Abstract
Objective.
As many as 65% of people with multiple sclerosis (MS) have clinically significant memory impairment, but the nature of this deficit is controversial. Some investigations suggest that an inability to retrieve newly-learned information from memory is prominent, whereas others imply that compromised acquisition accounts for impairment. Prior research has not simultaneously evaluated acquisition and retrieval processes in MS, and fewer have attempted to account for initial acquisition when studying retrieval. The Item Specific Deficit Approach (ISDA: Wright, Woo, Schmitter-Edgecombe, Hinkin, Miller, & Gooding, 2009) offers a method of quantifying acquisition, retrieval, and retention processes, with the latter two mechanisms being adjusted for initial acquisition. To simultaneously quantify acquisition and retrieval abilities, the ISDA was applied to list learning performance in two independent samples of people with MS and corresponding healthy comparison groups.
Participants and Methods.
Study 1 included 85 people with MS and 47 healthy individuals. Study 2 involved a separate sample of 79 people with MS and 22 healthy people. They were administered neuropsychological batteries, and participants with MS were classified as globally impaired or unimpaired. The California Verbal Learning Test-II was administered to assess new-learning in both studies, and responses were scored using the ISDA.
Results.
Both studies revealed that cognitively impaired people with MS manifest weaknesses involving acquisition and retrieval. Nearly identical effect sizes emerged across samples, with cognitive impairment achieving a medium effect upon acquisition and a large effect upon retrieval.
Conclusions.
These findings accord well with previous research showing diminished acquisition and retrieval among people with MS. The results may also reconcile contradictory findings in the extant literature by showing that memory impairment in MS is not exclusively attributable to either acquisition or retrieval. Rather, both processes may manifest across people with MS. The replication across samples with nearly identical effect sizes implies that these effects are reliable and possess external validity. These data hold implications for memory rehabilitation interventions involving people with MS, and suggest that acquisition and retrieval processes should be addressed in treatment.
Keywords: new-learning, memory, acquisition, retrieval, retention, multiple sclerosis
Multiple sclerosis (MS) is a central nervous system disease characterized by multiple demyelinating lesions, especially in the periventricular region of the brain (Grzegorski & Losy, 2017). Cognitive deficits occur commonly among people with multiple sclerosis (MS), with as many as 66% of patients demonstrating impairment on formal testing. The affected neurocognitive domains may vary between individuals and across time, but impairments involving explicit memory rank among the most common. Estimates vary, but memory impairment occurs in 22-65% of people with MS (Chiaravalloti & DeLuca, 2008; Grzegorski & Losy, 2017; Sumowski, Benedict, Enzinger, Fillippi, Geurts, Hamalainen, Hulst, Inglese, Leavitt, Rocca, Rosti-Otajarvi, & Rao, 2018). Memory impairment may exert a salient adverse effect upon quality of life, occupational and social role fulfillment, and activities of daily living (Bobholz, Gleason, & Miller, 2008). Owing to its prevalence and impact on functional outcomes, interventions have been developed to ameliorate forgetfulness. These interventions typically seek to enhance the amount of information acquired during learning (e.g., Basso, Lowery, Ghormley, Combs, & Johnson, 2006), or they facilitate retrieval of memories (e.g., Cicerone, Goldin, Ganci, Rosenbaum, Wethe, Langenbahn, Malec, Bergquist, Kingsley, Nagele, Trexler, Fraas, Bodanova, & Harley, 2019). Delineating the nature of impairment in MS can help clinicians to focus on the most affected memory processes in order to optimize rehabilitation methods.
In this vein, research has attempted to delineate whether forgetfulness in MS reflects compromised acquisition, retrieval, or retention of new memories. There is a general consensus that MS is not associated with impaired retention, thereby distinguishing its amnestic profile from Alzheimer’s disease and other conditions affecting the medial temporal lobes. Nonetheless, opinions vary as to whether MS corresponds with impaired acquisition or retrieval, and this has been a source of controversy in the field.
Early studies indicated that poor memory performance in MS was due to impaired retrieval. For instance, investigations by Beatty and Rao (Beatty, 1993; Beatty, Wilbanks, Blanco, Hames, Tivis, & Paul, 1996; Rao, Grafman, DiGiulio, Mittenberg, Bernardin, Leo, Luchetta, & Unverzagt, 1993) showed that people with MS achieved worse immediate and delayed recall than healthy individuals, but their recognition memory was essentially normal. This implied that information was acquired and retained normally, but people with MS struggled to retrieve the information from long-term memory. This pattern was observed repeatedly (Armstrong, Onishi, Robinson, D’Esposito, Thompson, Rostami, & Grossman, 1996; Brissart, Morele, E., Baumann, & Debouverie, 2012; Caine, Bamford, Schiffer, Shoulson, & Levy, 1986; Coolidge, Middleton, Griego, & Schmidt, 1996; Rao, Leo, & Aubin-Faubert, 1989), and a number of quantitative reviews concluded that memory impairment in MS manifested primarily with deficient retrieval (Prakash, Snook, Lewis, Motl, & Kramer, 2008; Wishart & Sharpe, 1997; Zakzanis, 2000).
Although such outcomes are compelling, they have been criticized for neglecting to address a potential confound. In particular, initial acquisition of information was not directly assessed (cf. DeLuca, Barbieri-Berger, & Johnson, 1994). Towards this end, DeLuca et al. (1994) employed an innovative modification to the Selective Reminding Test. On this task, a list of 10 words is presented repeatedly. After each presentation, participants are reminded only of words they had not recalled. Repetition of the word list normally concludes after participants display criterion performance (i.e., recall the entire list twice consecutively without reminders) or after 10 presentations. Deluca et al. reasoned that people with MS struggle to acquire information quickly, and permitted 15 repetitions of the list. They administered it to 25 patients with MS and 23 healthy individuals. Notably, two individuals with MS could not achieve criterion acquisition after 15 presentations of the list, and they were excluded from subsequent analyses. After 30-minutes, participants recalled the list, and a recognition trial was presented. Healthy individuals required an average of 5 presentations to achieve criterion, whereas persons with MS required an average of 8 trials. The groups achieved equivalent recall and recognition performance after a 30-minute delay. DeLuca et al. surmised that previous investigations had not controlled for poor acquisition. After ensuring that the patients with MS had acquired as much information as the healthy group, the MS sample displayed normal recall and recognition. Other studies utilizing this learning task have reported similar outcomes (DeLuca, Leavitt, Chiaravalloti, & Wylie, 2013; Demaree, Gaudino, DeLuca, & Ricker, 2000; Gaudino, Chiarvalloti, DeLuca, & Diamond, 2001), leading to the conclusion that poor delayed recall reflected compromised initial acquisition rather than diminished retrieval.
It should be noted, however, that these experiments (DeLuca et al., 1994; DeLuca et al., 2013; Demaree et al., 2000; Gaudino et al., 2001), manipulated acquisition only. They did not compare learning effects of experimentally manipulated retrieval and retention as well as acquisition. Consequently, these experiments were unable to compare the simultaneous and relative impact of MS on acquisition, retrieval, or retention. Additionally, these experiments probably do not represent the typical learning context of people with MS. In particular, few people with MS may be anticipated to study information until they have achieved perfect immediate free recall. As such, these experiments probably do not offer a naturalistic depiction of how people with MS acquire, retrieve, and retain information from memory. Furthermore, these experiments assessed learning of only 10 words, potentially imposing a ceiling effect upon performance. In turn, relative retrieval or retention effects may have been attenuated within subjects. Moreover, people with MS who failed to achieve criterion acquisition within 15 trials were eliminated from analyses. This might have also limited between subject effects on memory performance. Thus, although these experiments demonstrated attenuated acquisition in MS (DeLuca et al., 1994; DeLuca et al., 2013; Demaree et al., 2000; Gaudino et al., 2001), they do not conclusively negate the previously observed finding of poor retrieval (cf. Prakash et al., 2008; Wishart & Sharpe, 1997; Zakzanis, 2000).
The Item Specific Deficit Approach (ISDA) may be useful in simultaneously examining acquisition, retrieval, and retention among people with MS. Wright and colleagues (Wright, Woo, Schmitter-Edgecombe, Hinkin, Miller, & Gooding, 2009) derived the ISDA to quantify acquisition, retrieval, and retention deficits, with the latter two processes being corrected for initial acquisition. In this rubric, impaired acquisition is operationalized as the number of items that are not recalled during half of the learning trials. Higher numbers indicate worse acquisition. The retrieval index is depicted as a ratio. The numerator consists of words that were recalled at least once during learning trials but were recalled inconsistently across delayed recall trials. The denominator consists of words recalled at least once during learning trials. Higher values reflect inconsistent retrieval. Retention is also a ratio. The numerator reflects items that were initially recalled during learning trials but were not remembered during delayed recall. The denominator reflects the number of items that were recalled at least once during learning trials. Higher values reflect worse retention over time.
In an initial validation study, Wright et al. (2009) computed the ISDA indices based upon the original California Verbal Learning Test (CVLT: Delis, Kramer, Kaplan, & Ober, 1987) performance of 53 healthy individuals, 37 people infected with HIV, and 42 people who had sustained a traumatic brain injury. Wright et al. found that the ISDA indices were weakly correlated with each other, implying unique variance associated with each index. Furthermore, the indices discriminated between the patient and healthy groups, with poor acquisition and retrieval especially contributing to this distinction. These indices achieved better discriminant validity than traditional CVLT indices. Similar ISDA findings involving HIV have been shown with the CVLT-II in at least two other studies (Cattie, Woods, Arce, Weber, Delis, Grant, et al., 2012; Litvin, Siders, Waite, Woo, Romero, Foley, et al., in press).
