normalization of uncertainty and the associated emotional consequences is important in both ourselves and in our patients’ families.63 Approaching this uncertainty with honesty and transparency, particularly regarding overall prognosis and likelihood of long-term remission, is likely to lead to less anxiety for families, rather than more.
Penny receives the infusion of her CAR T-cells without any complications. On the fourth day following the infusion, she develops high fevers. The fevers become more persistent, her blood pressure begins to drop, and she develops an oxygen requirement. She is transferred to the intensive care unit (ICU) for close monitoring. This is her first admission to the ICU. After a couple of days, Penny’s clinical status improves and she is transferred back to the oncology unit. The following morning, Penny’s mother mentions on rounds that Penny has been uncharacteristically quiet and had difficulty getting up to go to the bathroom. Later that day, Penny has a seizure. Concerned about development of immune effector cell associated neurotoxicity syndrome (ICANS), emergent head imaging is completed and she is re-admitted to the ICU.
Domain 3: Symptom Management
Compared to more well-established therapies for relapsed or refractory leukemia, such as hematopoietic stem cell transplant, CAR T-cell therapies carry an increased degree of uncertainty and unpredictability regarding symptoms and toxicities. In particular, and especially relevant for pediatric patients, the treatment course with CAR T-cell therapies may be the first time a patient experiences severe treatment-related toxicity and may mark their first admission to the intensive care unit. For other families, admission to the intensive care unit may conjure memories of prior traumatic treatment experiences. In such cases, anticipatory discussion of indications for transfer to higher level care may be helpful in preventing tension between the family and the medical team. Families may associate the presence of symptoms with likelihood of treatment success, further complicating the relationship between symptoms and distress. These considerations may produce significant distress for the patient and the family, making it imperative to take an interdisciplinary approach to comprehensive care with attention to providing psychosocial symptom support to both the patient and the family. Establishing an interdisciplinary team evaluation plan that includes both early introduction and ongoing availability of comprehensive care team members can aid in rapidly escalating supportive care in times of crisis. This plan should include details regarding when representatives from each care team/support service are involved (i.e. social work, palliative care, psychology, pain team, etc.).
Two main severe toxicity events have been reported as part of CAR T-cell therapy: cytokine release syndrome (CRS) and neurotoxicity.75 Although there is great variability in the development, progression, and severity of symptoms, both CRS and neurotoxicity typically manifest within the first 14 days following infusion of the cellular product.76 Risk factors for the development and severity of toxicity events remain the subject of investigation. Consensus guidelines have been published for the definition and grading and management of acute toxicity events.6,75 Notably, mental status examination of the pediatric patient is highly dependent on the developmental stage of the child. Accordingly, the Cornell Assessment of Pediatric Delirium (CAPD) has been recommended as a tool to guide the diagnosis and grading of neurologic toxicity until more sensitive mechanisms are identified.6 As symptoms of neurologic toxicity may develop and progress rapidly, systematic evaluation of cognitive function and neurologic symptoms at baseline and along the treatment trajectory is necessary and may lead to earlier identification and intervention.6,77 Long-term sequelae of CAR T-cell therapy, including neurologic toxicity, are not yet well described. Acute neurotoxicity may be a risk factor for later neuropsychiatric symptoms.78 Until specific risk factors are clearly defined, ongoing neurocognitive evaluation following therapy is likely to be important.77,79
Engaging patients and caregivers in care can lead to decreased distress and improved quality of life.80,81 Until the symptom experience of CAR T-cells is better understood, we must heavily rely on partnership with the patient and the family as part of the care team.6 This is especially important as some of the symptoms of CRS and neurotoxicity are subtle and best recognized by the parents and family caregivers.82 The involvement of palliative care services for the purpose of symptom assessment and intervention is recommended as a standard of care.18 Another way to systematically incorporate the patient’s voice into routine clinical practice is the use of patient-reported outcomes (PROs); CAR T-cell therapy represents a population that may benefit from this type of assessment.83,84 Recently published data from the ELIANA trial described the trajectory of HRQOL following CAR T-cell therapy, strengthening our understanding of the patient experience and helping to identify areas in need of further investigation.85 Moreover, this study demonstrates the collection of PRO data in this population is feasible in the acute phase of treatment.85 Whenever possible, direct elicitation of the child’s voice is ideal. Caregiver proxy assessment of PROs, although an imperfect interpretation of the patient’s experience, can be helpful in times of critical illness when the patient is unable to complete assessments.
