Abstract
Introduction
Understanding the mechanisms underlying the differences in renal decline between men and women may improve sex-specific clinical monitoring and management. To this end, we aimed to compare the slope of renal function decline in older men and women in chronic kidney disease (CKD) Stages 4 and 5, taking into account informative censoring related to the sex-specific risks of mortality and dialysis initiation.
Methods
The European QUALity Study on treatment in advanced CKD (EQUAL) study is an observational prospective cohort study in Stages 4 and 5 CKD patients ≥65 years not on dialysis. Data on clinical and demographic patient characteristics were collected between April 2012 and December 2018. Estimated glomerular filtration rate (eGFR) was calculated using the CKD Epidemiology Collaboration equation. eGFR trajectory by sex was modelled using linear mixed models, and joint models were applied to deal with informative censoring.
Results
We included 7801 eGFR measurements in 1682 patients over a total of 2911 years of follow-up. Renal function declined by 14.0% [95% confidence interval (CI) 12.9–15.1%] on average each year. Renal function declined faster in men (16.2%/year, 95% CI 15.9–17.1%) compared with women (9.6%/year, 95% CI 6.3–12.1%), which remained largely unchanged after accounting for various mediators and for informative censoring due to mortality and dialysis initiation. Diabetes was identified as an important determinant of renal decline specifically in women.
Conclusion
In conclusion, renal function declines faster in men compared with women, which remained similar after adjustment for mediators and despite a higher risk of informative censoring in men. We demonstrate a disproportional negative impact of diabetes specifically in women.
Keywords: EQUAL, renal function decline, sex disparities
KEY LEARNING POINTS
What is already known about this subject?
It is known that the epidemiology of chronic kidney disease (CKD) differs by sex; however, the current evidence on sex-specific slopes of renal decline in advanced CKD remains inconclusive.
Studying renal function decline by sex is complicated by informative censoring caused by sex-specific risks of mortality and dialysis initiation.
What this study adds?
Men progress faster than women, even after adjustment for important mediators, and despite having a higher risk of censoring.
Diabetes is an important determinant of renal decline, with a disproportional negative impact specifically in women.
What impact this may have on practice or policy?
Our results improve understanding of the mechanisms underlying the differences in renal function decline between the sexes and help achieve individualized and sex-specific management and treatment in advanced CKD.
INTRODUCTION
The epidemiology of chronic kidney disease (CKD) differs by sex. Population-based studies across the globe consistently show a higher prevalence of CKD in women compared with men [1–7], yet ∼60% of those starting renal replacement therapy for end-stage kidney disease (ESKD) are men [8, 9]. This paradox has several potential explanations [10]. First, the longer life expectancy in women along with the natural decline of glomerular filtration rate (GFR) with age may partly explain the higher prevalence of CKD in women. Secondly, several (population-based) studies [11–14] as well as a large meta-analysis of studies in non-diabetic CKD patients [15], point towards a faster decline of renal function in men. In contrast, others have demonstrated a more rapid progression in women in various (sub-)populations [16–18], whereas some found no difference between the sexes at all [19, 20]. A meta-analysis of randomized controlled trials (RCTs) found that women progress at an equal speed as men, with adjusted analyses even suggesting a faster progression in women [21]. Given these inconclusive results, it is clear that the estimated sex-specific decline in renal function depends on the population studied; CKD stage, the presence of diabetes mellitus (postmenopausal), age, and population-based cohorts versus referred patients, are all factors that likely contribute to the variation in current evidence.
Studying renal function decline by sex is complicated by a sex-specific selection process caused by a higher mortality risk in men across all ranges of pre-ESKD estimated GFR (eGFR) [22, 23]. The effect of eGFR decline and albuminuria on mortality risk seems stronger in women, adding complexity to the selection process [22]. Furthermore, as men and women start dialysis at different levels of eGFR [24], censoring at dialysis initiation may be deemed informative when studying CKD progression. Consequently, it is important when investigating this topic to take into account informative censoring caused by mortality and dialysis initiation, as the estimated slopes of renal function decline by sex may otherwise be biased.
Understanding the mechanisms underlying the differences in renal function decline between the sexes may aid sex-specific clinical monitoring and management. To date, very few studies have investigated renal function decline by sex specifically during pre-dialysis Stages 4 and 5 in referred CKD patients of older age, and none has taken into account the potential bias caused by the sex-specific risk of mortality and dialysis initiation [25]. Consequently, here we aim to compare the slope of renal function decline in older men and women with advanced CKD, taking into account informative censoring due to mortality and dialysis. As a secondary aim, we will explore sex-specific determinants of renal function decline in this population.
MATERIALS AND METHODS
Study design and population
The European QUALity Study on treatment in advanced CKD (EQUAL) is an ongoing observational cohort study including Stages 4 and 5 CKD patients not on dialysis receiving routine medical care in Germany, Italy, the Netherlands, Poland, Sweden and the UK. Patients of ≥65 years of age were included with an incident eGFR <20 mL/min/1.73 m2 calculated by the Modification of Diet in Renal Disease equation. Patients were excluded if the drop in eGFR resulted from an acute event or if they had previously received dialysis or a kidney transplant. Approval was obtained from the medical ethical committees in each country. Informed consent was obtained from all patients. A full description of the study has been published elsewhere [26].
