Abstract
Diabetes mellitus is a major public health concern associated with high mortality and reduced life expectancy. The alarming rise in the prevalence of diabetes is linked to several factors including sedentary lifestyle and unhealthy diet. Nutritional intervention and increased physical activity could significantly contribute to bringing this under control. Food-derived bioactive peptides and protein hydrolysates have been associated with a number health benefits. Several peptides with antidiabetic potential have been identified that could decrease blood glucose level, improve insulin uptake and inhibit key enzymes involved in the development and progression of diabetes. Dietary proteins, from a wide range of food, are rich sources of antidiabetic peptides. Thus, there are a number of benefits in studying peptides obtained from food sources to develop nutraceuticals. A deeper understanding of the underlying molecular mechanisms of these peptides will assist in the development of new peptide-based therapeutics. Despite this, a comprehensive analysis of the antidiabetic properties of bioactive peptides derived from various food sources is still lacking. Here, we review the recent literature on food-derived bioactive peptides possessing antidiabetic activity. The focus is on the effectiveness of these peptides as evidenced by in vitro and in vivo studies. Finally, we discuss future prospects of peptide-based drugs for the treatment of diabetes.
Keywords: diabetes, bioactive peptides, food peptides, α-amylase, α-glucosidase, dipeptidyl peptidase IV
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by persistent hyperglycaemia accompanied by an array of metabolic dysfunctions. It is one of the largest global public health concerns causing high mortality and reduced life expectancy [1]. The three prevalent forms of this disorder are type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM) and gestational diabetes mellitus (GDM). Out of these, T2DM accounts for around 90% of the reported cases, 5–7% accounts for T1DM and 2–3% for GDM. T1DM involves autoimmune destruction of pancreatic β-cell that eventually leads to insulin deficiency. The progressive loss of β-cell insulin secretion along with insulin resistance accounts for T2DM. GDM is diagnosed during the second or third trimester of pregnancy [2]. Globally, the incidence of diabetes is rising at an alarming rate. The International Diabetes Federation (IDF) has estimated the global prevalence of diabetes in 2019 to be 9.3% (463 million people), with a projected increase to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045 [3]. Individuals with T2DM carry a high risk of developing other conditions such as cardiovascular disease, hypertension, stroke, chronic liver disease, chronic kidney disease and cancer [4]. The number of diabetes-related deaths between 2000 and 2019 has risen by almost 70%. According to the World Health Organisation (WHO), diabetes is now on the list of the top 10 causes of death in adults and imposes a heavy burden on public health systems [5]. This rising prevalence of diabetes is linked to several factors such as sedentary lifestyle, ageing, increased urbanisation, unhealthy diet and ubiquitous increase in body-mass index.
Various strategies have been employed to control and manage diabetes including medication, healthy diet, regular physical activity and changing lifestyle. The vast majority of widely used antidiabetic medications mainly focus on stimulating the release of insulin from the pancreas or improving insulin-stimulated glucose uptake [6]. The major classes of conventional antidiabetic drugs include sulfonylureas, biguanides, α-glucosidase inhibitors, peroxisome proliferator-activated receptor-γ (PPARγ) agonists, dipeptidyl peptidase IV (DPP IV) inhibitors, and sodium-glucose co-transporter-2 (SGLT2) inhibitors [7]. These treatment strategies are generally cost-effective. However, long-term use of these drugs could lead to severe complications including hypoglycaemia, vomiting, bloating, potential weight gain, oedema, and problems in the cardiovascular and central nervous systems [8,9]. Nearly half a billion people around the world are living with diabetes. Left unchecked, this situation will inevitably worsen. Hence, there is an urgent need for identifying, developing and implementing safer alternatives to tackle this disorder [3].
Since diabetes is closely linked with lifestyle, not surprisingly, nutritional intervention and increased physical activity could play a vital role in attenuating the problems related to diabetes. Muscular contractions induced by the physical exercises stimulate insulin-independent muscle blood glucose transport and also improves insulin sensitivity [10]. A large body of evidence from clinical trials and observational studies have emphasized the paramount significance of food and dietary patterns in the prevention and management of diabetes [11,12]. Nutraceuticals or functional foods have received significant attention recently due to their multifunctional health benefits.
Cryptides or bioactive peptides are defined as peptide fragments that are generated during proteolytic cleavage or maturation of functional proteins. These fragments may exhibit similar or completely unrelated biological properties in comparison to the parent protein [13]. Food-derived bioactive peptides are short sequences of 2–50 amino acids that are embedded within the primary structure of dietary proteins [14]. Normally, these peptide sequences are inactive in the precursor protein and are activated during food processing or enzymatic hydrolysis [8]. The precise function of these peptides depends on their amino acid composition, sequence and length [15]. A growing body of scientific evidence now indicates that peptides regulate a number of physiological processes and could act as antidiabetic, antihypertensive, antimicrobial, opioid, antioxidant, anticancer, and immunomodulatory agents [16,17,18]. To date, more than 80 peptide drugs have reached the market for various disorders including diabetes, cancer, osteoporosis, and multiple sclerosis. Additionally, several are in clinical and preclinical trials [19]. In this context, a large number of bioactive peptides possessing antidiabetic activity have been derived from various food sources including milk, egg, fish, pulses, legumes, and cereals [18,20]. These peptides regulate blood glucose levels by inhibiting major enzymes such as α-amylase, α-glucosidase, and DPP IV as well as acting as an agonist of glucagon-like peptide 1 (GLP-1) [21].
The focus of this review is to comprehensively evaluate the current knowledge of food-derived bioactive peptides possessing antidiabetic activity. Specific attention has been paid to identify the effectiveness of these peptides as evident from in vitro and in vivo studies. Furthermore, this review discusses the challenges involved in the development of and future prospects of peptide-based drugs for the treatment of diabetes.
2. Functional Foods and Bioactive Peptides
Functional foods refer to foods that provide potential health benefits beyond serving as a nutritional source. Epidemiological studies have highlighted the importance of dietary foods in managing and reducing the complications associated with diabetes [22]. Regular intake of these, including whole foods, enriched foods, and probiotics, could help with glycaemic control, pancreatic β-cell function, insulin secretion, regulation of lipid metabolism, and weight management.
As endogenous signalling molecules for several intracellular processes, peptides represent an excellent starting point for designing novel drug molecules. Furthermore, peptides are highly selective molecules with an exquisite potency and safety profiles in humans. The generally favourable pharmacological profile of peptides makes them a unique class of pharmaceutical compounds [23]. This has led to a significant growth in the utilization of bioactive peptides for managing chronic disorders in recent years. Functional foods are packed with phytochemicals, vitamins, minerals, fibre, antioxidants, fatty acids, and bioactive peptides. While many of these constituents have been widely studied, food-derived bioactive peptides have come into focus due to their nutritional capabilities and health benefits. These peptides are usually encrypted within the sequence of their parent protein and are released during maturation, or chemical, microbial, or enzymatic hydrolysis [13]. In a large number of instances, bioactive peptides liberated from the parent protein were shown to possess bioactivity that may or may not be similar to the parent protein. A myriad of bioactive peptides with multiple health benefits have been identified from various food sources. The most widely explored food sources from animals include milk, egg, and meat proteins. Bioactive peptides have also been isolated from vegetable proteins such as pulses, lentils, wheat, rice, oat, and flaxseeds. Proteins from marine organisms such as fish, tuna, salmon, squid, oyster, and crabs are also excellent sources of bioactive peptides [24,25,26]. Bioactive peptides from food sources are mainly produced by hydrolysis using digestive enzymes such as trypsin and pepsin, microbial fermentation, and food processing by pickling, canning, drying, and smoking. By far the most effective methods employed for producing peptides from food sources includes microbial fermentation and enzymatic digestion [27]. Fermentation involves the culturing of proteolytic microbes on a protein that helps in yielding shorter peptides. It represents a cost-effective method for producing natural bioactive peptides, especially in the dairy industry. Some preferred starter cultures used for the fermentation process include yeast, fungi, and microbes from the Lactobacillus genus [28]. Apart from obtaining bioactive peptides, studies have shown that fermentation improves the functional and nutritional quality of food, which in turn provides numerous health benefits [29]. Proteolytic enzyme digestion of food proteins is another efficient approach to release potentially active peptides. Some commonly used enzymes used for hydrolysis include pepsin, trypsin, chymotrypsin, alcalase, and flavourzyme. These enzymes hydrolyse peptide bonds and release peptides of different sizes and properties. Compared to microbial fermentation, enzymatic hydrolysis produces bioactive peptides in a short time with a high scalability. In both methods, bioactivity, size, and bioavailability of the released peptides largely depend on time, degree of hydrolysis, processing conditions, enzyme-substrate ratio, and pre-treatment of proteins [30]. Peptide fragments released from hydrolysed food proteins typically contain several biologically active peptides that could positively alter physiological function and reduce disease risk. They could also impart beneficial effects on endocrine, cardiovascular, digestive, nervous, and immune systems [31].
3. Current Diabetes Targets
Several therapeutic approaches are currently being employed for managing blood sugar levels and increasing insulin sensitivity. Carbohydrate metabolism is managed by a complex interplay of organs, glands, and secretions [32]. Blood sugar levels are largely regulated by the action of the two peptide hormones insulin and glucagon [33]. The use of insulin from animal pancreas began in the 1920s and is often regarded as a pioneering step in peptide therapeutics that revolutionised the treatment of T1DM [34].
Since the 1990s, α-glucosidase and α-amylase inhibitors have been considered first-line drugs for the treatment and management of T2DM [35,36]. As these digestive enzymes are involved in the catabolism of complex carbohydrates into glucose, inhibiting these enzymes is considered an effective strategy for decreasing blood sugar levels [37]. Another widely used treatment option is the inhibition of DPP IV and the activation of GLP-1 receptors using GLP-1 analogues and incretin mimetics [37,38]. GLP-1 is an important incretin hormone that helps in managing blood glucose levels by stimulating insulin release, inhibiting glucagon production, and slowing gastric emptying. However, this endogenous hormone is rapidly degraded and inactivated by DPP IV [39]. Given the significance of these molecules in managing diabetes, synthetic DPP IV inhibitors and GLP-1 receptor agonists were developed to regulate these pathways [40]. Other oral hypoglycaemic drugs include SGLT2 inhibitors, which reduce blood sugar levels by preventing reabsorption of renal glucose, and insulin sensitizers such as biguanides that improve insulin sensitivity, increase glucose uptake and prevent the production of glucose in the liver [41]. Insulin secretagogues, which stimulate insulin secretion, and thiazolidinediones (TZD), which reduce the insulin resistance in adipocytes, liver and muscles, are other treatment options used for managing diabetes [42]. Even though the number of treatment options has significantly grown over the past few decades, none of these drugs are capable of stopping the progressive decline of β-cell function [43]. The challenges and limitations of existing therapeutics have prompted the search for developing alternative or complementary drugs that are effective and have fewer, lower, or no side effects.
4. Peptide Drugs and Diabetes
In spite of the development of the therapeutic use of insulin in the early 1920s, peptide therapeutics have evolved at a slower pace over time. Even though peptides act as key biological modulators with remarkable potency, low toxicity and selectivity, limitations such as low oral bioavailability, stability, short circulation time, and cost have limited the interest of pharmaceutical companies in developing peptide-based drugs. The introduction of recombinant technology has provided a reliable and efficient option for the production of peptide drugs. The development of human insulin using recombinant technology (1982), as well as other synthetic hormones, have firmly established the market for peptide-based drugs [44]. Along with this, the discovery of natural peptide leads, from various sources including plants, animals, and microorganisms, has opened up several avenues to pursue the development of more potent antidiabetic drugs. Moreover, advancements in peptide synthesis, formulation, and drug delivery could encourage the industry to invest in peptide-based drug research and development.
