Abstract
Background & Aims:
This study compared the effectiveness of the Specific Carbohydrate Diet (SCD) to the Mediterranean Diet (MD) as treatment for Crohn’s disease (CD) with mild to moderate symptoms.
Methods:
Adult patients with CD and with mild-moderate symptoms were randomly assigned 1:1 to consume the MD or SCD for 12 weeks. For the first 6-weeks, participants received prepared meals and snacks according to their assigned diet. After 6-weeks, participants were instructed to follow the diet independently. The primary outcome was symptomatic remission at week 6. Key secondary outcomes at week 6 included: fecal calprotectin (FC) response (FC <250 μg/g and reduction by >50% among those with baseline FC >250 μg/g) and C-Reactive Protein (CRP) response (high-sensitivity CRP (hsCRP) <5 mg/L and >50% reduction from baseline among those with hsCRP >5mg/L).
Results:
194 patients were randomized, and 191 were included in the efficacy analyses. The percentage of participants who achieved symptomatic remission at week 6 was not superior with SCD (SCD 46.5%, MD 43.5%; P = .77). FC response was achieved in 8/23 participants (34.8%) with SCD and 4/13 participants (30.8%) with MD (P = .83). CRP response was achieved in 2/37 participants (5.4%) with SCD and 1/28 participant (3.6%) with MD (P = .68).
Conclusions:
SCD was not superior to MD to achieve symptomatic remission, FC response and CRP response. CRP response was uncommon. Given these results, the greater ease of following the MD, and other health benefits associated with MD, the MD may be preferred to the SCD for most patients with CD with mild to moderate symptoms.
ClinicalTrials.gov Identifier:
Keywords: nutrition, Mediterranean diet, clinical trial, randomization, comparative effectiveness
Lay Summary
In this randomized controlled trial of patients with Crohn’s disease and mild to moderate symptoms, the Specific Carbohydrate Diet was not superior to a Mediterranean Diet to achieve symptomatic remission.
Graphical Abstract
Background
Currently available Crohn’s disease (CD) therapies are approved by the United States Food and Drug Administration to treat of patients with moderate to severely active disease. Optimal management of patients with mild to moderate symptoms is uncertain. Many patients desire alternatives to chronic immunosuppressive therapy, such as therapeutic diets. High quality data to guide dietary recommendations are lacking. A Cochrane systematic review on diet for induction of remission in CD concluded that all studies provided low or very low quality evidence.1 Major medical society guidelines generally lack guidance on the use of therapeutic whole food diets as treatment for CD (summarized in2).
Several studies have identified a lower risk of CD among populations consuming a diet consistent with the traditional Mediterranean diet (MD) (i.e. high in fresh fruits, vegetables, nuts, fish and whole grains, and use of olive oil as the predominant fat source).3, 4 Consumption of a MD has also been associated with reduced symptoms and improved quality of life after CD diagnosis.5 Numerous cohort studies, randomized controlled trials, and systematic reviews have also documented the efficacy of this diet to reduce inflammation6, cardiovascular disease7, 8, cancer9, and mortality10. Thus, there are many reasons to recommend a MD diet for patients with CD.11, 12
One of the most commonly used therapeutic diets is the Specific Carbohydrate Diet (SCD). The SCD was popularized by Elaine Gottschall in the book Breaking the Vicious Cycle.13 The SCD is characterized by allowed and excluded foods. Fresh fruits and vegetables are universally acceptable with the exception of certain starchy vegetables such as potatoes and yams. Certain legumes (i.e. lentils, split peas) are permitted, however others (i.e. chickpeas, soybeans) are not. No grains are permitted in the SCD. Saccharin and honey are allowed as sweeteners. Canned fruits and vegetables are not allowed due to possible added sugars and starches. Unprocessed meats are permitted in the SCD without limitation. However, processed, canned, and most smoked meats are restricted due to possible sugars and starches used in additives. Milk is not permitted in the SCD due to lactose content. However, certain cheeses with minimal lactose content are permitted as is homemade yogurt fermented for 24-hours.
Small studies have provided preliminary evidence that use of the SCD or modified SCD both improves symptoms and reduces bowel inflammation14–17. For example, In a small randomized trial of SCD, modified SCD and a whole food diet, there was evidence of improved CD symptoms and markers of systemic inflammation with both SCD and modified SCD, however fecal calprotectin concentration did not improve in the SCD group by week 1216. Cohen et al. studied 10 children who received SCD as primary therapy.14 Four achieved complete endoscopic healing by video capsule endoscopy and six achieved clinical remission. However, large controlled trials demonstrating efficacy or superiority to other therapeutic diets have not been published. Similarly, to our knowledge, the SCD has not been thoroughly assessed for the other health benefits that have been demonstrated with the MD.
The inspiration for this study was derived in part by crowd sourcing ideas from members of the IBD Partners Patient Powered Research Network (PPRN) who identified research on the role of diet to manage IBD as a top priority. Thus, this randomized controlled trial was designed to compare the efficacy of a MD versus SCD for treatment of CD patients with mild to moderate symptoms. The hypotheses being tested were whether the SCD was superior to the MD to achieve symptomatic remission, clinical remission and reduction in biomarkers of inflammation among patients with CD who have mild to moderate symptoms.
Methods
Study Setting and Design
The Diet to INducE Remission in Crohn’s Disease (DINE-CD) study was a parallel group randomized trial comparing the effectiveness of the MD and SCD to resolve symptoms and inflammation among patients with CD. The study was conducted in 33 sites across the United States (Appendix 1). Enrollment in the trial occurred between September 29, 2017 and October 8, 2019.
Inclusion and Exclusion Criteria
Adult patients (age >18 years) with CD established by usual endoscopic, histologic and radiologic criteria and with mild to moderate symptoms defined as a short Crohn’s Disease Activity Index (sCDAI) score >175 and <400 were potentially eligible for inclusion. The sCDAI is a weighted index derived from the original CDAI and includes three patient reported symptoms: number of mostly liquid bowel movements per day, severity of abdominal pain (assessed from none to severe), and general wellbeing (assessed from generally well to terrible). Participants were required to have internet access to complete daily online symptom surveys. Exclusion criteria were pregnancy, hospitalized patients or surgery planned within 6 weeks, ostomy or known symptomatic intestinal stricture, use of the SCD within 4 weeks of screening, start or change in dose of thiopurines, natalizumab, vedolizumab or methotrexate within 12 weeks or anti-tumor necrosis factor alpha (anti-TNF) or ustekinumab within 8 weeks of screening, start or change in dose of any 5-ASA medication within 2 weeks of screening, use of antibiotics within 2 weeks of screening, start or change in corticosteroid dose within 1 week of screening or dose >20mg prednisone or equivalent, self-reported stool frequency >4 bowel movements/day when well, body mass index <16 or ≥40, celiac disease, recent Clostridioides difficile infection, diabetes, or albumin<2.0mg/dl, if measured as part of routine clinical care. For patients who had recently discontinued any of the following medications, a wash out period before screening was required to be a minimum of 4 weeks for thiopurine or methotrexate and 8 weeks for natalizumab, vedolizumab, anti-TNF, or ustekinumab.
Randomization and Intervention
A computer-generated randomization order stratified by whether the participant was currently receiving therapy with a biologic drug was used to allocate participants in a 1:1 ratio to either MD or SCD using random block sizes of 2 and 4. The randomization sequence was generated by the Biostatistics Analysis Center. Investigators and participants were not aware of which diet would be assigned next (i.e. blinded allocation). For the first six weeks of the trial, participants received weekly delivery of prepared meals consistent with their assigned diet (breakfast, lunch, dinner and two snacks per day). Meals were prepared by Healthy Chef Creations (Orlando, FL) based on menus developed by the food vendor in consultation with study dietitians. Women were provided 2500 kcal per day and men 3000 kcal per day. Participants were not required to consume all of the food. Participants assigned to the SCD received a three-day starter diet as recommended in Breaking the Vicious Cycle.13 Meals were designed to be heated in an oven or microwave. No other preparation was required. Example menus are included in the appendix of the supplemental materials.
After the first 6 weeks, participants were instructed on how to purchase and prepare their own meals and snacks. Participants were provided with meal planning guidance via the DINE-CD study website. Separate websites were created for each study arm. A dietitian was available to answer questions. Participants had the option of purchasing some or all of their meals from Healthy Chef Creations.