Data collection
Clinical data were collected between April 2012 and December 2018 on patient demographics, primary renal disease, laboratory data and cardiovascular risk factors [smoking status, body mass index (BMI), haemoglobin, blood pressure, cholesterol and diabetes mellitus]. Data on the following pre-existing cardiovascular co-morbid conditions were also collected (definitions provided in the Supplementary data): cerebrovascular disease, peripheral vascular disease, myocardial infarction, angina pectoris, congestive heart failure, left ventricular hypertrophy, hypertension and cardiac arrhythmias. Study visits were scheduled at 6-month intervals, and patients were followed until dialysis initiation, kidney transplantation, death, refusal for further participation, loss to follow-up or end of follow-up. The eGFR was calculated from serum creatinine level standardized to isotope dilution mass spectrometry using the CKD Epidemiology Collaboration (CKD-EPI) equation [27]. In addition, GFR was estimated during follow-up from routine 24-h urine collection by taking the average of creatinine clearance and urea clearance, normalized to body surface area following the Dubois and Dubois formula. Albumin–creatinine ratio (ACR) was also determined following routine 24-h urine collection, or a single sample if 24-h urinary collection, was unavailable. Primary kidney disease was classified using the codes of the ERA-EDTA and grouped as glomerulonephritis, diabetes mellitus, tubulo-interstitial disease, hypertension and miscellaneous kidney diseases.
Statistical analysis
Patient characteristics were reported by sex as mean values with standard deviations (SDs) for normally distributed continuous variables, as medians with interquartile ranges (IQRs) for skewed continuous variables and as proportions for categorical variables. Linear mixed models (LMMs) were used to model the eGFR trajectory. A random intercept was included to capture the variation in eGFR baseline value between patients and a random slope for time to capture variability in the patient’s eGFR trajectory. Due to nonlinear patient trajectories of eGFR, the latter was included as a cubic B-spline with two equally spaced knots positioned between the minimum and the maximum of follow-up. The unadjusted model includes time, sex and their interaction, and describes the sex-specific trajectory of eGFR over time. In subsequent models, we investigate to which extent the effect of sex on the eGFR trajectory is mediated by various groups of a priori defined covariates (i.e. mediators). All models were adjusted for baseline eGFR and age at inclusion.
We followed patients until death or dialysis initiation. Missing eGFR values may be introduced when patients drop out of the study due to mortality or are censored due to dialysis initiation. As the level of renal function is related to these events, drop out is deemed informative [28–30]. We applied joint models (JMs) for longitudinal and time-to-event data to avoid biased estimates of eGFR decline [31]. The JM links the LMM described above to a Cox survival model, which captures the risk of either mortality or dialysis. In this manner, the JM informs the longitudinal eGFR trajectory on missingness caused by either of these events. To determine whether the difference in eGFR slope between men and women had changed after taking into account informative censoring due to mortality or dialysis, we tested for equality between the time–sex interaction coefficients in the LMM and JM using a Z-score test [32].
Sex-specific determinants of eGFR decline were studied through effect modification using interaction analyses, specifically through three-way interactions between sex, time and the characteristics of interest. Q–Q plots were used to check whether the residuals were normally distributed, and eGFR was log-transformed to fulfil this assumption. Consequently, regression coefficients were exponentiated and interpreted as the mean percent change in eGFR per year. Only complete cases were analysed, and missing values are reported in the Supplementary data. All analyses were performed with SAS version 9.4 and R version 3.4.1.
Sensitivity analyses
We performed a number of sensitivity analyses. First, in addition to the CKD-EPI equation, we also repeated the analyses using the full age spectrum equation and the revised Lund–Malmö equation to estimate GFR [33, 34]. Secondly, we studied the association between sex and GFR decline estimated from 24-h urine collection. Thirdly, as age is an important variable in all estimating GFRs, we also considered the relationship between sex and 1/creatinine over time. Lastly, due to the wide range in individual follow-up time, we also repeated the analyses in patients with at least 1 year of follow-up.
RESULTS
Patient characteristics
Table 1 describes the baseline characteristics of 1682 patients by sex. On average, patients were 76 years old at inclusion (IQR 71–81), two-thirds were men and the eGFR at baseline was 17.0 mL/min/1.73 m2 (IQR 14.5–20.4). Women were older, had a slightly higher BMI, higher values of serum calcium, cholesterol and potassium, but lower levels of haemoglobin. Diabetes and glomerular disease accounted for a lower proportion of primary renal disease in women compared with men, whereas tubulo-interstitial disease and hypertension were more common in women. Women had higher baseline renal function and lower ACR. Regarding co-morbidity, more men had diabetes, peripheral vascular disease, myocardial infarction and angina pectoris.
Table 1.