In recent years, peptide drugs have received significant attention as potential leads due to their beneficial properties. Compared to small molecule drugs, peptides could be more potent and tissue-specific with negligible side effects. In the last few years (2015–2019), the US Food and Drug Administration (FDA) has approved 15 peptide drugs that accounted for 7% of the total drugs approved during this period [45]. Recent advancements in peptide screening and computational biology approaches have assisted in the development of novel peptide drugs. Peptide drugs that are currently used for the treatment of diabetes are derived from a number of different sources. One of the most promising GLP-1 receptor agonists currently used for the treatment of diabetes is exedin-4. It was derived from the venom of Heloderma suspectum (Gila monster) and approved by the FDA in 2005. A more potent synthetic version, exenatide, was also introduced [46]. Despite various oral medications available for T2DM, the market for injectable exedin-4 has continued to grow since its approval. Following this, various other peptide drugs such as liraglutide and semaglutide with increased stability and improved half-life were approved by the FDA [47]. Protein hydrolysates and peptides from food sources have already been commercialized and are being used as health promoting agents. For instance, NutripeptinTM is a marine bioactive peptide obtained from the proteolytic hydrolysis of cod fish fillets. It lowers the glycaemic index of foods, which in turn reduces the risk of T2DM. The growing interest of bioactive peptides as a safer and promising antidiabetic agent has spearheaded the search for finding more peptide-based lead molecules for the treatment of diabetes.
5. Antidiabetic Peptides from Food Sources
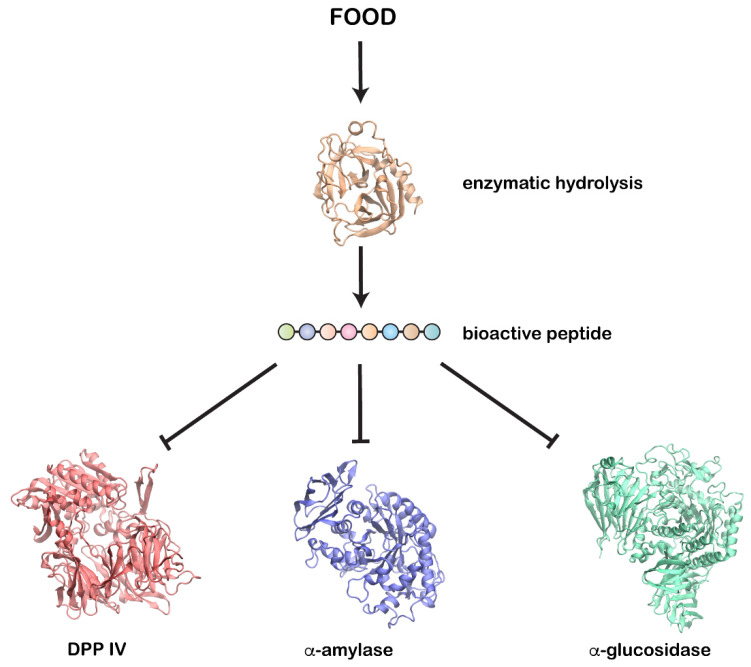
Bioactive peptides from several food sources have been reported to show antidiabetic activity by inhibiting carbohydrate digesting enzymes (α-amylase and α-glucosidase) and DPP IV (Figure 1), enhancing pancreatic insulin secretion, controlling satiety, and reducing glucose absorption from the gut [18].
Figure 1.
Enzymatic hydrolysis of food proteins produces bioactive peptides that could inhibit key enzymes involved in diabetes—DPP IV, α-amylase, and α-glucosidase.
5.1. Animal Products
5.1.1. Dairy Foods
Dairy products such as milk, cheese, yoghurt, and other cultured dairy foods are dominant sources of bioactive peptides. Milk proteins are a versatile source of bioactive peptides as they possess numerous health benefits including antidiabetic, antioxidant, antihypertensive, antimicrobial, and immunomodulatory properties [48]. Animal milk is a nutrient-rich fluid secreted by the epithelial cells of the mammary glands. The two major proteins present in milk are casein and whey. Both of these have been identified as rich sources of bioactive peptides [49]. Since peptides and hydrolysates from milk could act as antidiabetic agents, they have attracted significant interest from the scientific community [50]. Perhaps the most widely consumed animal milk originates from cows. Therefore, antidiabetic potential of cow milk proteins and their hydrolysates has been investigated extensively [51]. Oral administration of cow milk proteins and their hydrolysates on diabetic rats significantly reduced the concentration of blood glucose, total lipids, triglycerides, and cholesterol as well as increased the concentration of globulin and high-density lipoproteins (HDL) [52]. Administration of donkey milk has been demonstrated to improve the viability of damaged pancreatic β-cells in mouse insulinoma beta-pancreatic (MIN6) cells. Furthermore, diabetic rats fed with donkey milk powder for four weeks showed a significant decrease in blood glucose levels, increased insulin sensitivity and improved insulin resistance. It has been suggested that donkey milk could be used as an antidiabetic agent as it downregulated phosphoenolpyruvate carboxykinase 1 (PCK1) and glucose-6-phosphatase (G6Pase), enzymes that are involved in hepatic gluconeogenesis [53]. The peptides SDIPNPIGSE, NPWDQVKR, SLSSSEESITH, and QEPVLGPVRGPFP isolated from goat milk casein hydrolysates showed a significant improvement in glucose metabolism in insulin-resistant HEPG-2 cells. Interestingly, some of these casein-derived sequences are also conserved in sheep, buffalo, and cow. However, more work needs to be done to establish if these peptides are indeed released and are functional. Goat milk was also observed to decrease the mRNA level of PCK1 and G6Pase and in turn improved glycogen concentration. Thus, these findings suggest that peptides from goat milk could ameliorate insulin resistance and manage type 2 diabetes [54]. Zhang et al. identified novel DPP IV inhibitory peptides from trypsin- and chymotrypsin-treated goat milk casein. Out of the five new identified peptides (MHQPPQPL, SPTVMFPPQSVL, VMFPPQSVL, INNQFLPYPY and AWPQYL), the peptide INNQFLPYPY derived from the κ-casein displayed highest inhibitory activity with an IC50 value of 40.08 μM [55]. Studies have also identified camel milk peptides that could potentially inhibit key enzymes like DPP IV and amylase [56]. Trypsin-digested camel milk proteins were shown to produce the peptides FLQY, FQLGASPY, ILDKEGIDY, ILELA, LLQLEAIR, LPVP, LQALHQGQIV, MPVQA, and SPVVPF that could inhibit DPP IV [57]. Thus, a range of studies involving widely consumed animal milk have underscored the importance of milk proteins and hydrolysates in managing diabetes (Table 1).
Table 1.
Antidiabetic action of peptides and protein hydrolysates identified from animal food sources.
| Source | Enzyme(s) Used | Substrate | Protein Hydrolysate | Peptide(s) Identified | Mechanism of Action | IC50 | Reference |
|---|---|---|---|---|---|---|---|
| Cow milk | Trypsin | Milk protein | Milk protein hydrolysates | Reduced blood plasma glucose level in vivo | [52] | ||
| Donkey milk | Pepsin and pancreatin | Milk protein hydrolysates | Improved damaged β-cells viability in vitro, reduced blood glucose level and improved insulin resistance in vivo |
[53] | |||
| Goat milk | Flavourzyme | Casein | Casein hydrolysate | SDIPNPIGSE | Ameliorated insulin resistance in HepG2 cells | [54] | |
| NPWDQVKR | |||||||
| SLSSSEESITH | |||||||
| QEPVLGPVRGPFP | |||||||
| Goat milk | Trypsin and chymotrypsin | Casein | Casein hydrolysate | MHQPPQPL | Inhibited DPP IV in vitro | 350.41 ± 4.1 μM | [55] |
| SPTVMFPPQSVL | 676.31 ± 12.6 μM | ||||||
| VMFPPQSVL | Nd | ||||||
| INNQFLPYPY | 40.08 ± 5.0 μM | ||||||
| AWPQYL | Nd | ||||||
| Camel milk | Alcalase, bromelain, and papain | Milk proteins | Milk protein hydrolysates | MPSKPPLL | Inhibited pancreatic α-amylase in vitro | [56] | |
| KDLWDDFKGL | |||||||
| Camel milk | Trypsin | Milk proteins | Milk protein hydrolysates | FLQY | Inhibited DPP IV in vitro | >1000 μM | [57] |
| FQLGASPY | >1000 μM | ||||||
| ILDKEGIDY | 347.8 ± 42.8 μM | ||||||
| ILELA | 721.1 ± 46.3 μM | ||||||
| LLQLEAIR | 177.8 ± 22.2 μM | ||||||
| LPVP | 87.0 ± 3.2 μM | ||||||
| LQALHQGQIV | >1000 μM | ||||||
| MPVQA | 93.3 ± 8.0 μM | ||||||
| SPVVPF | 214.1 ± 16.7 μM | ||||||
| Gouda cheese | Microbial fermentation | Water-soluble extracts (WSEs) | LPQNIPPL | Reduced glucose level in vivo and inhibited DPP IV in vivo and in vitro | 46 μM | [60] | |
| LPQ | 82 μM | ||||||
| VPITPTL | 110 μM | ||||||
| VPITPT | 130 μM | ||||||
| Egg | Alcalase | Egg white protein | Egg white protein hydrolysates | RVPSLM | Inhibited α-glucosidase in vitro | 23.07 μmol/L | [61] |
| TPSPR | 40.02 μmol/L | ||||||
| DLQGK | >150.0 μmol/L | ||||||
| AGLAPY | >150.0 μmol/L | ||||||
| RVPSL | >150.0 μmol/L | ||||||
| DHPFLF | >150.0 μmol/L | ||||||
| HAEIN | >150.0 μmol/L | ||||||
| QIGLF | >150.0 μmol/L | ||||||
| Egg | Pepsin | Egg yolk protein | Egg yolk protein hydrolysates | YINQMPQKSRE, | Inhibited DPP IV in vitro | 222.8 μg/mL | [62] |
| VTGRFAGHPAAQ | Inhibited α-glucosidase in vitro | 365.4 μg/mL | |||||
| Turtle egg | Trypsin, pepsin, α-chymotrypsin, thermolysin, and GI enzyme | Egg yolk protein | Egg yolk protein hydrolysates | LPSW | Inhibited DPP IV in vitro and in silico | 289.2 ± 11.85 μM | [63] |
| WLQL | 269.7 ± 15.91 μM | ||||||
| LPLF | 463.6 ± 5.52 μM | ||||||
| VPGLAL | >2000 | ||||||
| LVGLPL | 432.5 ± 40.31 μM | ||||||
| Chicken egg | Pepsin and trypsin | Myosin and lysozyme | ADF | Inhibited DPP IV in vitro and in silico | 16.83 mM | [64] | |
| MIR | 4.86 mM | ||||||
| FGR | 46.2 mM | ||||||
| Cooked meat (pork, beef, chicken, and turkey) | Amylase, pepsin and trypsin | Meat proteins | Meat protein hydrolysates | IPI | Inhibited DPP IV in vitro | 3.5 μmol/L | [65] |
| WL | 44 μmol/L | ||||||
| LPL | 241 μmol/L | ||||||
| WI | 89 μmol/L | ||||||
| FL | 400 μmol/L | ||||||
| LW | 993 μmol/L | ||||||
| Cooked meat (Pork, chicken and turkey) | VL | 74 μmol/L | |||||
| WM | 243 μmol/L | ||||||
| ML | 91 μmol/L | ||||||
| Cooked meat (Pork and beef) | IP | 150 μmol/L | |||||
| LP | 712 μmol/L | ||||||
| AL | 882 μmol/L | ||||||
| Cooked meat (Chicken and turkey) | FP | 363 μmol/L | |||||
| Cooked meat (Pork) | IPM | 70 μmol/L | |||||
| Dry cured ham | KA | Inhibited DPP IV in vitro | 6.27 ± 0.59 mM | [66] | |||
| AA | 9.40 ± 0.10 mM | ||||||
| GP | 9.69 ± 0.49 mM | ||||||
| PL | >10 mM | ||||||
| AAATP | 6.47 ± 0.20 mM | ||||||
| AAAAG | 8.13 ± 0.48 mM | ||||||
| ALGGA | >10 mM | ||||||
| LVSGM | >10 mM | ||||||
| Chicken feet | Neutrase and protamex | Feet protein | Feet protein hydrolysates | Stimulated GLP-1 release in vivo and inhibited DPP IV in vitro | 4.42 ± 0.1 μL | [67] | |
| Chicken by product | Flavourzyme and corolase | Mechanical chicken deboning residue | Protein hydrolysates | TL | Promoted glucose uptake and inhibited DPP IV in vitro | [68] | |
| HT | |||||||
| LA | |||||||
| LADVEVDLL | |||||||
| LL | |||||||
| ETGKGEDGE | |||||||
| FL | |||||||
| LFFSMLLML | |||||||
| LF |
Nd—Not determined.