Apart from HIV, Wright, Schmitter-Edgecombe, and Woo (2010) applied the ISDA to CVLT responses of individuals who had sustained a severe traumatic brain injury. Compared to healthy individuals, the patients displayed marked deficits on the acquisition and retention indices, consistent with extant research concerning memory impairment among people with traumatic brain injury (cf. DeLuca, Schultheis, Madigan, Christodoulou, & Averill, 2000; Vanderploeg, Crowell, & Curtiss, 2001). In people diagnosed with Alzheimer’s disease or mild cognitive impairment (Andres, Vico, Yanez, Siquier, Ferrer, 2019; Oltra Cucarella, Perez-Elvira, & Duque, 2014), the ISDA had been adapted for use with a version of the Selective Reminding Test. Consistent with much of the existing clinical literature, the ISDA revealed evidence of deficient retention and acquisition, but retrieval was unaffected. In these studies, the ISDA indices achieved salient discriminant validity, and were better able than Selective Reminding Test scores to distinguish patients from healthy individuals.
Christidi, Zalonis, Symyrnis, and Evdokimidis (2012) applied the ISDA to study memory among people with amyotrophic lateral sclerosis (ALS) and a healthy comparison group. Using the Auditory Verbal Learning Test instead of the CVLT-II, Christidi et al. found that ALS corresponded with prominent retention and acquisition deficits, and retention emerged as a salient predictor of overall memory impairment.
In addition to discriminating between patient and healthy groups, the ISDA indices possess some ecological validity. Wright and colleagues (Wright, Woo, Foley, Ettenhofer, Cottingham, Gooding, Jang, Kim, Castellon, Miller, & Hinkin, 2011) examined the relationship between ISDA indices and treatment adherence in people infected with HIV. CVLT-derived ISDA acquisition and retrieval scores were better in patients who adhered to their treatment regimen compared to those who did not.
Collectively, these findings imply that the ISDA indices hold promise in identifying relative memory dysfunction associated with acquisition, retrieval, and retention, and they achieve this objective while accounting for differential acquisition between individuals. Their utility has been demonstrated among individuals with diverse pathologies, including HIV infection, substance use disorder, ALS, traumatic brain injury, mild cognitive impairment, and Alzheimer’s disease. In particular, ALS and traumatic brain injury tend to coincide with significant white matter damage (e.g., Rutgers, Toulgoat, Cazejust, Fillard, Lasjaunias, & Ducreux, 2008; Zhou, Ahmad, Gozda, Truong, Kong, & Namaka, 2017). Owing to somewhat similar pathophysiology, the ISDA indices may also help to elaborate memory dysfunction in people with MS. Specifically, they may help to clarify the relative pattern of memory dysfunction in MS while accounting for initial acquisition. Consistent with recommendations regarding replicability in psychological science (e.g., Lindsay, 2015), we evaluate this matter in two separate samples of persons with MS and healthy adults using parallel methods. ISDA indices were compared between healthy people and individuals with MS who were cognitively unimpaired or cognitively impaired. Cognitive impairment was defined by aggregate performance on brief neuropsychological test batteries.
Study 1
Methods
Participants
Participants were recruited from support groups and advertisements in the local National Multiple Sclerosis Society Newsletter. Individuals were paid an honorarium for their participation. They were informed that their data would be de-identified and embargoed from release. Thus, their data could not be used for clinical purposes. A board certified neurologist made diagnoses in accordance with the Polman et al. (2005) criteria. Patients were excluded if they had a psychiatric disorder that preceded onset of MS, current or past substance use disorder, history of learning or developmental disorders, or any neurological disease or injury besides MS. Because the instruments assessed language-mediated abilities, non-English speakers were excluded. Primary language was determined by self-report of participants. All healthy participants were free of neurological, substance, or psychiatric disorder, and none had a history of learning or developmental disorder. The study was approved by the local Institutional Review Board. Participants included 47 healthy individuals and 85 people with MS. Demographic information concerning the sample appears in Table 1.
Table 1.
Study 1: Demographics for Sex, Age, and Education
| Healthy (n=47) | MS Unimpaired (n=59) | MS Impaired (n=26) | p-value | |
|---|---|---|---|---|
| Sex | .05 | |||
| Female | 32 (68.0%) | 51 (86.4%) | 18 (69.2%) | |
| Male | 15 (32.0%) | 8 (13.6%) | 8 (30.7%) | |
|
| ||||
| Age | .28 | |||
| Mean (SD) | 42.55 (11.36) | 44.31 (10.71) | 48.12 (9.33) | |
|
| ||||
| Education | .08 | |||
| Mean (SD) | 14.32 (2.22) | 14.73 (2.07) | 13.73 (2.72) | |
|
| ||||
| MS Subtype | <.01 | |||
| 42 Relapsing | 9 Relapsing | |||
| Remitting | Remitting | |||
| 6 Secondary | 3 Secondary | |||
| Progressive | Progressive | |||
| 2 Progressive | 2 Primary | |||
| Relapsing | Progressive | |||
| 9 Uncertain | 1 Progressive | |||
| Relapsing | ||||
| 11 Uncertain | ||||
Materials and Procedures
To classify participants according to presence of neuropsychological impairment, a test battery was administered. The measures assessed concept formation, mental flexibility, ideational fluency, working memory, and confrontation naming, each of which may be impaired among people with MS (Sumowski et al., 2018). Scores from individual tests were compared to relevant normative references, and impaired scores were compiled to determine whether participants were impaired. Memory performance did not contribute to the determination of impairment.
The Verbal Concept Attainment Test (VCAT; Rosen, 1962).
The VCAT is a 23 item test that assesses abstract reasoning, hypothesis testing, and concept formation. It relies exclusively upon verbal stimuli. Examinees are instructed to select combinations of words that are all alike in some way. Examinees have 30 minutes to complete the test. A total raw score was examined in analyses.
The Wisconsin Card Sorting Test (WCST; Heaton, Chelune, Tallen, Kay, & Curtiss, 1993).
The WCST assesses abstract reasoning and non-verbal concept formation, and it is sensitive to frontal lobe dysfunction. Examinees match stimulus cards to a criterion card according to color, shape, or number of shapes stimulus card. After achieving ten consecutive correct sorts, the correct matching principle changes without warning. Examinees must determine the new matching principle and adjust their responses accordingly. Raw number of perseverative errors and conceptual level responses were examined.
The Delis Kaplan Executive Function System-Verbal Fluency (DKEFS; Kaplan et al., 2001).
The Verbal Fluency subtests of the DKEFS was administered. It consists of three components, phonemic (i.e. identifying words that start with a specified letter), semantic (i.e. sorting words by a given category), and semantic category switching abilities. Age corrected scaled scores for letter fluency, category fluency, and category switching accuracy were analyzed.
The Paced Auditory Serial Addition Test (PASAT; Rao, 1990).
The PASAT is a measure of information processing speed and auditory working memory. The PASAT presents a series of 60 digits at 3- and 2-second intervals. Examinees attempt to state consecutive sums of two digits, and the total number of correct sums is assessed. Norm-referenced T-scores for Trial 1 was examined.
The Symbol Digit Modalities Test (SDMT; Smith, 1991).
The SDMT assesses speed of information processing and visual working memory. Participants view a set of nine abstract symbols that are uniquely associated with digits. Beneath this set of stimuli, a matrix of symbols without digits appears, and participants write digits associated with the symbols over a 90 second interval. Afterward, participants perform the same task, but they verbalize the number associated with each symbol rather than write it. Number of oral responses was recorded. Norm referenced T-scores were computed.
The Action Fluency Test (Piatt et al., 1999a).
The Action Fluency Test is a verbal fluency measure in which participants name as many action words as possible in a one-minute interval. It possesses satisfactory reliability and validity as an executive function measure (Piatt et al., 1999b; Woods et al., 2005). The total raw score was analyzed.
Wechsler Adult Intelligence Scale IV-Digit Span (WAIS-IV; Wechsler, 2008).
The Digit Span subtest of the WAIS-IV assessed auditory working memory. Sequences of digits are read aloud at a rate of one per second, and participants recited the sequences. An age corrected scaled score was analyzed.
The Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 2000).
BNT assesses word retrieval abilities, and is sensitive to language impairment. Participants view illustrations of common objects and attempt to name them aloud. Raw total number of correct responses was evaluated.
Impairment Index.
An overall impairment index was calculated to quantify the degree of neuropsychological deficit. Scores from the following indices comprised the impairment index: Digit Span Age Corrected Scaled Scores, VCAT total score, WCST Percent Preservative Errors and Percent Conceptual Level Responses, BNT Total Raw Score, DKEFS Letter Fluency and Category Fluency, PASAT Total Correct T-Score, Symbol Digit Modalities Test Oral Scale Z-Score, and Action Fluency Total Raw Score. Notably, because new-learning performance served as the dependent variable in this study, scores on memory tests did not contribute to the impairment index. Scores were compared to relevant test norms for each of the 11 neuropsychological indices. Performance at or below the 16th percentile was considered impaired (e.g., Heaton et al. 1991). The number of impaired scores was summed. If a participant scored in the impaired range on one third or more of the index scores (i.e., 4 or more), they were classified as impaired. A similar criterion has been widely used in clinical practice (cf. Reitan & Wolfson, 1993) and in research concerning people with MS (cf. Baughman et al., 2015; Rao et al., 1991).
The California Verbal Learning Test-II (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000).
CVLT-II assesses memory for a 16-item word list that is read aloud five times to the examinee. Recall is assessed after a 20-minute delay, and is supplemented by semantic cueing. A recognition trial follows this delayed cued recall trial. Norm referenced scores were assessed.
Item Specific Deficit Approach (ISDA: Wright et al., 2009).
