Abstract
Abiotic stress factors, such as drought and salinity, are known to negatively affect plant growth and development. To cope with these adverse conditions, plants have utilized certain defense mechanisms involved in various aspects, including morphological, biochemical and molecular alterations. Particularly, a great deal of evidence for the biological importance of the plant-specific NAM, ATAF1/2, CUC2 (NAC) transcription factors (TFs) in plant adaptation to abiotic stress conditions has been reported. A previous in planta study conducted by our research group demonstrated that soybean (Glycine max) GmNAC085 mediated drought resistance in transgenic Arabidopsis plants. In this study, further characterization of GmNAC085 function in association with salt stress was performed. The findings revealed that under this condition, transgenic soybean plants overexpressing GmNAC085 displayed better germination rates than wild-type plants. In addition, biochemical and transcriptional analyses showed that the transgenic plants acquired a better defense system against salinity-induced oxidative stress, with higher activities of antioxidant enzymes responsible for scavenging hydrogen peroxide or superoxide radicals. Higher transcript levels of several key stress-responsive genes involved in the proline biosynthetic pathway, sodium ion transporter and accumulation of dehydrins were also observed, indicating better osmoprotection and more efficient ion regulation capacity in the transgenic lines. Taken together, these findings and our previous report indicate that GmNAC085 may play a role as a positive regulator in plant adaptation to drought and salinity conditions.
Keywords: GmNAC085, ROS-scavenging system, salt tolerance, stress-related genes, transcription factor
1. Introduction
In recent years, soil salinization has emerged as one of the most serious abiotic stress factors, narrowing cultivable areas and threatening agricultural production [1]. According to a report in 2020, nearly 800 million hectares of land have been faced with saline problems [2]. Unfortunately, it is predicted that salinity will continue to spread to other parts of the world in the near future as a consequence of climate change and inappropriate farming practices [3]. It is known that under stress conditions, various biological processes in plants are negatively affected, including reduction in shoot growth, photosynthesis and biomass accumulation; promotion of senescence; and decrease in seed quantity and quality [4,5,6]. From an agricultural perspective, yield loss not only threatens global food security but also reduces the income of farmers.
A previous review on plant defense against salinity indicated that various synchronous mechanisms have been employed [7]. In response to external changes in general, salinity in particular, transcription factors (TFs) play important roles, as they can directly regulate gene expression [8]. Salinity-related TFs that have been identified are members of various TF families such as NAM, ATAF1/2, CUC2 (NAC), APETALA2/ethylene-responsive element-binding factor (AP2/EREBP), basic leucine zipper (bZIP), myeloblastosis (MYB) and WRKY [9,10]. Among these, the NAC TF family has been highlighted to perform pivotal functions in regulating different biological aspects of plant growth, development and plant responses to biotic and abiotic stresses [11,12,13,14]. In soybean (Glycine max), from the first large-scale examination of NAC gene expression, 9 out of 31 GmNAC genes in the soybean cv. Maverick displayed transcriptional induction upon various abiotic stress treatments, including dehydration, salinity and low temperature [15]. A subsequent investigation of the dehydration stress effect on expression of 152 full-length GmNACs identified from the genome of soybean cv. Williams 82 (W82) revealed more dehydration-related GmNAC genes, of which 25 genes were upregulated and 6 genes were downregulated [16]. Following this study, a subset of these dehydration-responsive genes was selected for further expression profiling under drought stress conditions using two local soybean varieties (DT51 and MTD720) with contrasting drought-tolerant phenotypes [12,17]. Differential expression analyses of these genes helped the identification of certain members that might be associated with the drought tolerance capacity of soybean, including GmNAC043, 085, 092, 095, 101 and 109. A similar investigation carried out on drought-sensitive (B217 and H228) and drought-tolerant (Jindou74 and 78) soybean cultivars identified eight GmNAC genes with differential expression (GmNAC004, 021, 065, 066, 073, 082, 083 and 087) between the two studied groups of soybeans under drought stress conditions, whereby the tolerant cultivars displayed higher gene expression levels [18]. These findings indicate expression of drought-associated GmNAC genes is genotype-dependent.
Particularly, GmNAC085 has been recognized as an important drought-related NAC gene, as its expression was the most induced by dehydration in both shoot and root tissues among the 25 GmNAC genes with upregulated expression in the study of Le et al. (2011) [16]. Expression of GmNAC085 was also found to be significantly higher in the drought-tolerant cultivar than the drought-sensitive soybean cultivar under drought conditions [12,17]. Subsequent in planta functional characterization of GmNAC085 highlighted the positive regulatory role of this TF in mediating plant response to drought. Transgenic Arabidopsis plants harboring GmNAC085 acquired better drought tolerance with better reactive oxygen species (ROS) detoxification capacity owing to higher activities of the antioxidant enzymes superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione peroxidase (GPX), glutathione-S-transferase (GST) and glutathione reductase (GR) [19,20]. Furthermore, bioinformatic analyses revealed that the amino acid sequence of GmNAC085 protein was 39% identical to that of the rice (Oryza sativa) stress-responsive NAC1 (SNAC1/ONAC2) [16], which was found to improve the plant’s tolerance toward not only drought but also high salinity in different transgenic crop plants, including rice [21], wheat (Triticum aestivum) [22], cotton (Gossypium hirsutum) [23] and ramie (Boehmeria nivea) [24]. Similar to drought, salinity also triggers osmotic and oxidative stresses in plants [25,26]. Under these adverse environmental conditions, plants have difficulties in absorbing sufficient water due to decreased soil water potential, whereas excessive levels of endogenous ROS can trigger cellular damage and inhibition of metabolic activities [27]. Therefore, from the lines of evidence for the important role of GmNAC085 in mediating plant tolerance to drought, in this study, we further investigated the biological role of GmNAC085 in plant response to salinity to find out whether this TF is a plant regulator for multi-abiotic stress factors. To do this, effects of salinity on germination rate, antioxidant enzyme activities and expression of several key salinity-related genes were compared between transgenic soybean plants overexpressing GmNAC085 and wild-type (WT) plants.