Outcome Measures
The primary and key secondary outcomes were measured after 6 and 12 weeks on the diet. The primary outcome measure was symptomatic remission at week 6 defined as sCDAI <150 in the absence of initiation or increase of any CD medications. Key secondary outcomes were fecal calprotectin (FC) response (reduction of FC to less than 250 μg/g and by greater than 50% from screening among those with screening FC >250 μg/g) and C-Reactive Protein (CRP) response (reduction in high-sensitivity CRP (hsCRP) to <5 mg/L and >50% reduction from screening among those with screening hsCRP >5mg/L). FC and hsCRP assays were conducted by LabCorp.
Additional dichotomous outcome measures at week 6 and 12 included CDAI<150 (in addition to the components of the sCDAI, the full CDAI also includes hematocrit, use of anti-diarrhea medications, body weight, abdominal mass on exam and extra-intestinal manifestations of CD), PRO2 remission (mean daily liquid or very soft stool frequency ≤1.5 and abdominal pain ≤1 without either being worse than baseline), combined symptomatic remission and FC response, combined symptomatic remission and CRP response, combined PRO2 remission and FC response, and combined PRO2 remission and CRP response. Additional continuous outcome measures included sCDAI, CDAI, the Short Inflammatory Bowel Disease Questionnaire (sIBDQ)18, Patient-Reported Outcomes Measurement Information System (PROMIS®) measures for fatigue, social isolation, pain interference, and sleep19, RAPID-3 (a measure of arthritis symptoms) and Bath Ankylosing Spondylitis Functional Index among those with inflammatory back pain screening score of 4 or 5 out of 5 per the guidance of the Assessment of SpondyloArthritis International Society20. Details of PROMIS measures are included in Supplemental Materials. The primary and secondary outcomes were assessed again at week 12 although the week 6 outcomes were considered the primary analysis.
For all dichotomous outcomes, a participant who withdrew prior to time of assessment or was lost to follow-up was categorized as a treatment failure. For continuous outcomes, missing data were imputed using first observation carried forward.
Assessment of Inflammation at Screening
Confirmed inflammation at screening was defined as hsCRP >5 mg/L, FC >250 μg/g, or presence of inflammation on endoscopy in the 3 months prior to screening based on retrospective review of procedure reports by the local investigator. All procedure reports were also reviewed by the data coordinating center (AS) and any disagreement was resolved by the study PI (JDL) without knowledge of treatment group or outcomes. Colonoscopy was not a required screening procedure. Rather, data from endoscopic procedures performed for clinical purposes in the 3 months prior to screening were included.
Diet Adherence Assessments
Adherence was measured at week 3, 6, 9 and 12 by asking participants to self-report their adherence to the study diet during the past week on the following scale: followed the diet all of the time, some of the time, or none of the time. Participants completed a 24-hour dietary recall at baseline, during week 6 and during week 12. These data were used to compute the Alternate Mediterranean Diet Score (AMeD)21, where a higher score implies greater consistency with a MD, and the Healthy Eating Index 2015 (HEI-2015), where higher scores imply a healthier diet.22 Details of AMeD scoring are included in the Supplemental Materials.
Adverse Events
Adverse events were assessed at each study visit and were categorized according to the Common Toxicity Criteria for Adverse Events. Serious adverse events included: death, life threatening adverse event, inpatient hospitalization or prolongation of stay, persistent or significant disability, congenital anomaly or birth defect, or other medically significant event as deemed such by the investigator.
Protocol modifications, deviations and monitoring
The supplemental methods describe the protocol modifications, deviations and study monitoring.
Statistical Methods and Power
Except where specified, all analyses were conducted following the principle of intention to treat such that participants were considered in the group that they were randomly assigned regardless of whether they followed the diet. A P value <0.05 was considered statistically significant for the primary and key secondary outcomes. Descriptive data are reported as median and interquartile range (IQR) or counts and percentage. Comparisons between the treatment groups employed standardized mean differences (SMD) where a SMD 0.20–0.49 is considered small, 0.50–0.79 medium, and >0.79 large23. Within group analysis of change in continuous outcome measures used paired t-test, and comparison of magnitude of change between groups used unpaired t-tests for symptoms and PROMIS measures. Wilcoxon sign rank and rank sum tests were used for within and between group comparisons of change in concentration of FC and CRP, respectively. Dichotomous outcomes were compared between treatment groups using Cochrane-Mantel-Haenszel chi squared test. Continuous variables were compared between groups using linear regression adjusted for the stratification factor. Although this was a randomized trial, we pre-specified that we would test for residual confounding by the following variables for the primary and key secondary outcomes using logistic regression: age, sex, smoking status, duration of CD, evidence of ongoing inflammation at screening defined as hsCRP greater than 5 mg/L, FC greater than 250 μg/g, or colonoscopy demonstrating mucosal breaks within 3 months prior to screening, duration of CD, presence of colonic and/or rectal disease, and use of corticosteroids during the trial and use of immunomodulator drugs during the trial. After seeing that most patients had prior biologic drug exposure, a post hoc analysis was conducted stratifying by whether the participant had ever used anti-TNF drugs. As an exploratory analysis, we examined the proportion of participants with a rise in FC above 250 μg/g and hsCRP above 5 mg/L at week 6 and week 12.
Pre-specified tests for heterogeneity of effect evaluated sex, presence or absence of evidence of ongoing inflammation at screening, duration of CD, presence of colonic and/or rectal disease, use of corticosteroids during the trial, use of biologic therapy during the trial, number of prior biologic therapies, and prior surgery for CD. Analyses for treatment effect heterogeneity used conditional logistic regression to account for stratified randomization and an interaction term between treatment and the potential effect modifier. See Supplemental Materials for additional details.
A pre-specified per protocol analysis was limited to participants who reported that they attempted to follow the diet all the time in the week prior to the week 6 visit. The efficacy in this group was compared to those who reported use of the diet less than all of the time and those with missing data for adherence to the diet.
Although it was not possible to blind the study participants or the evaluators to the treatment assignment, all primary analyses were finalized prior to revealing the treatment assignment to the steering committee.
With 97 p0061rticipants per group, the study had 80% to 90% power with a type 1 error of 5% to detect a 20% absolute difference in effectiveness of the two diets depending on the success rate in the reference arm. Our PPRN Patient Governance Council opined that a smaller difference is unlikely to justify the challenges of following a strict restriction diet.
Stool Microbiome Analysis
Details of DNA isolation and bioinformatics methods are described in the Supplemental Materials. Sample similarity was assessed by Bray-Curtis and Jaccard distances, and community-level differences between groups were assessed using the PERMANOVA test. The abundance of genes and taxa were analyzed at a community level using pairwise distance between samples and visualized with Principal Coordinates Analysis. Linear mixed-effects models were used to detect differences in taxon abundance between sample groups. P values from multiple testing procedures were corrected to control for a specified false discovery rate or Bonferroni method. CRP and FC values were log transformed for inclusion in linear mixed-effects models.
Author participation:
All authors had access to the study data and reviewed and approved the final manuscript.