Discussion
In 2008, the Institute of Medicine called for a more holistic approach toward cancer care, reinforcing the importance of providing comprehensive care that addresses psychosocial needs.8 Seeing this as a priority, the American Society of Pediatric Hematology/Oncology published the Standards for Psychosocial Care for Children with Cancer and Their Families in 2015, a series of systematic reviews providing an interdisciplinary set of guidelines for approaching psychosocial care in pediatric oncology.11,12,16,18,86,87 Representing a new paradigm of treatment, CAR T-cell therapy is associated with unique challenges and opportunities for high-quality comprehensive care, for which we propose the following recommendations (Table 3). For individuals whose prior treatment was unsuccessful, hopes for cure are complicated by tremendous uncertainty. Incorporating comprehensive interdisciplinary care throughout the experience is important to explore these and other hopes and worries. Comprehensive care teams also optimize health outcomes, minimize distress, and promote quality of life. Empathic and honest communication is necessary, particularly in discussing prognosis and the anticipated treatment course. Early and ongoing psychosocial and symptom assessment and partnership with both the family and primary oncology team are paramount to providing high quality care and successful outcomes. As the field continues to make progress in developing innovative approaches to treatment of childhood cancers, approaches to comprehensive care must keep step to ensure that cures and patient-centeredness go hand in hand.
TABLE 3.
Checklist for Comprehensive Care for Children Receiving CAR T-cell Therapy.
| Action Items | Additional Considerations | |
|---|---|---|
| Prior to leukapheresis (or arrival to referral center) | ||
| Evaluate understanding of treatment course, symptoms, prognosis | ||
| Explore expectations, including hopes and worries and perceived needs for additional support | ||
| Identify learning needs and determine educational and support resources previously available and helpful to the family | Provide multimedia learning aids based on learning preferences | |
| Understand child/family coping strategies | ||
| Identify psychosocial risk factors | •Financial insecurity •Housing •Material hardship •Psychiatric history •Family/support network dynamics •Health literacy |
|
| Introduce interdisciplinary team model | •Social work •Palliative care •Psychology/Psychiatry •Translators/cultural navigators |
|
|
For patient’s referred for CAR T-cell therapy: Establish a key contact from the referring team to include in future important discussions, provide patient-care updates, and to help coordinate care back to referring center. Understand historical experiences, medical/psychosocial needs, and potential differences in available support Hospital tour |
||
| Pre-treatment | ||
| Review types of symptoms/toxicities and anticipated timing | ||
| Review timing of disease response assessments | ||
| Review anticipated course if treatment is successful | ||
| Review hypothetical next steps/prognosis if remission is not achieved | ||
| Conduct baseline full neurologic assessment | Including full neurologic exam with mental status exam, evaluation of baseline cognitive function and neurologic symptom assessment | |
| During Treatment | ||
| Re-assess patient/family coping regularly | ||
| Conduct serial somatic symptom assessments | Consider patient-reported outcome measures | |
| Conduct serial neurocognitive assessments | Include full neurologic exam with mental status exam, cognitive function, and neurologic symptom assessment | |
| Update primary/referring team regularly | Consider communicating weekly | |
| Post-treatment | ||
| Involve referring team early if transferring care back to home institution | ||
| Conduct ongoing symptom assessment and neurocognitive evaluation | ||
Acknowledgments:
This work was conducted with support from a T32 Training Grant (5T32CA009351-40) awarded to A. Steineck. This work was supported, in part, by the Intramural Program of the National Cancer Institute, NIH”.
Abbreviations:
- ALL
Acute Lymphoblastic Leukemia
- CAR
Chimeric Antigen Receptor
- CRS
Cytokine release syndrome
- ICANS
Immune effector cell-associated neurotoxicity syndrome
- PRO
Patient-reported outcome
Footnotes
Conflicts of Interest Statement: The authors have no conflicts of interest to disclose.
References
- 1.Maude SL. Tisagenlecleucel in pediatric patients with acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2018;16(10):664–666. [PubMed] [Google Scholar]
- 2.Annesley CE, Summers C, Ceppi F, Gardner RA. The Evolution and Future of CAR T Cells for B-Cell Acute Lymphoblastic Leukemia. Clin Pharmacol Ther. 2018;103(4):591–598. [DOI] [PubMed] [Google Scholar]
- 3.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Find A KYMRIAH Treatment Center. 2019; https://www.us.kymriah.com/treatment-center-locator/#find-a-kymriah-treatment-center.Accessed January 24, 2020.