2. Results and Discussion
2.1. GmNAC085 Expression Is Inducible by Various Abiotic Stress Conditions
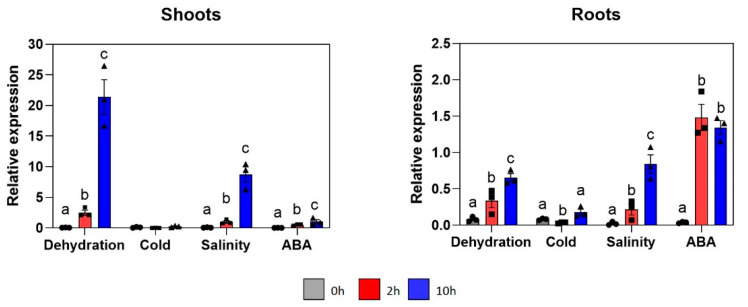
Environmental stress factors, such as drought, salinity and heat, seriously affect plant growth and development [28]. Under these adverse conditions, transcriptional regulation plays a crucial role in plant stress adaptation and tolerance [29]. Following our previous findings on the drought-responsive feature of GmNAC085 using local soybean DT51, which is a drought-tolerant cultivar [12,17], we further investigated the expression patterns of this TF-encoding gene in this cultivar that had been exposed to either dehydration, salinity, low temperature or abscisic acid (ABA). The obtained results showed that except cold treatment, GmNAC085 expression was significantly upregulated over the course of dehydration, salinity and ABA challenge in both root and shoot tissues (Figure 1). These findings were in agreement with previous studies, as expression of several genes, including the Arabidopsis Dehydration-responsive element (DRE)-binding factor 2 (DREB2) [30], Galactinol synthase 1 (AtGolS1), AtGolS2 [31] and the rice OsNAC10 [32], was induced by drought and salinity but not by cold stress. Analysis of the GmNAC085 regulatory region [12] also revealed that the promoter sequence did not contain (i) DRE cis-acting element, which has been known to involve in drought and cold response in an ABA-independent manner [33], or (ii) induction of C-repeat binding factor (CBF) expression regions (ICEr1/ICEr2), C-repeat (CRT) and low-temperature-responsive element (LTRE), which are cold-responsive cis-motifs [34]. In particular, transcript abundance of GmNAC085 increased by 30-fold in the shoots and 5-fold in the roots after 2 h of dehydration, and 261-fold in the shoots and 8-fold in the roots after 10 h of dehydration (Figure 1). Upregulation of GmNAC085 in the dehydration-treated W82 variety was also reported, with a higher induction level in the shoots than in the roots [16].
Figure 1.
Expression profiles of GmNAC085 in shoot and root tissues of soybean cultivar DT51 under dehydration, cold, salt and abscisic acid (ABA) treatments. Each value represents the mean ± SE (n = 3). Significance in transcriptional changes over the course of each treatment was analyzed by ANOVA and Tukey’s honestly significant difference and indicated by different letters (p < 0.05).
Under salinity conditions, transcript abundance of GmNAC085 was enhanced by 115-fold and 50-fold in shoot and root tissues, respectively, after 10 h of treatment (Figure 1). GmNAC085 expression was also ABA-inducible but at a higher level in the roots (16-fold) than in the shoots (9-fold) (Figure 1). Previously, the upregulation of the Nine-cis-epoxycarotenoid dioxygenase 3 (NCED3) gene, whose product is a key enzyme in the ABA biosynthetic pathway, in Arabidopsis ectopically expressing GmNAC085 was reported [19]. In addition, ABA-related cis-elements, including ABA-responsive element 2 (ABRE2) and the MYB recognition (MYBR) site, were also found within the promoter region of GmNAC085 [12,35], suggesting an interaction between ABA and GmNAC085 activities. Furthermore, it is noteworthy that dehydration and salinity induced GmNAC085 more than ABA treatment in the shoot tissues, which was also observed in a study of transgenic Arabidopsis ectopically expressing the pearl millet (Pennisetum glaucum) PgNAC21 [36]. A hypothesis proposed by Shinde et al. (2019) for this finding was a possible regulation of GmNAC085 expression via an ABA-independent yet stress-dependent route [36]. Collectively, it is suggested that GmNAC085 might function in plant responses to various abiotic stress factors, and its role might vary in different tissues.
Importantly, amino acid sequence analysis revealed that GmNAC085 displayed 39% identity and 50% similarity to the well-known SNAC1 in rice, which functions as a positive regulator for plant response to drought [16]. In addition, both GmNAC085 and SNAC1 harbor sequences with transcriptional activation potential in the C-terminal region, as shown by the yeast one-hybrid assay [19,21], and are induced by dehydration, salinity and ABA treatments (Figure 1) [37]. Overexpression of SNAC1 in rice resulted in enhanced tolerance toward drought and salinity [21,23]. GmNAC085, therefore, appears to be an excellent candidate to enhance salt stress tolerance of crop plants by genetic engineering.