Results
Participants
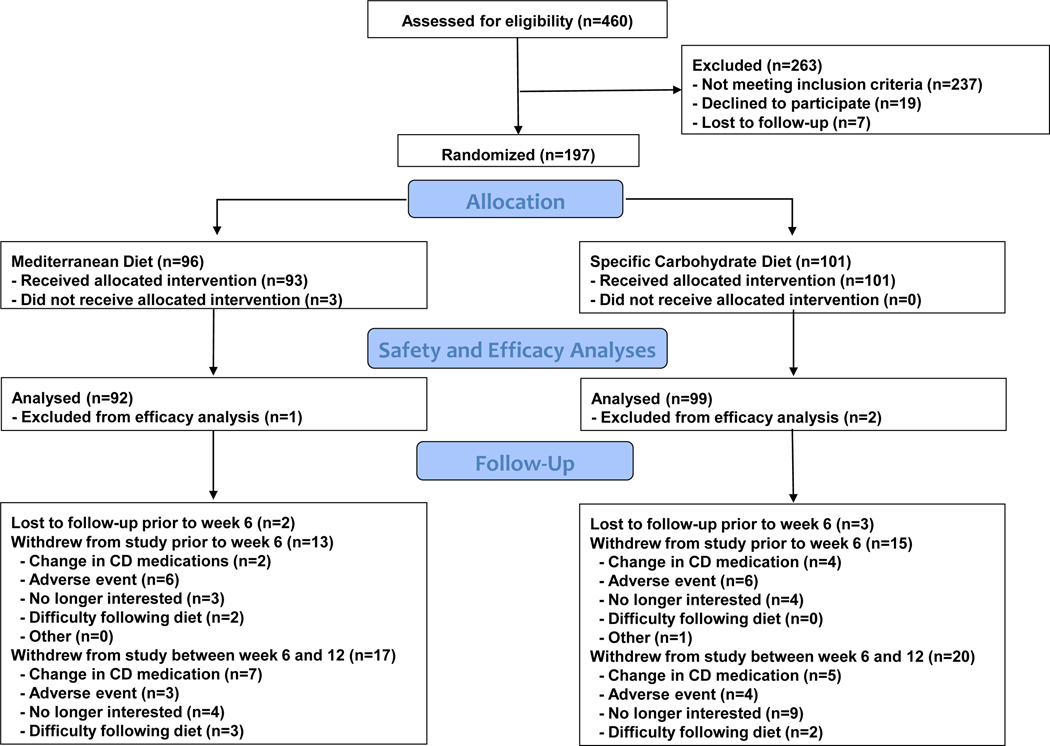
We screened 460 potential participants, of whom 263 failed to meet the eligibility criteria and 3 elected not to participate in the study after meeting the eligibility criteria but before being assigned to a diet. Figure 1 summarizes recruitment, diet allocation and early discontinuation. Ninety-three participants were randomly assigned to consume a MD and 101 to SCD. Due to baseline sCDAI <150, one participant who received the MD and two who received the SCD were excluded from the efficacy analysis. Thirty-three participants withdrew from the study prior to week 6 and 37 participants withdrew from the study between weeks 6 and 12.
Figure 1.
Study enrollment, allocation of diets and follow-up
Overall, 63% of participants were female, 91% white and 4% Hispanic ethnicity. The median BMI at screening was 25.1 (IQR 21.8–29.4). Non-stricturing and non-penetrating disease behavior was reported for 61% of participants. At screening, 57% of participants were taking a biologic medication, 67% had used one or more anti-TNF therapy in the past and 11% had used 3 or more. Confirmed evidence of inflammation at baseline by hsCRP, calprotectin or colonoscopy was documented in 47% of participants.
Covariates were generally well balanced between treatment groups (Table 1) although the MD group were more likely to be women (68.5% vs 58.6%, SMD 0.21). In contrast, the participants in the MD group were less likely to have FC >250 μg/g (14.6% vs 24.0%, SMD 0.24) and to be currently taking oral mesalamine (5ASA) (9.8% vs. 17.2%, SMD 0.22).
Table 1.
Baseline characteristics of participants in the efficacy analyses
| Characteristic | Specific Carbohydrate Diet (n=99) | Mediterranea n Diet (n=92) | SMD |
|---|---|---|---|
| Age at randomization (median years, IQR) | 36.0 (27.0–46.0) | 37.0 (29.5–53.0) | .21 |
| Female Sex | 58 (58.6) | 63 (68.5) | .21 |
| Race | |||
| Asian | 2 (2.0) | 0 (0.0) | .20 |
| Black | 5 (5.1) | 2 (2.2) | .15 |
| Multi | 1 (1.0) | 1 (1.1) | .01 |
| Other | 1 (1.0) | 3 (3.3) | .16 |
| Unknown | 2 (2.0) | 0 (0.0) | .20 |
| White | 88 (88.9) | 86 (93.5) | .16 |
| Ethnicity | |||
| Hispanic | 4 (4.0) | 4 (4.3) | .02 |
| Not Hispanic | 92 (92.9) | 88 (95.7) | .12 |
| Unknown | 3 (3.0) | 0 (0.0) | .25 |
| BMI at baseline (Median, Q1-Q3) | 25.6 (22.5–29.0) | 25.1 (21.3–29.9) | .02 |
| Smoking status | |||
| Never | 79 (79.8) | 68 (73.9) | .14 |
| Past | 20 (20.2) | 21 (22.8) | .06 |
| Current | 0 (0.0) | 3 (3.3) | .26 |
| Years since CD diagnosis (Median, Q1-Q3) | 10.0 (3.0–16.0) | 10.0 (5.0–21.0) | .17 |
| CD behavior | |||
| Non-stricturing, nonpenetrating | 60 (60.6) | 56 (60.9) | .01 |
| Stricturing | 17 (17.2) | 19 (20.7) | .09 |
| Penetrating | 13 (13.1) | 9 (9.8) | .11 |
| Stricturing and Penetrating | 9 (9.1) | 8 (8.7) | .01 |
| CD disease distributiona | |||
| Ileum alone | 30 (30.6) | 22 (23.9) | .15 |
| Colon alone | 13 (13.3) | 17 (18.5) | .14 |
| Ileum and Colon | 55 (56.1) | 53 (57.6) | .03 |
| History of perianal fistula | 20 (20.2) | 24 (26.1) | .14 |
| History of intestinal surgery | 29 (29.3) | 34 (37.0) | .16 |
| Current IBD Medications | |||
| Any biologic | 53 (53.5) | 55 (59.8) | .13 |
| Infliximab | 12 (12.1) | 10 (10.9) | .04 |
| Adalimumab | 10 (10.1) | 9 (9.8) | .01 |
| Certolizumab | 2 (2.0) | 3 (3.3) | .08 |
| Vedolizumab | 7 (7.1) | 11 (12.0) | .17 |
| Ustekinumab | 22 (22.2) | 22 (23.9) | .04 |
| Immunomodulators | 14(14.1) | 14 (15.2) | .03 |
| Oral 5ASA | 17 (17.2) | 9 (9.8) | .22 |
| Rectal 5ASA | 3 (3.0) | 1 (1.1) | .14 |
| Oral corticosteroids | 14(14.1) | 8 (8.7) | .17 |
| Rectal steroids | 2 (2.0) | 1 (1.1) | .08 |
| Number of prior or current anti-TNF medicationsb | |||
| 0 | 34 (34.3) | 28 (30.4) | .08 |
| 1 | 29 (29.3) | 22 (23.9) | .12 |
| 2 | 26 (26.3) | 32 (34.8) | .19 |
| 3 | 9 (9.1) | 9 (9.8) | .02 |
| 4 | 1 (1.0) | 1 (1.1) | .01 |
| Prior vedolizumab | 22 (22.2) | 27 (29.3) | .16 |
| Prior ustekinumab | 23 (23.2) | 22 (23.9) | .02 |
| hsCRP mg/L | 3.2 (1.4–8.1) | 2.5 (1.2–6.2) | .05 |
| hsCRP > 5 mg/L | 37 (37.4) | 28 (30.4) | .15 |
| FC μg/g | 107.5 (16.0–223.0) | 40.0 (16.0–185.0) | .02 |
| FC > 250 μg/g | 23 (24.0) | 13 (14.6) | .24 |
| Inflammation on colonoscopy | |||
| Not performed | 89 (89.9) | 80 (87.0) | .09 |
| Yes | 8 (8.1) | 8 (8.7) | .02 |
| Probably | 0 (0.0) | 1 (1.1) | .15 |
| Probably not | 1 (1.0) | 1 (1.1) | .01 |
| FC > 250 μg/g or hsCRP > 5 mg/L at | .21 | ||
| baseline or definite inflammation on colonoscopy | 50 (52.1) | 38 (41.8) | |
| sCDAI (Median, Q1-Q3) | 226.0 (197.0–263.8) | 217.7 (195.7–247.0) | .12 |
| CDAI (Median, Q1-Q3) | 210.0 (169.8–246.2) | 206.8 (179.3–248.0) | .02 |
| Short IBDQ (Median, Q1-Q3) | 40.0 (33.0–45.0) | 38.0 (33.0–44.0) | .06 |
| PROMIS measures (Median, Q1-Q3) | |||
| Fatigue | 58.9 (54.8–65.6) | 60.4 (55.9–65.9) | .10 |
| Pain interference | 61.3 (55.7–63.6) | 60.1 (55.7–63.8) | .07 |
| Sleep disturbance | 54.5 (49.4–60.2) | 57.3 (51.4–62.1) | .25 |
| Social isolation | 47.2 (34.8–53.8) | 48.4 (41.0–56.1) | .24 |
| Inflammatory back pain screenc | |||
| 0 | 40 (40.8) | 30 (33.0) | .18 |
| 1–3 | 40 (69.0) | 39 (61.9) | .15 |
| >3 | 1S (31.0) | 22 (34.9) | .0S |
| RAPID-3d | |||
| 0–12 | 56 (S3.6) | 49 (76.6) | .1S |