Due to high nutritional value and protein content, milk-derived food products such as yoghurt and cheese are often consumed as part of a balanced diet. In recent years, the health benefits of yoghurt have been studied extensively and it has been shown to reduce the risk of several metabolic disorders. Regular intake of yoghurt could modulate glucose metabolism and reduce the risk associated with diabetes [58]. Microbial fermentation that produces yoghurt generates bioactive peptides that have antidiabetic potential. Addition of aqueous berry extracts (salal berry and blackcurrant pomace) to yoghurt improved blood glucose regulation by the inhibition of α-amylase, α-glucosidase and DPP IV enzymes. Using liquid chromatography coupled with mass spectrometry (LC/MS), nearly 486 peptides were identified from yoghurt possessing diverse bioactivities [59]. Water-soluble extracts of gouda-type cheese has been shown to exhibit DPP IV inhibitory activity. Among several peptides that were identified, LPQNIPPL exhibited the highest DPP IV inhibitory activity. Interestingly, oral administration of this peptide to female rats reduced blood glucose concentration. Furthermore, the concentration of LPQNIPPL was found to increase 4.3-fold in cheese ripened for a year [60].
5.1.2. Eggs
As part of a balanced diet, eggs are generally recommended as a good source of protein and nutrients. Beyond serving as an excellent nutrient-dense food source, egg proteins harbour bioactive peptides that perform diverse biological activities [69]. Proteolysis of egg proteins using pepsin and alcalase have been reported to produce bioactive peptides with antidiabetic property. Hydrolysis of egg white protein using alcalase produced eight peptides. Among these, RVPSLM and TPSPR exhibited the highest α-glucosidase inhibitory activity [61]. Peptides YINQMPQKSRE and VTGRFAGHPAAQ from chicken egg yolk pepsin hydrolysate was shown to inhibit both α-glucosidase and DPP IV. Notably, compared to peptides produced by alcalase digestion, these peptides exhibited better antidiabetic activity [62]. Five novel DPP IV inhibitory peptides LPSW, WLQL, LPLF, VPGLAL, and LVGLPL, were identified from the egg yolk of the soft-shelled turtle. Out of these, LPSW competitively inhibited DPP IV with an IC50 of 269.7 ± 15.91 µM [63].
Often, in silico virtual screening is employed to identify novel peptides since this method is cost-effective, saves time and is dependable. In silico digestion of chicken egg proteins by pepsin and trypsin has been used to identify antidiabetic and antihypertensive peptides. The peptides ADF, MIR, and FGR obtained from myosin and lysozyme have been shown to be able to inhibit DPP IV [64] (Table 1). Additionally, whole peptidomics and bioinformatics study of chicken egg white has revealed the presence of 43 novel antidiabetic peptides based on homologous sequence motifs identified in other food-derived peptides [70].
5.1.3. Meat Products
Animal meat is widely consumed and serves as a rich dietary source of proteins, minerals, and vitamins. Bioactive peptides generated from the hydrolysis of meat from cattle, sheep, goats, pigs, and poultry have been identified to play a crucial role in various physiological processes [24]. Apart from meat, by-products such as blood, skin and bone could also serve as a source of peptides [71]. In vitro digestion of four types of cooked meat, including pork, beef, chicken, and turkey, using gastrointestinal enzymes producing peptides with antioxidant, angiotensin-converting enzyme (ACE) inhibitory and DPP IV inhibitory properties. Out of these, pork meat was found to be an excellent source of DPP IV inhibitory peptides [65]. Peptide fractions of digested meat yielded 23 DPP IV inhibitory peptides with the highest activity shown by the tripeptide IPI (diprotin A) with an IC50 of 3.5 μmol/L [65]. In silico digestion of pork meat proteins using gastrointestinal enzymes identified small dipeptides that could improve glucose absorption and DPP IV inhibition [72]. Processing of meat by drying, curing, ripening, and fermentation are some of the earliest approaches used for the preservation and enhancement of flavour. Such processing often releases encrypted bioactive peptides from parent proteins [71]. Dry-cured ham is an excellent source of bioactive peptides [73]. A regular intake of 80 g of Spanish dry-cured ham has been shown to be protective against cardiovascular disorders and could lower blood glucose levels [74]. Added to this, peptides from dry ham could also act as DPP IV and α-glucosidase inhibitors. Nine peptides were isolated from Spanish dry-cured ham and peptides KA and AAATP exhibited strong DPP IV inhibitory activity, with IC50 of 6.27 mM and 6.47 mM [66]. Similarly, 63 peptides were identified from Iberian dry-cured ham. Out of these, eight peptides (GGLGP, LGVGG, AEEEYPDL, EA, PP, VE, PE, AD) were previously reported to be bioactive and these were tested for α-glucosidase inhibitory activity. The results confirmed the multifunctionality of these peptides [75].
Bioactive peptides have also been isolated from poultry products. Protein hydrolysates from chicken feet showed high DPP IV inhibitory capacity with an IC50 of 300 μg/mL. Furthermore, glucose tolerance test in diet and age-induced glucose-intolerant Wistar rat models revealed that the peptides improved plasma glucose profile and stimulated GLP-1 release [67]. In another study, peptides isolated from chicken by-product, such as residue from mechanical deboning of chicken, exhibited potential antidiabetic activity by inhibiting DPP IV in vitro and promoting ex vivo cellular glucose uptake. Among the 14 DPP IV inhibitory peptides identified, the highest inhibitory activity was observed in peptides with an N-terminal leucine or isoleucine residue [68] (Table 1).
5.2. Fish Products
Fish is one of the healthiest food sources, owing to its low fat and high-quality protein content. It is loaded with minerals and vitamins and is also a great source of omega-3 fatty acids. Including fish as a part of a healthy diet could reduce the incidence of various metabolic disorders. Protein hydrolysates or peptides isolated from various fish species has the potential to significantly improve glucose metabolism (Table 2). Silver carp (Hypophthalmichthys molitrix Val.) is one of the major freshwater fish in China. Combining in vitro and in silico analysis, Zhang et al. investigated the DPP IV inhibitory activity of silver carp protein (SCP) hydrolysates. Four peptides—AGPPGPSG, ALAPSTM APGPAGP, and LPIIDI—were identified from the protein hydrolysates fraction that exhibited high DPP IV inhibitory activity. All these peptides were assumed to be good inhibitory agents due to the presence of proline in their sequence. Out of these peptides, LPIIDI and APGPAGP showed high DPP IV-inhibitory activity with an IC50 value of 105.44 and 229.14 μM [76]. Protein hydrolysates generated from the enzymatic digestion of silver carp swim bladder protein also exhibited DPP IV inhibitory activity. The protein fraction that exhibited the highest inhibitory action contained 40 different peptides of varying lengths. The most potent peptide WGDEHIPGSPYH inhibited DPP IV in an uncompetitive manner and docking analysis indicated that the peptide bound close to the catalytic site of the enzyme. Furthermore, this peptide and its hydrolysate IPGSPY exhibited strong DPP IV inhibition and promoted insulin secretion in Caco-2 cells [77]. Three peptides, PGVGGPLGPIGPCYE, CAYQWQRPVDRIR, and PACGGFWISGRPG, that produced in vitro DPP IV inhibitory activity with IC50 of 116.1, 78.0 and 96.4 μM, respectively, were identified from tuna cooking juice [78]. In vitro and in vivo analysis of blue whiting (Micromesistius poutassou) protein hydrolysate was found to inhibit DPP IV and promoted the release of insulin and GLP-1 in pancreatic BRIN-BD11 and GLUTag cells. Similarly, protein hydrolysates from salmon by-products such as skin and trimmings have exhibited DPP IV inhibitory activity and improved insulin and GLP-1 secretion in pancreatic BRIN-BD11 and GLUTag cells [79,80].
Table 2.
Antidiabetic action of peptides and protein hydrolysates identified from fish.
| Source | Enzyme(s) Used | Substrate | Proteins Hydrolysates | Peptide(s) Identified | Mechanism of Action | IC50 | Reference |
|---|---|---|---|---|---|---|---|
| Silver carp | Trypsin, neutrase, alcalase, papain, pepsin, and flavourzyme | Dorsal muscle | Muscle protein hydrolysates | LPIIDI | Inhibited DPP IV in vitro | 105.44 μM | [76] |
| APGPAGP | 229.14 μM | ||||||
| AGPPGPSG | Nd | ||||||
| ALAPSTM | Nd | ||||||
| Silver carp | Papain, bromelain, alcalase 2.4 L, neutrase, and flavourzyme | Swim bladder protein | Protein hydrolysates | WGDEHIPGSPYH | Inhibited DPP IV in vitro and improved glucose uptake in INS cells | 0.35 ± 0.01 mM | [77] |
| IAQPQEKAPDPFRH | 0.81 ± 0.01 mM | ||||||
| IAGPAGPRGPAGPN | 1.17 ± 0.04 mM | ||||||
| VAPEEHPTL | 1.93 ± 0.02 mM | ||||||
| YALPHAI | 2.27 ± 0.04 mM | ||||||
| EPGNPGPAGPA | 2.94 ± 0.01 mM | ||||||
| Tuna | Protease XXIII, orientase 90N | Cooking juice | Cooking juice hydrolysates | PGVGGPLGPIGPCYE | Inhibited DPP IV in vitro | 116.1 μM | [78] |
| CAYQWQRPVDRIR | 78 μM | ||||||
| PACGGFWISGRPG | 96.4 μM | ||||||
| Blue whiting | Alcalase and flavourzyme 500 | Fish meat | Meat protein hydrolysates | Inhibited DPP IV and mediated insulin and GLP-1 release from BRIN-BD11 and GLUTag cells | [79] | ||
| Salmon | Alcalase and Flavourzyme | Skin and trimmings | Protein hydrolysates | Inhibited DPP IV and mediated insulin and GLP-1 release from BRIN-BD11 and GLUTag cells | [80] |
Nd—Not determined.
5.3. Plant Products
A large number of bioactive peptides and protein hydrolysates with potential health benefits have been isolated and identified from plant-based food sources.