2.2. GmNAC085-Transgenic Soybean Lines Display Normal Phenotype
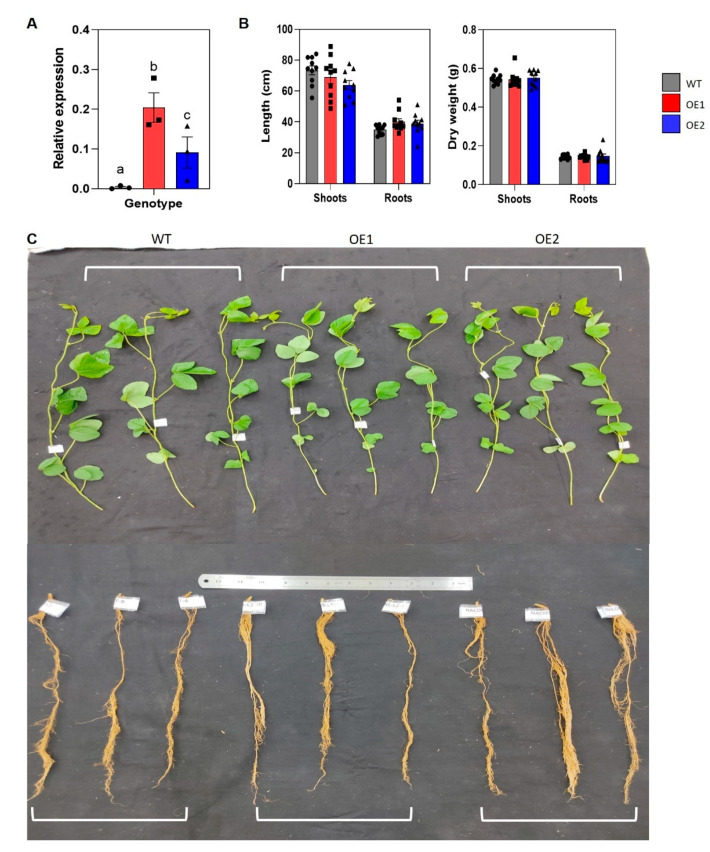
To verify the biological role of GmNAC085 in relation to salt tolerance in soybean, we performed an in planta study using two independent homologous transgenic soybean lines overexpressing GmNAC085 (OE1 and OE2). In comparison with WT plants, expression levels of GmNAC085 in OE1 and OE2 were significantly higher, by 170-fold in OE1 and 58-fold in OE2 (Figure 2A). Analysis of shoot- and root-related traits, including shoot and root lengths and shoot and root dry weights, indicated that these two transgenic lines and WT showed similar plant growth and development under normal growth conditions (Figure 2B,C). A number of studies have reported growth retardation under normal conditions in transgenic plants using the promoter 35S to drive expression of the transgene [38,39]. However, other overexpression studies reported no alteration in plant size due to activity of this constitutive promoter [40,41]. It has been suggested that although a smaller phenotype, including the transgenic Arabidopsis carrying 35S::GmNAC085, is considered a non-desirable agronomic trait under non-stressed conditions, this could help the plants become more resilient to water deficit due to the lower demand of water consumption and better prevention in water loss [19,27,42,43]. Meanwhile, 35S::GmNAC085-harboring transgenic soybean plants do not display this feature, as they share a similar morphology with WT plants under normal growth.
Figure 2.
Expression of GmNAC085 in 21-day-old GmNAC085-transgenic plants (OE1 and OE2) and their phenotype under normal growth conditions. (A) GmNAC085 expression levels in wild-type (WT), OE1 and OE2 plants (n = 3). (B) Phenotypic parameters, including shoot length, root length, shoot dry weight and root dry weight (n = 10). (C) Representative pictures of shoots and roots of the WT and transgenic plants. Each value represents the mean ± SE. Significant differences analyzed by ANOVA and Tukey’s honestly significant difference test were indicated by different letters (p < 0.05).
2.3. Transgenic Plants Have Higher Germination Rates under High Salinity Conditions
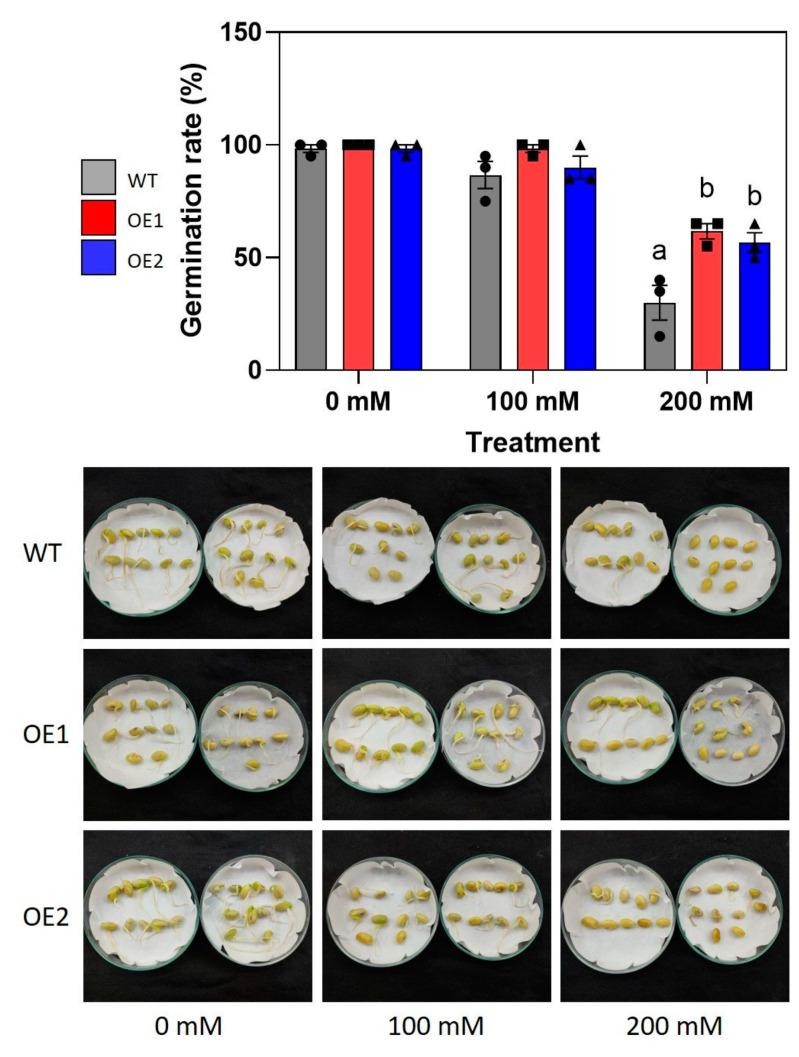
The importance of GmNAC085 in plant resistance to salinity was first evaluated using a germination assay. According to the obtained data, at a lower concentration of NaCl (100 mM), the germination rates of all three examined genotypes compared with their non-treated counterparts were similar (Figure 3). However, differential inhibitory effects of NaCl on soybean seed germination were clearly found under the high salt concentration of 200 mM. Under this stress condition, the germination rates of the soybean seeds were significantly reduced by 68% in WT, 38% in OE1 and 42% in OE2 compared with the control condition (Figure 3). This result indicated that GmNAC085-overexpressing plants maintained better germination rates than their non-transgenic counterparts under high salt concentrations. With influences on water uptake capacity and toxic ion disturbance on enzymatic activities, cellular metabolism and nutrient acquisition, salt stress is known to impair soybean seed germination and post-germinative growth, ultimately leading to yield loss [44,45]. Furthermore, NaCl treatment can cause oxidative stress, which is also detrimental to seed development and suppresses seed germination [46,47]. Therefore, the higher germination rates observed in the transgenic plants could be attributed to a better defensive capability against salt stress effects.