The ISDA derives Acquisition, Retrieval, and Retention indices based on CVLT-II performance. Higher values on these scales reflects worse performance. The Acquisition Index is computed by summing words from the 16-item list that were recalled less than three times across the five learning trials. The Retrieval Index is calculated by summing words that were recalled at least once during the five learning trials but were recalled inconsistently during delayed recall trials. To correct for initial acquisition effectiveness the sum of words recalled inconsistently across the short and delayed recall trials, was divided by the number of unique words that were recalled during the five learning trials. The Retention Index summed the words not recalled during any delayed recall trials but which were recalled at least once during the five learning trials. To correct for initial acquisition, the sum of such words was then divided by the number of unique words recalled during the learning trials. A blank scoring worksheet and an example scored case appear in Appendix 1.
Multiple Sclerosis Functional Composite Scale-Timed 25-Foot Walk (MSFC; Fischer et al., 1999).
The Timed 25-Foot Walk, a component of the Multiple Sclerosis Functional Composite Scale (MSFC; Cutter et al., 1999; Fischer et al., 1999) assesses disability severity. The time for a participant to walk 25 feet was measured.
The Chicago Multiscale Depression Inventory (CMDI; Nyenhuis et al., 1998).
The CMDI is a self-report measure of depression. The CMDI consists of 50 items evaluating aspects of depression such as mood, evaluation of self, and vegetative tendencies, and examinees rate how severely they have experienced the symptoms using a 5 point Likert scale. The mood scale was examined in the study. Research has shown this scale to be a valid and reliable measure of emotional distress among people with MS (Nyenhuis et al., 1998).
Procedure
After providing informed consent, participants answered questions concerning medical and psychiatric history. After completing the neuropsychological battery, self-report measures were completed. All instruments were administered in accordance with respective standardization instructions. The testing sessions occurred within a quiet laboratory space.
Results
Among the people with MS, 59 were classified as unimpaired and 26 were classified as impaired. Table 1 depicts the demographic characteristics of the groups. One-way analyses of variance (ANOVA) and a chi-square test showed that the healthy group, unimpaired MS group, and impaired MS group were not significantly different in age, education, or sex composition. The two groups of participants with MS differed with respect to disease course (X2(4)=14.22, p=.007). The impaired group included more people with an uncertain course than the unimpaired group.
The means and standard deviations for the neuropsychological measures, Timed 25-Foot Walk, and CMDI Mood Score appear in Table 2. Scores were analyzed using one-way ANOVAs, with participant group (Healthy, MS Unimpaired, MS Impaired) serving as the between groups factor. To control for Type I error, a conservative p<.01 was employed as a criterion for statistical significance. To follow-up significant effects of participant group, Tukey Honestly Significant Difference (HSD) contrasts were employed. This contrast was used to delineate differences between groups while offering additional protection against Type I error. As expected because of our post-hoc method of distinguishing patients with MS, Tukey HSD contrasts showed that the impaired MS participants performed worse than the healthy group (p’s < .01) and unimpaired MS sample (p’s < .01) on all neuropsychological measures (See Table 2). The persons with MS endorsed more depressed mood on the CMDI Mood scale than the healthy individuals, but the two patient groups were indistinguishable. CMDI scores did not correlate significantly with ISDA indices, with correlations ranging from .01 to .09.
Table 2.
Study 1: Means and Standard Deviations for Neuropsychological Tests
| HealthyA (n=47) | MS UnimpairedB (n=59) | MS ImpairedC (n=26) | η2 | HSD | |
|---|---|---|---|---|---|
| VCAT | 20.56 (2.34) [10 to 23] | 19.44 (2.72) [12 to 23] | 17.33 (2.76) [11 to 22] | .14 | A&B>C |
| WCST PE | 12.48 (6.18) [5.4 to 34.4] | 11.71 (6.03) [4.1 to 33.6] | 21.90 (12.06) [5.5 to 46.9] | .18 | A&B<C |
| WCST CR | 70.17 (15.78) [30.5 to 86.8] | 73.38 (15.45) [11.7 to 89.5] | 48.72 (21.95) [12.5 to 87.7] | .20 | A&B>C |
| Action Fluency | 19.59 (5.13) [10 to 34] | 18.76 (4.51) [7 to 29] | 13.79 (4.20) [8 to 23] | .19 | A&B>C |
| DKEFS LF | 10.32 (3.17) [4 to 15] | 10.63 (2.70) [5 to 17] | 7.25 (2.61) [2 to 13] | .19 | A&B>C |
| DKEFS CF | 12.51 (2.74) [6 to 18] | 12.08 (3.18) [4 to 19] | 9.00 (3.28) [3 to 17] | .18 | A&B>C |
| Digit Span | 11.41 (3.07) [6 to 17] | 10.36 (2.20) [6 to 17] | 8.50 (1.98) [6 to 13] | .16 | A&B>C |
| BNT | 56.83 (3.73) [41 to 60] | 56.66 (2.01) [52 to 60] | 53.54 (3.49) [44 to 58] | .13 | A&B>C |
| PASAT | 49.90 (9.98) [17.0 to 59.0] | 46.75 (9.77) [13.0 to 59.0] | 36.33 (12.58) [13.0 to 56.0] | .18 | A&B>C |
| SDMT Oral | 0.91 (1.28) [−1.15 to 3.85] | 0.20 (1.58) [−3.9 to 3.6] | −1.12 (1.79) [−3.5 to 5.5] | .19 | A&B>C |
| Timed 25-Foot Walk | 4.98 (1.13) [3.6 to 9.2] | 16.23 (38.45) [3.2 to 180.0] | 30.31 (58.05) [4.3 to 180] | .05 | NS |
| CMDI Mood | 46.84 (6.51) [39.8 to 61.5] | 55.89 (14.27) [39.8 to 99.6] | 55.26 (13.12) [39.8 to 81.5] | .11 | A<B&C |
Note: Standard deviations appear in parentheses. Minimum and maximum scores appear in brackets. For Timed 25-Foot Walk, p>.01. Other p-values <.001. NS: Not significant. HSD: Tukey Honestly Significant Difference significant contrasts. A: Healthy Group, B: MS Unimpaired Group, C: Impaired Group. VCAT: Verbal Concept Attainment Test. WCST PE: Wisconsin Card Sorting Test Percent Preservative Errors. WCST CR: Wisconsin Card Sorting Test Percent Conceptual Level Responses. BNT: Boston Naming Test Total Raw Score. DKEFS-LF: Delis Kaplan Executive Function System Letter Fluency. DKEFS-CF: Delis Kaplan Executive Function System Category Fluency. PASAT: Paced Auditory Serial Addition Test Composite Total Correct. SDMT: Symbol Digit Modalities Test Oral Scale Z-Score.
Regarding the CVLT-II, among the myriad indices available, we focused upon those that emphasize acquisition, retention, and retrieval. Specifically, the CVLT-II Total Recall T-score, Long Delayed Free and Cued Recall, and Recognition indices were examined. To specifically address retention and retrieval, the Long Delay Retention Index and Long Delay Free Recall versus Total Recognition Discriminability (Retrieval) index were analyzed, respectively. Table 3 shows that the groups did not differ in regard to the Long Delay Retention or Retrieval indices. Nonetheless, the impaired MS group performed worse than the healthy group on the Total Recall, Long Delay Free and Cued Recall indices, and number of words recognized (ps<.01). The unimpaired MS group performed better than the impaired group on the Long Delay Free and Cued Recall indices, and they were indistinguishable from the healthy comparison sample. Effect sizes for these significant group differences generally emerged as medium sized (cf. Cohen, 1988), and ranged from (η2=.07) for Total Recall and words recognized to (η2=.13) for the Long Delay Free Recall index.
Table 3.
Study 1: Means and Standard Deviations for Memory Indices
| HealthyA (n=47) | MS UnimpairedB (n=59) | MS ImpairedC (n=26) | p-value | η2 | HSD | |
|---|---|---|---|---|---|---|
| CVLT 2 | ||||||
| Acquisition | ||||||
| Total Recall T-Score | ||||||
| 49.98 (9.86) [31 to 76] | 46.70 (11.06) [25 to 67] | 41.81 (10.27) [23 to 63] | <.01 | .07 | A> C | |
| Retention | ||||||
| Long Delay Free Recall | ||||||
| −0.36 (1.10) [−3.50 to 1.50] | −0.64 (1.14) [−4.5 to 1.5] | −1.54 (1.10) [−3.5 to 0.5] | <.001 | .13 | A&B>C | |
| Long Delay Cued Recall | ||||||
| −0.05 (1.11) [−4.00 to 1.50] | −0.23 (1.01) [−3.5 to 2.0] | −1.10 (1.30) [−3.5 to 1.0] | =.001 | .10 | A&B>C | |
| Long Delay Retention | ||||||
| −0.19 (0.53) [−1.0 to 1.50] | −0.21 (0.77) [−1.5 to 1.5] | −0.15 (0.54) [−1.0 to 1.0] | >.05 | .001 | NS | |
| Retrieval | ||||||
| Long Delay Free Recall versus Total Recognition | ||||||
| Discriminability (Retrieval) | ||||||
| 0.28 (0.76) [−2.00 to 1.50] | 0.33 (0.89) [−1.5 to 3.0] | 0.42 (1.01) [−1.0 to 3.0] | >.05 | .004 | NS | |
| Recognition Hits | ||||||
| −0.39 (1.02) [−2.50 to 1.00] | −0.60 (1.03) [−4.0 to 1.0] | −1.27 (1.54) [−5.0 to 0.5] | <.01 | .07 | A>C | |
| ISDA | ||||||
| Acquisition Index | 5.10 (2.61) [0 to 10] | 5.20 (2.50) [1.0 to 13] | 7.38 (2.31) [3.0 to 12.0] | <.001 | .11 | A&B<C |
| Retention Index | 0.12 (0.11) [0.0 to 0.38] | 0.13 (0.11) [0.0 to 0.43] | 0.20 (0.14) [0.0 to 0.45] | =.01 | .07 | NS |
| Retrieval Index | 0.26 (0.15) [0.0 to 0.60] | 0.27 (0.14) [0.0 to 0.60] | 0.40 (0.13) [0.18 to 0.64] | <.001 | .15 | A&B<C |
Note: Standard deviations appear in parentheses. Minimum and maximum scores appear in brackets. CVLT 2 Total Recall is a norm referenced T-score. Other CVLT-II scores reflect norm-referenced z-scores. HSD: Tukey Honestly Significant Difference significant contrasts. A: Healthy Group, B: MS Unimpaired Group, C: Impaired Group. NS: Not significant. ISDA: Item Specific Deficit Approach. Higher ISDA values reflect worse performance.