5.3.1. Pulses and Legumes
Pulses and legumes represent the cheapest source of essential proteins that are consumed all over the world. Soybeans are a rich source of high-quality proteins including all essential amino acids required for human health. Storage proteins abundantly found in soybean include β-conglycinin and glycinin [81]. Epidemiological studies have suggested that soy consumption could be associated with the alleviation of chronic disorders such as cardiovascular disease, diabetes, obesity, cancer and other immune disorders [81]. Diverse bioactive peptides isolated from soybeans and fermented or processed soybean products such as soymilk, tofu, soy sauce, tempeh, natto and soy paste are gaining interest due to their health benefits [82]. Several studies have also highlighted the importance of soy peptides in the management of diabetes and insulin resistance [83,84]. The pharmacological activity of aglycin, a 37 amino acid long peptide isolated from soybean has been investigated in vivo [85]. Oral administration of the peptide (50 mg/kg) to diabetic mice was shown to effectively control hyperglycaemia by the enhancement of the insulin-signalling pathway and an improvement in glucose uptake in peripheral tissues. Additionally, this natural peptide is highly stable and resistant to gastrointestinal digestive enzymes such as trypsin and pepsin [85]. Pharmacological and animal model studies have been performed using vglycin, another 37 amino acid long peptide isolated from soybean, to determine the effect on the proliferation and restoration of pancreatic β-cells. The results suggest that this peptide could improve glucose tolerance, restore pancreatic function and enhance insulin signalling by activating the insulin receptor (IR)/Akt signalling pathway [86,87]. Long term administration of soymorphin-5 (YPFVV), a soy-derived μ-opioid peptide derived from the β-subunit of β-conglycinin, improved glucose and lipid metabolism in diabetic KKAy mice by activating the adiponectin and peroxisome proliferator-activated receptor α system [88]. In vitro studies using germinated soybean peptides were found to modulate hyperglycaemia by inhibiting DPP IV, salivary α-amylase, and intestinal α-glucosidases enzymes [89]. The inhibitory activity of soy peptide IAVPTGVA and lupin bean peptide LTFPGSAED were investigated by performing DPP IV assays in situ on human intestinal Caco-2 cells and ex vivo on human serum. These peptides inhibited DPP IV with an IC50 of 223.2 and 207.5 µM. Furthermore, they displayed inhibitory action on circulating DPP IV with a slighter lower potency when compared to in vitro and in situ studies [90]. These results are in agreement with the inhibition of DPP IV activity in situ in human intestinal Caco-2 cells by soybean protein hydrolysates [91] (Table 3).
Table 3.
Antidiabetic action of peptides and protein hydrolysates identified from plant food sources.
| Source | Enzyme(s) Used | Substrate | Proteins Hydrolysate | Peptide(s) Identified | Mechanism of Action | IC50 | Reference |
|---|---|---|---|---|---|---|---|
| Soy bean | ASCNGVCSPFEMPPCGSSACRC IPVGLVVGYCRHPSG (aglycin) |
Enhanced insulin receptor signalling pathway and enhanced glucose uptake in vivo | [85] | ||||
| Soy bean | VSCNGVCSPFEMPPCGSSACR CIPYGLVVGNCRHPSG (vglycin) |
Enhanced insulin signalling by activating the insulin receptor (IR)/Akt signalling pathway | [86,87] | ||||
| Restored impaired insulin signalling, glucose tolerance and pancreatic function in vivo | |||||||
| Soy bean | β-conglycinin | YPFVV (soymorphin-5) | Improved glucose and lipid metabolism via activation of the adiponectin and PPARα system- in vivo | [88] | |||
| Soy bean | Pepsin and pancreatin | Germinated soybeans | Protein hydrolysates | Inhibited DPP IV, α-amylase and α-glucosidase in vitro | DPP-IV inhibition- 1.49 mg/mL) | [89] | |
| α-amylase inhibition- 1.70 mg/mL | |||||||
| Maltase and sucrase activities of α-glucosidase 3.73 and 2.90 mg/mL | |||||||
| Lupin bean | LTFPGSAED | Inhibited DPP IV in situ | 207.5 µM | [90] | |||
| Soy bean | IAVPTGV | 223.2 µM | |||||
| Fermented soy bean - Natto | Water soluble extract | KL | Inhibited DPP IV in vitro | 41.40 ± 2.68 μg/mL | [96] | ||
| LA | 598.02 ± 18.35 μg/mL | ||||||
| Black bean | Proteinase K, pepsin, trypsin, papain, alcalase, flavourzyme, themolysin, and chymotrypsin | Protein hydrolysates | EGLELLLLLLAG | Inhibited DPP IV in vitro and in silico | [97] | ||
| AKSPLF | |||||||
| FEELN | |||||||
| TTGGKGGK | Inhibited α-glucosidase in vitro and in silico | ||||||
| AKSPLF | |||||||
| WEVM | Inhibited α-amylase in vitro and in silico | ||||||
| Pinto beans | Protamex | Protein hydrolysates | PPHMLP | Inhibited α-amylase in vitro | 1.97 mg/mL | [98,99] | |
| PLPTGAGF | 8.96 mg/mL | ||||||
| PPHMGGP | 14.63 mg/mL | ||||||
| PLPLHMLP | 18.45 mg/mL | ||||||
| LSSLEMGSLGALFVCM | 20.56 mg/mL | ||||||
| Common beans | Pepsin, and pancreatin | Protein hydrolysates | Lowered blood glucose level by inhibiting α-glucosidase | [100] | |||
| Bambara bean | Alcalase and thermolysin | Protein hydrolysates | Inhibited DPP IV in vitro | 1.73 mg/mL | [101] | ||
| Cow pea | Peptides | Activated the insulin signalling pathway in vivo | [102] | ||||
| Chickpea | Pepsin, pancreatin and bromelain | Protein hydrolysates | PHPATSGGGL | Inhibited DPP IV in vitro | 245 μg/mL | [103] | |
| YVDGSGTPLT | |||||||
| Oats | Pepsin, trypsin, pancreatin, and alcalase | Globulin | LQAFEPLR | Inhibited DPP IV in vitro | 103.5 μM | [106] | |
| EFLLAGNNK | |||||||
| Wheat | Debitrase HYW20 | Gluten | Gluten hydrolysates | VPL WL |
Inhibited DPP IV in vitro | [107] | |
| Quinoa | Pepsin and pancreatin | Protein hydrolysates | GEHGSDGNV | Inhibited DPP IV and α-glucosidase in vitro | [109] | ||
| IQAEGGLT | Inhibited DPP IV and α-glucosidase in vitro | ||||||
| DKKYPK | Inhibited α-glucosidase in vitro | ||||||
| Quinoa | Papain, ficin and bromelain | Seed storage proteins | MAF | Inhibited DPPIV in vitro | [110] | ||
| NMF | |||||||
| HPF | |||||||
| MCG | |||||||
| Amaranthus | Alcalase | Albumin and globulin | Protein hydrolysates | Inhibited DPP IV in vitro and in vivo | 0.12 ± 0.01 mg/mL | [111] | |
| Amaranthus | Pepsin and pancreatin | Protein hydrolysates | FLISCLL | Inhibited α-amylase and DPP IV in vitro | [112] | ||
| SVFDEELS | |||||||
| DFIILE | |||||||
| NRPET | |||||||
| HVIKPPS | |||||||
| Walnut | Alcalase, Trypsin, Neutrase, Protamex and Flavourzyme | Protein hydrolysates | LPLLR | Inhibited both α-glucosidase and α-amylase in vitro | [115,116] | ||
| Cumin seeds | Protamex | Seed protein hydrolysates | FFRSKLLSDGAAAAKGALLPQYW | Inhibited porcine pancreatic α-amylase in vitro | [119] | ||
| Basil seeds | Alcalase | Seed protein hydrolysates | ACGNLPRMC | Inhibited α-amylase activity in vitro | [118] | ||
| ACNLPRMC | |||||||
| AGCGCEAMFAGA |
Apart from this, fermented soybean products are very popular in Asian countries including Japan, China, Korea, Thailand, and Indonesia. Fermentation results in the release of bioactive peptides by the action of proteolytic enzymes produced by microorganisms [92]. Interestingly, compared to unfermented soybeans, fermented soybean products have been shown to exhibit better antidiabetic properties in animal and human studies [93]. Fermented soybean fed to streptozotocin (STZ)-induced diabetic rats inhibited α-amylase and intestinal α-glucosidase. Fermented soymilk is an excellent source of bioactive peptides and fermentation of soymilk by kefir, which are colonies of microorganisms primarily involving lactic acid bacteria, was observed to inhibit α-amylase [94]. Clinical studies have also established that consumption of fermented soy foods (natto and miso) is associated with lowering the incidence of gestational diabetes [95]. DPP IV inhibitory dipeptides KL and LR have been isolated from water-soluble extract of natto [96].
Bioactive peptides from different varieties of bean have also been found to be beneficial for the treatment of diabetes. Biochemical and in silico analysis have been employed to identify potential bioactive peptides from black bean (Phaseolus vulgaris) protein. Molecular docking analysis suggested that the peptides EGLELLLLLLAG, AKSPLF, and FEELN could potentially inhibit DPP IV, TTGGKGGK could inhibit α-glucosidase, and AKSPLF and WEVM could inhibit α-amylase [97]. Using phage display approach 5 novel α-amylase inhibitory peptides, PPHMLP, PLPTGAGF, PPHMGGP, PLPLHMLP, and LSSLEMGSLGALFVCM were identified from pinto beans [98]. The effectiveness of these peptides was further evaluated in AR42J cells. The results demonstrated that these peptides could indeed inhibit α-amylase with LSSLEMGSLGALPVCM exhibiting the best inhibition with an IC50 of 0.31 mM [99]. Similarly, peptides and protein hydrolysates from easy-to-cook (ETC) and hard-to-cook (HTC) beans were also shown to lower blood glucose levels by inhibiting α-glucosidase [100]. Bambara bean (Vigna subterranean) protein hydrolysates digested using alcalase and thermolysin also exhibited significant DPP IV inhibitory properties [101]. Peptides from cowpea (Vigna unguiculata) have been demonstrated to activate the insulin signalling pathway in vivo in rat skeletal muscles. L6 rat skeletal muscles were treated with various doses of cowpea peptides (0.1, 1, 10 and 100 ng) for 20 h or insulin (100 nM) for 30 min. Administration of cowpea peptides induced phosphorylation of Akt (protein kinase B) that eventually activated the insulin signalling cascade [102]. Peptides produced from chickpea (Cicer arietinum) using a simulated digestive system and bromelain (enzyme derived from pineapple plant) hydrolysis were compared for their antidiabetic activity. Inhibition assays and molecular docking analysis indicated that the peptides PHPATSGGGL and YVDGSGTPLT had greater affinity for the catalytic site of DPP IV compared to peptides isolated from bromelain hydrolysate [103]. Evidently, protein-rich beans are a good source of bioactive peptides, many of which are yet to be fully explored.
5.3.2. Cereals and Pseudocereals
Since ancient times, cereal grains have been consumed as a staple around the world. Protein hydrolysates and peptides from cereals and pseudocereals have been shown to inhibit enzymes associated with diabetes and could maintain glucose homeostasis by improving insulin sensitivity [104]. Oral administration of dietary corn and wheat-based peptides to nonobese diabetic (NOD) mice have delayed the onset and reduced the incidence of type 1 diabetes by reducing the inflammation in mouse models [105]. Peptide hydrolysates from three cereals—oat, buckwheat and highland barley seeds—have been evaluated for DPP IV inhibitory activity. Hydrolysates of all three cereals strongly inhibited DPP IV activity (highland barley, IC50 of 3.91 mg/mL; buckwheat, IC50 of 1.98 mg/mL; oat, IC50 of 0.99 mg/mL). From the hydrolysates, peptides LQAFEPLR and EFLLAGNNK obtained from oat storage proteins showed the highest degree of DPP IV inhibition in molecular docking and in in vitro assays [106]. Nine protein hydrolysates from wheat gluten exhibited DPP IV inhibitory activity and the most effective hydrolysate was further processed to identify potent peptides. VPL, WL, and WP were subsequently shortlisted using liquid chromatography tandem mass spectrometry (LC-MS/MS) [107] (Table 3).
