Abstract
Knowledge of the isocitrate dehydrogenase (IDH) mutation status of glioma patients could provide insights for decision-making during brain surgery. However, pathology is not able to provide such information intraoperatively. Here we describe the first application of a miniature mass spectrometer (MS) to the determination of IDH mutation status in gliomas intraoperatively. The instrumentation was modified to be compatible with use in the operating room. Tandem MS was performed on the oncometabolite, 2-hydroxyglutarate, and a reference metabolite, glutamate, which is not involved in the IDH mutation. Ratios of fragment ion intensities were measured to calculate an IDH mutation score, which was used to differentiate IDH mutant and wild-type tissues. The results of analyzing 25 biopsies from 13 patients indicate that reliable determination of IDH mutation status was achieved (p = 0.0001, using the Kruskal-Wallis non-parametric test). With its small footprint and low power consumption and noise level, this application of miniature mass spectrometers represents a simple and cost-effective platform for an important intraoperative measurement.
Keywords: Molecular cancer diagnostics, Ambient ionization mass spectrometry, Isocitrate dehydrogenase mutation, Miniature mass spectrometry, Point-of-care diagnostics
Introduction
The accurate diagnosis of gliomas relies increasingly on molecular features [1]. However, the microscopic review of tissue biopsies, the principal source of intraoperative diagnostic information, does not provide any molecular or genetic information, including information on isocitrate dehydrogenase (IDH) mutation status. Conventionally, mutations in the enzyme IDH are determined using immunohistochemistry with results not available until some days after surgery (Fig. 1). Knowledge of IDH mutation status at the time of surgery could improve surgical outcomes because more aggressive tumor resection of IDH-mutated gliomas is associated with increased survival [2, 3]. IDH mutations alter enzymatic pathways and lead to the accumulation of the oncometabolite 2-hydroxyglutarate (2-HG) [4]. While 2-HG is present in very small concentrations in normal tissues, concentrations increase dramatically in tumors with mutations in IDH1 and IDH2, reaching levels up to 35 μmol per gram of tumor [5]. This feature can be used to assess mutations in IDH, as has been demonstrated using magnetic resonance spectroscopy and mass spectrometry (MS) [6, 7].
Fig. 1.
Intraoperative detection of IDH mutations with Mini MS determines mutation status in minutes as opposed to days by means of conventional genomic analysis or immunohistochemistry
MS is a highly sensitive technique that is capable of the qualitative and quantitative analysis of complex samples, including biological tissues. This application is most accurately done in conjunction with liquid or gas chromatography but doing so requires tedious sample preparation. Matrix-assisted laser desorption ionization (MALDI) is an alternative method which is important in tissue analysis [8]. MALDI has been used for the detection of 2-HG in frozen sections; however, the required sample preparation and instrumentation make it incompatible with use in the operating room (OR) [9]. By contrast, ambient ionization is well suited for intraoperative use, as it allows chemical information to be obtained by MS at atmospheric pressure for a variety of matrices, including tissues, thereby eliminating sample pretreatment steps and making possible analysis in the OR. The first ambient methods were desorption electrospray ionization mass spectrometry (DESI-MS) and direct analysis in real time [10, 11]. These experiments have stimulated the development of numerous ambient ionization methods, including air flow–assisted desorption electrospray ionization, matrix-assisted laser desorption electrospray ionization, liquid micro junction-surface sampling, paper spray, MasSpec Pen, and rapid evaporative ionization MS [12–18]. They have also prompted a reexamination of conventional tissue diagnosis.
We have previously measured the abnormal accumulation of 2-HG for the intraoperative diagnosis of IDH mutation status using DESI-MS in near real time [19]. However, this intraoperative application requires expensive instrumentation. By contrast, miniature MS systems have small footprints, have low energy consumption, and produce little noise [20]. Their use in the OR offers the prospect of intraoperative genetic diagnosis in a cost-efficient manner. In an earlier study, we established the fact that a miniature mass spectrometer can be used to determine IDH mutation status in banked brain tissue samples [21]. In this small-scale clinical study with 13 subjects, we report the first application of a miniature mass spectrometer to determine IDH-mutation status of glioma tissue intraoperatively, building on prior laboratory-based work [21, 22].
