Summary
Sneezing is a vital respiratory reflex frequently associated with allergic rhinitis and viral respiratory infections. However, its neural circuit remains largely unknown. A sneeze-evoking region was discovered in both cat and human brainstems, corresponding anatomically to the central recipient zone of nasal sensory neurons. Therefore, we hypothesized that a neuronal population postsynaptic to nasal sensory neurons mediates sneezing in this region. By screening major presynaptic neurotransmitters/neuropeptides released by nasal sensory neurons, we found that Neuromedin B (NMB) peptide is essential for signaling sneezing. Ablation of NMB-sensitive postsynaptic neurons in the sneeze-evoking region or deficiency in NMB receptor abolished the sneezing reflex. Remarkably, NMB-sensitive neurons further project to the caudal ventral respiratory group (cVRG). Chemical activation of NMB-sensitive neurons elicits action potentials in cVRG neurons and leads to sneezing behavior. Our study delineates a peptidergic pathway mediating sneezing, providing molecular insights into the sneezing reflex arc.
In Brief:
By using a newly developed mouse model, Li et al. were able to elucidate the peptidermic pathway that mediates the sneezing reflex, offering insight into how sneezing occurs
Graphical Abstract
Introduction
Sneezing is a basic reflex that expels irritants and pathogens from the airway. Although designed to be a defense mechanism, sneezing is associated with many viral respiratory infections (e.g., common cold and flu) and allergic rhinitis (a.k.a., hay fever), and severely impacts our quality of life and productivity (Songu and Cingi, 2009). Moreover, sneezing represents the most forceful and common mechanism to spread infectious droplets during viral respiratory infections. Studies indicate that a single sneeze can create 40,000 virus-containing droplets that reach a radius of 7 to 8 meters and suspend in the air for up to 10 minutes (Bourouiba, 2016, 2020; Bourouiba et al., 2014; Cole and Cook, 1998; Scharfman et al., 2016; Tang et al., 2006). In contrast, a cough produces 3,000 droplets at most, which is about the same number as talking continuously for five minutes (Cole and Cook, 1998; Tang et al., 2006). Hence, sneezing is an important mechanism behind the community spread of many respiratory viruses. From the perspective of either basic science or clinical medicine, it is of paramount importance to understand the mechanisms underlying sneezing.
A sneeze-evoking region was discovered in the ventromedial spinal trigeminal nucleus (SpV) of both cats and humans (Hersch, 2000; Nonaka et al., 1990; Seijo-Martinez et al., 2006). Electrical stimulations of this region evoke sneeze-like responses in cats (Nonaka et al., 1990). Stroke-induced lesions on this region lead to paroxysmal sneezing or loss of ability to sneeze in humans (Hersch, 2000; Seijo-Martinez et al., 2006). However, the lack of information on the molecular identities of the neurons mediating sneezing in this region and related synaptic pathway impedes further investigation.
The sneeze-evoking region corresponds anatomically to the central recipient zone of the ethmoidal nerve that provides sensory branches to the nasal mucosa (Hersch, 2000; Lucier and Egizii, 1986; Nonaka et al., 1990; Seijo-Martinez et al., 2006), suggesting that the region comprises the postsynaptic neurons of nasal sensory neurons. We therefore hypothesize that a specific population of postsynaptic neurons in the sneeze-evoking region mediates the sneezing reflex. Previous studies have shown that stimulations of nasal sensory fibers or ethmoidal nerve trigger sneezing in both cats and humans (Batsel and Lines, 1978; Geppetti et al., 1988; Hyden and Arlinger, 2007; Satoh et al., 1998; Taylor-Clark et al., 2005; Wallois et al., 1997; Wallois et al., 1991). However, it remains unclear which population of nasal sensory neurons initiates the sneezing reflex and which neurotransmitters/neuropeptides in nasal sensory neurons are required for transmitting sneeze signals. Lacking this information greatly impedes the study of the postsynaptic neurons in the sneeze-evoking region and hinders testing our hypothesis.
Sneezing is a reflex generated by central respiratory neurons. Nevertheless, the sneeze-evoking region is located within the ventromedial SpV, and does not overlap with any reported respiratory centers in the brainstem (Feldman et al., 2013). It is therefore reasonable to speculate that the sneeze-evoking region further projects to central respiratory groups. Studies have characterized sneeze-related electrical activities in different groups of central respiratory neurons in cats (Batsel and Lines, 1978; Jakus et al., 1985; Orem and Brooks, 1986; Wallois et al., 1997). As sneezes interrupt the normal pattern of respiration, whether these electrical activities are the “drivers” or “passengers” of sneezing and whether they are induced by inputs from the sneeze-evoking region remain unknown.
In this study, we reveal the molecular identities of the neurons mediating sneezing in the sneeze-evoking region and delineate their anatomical and functional connections with central respiratory neurons. We developed a two-step reverse screening strategy to identify the neuronal population essential for sneezing in the sneeze-evoking region. Firstly, we screened all major presynaptic neurotransmitters/neuropeptides released by nasal sensory neurons for sneeze signaling. After we identified the neuropeptide that is critically required for transmitting sneeze signals, secondly, we targeted the corresponding postsynaptic neurons in the sneeze-evoking region and studied their role in mediating sneezing. This strategy successfully helped us identify a highly restricted neuronal population that is key for the sneezing reflex in the sneeze-evoking region. To further determine which populations of respiratory neurons are the projection target(s) of the sneeze-evoking region, we conducted neuronal tracing, electrophysiology recording and behavioral studies. These studies reveal that the caudal ventral respiratory group is a direct projection target of the sneeze-evoking region and drives the generation of sneezes. Collectively, we delineate the peptidergic pathway from nasal sensory neurons to central respiratory neurons that mediates the sneezing reflex. This newly defined pathway greatly advances our understanding of the neural mechanism underlying the sneezing reflex and has important implications in the development of therapeutic strategies for pathological sneezing.
Results
Establishment of a sneezing model in mice
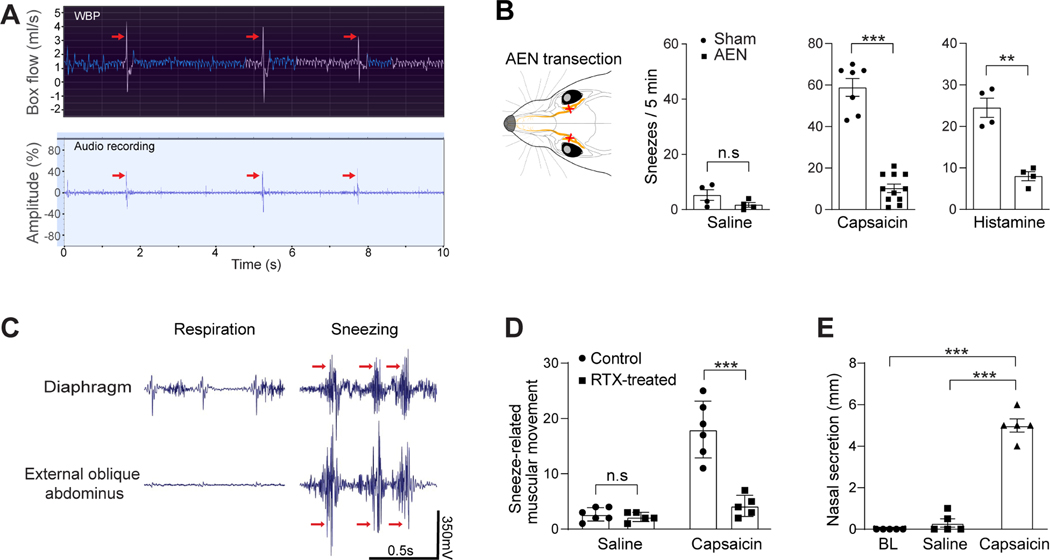
Sneezing is characterized by convulsive expulsion of air and the sound of “Achoo” in humans. However, most previous studies used anesthetized or decerebrate cats that could not make real sneezes, and monitored fictive sneezing represented by diaphragmatic and intercostal muscle activities and related nerve activity (Batsel and Lines, 1978; Nonaka et al., 1990; Satoh et al., 1998; Wallois et al., 1997; Wallois et al., 1991). To study the neural mechanism underlying the sneezing reflex, we established a behavioral model in freely-moving mice. Studies have shown that capsaicin (a pungent compound from chili pepper) and histamine elicit sneezes in humans (Doyle et al., 1990; Geppetti et al., 1988; Tonnesen and Mygind, 1985). By delivering aerosolized droplets containing capsaicin or histamine via a nebulizer to the mouse test chambers, we found that both compounds triggered sneeze-like responses in mice (Figure 1A-B). Specifically, the audio recording revealed sneeze-like episodes characterized by audio peaks, which comprise both sonic and ultrasonic components and are distinct from nociception-associated vocalization (Figure 1A, supplemental video S1 and Figure S1). Whole body plethysmography (WBP) showed repetitive sneeze-like respiratory patterns characterized by forceful and convulsive expiration (Figure 1A). The respiratory patterns and audio peaks correlate well, as revealed by simultaneous WBP and audio recordings (Figure 1A, supplemental table S1 and video S2). To confirm that the audio- and respiratory-responses are sneezes but not other respiratory reflexes, we examined whether these responses are mediated by sensory fibers innervating the nasal mucosa rather than those in the trachea, lung, or other sites. The anterior ethmoidal nerve (AEN) provides sensory branches to the nasal mucosa, and plays an essential role in triggering sneezes (Batsel and Lines, 1978; Satoh et al., 1998; Wallois et al., 1997; Wallois et al., 1991). After transecting AEN, we found that the audio- and respiratory-responses to both capsaicin and histamine were significantly reduced compared with the sham surgery group (Figure 1B), confirming that these responses are sneezes mediated by nasal sensory fibers.
Figure 1:
Establish the mouse sneeze model. (A) Representative traces showing the simultaneous Whole Body Plethysmography (WBP) and audio recordings of a freely-moving WT mouse upon exposure to aerosolized capsaicin solution (12 μM). The WBP and audio peaks (indicated by the arrows) correlate well and indicate sneezing-like responses. (B) Transection of the anterior ethmoid nerve (AEN), which provides sensory innervation to the nose, significantly reduced sneezing-like responses to aerosolized capsaicin (12 μM) and histamine (100 mM) solution. (C) Electromyography (EMG) recording showing the representative muscle contractions of diaphragm and external oblique abdominus of mice in eupnea and during sneezing responses to nasal challenge of capsaicin (12 μM). Muscular contractions that significantly increase over regular respiratory movement and interrupt the respiratory rhythm were counted as sneezing-related responses (indicated by red arrows). (D) Nasal instillation of resiniferatoxin (RTX, 50 ng in 2 μl/ nostril) ablated nasal Trpv1+ sensory fibers and eliminated sneezing-related muscular responses to capsaicin, as revealed by EMG recording. (E) Nasal secretion was significantly increased after capsaicin-induced sneezes, compared with baseline (BL) or saline control. Each dot represents an individual mouse (n=4–11 mice/group). Data are represented as mean ± SEM. **P≤0.01, ***P≤0.001, n.s. not significant. See also Figure S1, Videos S1 and S2, and Table S1.
