Abstract
C/EBPα and C/EBPβ are intronless genes that can produce several N-terminally truncated isoforms through the process of alternative translation initiation at downstream AUG codons. C/EBPβ has been reported to produce four isoforms: full-length 38-kDa C/EBPβ, 35-kDa LAP (liver-enriched transcriptional activator protein), 21-kDa LIP (liver-enriched transcriptional inhibitory protein), and a 14-kDa isoform. In this report, we investigated the mechanisms by which C/EBPβ isoforms are generated in the liver and in cultured cells. Using an in vitro translation system, we found that LIP can be generated by two mechanisms: alternative translation and a novel mechanism—specific proteolytic cleavage of full-length C/EBPβ. Studies of mice in which the C/EBPα gene had been deleted (C/EBPα−/−) showed that the regulation of C/EBPβ proteolysis is dependent on C/EBPα. The induction of C/EBPα in cultured cells leads to induced cleavage of C/EBPβ to generate the LIP isoform. We characterized the cleavage activity in mouse liver extracts and found that the proteolytic cleavage activity is specific to prenatal and newborn livers, is sensitive to chymostatin, and is completely abolished in C/EBPα−/− animals. The lack of cleavage activity in the livers of C/EBPα−/− mice correlates with the decreased levels of LIP in the livers of these animals. Analysis of LIP production during liver regeneration showed that, in this system, the transient induction of LIP is dependent on the third AUG codon and most likely involves translational control. We propose that there are two mechanisms by which C/EBPβ isoforms might be generated in the liver and in cultured cells: one that is determined by translation and a second that involves C/EBPα-dependent, specific proteolytic cleavage of full-length C/EBPβ. The latter mechanism implicates C/EBPα in the regulation of posttranslational generation of the dominant negative C/EBPβ isoform, LIP.
The CCAAT/enhancer binding proteins (C/EBPs) are transcription factors belonging to the bZIP family of proteins, which are characterized by the presence of a basic region of amino acids involved in DNA binding followed by a leucine zipper motif involved in homo- and heterodimerization with other family members (40). Several C/EBP family members have been described: C/EBPs α, β, δ, ɛ, γ, and ζ (9, 17, 26, 30, 43). C/EBPα is highly expressed in liver, adipose tissue, and in certain cells of the lungs and mammary glands (2, 28, 29, 33). It has been shown to be required for hepatic glycogen synthesis and gluconeogenesis; C/EBPα−/− mice die shortly after birth due to severe hypoglycemia (41). C/EBPα has been shown to play a key role in adipocyte differentiation and lipid accumulation in vitro (5, 45) and in vivo (7, 41) as well as in cellular growth arrest (37, 39). C/EBPβ is more widely expressed than C/EBPα (9). C/EBPβ−/− mice display several immunity defects (32, 35), and C/EBPβ has been implicated in the differentiation of myeloid and lymphoid cell lines (32) as well as in adipocyte differentiation (5, 36, 45). C/EBPβ−/− females are sterile due to a defect in folliculogenesis (34) and exhibit abnormal morphology in the mammary glands (29, 33).
It has been reported that although both C/EBPα and C/EBPβ lack introns, they each can produce several isoforms by the process of alternative translation (1, 10, 19, 25). This process has been suggested to occur through leaky ribosome scanning leading to initiation at downstream AUG codons and yielding several N-terminally truncated products. The production of N-terminally truncated C/EBP isoforms is highly conserved throughout vertebrate evolution (4, 21). The evolutionary similarity suggests that the smaller isoforms have physiological significance, and this suggestion is supported by observations of different functions of the full-length and truncated isoforms of both C/EBPα (19, 25) and C/EBPβ (10, 45). For C/EBPβ, four putative isoforms have been described: full-length C/EBPβ (38-kDa FL), 35-kDa LAP (liver-enriched transcriptional activating protein), 21-kDa LIP (liver-enriched transcriptional inhibitory protein), and a 14-kDa isoform (1). Due to the N-terminal truncation of the isoforms, both LIP and the 14-kDa isoform lack most of the transactivation domain, and LIP in particular has been proposed to act as a dominant negative inhibitor of C/EBP-mediated transcription (10). There are several lines of evidence that LIP can inhibit the transcriptional activities of LAP and other C/EBPs. Ectopic expression of LAP in 3T3-L1 cells stimulates adipocyte differentiation; however, overexpression of LIP in these cells inhibits preadipocyte conversion (45). This effect most likely occurs through heterodimerization of LIP with the other isoforms of C/EBPβ or with C/EBPδ, exerting a dominant negative effect on transcription. It has also been shown that LIP can attenuate the transcriptional activity of LAP at substoichiometric amounts (10). However, a recent report showed that overexpression of LIP in COS-1 cells leads to increased expression of a chloramphenicol acetyltransferase reporter construct containing the C/EBP binding site of the α1-acid glycoprotein promoter (13). Whether this mechanism involves direct transactivation of this promoter by LIP or an effect on a negative regulator of this promoter has not been determined. Although the targets of C/EBPβ LAP and LIP isoforms in vivo are not yet fully known, the fact that these isoforms have different functions suggests that the LIP/LAP ratio is important in C/EBP-mediated gene expression.
There are several situations in which the LIP/LAP ratio has been reported to change in the liver. The alteration of this ratio under certain conditions implies that it is regulated and indicates that the LIP/LAP ratio is functionally important for these processes. Examples include the acute-phase response to inflammation (1), liver development (11), and liver regeneration (38). In all of these cases, LIP levels increase transiently, resulting in an increase in the LIP/LAP ratio. In addition, we recently found that newborn C/EBPα−/− animals have a greatly altered LIP/LAP ratio in the liver (3). Wild-type newborn livers express high levels of the low-molecular-weight LIP isoform of C/EBPβ and low levels of the high-molecular-weight LAP isoform. On the contrary, newborn livers from C/EBPα−/− animals express a reduced amount of LIP and a high level of LAP. Given these observations, we decided to investigate the mechanism(s) by which C/EBPα may regulate C/EBPβ isoform generation in the liver. In this report, we present evidence for two mechanisms of regulation of the C/EBPβ LIP/LAP ratio in the liver: a mechanism that is dependent on alternative translation as well as a novel mechanism that is dependent on C/EBPα and involves proteolytic cleavage of C/EBPβ.
MATERIALS AND METHODS
Animals and tissue collection.
C/EBPα−/− (null) mice and wild-type littermates were sacrificed immediately after birth or at the appropriate gestational age. Liver, lung, and brown adipose tissues from the newborn animals were harvested and immediately frozen in liquid nitrogen. Tissues were stored at −80°C until proteins were isolated for analysis.
Isolation of cytoplasmic and nuclear proteins.
Nuclei were isolated as previously described (37, 39). In brief, the tissue was homogenized in buffer A (25 mM Tris-HCl [pH 7.5], 50 mM KCl, 2 mM MgCl2, 1 mM EDTA, 5 mM dithiothreitol [DTT]) containing 100 μM leupeptin, 2 μg of aprotinin per ml, and 1 mM phenylmethylsulfonyl fluoride (PMSF). In some experiments, 50 μM chymostatin was also included in buffer A. (Chymostatin was not included in nuclear extract isolations to be used as a source for the proteolytic cleavage of C/EBPβ, as it is an inhibitor of this process.) The nuclei were pelleted by centrifugation at 7,000 × g for 10 min and washed once with buffer A. The cytoplasmic extract (supernatant) was stored frozen at −80°C. Nuclear proteins were then isolated by one of two procedures. For isolation of total nuclear protein without extraction, the pelleted nuclei were directly resuspended in an equal volume of sodium dodecyl sulfate (SDS) loading buffer and heated to 95°C for 30 min. For preparation of a nuclear extract by our standard method of high-salt extraction (39), the pelleted nuclei were resuspended in buffer B (25 mM Tris-HCl [pH 7.5], 0.42 M NaCl, 1.5 mM MgCl2, 0.5 mM EDTA, 1 mM DTT, 25% sucrose) containing 100 μM leupeptin, 2 μg of aprotinin per ml, and 1 mM PMSF. This mixture was incubated for 15 min on ice and then centrifuged at 7,000 × g for 10 min. The nuclear extract (supernatant) was stored at −80°C.