Figure 3.
Germination of GmNAC085-transgenic (OE1 and OE2) and wild-type (WT) seeds under different concentrations of NaCl. The germination rates and representative pictures were taken after 3 days of incubating the seeds under dark conditions. Each value represents the mean ± SE (n = 3 replicates, 20 seeds/replicate). Significant differences among the genotypes in each treatment that were analyzed by ANOVA and Tukey’s honestly significant difference test were indicated by different letters (p < 0.05).
2.4. GmNAC085-Transgenic Plants Display Enhanced ROS-Scavenging Capacity
Prolonged salt stress triggers over-accumulation of ROS, leading to damage of cellular components, including DNA and proteins [48]. According to Sadak et al. (2020), salt stress significantly increases the hydrogen peroxide (H2O2) content in soybean leaves [49], which was also confirmed earlier in leaves of other plant species, such as sunflower (Helianthus annuus) [50] and wheat [51]. At appropriate concentrations, ROS can function as messenger molecules involved in acclimatory signaling to trigger plant tolerance against various abiotic stresses [52,53,54,55]. As ROS play dual roles in stress tolerance of plants, ROS synthesis and ROS-scavenging machineries are tightly regulated to maintain relevant levels of ROS at different plant developmental stages and under different growing environments [56]. In a previous study, transgenic Arabidopsis plants harboring GmNAC085 were shown to obtain improved drought tolerance due to, at least partly, enhanced expression of genes associated with the activities of antioxidant enzymes, including SOD, CAT and APX that are known as the major ROS scavengers [19]. Therefore, expression profile analysis of antioxidant enzyme-encoding genes, GmCAT, GmAPX1 and GmMnSOD, by quantitative real-time PCR (RT-qPCR) was carried out.
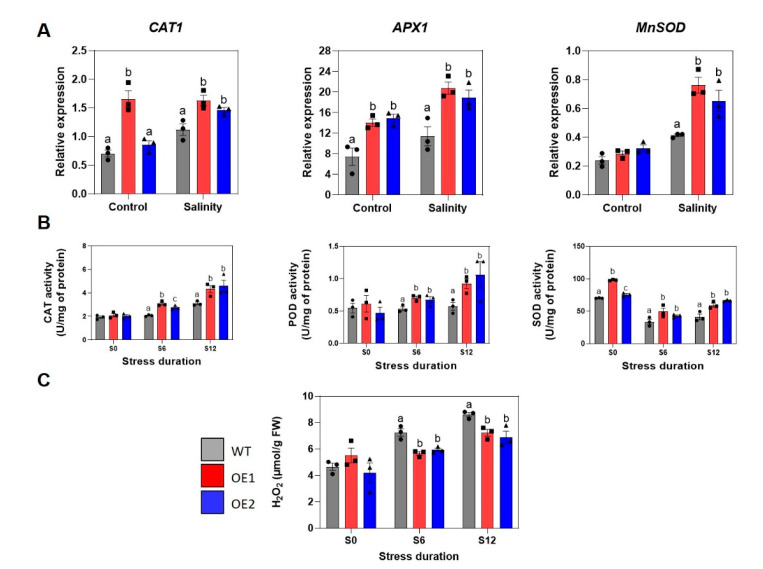
As shown in Figure 4A, GmNAC085 overexpression lines had increased transcript abundance of the examined antioxidant enzyme-encoding genes. Analyses of GmCAT expression patterns showed that only OE1 had significantly higher expression of this gene in non-stressed root tissue, probably due to higher GmNAC085 transcript abundance compared with that of the OE2 line (Figure 2A). This high expression level status was maintained in the stressed OE1 line, whereas a substantial upregulation by 2.2-fold of GmCAT in the OE2 line upon salt treatment was observed (Figure 4A). Under normal conditions, expression levels of GmAPX1 were higher in both transgenic lines compared with WT plants (by 2-fold in OE1 and 2.1-fold in OE2). After 10-day exposure to salinity, GmNAC085-overexpressing plants continuously outperformed their non-transgenic counterparts in GmAPX1 transcript abundance. Following this, a significant increase in expression of GmAPX1 was only observed in the transgenic lines, whereas the transcript level of this gene in the WT plants did not change much between the two conditions (Figure 4A). Regarding GmMnSOD, the transcript abundance of this gene was found almost identical among the three genotypes under normal conditions but at substantially higher levels under salt treatment in the transgenic lines (by 2.6-fold in OE1 and 1.6-fold in OE2 in comparison with the WT counterpart) (Figure 4A).