Convulsive expulsion of air and associated sound are generated by sudden and involuntary muscular movements during sneezing. By conducting electromyography (EMG) recording of the diaphragm and external oblique abdominus of lightly anesthetized mice, we observed sudden and involuntary muscular movements upon nasal challenge of capsaicin (Figure 1C), consistent with sneezing-related muscular movement in cats (Nonaka et al., 1990; Satoh et al., 1998). To confirm that the recorded muscular movements faithfully reflect sneezing in mice, we ablated nasal sensory fibers that express Trpv1, a capsaicin-sensitive cation channel. This ablation abolished capsaicin-induced muscular movements as revealed by EMG (Figure 1D). These results confirm that the recorded muscular movements are triggered by nasal sensory fibers and faithfully reflect sneezing but no other respiratory responses (e.g., coughing mediated by sensory fibers in the lower airway) in mice. In addition to muscular movement, nasal secretion is also significantly increased after capsaicin-induced sneezing (Figure 1E). Together, our studies firmly establish the mouse sneezing model.
Small-diameter sensory neurons mediate both chemical-induced and allergy-associated sneezing
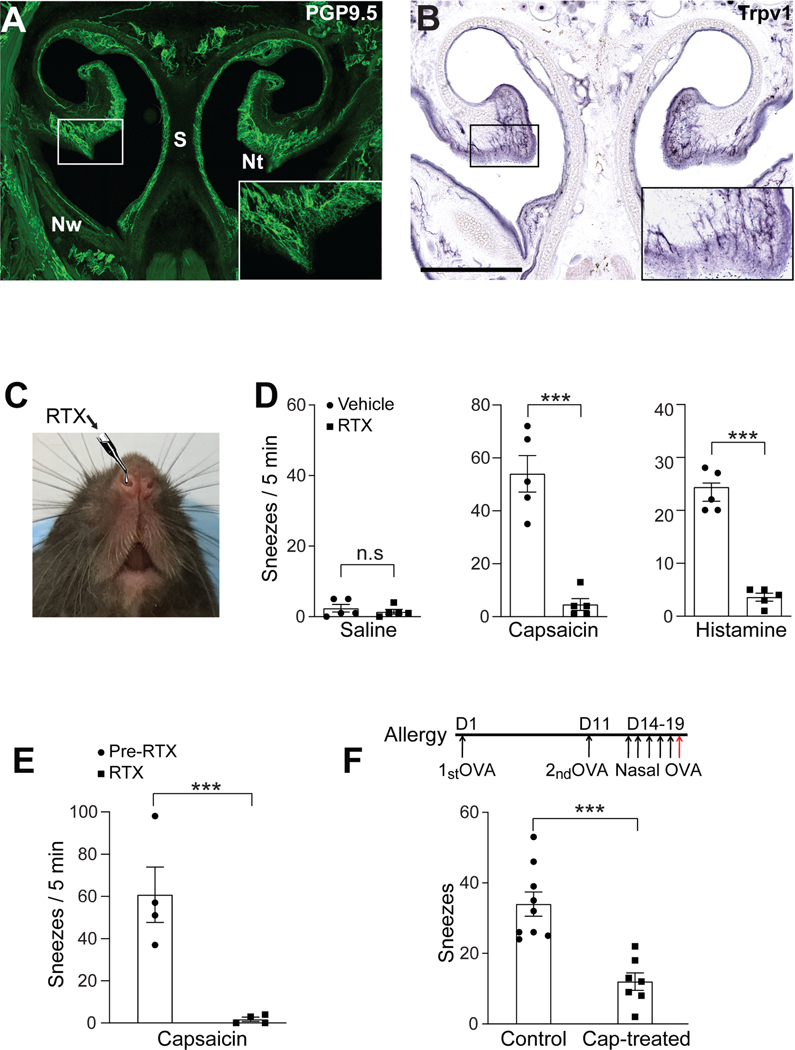
Sensory neurons are highly heterogeneous and can be grossly classified into two groups: myelinated large-diameter neurons that detect innocuous stimuli (e.g., light touch) and thinly myelinated or unmyelinated small-diameter neurons that detect noxious stimuli. To screen the presynaptic neurotransmitters / neuropeptides essential for sneeze signaling in an efficient and precise manner, we first sought to determine which group of sensory neurons mediates sneezing. As sneezes can be readily elicited by capsaicin and histamine in humans and mice (see Figure 1) (Doyle et al., 1990; Geppetti et al., 1988; Tonnesen and Mygind, 1985), we examined the nasal innervation of sensory neurons that express Trpv1, the capsaicin-sensitive cation channel that also serves as the downstream transduction channel of histamine H1r receptor (Caterina et al., 1997; Shim et al., 2007). Trpv1 is expressed by many thinly myelinated or unmyelinated small-diameter neurons. Utilizing Trpv1PLAP/+ reporter line (Cavanaugh et al., 2011), we found that Trpv1+ sensory fibers richly innervate the nasal mucosa and comprise a major population of nasal sensory fibers (Figure 2A-B).
Figure 2:
Trpv1-expressing nasal sensory fibers mediate both chemical-induced and allergy-associated sneezing. (A) The mouse nose is densely innervated by primary sensory fibers (green), as revealed by immunostaining for PGP9.5, a pan-neuronal marker. The inset shows a higher-magnification view of the boxed area. Nt, nasal turbinate; S, nasal septum; Nw, nasal wall. (B) Histochemistry for placental alkaline phosphatase (PLAP) revealed that Trpv1-expressing sensory fibers densely innervate the nasal mucosal membrane lining the nasal turbinate and septum in Trpv1PLAP/+ transgenic mice. The inset shows a higher-magnification view of the boxed area. (C) Nasal instillation of resiniferatoxin (RTX, 50 ng in 2 μl/ nostril) to desensitize/degenerate Trpv1-expressing sensory fibers in the nose. (D-E) Nasal instillation of RTX eliminated sneezing responses to aerosolized capsaicin (12 μM) and histamine (100 mM) solution, as revealed by (D) audio- and (E) WBP-recording. (F) Generation of allergic rhinitis mouse model. The red arrow indicates the intranasal instillation of allergen (ovalbumin, OVA, 0.2 μg in 2 μl PBS / nostril) for sneezing test after two systemic immunizations and five days of nasal sensitization. Allergen-induced sneezing response was significantly reduced in capsaicin-pretreated mice, compared with vehicle-treated control mice. All images shown are representatives of three biologically independent mice. Scale bars: 500 μm. Each dot represents an individual mouse (n=4–9 mice/group). Data are represented as mean ± SEM. ***P≤0.001, n.s. not significant.
To study the role of Trpv1+ small-diameter neurons in triggering sneezes, we instilled a small volume (2 μl) of resiniferatoxin solution (RTX) into the mouse nasal cavity to ablate Trpv1+ nasal sensory fibers (Figure 2C) (Karai et al., 2004). After RTX treatment, audio- and respiratory-recording revealed a significant reduction in the sneezing responses to capsaicin and histamine (Figure 2D-E). Furthermore, allergy-associated sneezing was also significantly reduced by desensitization of Trpv1+ nasal afferent fibers (Figure 2F). These results reveal the central role of Trpv1+ small-diameter neurons in mediating sneezing induced by both chemical irritants and allergens.
Neuromedin B (NMB) is required for signaling sneezing
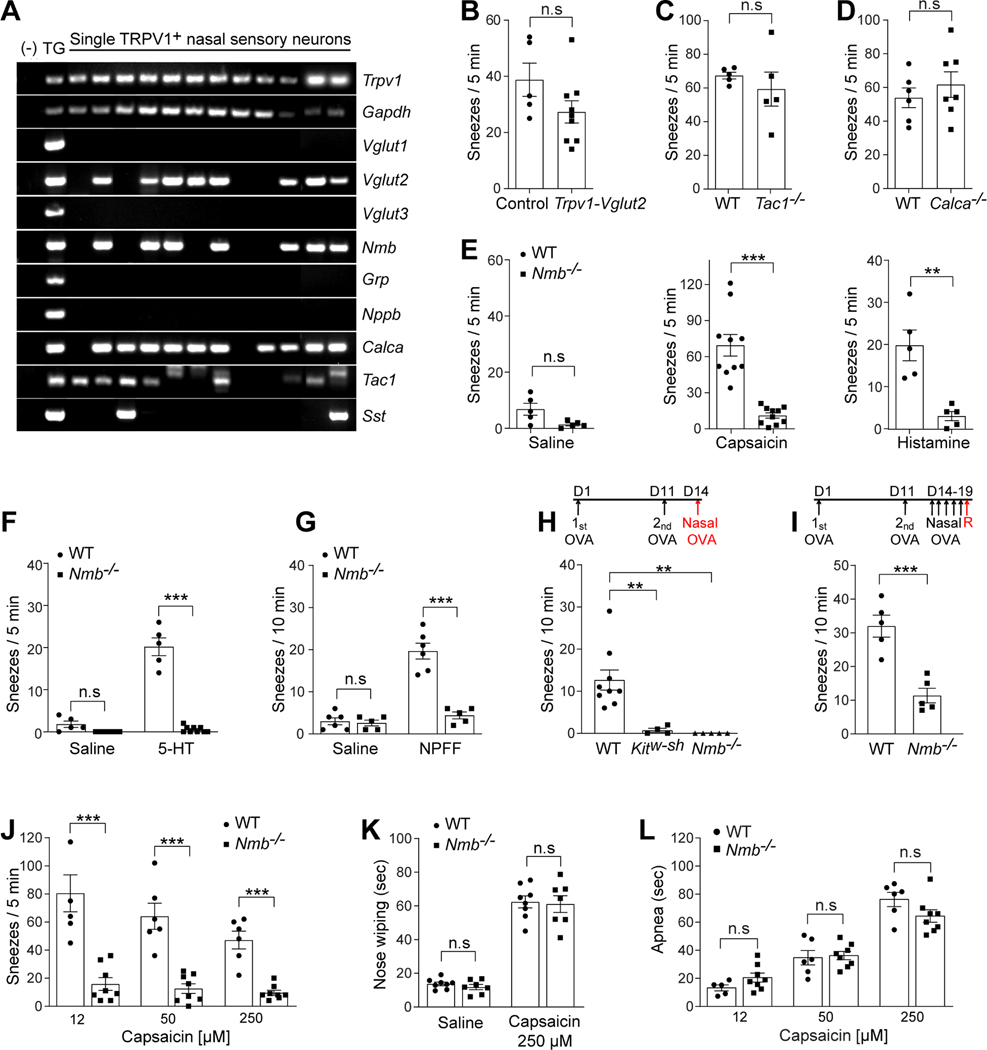
The identification of Trpv1+ nasal small-diameter sensory neurons in eliciting sneezing allows for targeted screening of neurotransmitters / neuropeptides essential for signaling sneezing. First, we investigated which neurotransmitters and neuropeptides are expressed in Trpv1+ nasal small-diameter neurons. Among all major neurotransmitters/neuropeptides identified in primary sensory neurons, Trpv1+ nasal sensory neurons selectively express vesicular glutamate transporter 2 (Vglut2), neuropeptides substance P (Tac1), CGRP (Calca), and neuromedin B (Nmb) as revealed by single-cell RT-PCR (Figure 3A). Second, we studied which neurotransmitters / neuropeptides are required for transmitting sneeze signals by examining the sneezing responses of mice deficient in each neurotransmitter or neuropeptide. Trpv1Cre/+;Vglut2F/F conditional knockout mice failed to show a significant reduction in their sneezing responses to capsaicin, compared with control littermates (Figure 3B), indicating that Vglut2-dependent glutamate signaling is dispensable for signaling sneezing. Furthermore, deficiency in the neuropeptide substance P or CGRP did not affect the sneezing responses either (Figure 3C-D).
