Abstract
Background:
The Dietary Approaches to Stop Hypertension (DASH) diet pattern has shown some promise for preventing heart failure (HF), but studies have been conflicting.
Objective:
To determine whether the DASH diet pattern was associated with incident HF in a large biracial and geographically diverse population.
Methods:
Among participants in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study of adults aged ≥45 years who were free of suspected HF at baseline in 2003–2007, DASH diet score was derived from the baseline food frequency questionnaire. The main outcome was incident HF defined as the first adjudicated HF hospitalization or HF death through December 31, 2016. We estimated hazard ratios (HR) for the associations of DASH diet score quartiles with incident HF, and incident HF with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF) using the Lunn-McNeil extension to the Cox model. We tested for several pre-specified interactions including with age.
Results:
Compared to the lowest quartile, individuals in the 2nd-4th DASH diet score quartiles had lower risk for incident HF after adjustment for sociodemographic and health characteristics: Q2 HR 0.70, 95%CI[0.56–0.87]; Q3 HR 0.65, 95%CI:[0.52–0.81]; Q4 HR 0.67, 95%CI:[0.52–0.85]. When stratifying results by age, quartiles 2–4 had a lower hazard for incident HF among age<65 years, quartiles 3–4 had a lower hazard among age 65–74, and the quartiles had similar hazard among age ≥75 years (p-for-interaction=0.003). We did not find a difference in the association of DASH diet with incident HFrEF vs. HFpEF (p=0.11).
Conclusions:
DASH diet adherence was inversely associated with incident HF, specifically among individuals <75 years old.
Keywords: heart failure, incident heart failure, DASH diet
INTRODUCTION:
Heart failure (HF) affects over 6 million people across the United States(1), and is responsible for a significant portion of healthcare costs, as well as considerable morbidity and mortality. Unfortunately, despite significant advances in HF care over the past 2 decades, outcomes for adults with HF remain poor, with a 5-year mortality above 50% irrespective of HF subtype.(2) Accordingly, the best strategy to improve outcomes is likely to prevent the development of HF.(3)
Behavior and lifestyle represent the cornerstones of primary prevention for many cardiovascular conditions including HF. Over the past several years, there has been increased attention on identifying diet patterns that may be protective against HF. A recent study from the REasons for Geographic And Racial Differences Study (REGARDS) cohort examining diet patterns derived from a factor analysis showed that a plant-based diet pattern was inversely associated with incident HF, while a Southern diet pattern was positively associated with incident HF.(4) Because hypertension is one of the most important modifiable risk factors for HF, diet patterns that reduce blood pressure are particularly appealing for the prevention of HF. The well-known Dietary Approaches to Stop Hypertension (DASH) diet was designed as a palatable, accessible style of eating for US adults that is low in sodium and high in potassium, rich in fruits and vegetables, moderate in low-fat dairy products, and low in red and processed meats, with a substantial amount of plant protein from legumes and nuts. Randomized controlled trials have shown that the DASH diet lowers blood pressure(5) and reduces low-density lipoprotein (LDL) cholesterol.(6) The DASH diet is also associated with favorable changes in left ventricular systolic parameters(7) and diastolic function.(8) While it shares some features with the plant-based diet pattern derived among REGARDS participants, the DASH diet pattern is distinct from the plant-based diet pattern in that it highlights several specific features, including low intake of red and processed meats, sodium, and sugar-sweetened beverages.
Prior studies examining the association of a DASH diet and HF have had conflicting results. Two studies from Sweden showed that the DASH diet was inversely associated with incident HF among women(9) and men.(10) In contrast to these studies, findings from the Cardiovascular Health Study showed no association between DASH diet and incident HF.(11) Importantly, these studies had limited generalizability, as they each examined cohorts predominantly comprised of white participants. Moreover, none of these studies examined specific subtypes of HF, which may be important given key differences in the pathophysiologies of HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF).(12) Thus, we examined the association between the DASH diet pattern and HF among participants in the REGARDS cohort, which is particularly well-suited for this inquiry because it is a geographically-diverse population that includes men and women, a broad range of ages, and a significant number of blacks (in addition to whites), and provides detail on HF subtypes (HFpEF and HFrEF).
METHODS:
Data Source
The study population was derived from the REGARDS cohort, which has been previously described.(13) Briefly, the REGARDS cohort is comprised of 30,239 community dwelling participants (42% blacks and 55% women) residing in the continental United States. Participants were recruited from 2003 to 2007 with ongoing follow-up. The cohort was initially designed to address geographic and racial differences in stroke mortality and therefore over-selected for populations with higher risk for stroke including black adults and those residing in the southeastern United States in areas known as the stroke belt (an area in the Southeastern U.S. with high stroke mortality that includes North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana) and stroke buckle (an area with even higher stroke mortality that includes the coastal plains of North Carolina, South Carolina, and Georgia).
Baseline data collection for REGARDS was completed using computer-assisted telephone interviews to collect medical history, functional status, health behaviors, and psychosocial measures. In-home examinations were conducted by trained health care professionals using standardized, quality-controlled protocols to collect physiologic and anthropometric measures (blood pressure, height, weight, waist circumference), blood and urine samples, electrocardiograms, and medication use by pill bottle review. Food frequency questionnaires were completed by participants following the in-home visit. Blood and urine samples were centrally analyzed at the University of Vermont.
The REGARDS study was previously approved by the Institutional Review Boards at the participating centers. This ancillary study was approved by the Weill Cornell Institutional Review Board. All participants provided written informed consent.