Baseline patient characteristics by sex
| Baseline patient characteristics | Overall (n = 1682) | Men (n = 1099) | Women (n = 583) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD) | 76.30 (6.76) | 75.97 (6.45) | 76.94 (7.28) | 0.006 |
| Primary renal disease, n (%) | ||||
| Diabetes | 341 (20.5) | 238 ( 21.9) | 103 ( 17.9) | 0.006 |
| Glomerular disease | 152 ( 9.1) | 111 ( 10.2) | 41 ( 7.1) | |
| Tubulo-interstitial disease | 138 ( 8.3) | 76 ( 7.0) | 62 ( 10.8) | |
| Hypertension | 596 (35.8) | 378 ( 34.7) | 218 ( 37.9) | |
| Miscellaneous renal disorders | 436 (26.2) | 285 ( 26.2) | 151 ( 26.3) | |
| Weight, mean (SD), kg | 79.70 (17.16) | 83.44 (16.10) | 72.51 (16.88) | <0.001 |
| Height, mean (SD), cm | 167.57 (9.94) | 172.17 (7.86) | 158.81 (7.25) | <0.001 |
| BMI, mean (SD), kg/m2 | 28.42 (5.34) | 28.20 (4.81) | 28.86 (6.23) | 0.023 |
| Blood chemistry | ||||
| Albumin, mean (SD), g/dL | 37.70 (5.91) | 37.66 (5.89) | 37.78 (5.97) | 0.708 |
| Calcium, mean (SD), mmol/L | 2.24 (0.32) | 2.23 (0.32) | 2.27 (0.33) | 0.013 |
| Cholesterol, mean (SD), mmol/L | 4.53 (1.28) | 4.34 (1.17) | 4.89 (1.41) | <0.001 |
| PO4, mean (SD), mmol/L | 1.30 (0.32) | 1.30 (0.33) | 1.31 (0.30) | 0.303 |
| Potassium, mean (SD), mmol/L | 4.64 (0.61) | 4.67 (0.62) | 4.60 (0.60) | 0.037 |
| Cardiovascular | ||||
| Systolic blood pressure, mean (SD), mmHg | 142.85 (21.96) | 143.31 (21.61) | 141.99 (22.61) | 0.245 |
| Diastolic blood pressure, mean (SD) | 73.83 (11.26) | 74.00 (11.35) | 73.49 (11.10) | 0.379 |
| Hb, mean (SD), g/dL | 0.72 (0.09) | 0.73 (0.10) | 0.71 (0.09) | <0.001 |
| Current smoker, n (%) | 119 ( 9.3) | 82 ( 9.7) | 37 ( 8.4) | 0.538 |
| Ex-smoker, n (%) | 752 (63.1) | 587 ( 74.5) | 165 ( 40.8) | <0.001 |
| Renal function | ||||
| CKD-EPI, median (IQR), mL/min/1.73 m2 | 17.01 (13.79–20.11) | 16.69 (13.67–19.63) | 17.63 (14.40–21.08) | <0.001 |
| MDRD, median (IQR), mL/min/1.73 m2 | 18.57 (15.27–21.92) | 18.45 (15.05–21.62) | 18.99 (15.54–22.62) | 0.036 |
| ACR, median (IQR) | 33.67 (4.90–154.67) | 41.36 (7.47–161.10) | 19.66 (2.99–119.00) | 0.002 |
| Comorbidities, n (%) | ||||
| Diabetes | 693 (42) | 480 ( 44.5) | 213 ( 37.4) | 0.006 |
| Chronic heart failure | 290 (18.1) | 195 ( 18.7) | 95 ( 17.0) | 0.443 |
| Cerebrovascular disease | 257 (15.7) | 171 ( 15.9) | 86 ( 15.3) | 0.781 |
| Peripheral vascular disease | 279 (17.2) | 203 ( 19.2) | 76 ( 13.5) | 0.005 |
| Myocardial infarction | 287 (17.4) | 222 ( 20.6) | 65 ( 11.4) | <0.001 |
| Angina pectoris | 239 (14.7) | 178 ( 16.8) | 61 ( 10.9) | 0.002 |
| Left ventricular hypertrophy | 349 (23.7) | 244 ( 25.3) | 105 ( 20.8) | 0.062 |
| Atrial fibrillation | 297 (18.2) | 190 ( 17.9) | 107 ( 18.9) | 0.644 |
| Hypertension | 1432 (89.1) | 935 ( 89.0) | 497 ( 89.4) | 0.860 |
MDRD, Modification of Diet in Renal Disease.