Experimental
Sampling method
Extraction nanoelectrospray ionization (nESI), a method characterized by minimal sample preparation, was used to generate ions [23]. We have previously established this method for rapid diagnosis of IDH mutation status based on measurement of 2-HG using banked tissue samples [21], so similar procedures were carried out in the OR. Biopsied tissue specimens from surgeon-defined positions within the tumor and at the walls of the resection cavity were collected intraoperatively. The number and location of the biopsies were determined according to the medical judgment of the attending surgeon. The biopsies were touched with a thin strip of Whatman 1 filter paper (ca. 0.5 mm wide and 15 mm long) that was then inserted into a nanotip (i.d. 0.86 mm, length ~ 4 cm) pre-filled with 20 μL methanol/water (9:1, v/v). The nanotip was subsequently placed in a custom 3D printed sample holder for MS analysis.
MS analysis
The experiment used the ion trap based PURSPEC Mini β, a stand-alone system that does not require external pumps or gas tanks. The Mini β, which measures 55 cm × 24 cm × 31 cm (L × W × H), was placed on an aluminum cart with all equipment required for its operation in the OR (Fig. S1, see Electronic Supplementary Material, ESM). An enclosed volume was employed to minimize any material transfer from the instrument. Prior to each surgery, the instrument was rolled into the OR and operated within the surgical suite for the duration of the surgery. The method is not quantitative, but we carried out calibration of the instrument before and after every surgical case to establish mass accuracy. This was done using a solution composed of aspartic acid, glutamate (GLU), 2-HG, and N-acetylaspartate using nESI in the negative ion mode; their molecular ions were found at m/z 132, 146, 147, and 174, respectively (Fig. S2, ESM). Ionizing voltage (− 1.5 kV) was applied to initiate nESI from a disposable acupuncture needle (diameter 0.3 mm, length 4 cm).
Tandem MS analysis of 2-HG was performed in the negative ion mode with GLU, an abundant metabolite not specifically associated with glioma, serving as an internal standard. The product ion abundances for the transitions m/z 147 → 129 for 2-HG and m/z 146 → 128 for GLU provided a relative measure of the concentration of 2-HG in tissue. The precursor ions were selected using a 2-Th-wide mass window. For each sample, at least three product ion spectra were recorded, each recorded spectrum being the average of three scans. Each scan took ~ 2 s and spectra were saved manually, making the total data acquisition time per sample ca. 1 min and the total analysis time including sample manipulation ca. 3 min. Postoperative analysis of the same biopsies was performed using the extraction nESI method on a Thermo TSQ to confirm intraoperative IDH mutation predictions. The TSQ analysis was performed using multiple reaction monitoring (MRM) mode; transitions selected were 2-HG (m/z 147 → 129) and GLU (m/z 146 → 128) (Figs. S3 and S4, ESM). With respect to each transition, optimized tube lens values of 67 and 69 V and nominal collision energies of 12 and 13 were applied.
Data analysis
De-identified clinical data, consisting of patient demographics and radiology, surgical, and pathology reports, were obtained for each patient for correlation with the data produced by the Mini β system. The Mini β data were analyzed within 30 s postoperatively in MATLAB using a custom program to subtract background intensities and to calculate IDH mutation scores. This calculation can be done intraoperatively. The TSQ data were analyzed using Xcalibur. An IDH mutation score was calculated as the ratio of the two product ion intensities after correcting for the isotopic contributions of the 13C glutamate fragment ion at m/z 129 (Eq. 1).