| >12 | 11 (16.4) | 15 (23.4) | .1S |
| AMeD Score | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | .27 |
missing data for 1 participant
prior or current use
missing data for 1 participant from each group
0–30 scale
SMD – Standardized mean difference
Primary and Secondary Outcomes
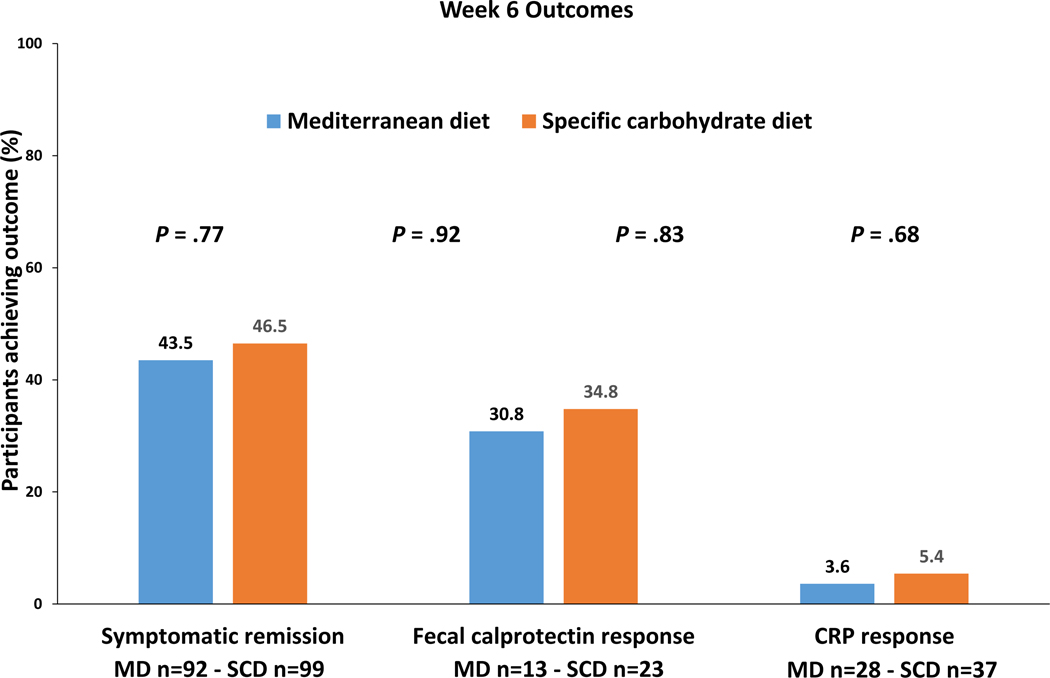
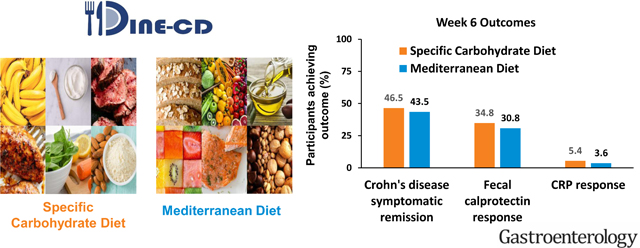
The percentage of participants who achieved the primary outcome, symptomatic remission at week 6 was not superior with SCD compared to MD, (SCD 46.5%, MD 43.5% , P = .77) (Figure 2).
Figure 2.
Primary and key secondary outcomes after 6 weeks of diet therapy.
Within the SCD and MD groups, from week 0 to week 6, there was improvement in the sCDAI, CDAI, short IBDQ, fatigue, sleep interference, pain and social isolation (p<0.02 for all outcomes in both arms). However, the magnitude of change with SCD was not superior to that with MD (p>0.2 for all comparisons) (Supplemental Table 1). Weights were available for 78 MD and 83 SCD participants at week 6. Mean percent change in weight was −2.6% (±3.3%) with MD and −2.6% (±3.7%) with SCD (P = .91).
High-sensitivity CRP did not change significantly in either group from screening to week 6. Reduction in FC concentration was significant only in the SCD group (median reduction (μg/g) −140, interquartile range (IQR) −321, 0, P = .0001 within group, P = .44 between groups) (Supplemental Table 2).
The percentage of patients achieving each of the key secondary outcomes was not superior with SCD at week 6 (Figure 2). Among those with elevated FC at screening, FC response was achieved in 8/23 participants (34.8%) with SCD and 4/13 participants (30.8%) with MD (P = .83). Among those with elevated hsCRP at screening, CRP response was achieved in only 2/37 participants (5.4%) with SCD and 1/28 participant (3.6%) with MD (P = .68). Because of the slight imbalance of several baseline characteristics, regression models were used to assess for residual confounding despite the randomized design. None of the factors tested altered the odds ratio for symptomatic remission by 10% or more. For FC response, the final model included duration of CD of greater or less than 10 years as a covariate. The results were comparable to the overall analysis with adjusted OR 1.55 (95% confidence interval 0.34–7.01, P = .57). There were too few patients achieving the 50% reduction in hsCRP to allow for adjusted analyses.
The percentage of participants achieving other secondary outcomes at week 6 did not differ between the two treatment groups (Table 2). Among the participants achieving FC response at week 6, one of four (25%) on MD also achieved symptomatic remission at week 6 as compared to six of eight on the SCD diet (75%). Overall, combined symptomatic remission and FC response rates were 26.1% with SCD and 7.7% with MD (P = .25).
Table 2.
Rates of achieving secondary outcome
| Specific Carbohydrate Diet (N) | Mediterranean Diet (N) | Specific Carbohydrate Diet (N, %) | Mediterranean Diet (N, %) | P value | |
|---|---|---|---|---|---|
| Week 6 | |||||
|
| |||||
| CDAI<150 | 99 | 92 | 48 (48.5) | 44 (47.8) | .92 |
| Symptomatic remission and FC response | 23 | 13 | 6 (26.1) | 1 (7.7) | .25 |
| Symptomatic remission and CRP response | 37 | 28 | 2 (5.4) | 0 (0.0) | .22 |
| PRO2 remission | 99 | 92 | 34 (34.3) | 25 (27.2) | .37 |
| PRO2 remission and FC response | 23 | 13 | 3 (13.0) | 0 (0.0) | .23 |
| PRO2 remission and CRP response | 37 | 28 | 2 (5.4) | 0 (0.0) | .22 |
|
| |||||
| Week 12 | |||||
|
| |||||
| CDAI<150 | 99 | 92 | 40 (40.4) | 43 (46.7) | .28 |
| Symptomatic remission and FC response | 23 | 13 | 3 (13.0) | 0 (0.0) | .23 |
| Symptomatic remission and CRP response | 37 | 28 | 4 (10.8) | 1 (3.6) | .27 |
| PRO2 remission | 99 | 92 | 33 (33.3) | 29 (31.5) | .91 |
| PRO2 remission and FC response | 23 | 13 | 3 (13.0) | 0 (0.0) | .23 |
| PRO2 remission and CRP response | 37 | 28 | 4 (10.8) | 1 (3.6) | .27 |
CDAI – Crohn’s Disease Activity Index; FC – fecal calprotectin; CRP – C-Reactive Protein; PRO2 – two component patient reported outcome
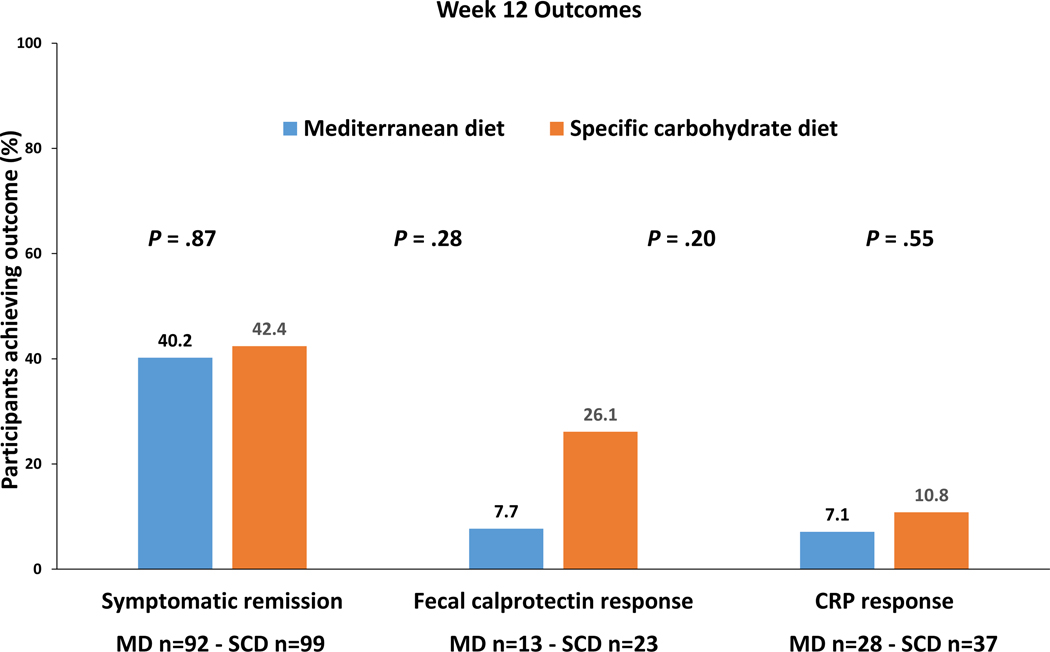
At week 12, the percentage of participants who achieved symptomatic remission was 42.4% with the SCD and 40.2% with the MD (P = .87) (Figure 3). Clinical remission at week 12 was achieved in 40.4% with SCD and 46.7% with MD (P = .28). Among those with elevated FC at screening, FC response was achieved in 6 participants (26.1%) with SCD and 1 participant (7.7%) with MD (P = .20). Among those with elevated hsCRP at screening, CRP response was achieved in 4 participants (10.8%) with SCD and 2 participants (7.1%) with MD (P = .55). Among the participants achieving FC response at week 12, three of six on the SCD diet (50%) achieved symptomatic remission as compared to zero of one (0%) on MD. Combined symptomatic remission and FC response rates at week 12 were 3/23 (13.0%) with SCD and 0/13 (0%) with MD (P = .23). Similarly, combined symptomatic remission and CRP response rates at week 12 were 4/37 (10.8%) with SCD and 1/28 (3.6%) with MD (P = .27) (Table 2). Change in CRP and FC between screening and week 12 among those with elevated concentrations at screening is summarized in Table 2.