Study Population
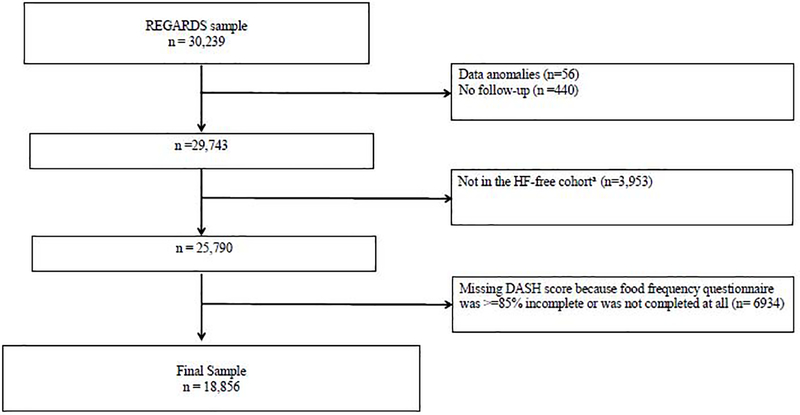
We studied participants without suspected baseline HF from the REGARDS cohort, who had completed food frequency questionnaires. This HF-free cohort was created based on the use of HF-specific medication, and has a negative predictive value of >95% compared to diagnoses in Medicare claims (14). Among 25,790 participants in the HF-free cohort, we excluded those with missing or incomplete (<85% completion) food frequency questionnaires and participants with biologically implausible energy intake (men with an intake of <3347 kJ/d or >20,920 kJ/d; and women with an intake of <2093 kJ/d or >18,841 kJ/d), as has been done in prior studies.(15) (Figure 1). Supplemental Table 1 shows differences between participants with complete dietary data (n=18,856) and those with missing dietary data (n=6934).
Figure 1:
Inclusion/Exclusion Cascade
Abbreviations: REGARDS- Reasons for geographic and racial differences in strokes; HF-heart failure
Primary Outcome:
The primary outcome was an adjudicated incident HF event, defined as an adjudicated HF hospitalization or HF death through December 31, 2016; adjudication was performed independently by 2 clinician investigators with disagreements resolved by discussion. Adjudication of a HF hospitalization was based on signs and symptoms, laboratory studies, electrocardiogram, and assessments of left ventricular function documented in the medical records.(16) Adjudication of a death with HF as the underlying cause was based on interviews with participant proxies, medical records for hospitalizations around the time of death, and death certificates. HF-subtypes were identified based on left ventricular ejection fraction (EF) obtained at the time of an incident event—HFrEF was defined as EF <50% or qualitative report of reduced EF; and HFpEF was defined as EF ≥50% or qualitative report of preserved EF.(17) Subtyping HF was not possible for incident events based on a HF-related death.
Key Explanatory Variable:
The primary exposure variable was DASH diet score. The DASH diet score was calculated as the sum of 8 component scores based on dietary intake data from the Block 98 Food Frequency Questionnaire across the following domains: (1) fruits, (2) vegetables, (3) nuts and legumes, (4) low-fat dairy products, (5) whole grains, (6) sodium, (7) sweetened beverages, and (8) red and processed meats.(18) Component scores for each domain were determined based on population-specific quintiles. For components 1 through 5, participants received a score of 5 if they were in the highest quintile and a score of 1 if they were in the lowest quintile. For components 6–8, participants received a score of 1 if they were in the highest quintile and a score of 5 if they were in the lowest quintile. The final DASH diet score ranged from 8–40, where 8 indicated the lowest adherence to the DASH diet and 40 indicated highest adherence to the DASH diet. We categorized each participant into a quartile based on their DASH diet score.
Covariates:
We chose covariates for our statistical models a priori based on the literature and clinical expertise. These variables included baseline total energy intake, sociodemographic characteristics, health behaviors, physiologic parameters, and cardiovascular comorbid conditions (diabetes, coronary heart disease, and atrial fibrillation). Given their association with incident HF, similar variables have been used in prior studies of incident HF using REGARDS.(16,19)
Statistical Analysis
We calculated descriptive statistics for baseline participant characteristics (mean with standard deviation [SD] for continuous variables or percentages for categorical variables). We then calculated the incident rates of HF, HFpEF, and HFrEF for each DASH score quartile as number of cases per 10,000-person years.
We used Cox proportional-hazards regression models to determine whether the DASH diet score was associated with incident HF events. Model 1 included total energy intake and socio-demographic factors (age, sex, and race); Model 2 included Model 1 plus annual family income, educational achievement, insurance type, marital status, geographic region, cigarette smoking, alcohol consumption (none, moderate [0–7 drinks per week for women, 0–14 drinks per week for men], or heavy [>7 drinks per week for women, >14 drinks for per week for men], defined according to the National Institute on Alcohol Abuse and Alcoholism), and physical activity (based on amount of exercise per week); and Model 3 included Model 2 plus potentially mediating physiologic parameters and comorbid conditions including systolic blood pressure, diastolic blood pressure, body mass index, high-density lipoprotein cholesterol, total cholesterol, triglycerides, estimated glomerular filtration rate, natural log-transformed C-reactive protein, urine albumin to creatinine ratio, left ventricular hypertrophy, diabetes, coronary heart disease, and atrial fibrillation.
A global test of violation of the proportion hazards assumption using Schoenfeld residuals was significant and individual tests of the proportional hazards assumption indicated that the associations of diabetes and age varied over follow-up. Therefore, in the adjusted models we allowed the baseline hazard to vary by age and diabetes.