As demonstrated in Table 3, the impaired MS group performed worse than the unimpaired MS group and the healthy individuals on the ISDA Acquisition and Retrieval indices, and there were no differences between the unimpaired MS patients and healthy individuals. The effect size for the Acquisition Index was medium (η2=.11), and the effect size for the Retrieval Index was large (η2=.15). Although the effect of group achieved a medium effect size and a p-value of .01 on the Retention Index, the Tukey HSD contrasts showed no significant differences between groups.
Discussion
The groups demonstrated significant differences on the ISDA indices. Specifically, the cognitively impaired participants with MS performed worse than the unimpaired MS group and healthy individuals on the Acquisition and Retrieval Indices. Regarding the Retention Index, follow-up contrasts showed no significant differences between the groups, and the effect size was small. This suggests that poor acquisition and retrieval are apt to occur among cognitively impaired patients with MS, but poor retention is less likely a problem.
Such a pattern accords well with existing research concerning memory function in people with MS. In particular, few investigations have demonstrated significant retention deficits in MS, whereas poor acquisition and retrieval have been reported in previous studies (Beatty et al., 1996; Deluca et al., 1994; 2013; Rao et al., 1993; Prakash et al., 2008; Wishart & Sharpe, 1997; Zakzanis, 2000). Because the current findings conform to the existing literature, they contribute to increasing evidence of construct validity for the ISDA indices (Andres et al., 2019; Cattie et al., 2012; Christidi et al., 2012; Litvin et al., in press; Oltra Cucarella et al., 2014; Wright et al., 2009; 2010; 2011).
The relative impact of acquisition and retrieval deficits is worthy of consideration. Previous research has reported variable patterns of memory dysfunction in MS, with some investigations showing pronounced acquisition problems (e.g., DeLuca et al., 1994; 2013; Demaree et al., 2000; Gaudino et al., 2001) whereas others reveal salient retrieval deficits (e.g., Prakash et al., 2008; Wishart & Sharpe, 1997; Zakzanis, 2000). In the current study, a medium effect size was observed on the Acquisition Index, and a large effect size appeared on the Retrieval Index. This occurred despite accounting for initial acquisition on the Retrieval Index. Indeed, the effect size of cognitive impairment on the Retrieval Index emerged as the largest of the three ISDA indices. Taken together, these findings suggest that both patterns of memory dysfunction occur in MS. Although the effect size was larger for the Retrieval Index than for the Acquisition Index, the relative difference was modest. Before more conclusive statements can be made, replication would be required.
Regarding the canonical CVLT-II indices, the impaired patients recalled fewer words across the five learning trials on the Total Recall T-score, implying poor acquisition and conforming to previous findings concerning MS. Additionally, compared to the healthy individuals and the unimpaired patients, the impaired patients recalled fewer words on the Long Delay Free and Cued Recall indices. They also recognized fewer words. In contrast, when examining CVLT-II indices that are specifically designed to assess retention and retrieval, including the Long Delay Retention Index and Long Delay Free Recall versus Total Recognition Discriminability Index, no significant differences emerged. Further, effect sizes on these indices were small, implying a negligible deficit involving retrieval or retention.
Although the pattern of CVLT-II performance suggests an exclusive acquisition deficit, uncertainties exist. The delayed, cued, and recognition indices do not reference initial acquisition performance. As such, consistent with criticisms of previous research leveled by DeLuca and colleagues (DeLuca et al., 1994; DeLuca et al., 2013; Demaree et al., 2000; Gaudino et al., 2001), they do not address loss of memory or inconsistent recall across time. As such they may not represent a comprehensive depiction of memory dysfunction in MS. Indeed, CVLT-II performances suggest that interventions should seek to improve poor acquisition among people with MS. In comparison, the ISDA indices offer a more nuanced representation of memory function in MS, implying that poor acquisition and retrieval should be addressed. If relying exclusively upon the standard CVLT-II scales, clinicians might make erroneous choices regarding memory remediation targets, and may focus upon acquisition exclusively. These findings insinuate that the ISDA indices may offer a useful augmentation to the canonical CVLT-II scales.
It should be noted that no measure of performance validity was addressed in this study. As such, it is unclear whether individuals may have exerted poor effort, thereby biasing the results. It should be noted that all participants were notified that their neuropsychological test scores would be de-identified and embargoed from release. Thus, if they had any ongoing efforts to obtain disability compensation, they would have had no incentive to perform poorly in the present study. Indeed, Galioto, Dhima, Berenholz, and Busch (in press) conducted a retrospective study of clinically-referred patients with MS. They noted that poor effort indexed by the Victoria Symptom Validity Test principally occurred only among those who had sought disability status. Nonetheless, it may be worthwhile to address this uncertainty in subsequent data collection.
To address the aforementioned uncertainties and determine the consistency of the present findings, it would be helpful to attempt a replication with an independent sample. Towards this end, data were collected from a separate sample of people with MS and another healthy comparison group. Paralleling this first study, performance on a neuropsychological battery was used to identify patients as cognitively-impaired. The ISDA indices and canonical CVLT-II indices were again scored to evaluate the relative presence of Acquisition, Retrieval, and Retention problems in people with MS. The CVLT-II forced choice index, a measure of performance validity, was administered and scored.
Study 2
Methods
Participants
As with the first study, notices were published in the newsletter of the local National Multiple Sclerosis Society chapter and in newspapers to recruit participants. An investigator also met with MS support groups. All persons volunteered to participate in the present study, and they received an honorarium for their participation. Ultimately, data were collected from 100 individuals, including 79 people with MS and 21 healthy participants without MS. Paralleling Study 1, participants were excluded if they had a psychiatric disorder that preceded onset of MS, current or past substance abuse or dependence, history of learning or developmental disorders, or any neurological disease or injury besides MS. As with Study 1, non-English speakers were excluded. The healthy group was screened for each of these characteristics, including current psychiatric illness.
Descriptive statistics concerning the MS and healthy groups appear in Table 4. Diagnoses were confirmed by a board-certified neurologist through chart review, including magnetic resonance imaging, laboratory studies, and physical examination.
Table 4.
Study 2: Demographics for Sex, Age, and Education
| Healthy(n=21) | MS Unimpaired(n=49) | MS Impaired(n=30) | p-value | |
|---|---|---|---|---|
| Sex | .39 | |||
| Female | 16 (76.2%) | 42 (85.7%) | 27 (90.0%) | |
| Male | 5 (23.8%) | 7 (14.3%) | 3 (10.0%) | |
|
| ||||
| Age | .35 | |||
| Mean (SD) | 41.62 (11.96) | 44.49 (9.53) | 47.57 (9.51) | |
|
| ||||
| Education | .15 | |||
| Mean (SD) | 15.59 (2.07) | 14.72 (2.07) | 14.68 (2.51) | |
|
| ||||
| MS Subtype | .05 | |||
| 23 Relapsing | 18 Relapsing | |||
| Remitting | Remitting | |||
| 12 Chronic | 1 Chronic | |||
| Progressive | Progressive | |||
| 14 Uncertain | 11 Uncertain | |||
Materials
Similar to Study 1, participants were classified according to presence of neuropsychological impairment with the use of a neuropsychological test battery. The measures assessed concept formation, mental flexibility, ideational fluency, and working memory, each of which are commonly impaired among people with MS (Sumowski et al., 2018). Individual test scores were compared to normative references, and impaired scores were summed to determine whether participants were impaired. Memory was excluded from this compilation of impaired scores.
WCST (Heaton et al., 1993).
The WCST was administered to assess abstract reasoning and concept formation. Number of perseverative errors and conceptual level responses were examined.
Controlled Oral Word Association Test (COWAT).
The COWAT was administered to assess verbal fluency (Benton & Hamsher, 1989). The COWAT is similar to the DKEFS Letter Fluency subtest. It consists of three word-naming trials where participants are given a letter (C, F, & L) and are asked to name as many words as they can think of that begin with that letter. The sum of the words generated across three 1-minute trials comprise the total score, which is adjusted for age, sex, and education.
Trail Making Tests (TMT).
The TMT measures scanning and visuo-motor tracking, divided attention, and cognitive flexibility (Reitan & Wolfson, 1993). Time to complete Test B was included in analyses.
Digit Span Test.
This measure from the Wechsler Adult Intelligence Scale–III was used to assess auditory attention span (Wechsler, 1997). The age corrected scaled score was considered in data analyses.
Letter–Number Sequencing.
Also found in the Wechsler Adult Intelligence Scale–III (Wechsler, 1997), the Letter–Number Sequencing task is a measure of auditory working memory. The age corrected scaled score was included in analyses.
Impairment Index.