Figure 3.
Primary and key secondary outcomes after 12 weeks of diet therapy
Exploratory analyses of worsening symptoms or increased inflammation based on a rise in FC or hsCRP by week 6 did not show significant differences between the treatment groups. Eighteen participants in the SCD group and 15 participants in the MD group withdrew from the study prior to week 6. Among participants with data available at week 6, only two participants had a rise in sCDAI of 100 points, both in the SCD group (P = .17) (Supplemental Table 3). The number of participants with a rise in FC above 250 μg/g at week 6 was 2 (4.3%) with SCD and 3 (6.3%) with MD (P = .67). The number of participants with a rise in hsCRP above 5 mg/L at week 6 was 9 (17.3%) with SCD and 8 (16.7%) with MD (P = .88).
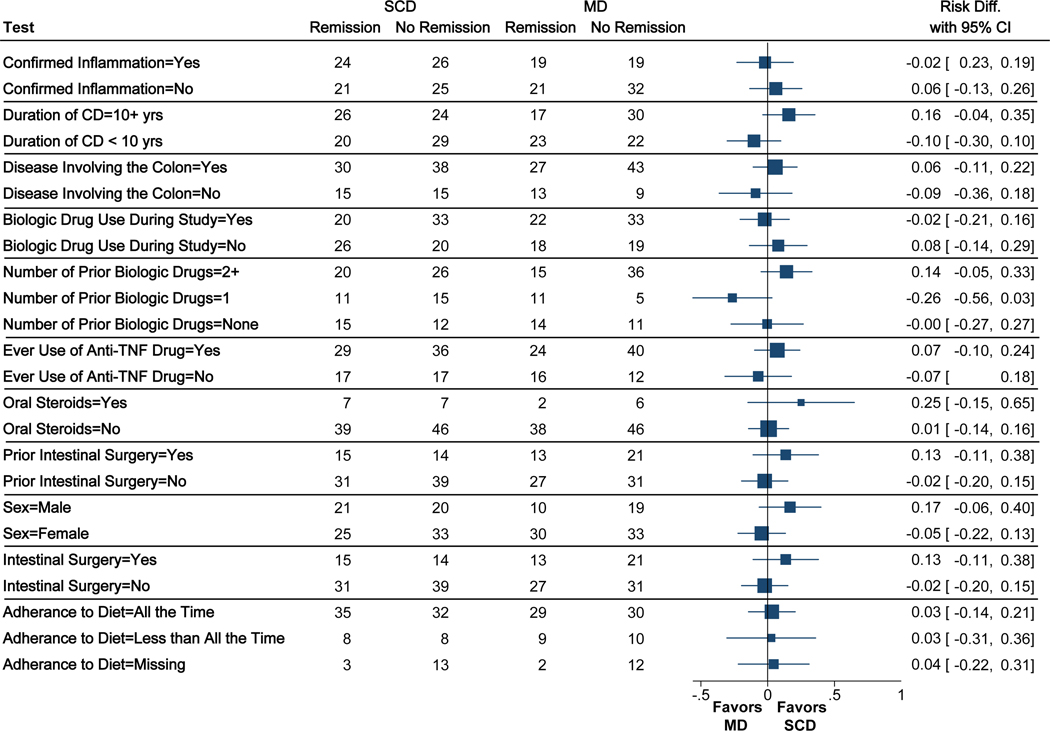
Stratified results to assess for heterogeneity of effect are summarized in Figure 4. A total of 88 participants had confirmed inflammation at screening defined as an elevated hsCRP or FC concentration or a colonoscopy demonstrating mucosal breaks (SCD n=50, MD n=38). Among these, symptomatic remission was achieved in 48% with SCD and 50% with MD (P = .86); while among those without documented inflammation, symptomatic remission was achieved in 46% with SCD and 40% with MD (P = .68; test for interaction P = .68). No significant treatment effect heterogeneity was detected for other variables, including duration of CD (P = .08), colonic involvement (P = .33), use of a biologic medication during the trial (P = .49), number of prior biologic medications used (P = .09), prior bowel resection surgery (P = .17), sex (P = .20) oruse of oral steroids (0.36).
Figure 4. Stratified analyses to assess for treatment effect heterogeneity.
Point estimates and 95% confidence intervals are risk differences from unadjusted models. Test for treatment effect heterogeneity using conditional logistic regression accounting for the stratified randomization all produced P values greater than 0.05.
Quality of Life Measures
The short IBDQ and PROMIS measures for fatigue, pain interference, social isolation, and sleep disturbance improved during the course of the trial regardless of the assigned diet (Supplemental Table 1). However, there was no significant difference between treatment groups at week 6 or 12 for any of these quality of life measures (p>0.3 for all comparisons) (Supplemental Table 4).
Back and Joint Symptoms
Most patients did not have severe back or joint symptoms at enrollment into the trial (Table 1). Among the patients with Inflammatory Back Pain Screen20 greater than 3, the median BASFI scores at week 6 and 12 were comparable between the groups (Supplemental Table 5). A sensitivity analysis comparing median BASFI among patients with an Inflammatory Back Pain Screen score of 3 or more produced similar results (week 6 P = .16; week 12 P = .23). Similarly, among the patients with RAPID3 score >12 at screening, there was no difference in the median RAPID3 at week 6 or 12 between the diet groups (Supplemental Table 5).
Adherence to Diet
Adherence to the study diet was based on self-report. Adherence to the diet all of the time in the 6th week of the study was reported by 67 (68%) of those on SCD and 59 (64%) of those on MD. Among those reporting adherence to the diet all of the time during the 6th week of the study, symptomatic remission was achieved in 52% of the SCD patients and 49% of the MD patients (P = .70). Similar comparative effectiveness was observed for those reporting adherence to the diet less than all of the time and those with missing adherence data (test for interaction P = .98) (Figure 4). By week 12, 40 (40%) and 39 (42%) of patients reported adherence all of the time to the SCD and MD, respectively. Among those on MD, 14 (15%) reported purchasing food from Healthy Chef Creations during the 12th week of the study as compared to 8 (8%) on the SCD.