- 6.Mahadeo KM, Khazal SJ, Abdel-Azim H, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol. 2019;16(1):45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmoudjafari Z, Hawks KG, Hsieh AA, Plesca D, Gatwood KS, Culos KA. American Society for Blood and Marrow Transplantation Pharmacy Special Interest Group Survey on Chimeric Antigen Receptor T Cell Therapy Administrative, Logistic, and Toxicity Management Practices in the United States. Biol Blood Marrow Transplant. 2019;25(1):26–33. [DOI] [PubMed] [Google Scholar]
- 8.Medicine Io. In: Adler NE, Page AEK, eds. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington (DC): The National Academies Press; 2008. [PubMed] [Google Scholar]
- 9.Medicine Io. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): The National Academies Press; 2001. [PubMed] [Google Scholar]
- 10.Eilertsen ME, Reinfjell T, Vik T. Value of professional collaboration in the care of children with cancer and their families. Eur J Cancer Care (Engl). 2004;13(4):349–355. [DOI] [PubMed] [Google Scholar]
- 11.Steele AC, Mullins LL, Mullins AJ, Muriel AC. Psychosocial Interventions and Therapeutic Support as a Standard of Care in Pediatric Oncology. Pediatr Blood Cancer. 2015;62Suppl 5:S585–618. [DOI] [PubMed] [Google Scholar]
- 12.Kazak AE, Abrams AN, Banks J, et al. Psychosocial Assessment as a Standard of Care in Pediatric Cancer. Pediatr Blood Cancer. 2015;62Suppl 5:S426–459. [DOI] [PubMed] [Google Scholar]
- 13.Menard C, Merckaert I, Razavi D, Libert Y. Decision-making in oncology: a selected literature review and some recommendations for the future. Curr Opin Oncol. 2012;24(4):381–390. [DOI] [PubMed] [Google Scholar]
- 14.Kusch M, Labouvie H, Ladisch V, Fleischhack G, Bode U. Structuring psychosocial care in pediatric oncology. Patient Educ Couns. 2000;40(3):231–245. [DOI] [PubMed] [Google Scholar]
- 15.Corrigan JJ, Feig SA, American Academy of P. Guidelines for pediatric cancer centers. Pediatrics. 2004;113(6):1833–1835. [DOI] [PubMed] [Google Scholar]
- 16.Wiener L, Kazak AE, Noll RB, Patenaude AF, Kupst MJ. Interdisciplinary Collaboration in Standards of Psychosocial Care. Pediatr Blood Cancer. 2015;62Suppl 5:S425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patenaude AF, Pelletier W, Bingen K. Communication, Documentation, and Training Standards in Pediatric Psychosocial Oncology. Pediatr Blood Cancer. 2015;62Suppl 5:S870–895. [DOI] [PubMed] [Google Scholar]
- 18.Weaver MS, Heinze KE, Kelly KP, et al. Palliative Care as a Standard of Care in Pediatric Oncology. Pediatr Blood Cancer. 2015;62Suppl 5:S829–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson JE, Puntillo KA, Pronovost PJ, et al. In their own words: patients and families define high-quality palliative care in the intensive care unit. Crit Care Med. 2010;38(3):808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of Inpatient Palliative Care on Quality of Life 2 Weeks After Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. JAMA. 2016;316(20):2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greer JA, Jackson VA, Meier DE, Temel JS. Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin. 2013;63(5):349–363. [DOI] [PubMed] [Google Scholar]
- 22.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 23.Burns JP, Mitchell C, Griffith JL, Truog RD. End-of-life care in the pediatric intensive care unit: attitudes and practices of pediatric critical care physicians and nurses. Crit Care Med. 2001;29(3):658–664. [DOI] [PubMed] [Google Scholar]
- 24.Phipps S, Dunavant M, Lensing S, Rai SN. Psychosocial predictors of distress in parents of children undergoing stem cell or bone marrow transplantation. J Pediatr Psychol. 2005;30(2):139–153. [DOI] [PubMed] [Google Scholar]
- 25.Humphrey L, Kang TI. Palliative care in pediatric patients with hematologic malignancies. Hematology Am Soc Hematol Educ Program. 2015;2015:490–495. [DOI] [PubMed] [Google Scholar]
- 26.Ferrell BR, Temel JS, Temin S, et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(1):96–112. [DOI] [PubMed] [Google Scholar]
- 27.Section On H, Palliative M, Committee On Hospital C. Pediatric Palliative Care and Hospice Care Commitments, Guidelines, and Recommendations. Pediatrics. 2013;132(5):966–972. [DOI] [PubMed] [Google Scholar]