The effect of sex on the eGFR trajectory
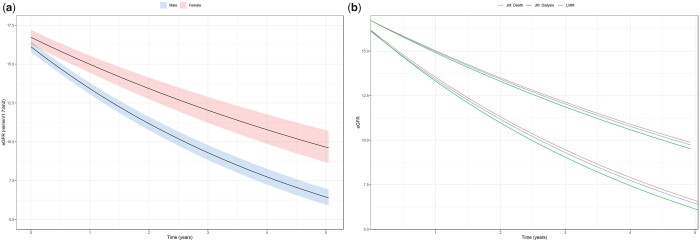
We included 7801 eGFR measurements over a total of 2911 years of follow-up, with a median (IQR) of 4 (2–7) measurements per patient, and a median follow-up time of 18.6 months (6.7–32.6). Renal function declined 14.0% [95% confidence intervals (CI) 12.9–15.1%] on average each year. Figure 1a shows a faster unadjusted annual decline in renal function in men (16.2%/year, 95% CI 15.9–17.1%) compared with women (9.6%/year, 95% CI 6.3–12.1%), with a difference of 6.6% (95% CI 4.3–9.1%). These estimates remained largely unchanged after accounting for various groups of mediators (Table 2). For the purpose of comparison with existing literature, we determined the linear sex-specific slopes of renal function decline, without log-transformation, as −1.82 (95% CI −1.63–2.01) mL/min/1.73 m2 per year for men and −0.91 (95% CI −0.40–1.43) mL/min/1.73 m2 per year for women. Sensitivity analyses using GFR estimated following routine 24-h urine collection, 1/creatinine and eGFR calculated using the full age spectrum and the revised Lund–Malmö equations provided similar results. Estimated renal function decline in a subgroup of patients with at least 1 year of follow-up was also similar to the main results (Supplementary data).
FIGURE 1.
(a) The average eGFR trajectory by sex with 95% CIs. (b) The average eGFR trajectory by sex (LMM) adjusted for censoring due to death (JM: Death) and dialysis (JM: Dialysis). The adjusted trajectories represent the average eGFR trajectory in the hypothetical situation that all patients had remained alive/had not started dialysis. The top group of lines corresponds to the eGFR trajectory in women and the bottom lines to that in men.
Table 2.
The average annual percent decline in eGFR by sex, adjusted for various groups of mediators
| Crude |
Adjusted |
|||
|---|---|---|---|---|
| Model | Estimates | P-value | Estimates | P-value |
| Unadjusted model (N = 1682, n = 7801) | ||||
| Slope men | 16.2 (14.9–17.4%) | <0.001 | – | – |
| Slope women | 9.6 (5.8–13.1%) | <0.001 | – | – |
| Difference | 6.6 (4.3–9.1%) | <0.001 | – | – |
| Adjusted for age (N = 1647, n = 7691) | ||||
| Slope men | 16.2 (15.0–17.5%) | <0.001 | 16.3 (15.0–17.6%) | <0.001 |
| Slope women | 9.6 (5.8–13.2%) | <0.001 | 9.6 (5.9–13.3%) | <0.001 |
| Difference | 6.7 (4.3 –9.1%) | <0.001 | 6.7 (4.3–6.7%) | <0.001 |
| Adjusted for ACR (N = 1006, n = 2956) | ||||
| Slope men | 14.3 (12.7–16.0%) | <0.001 | 14.2 (12.5–15.8%) | <0.001 |
| Slope women | 8.2 (3.3–12.9%) | <0.001 | 8.0 (3.1–12.6%) | <0.001 |
| Difference | 6.1 (3.1%–9.4%) | <0.001 | 6.2 (3.2–9.4%) | <0.001 |
| Adjusted for comorbiditiesa (N = 1386, n = 6547) | ||||
| Slope men | 17.1 (15.6–18.5%) | <0.001 | 17.0 (15.6–18.4%) | <0.001 |
| Slope women | 10.1 (6.0–14.1%) | <0.001 | 10.1 (5.9–14.1%) | <0.001 |
| Difference | 6.9 (4.3–9.7%) | <0.001 | 6.9 (4.3–9.7%) | <0.001 |
| Adjusted for CVD risk factorsb (N = 1158, n = 5444) | ||||
| Slope men | 16.8 (15.3–18.3) | <0.001 | 16.0 (14.5–17.5%) | <0.001 |
| Slope women | 9.9 (5.3–14.2%) | <0.001 | 9.6 (5.2–13.8%) | <0.001 |
| Difference | 6.9 (4.1–10.0%) | <0.001 | 6.4 (3.7–9.4%) | <0.001 |
| Adjusted for blood chemistryc (N = 937, n = 2633) | ||||
| Slope men | 14.9 (13.1–16.6%) | <0.001 | 12.3 (10.8–13.8%) | <0.001 |
| Slope women | 9.0 (3.7–14%) | <0.001 | 7.4 (2.8–11.4%) | <0.001 |
| Difference | 5.8 (2.6–9.4%) | <0.001 | 5.0 (2.2–7.9%) | <0.001 |
| Adjusted for PO4 (N = 1622, n = 6986) | ||||
| Slope men | 16.3 (15.0–17.6%) | <0.001 | 13.5 (12.3–14.6%) | <0.001 |
| Slope women | 9.6 (5.7–13.3%) | <0.001 | 7.9 (4.5–7.9%) | <0.001 |
| Difference | 6.7 (4.3–9.3%) | <0.001 | 5.5 (3.4–7.8%) | <0.001 |
| Adjusted for diabetes (N = 1645, n = 7687) | ||||
| Slope men | 16.3 (15.0–17.6%) | 16.3 (15.0–17.6%) | <0.001 | |
| Slope women | 9.6 (5.8–13.2%) | 9.6 (5.9–13.2%) | <0.001 | |
| Difference | 6.7 (4.3–9.2%) | 6.7 (4.3–9.2%) | <0.001 | |
Difference in annual percent decline between sex in bold. aDiabetes, chronic heart failure, cerebrovascular disease, peripheral vascular disease, myocardial infarction, angina pectoris, left ventricular hypertrophy, atrial fibrillation and hypertension.