Figure 3:
Neuromedin B (NMB) is required for signaling sneezing. (A) Single-cell RT-PCR was performed on individual Trpv1+ nasal sensory neurons that were labeled by intranasal application of fluorescent retrograde axonal tracer. Vesicular glutamate transporter 2 (Vglut2) and neuropeptides NMB (Nmb), CGRP (Calca), and substance P (Tac1) are selectively expressed by a majority of Trpv1+ nasal neurons. In contrast, Vesicular glutamate transporter 1 and 3 (Vglut1 and Vglut3), gastrin-releasing peptide (Grp), natriuretic peptide B (Nppb), and somatostatin (Sst) are expressed by few or no Trpv1+ nasal neurons. Whole trigeminal ganglia (TG) were used as a positive control. Genomic DNA served as a negative control (−) for intron-spanning primers used for RT-PCR. (B-D) Deficiency in VGLUT2 (Vglut2), substance P (Tac1), or CGRP (Calca) does not affect the sneezing response to aerosolized capsaicin solution (12 μM), compared with control mice. (E) Deficiency in the neuropeptide NMB (Nmb) abolishes the sneezing responses to aerosolized capsaicin (12 μM) and histamine (100 mM) solution. (F-G) Nmb deficiency abolishes the sneezing responses to serotonin (5-HT, aerosolized solution (1 mM), F) and neuropeptide FF solution (NPFF, 20 nmol in 2 μl, G). (H) In acute allergic model, the sneezing responses to allergen (ovalbumin, OVA, 0.2 μg in 2 μl PBS/nostril) were abolished in mast cell-deficient Kitw-sh mice, compared with WT controls. This mast cell-dependent allergic sneezing was eliminated in Nmb−/− mice. (I) Nmb−/− mice display reduced sneezing response in chronic allergic model, compared with WT controls. The red arrow indicates the intranasal instillation of allergen (ovalbumin, OVA, 0.2 μg in 2 μl PBS / nostril) for sneezing test after five days of intranasal sensitization. (J) Inverse dose-response relationship between capsaicin and sneezing responses in WT mice. Nmb−/− mice display reduced sneezing responses to capsaicin at all the doses tested. (K) Pain-related nose wiping behavior elicited by high-dose capsaicin (250 μM) remains intact in the Nmb−/− mice, as in WT mice. (L) Capsaicin induces mouse apnea responses in a dose-dependent manner. Each dot represents an individual mouse (n=5–10 mice/group). Data are represented as mean ± SEM. ** P≤0.01, ***P≤0.001, n.s. not significant. See also Figure S2.
Interestingly, deficiency in the neuropeptide NMB significantly reduced the sneezing responses to capsaicin (Figure 3E), without altering other respiratory parameters examined (supplemental Figure S2). NMB deficiency also resulted in an abolishment of sneezing responses to histamine (Figure 3E), one of principal mast cells mediators in allergic rhinitis (Uzzaman and Story, 2012). In addition to histamine, mast cells release many other mediators, including serotonin and various neuropeptides (Kushnir-Sukhov et al., 2007; Lee et al., 2008). However, the role of histamine-independent pathways in mediating sneezing remains poorly defined. We found that serotonin induces significant sneezing in mice (Figure 3F), correlating well with studies showing that serotonin induces sneezing in humans (Tonnesen and Mygind, 1985). Importantly, this sneezing response was abolished in Nmb-deficient mice (Figure 3F). Besides serotonin, neuropeptide FF (NPFF) is released from mast cells upon degranulation as shown by our previous study (Lee et al., 2008). Although NPFF has never been implicated in allergic sneezing, we newly found that NPFF induces significant sneezing responses in WT mice but not Nmb−/− mice (Figure 3G). These results reveal the essential role of NMB peptide in signaling both histamine-dependent and -independent sneezing responses and suggest its involvement in allergic sneezing. Further study indeed showed that NMB deficiency resulted in an abolishment of sneezing responses to mast cell-dependent allergy (Figure 3H-I). Together, these results demonstrate that NMB is critically required for signaling sneezing.
As nasal challenge of capsaicin evokes sneezes and burning pain in humans (Geppetti et al., 1988), we next investigated whether there is any dose-dependent effects of capsaicin in inducing sneezes and pain, and whether NMB is required for signaling pain. Interestingly, we found an inverse dose-response relationship between capsaicin doses and sneezing responses in wild-type (WT) mice. High dose capsaicin induced fewer sneezes than low doses (Figure 3J). In contrast, pain-related behavior is evoked by capsaicin in a positive dose-dependent manner. While high-dose capsaicin induced fewer sneezes, it evoked more nose wiping (stereotypic behavior indicative of pain, Figure 3K) and more apnea episodes to reduce capsaicin inhalation (avoidance behavior, Figure 3L), revealing an antagonism between pain and sneezing. Interestingly, NMB deficiency abolished sneezing responses to capsaicin at both low and high doses tested (Figure 3J), but did not reduce pain-related nose wiping behavior or apnea induced by capsaicin (Figure 3K-L). These results indicate that NMB selectively signals sneezing but not nasal pain-related behavior.
NMB is released from sensory neurons for signaling sneezing
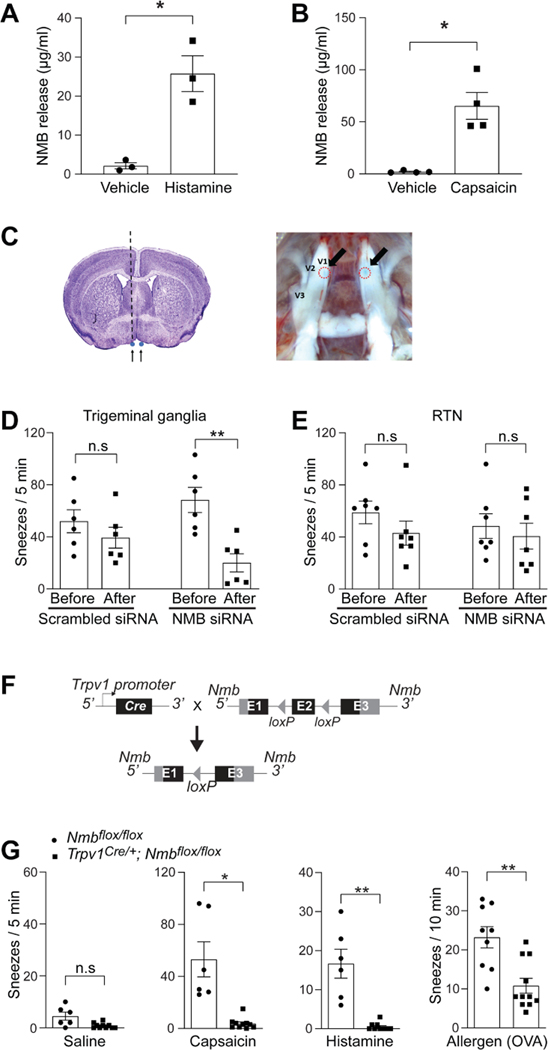
Although NMB is highly expressed in TG, it is also detected in several brain regions including the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) (Li et al., 2016; Wada et al., 1990). To determine whether NMB is released from primary sensory neurons to mediate sneezing, we first stimulated TG neurons with sneeze-inducing chemicals histamine and capsaicin and found that both compounds elicited significant NMB release from TG neurons (Figure 4A-B). We then performed microinjection of NMB-specific siRNA into the V1 division of TG, where most nasal sensory neurons are located (Figure 4C). The knockdown of Nmb in TG significantly reduced sneezing responses, compared with pre-treatments and scrambled siRNA controls (Figure 4D and supplemental Figure S3), indicating that NMB in sensory neurons is required for transmitting sneeze signals. In contrast, siRNA knockdown of Nmb in RTN did not affect sneezing responses (Figure 4E), suggesting that NMB plays distinct roles in the nasal sensory neurons and central RTN. To confirm the site of NMB action in signaling sneezing, we also generated Trpv1Cre/+; Nmbfl°x/fl°x mice in which Nmb was conditionally knocked out in Trpv1-lineage sensory neurons (Figure 4F). Trpv1Cre/+; Nmbfl°x/fl°x mice displayed significant reductions in their sneezing responses to capsaicin, histamine and allergens (Figure 4G). These results demonstrate that NMB is released from sensory neurons to signal sneezing.
Figure 4:
NMB is released from sensory neurons for signaling sneezing. (A-B) NMB is released from cultured trigeminal ganglia neurons of WT mice after vehicle or sneeze-inducing compounds treatment (histamine: 200 μM; capsaicin: 10 μM). Each dot represents one replicate. (C) Diagrams illustrating microinjection of siRNA into the V1 division of the trigeminal ganglia. The black dotted line represents the path of the microinjection needle. Arrows in the right panel point toward the injection sites, outlined by red dashed circles, in the trigeminal ganglia. (D) NMB-siRNA silencing in the trigeminal ganglia efficiently decreases the sneezing response to aerosolized capsaicin solution (12 μM), compared with the sneezing responses before microinjection or the non-targeting scrambled siRNA controls. (E) NMB-siRNA silencing in the retrotrapezoid nucleus (RTN) does not affect the sneezing response to aerosolized capsaicin solution (12 μM). (F) Schematic diagram illustrating the generation of Trpv1Cre/+; Nmbfl°x/fl°x mice in which Nmb was conditionally knocked out in Trpv1-lineage sensory neurons. (G) Trpv1Cre/+; Nmbfl°x/fl°x mice display significantly reduced sneezing responses to aerosolized capsaicin (12 μM), histamine (100 mM) solution and chronic allergy induced by ovalbumin (OVA, 0.2 μg in 2 μl PBS / nostril). Each dot represents an individual mouse (n=6–11 mice/group). Data are represented as mean ± SEM. * P≤0.05, ** P≤0.01, n.s. not significant. See also Figure S3.