| (1) |
Results and discussion
In this preliminary clinical study, we analyzed 25 biopsies from 13 subjects. The patient cohort is described in detail in Table 1. Of the 13 subjects, 4 were IDH mutant (7 biopsies) and 9 were IDH wild-type (18 biopsies), according to postoperative pathology reports based on immunohistochemistry. Representative product ion mass spectra from an IDH wild-type and an IDH mutant glioma are included in the ESM (Figs. S5 and S6). As shown in Fig. 2 (left), the IDH wild-type tissues all had IDH mutation status scores below 0.5, whereas all IDH mutant samples had scores above 1.7. The box represents the interquartile range with a median line and whiskers at ± 1.5 standard deviations. The clear differentiation between IDH wild-type and mutant (p = 0.0001) is consistent with our previous observations on banked tissue samples [21], which suggests that intraoperative diagnosis of IDH mutation status can be realized with the current methodology.
Table 1.
IDH mutation scores of tissue biopsies
| Subject | Biopsy | Type of glioma | WHO grade | IDH mutation status | IDH mutation score (Mini MS) | IDH mutation score (TSQ) |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 1 | Glioblastoma | IV | Wild-type | 0.06 | < 0.01 |
| 2 | 1 | Glioblastoma | IV | Wild-type | 0.02 | 0.02 |
| 3 | 1 | Astrocytoma | II | Mutant | 3.89 | 10.56 |
| 4 | 1 | Pleomorphic xantoastrocytoma | II | Wild-type | 0.33 | 0.03 |
| 2 | 0.18 | 0.02 | ||||
| 3 | 0.35 | 0.07 | ||||
| 5 | 1 | Glioblastoma | IV | Wild-type | 0.20 | 0.06 |
| 2 | 0.24 | 0.06 | ||||
| 3 | 0.33 | 0.12 | ||||
| 6 | 1 | Glioblastoma | IV | Wild-type | 0.35 | 0.04 |
| 2 | 0.33 | 0.04 | ||||
| 7 | 1 | Glioblastoma | IV | Wild-type | 0.28 | 0.07 |
| 2 | 0.20 | 0.09 | ||||
| 8 | 1 | Anaplastic astrocytoma | III | Mutant | 2.83 | 5.01 |
| 9 | 1 | Glioblastoma | IV | Wild-type | 0.11 | 0.05 |
| 10 | 1 | Glioblastoma | IV | Mutant | 3.20 | 35.51 |
| 2 | 1.73 | 99.33 | ||||
| 3 | 1.73 | 14.34 | ||||
| 11 | 1 | Glioblastoma | IV | Wild-type | 0.12 | 0.06 |
| 12 | 1 | III | Mutant | 2.07 | 6.73 | |
| 2 | 2.30 | 16.93 | ||||
| 13 | 1 | Glioblastoma | IV | Wild-type | 0.25 | 0.04 |
| 2 | 0.35 | 0.04 | ||||
| 3 | 0.50 | 0.07 | ||||
| 4 | 0.26 | 0.03 | ||||
Fig. 2.