Western blot analysis.
Western analysis was carried out as previously described (39). Protein (50 to 100 μg) was loaded onto a 12 to 15% polyacrylamide gel containing 0.1% SDS. The proteins were electroblotted onto a nitrocellulose membrane (0.22-μm-pore size; Bio-Rad) after electrophoresis. The blots were blocked with a solution of TBST (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.05% Tween 20) containing 2% bovine serum albumin and 10% nonfat dry milk for 1 h at room temperature. The blots were then washed several times with TBST plus 1% nonfat dry milk. Conditions for incubation with primary and secondary antibodies were optimized for each antibody. Primary antibodies used for Western analysis included serum specific for C/EBPβ (polyclonal serum C-19; Santa Cruz Biotechnology), serum specific for the FLAG epitope (polyclonal serum D-8; Santa Cruz), and serum specific for human C/EBPα (polyclonal serum B9 [39]). To examine the relative amounts of proteins loaded in the lanes, blots were reprobed with antibodies to β-actin (monoclonal serum AC-74; Sigma) after being stripped in a solution containing 100 mM Tris-HCl (pH 7.5), 2% SDS, and 14 mM β-mercaptoethanol. Secondary antibodies used were either goat anti-rabbit–horseradish peroxidase (HRP) or goat anti-mouse–HRP antibodies (both from Santa Cruz). Proteins were detected with an ECL kit from Amersham, and Western blots were quantitated with a Molecular Dynamics personal densitometer and Molecular Dynamics ImageQuant software.
In vitro transcription-translation.
In vitro transcription-translation of C/EBPβ and p21 was carried out in the presence of 35S-methionine by use of a TNT reticulocyte lysate kit from Promega. When needed, the translation system was altered by the addition of 0.2 to 1.0 μg of cytoplasmic or nuclear extracts from livers of C/EBPα wild-type or null mice or, alternatively, cytoplasmic extracts from regenerating mouse or rat liver. The following constructs were used in this system: pSCT-LAP, pSCT-LAPΔ1, pSCT-LAPΔ2, and pSCT-LAPΔ3 (generous gifts from U. Schibler and P. Descombes) and pBS-p21(sdi1) (39). For more sensitive detection of radiolabeled products, immunoprecipitation of C/EBPβ was carried out with antibodies specific for the C terminus of C/EBPβ to detect all isoforms (C-19). Alternatively, antibodies specific for the N terminus of C/EBPβ were used for detection of the FL isoform only (a gift from S.-C. Lee). For immunoprecipitation of in vitro-translated p21 protein, polyclonal antiserum H-164 (Santa Cruz) was used. Immunoprecipitation reactions were carried out by incubating diluted in vitro-translated proteins (10 mM Tris-HCl [pH 7.5], 125 mM NaCl) with specific antibodies and protein A-agarose overnight at 4°C. Following immunoprecipitation and several washes with buffer, proteins were run on SDS-polyacrylamide gels, transferred to membranes, and visualized by autoradiography.
In vitro cleavage assay.
For in vitro cleavage reactions, immunoprecipitated in vitro-translated full-length C/EBPβ or p21 protein was incubated with 0.2 to 1.0 μg of nuclear extract from C/EBPα wild-type or null livers for 30 min at 37°C, unless otherwise indicated. Other cleavage reactions were carried out with the same amount of nuclear extract from developing embryonic liver, newborn lung, newborn brown adipose tissue, or quiescent adult mouse or rat liver tissue. Alternatively, immunoprecipitated C/EBPβ was incubated with 0.001 to 0.70 U of m-calpain protease (Sigma). Certain cleavage reactions were carried out in the presence of protease inhibitors at the following concentrations: 100 μM leupeptin, 2 μg of aprotinin per ml, 1 mM PMSF, 50 μM chymostatin, 1 μM pepstatin A, 10 mM EDTA, 10 mM EGTA, 10 μM E-64, 10 μg of cathepsin inhibitor I per ml, 3.8 mg of N-acetyl-Leu-Leu-norleucinal (calpain inhibitor I) per ml, 20 mM lactacystin, 300 μM MG132, or 300 μM MG115. Following the incubation, the reaction was stopped by the addition of SDS loading buffer, and the samples were heated to 95°C for 5 min. Proteins were then run on SDS–12 to 15% polyacrylamide gels, transferred to membranes, visualized by autoradiography, and quantitated by densitometry as described above.
Cell culture and transfection.
HT-1 cells, which conditionally express C/EBPα under the control of the lac repressor system, were previously described (39). The B4 and B5 cell lines are mouse embryonic fibroblasts derived from C/EBPβ heterozygous or null mice, respectively, by previously described methods (14). Transient transfection was carried out at 30 to 50% confluency with the Fu gene transfection system (Boehringer Mannheim Biochemicals). Cells were transfected with either the wild-type C/EBPβ construct (FL; described above) or a LIP mutant construct (MT20-FLAG; a generous gift from John Papaconstantinou). For studies involving the effect of C/EBPα on the cleavage of C/EBPβ, HT-1 cells were treated with 10 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to induce C/EBPα at the time of transfection. Control cells were treated with 10 mM glucose. At 24 h after transfection, cells were collected by scraping into phosphate-buffered saline, followed by centrifugation at 400 × g for 5 min. When whole cells were collected, the cell pellet was directly resuspended in an equal volume of SDS loading buffer and heated to 95°C for 30 min. Total nuclear protein was isolated by direct lysis of nuclei in SDS loading buffer as described above.
Protein elution and band-shift assay.
To analyze the binding activities of truncated C/EBPβ isoforms, HT-1 cells were transfected with the C/EBPβ LIP mutant construct, and C/EBPα expression was induced by IPTG (39). Because maximal levels of C/EBPα expression in HT-1 cells are observed 8 to 12 h after IPTG stimulation, proteins were isolated 24 h after transfection and IPTG addition. Fractionation of the proteins was carried out as previously described (24). Briefly, 500 to 1,000 μg of protein was separated on an SDS–12% polyacrylamide gel and transferred to a nitrocellulose filter. The membrane was cut into fractions according to molecular weight, and proteins were eluted in 200 to 300 μl of elution buffer (25 mM Tris-HCl [pH 7.5], 50 mM KCl, 1% Triton X-100, 2 mM MgCl, 1 mM DTT). Each elution fraction (5 μl) was used for gel-shift analysis with a bZIP probe containing the C/EBP consensus binding site (44). The conditions for the gel-shift assay were described in our previous publications (39, 44). For the supershift assay, antibodies to C/EBPβ (C-19) or to the FLAG epitope (D-8) were added to the binding reaction mixture before the probe addition.
RESULTS
Analysis of C/EBPβ isoform production in a cell-free system.
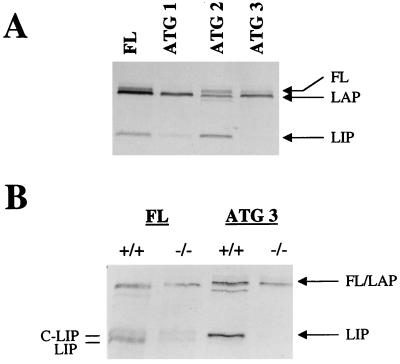
In order to carefully analyze the mechanism(s) of generation of C/EBPβ isoforms, as well as the putative role of C/EBPα in the regulation of this process, we used an in vitro coupled transcription-translation system in reticulocyte lysate. In vitro translation of a wild-type C/EBPβ construct (10) yielded three products: FL, LAP, and LIP (Fig. 1A, first lane). As expected, LAP and LIP were N-terminally truncated products, since they were immunoprecipitated with antibodies specific to the C/EBPβ C terminus (see Materials and Methods). The molecular weight of each C/EBPβ isoform produced in the reticulocyte lysate was consistent with that predicted from the amino acid sequence and was also consistent with the C/EBPβ of isoforms detected in the liver (3). When the C/EBPβ construct was not included in the reticulocyte lysate system, no C/EBPβ-immunoreactive products were produced, ruling out the possibility that endogenous C/EBPβ mRNAs are responsible for protein production in this system (data not shown). In vitro translation of various C/EBPβ mutant constructs (10) revealed that mutation of ATG 1, ATG 2, and ATG 3 led to the disappearance of FL, LAP, and LIP, respectively (Fig. 1A). It is apparent that mutation of ATG 2 led to the production of a band with a mobility slightly higher than that of LAP; however, the origin of this band is unknown and has not been investigated in this report. The disappearance of LIP with mutation of ATG 3 indicated that LIP is produced in the reticulocyte lysate system by AUG-dependent translation and not by a mechanism such as proteolysis of C/EBPβ.