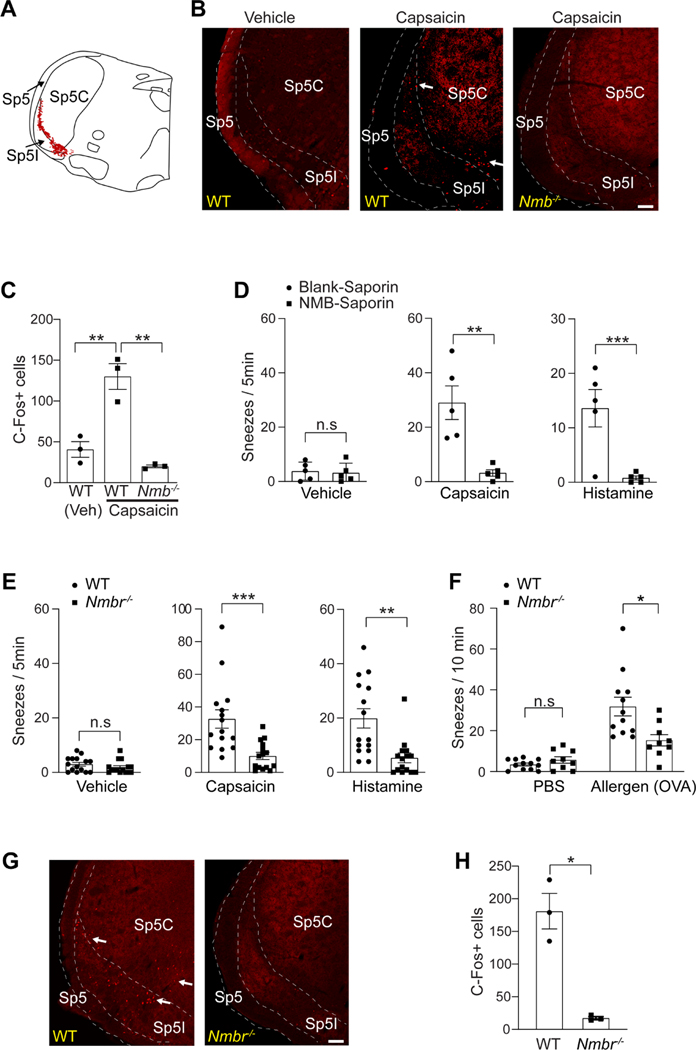
NMB-sensitive postsynaptic neurons in the sneeze-evoking region mediate sneezing
The essential role of NMB in signaling sneezing suggests the involvement of NMB-sensitive postsynaptic neurons within the sneeze-evoking region (i.e., the central recipient zone of nasal sensory neurons; see the diagram in Figure 5A). Indeed, capsaicin-induced sneezes led to a significantly increased expression of c-Fos within the sneeze-evoking region of WT mice but not Nmb-deficient mice (Figure 5B-C), suggesting NMB-dependent transmission to the sneeze-evoking region. We further examined the role of NMB-sensitive postsynaptic neurons in signaling sneezing. Studies have shown that NMB-saporin efficiently ablates NMB-sensitive neurons (Mishra et al., 2012; Wan et al., 2017). Microinjection of NMB-saporin into the sneeze-evoking region abolished the sneezing responses to capsaicin and histamine (Figure 5D), suggesting that NMB-sensitive postsynaptic neurons mediate sneezing.
Figure 5:
NMB-sensitive postsynaptic neurons in the sneeze-evoking region mediate sneezing. (A) Diagram showing the sneeze-evoking region (i.e., the central projection of nasal sensory neurons; indicated by red dots) in the spinal trigeminal nucleus (SpV) based on previous studies. Sp5, spinal trigeminal tract; Sp5C, spinal trigeminal nucleus caudalis; Sp5I, spinal trigeminal nucleus interpolar. (B) Capsaicin-induced sneezes lead to a significant increase in the expression of c-Fos within the sneeze-evoking region of WT (indicated by arrows), compared with the saline vehicle control. Nmb-deficient mice display a significantly reduced c-Fos signal. (C) Quantification of c-Fos+ neurons in the sneeze-evoking region after saline (Veh) or capsaicin treatments. (D) Microinjection of NMB-saporin into the sneeze-evoking region abolishes the sneezing responses to aerosolized capsaicin (12 μM) and histamine (100 mM) solution. (E-F) Nmbr−/− mice display significantly reduced sneezing responses to aerosolized capsaicin (12 μM), histamine (100 mM) solution and allergen (ovalbumin, OVA, 0.2 μg in 2 μl PBS / nostril) stimuli. (G-H) Capsaicin-induced sneezes lead to the expression of c-Fos in the sneeze-evoking region of WT (indicated by arrows) but not Nmbr−/− mice. All images shown are representatives of three biologically independent mice. Scale bars: 100 μm. The total number of c-Fos+ neurons in the sneeze-evoking region was counted from ten sections per mouse (n=3 / genotype). Each dot represents an individual mouse. n=5–15 mice/group for behavioral tests. Data are represented as mean ± SEM. * P≤0.05, ** P≤0.01, *** P≤0.001, n.s. not significant.
NMB acts by binding to its high affinity cell surface G protein-coupled receptor, neuromedin B receptor (NMBR) (Wada et al., 1991). We further tested whether NMBR is required for signaling sneeze. NMBR-deficient mice displayed significantly reduced sneezing responses to chemical and allergen stimuli (Figure 5E-F). Importantly, after sneeze-evoking stimulations, the c-Fos signal was barely detectable in the sneeze-evoking region of Nmbr−/− mice (Figure 5G-H), confirming the indispensable role of NMB-NMBR interaction in transmitting the sneeze-related signal.
NMBR+ neurons represent a highly restricted population in the sneeze-evoking region and project to the caudal ventral respiratory group (cVRG)
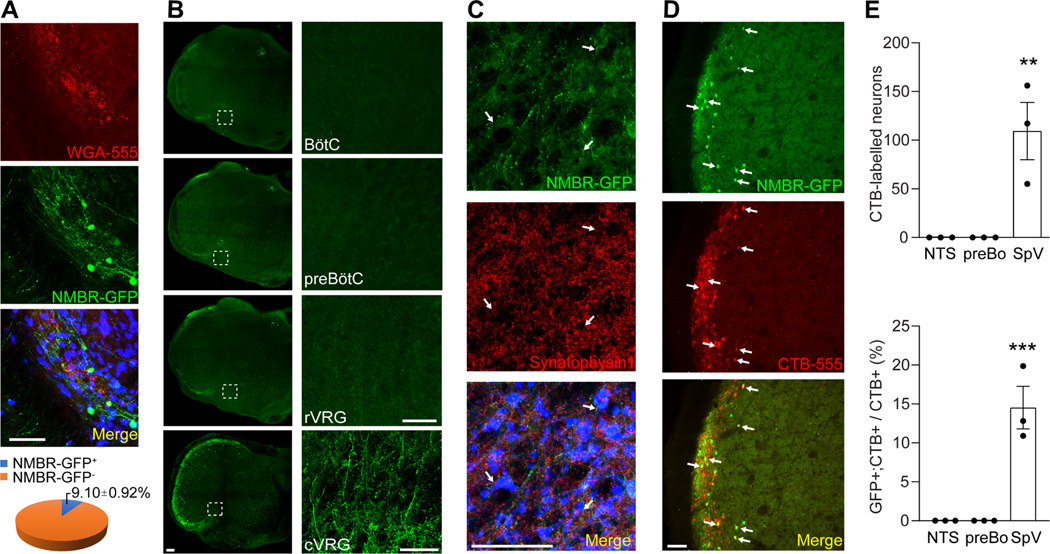
Sneezing is a reflex generated by central respiratory neurons. However, the sneeze-evoking region does not overlap with any reported respiratory regions in the brainstem. Hence, we hypothesize that NMBR+ neurons further project to the central respiratory groups. To identify the projection targets of NMBR+ neurons, we conducted axonal tracing of NMBR+ neurons utilizing NmbreGFP reporter line (Wan et al., 2017). NMBR+ neurons account for 9.1±0.92% of total neurons in the sneeze-evoking region and comprise a highly restricted population (Figure 6A). Strikingly, we found that NMBR-GFP+ axons richly innervate the caudal ventral respiratory group (cVRG) but no other respiratory regions including the rostral ventral respiratory group (rVGR), pre-BÖtzinger complex and BÖtzinger complex (Figure 6B and supplemental Figure S4). Immunostaining for the presynaptic markers synaptophysin −1 indicates that NMBR+ axons synapse with neurons in cVRG (Figure 6C). To confirm that NMBR-GFP+ axons in cVRG originate from NMBR+ neurons in the sneeze-evoking region but no other brain regions, we microinjected Alexa Fluor™ 555-conjugated Cholera Toxin Subunit B (CTB, neuronal tracer) into cVRG of NmbreGFP mice for retrograde axonal tracing. The CTB labeled NMBR-GFP+ neurons are localized within the sneeze-evoking region and absent from other brain regions including the pre-BÖtzinger complex and nucleus tractus solitarius (NTS) (Figure 6D-E). These anatomical studies reveal that NMBR+ neurons in the sneeze-evoking region project directly to cVRG and synapse with neurons within cVRG.
Figure 6:
The projection of NMBR+ neurons to the caudal ventral respiratory group (cVRG). (A) Representative imaging of brainstem coronal sections showing that NMBR+ neurons (green) comprise a highly restricted population in the sneeze-evoking region (red, labeled by WGA-555 applied to mouse nasal cavity). Neurons were marked by NeuN (blue). (B) Representative imaging showing that NMBR+ neurons selectively project to cVRG but no other respiratory regions including the rostral ventral respiratory group (rVRG), pre-BÖtzinger complex (preBÖtC) and BÖtzinger complex (BÖtC) as revealed by axonal tracing in NmbreGFP reporter line. Images on the right show higher-magnification view of each boxed region. (C) Representative imaging showing that NMBR-GFP+ nerve fibers (green) express the presynaptic marker synaptophysin 1 (red) and synapse with cVRG neurons (marked by NeuN, blue). (D-E) Retrograde axonal tracing by microinjection of CTB-555 into cVRG of NmbreGFP mice shows that CTB labeled NMBR-GFP+ neurons (indicated by arrows) are localized within the sneeze-evoking region in the spinal trigeminal nucleus and absent from other brain regions including the pre-BÖtzinger complex (preBÖ) and nucleus tractus solitarius (NTS). All images shown are representatives of three biologically independent mice. Scale bars: 100 μm. Each dot represents an individual mouse (n=3 mice/group). Data are represented as mean ± SEM. ** P≤0.01, *** P≤0.001. See also Figure S4.
The synaptic connections between NMBR+ neurons and cVRG neurons are essential for signaling sneezing
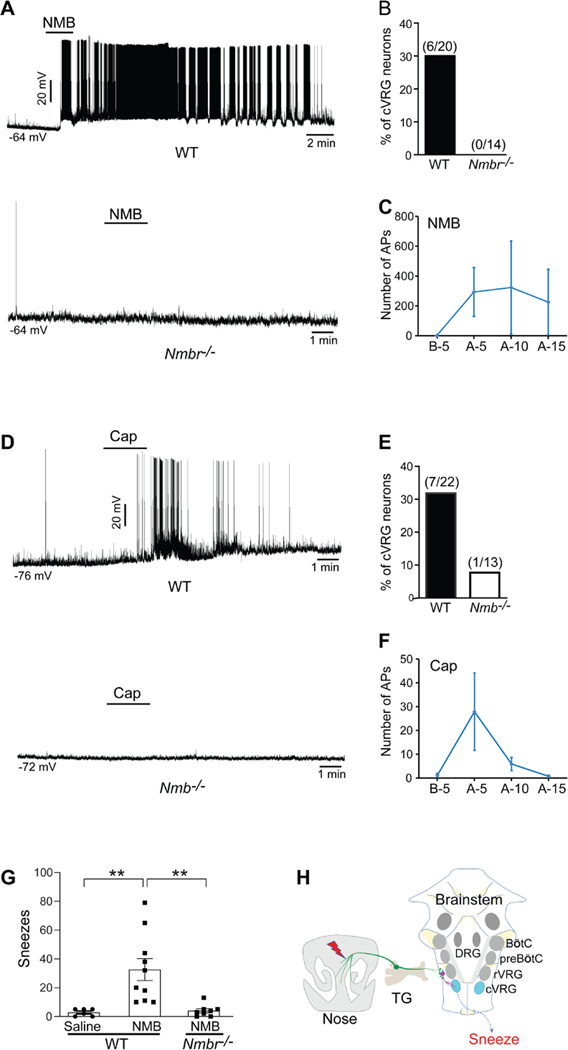
cVRG contains mostly expiratory neurons that transmit excitatory drive to spinal motor neurons that control internal intercostal and abdominal expiratory muscles (Ezure et al., 2003; Iscoe, 1998; Shiba et al., 2007). To confirm the functional connections between NMBR+ neurons and cVRG neurons, we tested whether cVRG neurons receive the synaptic input from NMBR+ neurons by conducting electrophysiological recording of brainstem slices. Pharmacological activation of NMBR+ neurons using NMB peptide evoked robust action potentials (AP) firing in 30% of cVRG neurons (6 out of 20 neurons recorded; Figure 7A-C). As a negative control, cVRG neurons in the brainstem slices from Nmbr−/− mice displayed no response to the same NMB treatment (0 out of 14 neurons recorded; Figure 7A-B). Our electrophysiological recordings confirm the functional connections between NMBR+ neurons and cVRG neurons.