Quinoa is a pseudocereal with a very high protein and amino acid content compared to other dietary grains. The antidiabetic properties of quinoa protein have been demonstrated [108]. Bioactive peptides from quinoa protein hydrolysates were found to be potent DPP IV inhibitors. Quinoa peptides GEHGSDGNV, IQAEGGLT, and DKKYPK exhibited antidiabetic activity by inhibiting DPP IV, α-amylase, or α-glucosidase [109]. In silico digestion of quinoa globulin protein yielded four promising tripeptides MAF, NMF, HPF, and MCG that exhibited DPP IV and ACE inhibitory activity [110]. Like quinoa, amaranth is another pseudocereal with high nutritional value and nutraceutical properties. Bioactive peptides isolated from enzymatic digestion of amaranth seed proteins were reported to inhibit DPP IV in in vitro, in vivo and in silico studies [111]. Hydrolysates of amaranth grain storage proteins albumin 1, globulin and glutelin hydrolysates (GluH) competitively inhibited DPP IV activity in STZ-induced diabetic mice. GluH was observed to improve glucose tolerance and plasma insulin [111]. Additionally, in vitro simulated gastrointestinal digestion (SGID) of Amaranthus caudatus proteins released multifunctional peptides that possessed DPP IV and α-amylase inhibitory properties. This included peptides FLISCLL, SVFDEELS, DFIILE, NRPET, and HVIKPPS that could potentially inhibit α-amylase by forming aromatic–aromatic interactions (Table 3). NRPET and VEEGNM were suggested to inhibit DPP IV due to the presence of proline in the first four positions and branched chain amino acids at the N-terminal end [112].
5.3.3. Plant Seeds and Nuts
Plant seeds and nuts are excellent sources of proteins, vitamins, minerals and unsaturated fats and is widely recommended as part of a balanced diet. Abundant evidence shows that regular adequate consumption of seeds and nuts impart significant health benefits and protects against several chronic diseases [113]. Peptides identified from various nuts and seeds have also been shown to regulate diabetes by improving glucose metabolism via different mechanisms [114]. Walnut (Juglans mandshurica) hydrolysed peptides (WHPs) have produced an α-glucosidase inhibitory rate of 61.73% in in vitro studies [115]. Additionally, administration of WHPs in streptozotocin-induced type 2 diabetic mice resulted in a reduction of fasting glucose level and increase in insulin secretion [115]. The peptide LPLLR, isolated from walnut protein hydrolysates, improved glucose metabolism by inhibiting both α-glucosidase and α-amylase in high glucose-induced insulin-resistant (IR) hepatic HepG2 cells with an inhibition rate of 50.12% and 39.08% respectively. It increased glycogen synthesis and glucose uptake, and decreased gluconeogenesis by activating IRS-1/PI3K/Akt and AMPK signalling pathways [116]. Cationic peptide fractions obtained from flaxseed protein hydrolysate have also demonstrated antidiabetic activity by promoting glucose uptake in L6 myoblast cells [117]. Peptides ACGNLPRMC, ACNLPRMC, and AGCGCEAMFAGA possessing α-amylase inhibitory activity were isolated from basil seeds (Ocimum basilicum) using in vitro assays. In silico molecular docking analysis indicated that these peptides bound by interacting with key residues in the active site of the enzyme [118]. An α-amylase inhibitory peptide has also been identified from cumin seeds, an important aromatic spice. Based on in vitro assays, the peptide FFRSKLLSDGAAAAKGALLPQYW inhibited porcine pancreatic α-amylase with an IC50 of 0.02 µM [119].
Integrated in silico bioinformatics-based approaches involving proteins from soybean, flaxseed, rapeseed, sunflower, and sesame have identified several potential peptides with DPP IV and ACE inhibitory activity [120]. However, additional in vitro or in vivo experiments would be required to validate these findings. Nonetheless, these approaches highlight the vast library of cryptic peptides that have the potential to inhibit key enzymes in pathways associated with diabetes.
6. Structural Characteristics of Antidiabetic Peptides from Food
Numerous studies have demonstrated that bioactive peptides derived from food exhibit physiological functions that could be attributed to their sequence and amino acid composition. The structure and function of the peptide produced is often determined by substrate pre-treatment, enzyme used and hydrolysis conditions. Several of the protein hydrolysates and peptides mentioned here exhibit their antidiabetic activity by inhibiting DPP IV [55,60,63,66,76,90,106]. Evidently, a number of animals, fish and plant proteins could be used as templates for the generation of DPP IV inhibitory peptides. Milk is one of the most extensively studied sources for the generation of peptides. Several milk protein hydrolysates and peptides with antidiabetic activity have been identified from cow, donkey, goat, and camel sources [52,53,54,56]. Other food sources such as egg, meat, pulse, and fish proteins could also potentially contribute antidiabetic peptides [64,72,77]. Interestingly, structure–activity relationship studies have shown that the presence of specific amino acids, as well as their position, could influence DPP IV inhibitory activity. Often, hydrophobic amino acids such as alanine, glycine, isoleucine, leucine, phenylalanine, proline, methionine, tryptophan, and valine are abundantly present in the DPP IV inhibitory peptides. Hydrophilic amino acids such as threonine, histidine, glutamine, serine, lysine, and arginine are also present in many of these peptides [121]. However, the precise role of these hydrophilic amino acids is not well understood. The presence of hydrophobic pockets in the active site of the DPP IV is thought to play an important role in the inhibition of the enzyme by peptides [122]. Moreover, several studies have reported that the presence of a proline residue at the N-terminal region could be a good indicator of DPP IV inhibition. Hatanaka et al. identified 14 different proline-containing dipeptides that could inhibit DPP IV. Interestingly, the position of residues could also determine the activity of a peptide. For instance, the dipeptide Pro-Ile did not inhibit DPP IV activity, while the reverse peptide Ile-Pro competitively inhibited DPP IV. It was also reported that proline-containing dipeptides and tripeptides are also resistant to gastrointestinal digestion [21]. Furthermore, peptides containing proline at the penultimate position was also shown to inhibit DPP IV by substrate type inhibition, where peptides bind to the active site and are subsequently degraded into smaller fragments [57]. Quantitative structure–activity relationship (QSAR) studies have also positively correlated the hydrophobicity of the two amino acids located at the N-terminal end of a peptide with its DPP IV inhibitory potency [57].
The presence of high molecular weight amino acids and aromatic residues such as phenylalanine, tryptophan, tyrosine, and arginine have been observed in peptides that inhibit α-amylase. This is due to the fact that substrate-binding pockets of the α-amylase enzyme has a number of aromatic residues. Hence, apart from interactions involving hydrogen bonds, electrostatic and van der Waals interactions, aromatic–aromatic interactions also play a crucial role in α-amylase inhibitory activity [112,119]. An analysis of 43 α-glucosidase inhibitory peptides suggested that the presence of hydroxyl or basic amino acids at the N-terminal could be responsible for the potency of these peptides. The presence of proline within the peptide and alanine and methionine at the C-terminal could also enhance the inhibitory potential of peptides [123].
7. Oral Bioavailability and Stability of Peptide Drugs
Due to their bioactivity, peptides are promising lead molecules for the development of nutraceuticals. Despite their potential, progress is limited due to their poor pharmacokinetics properties. The major challenges faced by peptide drugs include poor oral bioavailability and increased proteolytic instability when compared to small molecule drugs as well as manufacturing costs [124]. However, over the past few decades, new technologies and drug formulations have improved the clinical application of peptide drugs [19]. Oral administration is considered to be the most preferred and convenient way for delivering drugs [125]. Due to poor oral bioavailability, peptide drugs are commonly administered as intravenous, intraperitoneal, or intramuscular injections. However, long-term continuous injection could cause pain and discomfort among patients. Alternate routes are now being sought to deliver peptide drugs safely and effectively [126]. Physical and chemical methods could improve the oral bioavailability and stability of the peptides [127]. This includes pH modulation of intestinal enzymes by adding organic acids such as citric acid and enzyme inhibitors, enteric coating using polyacrylic polymers, and nanoparticles [128,129]. Nanoparticle-coated drugs have shown enhanced bioavailability and distribution [130]. An oral carrier of insulin composed of polysaccharides and nano-silica, which is now in Phase II clinical trials, has been shown to safely lower blood glucose levels [131]. Stability of peptides could also be improved by various chemical approaches such as peptide cyclization and conjugation. Cyclic and conjugated peptide drug candidates for various diseases are in clinical and preclinical trials [132,133]. For example, a PEGylated insulin analog that could be orally delivered is now in Phase II/III clinical trials [134]. The alkylated PEG and permeation enhancer in the insulin analog was shown to increase water solubility, stability, and to enhance absorption.
Despite these advances, bioavailability and stability of peptide drugs is still a major challenge [19]. Nonetheless, a growing number of technological advances in this field is likely to break some of these barriers.
8. Future Prospects of Peptide Drugs
Currently, more than 80 peptide drugs are on the market for the treatment of various endocrine, metabolic, and cardiovascular disorders as well as cancer. More than 150 peptides are in clinical trials and nearly 600 peptides are undergoing preclinical studies [19]. The remarkable increase in the number of approvals for peptide-based therapeutics in the last two decades bodes well for an expected acceleration in the near future. Peptide drugs with improved potency and specificity are expected to increase in near future. Additionally, use of conjugated peptide drugs and membrane-penetrating peptides in treating various metabolic syndromes including T2DM will become more common in the coming years. Interest in peptide therapeutics is expected to significantly increase once effective delivery platforms are in place. Furthermore, introduction of novel formulations and chemical methods to stabilize peptide drugs could improve their efficacy. As technology advances, this field is expected to open new horizons in the development of safe and more potent drugs for the treatment of several disorders including diabetes.
9. Conclusions
A mounting body of evidence supports the importance of functional foods and food-derived bioactive peptides in managing and treating T2DM. A myriad of bioactive peptides, obtained from various food sources, could be utilized as antidiabetic agents, food supplements or lead compounds in drug discovery. Here, we discuss the potential antidiabetic peptides isolated from a variety of food sources that are capable of inhibiting α amylase, α glucosidase, and DPP IV. Salient structural characteristics of these bioactive peptides have also been summarized, which should assist the design of more potent and specific bioactive peptides. Even though several studies have shown the importance of food-derived bioactive peptides in managing diabetes, their effect on humans is still being actively studied. There still exists a wide gap between scientific studies and commercialization of food-based bioactive peptides. Additional work is required to validate the antidiabetic properties of these peptides through cellular assays and clinical trials for ensuring their efficacy and safety. Much more work needs to be done in the field of peptide therapeutics and delivery systems for improving stability, promoting bioavailability, and enhancing half-life. These studies will eventually help in the commercial production of these peptides as nutraceuticals that could contribute to human health.
Author Contributions
R.V. and P.A. wrote the review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a UPAR grant (12S006) to RV from the United Arab Emirates University.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lin X., Xu Y., Pan X., Xu J., Ding Y., Sun X., Song X., Ren Y., Shan P.-F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020;10:14790. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association Classification and diagnosis of diabetes: Standards of medical care in diabetes—2020. Diabetes Care. 2020;43:S14–S31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 3.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.Bragg F., Holmes M.V., Iona A., Guo Y., Du H., Chen Y., Bian Z., Yang L., Herrington W., Bennett D., et al. Association between diabetes and cause-specific mortality in rural and urban areas of China. JAMA. 2017;317:280–289. doi: 10.1001/jama.2016.19720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization [(accessed on 10 June 2021)];Diabetes Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes.