To account for missing covariate values in regression analysis, we applied multiple imputation using the Markov Chain Monte Carlo algorithm designed for single-level and multi-level data.(20) The variables with the greatest percent missing were income (11.5%) and CRP (5.5%).
To ensure the robustness of our findings, we conducted several sensitivity analyses. Given the competing risk of mortality, we conducted a sensitivity analysis to examine the association between DASH diet score and incident HF using Fine and Gray competing risk models. For improved granularity regarding a potential threshold for an association between DASH diet score and incident HF, we also examined the association between DASH diet score and incident HF using DASH diet deciles instead of quartiles.
Given the potential influence of baseline coronary heart disease, hypertension (according to baseline measured systolic blood pressure [SBP]), and/or chronic kidney disease (according to baseline estimated glomerular filtration rate [eGFR]) on an association between DASH diet and incident HF, we also examined for possible interactions with these comorbid conditions by adding cross-product terms to the models and performing a Wald test. Given known sex and race-based differences in left ventricular remodeling in response to stressors, we also examined whether associations between the DASH diet score and incident HF differed according to sex and race. Finally, given the possibility that the benefits of the DASH diet might attenuate with increasing age, we examined whether associations between the DASH diet score and incident HF differed according to age <65 years, 65–74 years, or ≥75 years. For interactions where the p<0.10, we presented stratum-specific analyses.
Given well-described differences in the demographics, pathophysiology, treatment, and disease trajectory of HFrEF compared to HFpEF, we repeated the regression models to determine whether the DASH diet score was differentially associated with incident HFrEF and HFpEF using a Lunn-McNeil extension to the Cox proportional hazards model.(21)
We used two-sided hypothesis testing, and p-value <0.05 to determine statistical significance for main effects and p-value <0.10 to determine statistical significance of interaction terms. We managed the data in SAS version 9.4 (SAS Institute, Cary, NC) and performed statistical analysis using STATA version 14 (IBM corporation, Armonk, NY).
RESULTS:
Study Population
The mean age of participants was 64.0 (SD 9.2) years, 55.9% were female, and 32.5% were black (Table 1). The median DASH diet score was 24 with an interquartile range of 21–27. When compared to participants in the lowest DASH diet quartile, participants in DASH diet quartiles 2 through 4 were older, more frequently white, more likely to have completed high school, and had higher household incomes. Non-smokers and individuals with moderate alcohol use and more frequent exercise were more common among the higher DASH diet quartiles. Diabetes mellitus was more common among the higher DASH quartiles while hypertension and left ventricular hypertrophy were more common in the lowest DASH diet quartile. Intake of selected nutrients stratified by DASH diet quartile are shown in Supplemental Table 2. Notably, mean sodium intake was similar across quartiles, with a narrow range of 2212–2313 mg/d.
Table 1.
Participant characteristics according to DASH Diet Score Quartiles
| Characteristic | Total | Q1(≥ 20) | Q2(21–23) | Q3(24–27) |
|---|---|---|---|---|
| N | 18856 | 4203 | 4559 | 5764 |
| Age, mean (SD#) | 64 (9.2) | 62 (9) | 64 (9.2) | 65 (9.1) |
| Female | 10535 (55.9%) | 2348 (55.9%) | 2474 (54.3%) | 3271 (56.7%) |
| Black | 6130 (32.5%) | 1804 (42.9%) | 1619 (35.5%) | 1716 (29.8%) |
| Region | ||||