FIG. 1.
In vitro translation of C/EBPβ mRNA in the presence of C/EBPα wild-type liver extracts leads to an increase in LIP production. (A) Mutation of the first, second, or third ATG in the C/EBPβ construct leads to the disappearance of FL, LAP, and LIP, respectively. The positions of the isoforms are indicated at the right. (B) In vitro translation of C/EBPβ in the presence of cytoplasmic extracts from C/EBPα wild-type (+/+) or null (−/−) mice. Translation of the wild-type C/EBPβ construct (FL) is shown in the left two lanes, and translation of the ATG 3 (LIP mutant) construct is shown in the right two lanes. The regions of the expected isoforms are indicated at the right, and the doublet for LIP is labeled at the left as C-LIP and LIP (see the text). For these experiments, in vitro-translated products were immunoprecipitated with antibodies specific for the C terminus of C/EBPβ (C-19).
Addition of extracts from C/EBPα wild-type livers, but not from C/EBPα null livers, increases LIP production in a cell-free system.
To investigate the mechanism(s) of C/EBPβ isoform generation in the liver, we examined whether nuclear or cytoplasmic proteins present in the newborn liver could influence C/EBPβ isoform generation. Since very little LIP is observed in nuclei from livers of newborn C/EBPα−/− mice compared with their wild-type littermates (3), we investigated whether a factor present in the wild-type newborn liver cytoplasm could increase the generation of LIP. In vitro translation of C/EBPβ was carried out in the presence of cytoplasmic extracts from C/EBPα null or wild-type livers. C/EBPβ translation in the presence of wild-type cytoplasmic extracts led to higher levels of LIP than were produced in the presence of null cytoplasmic extracts (Fig. 1B, first and second lanes). Subsequently, we determined that the LIP-generating activity was even more pronounced with nuclear extracts. Due to the increased activity in nuclear extracts relative to cytoplasmic extracts, further experiments were carried out with nuclear extracts.
Our results indicated that a factor in both the cytoplasm and the nucleus of C/EBPα wild-type mouse livers could increase LIP production in vitro, but this factor was not active in the livers of C/EBPα null littermates. To determine whether this factor increases the production of LIP by a translational mechanism, we carried out the same experiments with a LIP ATG mutant construct (ATG 3). Unexpectedly, the increase in LIP production was not abrogated by mutation of the third ATG (Fig. 1B, third lane). This observation indicates that, unlike the production of LIP in the reticulocyte lysate, the addition of extracts from newborn mouse livers yielded an increase in LIP production by a mechanism other than AUG-dependent initiation of translation. This increase was not due to the addition of C/EBPβ mRNA present in liver extracts, since the addition of liver extracts from C/EBPβ-deficient animals gave the same result (data not shown). Furthermore, the increase in LIP production was C/EBPα dependent, because C/EBPα null liver extracts were not capable of upregulating LIP generation in vitro. This finding is consistent with our observations of altered C/EBPβ isoforms in the C/EBPα−/− mouse model (3).
FL and LAP can be proteolytically cleaved to generate C-LIP.
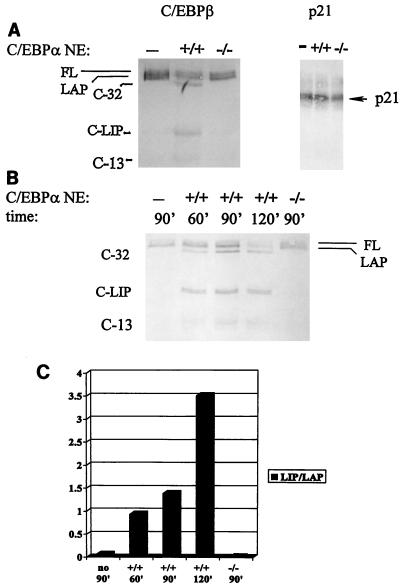
To determine if the C/EBPα-dependent increase in LIP production was due to proteolytic cleavage of C/EBPβ, we first carried out in vitro translation of C/EBPβ and then immunoprecipitated the product with antibodies specific for the C/EBPβ N terminus (18). FL and a small amount of LAP were the only isoforms detectable by this method (Fig. 2A, lane 1, and data not shown). When the immunoprecipitated products were incubated with liver extracts from wild-type newborn mice, proteolytic cleavage of the high-molecular-weight isoforms occurred, yielding a LIP-like molecule as a major cleavage product (C-LIP) as well as two additional cleavage products, C-32 and C-13 (Fig. 2A). Both FL and LAP could be substrates for the specific proteolytic cleavage. When compared directly, the mobility of the LIP isoform produced by translation in the in vitro system was very similar to that of C-LIP generated by proteolysis (Fig. 1B). Because the mobilities of LIP and C-LIP were also very similar when detected with antibodies specific for the C terminus of C/EBPβ (see below), it is likely that the site of cleavage was close to the methionine residue which would mark the N terminus of LIP. The pattern of cleavage products generated from FL immunoprecipitated following translation was identical to the pattern of isoforms detected with the addition of liver extracts directly to the in vitro translation system (compare Fig. 1B and 2A) and also matched the pattern of isoforms detected in vivo (3). Invariably, proteolytic activity was absent in C/EBPα null liver extracts (more than five animals were tested).
FIG. 2.
Nuclear extracts from newborn wild-type livers contain a C/EBPα-dependent specific proteolytic activity that produces C-LIP. (A) In vitro-translated FL is cleaved by nuclear extracts (NE) from livers of C/EBPα wild-type mice (+/+) but not by those from C/EBPα null mice (−/−). Three major cleavage products are observed: C-32, C-LIP, and C-13 (shown at the left). On the contrary, the stability of in vitro-translated p21 protein is not affected by incubation with either wild-type extracts or null extracts. (B) Representative experiment showing an analysis of the relative amount of C/EBPβ cleavage over time in the presence of no extract (−), nuclear extracts from C/EBPα wild-type mice (+/+), or nuclear extracts from C/EBPα null mice (−/−). (C) Densitometric analysis of C/EBPβ cleavage over time (shown as the LIP/LAP ratio). The graph is representative of three independent experiments.
To demonstrate that the proteolytic cleavage of C/EBPβ was not due to nonspecific, global protein degradation, we also carried out in vitro translation of an unrelated protein, p21sdi-1/cip-1/waf-1, and incubated the immunoprecipitated product with liver extracts. Figure 2A shows that, under conditions appropriate for the cleavage of C/EBPβ, p21 protein is stable in the presence of either wild-type or C/EBPα null liver extracts. This result suggested that the C/EBPα-dependent proteolysis of C/EBPβ was specifically targeted and did not represent global proteolysis. Prolonged incubation with C/EBPα wild-type extracts led to further cleavage of C/EBPβ (Fig. 2B). Although the lane containing C/EBPβ incubated with wild-type extracts for 90 min was slightly overloaded in Fig. 2B, a summary of experiments measuring the LIP/LAP ratio with increasing time of incubation is shown in Fig. 2C. For the purpose of quantitation, LIP/LAP ratios indicate the ratio of LIP to FL and LAP isoforms combined, since both of these latter isoforms are substrates for specific cleavage. It is notable that the addition of a mixture of C/EBPα null and wild-type extracts had the same effect on C/EBPβ isoforms as did the addition of wild-type extracts alone (data not shown). This result suggested that the C/EBPβ-specific protease was not present in the C/EBPα null extracts rather than that there was a lack of inhibitory activity for this protease in the wild-type extracts.
The proteolytic pathway of LIP generation is specific for prenatal and newborn livers.