- 6.Banu S., Bhowmick A. Therapeutic targets of type 2 diabetes: An overview. MOJ Drug Des. Dev. Ther. 2017;1:1–6. doi: 10.15406/mojddt.2017.01.00011. [DOI] [Google Scholar]
- 7.Padhi S., Nayak A.K., Behera A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020;131:110708. doi: 10.1016/j.biopha.2020.110708. [DOI] [PubMed] [Google Scholar]
- 8.Daliri E., Oh D., Lee B. Bioactive peptides. Foods. 2017;6:32. doi: 10.3390/foods6050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thrasher J. Pharmacologic management of type 2 diabetes mellitus: Available therapies. Am. J. Med. 2017;130:S4–S17. doi: 10.1016/j.amjmed.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Sylow L., Kleinert M., Richter E.A., Jensen T.E. Exercise-stimulated glucose uptake—regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017;13:133–148. doi: 10.1038/nrendo.2016.162. [DOI] [PubMed] [Google Scholar]
- 11.Forouhi N.G., Misra A., Mohan V., Taylor R., Yancy W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ. 2018;361:k2234. doi: 10.1136/bmj.k2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ley S.H., Hamdy O., Mohan V., Hu F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iavarone F., Desiderio C., Vitali A., Messana I., Martelli C., Castagnola M., Cabras T. Cryptides: Latent peptides everywhere. Crit. Rev. Biochem. Mol. Biol. 2018;53:246–263. doi: 10.1080/10409238.2018.1447543. [DOI] [PubMed] [Google Scholar]
- 14.Manzanares P., Gandía M., Garrigues S., Marcos J.F. Improving health-promoting effects of food-derived bioactive peptides through rational design and oral delivery strategies. Nutrients. 2019;11:2545. doi: 10.3390/nu11102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sánchez A., Vázquez A. Bioactive peptides: A review. Food Qual. Saf. 2017;1:29–46. doi: 10.1093/fqs/fyx006. [DOI] [Google Scholar]
- 16.Aslam M.Z., Aslam M.S., Firdos S., Ghous G., Firdos G., Hongfei Z., Bolin Z. Role of bioactive peptides in reducing the severity of hypertension with the inhibition of ACE. Int. J. Pept. Res. Ther. 2019;25:1639–1649. doi: 10.1007/s10989-018-09806-y. [DOI] [Google Scholar]
- 17.Tyagi A., Daliri E.B.-M., Kwami Ofosu F., Yeon S.-J., Oh D.-H. Food-derived opioid peptides in human health: A review. IJMS. 2020;21:8825. doi: 10.3390/ijms21228825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J., Zhao J., Yang R., Zhao W. Bioactive peptides with antidiabetic properties: A review. Int. J. Food Sci. Technol. 2019;54:1909–1919. doi: 10.1111/ijfs.14090. [DOI] [Google Scholar]
- 19.Muttenthaler M., King G.F., Adams D.J., Alewood P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021;20:309–325. doi: 10.1038/s41573-020-00135-8. [DOI] [PubMed] [Google Scholar]
- 20.Rivero-Pino F., Espejo-Carpio F.J., Guadix E.M. Antidiabetic food-derived peptides for functional feeding: Production, functionality and In Vivo evidences. Foods. 2020;9:983. doi: 10.3390/foods9080983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatanaka T., Inoue Y., Arima J., Kumagai Y., Usuki H., Kawakami K., Kimura M., Mukaihara T. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012;134:797–802. doi: 10.1016/j.foodchem.2012.02.183. [DOI] [PubMed] [Google Scholar]
- 22.Lindström J., Virtanen S.M. Functional Foods. Elsevier; Amsterdam, The Netherlands: 2011. Functional foods and prevention of diabetes; pp. 261–276. [Google Scholar]
- 23.Fosgerau K., Hoffmann T. Peptide therapeutics: Current status and future directions. Drug Discov. Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Bhat Z.F., Kumar S., Bhat H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015;52:5377–5392. doi: 10.1007/s13197-015-1731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazcano-Perez F., Roman-Gonzalez S.A., Sanchez-Puig N., Arreguin- Espinosa R. Bioactive peptides from marine organisms: A short overview. Protein Pept. Lett. 2012;19:700–707. doi: 10.2174/092986612800793208. [DOI] [PubMed] [Google Scholar]
- 26.Patil S.P., Goswami A., Kalia K., Kate A.S. Plant-derived bioactive peptides: A treatment to cure diabetes. Int. J. Pept. Res. Ther. 2020;26:955–968. doi: 10.1007/s10989-019-09899-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Udenigwe C.C., Aluko R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012;77:R11–R24. doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- 28.Chai K.F., Voo A.Y.H., Chen W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020;19:3825–3885. doi: 10.1111/1541-4337.12651. [DOI] [PubMed] [Google Scholar]
- 29.Raveschot C., Cudennec B., Coutte F., Flahaut C., Fremont M., Drider D., Dhulster P. Production of bioactive peptides by lactobacillus species: From gene to application. Front. Microbiol. 2018;9:2354. doi: 10.3389/fmicb.2018.02354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakrabarti S., Guha S., Majumder K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients. 2018;10:1738. doi: 10.3390/nu10111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korhonen H., Pihlanto A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006;16:945–960. doi: 10.1016/j.idairyj.2005.10.012. [DOI] [Google Scholar]
- 32.Aronoff S.L., Berkowitz K., Shreiner B., Want L. Glucose metabolism and regulation: Beyond insulin and glucagon. Diabetes Spectr. 2004;17:183–190. doi: 10.2337/diaspect.17.3.183. [DOI] [Google Scholar]
- 33.Galicia-Garcia U., Benito-Vicente A., Jebari S., Larrea-Sebal A., Siddiqi H., Uribe K.B., Ostolaza H., Martín C. Pathophysiology of type 2 diabetes mellitus. IJMS. 2020;21:6275. doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vecchio I., Tornali C., Bragazzi N.L., Martini M. The discovery of insulin: An important milestone in the history of medicine. Front. Endocrinol. 2018;9:613. doi: 10.3389/fendo.2018.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van de Laar F.A., Lucassen P.L., Akkermans R.P., van de Lisdonk E.H., Rutten G.E., van Weel C. Glucosidase inhibitors for patients with type 2 diabetes: Results from a cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 36.Bashary R., Vyas M., Nayak S.K., Suttee A., Verma S., Narang R., Khatik G.L. An insight of alpha-amylase inhibitors as a valuable tool in the management of type 2 diabetes mellitus. CDR. 2020;16:117–136. doi: 10.2174/1573399815666190618093315. [DOI] [PubMed] [Google Scholar]
- 37.Kalra S., Bhutani J. Diabetology: Type 2 Diabetes Mellitus. Jaypee Brothers Medical Publishers (P) Ltd.; New Delhi, India: 2014. Alpha-glucosidase Inhibitors; p. 55. [Google Scholar]
- 38.Kawamori R., Tajima N., Iwamoto Y., Kashiwagi A., Shimamoto K., Kaku K. Voglibose for prevention of type 2 diabetes mellitus: A randomised, double-blind trial in japanese individuals with impaired glucose tolerance. Lancet. 2009;373:1607–1614. doi: 10.1016/S0140-6736(09)60222-1. [DOI] [PubMed] [Google Scholar]
- 39.Kazakos K. Incretin effect: GLP-1, GIP, DPP4. Diabetes Res. Clin. Pract. 2011;93:S32–S36. doi: 10.1016/S0168-8227(11)70011-0. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert M.P., Pratley R.E. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: Review of head-to-head clinical trials. Front. Endocrinol. 2020;11:178. doi: 10.3389/fendo.2020.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao E.C., Henry R.R. SGLT2 inhibition—a novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 2010;9:551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 42.Shorr R.I., Ray W.A., Daugherty J.R., Griffin M.R. Individual sulfonylureas and serious hypoglycemia in older people. J. Am. Geriatr. Soc. 1996;44:751–755. doi: 10.1111/j.1532-5415.1996.tb03729.x. [DOI] [PubMed] [Google Scholar]
- 43.Kokil G.R., Veedu R.N., Ramm G.A., Prins J.B., Parekh H.S. Type 2 diabetes mellitus: Limitations of conventional therapies and intervention with nucleic acid-based therapeutics. Chem. Rev. 2015;115:4719–4743. doi: 10.1021/cr5002832. [DOI] [PubMed] [Google Scholar]
- 44.Lau J.L., Dunn M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic Med. Chem. 2018;26:2700–2707. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 45.De la Torre B.G., Albericio F. Peptide therapeutics 2.0. Molecules. 2020;25:2293. doi: 10.3390/molecules25102293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furman B.L. The Development of byetta (Exenatide) from the venom of the gila monster as an anti-diabetic agent. Toxicon. 2012;59:464–471. doi: 10.1016/j.toxicon.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Wajcberg E. Amatur amarah liraglutide in the management of type 2 diabetes. Drug Des. Dev. Ther. 2010;4:279–290. doi: 10.2147/DDDT.S10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohanty D.P., Mohapatra S., Misra S., Sahu P.S. Milk derived bioactive peptides and their impact on human health-a review. Saudi J. Biol. Sci. 2015;23:577–583. doi: 10.1016/j.sjbs.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCarthy R., Mills S., Ross R.P., Fitzgerald G.F., Stanton C. Bioactive peptides from casein and whey proteins. In: Kanekanian A., editor. Milk and Dairy Products as Functional Foods. John Wiley & Sons, Ltd; Chichester, UK: 2014. pp. 23–54. [Google Scholar]
- 50.Vargas-Bello-Pérez E., Márquez-Hernández R.I., Hernández-Castellano L.E. Bioactive Peptides from milk: Animal determinants and their implications in human health. J. Dairy Res. 2019;86:136–144. doi: 10.1017/S0022029919000384. [DOI] [PubMed] [Google Scholar]
- 51.Lucarini M. Bioactive peptides in milk: From encrypted sequences to nutraceutical aspects. Beverages. 2017;3:41. doi: 10.3390/beverages3030041. [DOI] [Google Scholar]
- 52.El-Sayed M.I., Awad S., Wahba A., El Attar A., Yousef M.I., Zedan M. In Vivo anti-diabetic and biological activities of milk protein and milk protein hydrolyaste. Adv. Dairy Res. 2016;4 doi: 10.4172/2329-888X.1000154. [DOI] [PubMed] [Google Scholar]
- 53.Li Y., Fan Y., Shaikh A.S., Wang Z., Wang D., Tan H. Dezhou donkey (Equus asinus) milk a potential treatment strategy for type 2 diabetes. J. Ethnopharmacol. 2020;246:112221. doi: 10.1016/j.jep.2019.112221. [DOI] [PubMed] [Google Scholar]
- 54.Gong H., Gao J., Wang Y., Luo Q.W., Guo K.R., Ren F.Z., Mao X.Y. Identification of novel peptides from goat milk casein that ameliorate high-glucose-induced insulin resistance in HepG2 cells. J. Dairy Sci. 2020;103:4907–4918. doi: 10.3168/jds.2019-17513. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y., Chen R., Ma H., Chen S. Isolation and identification of dipeptidyl peptidase IV-inhibitory peptides from trypsin/chymotrypsin-treated goat milk casein hydrolysates by 2D-TLC and LC–MS/MS. J. Agric. Food Chem. 2015;63:8819–8828. doi: 10.1021/acs.jafc.5b03062. [DOI] [PubMed] [Google Scholar]