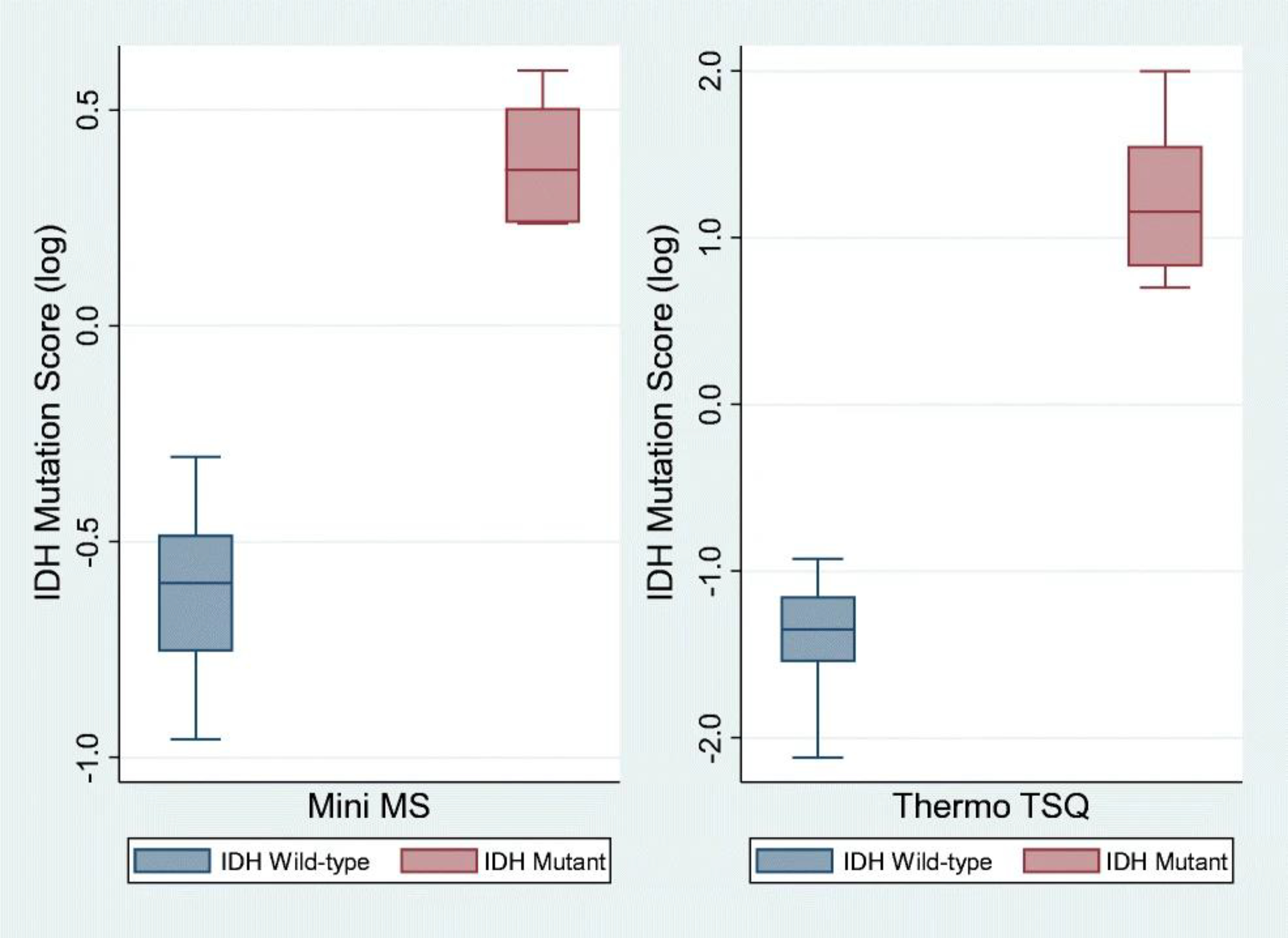
Box-and-whisker plot of IDH mutation scores in human glioma tissue specimens (n = 25) analyzed intraoperatively using a PURSPEC Mini β and in the laboratory using a Thermo Triple Quadruple TSQ. (The box represents the interquartile range with a median line and whiskers at ± 1.5 SD. Two outliers (IDH wild-type) with IDH mutation scores below 1.5 SD were excluded in the Mini MS plot. Population medians for IDH mutation scores for both methods were statistically different; p values for both are 0.0001, using the Kruskal-Wallis non-parametric test.)