Similar to the preceding study, an overall impairment index was computed, and patients were designated as impaired based on their performance. Scores from the following indices comprised the impairment index: COWAT total score, WCST Percent Preservative Errors and Percent Conceptual Level Responses, Trail Making Test B time to completion, and Digit Span and Letter Number Sequencing Age Corrected Scaled Scores. Scores were compared to relevant test norms for each of the eight neuropsychological indices. Performance at or below the 16th percentile was considered impaired (cf., Heaton et al. 1991). The number of impaired scores was summed. If a participant scored in the impaired range on one third or more of the index scores (i.e., two or more), they were classified as impaired. A similar criterion has been widely used in clinical practice (cf. Reitan & Wolfson, 1993) and in research concerning people with MS (cf. Baughman et al., 2015; Rao et al., 1991). Performance on the CVLT-II was not included.
California Verbal Learning Test-II (CVLT-II).
Similar to the preceding study, the CVLT-II was administered to assess verbal learning and memory (Delis et al., 2000). The ISDA Acquisition, Retention, and Retrieval indices were again computed based on CVLT-II performance (cf., Wright et al., 2009). Extending Study 1, the Forced Choice Index was employed to assess performance validity. No participant performed below established cutoff values for non-credible effort (cf., Schwartz et al., 2016).
Ambulation Index.
The Ambulation Index (Hauser, Dawson, Lehrich, Beal, Kevy, Propper, Mills, & Weiner, 1983) is a variant of the Timed 25-Foot Walk (cf. Fischer et al., 1999), and it assesses impaired mobility. Time to walk 25 feet is measured, and a Likert scale rating is assigned to reflect severity of impairment. This index has satisfactory reliability, validity, and sensitivity to clinically relevant change in MS patients, and it is correlated with indices of disease severity such as the Expanded Disability Status Scale (Fischer et al., 1999). This instrument was included to assess diminished mobility.
Results
Based on the Impairment Index, 49 people with MS were classified as cognitively unimpaired and 30 were designated as cognitively impaired. Table 4 reveals the demographic characteristics of the groups. One-way ANOVAs and a chi-square test showed that the healthy group, unimpaired MS group, and impaired MS group were not significantly different in age, education, or sex composition. The two groups of participants with MS did not differ with respect to disease course (X2(2)=6.05, p=.05). Nonetheless, disease course failed to correlate with performance on ISDA indices.
The means and standard deviations for the neuropsychological measures and Ambulation Index appear in Table 5. Scores were analyzed using one-way ANOVAs. Participant group (Healthy, MS Unimpaired, MS Impaired) served as the between groups factor. To control for Type I error, a conservative p<.01 was employed as a criterion for statistical significance. Tukey HSD contrasts were employed to elaborate significant effects of group and provide further protection against Type I error. As expected because of our post-hoc method of distinguishing people with MS, Tukey HSD contrasts showed that the impaired individuals with MS performed worse than the healthy group (ps < .01) and unimpaired MS sample (ps < .01) on all neuropsychological measures except for COWAT (See Table 5). Despite a medium effect size, the groups did not differ in verbal fluency. The two groups of MS participants had worse mobility as reflected by significantly worse scores on the Ambulation Index.
Table 5.
Study 2: Means and Standard Deviations for Neuropsychological Tests
| HealthyA(n=21) | MS UnimpairedB(n=49) | MS ImpairedC(n=30) | η2 | HSD | |
|---|---|---|---|---|---|
| COWAT | 44.19 (6.76) [31 to 58] | 39.24 (9.49) [24 to 67] | 36.43 (11.63) [15 to 62] | .08 | NS |
| WCST PE | 11.91 (7.53) [4.7 to 35.9] | 12.75 (6.65) [6.3 to 40.6] | 25.57 (13.59) [7.8 to 65.6] | .30 | A&B<C |
| WCST CR | 70.11 (21.11) [20.3 to 89.1] | 70.78 (15.40) [4.7 to 89.1] | 42.38 (21.79) [4.7 to 82.8] | .33 | A&B>C |
| Trail Making Test B | 53.08 (19.00) [28 to 99] | 73.04 (23.55) [40 to 145] | 124.65 (59.38) [40 to 308] | .36 | A&B>C |
| Digit Span | 11.09 (3.46) [5 to 19] | 10.57 (2.51) [7 to 18] | 8.63 (2.52) [5 to 14] | .12 | A&B>C |
| Letter Number | 11.47 (3.31) [6 to 18] | 10.90 (2.28) [6 to 16] | 8.90 (3.00) [3 to 15] | .13 | A&B>C |
| Ambulation Index | 0.09 (0.44) [0.0 to 2.0] | 2.42 (2.07) [0.0 to 8.0] | 2.70 (1.80) [1.0 to 8.0] | .25 | A>B&C |
Note: Standard deviations appear in parentheses. Minimum and maximum scores appear in brackets. For COWAT, p>.01. Other p-values <.01. NS: Not significant. HSD: Tukey Honestly Significant Difference significant contrasts. A: Healthy Group, B: MS Unimpaired Group, C: Impaired Group. COWAT: Controlled Oral Word Association Test Total Score. WCST PE: Wisconsin Card Sorting Test Percent Preservative Errors. WCST CR: Wisconsin Card Sorting Test Percent Conceptual Level Responses.
Regarding the CVLT-II, we again focused upon indices that emphasize acquisition, retention, and retrieval, especially the CVLT-II Total Recall T-score, Long Delayed Free and Cued Recall, Recognition Hits, the Long Delay Retention Index, and the Long Delay Free Recall versus Total Recognition Discriminability Index. As shown in Table 6, groups did not differ in regard to the Recognition, Long Delay Retention or the Long Delay Free Recall versus Total Recognition Discriminability indices. Nonetheless, the impaired MS group performed worse than the unimpaired MS group and the healthy comparison sample on the Total Recall, Long Delay Free and Long Delay Cued Recall indices (ps<.01). Effect sizes for these group differences generally emerged as large (cf. Cohen, 1988), and ranged from (η2=.17) for Total Recall to (η2=.21) for the Long Delay Free Recall index.
Table 6.
Means and Standard Deviations for Memory Indices
| HealthyA(n=21) | MS UnimpairedB(n=49) | MS ImpairedC(n=30) | p-value | η2 | HSD | |
|---|---|---|---|---|---|---|
| CVLT 2 | ||||||
| Acquisition | ||||||
| Total Recall T-Score | ||||||
| 59.19 (8.81) [44 to 75] | 54.31 (10.63) [26 to 76] | 46.63 (10.57) [28 to 67] | <.001 | .17 | A&B>C | |
| Retention | ||||||
| Long Delay Free Recall | ||||||
| 0.33 (0.79) [−1.0 to 1.5] | 0.19 (1.05) [−3.5 to 1.5] | −1.05 (1.43) [−5.0 to 1.5] | <.001 | .22 | A&B>C | |
| Long Delay Cued Recall | ||||||
| 0.33 (1.06) [−3.0 to 1.5] | 0.19 (0.92) [−3.0 to 1.5] | −0.87 (1.35) [−4.0 to 1.0] | <.001 | .18 | A&B>C | |
| Long Delay Retention | ||||||
| −0.21 (0.64) [−1.0 to 1.5] | 0.01 (0.67) [−1.5 to 1.5] | −0.47 (0.79) [−3.0 to 1.0] | >.01 | .08 | NS | |
| Retrieval | ||||||
| Long Delay Free Recall versus Total Recognition | ||||||
| Discriminability (Retrieval) | ||||||
| −0.14 (0.95) [−2.0 to 2.0] | 0.02 (1.04) [−5.0 to 2.0] | 0.33 (0.89) [−1.5 to 2.5] | >.05 | .03 | NS | |
| Recognition Hits | ||||||
| −0.38 (1.28) [−5.0 to 1.0] | −0.44 (1.25) [−5.0 to 1.0] | −0.65 (1.33) [−5.0 to 1.0] | >.05 | .01 | NS | |
| ISDA | ||||||
| Acquisition Index | 2.61 (2.22) [0.0 to 8.0] | 4.04 (3.05) [0.0 to 11.0] | 5.80 (3.28) [0.0 to 11.0] | =.001 | .13 | A<C |
| Retention Index | 0.08 (0.09) [0.0 to 0.27] | 0.10 (0.10) [0.0 to 0.33] | 0.17 (0.13) [0.0 to 0.46] | <.01 | .11 | A<C |
| Retrieval Index | 0.22 (0.15) [0.0 to 0.60] | 0.26 (0.19) [0.0 to 0.64] | 0.41(0.19) [0.0 to 0.78] | <.001 | .15 | A&B<C |
Note: Standard deviations appear in parentheses. Minimum and maximum scores appear in brackets. CVLT 2 Total Recall is a norm referenced T-score. Other CVLT-II scores reflect norm-referenced z-scores. HSD: Tukey Honestly Significant Difference significant contrasts. A: Healthy Group, B: MS Unimpaired Group, C: Impaired Group. NS: Not significant. ISDA: Item Specific Deficit Approach. Higher ISDA values reflect worse performance.
Regarding ISDA indices, Table 6 reveals that the impaired MS group performed worse than the healthy individuals on the Acquisition, Retention, and Retrieval Indices. Notably, the impaired group only performed worse than the unimpaired MS group on the Retrieval Index. Effect sizes were medium for the Retention (η2=.10) and Acquisition Indices (η2=.13), and the effect size for the Retrieval Index was large (η2=.15).