Figure 7:
The synaptic connection between NMBR+ neurons and caudal ventral respiratory group (cVRG) neurons is essential for signaling sneezing. (A) Representative traces showing that pharmacological activation of NMBR+ neurons using NMB peptide (4 μM) induces robust action potential (AP) firing in cVRG neurons of WT but not Nmbr−/− mice. (B) NMB peptide evokes robust AP firing in 30% of cVRG neurons recorded in the brainstem slices from WT mice. As a negative control, cVRG neurons of Nmbr−/− mice display no response to the same NMB treatment. (C) The number of APs fired by WT cVRG neurons 5 min before (B-5) and 0–5 min (A-5), 5–10 min (A-10), and 10–15 min (A-15) after NMB treatment. (D) Representative traces showing that capsaicin (5 μM) induces robust AP firing in cVRG neurons of WT but not Nmb−/− mice. (E) Capsaicin treatment elicits robust AP firing in 31.8% of cVRG neuron recorded from WT mice but weak or no AP firing from Nmb−/− mice. (F) The number of APs fired by WT cVRG neurons 5 min before (B-5) and 0–5 min (A-5), 5–10 min (A-10), and 10–15 min (A-15) after capsaicin treatment. (G) Microinjection of NMB peptide (1 pmol in 100 nl) into the cVRG region induces significant sneezing responses in WT but not Nmbr−/− mice. As a control, microinjection of saline does not induce sneezing. (H) Schematic diagram illustrating the neural pathway for sneezing (nasal NMB+ sensory neurons → NMBR+ neurons in the sneeze-evoking region → cVRG neurons). TG: trigeminal ganglia; rVRG: rostral ventral respiratory group; preBÖtC: pre-BÖtzinger complex; BÖtC: BÖtzinger complex; DRG: dorsal respiratory group. Each dot represents an individual mouse (n=6–10 mice/group). Data are represented as mean ± SEM. ** P≤0.01.
Since NMBR+ neurons receive the sensory inputs from nasal sensory neurons, we further tested whether cVRG neurons are excited by sneeze-inducing stimuli. We activated sensory neurons using capsaicin and found that it elicited robust AP firing in 31.8% of cVRG neurons from WT mice (7 out of 22 neurons recorded; Figure 7D-F) but weak or no AP firing from Nmb−/− mice (one neuron with weak AP firing out of 13 neurons recorded; Figure 7D-E).
To determine the behavioral consequence of activating cVRG neurons via NMBR+ axons, we microinjected NMB peptide directly into the cVRG region. In vivo activation of NMBR+ axons innervating cVRG induces significant sneezing responses compared with the vehicle control (Figure 7G). Importantly, this sneeze-inducing effect was not observed in Nmbr−/− mice (Figure 7G), confirming the specificity of NMB for NMBR and NMBR+ axons in vivo.
Discussion
The sneezing reflex is beneficial to our health by rapidly removing noxious environmental irritants and pathogens from our airway. However, in the context of diseases, it can be annoying (e.g., hay fever) and even life-threatening in patients who are at high risk for lung herniation, subarachnoid hemorrhage or aneurysm rupture (Baydin et al., 2005; Bradley et al., 1982; Brock and Heitmiller, 2000; Nomani et al., 2015). Sneezing also represents an important mechanism of disease transmission by generating and spreading huge numbers of infectious droplets. Despite its importance in medicine and public health, the neurophysiological mechanisms underlying sneezing have remained elusive.
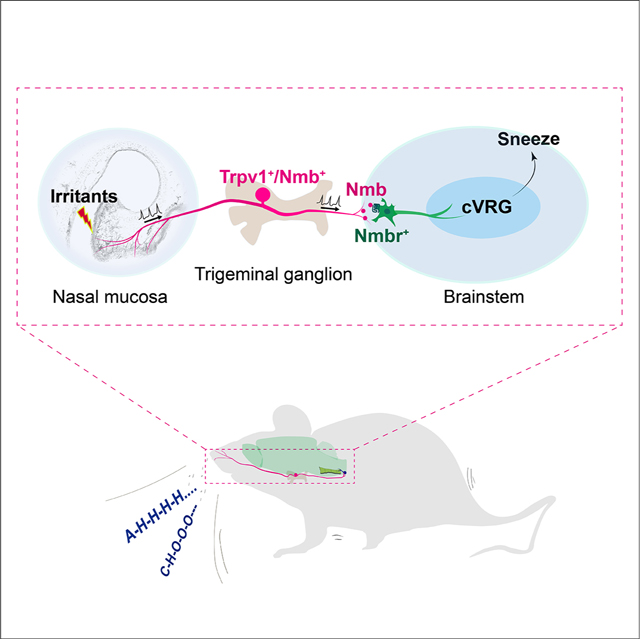
Our study identifies the peptidergic neurotransmission pathway mediating the sneezing reflex (Figure 7H). Although the anatomical location of the sneeze-evoking region has been defined in the brainstems of both cats and humans (Hersch, 2000; Nonaka et al., 1990; Seijo-Martinez et al., 2006), it was unknown which neuronal population in this region signals sneezing. Using the reverse screening strategy, we reveal that NMBR+ neurons are required for mediating sneezing induced by chemical irritants and allergens. This finding not only helps fill the gap in our knowledge of how the activation of nasal sensory fibers triggers the sneezing reflex, but also provides a non-respiratory neuronal target for suppressing intractable pathological sneezing, without affecting the normal respiration (Baydin et al., 2005; Bradley et al., 1982; Brock and Heitmiller, 2000; Nomani et al., 2015).
Although studies have shown the electrical activities of various respiratory neurons during fictive sneezing of cats (Batsel and Lines, 1978; Jakus et al., 1985; Orem and Brooks, 1986; Wallois et al., 1997), which respiratory population receives inputs from the sneeze-evoking region and drives sneezing remained unclear. Our neuronal tracing and electrophysiological recordings reveal that NMBR+ neurons synapse with cVRG expiratory neurons. Remarkably, in vivo activation of cVRG neurons via NMBR+ axons induces significant sneezing responses. These results demonstrate that cVRG expiratory neurons are the direct projection target of NMBR+ neurons in the sneeze-evoking region and are critically required for driving sneezing. However, the sneezing reflex involves both inspiratory and expiratory components. A future priority will be the integration of this peptidergic pathway with other respiratory neurons that influence sneezing. An interesting possibility is that NMBR+ neurons may synapse with as-yet-unidentified interneurons, which further regulate the activity of respiratory neurons controlling inspiration.
The newly defined sneezing pathway opens a door for future studies as to whether and how pathogens utilize our sneezing reflex to promote their propagation. We have shown that this peptidergic pathway mediates the sneezing responses to mast cell-dependent allergy (see Figure 3H-I). By exploring published transcriptome data of human mast cells (Motakis et al., 2014), we found that mast cells express multiple receptors for respiratory virus, including ICAM-1 (the receptor for the majority of human rhinovirus), CD13/aminopeptidase N for several coronaviruses (e.g., prevalent human coronavirus-229E that causes common cold), and dipeptidyl peptidase IV for Middle East respiratory syndrome coronavirus (MERS-CoV) (Dijkman and van der Hoek, 2009; Greve et al., 1989; Li, 2015). It would be interesting to test whether these receptors mediate mast cell degranulation upon viral binding and result in the release of histamine or other mediators to trigger the sneezing reflex via this neural pathway.
In summary, our findings delineate the peptidergic pathway for chemical- and allergy-induced sneezing (NMB+ nasal sensory neurons → NMBR+ neurons in the sneeze-evoking region → cVRG neurons), which have direct and immediate impact on treating pathological sneezing and important implications in the fight against infectious respiratory diseases.
Limitations of the Study
Our study reveals the neural pathway that mediates the sneezing reflex. A couple of limitations require further investigation. First, although we have shown that Trpv1+/NMB+ nasal sensory neurons initiate both chemical- and allergy-induced sneezing, TrpV1 and NMB are expressed in several transcriptionally and functionally defined subtypes of primary sensory neurons. It is unclear which subset of Trpv1+/NMB+ nasal sensory neurons specifically encode sneezing. We recently identified a subset of Trpv1+/NMB+ nasal sensory neurons that detect mast cell mediators in allergic rhinitis and trigger the sneezing reflex. We are currently testing whether they are the long-sought neuronal population mediating sneezing. Second, as a defense mechanism, the sneezing reflex is not only induced by irritative chemicals and allergens as shown in this study, but is also triggered by mechanical stimuli, strong light, cold air, and viral infection. We are establishing mouse models for these types of sneezing so that we can investigate whether the identified peptidergic pathway mediates other types of sneezing. Findings from these studies will help determine whether different types of sneezing are mediated by a common neural pathway.
STAR Methods
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Qin Liu (qinliu@wustl.edu)
Material availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate datasets/code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse lines
C57BL/6J wild-type (Stock#: 000664), Trpv1PLAP-nlacZ (Stock#: 017623), Vglut2FL°X (Stock#: 012898), Tac1−/− (Stock#: 004103) and KitW-sh/HNihrJaeBsm/J (Stock#: 005051) mice were ordered from the Jackson Laboratory. Trpv1Cre mice were gifted by Dr. Mark Hoon at the NIDCR (Mishra et al., 2011). Nmbfl°x/fl°x mice were generated using ES cells from the European Conditional Mouse Mutagenesis Program (EUCOMM) by the Molecular Genetic Service Core of the Department of Ophthalmology and Visual Science at Washington University in St. Louis. Calca−/− mice were gifted by Dr. Mark Zykla at the University of North Carolina (McCoy et al., 2012). Nmb−/−, Nmbr−/− and NmbreGFP/+ mice were provided by Dr. Zhou-Feng Chen at Washington University Center for the Study of Itch and Sensory Disorders (Wan et al., 2017; Zhao et al., 2014).
All experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at Washington University in Saint Louis, School of Medicine. All animals used in this study were housed on a 12:12 hour light cycle with ad libitum access to food and water. Three- to eight-week-old animals used for tissue collection, histology, and electrophysiological recordings. For behavioral experiments and electromyogram recording, all animals were between 8–12 weeks old at the time of testing. C57BL/6J wild-type mice were randomly assigned to control and treatment groups. Transgenic and control mice were assigned to groups based on their genotype. Researchers were blinded to treatment and mouse genotypes throughout testing and data analysis. Both male and female mice were used for electromyogram recording, tissue collection, histology, and electrophysiological recordings. No significant sex related differences were observed or noted.