| Nonbelt | 8313 (44.1%) | 1767 (42.0%) | 1953 (42.8%) | 2571 (44.6%) |
| Belt | 6452 (34.2%) | 1526 (36.3%) | 1615 (35.4%) | 1882 (32.7%) |
| Buckle | 4091 (21.7%) | 910 (21.7%) | 991 (21.7%) | 1311 (22.7%) |
| Married | 11797 (62.6%) | 2439 (58.0%) | 2820 (61.9%) | 3676 (63.8%) |
| Less than high school education | 1657 (8.8%) | 500 (11.9%) | 498 (10.9%) | 426 (7.4%) |
| Annual income Less than $20k | 2754 (16.5%) | 835 (22.1%) | 752 (18.6%) | 761 (15.0%) |
| No Health Insurance | 1180 (6.3%) | 406 (9.7%) | 332 (7.3%) | 283 (4.9%) |
| Smoking status | ||||
| Current | 2618 (13.9%) | 1087 (26.0%) | 768 (16.9%) | 547 (9.5%) |
| Past | 7585 (40.4%) | 1543 (36.9%) | 1808 (39.8%) | 2420 (42.1%) |
| Never | 8585 (45.7%) | 1555 (37.2%) | 1966 (43.3%) | 2783 (48.4%) |
| Alcohol use | ||||
| Heavy | 859 (4.6%) | 229 (5.6%) | 211 (4.7%) | 261 (4.6%) |
| Moderate | 6852 (37.0%) | 1371 (33.3%) | 1581 (35.3%) | 2223 (39.2%) |
| None | 10825 (58.4%) | 2519 (61.2%) | 2686 (60.0%) | 3194 (56.3%) |
| Times per week of exercise | ||||
| 4 or more per week | 5785 (31.1%) | 1012 (24.4%) | 1240 (27.6%) | 1820 (32.0%) |
| 1 to 3 time per week | 7039 (37.8%) | 1441 (34.7%) | 1674 (37.3%) | 2268 (39.9%) |
| None | 5789 (31.1%) | 1700 (40.9%) | 1571 (35.0%) | 1602 (28.2%) |
| Body mass index (kg/m2), mean (SD) | 29 (5.8) | 29 (6.2) | 29 (5.9) | 29 (5.7) |
| Waist circumference (cm), mean (SD) | 95 (15) | 96 (15) | 96 (15) | 94 (15) |
| Calories, median (IQR) | 1586 (1199, 2084) | 1589 (1187, 2118) | 1576 (1166, 2091) | 1570 (1188, 2080) |
| Diabetes | 2990 (16.4%) | 606 (14.9%) | 749 (17.0%) | 967 (17.3%) |
| Hypertension | 10349 (54.9%) | 2413 (57.4%) | 2603 (57.1%) | 3099 (53.8%) |
| SBP? (mmHg), mean (SD) | 126 (16) | 128 (16) | 127 (16) | 126 (16) |
| DBP† (mmHg), mean (SD) | 76 (9.4) | 77 (9.7) | 77 (9.5) | 76 (9.1) |
| Atrial Fibrillation | 1386 (7.4%) | 306 (7.3%) | 320 (7.0%) | 422 (7.3%) |
| History of Ischemic Heart Disease | 2647 (14.1%) | 548 (13.1%) | 643 (14.2%) | 809 (14.1%) |
| Left Ventricular Hypertrophy | 1548 (8.3%) | 366 (8.7%) | 404 (8.9%) | 473 (8.3%) |
| Dyslipidemia | 10588 (58.0%) | 2347 (57.7%) | 2628 (59.6%) | 3258 (58.4%) |
| Total Cholesterol (mg/dL), mean (SD) | 194 (39) | 196 (40) | 195 (40) | 193 (39) |
| HDL§ (mg/dL), mean (SD) | 53 (16) | 51 (16) | 52 (16) | 53 (17) |
| Triglycerides (mg/dL), median (IQR) | 110 (81, 157) | 114 (84, 163) | 114 (84, 162) | 109 (80, 157) |
| eGFR‡, mean (SD) | 86 (18) | 88 (20) | 86 (19) | 85 (18) |
| Urinary ACR* (mg/g) | ||||
| ACR<10 | 11886 (65.7%) | 2617 (65.3%) | 2817 (64.2%) | 3670 (66.0%) |
| ACR 10–29 | 4036 (22.3%) | 849 (21.2%) | 1035 (23.6%) | 1254 (22.6%) |
| ACR 30–300 | 1861 (10.3%) | 459 (11.4%) | 448 (10.2%) | 547 (9.8%) |
| ACR > 300 | 312 (1.7%) | 84 (2.1%) | 87 (2.0%) | 89 (1.6%) |
| C-reactive protein >3 | 6721 (37.7%) | 1838 (46.3%) | 1792 (41.6%) | 1959 (35.9%) |
Abbreviations:
ACR: Albumin to Creatinine Ratio
DBP: diastolic blood pressure
eGFR: estimated glomerular filtration rate
HDL: high-density lipoprotein
SBP: systolic blood pressure
SD: Standard deviation
Association Between DASH Diet and HF Events
Among this cohort, there were 767 incident HF events over a median of 10.1 years of follow-up, for an incidence rate of 44/10,000 person-years. Among the 767 events, 656 were HF hospitalizations and 111 were HF-related deaths.
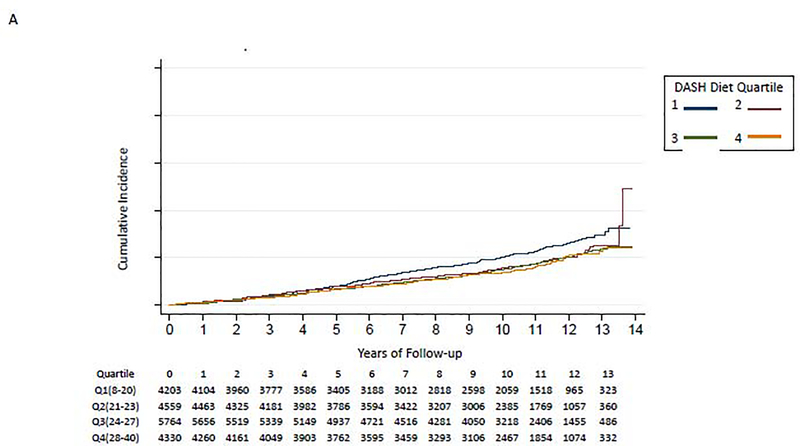
The incidence of HF was highest among those in the lowest DASH diet quartile (Table 2 and Figure 2A). Participants in quartiles 2–4 had a statistically significantly lower hazard for incident HF compared to the lowest quartile of DASH diet score in the fully adjusted model. These results were largely unchanged when using a Fine and Gray competing risk model to account for mortality (Supplemental Table 3).
Table 2.