Discussion
Paralleling Study 1, significant differences emerged on the ISDA indices. As depicted in Figure 1, the effect sizes on these indices were almost identical, and confidence intervals overlapped between the studies on the three ISDA indices. Consistent with Study 1, the impaired group retrieved less information than the healthy and unimpaired MS groups. In contrast to Study 1 in which the p-value was marginally significant (p=.01), groups in Study 2 differed on the Retention Index, and the effect size was comparable across the two studies (cf. Figure 1). Further differing from Study 1, the impaired MS group performed worse than the healthy group on the Acquisition and Retention Indices. In Study 1, the impaired group performed worse than the healthy and unimpaired MS groups on the Acquisition Index, and there was no significant difference on the Retention Index. Regardless of these differences, the results of both studies imply that cognitively-impaired patients with MS demonstrate diminished acquisition and retrieval of newly-learned information, especially compared to a healthy comparison group.
Figure 1. Effect Sizes of Group Differences for ISDA Indices Across Study 1 and Study 2.
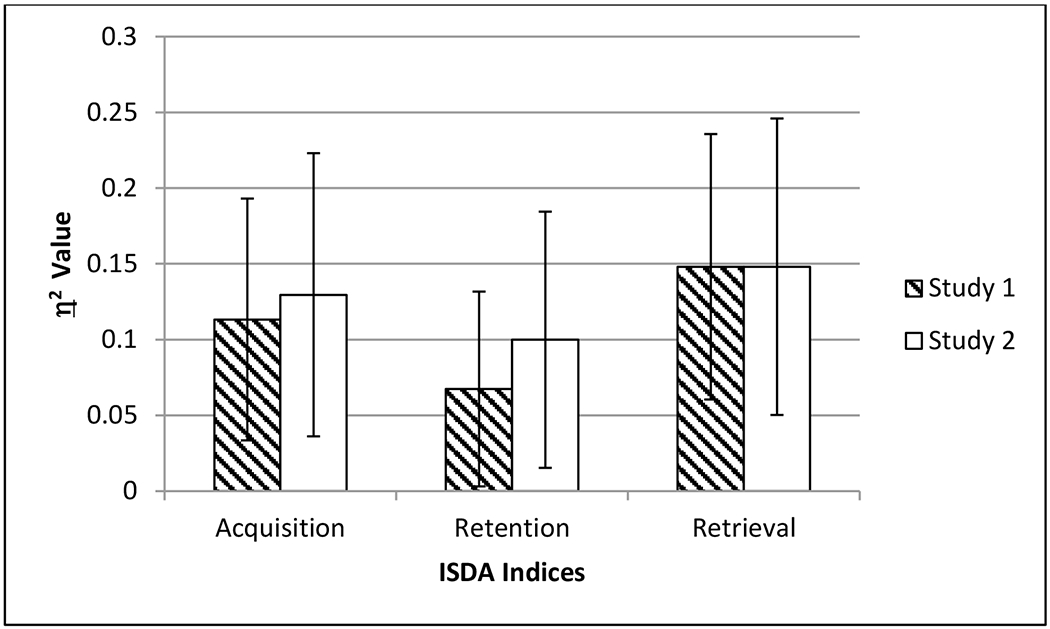
Note: Error bars reflect 95% confidence intervals.
With respect to the canonical CVLT-II indices, significant group differences emerged on the Total Recall T-score and Long Delay Free and Cued Recall indices. Similar to Study 1, the impaired MS group recalled less information than the healthy group. Additionally, as in Study 1, no differences emerged on the inherent CVLT-II retention and retrieval indices, namely the Long Delay Retention Index and Long Delay Free Recall versus Total Recognition Discriminability Index. In contrast to Study 1, the impaired MS group consistently recalled fewer words than the unimpaired MS group, whereas such a difference did not previously emerge on the Total Recall T-score. Additionally, in Study 1, the impaired MS group recognized fewer words than the healthy group, whereas no significant difference in recognition performance emerged in Study 2. Such minor differences notwithstanding, the data reveal that the CVLT-II recall indices consistently depict performance decrements among cognitively-impaired people with MS, whereas the CVLT-II indices pertaining to retention and retrieval seem insensitive to such problems.
Supplemental Cluster Analysis
In order to evaluate how acquisition, retrieval, and retention deficits manifest in discrete groups of patients, we conducted an exploratory cluster analysis. To increase reliability of the analyses, ISDA scores from participants in Study 1 and Study 2 were aggregated. Each ISDA index was transformed to a z-score that referenced all participants from the two studies, including people with MS and healthy individuals. Subsequent, these z-scores were entered into a cluster analysis.
In computing the cluster values, healthy individuals were excluded, and z-transformed ISDA scores of people with MS were entered as independent variables. Ward’s method was utilized to assess the overall structure of the data for the resulting cluster solutions, with squared Euclidian distance set as the dissimilarity metric (Everitt, Landau, & Leese, 2001; Ward, 1963). The structure of the dendogram suggested a 4-group solution was optimal (Aldenderfer & Blashfield, 1984; Seaton, 1999). A k-means algorithm and subsequent discriminant function analysis were employed to assess cluster stability (Lange, 2002). Results from the discriminant function indicated that over 98% of cases were correctly classified and there was sufficient group separation.
Figure 2 displays the 4-group cluster solution. People with MS were distinguished by discrete memory profiles. One cluster revealed a group of MS patients with entirely normal memory performance across all ISDA indices. A second cluster depicted a group with global impairment across the ISDA indices. A third cluster showed a group of MS patients with normal retrieval, but their acquisition and retention were diminished. A fourth cluster revealed a group of MS patients with relatively preserved acquisition and retention, but their retrieval was poor.
Figure 2. Cluster Analysis Results.
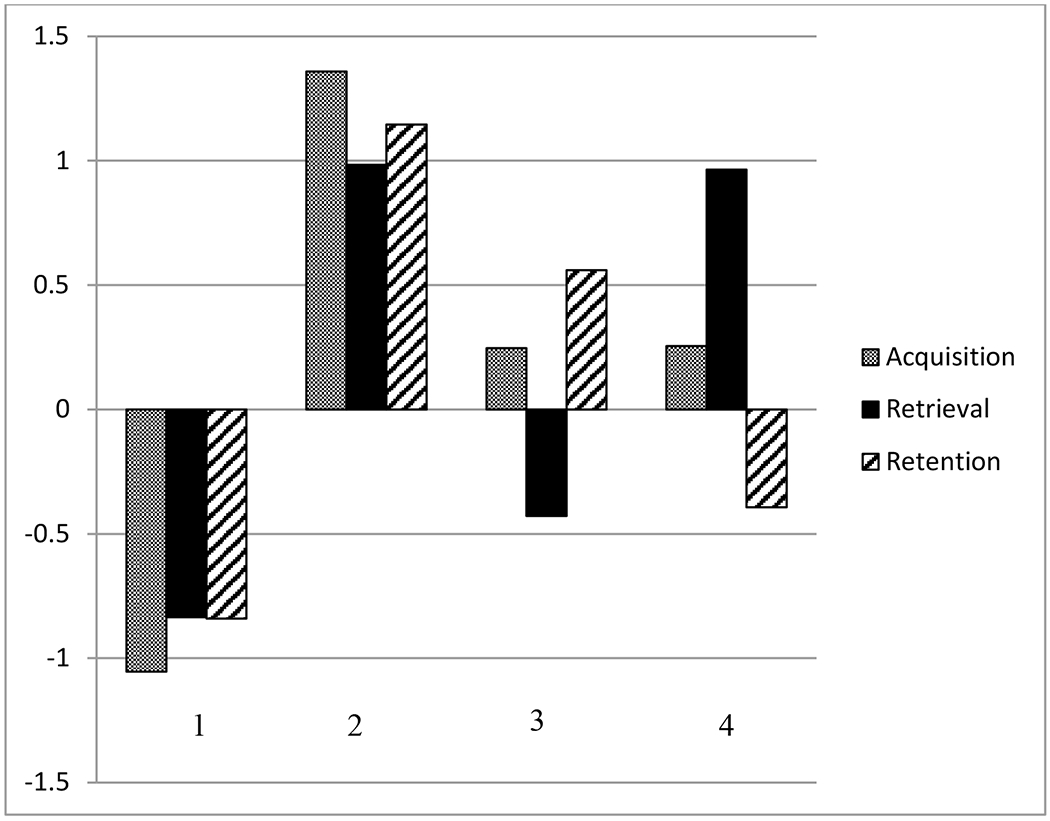
Note. Positive z-scores reflect worse ISDA performance. 1: Normal memory function. 2: Generally impaired memory. 3: Poor acquisition and retention. 4: Poor retrieval.
Summary and Concluding Discussion
These findings are novel in that they offer evidence of simultaneous acquisition and retrieval difficulties in MS. Indeed, this occurred after the ISDA Retrieval Index was adjusted for initial acquisition, and these difficulties emerged consistently across two independent samples with almost identical effect sizes.
Notably, memory performance differed between the samples. Ancillary analyses were performed, and CVLT-II scores of people with MS were compared between studies using ANOVA. In particular, patients in Study 1 performed better than those in Study 2 on the Total Recall T-score, Short Delayed Cued Recall, and Long Delayed Free Recall (all ps <.01, and η2=.03 to .07), but they did not differ on other indices. In Study 1, performance on the CVLT-II Total Recall T-score fell at the 34th percentile, whereas those in Study 2 achieved at the 63rd percentile. Regarding Short Delayed Cued Recall, patients in Study 1 achieved a score that fell at the 34th percentile, those in Study 2 performed at the 53rd percentile. Long Delayed Free Recall in Study 1 fell at the 23rd percentile, whereas patients in Study 2 performed at the 47th percentile. Despite these different levels of memory performance, the pattern of ISDA performance remained relatively consistent between studies.