- 56.Mudgil P., Kamal H., Yuen G.C., Maqsood S. Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chem. 2018;259:46–54. doi: 10.1016/j.foodchem.2018.03.082. [DOI] [PubMed] [Google Scholar]
- 57.Nongonierma A.B., FitzGerald R.J. Susceptibility of milk protein-derived peptides to dipeptidyl peptidase IV (DPP-IV) hydrolysis. Food Chem. 2014;145:845–852. doi: 10.1016/j.foodchem.2013.08.097. [DOI] [PubMed] [Google Scholar]
- 58.Baspinar B., Güldaş M. Traditional plain yogurt: A therapeutic food for metabolic syndrome? Crit. Rev. Food Sci. Nutr. 2020:1–15. doi: 10.1080/10408398.2020.1799931. [DOI] [PubMed] [Google Scholar]
- 59.Ni H., Hayes H.E., Stead D., Raikos V. Incorporating salal berry (Gaultheria Shallon) and blackcurrant (Ribes Nigrum) pomace in yogurt for the development of a beverage with antidiabetic properties. Heliyon. 2018;4:e00875. doi: 10.1016/j.heliyon.2018.e00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uenishi H., Kabuki T., Seto Y., Serizawa A., Nakajima H. Isolation and identification of casein-derived dipeptidyl-peptidase 4 (DPP-4)-inhibitory peptide LPQNIPPL from gouda-type cheese and its effect on plasma glucose in rats. Int. Dairy J. 2012;22:24–30. doi: 10.1016/j.idairyj.2011.08.002. [DOI] [Google Scholar]
- 61.Yu Z., Yin Y., Zhao W., Yu Y., Liu B., Liu J., Chen F. Novel peptides derived from egg white protein inhibiting alpha-glucosidase. Food Chem. 2011;129:1376–1382. doi: 10.1016/j.foodchem.2011.05.067. [DOI] [Google Scholar]
- 62.Zambrowicz A., Pokora M., Setner B., Dąbrowska A., Szołtysik M., Babij K., Szewczuk Z., Trziszka T., Lubec G., Chrzanowska J. Multifunctional peptides derived from an egg yolk protein hydrolysate: Isolation and characterization. Amino Acids. 2015;47:369–380. doi: 10.1007/s00726-014-1869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nong N.T.P., Chen Y.-K., Shih W.-L., Hsu J.-L. Characterization of novel dipeptidyl peptidase-iv inhibitory peptides from soft-shelled turtle yolk hydrolysate using orthogonal bioassay-guided fractionations coupled with In Vitro and In Silico study. Pharmaceuticals. 2020;13:308. doi: 10.3390/ph13100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao W., Zhang D., Yu Z., Ding L., Liu J. Novel membrane peptidase inhibitory peptides with activity against angiotensin converting enzyme and dipeptidyl peptidase iv identified from hen eggs. J. Funct. Foods. 2020;64:103649. doi: 10.1016/j.jff.2019.103649. [DOI] [Google Scholar]
- 65.Martini S., Conte A., Tagliazucchi D. Comparative peptidomic profile and bioactivities of cooked beef, pork, chicken and turkey meat after In Vitro gastro-intestinal digestion. J. Proteom. 2019;208:103500. doi: 10.1016/j.jprot.2019.103500. [DOI] [PubMed] [Google Scholar]
- 66.Gallego M., Aristoy M.-C., Toldrá F. Dipeptidyl peptidase iv inhibitory peptides generated in spanish dry-cured ham. Meat Sci. 2014;96:757–761. doi: 10.1016/j.meatsci.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 67.Casanova-Martí À., Bravo F.I., Serrano J., Ardévol A., Pinent M., Muguerza B. Antihyperglycemic effect of a chicken feet hydrolysate via the incretin system: DPP-IV-inhibitory activity and GLP-1 release stimulation. Food Funct. 2019;10:4062–4070. doi: 10.1039/C9FO00695H. [DOI] [PubMed] [Google Scholar]
- 68.De Cássia Lemos Lima R., Berg R.S., Rønning S.B., Afseth N.K., Knutsen S.H., Staerk D., Wubshet S.G. Peptides from chicken processing by-product inhibit DPP-IV and promote cellular glucose uptake: Potential ingredients for T2D management. Food Funct. 2019;10:1619–1628. doi: 10.1039/C8FO02450B. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y.-F., Oey I., Bremer P., Carne A., Silcock P. Bioactive peptides derived from egg proteins: A review. Crit. Rev. Food Sci. Nutr. 2018;58:2508–2530. doi: 10.1080/10408398.2017.1329704. [DOI] [PubMed] [Google Scholar]
- 70.Arena S., Renzone G., Scaloni A. A multi-approach peptidomic analysis of hen egg white reveals novel putative bioactive molecules. J. Proteom. 2020;215:103646. doi: 10.1016/j.jprot.2020.103646. [DOI] [PubMed] [Google Scholar]
- 71.Xing L., Liu R., Cao S., Zhang W., Guanghong Z. Meat protein based bioactive peptides and their potential functional activity: A review. Int. J. Food Sci. Technol. 2019;54:1956–1966. doi: 10.1111/ijfs.14132. [DOI] [Google Scholar]
- 72.Kęska P., Stadnik J., Bąk O., Borowski P. Meat proteins as dipeptidyl peptidase iv inhibitors and glucose uptake stimulating peptides for the management of a type 2 diabetes mellitus In Silico study. Nutrients. 2019;11:2537. doi: 10.3390/nu11102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toldrá F., Gallego M., Reig M., Aristoy M.-C., Mora L. Bioactive peptides generated in the processing of dry-cured ham. Food Chem. 2020;321:126689. doi: 10.1016/j.foodchem.2020.126689. [DOI] [PubMed] [Google Scholar]
- 74.Martínez-Sánchez S.M., Minguela A., Prieto-Merino D., Zafrilla-Rentero M.P., Abellán-Alemán J., Montoro-García S. The effect of regular intake of dry-cured ham rich in bioactive peptides on inflammation, platelet and monocyte activation markers in humans. Nutrients. 2017;9:321. doi: 10.3390/nu9040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mora L., González-Rogel D., Heres A., Toldrá F. Iberian dry-cured ham as a potential source of α-glucosidase-inhibitory peptides. J. Funct. Foods. 2020;67:103840. doi: 10.1016/j.jff.2020.103840. [DOI] [Google Scholar]
- 76.Zhang Y., Chen R., Chen X., Zeng Z., Ma H., Chen S. Dipeptidyl peptidase IV-inhibitory peptides derived from silver carp (Hypophthalmichthys molitrix Val.) proteins. J. Agric. Food Chem. 2016;64:831–839. doi: 10.1021/acs.jafc.5b05429. [DOI] [PubMed] [Google Scholar]
- 77.Hong H., Zheng Y., Song S., Zhang Y., Zhang C., Liu J., Luo Y. Identification and characterization of DPP-IV inhibitory peptides from silver carp swim bladder hydrolysates. Food Biosci. 2020;38:100748. doi: 10.1016/j.fbio.2020.100748. [DOI] [Google Scholar]
- 78.Huang S.-L., Jao C.-L., Ho K.-P., Hsu K.-C. Dipeptidyl-peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides. 2012;35:114–121. doi: 10.1016/j.peptides.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 79.Harnedy P.A., Parthsarathy V., McLaughlin C.M., O’Keeffe M.B., Allsopp P.J., McSorley E.M., O’Harte F.P.M., FitzGerald R.J. Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. J. Funct. Foods. 2018;40:137–145. doi: 10.1016/j.jff.2017.10.045. [DOI] [Google Scholar]
- 80.Harnedy P.A., Parthsarathy V., McLaughlin C.M., O’Keeffe M.B., Allsopp P.J., McSorley E.M., O’Harte F.P.M., FitzGerald R.J. Atlantic salmon (Salmo salar) co-product-derived protein hydrolysates: A source of antidiabetic peptides. Food Res. Int. 2018;106:598–606. doi: 10.1016/j.foodres.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 81.Chatterjee C., Gleddie S., Xiao C.-W. Soybean bioactive peptides and their functional properties. Nutrients. 2018;10:1211. doi: 10.3390/nu10091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanjukta S., Rai A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016;50:1–10. doi: 10.1016/j.tifs.2016.01.010. [DOI] [Google Scholar]
- 83.Lammi C., Zanoni C., Arnoldi A. Three peptides from soy glycinin modulate glucose metabolism in human hepatic HepG2 cells. Int. J. Mech. Sci. 2015;16:27362–27370. doi: 10.3390/ijms161126029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang R., Zhao H., Pan X., Orfila C., Lu W., Ma Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of A-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019;7:1848–1856. doi: 10.1002/fsn3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu J., Zeng Y., Hou W., Zhang S., Li L., Luo X., Xi W., Chen Z., Xiang M. The soybean peptide aglycin regulates glucose homeostasis in type 2 diabetic mice via IR/IRS1 pathway. J. Nutr. Biochem. 2012;23:1449–1457. doi: 10.1016/j.jnutbio.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 86.Jiang H., Feng J., Du Z., Zhen H., Lin M., Jia S., Li T., Huang X., Ostenson C.-G., Chen Z. Oral administration of soybean peptide vglycin normalizes fasting glucose and restores impaired pancreatic function in type 2 diabetic wistar rats. J. Nutr. Biochem. 2014;25:954–963. doi: 10.1016/j.jnutbio.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 87.Jiang H., Tong Y., Yan D., Jia S., Ostenson C.-G., Chen Z. The soybean peptide vglycin preserves the diabetic β-cells through improvement of proliferation and inhibition of apoptosis. Sci. Rep. 2015;5:15599. doi: 10.1038/srep15599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamada Y., Muraki A., Oie M., Kanegawa N., Oda A., Sawashi Y., Kaneko K., Yoshikawa M., Goto T., Takahashi N., et al. Soymorphin-5, a soy-derived μ-opioid peptide, decreases glucose and triglyceride levels through activating adiponectin and PPARα systems in diabetic KKAy mice. Am. J. Physiol. Endocrinol. Metab. 2012;302:E433–E440. doi: 10.1152/ajpendo.00161.2011. [DOI] [PubMed] [Google Scholar]
- 89.González-Montoya M., Hernández-Ledesma B., Mora-Escobedo R., Martínez-Villaluenga C. Bioactive peptides from germinated soybean with anti-diabetic potential by inhibition of dipeptidyl peptidase-IV, α-amylase, and α-glucosidase enzymes. Int. J. Mol. Sci. 2018;19:2883. doi: 10.3390/ijms19102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lammi C., Bollati C., Ferruzza S., Ranaldi G., Sambuy Y., Arnoldi A. Soybean- and lupin-derived peptides inhibit dpp-iv activity on in situ human intestinal caco-2 cells and Ex vivo human serum. Nutrients. 2018;10:1082. doi: 10.3390/nu10081082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lammi C., Arnoldi A., Aiello G. Soybean peptides exert multifunctional bioactivity modulating 3-hydroxy-3-methylglutaryl-coa reductase and dipeptidyl peptidase-IV targets in vitro. J. Agric. Food Chem. 2019;67:4824–4830. doi: 10.1021/acs.jafc.9b01199. [DOI] [PubMed] [Google Scholar]
- 92.Martinez-Villaluenga C., Peñas E., Frias J. Fermented Foods in Health and Disease Prevention. Elsevier; Amsterdam, The Netherlands: 2017. Bioactive peptides in fermented foods; pp. 23–47. [Google Scholar]
- 93.Kwon D.Y., Daily J.W., Kim H.J., Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010;30:1–13. doi: 10.1016/j.nutres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 94.Tiss M., Souiy Z., ben Abdeljelil N., Njima M., Achour L., Hamden K. Fermented soy milk prepared using kefir grains prevents and ameliorates obesity, type 2 diabetes, hyperlipidemia and liver-kidney toxicities in HFFD-rats. J. Funct. Foods. 2020;67:103869. doi: 10.1016/j.jff.2020.103869. [DOI] [Google Scholar]