- 28.Kaye EC, Friebert S, Baker JN. Early Integration of Palliative Care for Children with High-Risk Cancer and Their Families. Pediatr Blood Cancer. 2016;63(4):593–597. [DOI] [PubMed] [Google Scholar]
- 29.Kaye EC, Snaman JM, Baker JN. Pediatric Palliative Oncology: Bridging Silos of Care Through an Embedded Model. J Clin Oncol. 2017;35(24):2740–2744. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721–1730. [DOI] [PubMed] [Google Scholar]
- 31.Bakitas MA, Tosteson TD, Li Z, et al. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J Clin Oncol. 2015;33(13):1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dionne-Odom JN, Azuero A, Lyons KD, et al. Benefits of Early Versus Delayed Palliative Care to Informal Family Caregivers of Patients With Advanced Cancer: Outcomes From the ENABLE III Randomized Controlled Trial. J Clin Oncol. 2015;33(13):1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones BL. Companionship, control, and compassion: a social work perspective on the needs of children with cancer and their families at the end of life. J Palliat Med. 2006;9(3):774–788. [DOI] [PubMed] [Google Scholar]
- 34.Wolfe J, Hammel JF, Edwards KE, et al. Easing of suffering in children with cancer at the end of life: is care changing? J Clin Oncol. 2008;26(10):1717–1723. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt P, Otto M, Hechler T, Metzing S, Wolfe J, Zernikow B. Did increased availability of pediatric palliative care lead to improved palliative care outcomes in children with cancer? J Palliat Med. 2013;16(9):1034–1039. [DOI] [PubMed] [Google Scholar]
- 36.Snaman JM, Kaye EC, Lu JJ, Sykes A, Baker JN. Palliative Care Involvement Is Associated with Less Intensive End-of-Life Care in Adolescent and Young Adult Oncology Patients. J Palliat Med. 2017;20(5):509–516. [DOI] [PubMed] [Google Scholar]
- 37.Margolis R, Ludi E, Pao M, Wiener L. International adaptation: psychosocial and parenting experiences of caregivers who travel to the United States to obtain acute medical care for their seriously ill child. Soc Work Health Care. 2013;52(7):669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cawcutt KA, Wilson JW. Benefits and challenges of caring for international patients. Cleve Clin J Med. 2016;83(11):794–800. [DOI] [PubMed] [Google Scholar]
- 39.Martin DR. Challenges and opportunities in the care of international patients: clinical and health services issues for academic medical centers. Acad Med. 2006;81(2):189–192. [DOI] [PubMed] [Google Scholar]
- 40.Benedetti DJ, Golshan M, Kesselheim JC. Going the Distance: Ethical Issues Arising When Patients Seek Cancer Care From International Settings. J Glob Oncol. 2018;4:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bona K, London WB, Guo D, Abel G, Lehmann L, Wolfe J. Prevalence and impact of financial hardship among New England pediatric stem cell transplantation families. Biol Blood Marrow Transplant. 2015;21(2):312–318. [DOI] [PubMed] [Google Scholar]
- 42.Lin JK, Muffly LS, Spinner MA, Barnes JI, Owens DK, Goldhaber-Fiebert JD. Cost Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Multiply Relapsed or Refractory Adult Large B-Cell Lymphoma. J Clin Oncol. 2019;37(24):2105–2119. [DOI] [PubMed] [Google Scholar]
- 43.Guy GP Jr., Yabroff KR, Ekwueme DU, Rim SH, Li R, Richardson LC. Economic Burden of Chronic Conditions Among Survivors of Cancer in the United States. J Clin Oncol. 2017;35(18):2053–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guy GP Jr., Yabroff KR, Ekwueme DU, et al. Estimating the health and economic burden of cancer among those diagnosed as adolescents and young adults. Health Aff (Millwood). 2014;33(6):1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pelletier W, Bona K. Assessment of Financial Burden as a Standard of Care in Pediatric Oncology. Pediatr Blood Cancer. 2015;62Suppl 5:S619–631. [DOI] [PubMed] [Google Scholar]
- 46.Phipps S, Dunavant M, Lensing S, Rai SN. Acute health-related quality of life in children undergoing stem cell transplant: II. Medical and demographic determinants. Bone Marrow Transplant. 2002;29(5):435–442. [DOI] [PubMed] [Google Scholar]