We used 24-hour dietary recalls to further characterize change in diet based on the AMeD and HEI-2015. At screening, participants had relatively similar AMeD (SCD median 3, IQR 2, 4; MD 3, IQR 2, 4; SMD=0.27). The AMeD increased in both groups (P < .0001 for MD and SCD) such that the median AMeD was comparable between the groups at week 6 ()SCD 4 (IQR 3, 5), MD 5 (IQR 4, 5), P = .93). In both groups, there was a significant increase in consumption of fruits (P = SCD P < .0001; MD P = .002) and vegetables (P = SCD P = .0002; MD P = .03) from screening to week 6. Diet quality as assessed with HEI-2015 improved in both groups from screening to week 6 to similar extent (P = .43) (Supplemental Table 6).
Adverse Events
The diets were relatively well tolerated over the course of the study. Serious adverse events were reported by 2 participants in both arms of the trial (P=1.00) in the first 6 weeks. By week 12, serious adverse events were reported by 3 participants with SCD and 5 participants with MD (P = .48). Any adverse event was reported by 29 (29%) participants on SCD 22 (24%) on MD and in the first 6 weeks (P = .43) and by 40 (40%) participants on SCD and 34 (37%) on MD by 12 weeks (P = .77). Most adverse events were gastrointestinal related, with abdominal pain being the most common gastrointestinal complaint in both groups during the first 6 weeks (Table 3).
Table 3.
Adverse events
| By Week 6 | By Week 12 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| AE Type or Organ System | Specific Carbohydrate Diet (N, %) | Mediterranean Diet (N, %) | P value | Specific Carbohydrate Diet (N, %) | Mediterranean Diet (N, %) | P value |
| Gastrointestinal | 17 (17%) | 15 (16%) | 1.00 | 23 (23%) | 25 (27%) | .62 |
| Infection or infestation | 3 (3%) | 5 (5%) | .48 | 7 (7%) | 10 (11%) | .45 |
| Musculoskeletal | 5 (5%) | 1 (1%) | .21 | 6 (6%) | 1 (1%) | .12 |
| Nervous system | 4 (4%) | 1 (1%) | .37 | 6 (6%) | 1 (1%) | .12 |
Microbiome
Fecal microbiome was characterized in terms of alpha and beta diversity. Richness was assessed as number of species at a rarefying level of 1000 reads. The two diet groups had comparable richness and Shannon diversity indices and there was no significant change in diversity over the course of the study (Supplemental Figure 1). Beta diversity is characterized in Supplemental Figure 2 and demonstrates distinct gradients on PC1 and PC2 that were characterized by the increasing relative abundance of Bacteroides vulgatus on PC1 and Proteobacteria Enterobacteraceae on PC2. There was decreasing relative abundance of Firmicutes on PC2, including Faecalibacterium prausnitzii, Eubacterium eligens, and Eubacterium rectale. Beta diversity changed slightly over the course of the study, but this was independent of diet. Prior intestinal surgery was included in the PERMANOVA model as it was strongly related to beta diversity (PERMANOVA P values: Time in study 0.02, Diet 0.22, Interaction of time in study and diet 0.99, Prior surgery 0.001).
Neither alpha nor beta diversity at screening were associated with achieving symptomatic remission after adjusting for assigned diet (richness P = .88; Shannon diversity P = .90; beta diversity P = .94). Similarly, at week 6, neither alpha nor beta diversity were associated with symptomatic remission (richness P = .35; Shannon diversity P = .20; beta diversity P = .46) or assigned diet (richness P = .10; Shannon diversity P = .26; beta diversity P = .26). In analysis limited to those with elevated CRP at screening, beta diversity was not associated with log CRP at any time point (P>0.3 for all comparisons). In analysis limited to those with elevated FC concentration at screening, week 6 log FC concentrations were associated with beta diversity after adjusting for diet (PERMANOVA P = .02); significant associations between log FC concentrations and beta diversity were not evident screening or week 12 (P = .3; P = .12). The week 6 association was not significant after Bonferroni correction for 6 total comparisons.
Discussion
This randomized trial compared the effectiveness of SCD and MD to treat symptoms and inflammation in CD. While there was improvement in symptoms with both diets, we observed lack of superiority of SCD relative to MD to achieve symptomatic remission at 6 or 12 weeks and no evidence of treatment effect heterogeneity based on presence or absence of confirmed inflammation during screening. Among the subgroup with elevated FC at screening, FC response by week 6 was achieved by more than 30% of patients with both diets and was not superior with SCD. CRP response at week 6 or week 12 was achieved by fewer than 11% of patients with both diets. Thus, while symptomatic remission was common, few patients achieved combined symptomatic remission and resolution of inflammation. Based on the results of this trial, the greater ease of following the MD, and the other health benefits associated with the MD6–10, these results suggest that the MD would be preferred to the SCD for most patients with CD with mild to moderate symptoms.
Given the high rates of symptomatic remission, it is not surprising that participants in both treatment groups demonstrated improvement in quality of life measures. This included IBD specific quality of life and more general symptoms such as fatigue. We are not able to determine from this study whether the improvement represents regression to the mean, the impact of participating in a clinical trial or a true biological effect related to the diets. Although not directly comparable, several meta-analyses have estimated CD remission rates with placebo of approximately 18%.24, 25 If the remission was directly a consequence of the diets, perhaps it relates to the consumption of a diet sourced from fresh ingredients.
An important feature of the trial was the provision of prepared food for the first 6 weeks. This resulted in high self-reported adherence. The lower self-reported adherence in the second 6-weeks of the study suggests that adherence may decline significantly when patients are expected to buy and prepare meals themselves. Alternatively, this could reflect the challenge of maintaining a diet pattern that differs from one’s usual diet.
The MD and SCD diets used in this trial had some similarities, which included an emphasis on fresh fruits and vegetables. The result was that both groups had an increase in AMeD and HEI-2015 during the trial. The lack of difference in symptomatic outcomes and quality of life between the treatment arms could be from this or other similarities of the study diets, such as both being prepared with fresh ingredients.
Given the comparative effectiveness design, it is not possible to state whether either diet is better than continuing one’s usual diet. The two study diets differed from participants’ usual diet as evidence by the increase in the AMeD and HEI-2015 in both groups. The increase in AMeD score was mostly in response to increased fruit, vegetable and the ratio of monounsaturated to polyunsaturated lipid intake. The high percentage of participants who achieved symptomatic remission, suggests that it may not be necessary to recommend a low fiber diet for patients with mild to moderate symptoms of non-stricturing CD.
This trial included patients with and without documented inflammation at screening. In prior studies, the Crohn’s Disease Exclusion Diet (CDED) demonstrated significant benefit in patients with documented inflammation26 while the low FODMAP diet improved symptoms in patients without evidence of ongoing inflammation.27 There are some common features of the MD and the CDED, such as emphasis on consumption of chicken and fresh fruits and vegetables and limited intake of dairy and confectionaries. Some aspects of the SCD resemble a low FODMAP diet, such as avoidance of dairy, bread and grains and unlimited consumption of unprocessed meats. In this trial, when stratifying the analysis based on the presence or absence of documented inflammation at screening, we observed no evidence of treatment effect heterogeneity for the outcome of symptomatic remission.
The efficacy of nutrition therapy may be related to disease duration. For example, the CDED demonstrated efficacy in biologic naïve patients with a short duration of CD.26 In contrast, this study included patients with longstanding disease, many of whom had been treated with biologics.
The study diets appeared safe with few serious adverse events. The most common adverse events were gastrointestinal with both diets. Because clinicians may sometimes have concern that delaying changes to medical therapy while attempting diet-based therapy may predispose the patients to significant worsening of inflammation, we conducted an exploratory analysis of rise in CRP or FC. The results demonstrated little evidence of significant harm from a 6-week trial of diet-based therapy for CD patients with mild to moderate symptoms.
This study was not designed to assess endoscopic healing.28 Exclusive enteral nutrition has been demonstrated to induce endoscopic healing.29, 30 An uncontrolled study of the CDED documented endoscopic healing in more than 70% of patients who underwent colonoscopy, although not all patients had a colonoscopy.31 In contrast, a small study of modified SCD demonstrated that none of the seven patients had complete endoscopic healing.32 Whether the lack of endoscopic healing was due to the modified version of SCD, insufficient adherence to the diet, lack of therapeutic benefit of SCD for endoscopic healing of other factors could not be determined from that study. Additional studies using colonoscopy based outcomes are needed to further define the extent of endoscopic and histologic healing that can be expected with SCD.