- 95.Dong J.-Y., Kimura T., Ikehara S., Cui M., Kawanishi Y., Ueda K., Iso H., Yamazaki S., Ohya Y., Kishi R., et al. Soy consumption and incidence of gestational diabetes mellitus: The japan environment and children’s study. Eur. J. Nutr. 2020;60:897–904. doi: 10.1007/s00394-020-02294-1. [DOI] [PubMed] [Google Scholar]
- 96.Sato K., Miyasaka S., Tsuji A., Tachi H. Isolation and characterization of peptides with dipeptidyl peptidase IV (DPPIV) inhibitory activity from natto using DPPIV from aspergillus oryzae. Food Chem. 2018;261:51–56. doi: 10.1016/j.foodchem.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 97.Mojica L., de Mejía E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016;7:713–727. doi: 10.1039/C5FO01204J. [DOI] [PubMed] [Google Scholar]
- 98.Ngoh Y.-Y., Gan C.-Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from pinto beans (Phaseolus vulgaris Cv. Pinto) Food Chem. 2016;190:331–337. doi: 10.1016/j.foodchem.2015.05.120. [DOI] [PubMed] [Google Scholar]
- 99.Ngoh Y.-Y., Tye G.J., Gan C.-Y. The investigation of α-amylase inhibitory activity of selected pinto bean peptides via preclinical study using AR42J cell. J. Funct. Foods. 2017;35:641–647. doi: 10.1016/j.jff.2017.06.037. [DOI] [Google Scholar]
- 100.Valencia-Mejía E., Batista K.A., Fernández J.J.A., Fernandes K.F. Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy-to-cook and hard-to-cook beans (Phaseolus vulgaris L.) Food Res. Int. 2019;121:238–246. doi: 10.1016/j.foodres.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 101.Mune Mune M.A., Minka S.R., Henle T. Investigation on antioxidant, angiotensin converting enzyme and dipeptidyl peptidase iv inhibitory activity of bambara bean protein hydrolysates. Food Chem. 2018;250:162–169. doi: 10.1016/j.foodchem.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 102.Uruakp F. Influence of cowpea (Vigna unguiculata) peptides on insulin resistance. J. Nutr. Heal. Food Sci. 2015;3 doi: 10.15226/jnhfs.2015.00144. [DOI] [Google Scholar]
- 103.Chandrasekaran S., Luna-Vital D., de Mejia E.G. Identification and comparison of peptides from chickpea protein hydrolysates using either bromelain or gastrointestinal enzymes and their relationship with markers of type 2 diabetes and bitterness. Nutrients. 2020;12:3843. doi: 10.3390/nu12123843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cavazos A., Gonzalez de Mejia E. Identification of bioactive peptides from cereal storage proteins and their potential role in prevention of chronic diseases. Compr. Rev. Food Sci. Food Saf. 2013;12:364–380. doi: 10.1111/1541-4337.12017. [DOI] [PubMed] [Google Scholar]
- 105.Sun S., Zhang G., Mu H., Zhang H., Chen Y.Q. The mixture of corn and wheat peptide prevent diabetes in NOD mice. J. Funct. Foods. 2019;56:163–170. doi: 10.1016/j.jff.2019.03.020. [DOI] [Google Scholar]
- 106.Wang F., Yu G., Zhang Y., Zhang B., Fan J. Dipeptidyl peptidase IV inhibitory peptides derived from oat (Avena Sativa L.), buckwheat (Fagopyrum Esculentum), and highland barley (Hordeum Vulgare Trifurcatum (L.) trofim) proteins. J. Agric. Food Chem. 2015;63:9543–9549. doi: 10.1021/acs.jafc.5b04016. [DOI] [PubMed] [Google Scholar]
- 107.Nongonierma A.B., Hennemann M., Paolella S., FitzGerald R.J. Generation of wheat gluten hydrolysates with dipeptidyl peptidase IV (DPP-IV) Inhibitory properties. Food Funct. 2017;8:2249–2257. doi: 10.1039/C7FO00165G. [DOI] [PubMed] [Google Scholar]
- 108.Mudgil P., Kilari B.P., Kamal H., Olalere O.A., FitzGerald R.J., Gan C.-Y., Maqsood S. Multifunctional bioactive peptides derived from quinoa protein hydrolysates: Inhibition of α-glucosidase, dipeptidyl peptidase-IV and angiotensin I converting enzymes. J. Cereal Sci. 2020;96:103130. doi: 10.1016/j.jcs.2020.103130. [DOI] [Google Scholar]
- 109.Vilcacundo R., Martínez-Villaluenga C., Hernández-Ledesma B. Release of Dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods. 2017;35:531–539. doi: 10.1016/j.jff.2017.06.024. [DOI] [Google Scholar]
- 110.Guo H., Richel A., Hao Y., Fan X., Everaert N., Yang X., Ren G. Novel dipeptidyl peptidase-IV and angiotensin-I-converting enzyme inhibitory peptides released from quinoa protein by in silico proteolysis. Food Sci. Nutr. 2020;8:1415–1422. doi: 10.1002/fsn3.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jorge S.-S., Raúl R.-B., Isabel G.-L., Edith P.-A., Bernardo E.-B., César A.-P., Gerardo D.-G., Rubén R.-R. Dipeptidyl peptidase IV inhibitory activity of protein hydrolyzates from Amaranthus Hypochondriacus L. grain and their influence on postprandial glycemia in streptozotocin-induced diabetic mice. Afr. J. Trad. Compl. Alt. Med. 2015;12:90. doi: 10.4314/ajtcam.v12i1.13. [DOI] [Google Scholar]
- 112.Vilcacundo R., Martínez-Villaluenga C., Miralles B., Hernández-Ledesma B. Release of multifunctional peptides from kiwicha (Amaranthus caudatus) protein under in vitro gastrointestinal digestion: Multifunctional kiwicha-derived peptides. J. Sci. Food Agric. 2019;99:1225–1232. doi: 10.1002/jsfa.9294. [DOI] [PubMed] [Google Scholar]
- 113.Ros E., Hu F.B. Consumption of plant seeds and cardiovascular health: Epidemiological and clinical trial evidence. Circulation. 2013;128:553–565. doi: 10.1161/CIRCULATIONAHA.112.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kendall C.W.C., Esfahani A., Truan J., Srichaikul K., Jenkins D.J.A. Health benefits of nuts in prevention and management of diabetes. Asia Pac. J. Clin. Nutr. 2010;19:110–116. [PubMed] [Google Scholar]
- 115.Wang J., Du K., Fang L., Liu C., Min W., Liu J. Evaluation of the antidiabetic activity of hydrolyzed peptides derived from Juglans mandshurica maxim. Fruits in insulin-resistant HepG2 cells and type 2 diabetic mice. J. Food Biochem. 2018;42:e12518. doi: 10.1111/jfbc.12518. [DOI] [Google Scholar]
- 116.Wang J., Wu T., Fang L., Liu C., Liu X., Li H., Shi J., Li M., Min W. Anti-diabetic effect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting α-glucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods. 2020;69:103944. doi: 10.1016/j.jff.2020.103944. [DOI] [Google Scholar]
- 117.Doyen A., Udenigwe C.C., Mitchell P.L., Marette A., Aluko R.E., Bazinet L. Anti-diabetic and antihypertensive activities of two flaxseed protein hydrolysate fractions revealed following their simultaneous separation by electrodialysis with ultrafiltration membranes. Food Chem. 2014;145:66–76. doi: 10.1016/j.foodchem.2013.07.108. [DOI] [PubMed] [Google Scholar]
- 118.Nurul Hidayatul Afifah B.S.S., Gan C.-Y. Antioxidative and amylase inhibitor peptides from basil seeds. Int. J. Pept. Res. Ther. 2016;22:3–10. doi: 10.1007/s10989-015-9477-5. [DOI] [Google Scholar]
- 119.Siow H.-L., Gan C.-Y. Extraction, Identification, and structure–activity relationship of antioxidative and α-amylase inhibitory peptides from cumin seeds (Cuminum cyminum) J. Funct. Foods. 2016;22:1–12. doi: 10.1016/j.jff.2016.01.011. [DOI] [Google Scholar]
- 120.Han R., Maycock J., Murray B.S., Boesch C. Identification of angiotensin converting enzyme and dipeptidyl peptidase-IV inhibitory peptides derived from oilseed proteins using two integrated bioinformatic approaches. Food Res. Int. 2019;115:283–291. doi: 10.1016/j.foodres.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 121.Nongonierma A.B., FitzGerald R.J. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 2019;43:e12451. doi: 10.1111/jfbc.12451. [DOI] [PubMed] [Google Scholar]
- 122.Arulmozhiraja S., Matsuo N., Ishitsubo E., Okazaki S., Shimano H., Tokiwa H. Comparative binding analysis of dipeptidyl peptidase IV (DPP-4) with antidiabetic drugs–an Ab initio fragment molecular orbital study. PLoS ONE. 2016;11:e0166275. doi: 10.1371/journal.pone.0166275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ibrahim M.A., Bester M.J., Neitz A.W.H., Gaspar A.R.M. Structural properties of bioactive peptides with α-glucosidase inhibitory activity. Chem. Biol. Drug Des. 2018;91:370–379. doi: 10.1111/cbdd.13105. [DOI] [PubMed] [Google Scholar]
- 124.Otvos L., Wade J.D. Current challenges in peptide-based drug discovery. Front. Chem. 2014;2:62. doi: 10.3389/fchem.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brown T.D., Whitehead K.A., Mitragotri S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020;5:127–148. doi: 10.1038/s41578-019-0156-6. [DOI] [Google Scholar]
- 126.Ganesh A.N., Heusser C., Garad S., Sánchez-Félix M.V. Patient-centric design for peptide delivery: Trends in routes of administration and advancement in drug delivery technologies. Med. Drug Discov. 2021;9:100079. doi: 10.1016/j.medidd.2020.100079. [DOI] [Google Scholar]
- 127.Ibrahim Y.H.-E.Y., Regdon G., Hamedelniel E.I., Sovány T. Review of recently used techniques and materials to improve the efficiency of orally administered proteins/peptides. DARU J. Pharm. Sci. 2020;28:403–416. doi: 10.1007/s40199-019-00316-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zizzari A.T., Pliatsika D., Gall F.M., Fischer T., Riedl R. New perspectives in oral peptide delivery. Drug Discov. Today. 2021;26:1097–1105. doi: 10.1016/j.drudis.2021.01.020. [DOI] [PubMed] [Google Scholar]
- 129.Zhu Q., Chen Z., Paul P.K., Lu Y., Wu W., Qi J. Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharm. Sin. B. 2021 doi: 10.1016/j.apsb.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pudlarz A., Szemraj J. Nanoparticles as carriers of proteins, peptides and other therapeutic molecules. Open Life Sci. 2018;13:285–298. doi: 10.1515/biol-2018-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rachmiel M., Barash G., Leshem A., Sagi R., Doenyas-Barak K., Koren S. OR14-1 Pharmacodynamics, safety, tolerability, and efficacy of oral insulin formulation (Oshadi Icp) among young adults with type 1 diabetes: A summary of clinical studies phases I, Ib, and Ii. J. Endocr. Soc. 2019;3:OR14-1. doi: 10.1210/js.2019-OR14-1. [DOI] [Google Scholar]
- 132.Redko B., Tuchinsky H., Segal T., Tobi D., Luboshits G., Ashur-Fabian O., Pinhasov A., Gerlitz G., Gellerman G. Toward the development of a novel Non-RGD cyclic peptide drug conjugate for treatment of human metastatic melanoma. Oncotarget. 2017;8:757–768. doi: 10.18632/oncotarget.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alas M., Saghaeidehkordi A., Kaur K. Peptide–drug conjugates with different linkers for cancer therapy. J. Med. Chem. 2021;64:216–232. doi: 10.1021/acs.jmedchem.0c01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khedkar A., Lebovitz H., Fleming A., Cherrington A., Jose V., Athalye S.N., Vishweswaramurthy A. Impact of insulin tregopil and its permeation enhancer on pharmacokinetics of metformin in healthy volunteers: Randomized, open-label, placebo-controlled, crossover study. Clin. Transl. Sci. 2019;12:276–282. doi: 10.1111/cts.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]