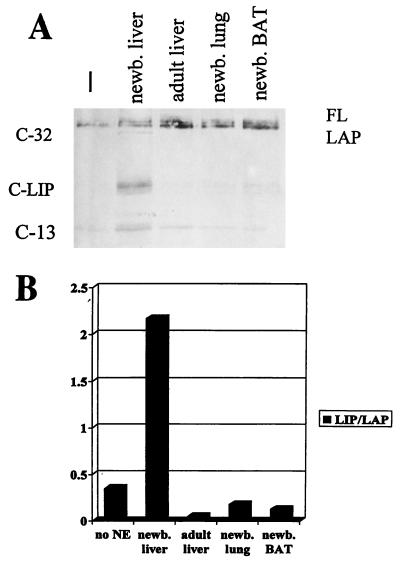
C/EBPα is a tissue-specific transcription factor, high levels of which are detected in liver, lung, and adipose tissues (2). We tested nuclear extracts from these tissues for the ability of C/EBPα to regulate the proteolytic cleavage of C/EBPβ (Figure 3). Newborn mouse liver was the only tissue tested in which significant proteolytic cleavage of in vitro-translated C/EBPβ was detected. Even though C/EBPα is expressed at high levels in quiescent mouse and rat liver tissues and in differentiated adipose tissue (2, 38), the factor which specifically cleaves C/EBPβ is not activated in these tissues. This result is supported by the fact that LIP/LAP ratios are not altered in lung and brown adipose tissues in C/EBPα−/− mice (3). These observations suggested that C/EBPα expression is necessary but not sufficient for the regulation of LIP production by specific cleavage and that temporal control during liver development is likely to play a role in the regulation of this activity.
FIG. 3.
Proteolytic cleavage of C/EBPβ is specific to the newborn liver. (A) C/EBPβ cleavage assay with no nuclear extract and nuclear extracts from newborn (newb.) liver, adult liver, newborn lung, and newborn brown adipose tissue (BAT). All of these tissues were from wild-type mice. (B) Densitometric analysis of the relative amount of cleavage of C/EBPβ in the tissues shown above. The graph is representative of three or more experiments done with tissues from different animals. NE, nuclear extract.
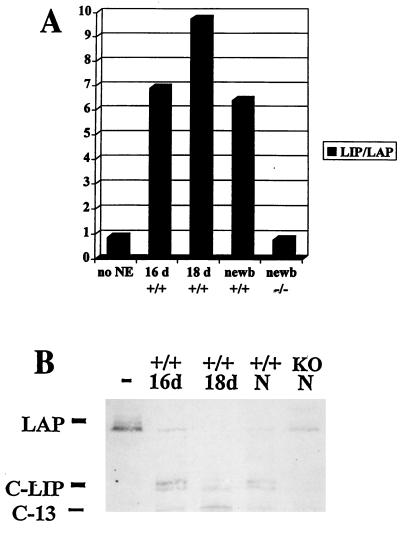
Since proteolytic cleavage of C/EBPβ in neonatal liver is C/EBPα dependent, we determined whether specific cleavage of C/EBPβ was activated in the developing liver when C/EBPα expression was induced. It has been reported that C/EBPα mRNA is expressed at relatively low levels at embryonic day 16, increases to a maximum at day 18, and then decreases slightly just before birth (2, 16). We tested liver nuclear extracts from wild-type animals during these stages of development for the ability to cleave C/EBPβ and found that the activation of specific cleavage occurred in the prenatal liver when C/EBPα expression was induced (Fig. 4). From these experiments, it is apparent that C-LIP can be resolved as a doublet, consistent with other observations made with cell lines indicating that there may be two C-LIP molecules with similar mobilities (see below). The proteolytic activity present at these stages of development yielded products identical to those produced by liver extracts from newborn animals (Fig. 4B). This observation was consistent with the hypothesis that C/EBPα is expressed at the proper time in development to contribute to regulation of the proteolytic cleavage of C/EBPβ.
FIG. 4.
Cleavage of C/EBPβ occurs in the liver before birth. (A) Densitometric analysis showing that cleavage is activated in the livers of C/EBPα wild-type (+/+) mice at the following times during development: 16 days of gestation (16 d), 18 days of gestation (18 d), and newborn animals (newb). No cleavage was detected in C/EBPα null (−/−) livers. The graph represents an average of three independent experiments. NE, nuclear extract. (B) Representative example of a C/EBPβ cleavage assay with tissues from wild-type mice at the same times during development. The positions of LAP, C-LIP, and C-13 are shown. N, newborn animals; KO, C/EBPα null extracts.
C/EBPα induces proteolytic cleavage of C/EBPβ to generate C-LIP in cultured cells.
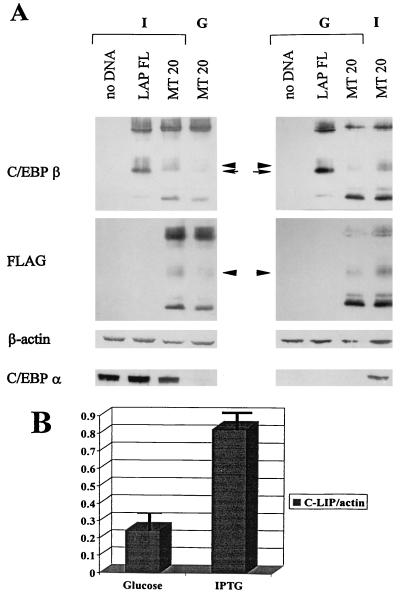
To further investigate the mechanisms of C/EBPβ isoform generation, as well as the role of C/EBPα in this process, we used a cell line in which both LAP and LIP are expressed and in which C/EBPα expression can be controlled. The HT-1 cell line is a stable clone (derived from human fibrosarcoma line HT1080) which conditionally expresses C/EBPα under the control of the lac repressor system. Cells treated with IPTG are induced to express C/EBPα at high levels, whereas glucose-treated control cells express little or no C/EBPα (39). We transfected HT-1 cells with wild-type or mutant C/EBPβ constructs and added glucose or IPTG to the media at the time of transfection. To distinguish the mechanisms of cleavage and translation, we used the MT20-FLAG construct bearing an ATG→TTG mutation at the LIP translation initiation site as well as a C-terminal FLAG epitope (13). Western blot analysis with antibodies specific for the C terminus of C/EBPβ showed that an immunoreactive band (C-LIP) generated by the mutant construct was present in nuclei of transfected cells (Fig. 5A, MT20). This band migrated with a mobility slightly lower than that of LIP produced from other constructs, partially due to the C-terminal FLAG epitope. Endogenous C/EBPβ did not contribute to the C/EBPβ isoforms observed here, as it was below the level of detection in these experiments (Fig. 5A, no DNA). Reprobing the same filter with antibodies to the FLAG epitope confirmed that this band was an N-terminally truncated protein specific for the MT20 C/EBPβ construct. Since this construct had no ATG for LIP production by translation, our data verified that C-LIP can be generated in cultured cells by an ATG-independent mechanism. This observation was in agreement with our finding that C/EBPβ can be proteolytically cleaved in vitro to generate C-LIP. All of these experiments were carried out by direct lysis of whole nuclei (isolated with chymostatin; see below) in SDS loading buffer, indicating that proteolysis of C/EBPβ occurs within the cells and not during the preparation of nuclear extracts. Additionally, cleavage of C/EBPβ to generate C-LIP was also observed in nuclear extracts, whole nuclei, and whole-cell preparations of mouse embryonic fibroblast cell lines derived from C/EBPβ heterozygous or null animals (B6 and B7 lines, respectively; data not shown).
FIG. 5.
C/EBPα induces specific proteolytic cleavage of C/EBPβ in cell cultures. (A) Western analysis of HT-1 whole nuclei. Cells were transfected with the wild-type (LAP FL) or the LIP mutant (MT20) construct. At the same time, cells were treated with 10 mM IPTG (I) to induce the expression of C/EBPα or with 10 mM glucose (G) as a control. The left and right columns represent two experiments showing that C/EBPα induces specific cleavage of C/EBPβ to generate C-LIP. (First [top] panel) Western analysis for C/EBPβ. The positions of LIP (arrow) and C-LIP (arrowhead) are shown in the center. C-LIP is slightly shifted, partially because of the presence of a FLAG epitope on the C terminus of the MT-20 construct. (Second panel) The same filter was reprobed with antibodies to FLAG. Again, the position of C-LIP is shown with an arrowhead. (Third panel) The same filter was reprobed again with antibodies to β-actin as a control for protein loading. (Fourth [bottom] panel) The same samples were loaded onto a second gel, and the filter was probed with antibodies specific for C/EBPα. (B) Densitometric analysis of three independent experiments shows a two- to fivefold induction of C/EBPβ cleavage with the overexpression of C/EBPα (IPTG-treated cells). Error bars indicate standard deviations.