This study demonstrated a significant reduction in FC at week 6 among those with elevated FC at screening with SCD. Although this finding might suggest the utility of SCD, we did not observe sustained improvement this at week 12. Moreover, the proportions of patients achieving FC response was not significantly different between the diets. Additionally, the low rates of CRP response overall and relative to the rates of symptomatic remission suggest that these diets may have a greater impact on symptoms than inflammation. This should be considered when deciding in what patients and how best to employ these diets.
The duration of the intervention was planned based on interviews with patients. These patients noted that they would be unlikely to continue a highly restrictive diet such as SCD if they did not experience symptomatic benefit within 6 weeks. Our analysis of outcomes at 12 weeks were similar to those at 6 weeks suggesting that trial duration was not a significant limitation. Moreover, reduction in CRP fecal calprotectin can be seen within 3–8 weeks of initiating therapy with exclusive enteral nutrition and CDED with partial enteral nutrition.33, 34
Strict adherence to a diet may be necessary to achieve optimal results. To explore the potential impact of adherence, our subgroup analysis among patients reporting the consumption of the diet all of the time demonstrated similar results to the primary analysis.
Diets vary by geography, cultures, and other factors. The generalizability of these results to other populations is unknown. Similarly, low symptom burden was the main reason for exclusion from the trial. The efficacy of these diets to prevent disease progression or improve mild CD symptoms remains to be determined.
The mechanisms whereby therapeutic diets may improve CD symptoms and inflammation remain to be fully elucidated. Among the hypothesized mechanisms are alterations in the gut microbiome, the metabolome, changing the ratio of omega-3 and omega-6 fatty acids, promoting short chain fatty acid production by intestinal bacterial fermentation and eliminating additives.11, 12 Both of the study diets were prepared from fresh ingredients by Healthy Chef Creations, thus were similar in terms of eliminating food additives. Because CD has been associated with alteration in the gut microbiome (dysbiosis) and some have hypothesized that effectiveness of SCD is mediated through alteration of the gut microbiome, we used whole genome sequencing to compare the fecal microbiome among those receiving the two diets. The richness and Shannon diversity were comparable between the groups and remained stable throughout the study. Beta diversity changed slightly over the course of the study. This was not related to the diet or symptomatic remission but was weakly associated with FC concentration.
In conclusion, the MD and SCD were well tolerated among patients with CD with mild to moderate symptoms. Symptomatic remission was common with both diets, was not superior with SCD relative to MD and did not appear to be influenced by the presence or absence of confirmed inflammation prior to randomization. Neither diet was associated with normalization of CRP concentration. Given the lack of improvement in CRP, additional controlled trials are needed to validate the reduction in FC observed after 6 weeks with SCD. For patients with CD with mild to moderate symptoms, MD may be preferred to SCD due to its well documented general health benefits6–10 and relative ease of implementation as compared to SCD.
Supplementary Material
What You Need to Know.
BACKGROUND AND CONTEXT:
Many patients with Crohn’s disease desire alternatives to chronic immunosuppressive therapy, such as therapeutic diets. This study compared the effectiveness of the Specific Carbohydrate Diet (SCD) and Mediterranean Diet (MD) as treatment for Crohn’s disease (CD) with mild to moderate symptoms.
NEW FINDINGS:
SCD was not superior to MD to achieve symptomatic remission, FC response and CRP response. CRP response was uncommon with both diets.
LIMITATIONS:
This study was not designed to assess endoscopic healing. Diets vary by geography, cultures, and other factors. The generalizability of these results to other populations is unknown.
IMPACT:
Given these results, the greater ease of following the MD, and other health benefits associated with MD, the MD may be preferred to the SCD for most patients with CD with mild to moderate symptoms.
Acknowledgments
We thank Ariel Myatt, MS for assistance in creating manuscript figures.
Funding Support:
PCORI Contract number PPRND-1507-31465 to Crohn’s & Colitis Foundation
Crohn’s & Colitis Foundation
5UL1TR001878
P30-DK050306
P30-DK034987
Sherman award to Dr. Lewis
Penn Center for Nutritional Science and Medicine
The funding sponsor (PCORI) was not involved in the study design in the collection, analysis, and interpretation of data
Potential conflict of interest
Dr. Lewis reports grants and personal fees from Janssen Pharmaceuticals, personal fees from Samsung Bioepis, personal fees from UCB, personal fees from Bristol-Myers Squibb, grants and personal fees from Nestle Health Science, personal fees from Merck, personal fees from Celgene, personal fees from Bridge Biotherapeutics, personal fees from Pfizer, personal fees from Gilead, personal fees from Arena Pharmaceuticals, personal fees from Protagonist Therapeutics, personal fees from Entasis Therapeutics, non-financial support from AbbVie, grants from Takeda Pharmaceuticals, outside the submitted work.
Dr. Cohen reports personal fees from Abbvie, personal fees from Celgene-Bristol Myers Squibb, personal fees and non-financial support from Pfizer, personal fees from Sublimity Therapeutics, personal fees from Target RWE, personal fees from Janssen, personal fees from Ferring, personal fees from AlphaSigma, outside the submitted work.
Dr. Suskind reports other from NiMBAL Health, Inc., outside the submitted work
Dr. Scherl reports grants and personal fees from Abbvie, Janssen, and Seres, grants from AstraZeneca, Pfizer, UCB, Genentech, and Celgene, personal fees from Entera Health, Evidera, Protagonist Therapeutics, Takeda Pharmaceuticals, and Bristol Myers Squibb, and other from Gilead, outside the submitted work.
Dr. Herfarth reports personal fees from Boehringer, personal fees from Alivio, personal fees from Finch, personal fees from Gilead, grants and personal fees from Pfizer, grants from Artizan, grants from Allakos, during the conduct of the study
Dr. Li reports personal fees from Eli Lilly, personal fees from AstraZeneca, outside the submitted work.
Dr. Hanson reports personal fees from AbbVie, personal fees from Pfizer, personal fees from Bristol Myers Squibb, personal fees from Target RWE, outside the submitted work
Dr. Valentine reports grants from AbbVie, grants from Bristol Myers Squibb/Celgene, grants from Janssen, grants from Roche/Genentech, grants from Takeda, personal fees from Bristol Myers Squibb, outside the submitted work.
Dr. Kappelman reports grants, personal fees and other from Janssen, grants and personal fees from Abbvie, grants and personal fees from Pfizer, grants and personal fees from Taleda, grants and personal fees from Lilly, grants from Bristol Myers Squibb, grants from Celltrion, grants from Arenapharm, grants from Boeringer Ingelheim, grants from Roche Genentech,outside the submitted work.
Dr. Fischer reports personal fees from BMS, personal fees from Takeda, personal fees from Janssen, personal fees from Eli Lilly, outside the submitted work
Dr. Saha, MD reports other from UCB and other from Eli Lilly outside the submitted work
Dr. Bewtra reports grants from GlaxoSmithKline, Takeda, Janssen, AbbVie, Bristol Myers Squibb, and Pfizer outside the submitted work.
Dr. Saxena reports grants from Pfizer outside the submitted work.
All remaining authors have nothing to disclose.
Author roles: See table on following page
APPENDIX
Appendix 1.