Postoperative analysis of the same biopsies was performed using extraction nESI on a Thermo TSQ. All IDH wild-type tissues had IDH mutation scores below 0.1 and all IDH mutant tissues had IDH mutation scores above 5.0 (Fig. 2, right) using this higher performance lab system. The higher sensitivity of the Thermo TSQ results in less noise and hence an increase in the absolute differences of the mutation scores. Identical conclusions can be drawn from both sets of results, as shown in Fig. 2. For both methods, the differences in population medians for the IDH mutation scores were statistically significant different (p values both 0.0001, using Kruskal-Wallis non-parametric test). Both instruments are capable of generating diagnostic information consistent with pathology. In this assay, the intraoperative application of a miniature mass spectrometer shows comparable performance with that of a conventional instrument.
Conclusion
We have successfully performed intraoperative diagnosis of IDH mutation using extraction nESI on Mini β. The instrumentation was modified to be compatible with use in the operating room. With its small footprint, low power consumption, minimal noise, and ability to generate reliable IDH mutation status predictions in near real time, the intraoperative use of a miniature mass spectrometer represents an advance towards improved glioma patient treatment. Further studies will be carried out with protocol standardization and automation to validate these preliminary findings and reduce the barrier for broader application.
Supplementary Material
Acknowledgments
The authors thank clinical research nurses Lauren Snyder and Heather Cero at Goodman Campbell Brain and Spin (Indianapolis, IN) for patient consent, providing clinical data, and IRB monitoring; Clint Alfaro and Tsdale Mehari for assistance in sample preparation; Robert Schrader for assistance with instrumentation; Zhuoer Xie for the table of contents figure; and PURSPEC Technologies Inc. for instrumentation support.
Funding information
This study received funding from the Purdue University Center for Cancer Research Small Grants Program; from National Institute of General Medical Sciences of the NIH under award number R44GM119584.
Footnotes
Conflict of interest Zheng Ouyang is the founder of PURSPEC Technologies Inc. All other authors declare that they have no conflict of interest.
Compliance with ethical standards Biopsies for tissue analysis were obtained from human subjects undergoing tumor resection for suspected glioma at IU Health Methodist Hospital, after they had provided written informed consent to participate in the research study, following an IUSMIRB approved protocol (IRB No. 1410342262). No results were shared with the neurosurgeons during the surgical resection so as not to affect the standard of care.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00216-019-02198-y) contains supplementary material, which is available to authorized users.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131(6):803–20. 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Chen R, Ravindra VM, Cohen AL, Jensen RL, Salzman KL, Prescot AP, et al. Molecular features assisting in diagnosis, surgery, and treatment decision making in low-grade gliomas. Neurosurg Focus. 2015;38(3):E2. 10.3171/2015.1.FOCUS14745. [DOI] [PubMed] [Google Scholar]
- 3.Patel T, Bander ED, Venn RA, Powell T, Cederquist GY, Schaefer PM, et al. The Role of Extent of Resection in IDH1 Wild-Type or Mutant Low-Grade Gliomas. Neurosurgery. 2018;82(6):808–14. 10.1093/neuros/nyx265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–44. 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller JJ, Shih HA, Andronesi OC, Cahill DP. Isocitrate dehydrogenase-mutant glioma: Evolving clinical and therapeutic implications. Cancer. 2017;123(23):4535–46. 10.1002/cncr.31039. [DOI] [PubMed] [Google Scholar]