We previously observed an increase in LIP production in HT-1 cells in which C/EBPα was induced by IPTG treatment (3). This finding further implicated C/EBPα in the regulation of C/EBPβ isoform production; however, the mechanism of LIP upregulation was unknown. Because we have evidence that C-LIP can be produced by proteolytic cleavage in vitro and in cell cultures and that C/EBPα may be involved in this process in vivo, we examined whether cleavage was responsible for the increased LIP production in IPTG-treated HT-1 cells. We found that when C/EBPα was induced by IPTG treatment, cleavage of C/EBPβ to C-LIP was increased compared to that in glucose-treated control cells (Fig. 5A, compare MT20 glucose with MT20 IPTG). Reprobing the same filter with antibodies to β-actin showed that total protein levels in the samples were similar; thus, the increase in LIP production was not due to aberrant protein loading.
Quantitation of these results by densitometry (ImageQuant) showed that the increase in LIP generation by cleavage was reproducibly two- to fivefold (Fig. 5B). It is also notable that in the HT-1 cell line, as well as in the B6 and B7 cell lines (data not shown), translation is likely to be a major mechanism by which LIP is generated, since the majority of LIP disappeared when the LIP ATG mutant construct was used (Fig. 5A, compare LAP FL with MT20). Taken together, the data obtained in the in vivo mouse model, the in vitro system, and cultured cell models implicate C/EBPα in the regulation of C/EBPβ LIP isoform production by specific proteolytic cleavage. It is interesting to note that when cleavage of C/EBPβ was activated in HT-1 cells by C/EBPα induction, the C/EBPα protein was stable (Fig. 5A) and no C/EBPα cleavage products were detectable (data not shown). These results indicate that the C/EBPα-dependent cleavage activity is specific for C/EBPβ; therefore, C/EBPα does not seem to regulate its own proteolysis. This notion is consistent with the observation that newborn mouse liver, which has high levels of proteolytic activity for C/EBPβ, did not display an increased ratio of 30-kDa to 42-kDa C/EBPα isoforms when compared to adult liver, in which proteolysis is not activated (data not shown).
C-LIP molecules generated by proteolytic cleavage bind to DNA and are able to form heterodimers with C/EBP family members.
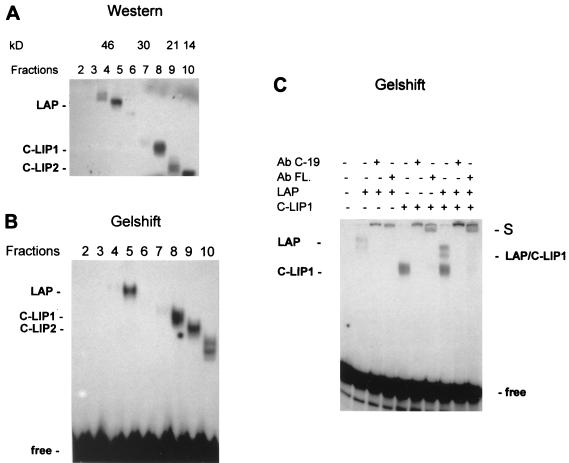
C-LIP molecules are C-terminal cleavage products of C/EBPβ, since they interact with antibodies specific for the C terminus of C/EBPβ. Like LIP, C-LIP presumably contains both the DNA binding domain and the leucine zipper region but lacks the transactivation domain due to N-terminal truncation. To examine whether C-LIP binds to the C/EBP consensus site and whether C-LIP forms heterodimers with FL and LAP, HT-1 cells were transfected with the MT20-FLAG construct. As described above, this construct cannot produce LIP molecules by translation but is able to generate the cleavage product, C-LIP. Whole nuclei were isolated from cells 24 h after transfection, and the proteins were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose filter. The membrane was cut crosswise in fractions on the basis of molecular weight, and proteins were eluted as described previously (24). Separation of the C/EBPβ proteins was examined by Western blotting with antibodies to C/EBPβ (C-19). Figure 6A shows that FL and LAP molecules were located in fractions 4 and 5, corresponding to 35 to 46 kDa. The truncated isoform generated by cleavage (C-LIP) was detected in fractions 8 and 9, corresponding to 21 to 24 kDa. These isoforms were designated C-LIP1 and C-LIP2. The presence of two cleavage products was in agreement with the pattern of cleavage activity in nuclear extracts from the developing liver (Fig. 4B). A 13- to 14-kDa band was also observed (see below).
FIG. 6.
Truncated C/EBPβ isoforms generated by cleavage bind to the C/EBP consensus site and form heterodimers with full-length C/EBPβ isoforms. (A) Western analysis of elution fractions containing proteins having different molecular masses. HT-1 cells were transfected with the MT20-FLAG construct, and nuclear proteins were separated on the basis of molecular mass as described in Materials and Methods. Each fraction (shown on the top) was analyzed by SDS–12% PAGE with antibodies to C/EBPβ (C-19). (B) Each fraction (5 μl) was analyzed by a gel-shift assay as described in Materials and Methods. (C) Gel-shift analysis of the C-LIP molecule. Antibodies (Ab) to C/EBPβ (C-19) or to the FLAG epitope (FL.) were added to the binding reaction mixture before the addition of the bZIP probe. Positions of supershifted complexes (S) and LAP–C-LIP1 heterodimers are indicated.
To examine the DNA binding activities of the fractionated proteins, gel-shift analysis of each fraction was carried out with a bZIP probe containing a high-affinity C/EBP binding site (44). Figure 6B shows that C-LIP1 and C-LIP2 (fractions 8 and 9) bound to the C/EBP consensus site. We also observed that the 13- to 14-kDa isoforms of C/EBPβ (fraction 10) were capable of binding to the consensus site, producing two complexes. These isoforms might be either translational or cleavage products, since the fourth AUG of C/EBPβ was retained in the construct used for these experiments. Initiation at this AUG would give a predicted product of 14 kDa (1). However, we found that a 13-kDa product was also generated by cleavage of C/EBPβ (C-13). Therefore, it is possible that a mixture of these products was represented by the appearance of two complexes from fraction 10.
To demonstrate that the binding activities in the eluted fractions represented C/EBPβ isoforms, antibodies specific for C/EBPβ (C-19) and the FLAG epitope were incorporated into the binding reaction mixture. Figure 6C shows that the LAP and C-LIP1 complexes were supershifted with both C/EBPβ and FLAG epitope antibodies. C-LIP2 complexes were also supershifted under the same conditions (data not shown). Mixing the fractions containing LAP and C-LIP formed complexes consisting of LAP–C-LIP heterodimers, which were also supershifted with antibodies to C/EBPβ and the FLAG epitope (Fig. 6C, lanes 8 to 10). Thus, we conclude that C-LIP molecules generated by cleavage are able to bind to the C/EBP consensus site and can also form heterodimers with LAP. These molecules can also form heterodimers with C/EBPα (data not shown). Therefore, it is likely that truncated C-LIP molecules have a function similar to that proposed for LIP: to operate as dominant negative isoforms that inhibit the transcriptional activities of the C/EBP proteins (10).
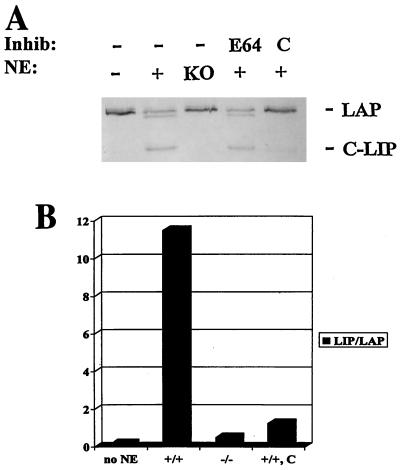
C/EBPα-dependent cleavage of C/EBPβ is a result of activation of a protease that is sensitive to chymostatin.