- 47.Blazin LJ, Cecchini C, Habashy C, Kaye EC, Baker JN. Communicating Effectively in Pediatric Cancer Care: Translating Evidence into Practice. Children (Basel). 2018;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernacki RE, Block SD, American College of Physicians High Value Care Task F. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003. [DOI] [PubMed] [Google Scholar]
- 49.Epstein R, Street RL. Patient-centered communication in cancer care : promoting healing and reducing suffering. Bethsda, MD: U.S. Dept. of Health and Human Services, National Institutes of Health, National Cancer Institute; 2007. [Google Scholar]
- 50.Jackson VA, Jacobsen J, Greer JA, Pirl WF, Temel JS, Back AL. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med. 2013;16(8):894–900. [DOI] [PubMed] [Google Scholar]
- 51.Masera G, Spinetta JJ, Jankovic M, et al. Guidelines for a therapeutic alliance between families and staff: a report of the SIOP Working Committee on Psychosocial Issues in Pediatric Oncology. Med Pediatr Oncol. 1998;30(3):183–186. [DOI] [PubMed] [Google Scholar]
- 52.Wiener L, Zadeh S, Wexler LH, Pao M. When silence is not golden: Engaging adolescents and young adults in discussions around end-of-life care choices. Pediatr Blood Cancer. 2013;60(5):715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zadeh S, Pao M, Wiener L. Opening end-of-life discussions: how to introduce Voicing My CHOiCES, an advance care planning guide for adolescents and young adults. Palliat Support Care. 2015;13(3):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schenker Y, White DB, Crowley-Matoka M, Dohan D, Tiver GA, Arnold RM. “It hurts to know... and it helps”: exploring how surrogates in the ICU cope with prognostic information. J Palliat Med. 2013;16(3):243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durall A, Zurakowski D, Wolfe J. Barriers to conducting advance care discussions for children with life-threatening conditions. Pediatrics. 2012;129(4):e975–982. [DOI] [PubMed] [Google Scholar]
- 56.Snaman JM, Feraco AM, Wolfe J, Baker JN. “What if?”: Addressing uncertainty with families. Pediatr Blood Cancer. 2019;66(6):e27699. [DOI] [PubMed] [Google Scholar]
- 57.Rosenberg AR, Feudtner C. What else are you hoping for? Fostering hope in paediatric serious illness. Acta Paediatr. 2016;105(9):1004–1005. [DOI] [PubMed] [Google Scholar]
- 58.Feudtner C The breadth of hopes. N Engl J Med. 2009;361(24):2306–2307. [DOI] [PubMed] [Google Scholar]
- 59.Contro N, Larson J, Scofield S, Sourkes B, Cohen H. Family perspectives on the quality of pediatric palliative care. Arch Pediatr Adolesc Med. 2002;156(1):14–19. [DOI] [PubMed] [Google Scholar]
- 60.Mack JW, Wolfe J, Grier HE, Cleary PD, Weeks JC. Communication about prognosis between parents and physicians of children with cancer: parent preferences and the impact of prognostic information. J Clin Oncol. 2006;24(33):5265–5270. [DOI] [PubMed] [Google Scholar]
- 61.Meert KL, Thurston CS, Sarnaik AP. End-of-life decision-making and satisfaction with care: parental perspectives. Pediatr Crit Care Med. 2000;1(2):179–185. [DOI] [PubMed] [Google Scholar]
- 62.Breen CM, Abernethy AP, Abbott KH, Tulsky JA. Conflict associated with decisions to limit life-sustaining treatment in intensive care units. J Gen Intern Med. 2001;16(5):283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith AK, White DB, Arnold RM. Uncertainty--the other side of prognosis. N Engl J Med. 2013;368(26):2448–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hill DL, Walter JK, Casas JA, DiDomenico C, Szymczak JE, Feudtner C. The codesign of an interdisciplinary team-based intervention regarding initiating palliative care in pediatric oncology. Support Care Cancer. 2018;26(9):3249–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hendricks-Ferguson VL, Kane JR, Pradhan KR, et al. Evaluation of Physician and Nurse Dyad Training Procedures to Deliver a Palliative and End-of-Life Communication Intervention to Parents of Children with a Brain Tumor. J Pediatr Oncol Nurs. 2015;32(5):337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dunlop JMH RJ. Hospital-based Palliative Care Teams: The hospital-hospice interface. Oxford Scholarship; 1998. [Google Scholar]
- 67.Murtagh F Can palliative care teams relieve some of the pressure on acute services? BMJ. 2014;348:g3693. [DOI] [PubMed] [Google Scholar]