In addition to differential memory performance across groups, cognitive impairment was designated differently between the studies. In both studies, executive function and working memory were assessed. Although the batteries overlapped, some different tests were administered between studies. Consequently, sensitivity to neurocognitive impairment may have varied between the two studies. Such differences notwithstanding, acquisition and retrieval deficits consistently emerged across samples. The replication of findings across studies is arguably a salient strength of this research.
Collectively, findings from these two studies suggest that acquisition and retrieval problems are apt to occur among cognitively-impaired people with MS. Indeed, effect size estimates on the ISDA Acquisition and Retrieval Indices were nearly identical across samples. In both studies, the Acquisition Index reliably displayed a medium effect size, and the Retrieval Index consistently revealed a large effect size. As such, these data suggest that both memory processes are diminished in MS, but retrieval problems may be slightly more prominent than acquisition weaknesses.
The replication of acquisition and retrieval weaknesses across samples bears relevance to the construct validity of the ISDA Indices. The ISDA Indices have been implemented in studies of neurodegenerative conditions, traumatic brain injury, HIV infection, and heterogeneous neuropsychiatric conditions (Andres et al., 2019; Cattie et al., 2012; Christidi et al., 2012; Litvin et al., in press; Olta Cucarella et al., 2014; Wright et al., 2009; 2010; 2011). Profiles of memory performance emerged that were consistent with preponderant literature regarding these conditions. The current data offer further evidence that the ISDA Indices assess memory processes in a manner that replicates across samples and which conforms to the established literature concerning MS (DeLuca et al., 1994; DeLuca et al., 2013; Demaree et al., 2000; Gaudino et al., 2001; Prakash et al., 2008; Wishart & Sharpe, 1997; Zakzanis, 2000).
Elaborating these mean differences on the ISDA indices, the cluster analysis distinguished four distinct patterns of memory impairment among people with MS. One group displayed normal acquisition, retrieval and retention, whereas another showed globally compromised ISDA scores. Notably, a third group demonstrated poor acquisition and retention with normal retrieval. Finally, a fourth group displayed poor retrieval only. As such, retrieval deficits may occur independently of poor acquisition and retention in some people with MS. Poor acquisition and retention may occur independently of compromised retrieval. Moreover, some people with MS show globally impaired acquisition, retention, and retrieval. Although ANOVAs demonstrated that poor acquisition and retrieval occur in neuropsychologically-impaired people with MS, these patterns of distinct memory deficits were only manifest with cluster analysis.
These data have implications for memory rehabilitation interventions for people with MS. Some people with MS may benefit from efforts to enhance acquisition, whereas others may benefit from treatments that emphasize cueing techniques such as memory retrieval aids. In this vein, successful efforts to optimize acquisition in people with MS have emphasized techniques such as self-generated encoding or self-testing (Basso et al., 2006; Basso, Ghormley, Lowery, Combs, & Bornstein, 2008; Goverover, Chiaravalloti, Genova, & DeLuca, 2018). The current findings suggest that such efforts may be incomplete. In particular, Cicerone and colleagues (Cicerone et al., 2019) demonstrated that external memory aids (e.g., task lists, daily planners, smart phones) which enhance retrieval, rank among the most powerful rehabilitation techniques among people with traumatic brain injury or stroke. Such aids have not been widely examined in MS (cf. Gentry, 2008). The observed weakness involving retrieval implies that such interventions may be especially relevant in people with MS.
These suggestions notwithstanding, it is important to recognize limitations of these data. Although the findings replicated across samples of varying levels of memory performance, the same memory test was employed in both studies. Additionally, the current results emphasize verbal declarative memory exclusively. It remains to be seen whether similar findings will emerge if a different verbal memory test or measures of non-verbal memory are employed. Although relative memory differences emerged with relative consistency across the two studies, implying that these findings are reliable, the clinical meaningfulness of the effect sizes is as yet unproven. Follow-up research would need to evaluate how the observed memory processes yield benefit in rehabilitation trials, and whether they correspond with functional outcomes such as activities of daily living. Furthermore, it is uncertain whether the emphasis upon acquisition and retrieval problems will remain as prominent in more severely amnestic patients with MS. Such caveats notwithstanding, these findings urge caution in asserting exclusive deficits of acquisition or retrieval among people with MS. Rather, these data suggest that both processes are affected by MS.
Supplementary Material
Key Points.
What is the key question this paper addresses?
This paper attempts to clarify the relative contribution of acquisition, retrieval, and retention processing to memory impairment in people with multiple sclerosis (MS).
What are the primary findings?
Across two independent samples of people with MS, acquisition and retrieval findings were prominent.
What are the key scientific and practical implications of the findings?
These results help to reconcile contradictory findings from previous research, and may also guide tailored memory rehabilitation techniques for people with MS.
What directions should be explored in future research?
Future research should determine whether these findings extend to non-verbal or non-declarative memory.
Contributor Information
Michael R. Basso, Mayo Clinic
Douglas Whiteside, University of Minnesota.
Dennis Combs, University of Texas.
Steven Paul Woods, University of Houston.
Jordan Hoffmeister, University of Tulsa.
Ryan Mulligan, University of Tulsa.
Peter Arnett, Pennsylvania State University.
Eva Alden, Mayo Clinic.
W. Oliver Tobin, Mayo Clinic.
References
- Aldenderfer MS, & Blashfield RK (1984). Cluster analysis. Sage University Paper Series On Quantitative Applications in the Social Sciences, 07–044. [Google Scholar]
- Andres P, Vico H, Yanez A, Siquier A, & Ferrer GA (2019). Quantifying memory deficits in amnestic mild cognitive impairment. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 11, 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C, Onishi K, Robinson K, D”Esposito M, Thompson H, Rostami A, & Grossman M (1996). Serial position and temporal cue effects in multiple sclerosis: Two subtypes of defective memory mechanisms. Neuropsychologia, 34, 853–862. [DOI] [PubMed] [Google Scholar]
- Basso MR, Lowery N, Ghormley C, Combs D, & Johnson J (2006). Self-generated learning in people with multiple sclerosis. Journal of the International Neuropsychological Society, 12, 640–648. [DOI] [PubMed] [Google Scholar]
- Basso MR, Ghormley C, Lowery N, Combs D, & Bornstein RA (2008). Self-generated learning in people with multiple sclerosis: An extension of Chiarvalloti and DeLuca (2002). Journal of Clinical and Experimental Neuropsychology, 30, 63–69. [DOI] [PubMed] [Google Scholar]
- Baughman BC, Basso MR, Sinclair RR, Combs DR, & Roper BL (2015). Staying on the job: The relationship between work performance and cognition in individuals diagnosed with multiple sclerosis. Journal of Clinical and Experimental Neuropsychology, 37, 630–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW (1993). Memory and “frontal lobe” dysfunction in multiple sclerosis. Journal of the Neurological Sciences, 115, S38–S41. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Wilbanks SL, Blanco CR, Hames KA, Tivis R, & Paul RH (1996). Memory Disturbance in Multiple Sclerosis: Reconsideration of Patterns of Performance on the Selective Reminding Test. Journal of Clinical and Experimental Neuropsychology, 18, 56–62. [DOI] [PubMed] [Google Scholar]
- Benton AL, & Hamsher K. deS. (1989). Multilingual Aphasia Examination. Iowa City, IA: AJA Associates. [Google Scholar]
- Bobholz JA, Gleason A, & Miller S (2008). Understanding and managing cognitive dysfunction in multiple sclerosis. Handbook of Clinical Neurology, 89, 705–717. [DOI] [PubMed] [Google Scholar]
- Brissart H, Morele E, Baumann C, & Debouverie M (2012). Verbal episodic memory in 426 multiple sclerosis patients: impairment in encoding, retrieval or both? Neurological Sciences, 33, 1117–1123. [DOI] [PubMed] [Google Scholar]
- Caine ED, Bamford KA, Schiffer RB, Shoulson I, & Levy S (1986). A Controlled Neuropsychological Comparison of Huntington’s Disease and Multiple Sclerosis. Archives of Neurology, 43, 249–254. [DOI] [PubMed] [Google Scholar]
- Cattie JE, Woods SP, Arce M, Weber E, Delis DC, Grant I, and The HIV Neurobehavioral Research Program Group. (2012). Construct Validity of the Item-Specific Deficit Approach to the California Verbal Learning Test (2nd ed) in HIV Infection. Journal of Clinical and Experimental Neuropsychology, 26, 288–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaravalloti ND, & DeLuca J (2008). Cognitive impairment in multiple sclerosis. Lancet Neurology, 7, 1139–1151. [DOI] [PubMed] [Google Scholar]
- Christidi F, Zalonis I, Smyrnis N, & Evdokimidis I (2012). Selective Attention and the Three-Process Memory Model for the Interpretation of Verbal Free Recall in Amyotrophic Lateral Sclerosis. Journal of the International Neuropsychological Society, 18, 809–818. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Goldin Y, Ganci K, Rosenbaum A, Wethe JV, Langenbahn DM, Malec JF, Bergquist TF, Kingsley K, Nageles D, Trexler L, Fraas M, Bogdanova Y, & Harley JP (2019). Evidence-based cognitive rehabiltion: systematic review of the literature from 2009 through 2014. Archives of Physical Medicine and Rehabilitation, 100, 1515–1533. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Coolidge FL, Middleton PA, Griego JA, & Schmidt MW (1996). The Effects of Interference on Verbal Learning in Multiple Sclerosis. Archives of Clinical Neuropsychology, 11, 605–611. [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (1987). The California Verbal Learning Test. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan Executive Function System: Technical Manual. San Antonio, TX: Harcourt Assessment Company. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BE (2000). California Verbal Learning Test – Second Edition Manual. Psychological Corporation, San Antonio, TX [Google Scholar]