Figure 4.
Evaluation of components of the reactive oxygen species-scavenging system in transgenic (OE1 and OE2) and wild-type (WT) plants under control and salinity conditions (S6 and S12 for 6-day and 12-day treatments, respectively). (A) Expression profile of antioxidant enzyme-encoding genes. (B) Enzymatic activities of catalase (CAT), peroxidase (POD) and superoxide dismutase (SOD). (C) Endogenous hydrogen peroxide (H2O2) content. Each value represents the mean ± SE (n = 3). Significant differences among the genotypes in each treatment that were analyzed by ANOVA and Tukey’s honestly significant difference test were indicated by different letters (p < 0.05).
Data from biochemical assays were also in agreement with the RT-qPCR analyses. Activities of CAT and peroxidase (POD), which are H2O2-scavenging enzymes, remained relatively low and similar among the three studied genotypes under normal conditions (Figure 4B). Under the applied stress condition, though all the three genotypes enhanced activities of these enzymes, the overexpression lines displayed their activities at remarkably higher levels. This partially explains the lower H2O2 accumulation after 6-day and 12-day stress application in the transgenic plants than in WT plants (Figure 4C).
Interestingly, activities of SOD, which is responsible for the dismutation of superoxide into H2O2, were found to be more active in the transgenic plants under both non-stressed and stressed conditions (at least by 1.4-fold and 1.3-fold higher in OE1 and OE2, respectively). It is also noted that under the stress conditions, molecular analyses revealed upregulation of GmMnSOD, whereas the biochemical data did not demonstrate this trend (Figure 4A,B). This could be due to alteration in expression of other genes encoding GmSOD isozymes, which remains to be explored. In addition, according to our data, it can be deduced that the increase in H2O2 over the course of stress treatment in general could be due to the over-production of this ROS from various sources that employ different enzymes, such as photorespiration, electron transport chain and redox reactions in the apoplast [57,58], rather than depending on the superoxide conversion into H2O2 by SOD enzyme activity (Figure 4B,C).
Many studies have shown that overexpression of CAT can increase plant resistance to abiotic and biotic stresses [59,60], acknowledging its indispensable role in alleviating oxidative stress [61,62,63]. With APX, this is a group of enzymes that belongs to the POD superfamily and plays a central role in the ascorbate-glutathione cycle that has evolved in plants to scavenge H2O2 from plant chloroplasts and cytosol [64]. Regarding SOD genes, they can be divided into four subfamilies, among which three (MnSOD, FeSOD and Cu/ZnSOD) are widely found in plants and one (NiSOD) is present in streptomyces [65,66]. Frequently, members of different SOD subfamilies are localized to different cellular compartments, including mitochondria (MnSOD), peroxisomes (MnSOD and Cu/ZnSOD), chloroplasts and cytosol (FeSOD, Cu/ZnSOD) [67]. The important role of the APX and SOD gene families in antioxidative stress has now been demonstrated in a variety of plants. For example, transgenic cassava (Manihot esculenta) co-expressing cytoplasmic MeCu/ZnSOD and MeAPX2 displayed high levels of SOD and APX antioxidant enzyme activities, thus improving their tolerance to cold stress [68]. In another report, ectopic expression of MnSOD gene from Tamarix androssowii conferred salinity and oxidative stress tolerance in the transgenic poplar (Populus davidiana x P. bolleana) [69]. Furthermore, various studies have reported that the enhanced stress tolerance in plants harboring a regulatory transgene (e.g., NAC and MYB) such as GmNAC085-transgenic Arabidopsis [19], GmNAC20-transgenic rice [70] and SlMYB102-transgenic tomato (Solanum lycopersicum) [71] or a non-antioxidant functional transgene (e.g., Sodium–proton antiporter (NHX) and Salt overly sensitive (SOS)) such as TaNHX2-transgenic sunflower [72], AtNHX1-transgenic mung bean (Vigna radiata) [73] and GmsSOS1-transgenic Arabidopsis [74] was also associated with higher expression levels of antioxidant enzyme-encoding genes and/or higher activities of antioxidant enzymes.
Taken these lines of evidence together, the higher activities of CAT, POD and SOD and higher transcript abundance of antioxidant enzyme-encoding genes in transgenic plants indicated their better defense capability against oxidative stress compared with the non-transgenic counterpart. As oxidative stress was found to be associated with seed germination [46], these results may also explain the higher germination rates observed in the transgenic plants compared with WT plants under NaCl treatment (Figure 3 and Figure 4).