METHOD DETAILS
Sneezing behavior study
Sneezing responses in mice were recorded using Buxco Small Animal Whole Body Plethysmography System (DSI, 601-1400-001 Rev11) and analyzed using the FinePointe™ Software (DSI, 007898-001 Rev03). Test animals were placed in the recording chamber for 10 minutes for acclimation and baseline testing. For chemical-induced sneezing, 0.3 ml of test solution were delivered using an ultrasonic nebulizer (DSI, 08271-001) over 2 min. Reagents used include histamine (Sigma, H7250), capsaicin (Sigma, M2028), and serotonin (Sigma, H9523). NPFF (BACHEM, H-5655.0001, 20 nmol in 2 μl saline) was instilled into each nostril of test mice. Sneezing behavior was recorded for 5 to 10 min. Sneezes, characterized by forceful expirations, were identified from the respiratory trace generated by the FinePointe Software of the WBP system. Apnea was defined as an end-expiratory pause of ≥2 average breath durations as in previous studies (Han et al., 2002; Yamauchi et al., 2008a; Yamauchi et al., 2008b). For video and audio recordings, a video camera (Canon, VIXIA HFG10) was placed on the top of the recording chamber. For simultaneous WBP and audio recordings, a mini audio recorder (aTTo digital, 16 kHz) was placed into the WBP recording chamber. Audio component was extracted from video files and analyzed using the software Audacity.
Ultrasonic recordings were conducted inside an audiometric booth. For mouse sneeze recordings, Microphone (Bruel & Kjaer, type 4134) was adjusted near the snout of the test mouse. For tail snip induced vocalization, mice were gently restrained close to the microphone (Williams et al., 2008). All sound recordings were collected using the BNC-2110 recorder (National Instruments), which is sensitive to frequencies up to 100 kHz, and were written in LabVIEW (National Instruments) consisted of I/O ports of data acquisition cards (PCI-6731 and PCI-4052e, National Instruments) and peripheral circuits. Sound signals were analyzed using Matlab.
Mast cell-dependent allergy in mice was adapted from our previous study(Han et al., 2013). In brief, 0.02% ovalbumin (OVA, Sigma, A5503) in sterile PBS (w/vol) was emulsified with an equal volume of Imject Alum (Thermo Scientific, 77161). Mice received i.p. injection of 200 μl OVA/Imject Alum mixture on the 1st and 11th day. For acute allergic model, sneezing responses were recorded for 10 min after nasal challenge with OVA (2 μl, 10%) on day 14. For chronic allergic model, sensitized animals were challenged daily with intranasal instillation of OVA (2 μl, 10%) under isoflurane anesthesia for five days (from day 14 to 18). Sneezing responses were tested immediately after nasal challenge with OVA on day 19.
Anterior ethmoidal nerve (AEN) bilateral rhizotomies
Rhizotomy of mouse AEN was performed using an intra-orbital approach as previously described (Panneton et al., 2006). Briefly, anesthetized adult mice were secured in a custom-made stereotaxic apparatus to immobilize the head during the procedure. A dorsal incision was made in the skin from bregma to the anterior of the nose to expose both sides of the medialis orbits. The AEN was seen entering the anterior ethmoidal foramen medially after retracting the eyeball laterally. Rhizotomy was accomplished after careful isolation of the AEN nerve. In the sham group, AEN was isolated without rhizotomy. After the surgery, mice were provided buprenorphine-SR (0.5 mg/kg s.c, once) and carprofen (5 mg/kg i.p, every eight hours for one day) for pain relief. Sneezing reflexes were tested two weeks after surgery.
Electromyogram recording of sneeze-related muscle movement
Electromyogram (EMG) recording of sneeze-related muscle movement was conducted according to the protocol adapted from a previous study (Satoh et al., 1998). Using IX-BIO8 biopotential recorder and LabScribe software (iWorx Systems Inc., Sn#R27196), EMGs were recorded in anesthetized adult mice via bipolar stainless steel wire electrodes implanted into the diaphragm and external oblique abdominus, respectively. Anesthesia depth was set at a level where animals were fully restrained but still produced sneezing-related muscle movements in response to nasal mechanical stimulation. Muscle movements were recorded for three minutes after saline or capsaicin (12μM) challenges. To measure muscle activity, EMG signals sampled at 1000 Hz were digitally rectified and integrated. Muscular contractions that significantly increase over regular respiratory movement and interrupt the respiratory rhythm were counted as sneezing-related responses as described in previous study (Satoh et al., 1998).
Chemical ablation or desensitization of Trpv1+ nasal sensory fiber
To degenerate or desensitize Trpv1+ fibers from the nose, resiniferatoxin (Sigma, R8756, 50 ng in 2 μl saline) or capsaicin (Sigma, M2028, 60 ng in 2 μl saline) was instilled into each nostril of ketamine/xylazine anesthetized mice. Sneezing behavior was tested five days after resiniferatoxin treatments and two days after capsaicin treatments.
Nasal secretion
Nasal secretions of adult mice were tested using phenol red thread (PRT) tests. Three minutes after saline or capsaicin (12 μM) challenge, Diagnostic threads (Zone-Quick, Menicon Co., Ltd) were placed inside the nostril for 15 seconds. The absorption of nasal mucus fluid by the thread was measured by the length of the color change (due to pH change) as indicated by the manual.
Histochemistry study of nasal sensory fibers
Adult mice were anesthetized with ketamine/xylazine cocktail and transcardially perfused with PBS, followed by 4% paraformaldehyde (PFA) in PBS (w/vol). The nose was dissected by trimming away the lower jaw and surrounding tissues, then post-fixed in 4% PFA on ice for 2 hours. The nasal tissue was incubated in a decalcification buffer (250 mM EDTA in 30% sucrose/PBS) for two days at 4°C, with buffer changes every 12 hours. After decalcification, the nose was immersed in OCT (Sakura, 4583) and placed in a vacuum chamber for 2 hours to allow OCT to fill the nasal cavity. Afterwards, embedded nose tissues were frozen for sectioning. For PGP9.5 staining, nose sections were blocked in 10% goat serum (vol/vol) for 1 hour and incubated with the primary antibody rabbit anti-PGP9.5 (Abcam, ab10404; 1:1000 in 0.2% PBST) solution overnight at 4°C. After rinse, the sections were incubated with the secondary antibody anti-Rabbit IgG - Alexa Fluor™ 488 (Invitrogen, A11008, Lot # 1797971; 1:500 in 0.2% PBST) for 2 hours at room temperature.
Trpv1-PLAP staining was adapted from our previous study (Liu et al., 2007). Briefly, nose sections were fixed in 4% PFA for 10 min on ice and rinsed with HBSS before PLAP staining. Endogenous alkaline phosphatase activity was blocked by incubation at 65°C in HBSS for 2 hours. After incubation, slides were washed in sequence with HBSS, B1 buffer (0.1 M Tris pH 7.5, 0.15 M NaCl), and freshly prepared B3 buffer (0.1 M Tris pH 9.5, 0.1 M NaCl, 50 mM MgCl2). PLAP activity was then visualized by incubation with 37.5 μg/ml nitrotetrazolium blue chloride (Sigma, N6876), 175 μg/ml 5–bromo-4-chloro-3-indolyl phosphate disodium salt (Sigma, B6149) in B3 buffer at room temperature until signals appeared. PLAP reaction was stopped by rinsing the sections in HBSS and fixed in 4% PFA at 4°C for 4 hours.
Retrograde labeling and single cell RT-PCR
Adult mice were anesthetized using ketamine/xylazine cocktail and were placed under a stereoscopic microscope. Intranasal instillation of WGA-Alexa Fluor® 488 (10 μg in 2 μl PBS, Molecular Probes, W11261) was performed using a fine glass capillary. Trigeminal ganglia were dissected and dissociated two days after instillation. Neurons were purified using a 15% BSA density gradient column as previously described(Li et al., 2019). WGA labeled cells were isolated manually using a custom made cell-picking setup comprising of a Leica DMi6000 inverted epifluorescence microscope (Buffalo Grove, IL) and a Narishige MMO-202ND micromanipulator (Amityville, NY). WGA labeled cells were identified visually under the microscope, captured, ejected into PCR tubes containing 10 μl of lysis buffer and RNase inhibitor (Invitrogen, 18080200), and flash frozen and stored at −80°C until cDNA synthesis.
cDNA was generated using Invitrogen SuperScript III CellsDirect cDNA Synthesiss Kit (Invitrogen, 18080200) according to the manufacturer’s recommended protocol. DNase digestion was performed for all samples. RT-PCR was performed using 2 μl of cDNA and Qiagen HotStarTaq Polymerase (Qiagen, 203203). Genomic DNA, obtained from single neuron, was used as the negative control. cDNA from whole trigeminal ganglion lysate was used as the positive control. Gene specific primers and annealing temperatures are listed in the supplemental table S2.
Capsaicin pain behavior
Mice were acclimated in the recording chamber for 30 min before treatment. Capsaicin solution (0.5 nmol in 2 μl saline) was instilled into each nostril. Wiping behavior was videotaped for 5 min immediately after instillation.
Enzyme-Linked Immunosorbent Assay (ELISA)
To detect NMB release from sensory neurons, TG neurons were dissociated and seeded at high density on the 7 mm glass window of a glass-bottomed culture dish (MatTek Corporation). Neurons were cultured at 37 °C for 24 hours and were gently rinsed twice with warm Ca2+- and Mg2+-free HBSS before test. To activate the neurons, 50 μL of CIB containing vehicle or stimulants were gently added onto the neurons and incubated at 37 °C for 5 min before the supernatant was collected. NMB concentration in the supernatant was then measured by ELISA (LifeSpan Biosciences, Cat#:LS-F4262) using the manufacturer’s supplied standards and their recommended protocol.
NMB-siRNA study
siRNA knockdown efficiency was evaluated using HEK-293 cells transfected with Nmb plasmid (OriGene Technologies, MR200598). 200ng of plasmid with either Nmb-siRNA (15pmol in 250μl, M-046334-01-0005, Dharmacon™) or scramble-siRNA (15pmol in 250μl, D-001206-14-05, Dharmacon™) were co-transfected into HEK293 cells. 24 hours after transfection, Nmb mRNA expression was quantified by RT-qPCR.
In vivo knockdown of Nmb was accomplished using siRNA microinjections. All injections were performed in ketamine/xylazine anesthetized mice using a Digital Stereotaxic apparatus (Leica, Model# 39477001). 5 μl of 0.4 μg/μl siRNA targeting Nmb (Dharmacon, M-046334-01-0005) or nontargeting scrambled controls (Dharmacon, D-001206-14-05) in nuclease free water were mixed with 5 μl of 1.45 μg/μl RVG-9R (Bachem, H-7502.0500) (Kumar et al., 2007) in 10% glucose and incubated at room temperature for 20 min. After incubation, the siRNA/RVG mixture (200 nl) was injected into the V1 division of trigeminal ganglion or retrotrapezoid nucleus region via a custom-made 32 gauge, 500 nl neuros-syringe (Hamilton Co.) controlled by a SMARTouch controller (World Precision Instruments, Model 40220); and repeated on the contralateral side. Coordinates for V1 division were: 0.7 mm anterior to bregma, 6.0 mm ventral from surface, ± 1.5 mm from midline. Coordinates for retrotrapezoid nucleus region were: 6.4 mm posterior to bregma, 4.75 mm ventral from surface, ± 1.3 mm from midline. Sneezing responses were tested 4 days after siRNA injection.