To characterize the proteolytic activity present in wild-type liver extracts, we carried out cleavage assays of C/EBPβ in the presence of several protease inhibitors. Results from these studies showed that complete inhibition of C/EBPβ proteolysis was achieved with chymostatin, a serine and cysteine protease inhibitor (Fig. 7). We also observed inhibition of C/EBPβ cleavage with relatively high concentrations of LLnL, also referred to as calpain inhibitor I (3.8 mg/ml; data not shown). No significant inhibition was observed with the protease inhibitors leupeptin, aprotinin, PMSF, pepstatin A, E-64, cathepsin inhibitor I, MG132, MG115, and lactacystin (Fig. 7 and data not shown). This result indicated that inhibitors of lysosomal proteases and specific inhibitors of the proteasome are not effective in protecting C/EBPβ from cleavage. The addition of either EDTA or EGTA also did not inhibit the proteolytic activity (data not shown). These results indicated that C/EBPβ is cleaved by a C/EBPα-dependent protease that is sensitive to chymostatin and, to a lesser extent, to LLnL. To further characterize the protease activity, we carried out cleavage assays of C/EBPβ under several different sets of conditions. Although the protease was active under a range of pH conditions, optimal activity was observed at pH 7.5 to 8.8 (data not shown). At pH 7.5, the cleavage activity was not detectable at 4°C but was substantial from 25 to 55°C. Incubation of the extract at 65°C or higher eliminated all activity (data not shown). Proteolytic activity was also stable over a range of salt conditions, with optimal activity at 250 to 400 mM NaCl (data not shown).
FIG. 7.
The protease responsible for the cleavage of C/EBPβ is sensitive to chymostatin. (A) C/EBPβ cleavage assay in the presence of C/EBPα wild-type or null nuclear extracts (NE) and protease inhibitors (Inhib). First lane, no extract or inhibitor added. Second lane, wild-type extract only. Third lane, null (KO) extract only. Fourth lane, wild-type extract plus 10 μM E-64. Fifth lane, wild-type extract plus 50 μM chymostatin (C). (B) Densitometric analysis of C/EBPβ cleavage in the absence and presence of the protease inhibitor chymostatin (C). The graph is representative of five independent experiments. +/+, C/EBPα wild-type extracts; −/−, C/EBPα null extracts.
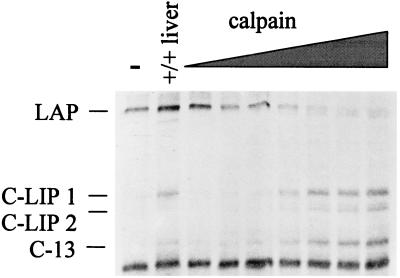
Since the proteolytic activity described in this report is sensitive to both chymostatin and LLnL (calpain inhibitor I) but not to specific inhibitors of the proteosome, such as lactacystin, we decided to investigate whether calpains could be involved in the cleavage of C/EBPβ. For these experiments, we used a commercially available preparation of m-calpain (80K subunit purified from rabbit skeletal muscle). The addition of increasing concentrations of m-calpain to C/EBPβ cleavage assay mixtures resulted in the accumulation of C/EBPβ cleavage products identical to those produced from the C/EBPα-dependent proteolytic activity found in newborn mouse liver (Fig. 8). We also observed that the m-calpain activity was inhibited by both chymostatin and LLnL (data not shown). These results indicated that this preparation of m-calpain contains an activity that is similar to the proteolytic activity found in newborn mouse liver.
FIG. 8.
A calpain protease can cleave C/EBPβ to generate C-LIP. C/EBPβ cleavage assay in the presence of increasing amounts of the 80K subunit of m-calpain. First lane, no protease added. Second lane, wild-type liver nuclear extract. Third through ninth lanes, 0.001, 0.003, 0.009, 0.025, 0.08, 0.20, and 0.70 U of m-calpain, respectively. LAP and cleavage products are labeled at the left.
The C/EBPβ LIP isoform is generated by a translation-dependent mechanism during liver regeneration.
In this report, we have shown with several different systems that C/EBPα is involved in the activation of a pathway of cleavage, possibly through the activation of a calpain protease, that leads to the conversion of high-molecular-weight isoforms of C/EBPβ to C-LIP. However, our experiments with cell cultures indicated that a translational mechanism for generating LIP also exists (Fig. 5A) and is, in fact, the major mechanism in these cell lines. LIP generation by translation is also in agreement with other reports (10, 13). Therefore, we examined whether there are other situations in the liver in which the upregulation of LIP occurs by a translation-dependent mechanism. Since we can distinguish the processes of translation and cleavage in vitro by using the LIP mutant construct, we tested another system in which C/EBPβ isoforms are altered: the process of liver regeneration. C/EBPβ is required for normal liver regeneration following partial hepatectomy (PH) (12). During the regeneration process, there is a transient increase in LIP production, leading to an increase in the LIP/LAP ratio (38).
To determine if a factor that could increase the translation of LIP is activated during liver regeneration we examined cytoplasmic extracts from regenerating rat and mouse livers by using the in vitro translation system. To distinguish translational control from the cleavage pathway, wild-type C/EBPβ and LIP mutant constructs were used in these experiments. The addition of cytoplasmic extracts from regenerating rat or mouse livers during translation led to the elevation of LIP levels relative to LAP levels with kinetics similar to those described in vivo (38). The production of LIP was induced by the addition of cytoplasmic extracts isolated 6 h after PH from rat livers (Fig. 9A) and 36 h after PH from mouse livers (Fig. 9B). Replacement of the wild-type C/EBPβ construct with the LIP ATG mutant construct (ATG 3) completely inhibited LIP induction. Furthermore, no protease activity was detectable when regenerating liver extracts were added to the in vitro C/EBPβ cleavage assay mixture (data not shown). These data clearly demonstrated the activation in the cytoplasm of regenerating liver cells of a factor that upregulates LIP by a translation-dependent mechanism. Taken together, our results suggest that there are two mechanisms for the generation of LIP: a translational mechanism and a novel cleavage pathway that is dependent on C/EBPα. Our data implicate C/EBPα as an important regulator of the C/EBPβ LIP/LAP ratio in the livers of newborn animals.
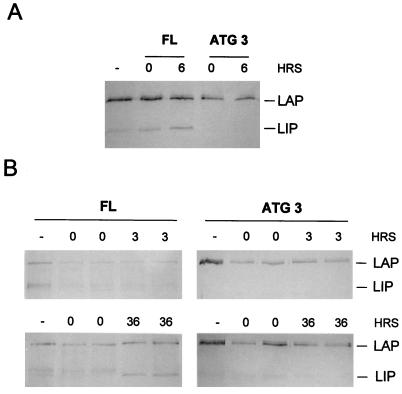
FIG. 9.
Induction of LIP during liver regeneration occurs by a translational mechanism. (A) In vitro translation of C/EBPβ in the absence (−) or presence of cytoplasmic extracts (CE) from regenerating rat livers. Extracts were added from rat livers at 0 or 6 h (HRS) after PH (times indicated above the gel). The left three lanes contain C/EBPβ translated from the wild-type C/EBPβ construct (FL), and the right two lanes contain C/EBPβ translated from the LIP mutant construct (ATG 3). The positions of LAP and LIP are indicated at the right. (B) In vitro translation of C/EBPβ in the presence of CE from regenerating mouse livers. Extracts were added from mouse livers at 0, 3, or 36 h after PH (times indicated above the gel). CE from two different animals were used in parallel. The left column depicts the use of the wild-type C/EBPβ construct (FL), and the right column depicts the use of the LIP mutant construct (ATG 3). The positions of LAP and LIP are indicated at the right.