- DeLuca J, Barbieri-Berger S, & Johnson SK (1994). The nature of memory impairments in multiple sclerosis: Acquisition versus retrieval. Journal of Clinical and Experimental Neuropsychology, 16, 183–189. [DOI] [PubMed] [Google Scholar]
- DeLuca J, Schultheis MT, Madigan NK, Christodoulou C, & Averill A (2000). Acquisition Versus Retrieval Deficits in Traumatic Brain Injury: Implications for Memory Rehabilitation. Archives of Physical Medicine and Rehabilitation, 81, 1327–1333. [DOI] [PubMed] [Google Scholar]
- DeLuca J, Leavitt VM, Chiaravalloti N, & Wylie G (2013). Memory impairment in multiple sclerosis is due to a core deficit in initial learning. Journal of Neurology, 260, 2491–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaree HA, Gaudino EA, DeLuca J, & Ricker JH (2000). Learning Impairment is Associated With Recall Ability in Multiple Sclerosis. Journal of Clinical and Experimental Neuropsychology, 22, 865–873. [DOI] [PubMed] [Google Scholar]
- Everitt SE, Landau S, & Leese M (2001). Cluster Analysis. Arnold, London. [Google Scholar]
- Fischer JS, Rudick RA, Cutter GR, Reingold SC, & The National M.S. Society Clinical Outcomes Assessment Task Force (1999). The Multiple Sclerosis Functional Composite Measure: An integrated approach to M.S. clinical outcome assessment. Multiple Sclerosis, 5, 244–250. [DOI] [PubMed] [Google Scholar]
- Galioto R, Dhima K, Berenholz O, & Busch R (in press). Performance validity in multiple sclerosis. Journal of the International Neuropsychological Society. [DOI] [PubMed] [Google Scholar]
- Gaudino EA, Charavalloti ND, DeLuca J, & Diamond BJ (2001). A Comparison of Memory Performance in Relapsing–Remitting, Primary Progressive and Secondary Progressive, Multiple Sclerosis. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 14, 32–44. [PubMed] [Google Scholar]
- Gentry T (2008). PDAs as cognitive aids for people with multiple sclerosis. American Journal of Occupational Therapy, 62:18–27. [DOI] [PubMed] [Google Scholar]
- Goverover Y, Chiaravalloti ND, O’Brien AR, & DeLuca J (2018). Evidence-based cognitive rehabilitation for persons with multiple sclerosis: An updated review of the literature from 2007 to 2016. Archives of Physical Medicine and Rehabilitation, 99, 390–407. [DOI] [PubMed] [Google Scholar]
- Grzegorski T, & Losy J (2017). Cognitive impairment in multiple sclerosis - a review of current knowledge and recent research. Reviews in the Neurosciences, 28, 845–860. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Dawson DM, Lehrich JR, Beal MF, Kevy SV, Propper RD, Mills JA, & Weiner HL (1983). Intensive immunosuppression in progressive multiple sclerosis: A randomized, three-arm study of high-dose intravenous cyclophosphamide, plasma exchange and ACTH. New England Journal of Medicine, 308, 173–180. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Tallen JL, Kay GG, Curtiss G (1993). Wisconsin Card Sorting Test Manual Revised and Expanded. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (2000). The Boston Naming Test - Second Edition (2nd ed.). Philadelphia: Lea & Febiger. [Google Scholar]
- Lange R, Iverson G, Senior G, & Chelune G (2002). A primer on cluster analysis applications to cognitive rehabilitation research. Journal of Cognitive Rehabilitation, 20, 16–33. [Google Scholar]
- Lindsay DS (2015). Replication in psychological science. Psychological Science, 26, 1827–1832. [DOI] [PubMed] [Google Scholar]
- Litvin PY, Siders CA, Waite EN, Woo E, Romero E, Foley J, Ettenhofer ML, Gooding AL, Castellon S, Hinkin C, & Wright MJ (in press). Recent cocaine use and memory impairment in HIV. Applied Neuropsychology: Adult. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyenhuis DL, Luchetta T, Yamamoto C, Terrien A, Bernardin L, Rao M, & Garron D (1998). The Development, Standardization, and Initial Validation of the Chicago Multiscale Depression Inventory. Journal of Personality Assessment, 70, 386. [DOI] [PubMed] [Google Scholar]
- Oltra-Cucarella J, Perez-Elvira R, & Duque P (2014). Benefits of deep encoding in Alzheimer’s disease. Analysis of performance in a memory task using the Item Specific Deficit Approach. Neurologia, 29, 286–293. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo A, & Troster AI (1999a). Action (Verb Naming) fluency as a unique executive function measure: Convergent and divergent evidence of validity. Neuropsychologia, 37, 1499–1503. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo A, Koller WC, & Troster AI (1999b). Lexical, semantic, and action fluency in Parkinson’s disease with and without dementia. Journal of Clinical and Experimental Neuropsychology, 21, 435–443. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Edan G, et al. (2005). Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Annals of Neurology, 58, 840–846. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Snook EM, Lewis JM, Motl RW, Kramer AF (2008). Cognitive impairments in relapsing-remitting multiple sclerosis: a meta-analysis. Multiple Sclerosis, 14, 1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM (1990). A Manual for the Brief Repeatable Battery of Neuropsychological Tests in Multiple Sclerosis. Cognitive Function Study Group of the National Multiple Sclerosis Society. National Multiple Sclerosis Society, New York. [Google Scholar]
- Rao SM, Leo GJ, Aubin-Faubert P (1989). On the nature of memory disturbance in multiple sclerosis. Journal of Clinical and Experimental Neuropsychology, 11, 699–712. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, Bernardin L, & Unverzagt F (1991). Cognitive dysfunction in multiple sclerosis: I. Frequency, patterns, and prediction. Neurology, 41, 685–691. [DOI] [PubMed] [Google Scholar]
- Rao SM, Grafman J, DiGiulio D, Mittenberg W, Bernardin L, Leo GJ, Luchetta T, & Unverzagt F (1993). Memory Dysfunction in Multiple Sclerosis: Its Relation to Working Memory, Semantic Encoding, and Implicit Learning. Neuropsychology, 7, 364–374. [Google Scholar]
- Reitan RM, & Wolfson D (1993). The Halstead–Reitan Neuropsychological Test Battery (2nd ed.). Tucson, AZ: Neuropsychology Press. [Google Scholar]
- Rosen HE (1962). The verbal concept attainment test. Detroit, MI: Wayne State University. [Google Scholar]
- Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, & Ducreux D (2008). White matter abnormalities in mild traumatic brain injury: A diffusion tensor imaging study. American Journal of Neuroradiology, 29, 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ES, Erdodi L, Rodriguez N, Ghosh JJ, Curtain JR, Flashman LA, & Roth RM (2016). CVLT-II forced choice recognition trial as an embedded validity indicator: A systematic review of the evidence. Journal of the International Neuropsychological Society, 22, 851–858. [DOI] [PubMed] [Google Scholar]
- Smith A (1991). Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Sumowski JF, Benedict R, Enzinger C, Filippi M, Geurts JJ, Hamalainen P, Hulst H, Inglese M, Leavitt VM, Rocca MA, Rosti-Otajarvi EM, & Rao S (2018) Cognition in multiple sclerosis: State of the field and priorities for the future. Neurology, 90, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, & Dede AJO (2015). Conscious and unconscious memory systems. Cold Spring Harbor Perspectives in Biology, 7, a021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderploeg RD, Crowell TA, & Curtiss G (2001). Verbal Learning and Memory Deficits in Traumatic Brain Injury: Encoding, Consolidation, and Retrieval. Journal of Clinical and Experimental Neuropsychology, 23, 185–195. [DOI] [PubMed] [Google Scholar]
- Ward J (1963). Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association, 58, 236–244. [Google Scholar]
- Wechsler D (1997). Wechsler Adult Intelligence Scale-III. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (2008). WAIS-IV technical and interpretive manual. San Antonio, TX: Pearson. [Google Scholar]
- Wishart H, & Sharpe D (1997). Neuropsychological aspects of multiple sclerosis: A quantitative review. Journal of Clinical and Experimental Neuropsychology, 19, 810–824. [DOI] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Sires DA, Grant I. Heaton RK, Troster A, and the HIV Neurobehavioral Research Center Group (2005). Action (verb) fluency: Test-retest reliability, normative standards, and construct validity. Journal of the International Neuropsychological Society, 11, 408–415. [PubMed] [Google Scholar]
- Wright MJ, Woo E, Schmitter-Edgecombe M, Hinkin CH, Miller EN, & Gooding AL (2009). The Item-Specific Deficit Approach to evaluating verbal memory dysfunction: Rationale, psychometrics, and application. Journal of Clinical and Experimental Neuropsychology, 31, 790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Schmitter-Edgecombe M, & Woo E (2010). Verbal Memory Impairment in Severe Closed-Head Injury: The Role of Encoding and Consolidation. Journal of Clinical and Experimental Neuropsychology, 32, 728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Woo W, Foley J, Ettenhofer ML, Cottingham ME, Gooding AL, Jang J, Kim MS, Castellon SA, Miller EN, & Hinkin CH (2011). Antiretroviral Adherence and the Nature of HIV Associated Verbal Memory Impairment. The Journal of Neuropsychiatry and Clinical Neurosciences, 23, 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK (2000). Distinct Neurocognitive Profiles in Multiple Sclerosis Subtypes. Archives of Clinical Neuropsychology, 15, 115–136. [PubMed] [Google Scholar]
- Zhou T, Ahmad TK, Gozda K, Truong J, Kong J, & Namaka M (2017). Implications of white matter damage in amyotrophic lateral sclerosis. Molecular Medicine Reports, 16, 4379–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