Immunofluorescence staining of the brainstem
Brainstems of mice were collected after 4% PFA perfusion and post-fixed for 4 hours on ice. After cryoprotected in 30% (w/v) sucrose for two nights at 4 °C, brainstem was immerged into OCT and sectioned at 50 μm using the cryostat for free-floating section staining. Sectioned tissues were blocked with 10% goat serum in PBST (PBS containing 0.1% Triton X-100) for 1 hour and incubated with primary antibodies at 4 °C overnight. After washing with PBST, secondary antibodies were applied for 2 hours at room temperature. Fluorescence images were taken using Nikon C2 confocal system. Primary antibodies including: rabbit anti-GFP (Invitrogen, A11122, Lot# 1925070; 1:1000), rabbit anti-c-Fos (Santa Cruz Biotechnology, sc-52, Lot# B0112; 1:1000), mouse anti-NeuN (MilliporeSigma, MAB377, Lot#3205920; 1:1000), goat anti-WGA (Vector Laboratories, AS-2024, Lot#T1112; 1:1000), guinea pig anti-Synaptophysin 1 (Synaptic System, 101 004; 1:200). Secondary antibodies including: goat anti-rabbit IgG-Alexa Fluor-488 (Invitrogen, A11008, Lot # 1797971; 1:500), goat anti-rabbit IgG-Alexa Fluor-555 (Invitrogen, A21429, Lot # 1683674; 1:500), Goat anti-mouse IgG-Alexa Fluor-Cyanine5 (Invitrogen, A10524, 1:500), Cy™3 AffiniPure Donkey Anti-Goat IgG (Jackson ImmunoResearch, 705165147, Lot # 148575; 1:500), goat anti-guinea pig IgG-Alexa Fluor-555 (Invitrogen, A21435; 1:500).
Neuronal ablation by NMB-saporin
WT mice were anesthetized by ketamine / xylazine and secured in the stereotaxic apparatus (Leica, Model# 39477001). NMB-saporin (Advanced Targeting Systems, Cat. #IT-70; 50 ng in 50 nl) or blank-saporin (Advanced Targeting Systems, Cat. #IT-21; 50 ng in 50 nl) was injected into the spinal trigeminal nucleus region via the coordinates (−7.60 mm posterior to bregma, −6.70 mm ventral from surface, ± 1.50 mm from midline).
Retrograde neuronal tracing from the caudal ventral respiratory group (cVRG)
Alexa 555 conjugated cholera toxin subunit B (CTB-555, Invitrogen, C34776, Lot# 2086750; 100 ng in 50 nl) was injected into the cVRG of NmbreGFP/+ mice. The coordinates were: −5.60 mm posterior to lambda, −1.30 mm ventral from the brainstem surface, ± 1.30 mm from midline. Five to seven days after injection, brainstems were collected for sectioning and immunofluorescence staining.
Brainstem preparation and electrophysiological recordings
Male mice (14–21 days old) were deeply anesthetized with isoflurane and decapitated at the cervical spinal level. The brainstem was removed and placed in ice cold solution containing (in mM): Sucrose 209, KCl 2, NaH2PO4 1.25, MgCl2 5, CaCl2 0.5, NaHCO3 26 and glucose 10, saturated with 95% O2, 5% CO2. Brainstem was vertically embedded in agar and transverse sliced (350 μm per slice) from caudal to rostral using a Vibratome (Vibratome Series 3000 Plus Tissue Sectioning System). Slices were allowed to recover at 34°C for 30 min in holding artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 92, KCl 2.5, NaH2PO4 1.25, NaHCO3 30, MgCl2 2, CaCl2 2, glucose 25 and HEPES 20, saturated with 95% O2, 5% CO2. Slices were placed at room temperature for one hour prior to recording. After incubation, slices were placed in a recording chamber continuously superfused with oxygenated recording ACSF containing (in mM): NaCl 124, KCl 2.5, NaH2PO4 1.25, NaHCO3 24, MgCl2 1, CaCl2 2, glucose 12.5 and HEPES 5. Whole-cell current-clamp recordings were performed on cVRG region of brainstem slices using a MultiClamp 700B amplifier and pCLAMP 10.5 software (Molecular Devices). The internal solution contained the following (in mM): K+-gluconate 130, NaCl 10, MgCl2 1, EGTA 0.2, HEPES10, Mg-ATP 1, Na-GTP 5 with an osmolarity of 290–300 mOsm and a pH that was adjusted to 7.25 using KOH.
NMB peptide injection into the caudal ventral respiratory group (cVRG)
WT and Nmbr−/− mice were anesthetized with 2% isoflurane and secured in the stereotaxic apparatus (Leica, Model# 39477001). NMB peptide (TOCRIS, 1908; 1 pmol in 100 nl) was injected into the cVRG region. The coordinates were: −5.60 mm posterior to lambda, −1.30 mm ventral from the brainstem surface, ± 1.30 mm from midline. Sneezing test was performed 10 min after injection, which allowed recovery from isoflurane anesthesia. Mice were euthanized after test and injection sites were checked to exclude the off-target injections.
QUANTIFICATION AND STATISTICAL ANALYSIS
All data are presented as the mean ± standard error of the mean. Sample sizes were chosen based on the “Sample size determination” (Dell et al., 2002), analysis of relevant prior studies and our pilot studies, and considerations including technical restrains, resource availability, and ethical use. No datapoint or result from a successful experiment was excluded from analysis. Unless otherwise defined n numbers in figure legends represent biological replicates (the number of mice). Statistical significance for the two groups was determined using a two-tailed Student’s t test. For three or more groups, statistical significances were tested using a one-way ANOVA followed by Tukey-Kramer post hoc tests. Difference between groups were considered statistically significant if P≤0.05. All statistical testing were performed using Prism 8 (GraphPad, San Diego, CA).
Supplementary Material
Supplemental videos S2: Video recording showing the simultaneous WBP and audio recordings of mouse sneezing responses to capsaicin, related to Figure 1.
Supplemental videos S1: Video recording of the mouse sneezing responses to capsaicin, related to Figure 1.
Figure S3. siRNA-mediated knockdown of Nmb expression, related to Figure 4. The efficiency of Nmb siRNA was tested by co-transfection of HEK293 cells with Nmb siRNA and the expression constructs of Nmb. Real-time quantitative RT-PCR shows that Nmb siRNA efficiently knocked down the expression of Nmb, compared with cells co-transfected with Nmb expression constructs and scrambled siRNA (scrambled siRNA) or Nmb constructs alone (untreated). Naïve HEK293 cells, which do not express Nmb mRNA, were used as a negative control (naïve cells). Three replicates / group; each dot represents one replicate. Data are represented as mean ± SEM. * P≤0.05, ** P≤0.01.
Figure S2. Deficiency in the neuropeptide NMB does not alter eupneic breathing of freely-moving mice, related to Figure 3. Whole body plethysmography recording revealed comparable respiratory parameters between WT and Nmb−/− mice: (A) respiratory frequency, (B) tidal volume, (C) minute ventilation, and (D) enhanced pause. Due to the prolonged recording period, mice fell asleep and resulted in the gradual decreases in respiratory frequency, tide volume and minute ventilation with time.
Figure S1: Characterization of sneeze sound in mice, related to Figure 1. (A) Nociception-associated vocalization was observed in 29.2% of mice (7 out of 24 mice) that received a painful tail snip. (B) The duration of sneeze sound (n=119 sneezes recorded from 11 mice) is significant shorter than nociception-associated vocalization (n=7 mice). (C) The sound level of mouse sneezes (n=59 sneezes recorded from 6 mice) is significantly lower than nociception-associated vocalization (n=4 mice). (D) Representative oscillogram and spectrogram of mouse sneeze sound (indicated by a red arrow). (E) Representative oscillogram and spectrogram of mouse nociception-associated vocalization, which is characterized by broadband consisting of several harmonics (indicated by arrows). Data are represented as mean ± SEM. ** P<0.01; ***P≤0.001
Figure S4: NMBR+ neurons selectively project to the caudal ventral respiratory group (cVRG), related to Figure 6. (A) Representative sagittal image showing that NMBR+ neurons selectively project to cVRG (the region around the red box), as revealed by axonal tracing in NmbreGFP reporter line. LRt, lateral reticular nucleus; VII, facial motor nucleus. (B) Higher magnification view of the boxed region in A. Scale bars: 100 μm
Highlights.
The first peptidergic pathway for chemical- and allergy-induced sneezing.
Nasal sensory neurons release neuromedin B (NMB) to signal sneezing.
NMB activates central NMBR+ neurons in the sneeze-evoking region of the brainstem.
NMBR+ neurons synapse in the caudal ventral respiratory group to drive sneezing.
Acknowledgments
We thank Qiufu Ma, Michael Panneton, Ru-Rong Ji and Guo Huang for their helpful comments on the manuscript, Feixue Liang and Fengrui Zhang for their help with audio recording. This work was supported by the National Institutes of Health (R01AI125743 and R01EY024704) and the Pew Scholar Award (to Q.L.). B.S. K. is supported by NIAMS grants (K08-AR065577, R01-AR070116 and R01 AR077007), the American Skin Association, and the Doris Duke Charitable Foundation.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Non-competing financial interests (unrelated to this work)
B.S.K. has served as a consultant for AbbVie, ABRAX Japan, Almirall, Cara Therapeutics, Maruho, Menlo Therapeutics, Pfizer, and Third Rock Ventures. He has also participated on the advisory board for Almirall, Boehringer Ingelheim, Cara Therapeutics, Kiniksa Pharmaceuticals, Menlo Therapeutics, Regeneron Pharmaceuticals, Sanofi Genzyme, and Trevi Therapeutics. He is also Founder, Chief Scientific Officer, and stockholder of Nuogen Pharma, Inc. He is stockholder of Locus Biosciences.