Hazard Ratios for incident HF event according to DASH diet score
| A. All | ||||
| Model | Q1 (Score 8–20) | Q2 (Score 21–23) | Q3 (Score 24–27) | Q4 (Score 28–40) |
| N (events) | 4,203 (200) | 4,559 (179) | 5,764 (224) | 4,330 (164) |
| Event Rate, person-years | 53/10,000 | 43/10,000 | 41/10,000 | 40/10,000 |
| 1* | 1.00 (ref) | 0.68 (0.55,0.83) | 0.62 (0.51,0.75) | 0.55 (0.45,0.68) |
| 2† | 1.00 (ref) | 0.73 (0.59,0.89) | 0.75 (0.61,0.91) | 0.70 (0.57,0.88) |
| 3‡ | 1.00 (ref) | 0.69 (0.56,0.85) | 0.71 (0.58,0.87) | 0.73 (0.58,0.92) |
| B. Age<65 | ||||
| Model | Q1 (Score 8–20) | Q2 (Score 21–23) | Q3 (Score 24–27) | Q4 (Score 28–40) |
| N (events) | 2,624 (82) | 2,427 (45) | 2,865 (44) | 1,927 (22) |
| Event Rate, person-years | 35/10,000 | 20/10,000 | 16/10,000 | 12/10,000 |
| 1* | 1.00 (ref) | 0.54 (0.38,0.78) | 0.43 (0.30,0.62) | 0.31 (0.19,0.49) |
| 2† | 1.00 (ref) | 0.60 (0.41,0.86) | 0.53 (0.36,0.77) | 0.42 (0.26,0.67) |
| 3‡ | 1.00 (ref) | 0.56 (0.39,0.81) | 0.54 (0.37,0.79) | 0.48 (0.30,0.78) |
| C. Age 65–74 | ||||
| Model | Q1 (Score 8–20) | Q2 (Score 21–23) | Q3 (Score 24–27) | Q4 (Score 28–40) |
| N (events) | 1,145 (7 ) | 1,457 (80) | 1,941 (77) | 1,579 (62) |
| Event Rate, person-years | 77/10,000 | 60/10,000 | 41/10,000 | 40/10,000 |
| 1* | 1.00 (ref) | 0.78 (0.57,1.07) | 0.54 (0.39,0.74) | 0.51 (0.37,0.72) |
| 2† | 1.00 (ref) | 0.87 (0.63,1.18) | 0.65 (0.48,0.90) | 0.67 (0.48,0.94) |
| 3‡ | 1.00 (ref) | 0.79 (0.58,1.08) | 0.57 (0.42,0.79) | 0.65 (0.46,0.92) |
| D. Age≥ 75 | ||||
| Model | Q1 (Score 8–20) | Q2 (Score 21–23) | Q3 (Score 24–27) | Q4 (Score 28–40) |
| N (events) | 434 (39) | 675 (54) | 958 (103) | 824 (80) |
| Event Rate, person-years | 113/10,000 | 98/10,000 | 126/10,000 | 113/10,000 |
| 1* | 1.00 (ref) | 0.83 (0.55,1.25) | 1.07 (0.74,1.55) | 0.96 (0.66,1.42) |
| 2† | 1.00 (ref) | 0.81 (0.54,1.23) | 1.19 (0.82,1.72) | 1.13 (0.77,1.66) |
| 3‡ | 1.00 (ref) | 0.81 (0.54,1.24) | 1.21 (0.83,1.76) | 1.12 (0.75,1.66) |
Model 1 adjusts for total energy intake, age, sex, and race
Model 2 adjusts for Model 1 plus other socio-demographic factors such as geographic region, annual family income (< vs. ≥ $20,000/year), educational achievement (< vs. ≥ high school diploma), health insurance, marital status, cigarette smoking, alcohol consumption, physical activity
Model 3 adjusts for Model 2 plus potentially-mediating physiologic parameters such as systolic blood pressure, diastolic blood pressure, body mass index, high-density lipoprotein cholesterol, total cholesterol, triglycerides, estimated glomerular filtration rate, natural log-transformed C-reactive protein, urine albumin-to-creatinine ratio, diabetes, history of coronary heart disease, history of atrial fibrillation, and left ventricular hypertrophy
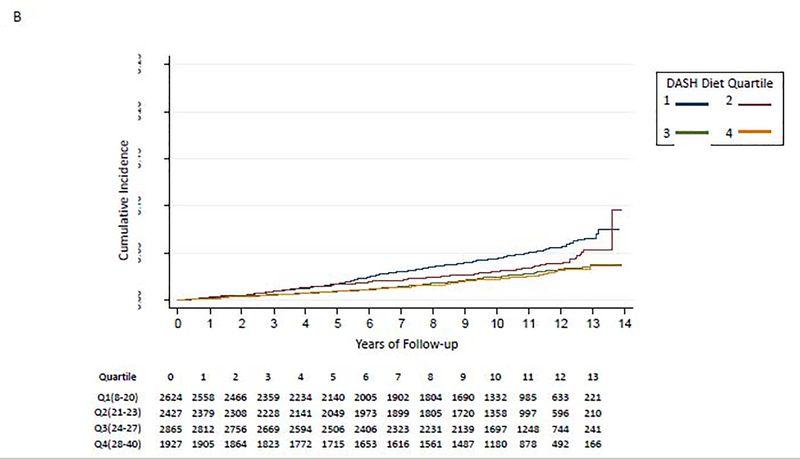
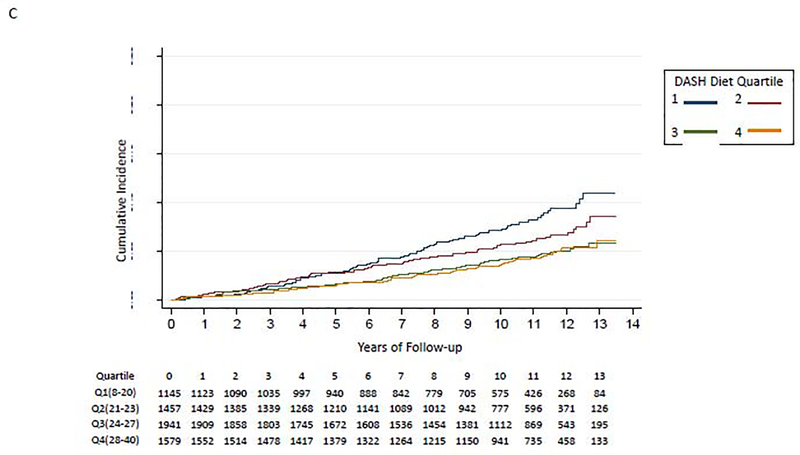
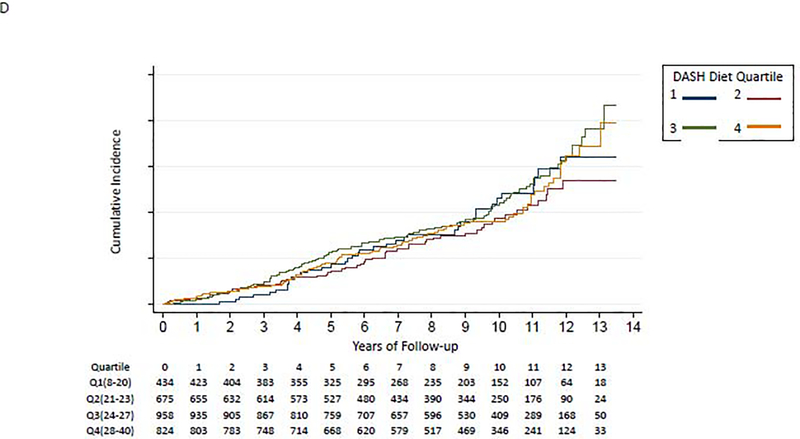
Figure 2:
Kaplan-Meier Curve for Association of DASH Diet quartiles and incident HF
A. All
B. Age <65 years
C. Age 65–74 years
D. Age ≥75 years
When examining the association of DASH diet score deciles and incident HF, we found that the 3rd through 10th decile (DASH score > 20) were associated with a significantly lower incident HF compared to the 1st decile (Supplemental Table 4).