- 6.Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med 2012;4(116):116ra114. 10.1126/scitranslmed.3002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santagata S, Eberlin LS, Norton I, Calligaris D, Feldman DR, Ide JL, et al. Intraoperative mass spectrometry mapping of an oncometabolite to guide brain tumor surgery. Proc Natl Acad Sci U S A. 2014;111(30):11121–6. 10.1073/pnas.1404724111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonnell LA, Heeren RM. Imaging mass spectrometry. Mass Spectrom Rev 2007;26(4):606–43. 10.1002/mas.20124. [DOI] [PubMed] [Google Scholar]
- 9.Longuespee R, Wefers AK, De Vita E, Miller AK, Reuss DE, Wick W, et al. Rapid detection of 2-hydroxyglutarate in frozen sections of IDH mutant tumors by MALDI-TOF mass spectrometry. Acta Neuropathol Commun 2018;6(1):21. 10.1186/s40478-018-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takats Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306(5695):471–3. 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 11.Cody RB, Laramee JA, Durst HD. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem 2005;77(8):2297–302. 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 12.Brown HM, Pirro V, Cooks RG. From DESI to the MasSpec Pen: Ambient Ionization Mass Spectrometry for Tissue Analysis and Intrasurgical Cancer Diagnosis. Clin Chem 2018;64(4):628–30. 10.1373/clinchem.2017.281923. [DOI] [PubMed] [Google Scholar]
- 13.He J, Sun C, Li T, Luo Z, Huang L, Song X, et al. A Sensitive and Wide Coverage Ambient Mass Spectrometry Imaging Method for Functional Metabolites Based Molecular Histology. Adv Sci 2018;5(11):1800250. 10.1002/advs.201800250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robichaud G, Barry JA, Muddiman DC. IR-MALDESI mass spectrometry imaging of biological tissue sections using ice as a matrix. J Am Soc Mass Spectrom 2014;25(3):319–28. 10.1007/s13361-013-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Berkel GJ, Kertesz V, Koeplinger KA, Vavrek M, Kong AN. Liquid microjunction surface sampling probe electrospray mass spectrometry for detection of drugs and metabolites in thin tissue sections. J Mass Spectrom 2008;43(4):500–8. 10.1002/jms.1340. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Manicke NE, Yang Q, Zheng L, Shi R, Cooks RG, et al. Direct Analysis of Biological Tissue by Paper Spray Mass Spectrometry. Anal Chem 2011;83(4):1197–201. 10.1021/ac103150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Rector J, Lin JQ, Young JH, Sans M, Katta N, et al. Nondestructive tissue analysis for ex vivo and in vivo cancer diagnosis using a handheld mass spectrometry system. Sci Transl Med 2017;9(406). 10.1126/scitranslmed.aan3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balog J, Sasi-Szabo L, Kinross J, Lewis MR, Muirhead LJ, Veselkov K, et al. Intraoperative Tissue Identification Using Rapid Evaporative Ionization Mass Spectrometry. Sci Transl Med 2013;5(194):194ra193. 10.1126/scitranslmed.3005623. [DOI] [PubMed] [Google Scholar]
- 19.Alfaro CM, Pirro V, Keating MF, Hattab EM, Cooks RG, Cohen-Gadol AA. Intraoperative assessment of isocitrate dehydrogenase mutation status in human gliomas using desorption electrospray ionization-mass spectrometry. J Neurosurg 2019:1–8. 10.3171/2018.8.JNS181207. [DOI] [PubMed] [Google Scholar]
- 20.Austin DE, Lammert SA. Mass Analyzer Miniaturization. In: The Encyclopedia of Mass Spectrometry, vol. 7. 1st ed: Elsevier; 2009. [Google Scholar]
- 21.Pu F, Alfaro CM, Pirro V, Xie Z, Ouyang Z, Cooks RG. Rapid determination of isocitrate dehydrogenase mutation status of human gliomas by extraction nanoelectrospray using a miniature mass spectrometer. Anal Bioanal Chem 2019;411(8):1503–8. 10.1007/s00216-019-01632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou R, Cao W, Chong L, Hua W, Xu H, Mao Y, et al. Point-of-Care Tissue Analysis Using Miniature Mass Spectrometer. Anal Chem 2019;91(1):1157–63. 10.1021/acs.analchem.8b04935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Y, Liu J, Li L, McLuckey MN, Ouyang Z. Direct Mass Spectrometry Analysis of Untreated Samples of Ultralow Amounts Using Extraction Nano-Electrospray. Anal Methods. 2013;5(23). 10.1039/C3AY41149D. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