ADDITIONAL RESOURCES
N/A
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batsel HL, and Lines AJ (1978). Discharge of respiratory neurons in sneezes resulting from ethmoidal nerve stimulation. Exp Neurol 58, 410–424. [DOI] [PubMed] [Google Scholar]
- Baydin A, Nural MS, Guven H, Deniz T, Bildik F, and Karaduman A. (2005). Acute aortic dissection provoked by sneeze: a case report. Emerg Med J 22, 756–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouiba L. (2016). IMAGES IN CLINICAL MEDICINE. A Sneeze. N Engl J Med 375, e15. [DOI] [PubMed] [Google Scholar]
- Bourouiba L. (2020). Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA 323, 1837–1838. [DOI] [PubMed] [Google Scholar]
- Bourouiba L, Dehandschoewercker E, and M.B.J.W. (2014). Violent expiratory events: on coughing and sneezing. J Fluid Mech 745, 27. [Google Scholar]
- Bradley WG Jr., Bank WO, and Fischbeck KH (1982). Sneeze-induced hemiparesis from unruptured intracranial aneurysm. J Neuroradiol 9, 323–327. [PubMed] [Google Scholar]
- Brock MV, and Heitmiller RF (2000). Spontaneous anterior thoracic lung hernias. J Thorac Cardiovasc Surg 119, 1046–1047. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, and Julius D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, et al. (2011). Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31, 5067–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole EC, and Cook CE (1998). Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. Am J Infect Control 26, 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell RB, Holleran S, and Ramakrishnan R. (2002). Sample size determination. ILAR J 43, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkman R, and van der Hoek L. (2009). Human coronaviruses 229E and NL63: close yet still so far. J Formos Med Assoc 108, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle WJ, Boehm S, and Skoner DP (1990). Physiologic responses to intranasal dose-response challenges with histamine, methacholine, bradykinin, and prostaglandin in adult volunteers with and without nasal allergy. J Allergy Clin Immunol 86, 924–935. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, and Saito Y. (2003). Brainstem and spinal projections of augmenting expiratory neurons in the rat. Neurosci Res 45, 41–51. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA, and Gray PA (2013). Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75, 423–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P, Fusco BM, Marabini S, Maggi CA, Fanciullacci M, and Sicuteri F. (1988). Secretion, pain and sneezing induced by the application of capsaicin to the nasal mucosa in man. Br J Pharmacol 93, 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, Marlor CW, Kamarck ME, and McClelland A. (1989). The major human rhinovirus receptor is ICAM-1. Cell 56, 839–847. [DOI] [PubMed] [Google Scholar]
- Han F, Subramanian S, Price ER, Nadeau J, and Strohl KP (2002). Periodic breathing in the mouse. J Appl Physiol (1985) 92, 1133–1140. [DOI] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. (2013). A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16, 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch M. (2000). Loss of ability to sneeze in lateral medullary syndrome. Neurology 54, 520–521. [DOI] [PubMed] [Google Scholar]
- Hyden D, and Arlinger S. (2007). On the sneeze-reflex and its control. Rhinology 45, 218–219. [PubMed] [Google Scholar]
- Iscoe S. (1998). Control of abdominal muscles. Prog Neurobiol 56, 433–506. [DOI] [PubMed] [Google Scholar]
- Jakus J, Tomori Z, and Stransky A. (1985). Activity of bulbar respiratory neurones during cough and other respiratory tract reflexes in cats. Physiol Bohemoslov 34, 127–136. [PubMed] [Google Scholar]
- Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, and Iadarola MJ (2004). Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest 113, 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, Lee SK, Shankar P, and Manjunath N. (2007). Transvascular delivery of small interfering RNA to the central nervous system. Nature 448, 39–43. [DOI] [PubMed] [Google Scholar]
- Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, and Metcalfe DD (2007). Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol 119, 498–499. [DOI] [PubMed] [Google Scholar]
- Lee MG, Dong X, Liu Q, Patel KN, Choi OH, Vonakis B, and Undem BJ (2008). Agonists of the MAS-related gene (Mrgs) orphan receptors as novel mediators of mast cell-sensory nerve interactions. J Immunol 180, 2251–2255. [DOI] [PubMed] [Google Scholar]
- Li F. (2015). Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J Virol 89, 1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yang W, Jiang H, Guo C, Huang AJW, Hu H, and Liu Q. (2019). TRPV1 activity and substance P release are required for corneal cold nociception. Nat Commun 10, 5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Janczewski WA, Yackle K, Kam K, Pagliardini S, Krasnow MA, and Feldman JL (2016). The peptidergic control circuit for sighing. Nature 530, 293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Vrontou S, Rice FL, Zylka MJ, Dong X, and Anderson DJ (2007). Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci 10, 946–948. [DOI] [PubMed] [Google Scholar]
- Lucier GE, and Egizii R. (1986). Central projections of the ethmoidal nerve of the cat as determined by the horseradish peroxidase tracer technique. J Comp Neurol 247, 123–132. [DOI] [PubMed] [Google Scholar]
- McCoy ES, Taylor-Blake B, and Zylka MJ (2012). CGRPalpha-expressing sensory neurons respond to stimuli that evoke sensations of pain and itch. PLoS One 7, e36355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Holzman S, and Hoon MA (2012). A nociceptive signaling role for neuromedin B. J Neurosci 32, 8686–8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Tisel SM, Orestes P, Bhangoo SK, and Hoon MA (2011). TRPV1-lineage neurons are required for thermal sensation. EMBO J 30, 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, Lassmann T, Carninci P, Hayashizaki Y, Zuberbier T, et al. (2014). Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 123, e58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomani AZ, Rajput HM, Iqbal M, Jan Z, Irshad M, Badshah M, and Khan RS (2015). Subarachnoid hemorrhage secondary to forceful sneeze. Case Rep Neurol Med 2015, 896732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Unno T, Ohta Y, and Mori S. (1990). Sneeze-evoking region within the brainstem. Brain Res 511, 265–270. [DOI] [PubMed] [Google Scholar]
- Orem J, and Brooks EG (1986). The activity of retrofacial expiratory cells during behavioral respiratory responses and active expiration. Brain Res 374, 409–412. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Gan Q, and Juric R. (2006). Brainstem projections from recipient zones of the anterior ethmoidal nerve in the medullary dorsal horn. Neuroscience 141, 889–906. [DOI] [PubMed] [Google Scholar]
- Satoh I, Shiba K, Kobayashi N, Nakajima Y, and Konno A. (1998). Upper airway motor outputs during sneezing and coughing in decerebrate cats. Neurosci Res 32, 131–135. [DOI] [PubMed] [Google Scholar]
- Scharfman BE, Techet AH, Bush JWM, and Bourouiba L. (2016). Visualization of sneeze ejecta: steps of fluid fragmentation leading to respiratory droplets. Exp Fluids 57, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seijo-Martinez M, Varela-Freijanes A, Grandes J, and Vazquez F. (2006). Sneeze related area in the medulla: localisation of the human sneezing centre? J Neurol Neurosurg Psychiatry 77, 559–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K, Nakazawa K, Ono K, and Umezaki T. (2007). Multifunctional laryngeal premotor neurons: their activities during breathing, coughing, sneezing, and swallowing. J Neurosci 27, 5156–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, and Oh U. (2007). TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci 27, 2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songu M, and Cingi C. (2009). Sneeze reflex: facts and fiction. Ther Adv Respir Dis 3, 131–141. [DOI] [PubMed] [Google Scholar]
- Tang JW, Li Y, Eames I, Chan PK, and Ridgway GL (2006). Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect 64, 100–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Kollarik M, MacGlashan DW Jr., and Undem BJ (2005). Nasal sensory nerve populations responding to histamine and capsaicin. J Allergy Clin Immunol 116, 1282–1288. [DOI] [PubMed] [Google Scholar]
- Tonnesen P, and Mygind N. (1985). Nasal challenge with serotonin and histamine in normal persons. Allergy 40, 350–353. [DOI] [PubMed] [Google Scholar]
- Uzzaman A, and Story R. (2012). Chapter 5: Allergic rhinitis. Allergy Asthma Proc 33 Suppl 1, S15–18. [DOI] [PubMed] [Google Scholar]
- Wada E, Way J, Lebacq-Verheyden AM, and Battey JF (1990). Neuromedin B and gastrin-releasing peptide mRNAs are differentially distributed in the rat nervous system. J Neurosci 10, 2917–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Way J, Shapira H, Kusano K, Lebacq-Verheyden AM, Coy D, Jensen R, and Battery J. (1991). cDNA cloning, characterization, and brain region-specific expression of a neuromedin-B-preferring bombesin receptor. Neuron 6, 421–430. [DOI] [PubMed] [Google Scholar]
- Wallois F, Bodineau L, Macron JM, Marlot D, and Duron B. (1997). Role of respiratory and non-respiratory neurones in the region of the NTS in the elaboration of the sneeze reflex in cat. Brain Res 768, 71–85. [DOI] [PubMed] [Google Scholar]
- Wallois F, Macron JM, Jounieaux V, and Duron B. (1991). Trigeminal afferences implied in the triggering or inhibition of sneezing in cats. Neurosci Lett 122, 145–147. [DOI] [PubMed] [Google Scholar]
- Wan L, Jin H, Liu XY, Jeffry J, Barry DM, Shen KF, Peng JH, Liu XT, Jin JH, Sun Y, et al. (2017). Distinct roles of NMB and GRP in itch transmission. Sci Rep 7, 15466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WO, Riskin DK, and Mott AK (2008). Ultrasonic sound as an indicator of acute pain in laboratory mice. J Am Assoc Lab Anim Sci 47, 8–10. [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Dostal J, Kimura H, and Strohl KP (2008a). Effects of buspirone on posthypoxic ventilatory behavior in the C57BL/6J and A/J mouse strains. J Appl Physiol (1985) 105, 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Ocak H, Dostal J, Jacono FJ, Loparo KA, and Strohl KP (2008b). Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir Physiol Neurobiol 162, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Wan L, Liu XY, Huo FQ, Li H, Barry DM, Krieger S, Kim S, Liu ZC, Xu J, et al. (2014). Cross-inhibition of NMBR and GRPR signaling maintains normal histaminergic itch transmission. J Neurosci 34, 12402–12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental videos S2: Video recording showing the simultaneous WBP and audio recordings of mouse sneezing responses to capsaicin, related to Figure 1.
Supplemental videos S1: Video recording of the mouse sneezing responses to capsaicin, related to Figure 1.
Figure S3. siRNA-mediated knockdown of Nmb expression, related to Figure 4. The efficiency of Nmb siRNA was tested by co-transfection of HEK293 cells with Nmb siRNA and the expression constructs of Nmb. Real-time quantitative RT-PCR shows that Nmb siRNA efficiently knocked down the expression of Nmb, compared with cells co-transfected with Nmb expression constructs and scrambled siRNA (scrambled siRNA) or Nmb constructs alone (untreated). Naïve HEK293 cells, which do not express Nmb mRNA, were used as a negative control (naïve cells). Three replicates / group; each dot represents one replicate. Data are represented as mean ± SEM. * P≤0.05, ** P≤0.01.
Figure S2. Deficiency in the neuropeptide NMB does not alter eupneic breathing of freely-moving mice, related to Figure 3. Whole body plethysmography recording revealed comparable respiratory parameters between WT and Nmb−/− mice: (A) respiratory frequency, (B) tidal volume, (C) minute ventilation, and (D) enhanced pause. Due to the prolonged recording period, mice fell asleep and resulted in the gradual decreases in respiratory frequency, tide volume and minute ventilation with time.
Figure S1: Characterization of sneeze sound in mice, related to Figure 1. (A) Nociception-associated vocalization was observed in 29.2% of mice (7 out of 24 mice) that received a painful tail snip. (B) The duration of sneeze sound (n=119 sneezes recorded from 11 mice) is significant shorter than nociception-associated vocalization (n=7 mice). (C) The sound level of mouse sneezes (n=59 sneezes recorded from 6 mice) is significantly lower than nociception-associated vocalization (n=4 mice). (D) Representative oscillogram and spectrogram of mouse sneeze sound (indicated by a red arrow). (E) Representative oscillogram and spectrogram of mouse nociception-associated vocalization, which is characterized by broadband consisting of several harmonics (indicated by arrows). Data are represented as mean ± SEM. ** P<0.01; ***P≤0.001
Figure S4: NMBR+ neurons selectively project to the caudal ventral respiratory group (cVRG), related to Figure 6. (A) Representative sagittal image showing that NMBR+ neurons selectively project to cVRG (the region around the red box), as revealed by axonal tracing in NmbreGFP reporter line. LRt, lateral reticular nucleus; VII, facial motor nucleus. (B) Higher magnification view of the boxed region in A. Scale bars: 100 μm
Data Availability Statement
This study did not generate datasets/code.