We did not find evidence to support interaction of the DASH diet score with baseline coronary heart disease (p-for-interaction=0.38), baseline hypertension (baseline SBP≥130: p-for-interaction=0.49; SBP≥140: p-for-interaction=0.63), baseline chronic kidney disease (baseline eGFR<60: p-for-interaction=0.93; eGFR<45: p-for-interaction=0.15), sex (p-for-interaction= 0.41), or race (p-for-interaction=0.54). The p-for-interaction for age was 0.003. Participants in lower DASH diet score quartiles had a higher risk for developing incident HF among individuals aged <65 years (Figure 2B) and aged 65–74 years (Figure 2C); those aged ≥75 years had similar risk of HF irrespective of DASH diet quartile (Figure 2D). In a fully-adjusted model, participants in quartiles 2–4 had a lower hazard for incident HF compared to the lowest quartile of DASH diet score among those aged <65 years, participants in quartiles 3–4 had a lower hazard for incident HF compared to the lowest quartile of DASH diet score among those aged 65–74 years; and the quartiles had similar hazard for incident HF among those aged ≥75 years old (Table 2).
Among 767 incident events, 574 had data on EF at the time of the event. There were 321 incident cases of HFrEF and 253 cases of incident HFpEF. Using a Lunn-McNeil model, we did not find a statistical difference in the association of DASH diet quartiles with incident HFrEF compared to incident HFpEF (p=0.11). Supplemental Table 5 shows hazard ratios for HFrEF and HFpEF separately.
DISCUSSION:
In this real-world, geographically diverse cohort of 18,856 participants, we found that the DASH diet was inversely associated with incident HF, specifically among those younger than 75 years. Given the ongoing HF epidemic, there is a need to identify population-based strategies to curb the incidence of HF. While control of traditional risk factors including hypertension, diabetes, and coronary artery disease has been beneficial, all cases of HF may not be fully explained by these traditional risk factors.(22) Thus, there may be value in identifying risk factors that are upstream from traditional risk factors which can subsequently be targeted. Diet has been identified as an important component of primary prevention for other cardiovascular conditions like hypertension and ischemic coronary disease.(15,18,23) Our study shows that, even when controlling for these other precipitating conditions and contributing factors like exercise, the DASH diet was associated with lower risk of incident HF. Moreover, these findings suggest that the benefit does not require extremely high DASH scores, and that avoiding low DASH scores is sufficient to reduce risk. Several other diets such as a plant-based diet and a Mediterranean diet have previously been studied. A recent retrospective study demonstrated that the highest quartile of following a plant-based diet was inversely associated with incident HF(4); and randomized controlled trials have shown that the Mediterranean diet may prevent incident HF but those studies were underpowered to make definitive conclusions(24). Given the potential benefit of these other diets which have some overlapping features with the DASH diet, future studies examining the interplay between specific macro or micronutrient components of these diets and incident HF may be helpful to better understand the role of diet in preventing HF.