- 68.Germino BB, Mishel MH, Crandell J, et al. Outcomes of an uncertainty management intervention in younger African American and Caucasian breast cancer survivors. Oncol Nurs Forum. 2013;40(1):82–92. [DOI] [PubMed] [Google Scholar]
- 69.Gil KM, Mishel MH, Belyea M, Germino B, Porter LS, Clayton M. Benefits of the uncertainty management intervention for African American and White older breast cancer survivors: 20-month outcomes. Int J Behav Med. 2006;13(4):286–294. [DOI] [PubMed] [Google Scholar]
- 70.Mishel MH, Germino BB, Gil KM, et al. Benefits from an uncertainty management intervention for African-American and Caucasian older long-term breast cancer survivors. Psychooncology. 2005;14(11):962–978. [DOI] [PubMed] [Google Scholar]
- 71.Mishel MH, Germino BB, Lin L, et al. Managing uncertainty about treatment decision making in early stage prostate cancer: a randomized clinical trial. Patient Educ Couns. 2009;77(3):349–359. [DOI] [PubMed] [Google Scholar]
- 72.Epstein RM. Mindful practice. JAMA. 1999;282(9):833–839. [DOI] [PubMed] [Google Scholar]
- 73.Mishel MH, Germino BB, Belyea M, et al. Moderators of an uncertainty management intervention: for men with localized prostate cancer. Nurs Res. 2003;52(2):89–97. [DOI] [PubMed] [Google Scholar]
- 74.Jiang X, He G. Effects of an uncertainty management intervention on uncertainty, anxiety, depression, and quality of life of chronic obstructive pulmonary disease outpatients. Res Nurs Health. 2012;35(4):409–418. [DOI] [PubMed] [Google Scholar]
- 75.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. [DOI] [PubMed] [Google Scholar]
- 76.Frey N, Porter D. Cytokine Release Syndrome with Chimeric Antigen Receptor T Cell Therapy. Biol Blood Marrow Transplant. 2019;25(4):e123–e127. [DOI] [PubMed] [Google Scholar]
- 77.Shalabi H, Wolters PL, Martin S, et al. Systematic Evaluation of Neurotoxicity in Children and Young Adults Undergoing CD22 Chimeric Antigen Receptor T-Cell Therapy. J Immunother. 2018;41(7):350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruark J, Mullane E, Cleary N, et al. Patient-Reported Neuropsychiatric Outcomes of Long-Term Survivors after Chimeric Antigen Receptor T Cell Therapy. Biol Blood Marrow Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Annett RD, Patel SK, Phipps S. Monitoring and Assessment of Neuropsychological Outcomes as a Standard of Care in Pediatric Oncology. Pediatr Blood Cancer. 2015;62Suppl 5:S460–513. [DOI] [PubMed] [Google Scholar]
- 80.Bombard Y, Baker GR, Orlando E, et al. Engaging patients to improve quality of care: a systematic review. Implement Sci. 2018;13(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Committee On Hospital C, Institute For P, Family-Centered C. Patient- and family-centered care and the pediatrician’s role. Pediatrics. 2012;129(2):394–404. [DOI] [PubMed] [Google Scholar]
- 82.Power N, Franck L. Parent participation in the care of hospitalized children: a systematic review. J Adv Nurs. 2008;62(6):622–641. [DOI] [PubMed] [Google Scholar]
- 83.Chakraborty R, Sidana S, Shah GL, Scordo M, Hamilton BK, Majhail NS. Patient-Reported Outcomes with Chimeric Antigen Receptor T Cell Therapy: Challenges and Opportunities. Biol Blood Marrow Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leahy AB, Feudtner C, Basch E. Symptom Monitoring in Pediatric Oncology Using Patient-Reported Outcomes: Why, How, and Where Next. Patient. 2018;11(2):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laetsch TW, Myers GD, Baruchel A, et al. Patient-reported quality of life after tisagenlecleucel infusion in children and young adults with relapsed or refractory B-cell acute lymphoblastic leukaemia: a global, single-arm, phase 2 trial. Lancet Oncol. 2019;20(12):1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wiener L, Kazak AE, Noll RB, Patenaude AF, Kupst MJ. Standards for the Psychosocial Care of Children With Cancer and Their Families: An Introduction to the Special Issue. Pediatr Blood Cancer. 2015;62Suppl 5:S419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kearney JA, Salley CG, Muriel AC. Standards of Psychosocial Care for Parents of Children With Cancer. Pediatr Blood Cancer. 2015;62Suppl 5:S632–683. [DOI] [PMC free article] [PubMed] [Google Scholar]