DISCUSSION
That several protein isoforms can be generated from a single gene through the process of alternative splicing has been well described (for a recent review, see reference 8). However, some intronless genes produce different protein isoforms from a single mRNA species. In this paper, we provide evidence for two specific mechanisms for the production of the low-molecular-weight C/EBPβ isoform LIP. The first mechanism is dependent on translation initiation at the third AUG codon in C/EBPβ mRNA. This mechanism seems to be the major pathway by which LIP is generated in the cultured cell lines that we tested, since mutation of this ATG greatly reduced the amount of LIP produced. Similarly, mutation of this ATG completely inhibited the upregulation of LIP seen with the addition of cytoplasmic extracts from regenerating liver to an in vitro translation system. Our observations with both of these systems verified that LIP can be generated via translation initiation at the third AUG codon of C/EBPβ. Generation of the LIP isoform by translation is also in agreement with other reports in which C/EBPβ isoforms were examined with cultured cells (10, 13).
Our data showed that LIP can also be generated by a second mechanism: C/EBPα-dependent proteolytic cleavage of FL and LAP. We designated this product C-LIP to distinguish it from the LIP product that is generated by the translation-dependent mechanism. LIP and C-LIP have virtually indistinguishable properties. Like LIP, C-LIP is an N-terminally truncated isoform; they have similar molecular weights. Furthermore, C-LIP can form heterodimers with other C/EBP family members and bind to the C/EBP consensus site. For these reasons, it is likely that the function of C-LIP is equivalent to that of LIP. We also determined that the generation of C-LIP by specific proteolytic cleavage of C/EBPβ occurs within cells and is very unlikely to result from the isolation procedure, as was recently proposed (21).
We examined the proteolytic pathway of C-LIP generation and found that a key component of this mechanism is a dependence on C/EBPα. In several different systems, C/EBPα contk;1trolled production of the C-LIP molecule. First, C/EBPα−/− mice expressed reduced levels of LIP in the liver compared to their wild-type littermates (3). Second, extracts from C/EBPα wild-type livers but not C/EBPα null livers could cleave C/EBPβ to produce C-LIP in vitro. Third, overexpression of C/EBPα in cell cultures led to increased cleavage of FL and LAP to produce C-LIP. We also found that the specific cleavage activity occurred in the prenatal liver when C/EBPα was expressed during development. This correlation was consistent with the observation that C/EBPα regulated this process. Although C/EBPα is highly expressed in several tissues, including liver, lung, and adipose tissues (2, 38), the cleavage activity was found to be quite specific for prenatal and newborn livers. This finding suggested that C/EBPα is necessary but not sufficient for activation of the cleavage of C/EBPβ and that regulation of the protease responsible for this cleavage may require other transcription factors that are present in neonatal and newborn livers. The specificity of the cleavage activity for the newborn liver suggested that C/EBPβ isoforms have important roles during development. It is interesting that no cleavage activity was detectable in quiescent adult liver, despite high levels of C/EBPα in this tissue (38). Whether the cleavage of C/EBPβ leading to an increase in LIP production is involved in the proliferation of hepatocytes in the developing neonatal liver remains to be determined. However, high levels of LIP have been associated with mammary neoplasias and may contribute to cellular proliferation (27). Unlike the mechanism of alternative translation, the cleavage mechanism simultaneously decreases levels of LAP and FL while increasing levels of LIP. It is possible that these activities constitute fine-tuning of C/EBP-mediated transcription in the newborn liver. We also demonstrated that an approximately 13-kDa isoform of C/EBPβ can be generated by cleavage. This isoform may be similar to the previously reported 14-kDa isoform of C/EBPβ (1). We showed that this molecule can bind to DNA; however, because of N-terminal truncation, this isoform lacks the transactivation domain and may have a dominant negative function similar to that of LIP.
Identification of in vivo targets of all of these isoforms will aid in understanding the importance of the specific cleavage of C/EBPβ. The fact that this mechanism is controlled by another C/EBP family member, C/EBPα, implies a possible feedback mechanism for balancing transcriptional control in the newborn liver. It is also interesting to note that during liver regeneration following PH, when we observed only translation-dependent upregulation of LIP, C/EBPα was reduced to low levels (38). This finding correlated with the lack of C/EBPα-dependent cleavage activity at this time.
Although the activation of various transcription factors by posttranslational modification such as phosphorylation or dephosphorylation has been well investigated, little has been described about the regulation of transcription factors by proteolytic processing. One of the most well-characterized pathways in which specific proteolytic processing plays a major role in regulating the activity of a transcription factor is the NF-κB pathway. The p50 subunit is generated from a p105 precursor by a ubiquitin-mediated proteolytic processing event in the cytoplasm of most cells (6), and it has been reported that this processing event is cotranslational (20). Additionally, the NF-κB complex (p50-p65) is sequestered in the cytoplasm by inhibitory IκB proteins, and it is the proteolytic degradation of IκB that releases NF-κB proteins for subsequent nuclear localization and activation of target genes (22, 23, 31). It has been reported that targeted proteolysis of the nuclear receptor corepressor N-CoR is a cell-specific mechanism for regulating gene transcription (15). Although both of these pathways involve the regulation of transcriptional activators or repressors by pathways of proteasomal degradation, we report in this paper that the specific cleavage of C/EBPβ is not prevented by inhibitors of the proteasome, such as lactacystin, MG132, and MG115. This result suggested that the C/EBPα-dependent cleavage of C/EBPβ occurs by a mechanism independent of the proteasome. Nonproteosomal cleavage of transcription factors has also been reported to occur. For example, the bHLHzip factor USF, as well as a number of other transcription factors, is specifically cleaved by m-calpain, leaving the DNA binding and dimerization domains intact. The resultant protein can bind to DNA but can no longer activate transcription (42). In this report, we show that the cleavage of C/EBPβ also yields a product, C-LIP, that can bind to DNA but that lacks a transactivation domain. Taken together, these results suggest that targeted proteolysis of certain transcription factors may be an important mechanism for rapidly regulating the expression of target genes.
Although the enzyme responsible for C/EBPβ cleavage has not yet been purified, we have determined that C-LIP is generated by a protease that is sensitive to both chymostatin and LLnL. We have also shown that a commercially available preparation of m-calpain can cleave C/EBPβ to generate C-LIP. These data suggested that the C/EBPα-dependent cleavage of C/EBPβ may be due to the activation of a calpain protease in liver. Whether the proteolytic activity in the newborn liver is identical to that found in the calpain preparation is currently under investigation. The purification and identification of the enzyme from liver will provide a greater understanding of the mechanism of cleavage, as well as the regulation of the protease activity by C/EBPα.
ACKNOWLEDGMENTS
We thank B. Burgess-Beusse and other members of the Darlington laboratory for helpful discussions and critical reading of the manuscript. Additionally, we thank P. Descombes, U. Schibler, and J. Papaconstantinou for generously providing the constructs used in these experiments as well as J. Albrecht for providing mouse livers isolated after partial hepatectomy.
This work was supported by NIH grants GM55188 and AG00766 (to N.A.T.) and R01-DK53045 (to G.J.D.).