Systolic and diastolic blood pressure, smoking status and history, and haemoglobin.
Serum albumin, cholesterol, calcium, phosphate and potassium.
The effect of sex on the eGFR trajectory adjusted for informative censoring
Figure 1b shows the eGFR trajectory in men and women after accounting for informative censoring due to death or dialysis initiation. The adjusted trajectories represent the average eGFR trajectory in the hypothetical situation that all patients had remained alive/had not started dialysis. After accounting for death (5-year cumulative incidence of 21.4% in men and 19.6% in women, P = 0.32), the difference in renal decline between men (16.1%/year, 95% CI 15.0–17.1%) and women (9.5%/year, 95% CI 6.3–12.6%) remained 6.6% (P-value for change in coefficient = 0.97). Accounting for drop out due to dialysis initiation (5-year cumulative incidence of 31.9% in men and 21.4% in women, P-value for difference <0.0001) also had little effect on the difference in renal function decline between men (17.2%/year, 95% CI 16.1–18.5%) and women (10.4%/year, 95% CI 6.9–14.2%), increasing the difference in slope between men and women marginally from 6.6% to 6.8% (P-value for change in coefficient = 0.81).
The sex-specific determinants of the eGFR trajectory
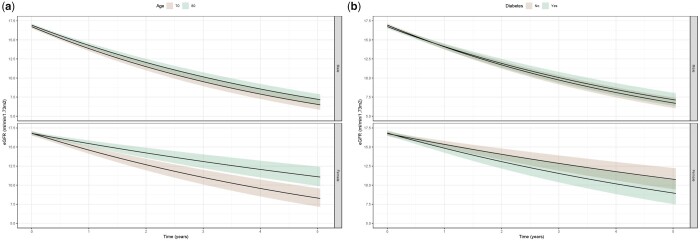
We identified effect modification by age, diabetes and myocardial infarction at inclusion on the slope of renal function decline by sex. We found that women of older age had slower declines in renal function compared with younger women (Figure 2a), whereas age had little effect on renal decline in men (P-value for interaction = 0.03). In addition, women with diabetes had significantly faster declines in renal function compared with non-diabetics, whereas this was not the case in men (Figure 2b, P-value for interaction = 0.05). The differential effect of diabetes seemed more pronounced in women under the age of 82 years (P-value for interaction = 0.02, Supplementary data, Figure S1). Other baseline characteristics did not differentially affect the slope of renal decline in men and women.
FIGURE 2.
(a) Effect modification by age on renal function decline by sex. (b) Effect modification by diabetes on renal function decline by sex.
DISCUSSION
In our population of elderly CKD Stages 4 and 5 patients not on dialysis, we demonstrate a faster decline of renal function in men compared with women, which persisted after taking into account important mediators. By applying JMs to account for the sex-specific risks of informative censoring due to death and dialysis, we demonstrate that men progress faster than women despite having a higher risk of drop out. Furthermore, we identified diabetes as an important determinant of renal decline specifically in women, demonstrating that renal function in female diabetics deteriorated at a similar pace as in men. Interestingly, older women had slower declines of renal function, indicative of a certain degree of selection bias in our cohort.
To our knowledge, this is the first study to explore renal decline by sex in a referred cohort of incident CKD patients with an eGFR of <20 mL/min/1.73 m2. We found that renal function in men declined approximately twice as fast as in women (−1.82 mL/min/1.73 m2 per year and −0.89 mL/min/1.73 m2 per year, respectively), which remained similar after adjustment for various mediators and informative censoring. Comparable studies on sex-specific renal decline during the transition period from Stages 4 and 5 CKD to dialysis are scarce [11, 25]. Nonetheless, our results are in line with studies published in cohorts consisting of patients in earlier stages of CKD [15]; a Swedish population-based study of CKD Stage 3 patients estimated similar differences in renal declines between men (−1.26 mL/min/1.73 m2 per year for a 70 year old) and women (−0.76 mL/min/1.73 m2 per year) [13]. More recently, in a referred cohort of CKD Stages 2 and 3 patients, the CRIC study also found faster declines in men (−1.43 mL/min/1.73 m2 per year) compared with women (−1.09 mL/min/1.73 m2 per year), although this difference was somewhat smaller compared with our estimates [12]. Even in the ‘healthy’ general population (cohort baseline eGFR of 80.7 mL/min/1.73 m2), the PREVEND study found an eGFR slope of −0.55 mL/min/year/1.73 m2 in men and −0.33 mL/min/year/1.73 m2 in women [14]. Altogether, most available evidence points towards a faster decline of renal function in men, seemingly regardless of CKD stage. Nonetheless, a handful of studies exist that have found either a faster progression in women [16] or no difference at all between the sexes [19, 20]. One of these studies, a large meta-analysis of RCTs, found that women progress at an equal speed as men, with adjusted analyses (baseline creatinine, blood pressure, urinary protein, age and treatment assignment), suggesting a faster progression in women [21], although this discrepancy may be attributed to stringent patient selection common to RCTs and erroneous adjustment within the causal pathway.