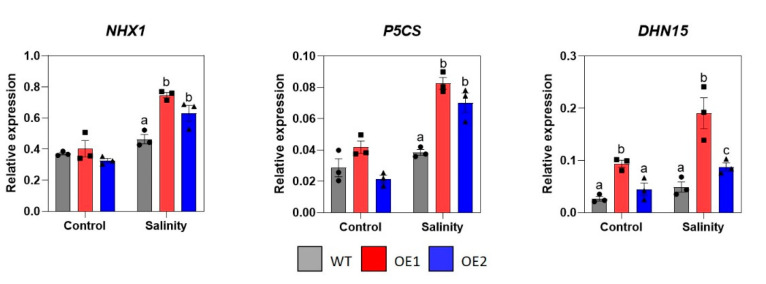
2.5. Expression Levels of Other Stress-Related Genes Are Also Enhanced in Transgenic Plants under Salinity Conditions
In addition to antioxidant genes, expression of the Na+/H+ antiporter-encoding gene (GmNHX1), as well as two osmoprotectant-related genes Delta-1-pyrroline-5-carboxylase synthase (GmP5CS) and Dehydrin 15 (GmDHN15), was also analyzed. Generally, the expression of these genes was upregulated upon stress exposure, which is consistent with findings from previous studies [75,76,77]. However, both transgenic lines displayed higher induction levels than WT plants (Figure 5). It is noticed that the GmNHX1 and GmP5CS shared similar expression patterns to that of GmMnSOD (Figure 4A and Figure 5). In particular, transcript abundance of these genes was at the same level among the three examined genotypes under normal conditions but remarkably higher in the overexpression lines upon stress application (at least 1.5-fold higher for NHX1 and 1.8-fold higher for P5CS). Meanwhile, DHN15 had the highest expression activity in the OE1 in both non-stressed (3.6-fold higher compared to WT) and stressed conditions (3.9-fold higher compared to WT) (Figure 5).
Figure 5.
Expression profiles of three stress-related genes in root tissues of GmNAC085-transgenic soybean lines (OE1 and OE2) and wild-type (WT) plants under salt stress treatments, including GmNHX1, GmP5CS and GmDHN15. Each value represents the mean ± SE (n = 3). Significant differences among the genotypes in each treatment that were analyzed by ANOVA and Tukey’s honestly significant difference test were indicated by different letters (p < 0.05).
Salinity, along with drought, cause changes in osmotic pressure and oxidative stress that trigger cellular damage and dehydration [78]. To deal with this, several strategies can be used by plants, including accumulation of osmolytes to lower cellular osmotic potential, activation of antioxidant systems for ROS removal and increase in chaperone activities for protein protection [79]. For example, synthesis of proline, an amino acid that can function as an osmolyte and osmoprotectant, is usually promoted under the stress condition by enhancement activities of P5CS, the rate-limiting enzyme in the proline biosynthetic pathway [75,80]. Similarly, increased synthesis of dehydrin and late embryogenesis abundant (LEA) proteins that play important roles in protein protection is also observed [77,81]. In this study, the upregulated expression of P5CS and DHN15 would bring certain advantages for the GmNAC085-transgenic soybean lines in mitigating the salinity effects. It is also known that the reluctant accumulation of cellular Na+ under salinity conditions leads to the disruption of ion balance and cellular metabolism [82,83]. Compartmentalization of Na+ ions in vacuole by activity of vacuolar Na+/H+ antiporter in replacement of Na+ for H+ has long been proposed as an effective mechanism for salt tolerance [84]. This helps avoid deleterious effects of excessive Na+ in the cytosol, while the osmotic balance in the vacuole can be maintained by using Na+ as an ionic osmolyte [85,86,87]. Furthermore, it has been evidenced that overexpression of Arabidopsis vacuolar NHX1 could confer improved salt tolerance in transgenic tomato plants [88]. Similar findings were also reported for other transgenic crop species overexpressing NHX-encoding genes, including rice [89,90,91], wheat [92], barley (Hordeum vulgare) [93], cowpea (Vigna unguiculata) [94] and mung bean [73]. Therefore, enhanced transcriptional activities of GmNHX1 observed in the GmNAC085-transgenic plants would contribute to the maintenance of normal metabolism under salt stress. Additionally, transcriptional activation assay in yeast demonstrated that the C-terminal transcriptional regulatory region of GmNAC085 possesses a transcriptional activation domain that enables the protein to function as a transcriptional activator [19]. Therefore, higher expression levels of the examined genes observed in this study could be the results of direct and/or indirect regulation of this NAC TF.
3. Materials and Methods
3.1. Plant Materials and Plant Growth Conditions
Seeds of soybean varieties W82 and DT51 were obtained from RIKEN Center (Yokohama, Japan) and Legumes Research and Development Center (Hanoi, Vietnam), respectively. The transgenic soybean (W82 background) lines were generated by the service at Iowa State University (Ames, IA, USA) using the Agrobacterium tumefaciens-mediated transformation method. Cassette P35S-GmNAC085-NOS from a pGKX vector constructed previously [19,95] was cloned into pENTR Direction TOPO using the following primers: 5′-CACCGAGCTTGCCAACATGGTGGAG-3′ (forward) and 5′-CGATCTAGTAACATAGATGAC-3′ (reverse). Subsequently, the cassette was transferred into a pTF101.1gw1 vector and then used for transformation. The homozygous transgenic progenies used in this study were verified as independent lines according to Mendelian segregation analyses for the ratio of Basta-resistant/Basta-sensitive phenotypes, followed by molecular confirmation [96,97]. The plants were grown in plastic pots containing soil, coir, husk ash and compost (Tribat soil, Saigon Xanh Biotechnology Ltd. Company, Ho Chi Minh City, Vietnam) and under net house conditions (28–33 °C, 60–70% humidity and natural photoperiod) [12].
3.2. Abiotic Stress Assays for Analyses of GmNAC085 Expression
Local soybean variety DT51 was used for various stress challenges, as described by Tran et al. (2009) [15]. For dehydration treatment, 12-day-old plants were carefully pulled from the container and washed to remove soil attached to the root surfaces. The plants were then placed on filter papers and allowed to dehydrate in a controlled growth chamber (60% relative humidity, 28 °C day/night temperature and 200 µmol m–2 s–1 light intensity). For salinity and ABA treatments, plants were transferred to 250 mM NaCl and 100 µM ABA solution, respectively, under laboratory conditions. Low-temperature treatment was conducted by keeping the seedlings in distilled water maintained at 4 °C. During the assays, the roots and shoots tissues of treated plants were collected at 0, 2- and 10-h timepoints for expression analysis of GmNAC085. All experiments were carried out with three biological replicates.