DINE-CD Study Group Participating Sites and Local Principal Investigators
| Site | Principal Investigator |
|---|---|
| Atlanta Gastroenterology Associates | Douglas C. Wolf, MD |
| Boston Children’s Hospital | Bridget Hron, MD, MMSc |
| Carolinas Healthcare | John S. Hanson, MD |
| Charlotte Gastroenterology | Sanjib P. Mohanty, MD |
| Clinical Research Institute of Michigan | Ronald P. Fogel, MDCM, MHSA |
| Dartmouth-Hitchcock Medical Center | L. Campbell Levy, MD |
| Emory University | Heba N. Iskandar, MD, MSc |
| Indiana University Health | Monika Fischer, MD |
| Icahn School of Medicine at Mt. Sinai | Benjamin Cohen, MD, MAS |
| Lenox Hill Hospital | Arun Swaminath, MD |
| Mayo Clinic Rochester | Sunanda Kane, MD |
| Minnesota Gastroenterology, PA | Robert P. McCabe, Jr., MD, AGAF |
| NorthShore University Health System | Eugene F. Yen, MD |
| Northwestern University | Stephen B. Hanauer, MD |
| NYU-Langone Health | David P. Hudesman, MD |
| The Ohio State University | Anita Afzali, MD, MPH, FACG |
| The Miriam Hospital Women’s Medical Collaborative |
Colleen Kelly, MD, FACG |
| Troy Gastroenterology | John R. Weber, MD |
| University of California San Francisco | Uma Mahadevan, MD |
| University of North Carolina Chapel Hill | Hans Herfarth, MD, PhD |
| University Health Case Medical Center | Jeffery Katz, MD |
| University of Arizona | Sasha Taleban, MD |
| University of Chicago | David T. Rubin, MD |
| University of Cincinnati | Bruce Yachyshyn, MD and Gorman J. Reynolds, MD |
| University of Colorado, Denver | Mark Gerich, MD |
| University of Louisville | Gerald W. Dryden, MD, PhD, MS, MSPH |
| University of Maryland | Sandra Quezada, MD, MS |
| University of Michigan | Peter D.R. Higgins, MD, PhD, MSc |
| University of Minnesota | Eugenia Shmidt, MD |
| University of Pennsylvania | James D. Lewis, MD, MSCE |
| University of Pittsburgh Medical Center | Marc B. Schwartz, MD |
| University of Utah | Ann D. Flynn, MD |
| University of Wisconsin | Sumona Saha, MD |
| Vanderbilt University Medical Center | Sara N. Horst, MD, MPH |
| Virginia Mason Medical Center | Michael Chiorean, MD |
| Wake Forest University | Patrick D. Green, MD |
| Weill Cornell Medicine | Ellen J. Scherl, MD |
APPENDIX
| Author | Study concept and design | Acquisition of data | Analysis and interpretation | Drafting manuscript | Critical revisions | Statistical analysis | Obtaining funding |
|---|---|---|---|---|---|---|---|
| Lewis | X | X | X | X | X | X | |
| Sandler | X | X | X | X | |||
| Brotherton | X | X | X | ||||
| Brensinger | X | X | X | ||||
| Li | X | X | X | ||||
| Kappelman | X | X | X | X | |||
| Daniel | X | X | X | ||||
| Bittinger | X | X | X | ||||
| Albenberg | X | X | X | ||||
| Valentine | X | X | X | X | |||
| Hanson | X | X | X | X | |||
| Suskind | X | X | X | ||||
| Meyer | X | X | X | ||||
| Compher | X | X | X | ||||
| Bewtra | X | X | X | ||||
| Saxena | X | X | X | ||||
| Dobes | X | X | X | ||||
| Cohen | X | X | |||||
| Flynn | X | X | |||||
| Fischer | X | X | |||||
| Saha | X | X | |||||
| Swaminath | X | X | |||||
| Yacyshyn | X | X | |||||
| Scherl | X | X | |||||
| Horst | X | X | |||||
| Curtis | X | X | X | ||||
| Braly | X | X | |||||
| Nessel | X | X | X | ||||
| McCauley | X | X | |||||
| McKeever | X | X | X | ||||
| Herfarth | X | X | X |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Limketkai BN, Iheozor-Ejiofor Z, Gjuladin-Hellon T, et al. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst Rev 2019;2:CD012839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limketkai BN, Gordon M, Mutlu EA, et al. Diet Therapy for Inflammatory Bowel Diseases: A Call to the Dining Table. Inflamm Bowel Dis 2020;26:510–514. [DOI] [PubMed] [Google Scholar]
- 3.Khalili H, Hakansson N, Chan SS, et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: results from two large prospective cohort studies. Gut 2020;69:1637–1644. [DOI] [PubMed] [Google Scholar]
- 4.Ananthakrishnan AN, Khalili H, Song M, et al. High School Diet and Risk of Crohn’s Disease and Ulcerative Colitis. Inflamm Bowel Dis 2015;21:2311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papada E, Amerikanou C, Forbes A, et al. Adherence to Mediterranean diet in Crohn’s disease. Eur J Nutr 2020;59:1115–1121. [DOI] [PubMed] [Google Scholar]
- 6.Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis 2014;24:929–39. [DOI] [PubMed] [Google Scholar]
- 7.Hoevenaar-Blom MP, Nooyens AC, Kromhout D, et al. Mediterranean style diet and 12-year incidence of cardiovascular diseases: the EPIC-NL cohort study. PLoS One 2012;7:e45458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 9.Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. Int J Cancer 2014;135:1884–97. [DOI] [PubMed] [Google Scholar]
- 10.Sofi F, Cesari F, Abbate R, et al. Adherence to Mediterranean diet and health status: meta-analysis. BMJ 2008;337:a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis JD, Abreu MT. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017;152:398–414 e6. [DOI] [PubMed] [Google Scholar]
- 12.Levine A, Rhodes JM, Lindsay JO, et al. Dietary Guidance From the International Organization for the Study of Inflammatory Bowel Diseases. Clinical Gastroenterology and Hepatology 2020;18:1381–1392. [DOI] [PubMed] [Google Scholar]
- 13.Gottschall E. Breaking the Vicious Cycle. Baltimore, ON: Kirkton Press, Limited, 1994. [Google Scholar]
- 14.Cohen SA, Gold BD, Oliva S, et al. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J Pediatr Gastroenterol Nutr 2014;59:516–21. [DOI] [PubMed] [Google Scholar]
- 15.Suskind DL, Cohen SA, Brittnacher MJ, et al. Clinical and Fecal Microbial Changes With Diet Therapy in Active Inflammatory Bowel Disease. J Clin Gastroenterol 2018;52:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suskind DL, Lee D, Kim YM, et al. The Specific Carbohydrate Diet and Diet Modification as Induction Therapy for Pediatric Crohn’s Disease: A Randomized Diet Controlled Trial. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suskind DL, Wahbeh G, Gregory N, et al. Nutritional therapy in pediatric Crohn disease: the specific carbohydrate diet. J Pediatr Gastroenterol Nutr 2014;58:87–91. [DOI] [PubMed] [Google Scholar]
- 18.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol 1996;91:1571–8. [PubMed] [Google Scholar]
- 19.Kappelman MD, Long MD, Martin C, et al. Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12:1315–23 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Annals of the Rheumatic Diseases 2009;68:784–8. [DOI] [PubMed] [Google Scholar]
- 21.Fung TT, McCullough ML, Newby PK, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005;82:163–73. [DOI] [PubMed] [Google Scholar]
- 22.Reedy J, Lerman JL, Krebs-Smith SM, et al. Evaluation of the Healthy Eating Index-2015. J Acad Nutr Diet 2018;118:1622–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical power analysis for the behavioral sciences. United States of America: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 24.Su C, Lichtenstein GR, Krok K, et al. A meta-analysis of the placebo rates of remission and response in clinical trials of active Crohn’s disease. Gastroenterology 2004;126:1257–69. [DOI] [PubMed] [Google Scholar]
- 25.Jairath V, Zou G, Parker CE, et al. Systematic review with meta-analysis: placebo rates in induction and maintenance trials of Crohn’s disease. Aliment Pharmacol Ther 2017;45:1021–1042. [DOI] [PubMed] [Google Scholar]
- 26.Levine A, Wine E, Assa A, et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019;157:440–450 e8. [DOI] [PubMed] [Google Scholar]
- 27.Cox SR, Lindsay JO, Fromentin S, et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients With Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020;158:176–188 e7. [DOI] [PubMed] [Google Scholar]
- 28.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2020. [DOI] [PubMed] [Google Scholar]
- 29.Grover Z, Muir R, Lewindon P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J Gastroenterol 2014;49:638–45. [DOI] [PubMed] [Google Scholar]
- 30.Borrelli O, Cordischi L, Cirulli M, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol 2006;4:744–53. [DOI] [PubMed] [Google Scholar]
- 31.Sigall-Boneh R, Pfeffer-Gik T, Segal I, et al. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm Bowel Dis 2014;20:1353–60. [DOI] [PubMed] [Google Scholar]
- 32.Wahbeh GT, Ward BT, Lee DY, et al. Lack of Mucosal Healing From Modified Specific Carbohydrate Diet in Pediatric Patients With Crohn Disease. J Pediatr Gastroenterol Nutr 2017;65:289–292. [DOI] [PubMed] [Google Scholar]
- 33.Lee D, Baldassano RN, Otley AR, et al. Comparative Effectiveness of Nutritional and Biological Therapy in North American Children with Active Crohn’s Disease. Inflamm Bowel Dis 2015;21:1786–93. [DOI] [PubMed] [Google Scholar]
- 34.Sigall Boneh R, Van Limbergen J, Wine E, et al. Dietary Therapies Induce Rapid Response and Remission in Pediatric Patients With Active Crohn’s Disease. Clinical Gastroenterology and Hepatology 2021;19:752–759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.