This sex difference in renal decline has several potential explanations related to biological and/or sociocultural aspects [10]. Risk factors related to lifestyle, such as a poor diet and smoking, may partly be responsible for faster decline as seen in men [18, 35]. Although more men had a history of smoking and a higher burden of cardiovascular co-morbidities in our cohort, adjustment for these factors had little effect on the sex difference in renal decline. Others have demonstrated differential effects of albuminuria, cholesterol, blood pressure and glycaemic control, on renal function decline in men and women [14, 16, 35], although most of these studies applied methodology corresponding to prognostic research, thus not contributing to mechanistic evidence. Lastly, sex hormones also likely play a role, as animal studies have demonstrated renoprotective effects of oestrogens and damaging effects of testosterone [25, 36–38].
We demonstrate that diabetes has a stronger effect on renal decline in women compared with men, to the extent that renal decline was similar between the sexes in those with diabetes. The literature surrounding this topic is inconsistent, with some reporting faster declines in diabetic men [39, 40], and others finding no differences between the sexes [41]. In line with our findings, a Japanese cohort of Type 2 diabetics described faster declines in women (−3.5 per year) compared with men (−2.0 per year), attributing this finding to a poorer metabolic control in women [42]. Similarly, a UK RCT in Type 2 diabetics found that women had an 88% increased risk over men of declining to <60 mL/min/1.73 m2 [43]. Moreover, excess mortality risk in diabetic women has been described in the dialysis population [44] as well as in non-renal cohorts [45, 46], confirming a disproportional negative impact of diabetes in women. Diminished protection of oestrogens in the hyperglycaemic state may explain this disparate effect, even though the women in our population were likely post-menopausal [47].
Missing eGFR values are introduced over time as patients are censored due to dialysis initiation or death. As the level of renal function is related to these events, censoring is deemed informative [28]. More importantly, as the risks of dialysis initiation or death are specific to men and women, informative censoring may affect the estimated slopes for men and women differentially, potentially introducing bias. We are unaware of any previous studies that have taken into account the sex-specific risks of drop out when studying renal decline by sex. Here, we were able to account for this issue by modelling both eGFR decline and the risk of drop out simultaneously, providing eGFR slopes corrected for both censoring due to death and dialysis. As the risk of death did not differ substantially between men and women in our cohort, adjustment had little effect on the difference in slopes between men and women. However, as Nitsch et al. demonstrated in their meta-analysis, the mortality risk difference between men and women is far larger in earlier stages of CKD [22]. In such populations, accounting for mortality would have likely had a larger effect on the difference in renal slopes between men and women, accounting for more of the difference in renal decline compared with our cohort. Conversely, accounting for censoring caused by dialysis initiation led to marginally steeper adjusted slopes, reflecting the faster renal decline in patients that were censored due to dialysis initiation. As the risk of dialysis was higher in men, the unbiased difference in renal function decline between the sexes was amplified slightly after accounting for this event, although this change in effect was not statistically significant.
Studying renal decline by sex is complicated by a sex-specific selection process throughout the pre-dialysis period. Contrary to our expectations, we found slower renal declines in older women. The literature on the effect of age on renal decline is inconsistent, with some reporting faster renal declines with increasing age [17, 48], and others reporting the opposite [49, 50]. Potential explanations for our findings may be a differential mortality rate in men and women (prior to inclusion), which may be inclined to select the healthier surviving women with slowly progressing CKD. One may also hypothesize that this finding may be caused by a sex-dependent decrease in muscle mass with age, biasing our eGFRs. Lastly, considering all patients in the EQUAL cohort are referred, there may be selection mechanisms at play in the referral patterns.
The main strength of our study is that we apply JMs to deal with informative censoring caused by mortality and dialysis initiation, providing unbiased estimates of renal decline. Furthermore, patients in our cohort were prospectively included when their eGFR dropped below the pre-defined level of 20 mL/min/1.73 m2, thus minimizing the risk of survivor bias. Our study is also subject to several limitations. Preferably, we would have used measured GFR by a reference method to estimate the slope of renal decline; however, measuring GFR with a tracer technology was unfortunately not feasible in a cohort study of the size of EQUAL. The use of eGFR in the main analysis may partly reflect muscle mass, which may disproportionately bias eGFR estimates in women [51]. Nonetheless, others have shown mGFR to perform similarly to eGFR. Lastly, due to the observational nature of our study, residual confounding may play a role, and therefore the results should be interpreted accordingly.