REFERENCES
- 1.An M R, Hsieh C C, Reisner P D, Rabek J P, Scott S G, Kuninger D T, Papaconstantinou J. Evidence for posttranscriptional regulation of C/EBPα and C/EBPβ isoform expression during the lipopolysaccharide-mediated acute-phase response. Mol Cell Biol. 1996;16:2295–2306. doi: 10.1128/mcb.16.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkenmeier E H, Gwynn B, Howard S, Jerry J, Gordon J I, Landschulz W H, McKnight S L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989;3:1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- 3.Burgess-Beusse B L, Timchenko N A, Darlington G J. CCAAT/enhancer binding protein α (C/EBPα) is an important mediator of mouse C/EBPβ protein isoform production. Hepatology. 1999;29:597–601. doi: 10.1002/hep.510290245. [DOI] [PubMed] [Google Scholar]
- 4.Calkhoven C F, Bouwman P R J, Snippe L, Geert A B. Translation start site multiplicity of the CCAAT/enhancer binding protein α mRNA is dictated by a small 5′ open reading frame. Nucleic Acids Res. 1994;22:5540–5547. doi: 10.1093/nar/22.25.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Z, Umek R M, McKnight S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 6.Coux O, Goldberg A L. Enzymes catalyzing ubiquitination and proteolytic processing of the p105 precursor of nuclear factor κB1. J Biol Chem. 1998;273:8820–8828. doi: 10.1074/jbc.273.15.8820. [DOI] [PubMed] [Google Scholar]
- 7.Darlington G, Wang N, Hanson R W. C/EBP alpha: a critical regulator of genes governing integrative metabolic processes. Curr Opin Genet Dev. 1995;5:565–570. doi: 10.1016/0959-437x(95)80024-7. [DOI] [PubMed] [Google Scholar]
- 8.Day D A, Tuite M F. Post-transcriptional gene regulatory mechanisms in eukaryotes—an overview. J Endocrinol. 1998;157:361–371. doi: 10.1677/joe.0.1570361. [DOI] [PubMed] [Google Scholar]
- 9.Descombes P, Chojkier M, Lichtsteiner S, Falvey E, Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990;4:1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- 10.Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- 11.Diehl A M, Michaelson P, Yang S Q. Selective induction of CCAAT/enhancer binding protein isoforms occurs during rat liver development. Gastroenterology. 1994;106:1625–1637. doi: 10.1016/0016-5085(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 12.Greenbaum L E, Li W, Cressman D E, Peng Y, Ciliberto G, Poli V, Taub R. CCAAT enhancer-binding protein beta is required for normal hepatocyte proliferation in mice after partial hepatectomy. J Clin Invest. 1998;102:996–1007. doi: 10.1172/JCI3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh C-C, Xiong W, Xie Q, Rabek J P, Scott S G, An M R, Reisner P D, Kuninger D T, Papaconstantinou J. Effects of age on the posttranscriptional regulation of CCAAT/enhancer binding protein β isoform synthesis in control of LSP-treated livers. Mol Biol Cell. 1998;9:1479–1494. doi: 10.1091/mbc.9.6.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu E, Mueller E, Oliviero S, Papaioannou V E, Johnson R, Spiegelman B M. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994;13:3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinsong Z, Guenther M G, Carthew R W, Lazar M A. Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes Dev. 1998;12:1775–1780. doi: 10.1101/gad.12.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochersperger L M, Parker E L, Siciliano M, Darlington G J, Denney R M. Assignment of genes for human monoamine oxidases A and B to the X chromosome. J Neurosci Res. 1986;16:601–616. doi: 10.1002/jnr.490160403. [DOI] [PubMed] [Google Scholar]
- 17.Landschulz W H, Johnson P F, Adashi E Y, Graves B J, McKnight S L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988;2:786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y-M, Miau L-H, Chang C-J, Lee S-C. Transcriptional induction of the alpha-1-glycoprotein (AGP) gene by synergistic interaction of two alternative activator forms of AGP/enhancer-binding protein (C/EBP beta) and NF-κB or Nopp140. Mol Cell Biol. 1996;16:4257–4263. doi: 10.1128/mcb.16.8.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin F-T, MacDougald O A, Diehl A M, Lane M D. A 30-kDa alternative translation product of the CCAAT/enhancer binding protein alpha messages: transcriptional activator lacking antimitotic activity. Proc Natl Acad Sci USA. 1993;90:9606–9610. doi: 10.1073/pnas.90.20.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin L, DeMartino G N, Greene W C. Cotranslational biogenesis of NF-κB p50 by the 26S proteasome. Cell. 1998;92:819–828. doi: 10.1016/s0092-8674(00)81409-9. [DOI] [PubMed] [Google Scholar]
- 21.Lincoln A J, Monczak Y, Williams S C, Johnson P F. Inhibition of CCAAT/enhancer-binding protein α and β translation by upstream open reading frames. J Biol Chem. 1998;273:9552–9560. doi: 10.1074/jbc.273.16.9552. [DOI] [PubMed] [Google Scholar]
- 22.May M J, Ghosh S. Rel/NF-kappa-B and I-kappa-B proteins—an overview. Semin Cancer Biol. 1997;8:63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto S, Seufzer B J, Shumway S D. Novel IκBα proteolytic pathway in WEHI231 immature B cells. Mol Cell Biol. 1998;18:19–29. doi: 10.1128/mcb.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien R M, Lucas P C, Yamasaki T, Noisin E L, Granner D K. Potential convergence of insulin and cAMP signal transduction systems at the phosphoenolpyruvate carboxykinase (PEPCK) gene promoter through CCAAT/enhancer binding protein (C/EBP) J Biol Chem. 1994;269:30419–30428. [PubMed] [Google Scholar]
- 25.Ossipow V, Descombes P, Schibler U. CCAAT/enhancer binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc Natl Acad Sci USA. 1993;90:8219–8223. doi: 10.1073/pnas.90.17.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poli V, Mancini F P, Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990;63:643–653. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- 27.Raught B, Gingras A-C, James A, Medina D, Sonenberg N, Rosen J M. Expression of a translationally regulated, dominant-negative CCAAT/enhancer-binding protein βisoform and up-regulation of the eukaryotic translation initiation factor 2α are correlated with neoplastic transformation of mammary epithelial cells. Cancer Res. 1996;56:4382–4386. [PubMed] [Google Scholar]
- 28.Raught B, Liao W S, Rosen J M. Developmentally and hormonally regulated CCAAT/enhancer-binding protein isoforms influence β-casein gene expression. Mol Endocrinol. 1995;9:1223–1232. doi: 10.1210/mend.9.9.7491114. [DOI] [PubMed] [Google Scholar]
- 29.Robinson G W, Johnson P F, Hennighausen L, Sterneck E. The C/EBP beta transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 1998;12:1907–1916. doi: 10.1101/gad.12.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ron D, Habener J F. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 31.Schulzeosthoff K, Ferrari D, Riehemann K, Wesselborg S. Regulation of NF-kappa-B activation by MAP kinase cascades. Immunobiology. 1997;198:35–49. doi: 10.1016/s0171-2985(97)80025-3. [DOI] [PubMed] [Google Scholar]
- 32.Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, Bistoni F, Frati L, Cortese R, Gulino A, Ciliberto G, Costantini F, Poli V. Lymphoproliferative disorder and imbalanced T-helper response in C/EBPβ-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seagroves T N, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington G J, Rosen J M. C/EBP beta, but not C/EBP alpha, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 1998;12:1917–1928. doi: 10.1101/gad.12.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterneck E, Tessarollo L, Johnson P F. An essential role for C/EBP beta in female reproduction. Genes Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBP beta and/or C/EBP gamma gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Timchenko N A, Harris T E, Wilde M, Bilyeu T A, Burgess-Beusse B L, Finegold M J, Darlington G J. CCAAT/enhancer binding protein alpha regulates p21 protein and hepatocyte proliferation in newborn mice. Mol Cell Biol. 1997;17:7353–7361. doi: 10.1128/mcb.17.12.7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timchenko N A, Wilde M, Kosai K, Heydari A R, Bilyeu T A, Finegold M J, Mohamedali K, Richardson A, Darlington G J. Regenerating livers of old rats contain high levels of C/EBPα that correlate with altered expression of cell cycle associated proteins. Nucleic Acids Res. 1998;26:3293–3299. doi: 10.1093/nar/26.13.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timchenko N A, Wilde M, Nakanishi M, Smith J R, Darlington G J. CCAAT/enhancer binding protein alpha (C/EBP alpha) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev. 1996;10:804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- 40.Vinson C R, Sigler P B, McKnight S L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989;246:911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- 41.Wang N D, Finegold M J, Bradley A, Ou C N, Abdelsayed S V, Wilde M D, Taylor L R, Wilson D R, Darlington G J. Impaired energy homeostasis in C/EBP alpha knock-out mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 42.Watt F, Molloy P L. Specific cleavage of transcription factors by the thiol protease, m-calpain. Nucleic Acids Res. 1993;21:5092–5100. doi: 10.1093/nar/21.22.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams S C, Cantwell C A, Johnson P F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991;5:1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- 44.Wilson D R, Juan T S, Wilde M D, Fey G H, Darlington G J. A 58-base-pair region of the human C3 gene confers synergistic inducibility by interleukin-1 and interleukin-6. Mol Cell Biol. 1990;10:6181–6191. doi: 10.1128/mcb.10.12.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeh W-C, Cao Z, Classon M, McKnight S L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]