Prior work in this area has been limited to select populations and has consequently revealed conflicting results. In Sweden, DASH diet correlated with a 37% lower incidence of HF in women(9) and 22% lower incidence in men(10) comparing the top to bottom quartiles. However, these studies examined a homogenous white population. A study of 4,490 individuals from the Cardiovascular Health Study showed no benefit; importantly, this study included an older population (mean age 72 years) that was again predominantly (89%) white. A recent study of 4,478 participants from Multi-Ethnic Study of Atherosclerosis (25) showed lower incident HF events in those less than 75 years old, but there were only 179 total events, yielding wide confidence intervals.(25) In contrast to prior studies, our study examined a geographically diverse United States population, which notably included 32.5% blacks and an age range of 45–98 years. Accordingly, our study helps to reconcile some of the conflicting findings of prior studies. In particular, we found that the DASH diet was potentially protective among those younger than 75 years, but not in individuals 75 years or older. This extends findings from the prior Multi-Ethnic Study of Atherosclerosis study and may help explain the differences in findings between the Swedish cohorts and the Cardiovascular Health Study (mean ages 61 years in the Swedish women and 59 years in the Swedish men, compared to 72 years in the Cardiovascular Health Study).(11,25) These findings suggest that the relative importance of diet may be less important with advancing age, perhaps due to the accumulation of other risk factors which may supersede the benefits of diet. Future research is needed to confirm the interaction between DASH diet and age and to elucidate potential mechanisms. Our study also showed that the DASH diet was similarly protective in both men and women, and in both white and black participants. We had hypothesized that the DASH diet would demonstrate a stronger effect in black persons given prior data demonstrating that DASH had an especially potent effect on blood pressure in blacks persons compared to white persons.(26) Our observation here of a similar association in both black and white persons suggests that the mechanism of protection may extend beyond blood pressure control. Taken together, our findings support the importance of implementing strategies for primary prevention earlier in the lifespan, irrespective of other demographic factors like sex or race.
This is the first study to our knowledge that examined the association of the DASH diet pattern with HF subtypes. The potential protective nature of a DASH diet appeared stronger for HFrEF than HFpEF, though the difference was not statistically significant. This was not surprising given the effect of the DASH diet on several shared pathophysiologic mechanisms of HFrEF and HFpEF. Indeed, the DASH diet can decrease blood pressure (5,27), LDL cholesterol,(6) serum inflammatory biomarkers,(28,29) and activation of the renin-angiotensin system,(30,31), and improve vascular and endothelial function(32)—factors that have been implicated in the pathophysiology of HFrEF and/or HFpEF. Relatedly, the DASH diet has also been associated with better left ventricular parameters including systolic and diastolic function.(33) Thus, our findings now reinforce a biologically plausible link between the DASH diet and both incident HFrEF and incident HFpEF independent of hypertension. Whether gut microbiota, which is an emerging area of inquiry in HF(34), mediates these associations is unclear and merits further investigation.
Strengths and Limitations:
There were several strengths of this study including the large sample size, geographic and racial diversity, and adjudicated HF outcomes which were subtyped into HFrEF and HFpEF. We also incorporated a broad array of covariates into the statistical models which controlled for several domains including socio-demographics, healthy behaviors, and physiologic parameters; and conducted several sensitivity analyses to maximize the robustness of findings. There were also several important limitations that merit attention. First, diet was self-reported using a food-frequency questionnaire, an approach with known limitations.(35) We also only included participants with at least 85% completed dietary data, which is a somewhat selected population. For example, a significant number of black participants were excluded due to missing dietary data. Although we did not observe an interaction with race, missing data could have influenced our findings. Second, the food frequency questionnaire was completed at a single time point, and may not reflect DASH diet adherence over the course of years. This concern is somewhat mitigated by the observation that dietary patterns are often stable over time.(32) Third, we lacked detailed information on cardiac function at baseline and other potential confounders and mediators. For example, we did not have data on ventricular function and used a proxy for HF diagnosis prior to enrollment. Although we adjusted for multiple confounders, there was the risk of residual confounding. Models adjusted for potential mediators through which the DASH diet may influence HF risk did not show substantial attenuation of the association. This may be because habitual diet patterns influence life-long risk factor burden, and this study included a single time point measurement of blood pressure and other HF risk factors. There are likely other mediators contributing to our finding that we did not capture—for example, our multivariable models did not include intervening cardiac events such as myocardial infarction or incident atrial fibrillation which could have played a role in the development of HF. Future work to better understand mediators of the association observed here between DASH diet and incident HF may be warranted. HF events were adjudicated based on hospitalizations and death, and thus did not capture incident HF diagnosed during an ambulatory visit. Although about a third of incident HF may be diagnosed in the ambulatory setting, a substantial proportion of individuals diagnosed with HF in the ambulatory setting are hospitalized within a year of diagnosis and would have thus been captured as an incident HF event at that time.(36) Finally, we observed a total of 574 events that were categorized as either HFrEF (n=321) or HFpEF (n=253), which limited power to detect subtle differences in the association of DASH diet with these HF subtypes.
CONCLUSIONS:
Adherence to the DASH diet pattern was associated with a reduced risk of incident HF for adults aged less than 75 years old, supporting its use for primary prevention of HF at a younger age.
Supplementary Material
ACKNOWLEDGEMENTS
This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at: https://www.uab.edu/soph/regardsstudy/.
Funding source:
This research project was supported by R01HL8077 (PI: Safford) from the National Heart, Lung, and Blood Institute (Bethesda, MD). The National Heart, Lung, and Blood Institute had no role in the collection, analysis, or interpretation of the data; and had no role in preparation or approval of the manuscript.
Sources of Funding: This research project was additionally supported by R01HL8077 (PI: Safford) from the National Heart, Lung, and Blood Institute (Bethesda, MD).
Disclosures:
Dr. Goyal was supported by the National Institute on Aging grant R03AG056446; Dr. Goyal also receives personal fees for medicolegal consulting in heart failure.
Dr. Safford has received research support from Amgen.
Dr. Levitan receives research support from Amgen, has served on Amgen advisory boards, and as a scientific consultant for a research project funded by Novartis.