3.3. Morphological Analysis of Transgenic Plants under Normal Conditions
Root and shoot growth of V4-stage seedlings (i.e., 21-day-old) were evaluated for length and dry biomass. The seedlings were grown in plastic pots (10 cm in diameter and 80 cm in height, one plant per pot) with normal irrigation until they were harvested for the measurement (n = 10) [98].
3.4. Analysis of Seed Germination Rate
For this experiment, seeds were first sterilized using 2% sodium hypochlorite (NaOCl) for 10 min before they were rinsed with distilled water for chemical removal. Next, the seeds were incubated between two layers of filter paper placed in a Petri dish (9 cm in diameter) supplied with 10 mL of NaCl solution with different concentrations (0, 100 and 200 mM) [99,100]. After keeping the plates in dark conditions at room temperature for three days, the germination rates were recorded. Seeds were considered successfully germinated if the length of rising radicles was at least greater than half of the seed length [101]. For each genotype, three replications per sodium chloride concentration were used, of which each replication was one plate with 20 seeds.
3.5. Biochemical Analyses for Endogenous Hydrogen Peroxide Content and Antioxidant Enzyme Activities
To initiate salt stress, 12-day healthy seedlings of both transgenic and non-transgenic plants were irrigated with NaCl solution (100 mM) (100 mL/plant) every two days. For each genotype, the leaf tissues of three individual plants (n = 3, 0.2 g/replicate) were collected on days 0, 6th and 12th during the stress application. Previously described methods for determination of contents of H2O2 [43,102] and soluble proteins [103], as well as activities of CAT [104], SOD [105] and POD [106] enzymes, were used.
3.6. Gene Expression Analysis by RT-qPCR
Expression analysis of GmNAC085 in DT51 root and shoot tissues that were exposed to various abiotic stress conditions (Section 3.2) and expression analysis of stress-related genes in root tissues of transgenic and WT plants subjected to salinity (Section 3.5) for ten days were conducted using RT-qPCR. The primer sets used for this assay are provided in Table 1. Total RNA extraction and purification, cDNA synthesis and RT-qPCR were carried out using commercial kits (Thermo Scientific, Waltham, MA, USA) and following the guidelines provided by the manufacturer [97]. NanoDrop OneC Microvolume UV-Vis Spectrophotometer (ND-ONEC-W, Thermo Scientific, MA, USA) was used to determine the concentrations and quality of total RNA extracts. cDNA synthesis was carried out using the same amount of total RNA from each sample. Preparation for reactions, thermal profile and melting curve analysis in RT-qPCR assay (Mastercycler® ep realplex, Eppendorf, Hamburg, Germany) was described in our previous study [97]. Fbox [107] was used as the reference gene for normalization based on the 2−ΔCt method [108]. LinRegPCR software (version 2020.2, Academic Medical Center, Amsterdam, The Netherlands) was used to calculate the efficiency of PCR reactions.
Table 1.
Information of primers that were used in gene expression analysis.
| Genes | ID | Primer Type | Primer Sequence (5′-3′) | Amplicon Size (bp) | References |
|---|---|---|---|---|---|
| GmNAC085 | Glyma12g22880 | Forward | GGCTAGACACATACAATGAATCGG | 92 | [16] |
| Reverse | TGCGGTGCTGTGGTGAAA | ||||
| GmFbox | Glyma12g051100 | Forward | AGATAGGGAAATTGTGCAGGT | 93 | [107] |
| Reverse | CTAATGGCAATTGCAGCTCTC | ||||
| GmCAT | Glyma06g017900 | Forward | CCACAGCCATGCCACTCAAG | 184 | [109] |
| Reverse | CAGGACCAAGCGACCAACAG | ||||
| GmAPX1 | Glyma12g073100 | Forward | AGTTGGCTGGCGTTGTTG | 86 | [109] |
| Reverse | TGGTGGCTCAGGCTTGTC | ||||
| GmMnSOD | Glyma04g221300 | Forward | GCACCACCAGACTTACATCAC | 88 | [109] |
| Reverse | AACGACGGCGGAGGAATC | ||||
| GmNHX1 | Glyma20g229900 | Forward | CTTTCCACTCCAACACACAC | 110 | [76] |
| Reverse | GGTGAGCCAGGTTCTATAGG | ||||
| GmP5CS | Glyma18g034300 | Forward | TGTCTCTCAGATCAAGAGTTCCAC | 144 | [110] |
| Reverse | CAGCCTGCTGGATAGTCTATTTTT | ||||
| GmDHN15 | Glyma11g149900 | Forward | TTTTGTTTTGTTGTATTGTGTAG | 150 | [77] |
| Reverse | GAAAAATCCTCCACCTGACGA |
3.7. Statistical Analyses
Data were analyzed using one-way ANOVA and Tukey’s honestly significant difference test for comparison among the examined genotypes under the same treatment to identify statistically significant differences (p < 0.05).
4. Conclusions
The results from this study showed that GmNAC085 functions as a positive regulator for plant response to salinity, in addition to a previous report on its contribution to plant resistance to drought. Under high salinity conditions, GmNAC085-overexpressing soybean plants maintained better germination rates and had more robust antioxidant enzyme activities. Gene expression profiling data also indicate that the enhanced salinity tolerance mediated by GmNAC085 comes from the increased biosynthesis of osmoprotectants proline and dehydrin, as well as effective sequestration of excessive cytosolic Na+ using the vacuolar Na+/H+ antiporter. Therefore, the findings presented here, together with our previous report, should lay a solid foundation for further study into the molecular mechanisms by which GmNAC085 mediates multi-responses to different types of osmotic stress, as well as for the development of stress-tolerant crops based on GmNAC085 manipulation.