In older patients with advanced CKD, we demonstrate faster declines in renal function in men compared with women, even after adjustment for multiple groups of mediators. Importantly, informative events such as death and dialysis initiation explained little of the difference in renal decline between the sexes in our advanced CKD cohort. In diabetics, however, both men and women declined at a similar rate, demonstrating a disproportional negative impact of diabetes in women. Our results improve understanding of the mechanisms underlying the differences in renal function decline between the sexes and warrant further research to develop the sex-specific interventions needed to achieve individualized management and treatment.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all the patients and health professionals participating in the EQUAL study.
FUNDING
Funding was received from the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA), the Swedish Medical Association (SLS), the Stockholm County Council ALF, Njurfonden (Sweden), the Italian Society of Nephrology (SIN-Reni), the Dutch Kidney Foundation (SB 142), the Young Investigators grant in Germany and the National Institute for Health Research (NIHR) in the UK.
CONFLICT OF INTEREST STATEMENT
M.E. received payment for lectures and participated in advisory board meetings for Astellas, received lecture honoraria from Vifor, participated in the advisory board and received study grants for AstraZeneca.
REFERENCES
- 1.Roth M, Roderick P, Mindell J.. Kidney disease and renal function. In: Craig R, Mindell J, eds. Health survey for England 2010. Leeds: NHS Information Centre, 2011. Chapter 8, 1–27 [Google Scholar]
- 2.Murphy D, McCulloch CE, Lin F et al.; for the Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 2016; 165: 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong-Ajyooth L, Vareesangthip K, Khonputsa P. et al. Prevalence of chronic kidney disease in Thai adults: a national health survey. BMC Nephrol 2009; 10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasparini A, Evans M, Coresh J. et al. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 2016; 31: 2086–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanamas SK, Magliano DJ, Lynch B. et al. AUSDIAB 2012: the Australian diabetes, obesity and lifestyle study. Melbourne: Baker IDI Heart and Diabetes Institute, 2013: 1–92
- 6.Foley RN, Chen S-C, Solid CA. et al. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int 2014; 86: 1–7 [DOI] [PubMed] [Google Scholar]
- 7.United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2018
- 8.ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2016. Amsterdam UMC, location AMC, Department of Medical Informatics, Amsterdam, the Netherlands, 2018
- 9.Sparke C, Moon L, Green F. et al. Estimating the total incidence of kidney failure in Australia including individuals who are not treated by dialysis or transplantation. Am J Kidney Dis 2013; 61: 413–419 [DOI] [PubMed] [Google Scholar]
- 10.Carrero JJ, Hecking M, Chesnaye NC. et al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018; 14: 151–164 [DOI] [PubMed] [Google Scholar]
- 11.Levin A, Djurdjev O, Beaulieu M. et al. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis 2008; 52: 661–671 [DOI] [PubMed] [Google Scholar]
- 12.Ricardo AC, Yang W, Sha D et al.; on behalf of the CRIC Investigators. Sex-related disparities in CKD progression. J Am Soc Nephrol 2019; 30: 137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksen BO, Ingebretsen OC.. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 2006; 69: 375–382 [DOI] [PubMed] [Google Scholar]
- 14.Halbesma N, Brantsma AH, Bakker SJL. et al. Gender differences in predictors of the decline of renal function in the general population. Kidney Int 2008; 74: 505–512 [DOI] [PubMed] [Google Scholar]
- 15.Neugarten J, Acharya A, Silbiger SR.. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol 2000; 11: 319–329 [DOI] [PubMed] [Google Scholar]
- 16.Chang PY, Chien LN, Lin YF. et al. Risk factors of gender for renal progression in patients with early chronic kidney disease. Medicine (Baltimore) 2016; 95: e4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai E, Horio M, Yamagata K. et al. Slower decline of glomerular filtration rate in the Japanese general population: a longitudinal 10-year follow-up study. Hypertens Res 2008; 31: 433–441 [DOI] [PubMed] [Google Scholar]
- 18.Coggins CH, Lewis JB, Caggiula AW. et al. Nephrology dialysis transplantation differences between women and men with chronic renal disease. Nephrol Dial Transplant 1998; 13: 1430–1437 [DOI] [PubMed] [Google Scholar]
- 19.Norris KC, Greene T, Kopple J. et al. Baseline predictors of renal disease progression in the African American study of hypertension and kidney disease. J Am Soc Nephrol 2006; 17: 2928–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John R, Webb M, Young A. et al. Unreferred chronic kidney disease: a longitudinal study. Am J Kidney Dis 2004; 43: 825–835 [DOI] [PubMed] [Google Scholar]
- 21.Jafar TH, Schmid CH, Stark PC. et al. The rate of progression of renal disease may not be slower in women compared with men: a patient-level meta-analysis. Nephrol Dial Transplant 2003; 18: 2047–2053 [DOI] [PubMed] [Google Scholar]