Footnotes
The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics−−2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 2.Shah KS, Xu H, Matsouaka RA et al. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 3.Butler J, Khan MS. Heart Failure Prevention for All: Treatment Is Good, Prevention Is Better. J Am Coll Cardiol 2020. [DOI] [PubMed] [Google Scholar]
- 4.Lara KM, Levitan EB, Gutierrez OM et al. Dietary Patterns and Incident Heart Failure in U.S. Adults Without Known Coronary Disease. J Am Coll Cardiol 2019;73:2036–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel LJ, Moore TJ, Obarzanek E et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 6.Obarzanek E, Sacks FM, Vollmer WM et al. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr 2001;74:80–9. [DOI] [PubMed] [Google Scholar]
- 7.Hummel SL, Seymour EM, Brook RD et al. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension 2012;60:1200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hummel SL, Seymour EM, Brook RD et al. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail 2013;6:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitan EB, Wolk A, Mittleman MA. Consistency with the DASH diet and incidence of heart failure. Arch Intern Med 2009;169:851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitan EB, Wolk A, Mittleman MA. Relation of consistency with the dietary approaches to stop hypertension diet and incidence of heart failure in men aged 45 to 79 years. Am J Cardiol 2009;104:1416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Gobbo LC, Kalantarian S, Imamura F et al. Contribution of Major Lifestyle Risk Factors for Incident Heart Failure in Older Adults: The Cardiovascular Health Study. JACC Heart Fail 2015;3:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 13.Howard VJ, Cushman M, Pulley L et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 14.Goyal P, Mefford MT, Chen L et al. Assembling and validating a heart failure-free cohort from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. BMC Med Res Methodol 2020;20:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta R, Wood DA. Primary prevention of ischaemic heart disease: populations, individuals, and health professionals. Lancet 2019;394:685–696. [DOI] [PubMed] [Google Scholar]
- 16.Bailey LN, Levitan EB, Judd SE et al. Association of Urine Albumin Excretion With Incident Heart Failure Hospitalization in Community-Dwelling Adults. JACC Heart Fail 2019;7:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler J, Anker SD, Packer M. Redefining Heart Failure With a Reduced Ejection Fraction. JAMA 2019. [DOI] [PubMed] [Google Scholar]
- 18.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 19.Pinheiro LC, Reshetnyak E, Sterling MR, Levitan EB, Safford MM, Goyal P. Multiple Vulnerabilities to Health Disparities and Incident Heart Failure Hospitalization in the REGARDS Study. Circ Cardiovasc Qual Outcomes 2020;13:e006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter JRKM. Multiple Imputation and its Application. Hoboken, NJ: John Wiley & Sons, Ltd, 2013. [Google Scholar]
- 21.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics 1995;51:524–32. [PubMed] [Google Scholar]
- 22.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail 2009;2:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 24.Sanches Machado d’Almeida K, Ronchi Spillere S, Zuchinali P, Correa Souza G. Mediterranean Diet and Other Dietary Patterns in Primary Prevention of Heart Failure and Changes in Cardiac Function Markers: A Systematic Review. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campos CL, Wood A, Burke GL, Bahrami H, Bertoni AG. Dietary Approaches to Stop Hypertension Diet Concordance and Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. Am J Prev Med 2019;56:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svetkey LP, Simons-Morton D, Vollmer WM et al. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med 1999;159:285–93. [DOI] [PubMed] [Google Scholar]
- 27.Sacks FM, Svetkey LP, Vollmer WM et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 28.Soltani S, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: A systematic review and meta-analysis of randomized trials. Clin Nutr 2018;37:542–550. [DOI] [PubMed] [Google Scholar]
- 29.Ergatoudes C, Thunstrom E, Hansson PO et al. Natriuretic and Inflammatory Biomarkers as Risk Predictors of Heart Failure in Middle-Aged Men From the General Population: A 21-Year Follow-Up. J Card Fail 2018;24:594–600. [DOI] [PubMed] [Google Scholar]
- 30.Maris SA, Williams JS, Sun B, Brown S, Mitchell GF, Conlin PR. Interactions of the DASH Diet with the Renin-Angiotensin-Aldosterone System. Curr Dev Nutr 2019;3:nzz091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conlin PR, Division of Endocrinology DaH, Brigham and Women’s Hospital, Boston, Massachusetts, USA, Veterans Affairs Boston Healthcare System and Harvard Medical School B, Massachusetts, USA et al. The DASH diet enhances the blood pressure response to losartan in hypertensive patients. American Journal of Hypertension 2019;16:337–342. [DOI] [PubMed] [Google Scholar]
- 32.Maddock J, Ziauddeen N, Ambrosini GL, Wong A, Hardy R, Ray S. Adherence to a Dietary Approaches to Stop Hypertension (DASH)-type diet over the life course and associated vascular function: a study based on the MRC 1946 British birth cohort. Br J Nutr 2018;119:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen HT, Bertoni AG, Nettleton JA, Bluemke DA, Levitan EB, Burke GL. DASH Eating Pattern Is Associated with Favorable Left Ventricular Function in the Multi-Ethnic Study of Atherosclerosis. J Am Coll Nutr 2012;31:401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol 2019;16:137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cobb LK, Anderson CA, Elliott P et al. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation 2014;129:1173–86. [DOI] [PubMed] [Google Scholar]
- 36.Chamberlain AM, Dunlay SM, Gerber Y et al. Burden and Timing of Hospitalizations in Heart Failure: A Community Study. Mayo Clin Proc 2017;92:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.