- 22.Nitsch D, Grams M, Sang Y. et al. ; for the Chronic Kidney Disease Prognosis Consortium. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ 2013; 346: f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravani P, Fiocco M, Liu P. et al. Influence of mortality on estimating the risk of kidney failure in people with Stage 4 chronic kidney disease J Am Soc Nephrol 2019; 30: 2219–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright S, Klausner D, Baird B. et al. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol 2010; 5: 1828–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neugarten J, Golestaneh L.. Influence of sex on the progression of chronic kidney disease. Mayo Clin Proc 2019; 94: 1339–1356 [DOI] [PubMed] [Google Scholar]
- 26.Jager KJ, Ocak G, Drechsler C. et al. The EQUAL study: a European study in chronic kidney disease stage 4 patients. Nephrol Dial Transplant 2012; 27 (Suppl 3): 27–31 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH et al.; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson R, Diggle P, Dobson A.. Joint modelling of longitudinal measurements and event time data. Biostatistics 2000; 1: 465–480 [DOI] [PubMed] [Google Scholar]
- 29.Hu C, Sale ME.. A joint model for nonlinear longitudinal data with informative dropout. J Pharmacokinet Pharmacodyn 2003; 30: 83–103 [DOI] [PubMed] [Google Scholar]
- 30.Wu MC, Carroll RJ.. Estimation and comparison of changes in the presence of informative right censoring by modeling the censoring process. Biometrics 1988; 44: 175. [PubMed] [Google Scholar]
- 31.Rizopoulos D.The R Package JMbayes for fitting joint models for longitudinal and time-to-event data using MCMC. J Stat Softw 2016; 72: 1–46; doi:10.18637/jss.v072.i07 [Google Scholar]
- 32.Paternoster R, Brame R, Mazerolle P. et al. Using the correct statistical test for the equality of regression coefficients. Criminology 1998; 36: 859–866 [Google Scholar]
- 33.Pottel H, Hoste L, Dubourg L. et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 2016; 31: 798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyman U, Grubb A, Larsson A. et al. The revised Lund-Malmö GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med 2014; 52: 815–824 [DOI] [PubMed] [Google Scholar]
- 35.Briganti EM, Branley P, Chadban SJ. et al. Smoking is associated with renal impairment and proteinuria in the normal population: the AusDiab kidney study. Am J Kidney Dis 2002; 40: 704–712 [DOI] [PubMed] [Google Scholar]
- 36.Rk D, Ek J.. Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. Am J Physiol Ren Physiol 2001; 280: 365–388 [DOI] [PubMed] [Google Scholar]
- 37.Carrero JJ.Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res 2010; 33: 383–392 [DOI] [PubMed] [Google Scholar]
- 38.Silbiger S, Neugarten J.. Gender and human chronic renal disease. Gend Med 2008; 5 (Suppl 1): 3–10 [DOI] [PubMed] [Google Scholar]
- 39.Hemmelgarn BR, Zhang J, Manns BJ. et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int 2006; 69: 2155–2161 [DOI] [PubMed] [Google Scholar]
- 40.de Hauteclocque A, Ragot S, Slaoui Y et al.; The SURDIAGENE Study group. The influence of sex on renal function decline in people with Type 2 diabetes. Diabet Med 2014; 31: 1121–1128 [DOI] [PubMed] [Google Scholar]
- 41.Krolewski AS, Skupien J, Rossing P. et al. Fast renal decline to end-stage renal disease: an unrecognized feature of nephropathy in diabetes. Kidney Int 2017; 91: 1300–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kajiwara A, Kita A, Saruwatari J. et al. Sex differences in the renal function decline of patients with type 2 diabetes. J Diabetes Res 2016; 2016: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Margaret K, Yu Lyles CR, Bent-Shaw LA. et al. Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: the Pathways Study. Am J Nephrol 2012; 36: 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carrero JJ, de Mutsert R, Axelsson J et al.; for the NECOSAD Study Group. Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant 2011; 26: 270–276 [DOI] [PubMed] [Google Scholar]
- 45.Huxley RR, Peters SAE, Mishra GD. et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2015; 3: 198–206 [DOI] [PubMed] [Google Scholar]
- 46.Xu G, You D, Wong L. et al. Risk of all-cause mortality in women versus men with type 2 diabetes: a systematic review and meta-analysis. Eur J Endocrinol 2019; 180: 243–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maric C, Sullivan S.. Estrogens and the diabetic kidney. Gend Med 2008; 5: S103–S113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen E, Nardi Y, Krause I. et al. A longitudinal assessment of the natural rate of decline in renal function with age. J Nephrol 2014; 27: 635–641 [DOI] [PubMed] [Google Scholar]
- 49.O'Hare AM, Choi AI, Bertenthal D. et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 2007; 18: 2758–2765 [DOI] [PubMed] [Google Scholar]
- 50.Evans M, Fryzek JP, Elinder CG. et al. The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis 2005; 46: 863–870 [DOI] [PubMed] [Google Scholar]
- 51.Zaman T, Filipowicz R, Beddhu S.. Implications and importance of skeletal muscle mass in estimating glomerular filtration rate at dialysis initiation. J Ren Nutr 2013; 23: 233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.