Author Contributions
X.L.T.H. was responsible for supervision, conceptualization, investigation, methodology, writing and review. N.N.C. was responsible for the investigation, data curation, formal analysis, visualization, and writing original draft preparation. T.T.K.H., H.D., P.H.P.V., L.D.M.T. and P.N.T.H. were involved in the investigation. D.T.L. was involved in material preparation and review. L.-S.P.T. was responsible for materials, conceptualization and review. N.P.T. was responsible for project administration, supervision, conceptualization, validation, review and funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Ministry of Science and Technology (MOST), Vietnam, under grant number ĐTĐL.CN-12/19.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Qados A.M.S.A. Effect of Salt Stress on Plant Growth and Metabolism of Bean Plant Vicia faba (L.) J. Saudi Soc. Agric. Sci. 2011;10:7–15. [Google Scholar]
- 2.Krishnamurthy S.L., Pundir P., Warraich A.S., Rathor S., Lokeshkumar B.M., Singh N.K., Sharma P.C. Introgressed Saltol QTL Lines Improves the Salinity Tolerance in Rice at Seedling Stage. Front. Plant Sci. 2020;11:833. doi: 10.3389/fpls.2020.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekmirzaev G., Ouddane B., Beltrao J., Fujii Y. The Impact of Salt Concentration on the Mineral Nutrition of Tetragonia tetragonioides. Agriculture. 2020;10:238. doi: 10.3390/agriculture10060238. [DOI] [Google Scholar]
- 4.Manchanda G., Garg N. Salinity and Its Effects on the Functional Biology of Legumes. Acta Physiol. Plant. 2008;30:595–618. doi: 10.1007/s11738-008-0173-3. [DOI] [Google Scholar]
- 5.Al-shareef N.O., Tester M. Plant Salinity Tolerance. eLS. 2019:1–6. [Google Scholar]
- 6.Li M., Chen R., Jiang Q., Sun X., Zhang H., Hu Z. GmNAC06, a NAC Domain Transcription Factor Enhances Salt Stress Tolerance in Soybean. Plant Mol. Biol. 2021;105:333–345. doi: 10.1007/s11103-020-01091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rejeb I.B., Pastor V., Mauch-Mani B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants. 2014;3:458–475. doi: 10.3390/plants3040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan S.-A., Li M.-Z., Wang S.-M., Yin H.-J. Revisiting the Role of Plant Transcription Factors in the Battle against Abiotic Stress. Int. J. Mol. Sci. 2018;19:1634. doi: 10.3390/ijms19061634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoang X.L.T., Nhi D.N.H., Thu N.B.A., Thao N.P., Tran L.-S.P. Transcription Factors and Their Roles in Signal Transduction in Plants under Abiotic Stresses. Curr. Genomics. 2017;18:483–497. doi: 10.2174/1389202918666170227150057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao C., Zhang H., Song C., Zhu J.-K., Shabala S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innov. 2020;1:100017. doi: 10.1016/j.xinn.2020.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran L.-S.P., Nakashima K., Sakuma Y., Simpson S.D., Fujita Y., Maruyama K., Fujita M., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. Isolation and Functional Analysis of Arabidopsis Stress-Inducible NAC Transcription Factors That Bind to a Drought-Responsive cis-Element in the Early Responsive to Dehydration Stress 1 Promoter. Plant Cell. 2004;16:2481–2498. doi: 10.1105/tpc.104.022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thao N.P., Thu N.B.A., Hoang X.L.T., van Ha C., Tran L.S.P. Differential Expression Analysis of a Subset of Drought-Responsive GmNAC Genes in Two Soybean Cultivars Differing in Drought Tolerance. Int. J. Mol. Sci. 2013;14:23828–23841. doi: 10.3390/ijms141223828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erpen L., Devi H.S., Grosser J.W., Dutt M. Potential Use of the DREB/ERF, MYB, NAC and WRKY Transcription Factors to Improve Abiotic and Biotic Stress in Transgenic Plants. Plant Cell Tissue Organ Cult. 2018;132:1–25. doi: 10.1007/s11240-017-1320-6. [DOI] [Google Scholar]
- 14.Sun H., Hu M., Li J., Chen L., Li M., Zhang S., Zhang X., Yang X. Comprehensive Analysis of NAC Transcription Factors Uncovers Their Roles during Fiber Development and Stress Response in Cotton. BMC Plant Biol. 2018;18:150. doi: 10.1186/s12870-018-1367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran L.-S.P., Quach T.N., Guttikonda S.K., Aldrich D.L., Kumar R., Neelakandan A., Valliyodan B., Nguyen H.T. Molecular Characterization of Stress-Inducible GmNAC Genes in Soybean. Mol. Genet. Genomics. 2009;281:647–664. doi: 10.1007/s00438-009-0436-8. [DOI] [PubMed] [Google Scholar]
- 16.Le D.T., Nishiyama R.I.E., Watanabe Y., Mochida K., Yamaguchi-Shinozaki K., Shinozaki K., Tran L.-S.P. Genome-Wide Survey and Expression Analysis of the Plant-Specific NAC Transcription Factor Family in Soybean during Development and Dehydration Stress. DNA Res. 2011;18:263–276. doi: 10.1093/dnares/dsr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thu N.B.A., Hoang X.L.T., Doan H., Nguyen T.-H., Bui D., Thao N.P., Tran L.-S.P. Differential Expression Analysis of a Subset of GmNAC Genes in Shoots of Two Contrasting Drought-Responsive Soybean Cultivars DT51 and MTD720 under Normal and Drought Conditions. Mol. Biol. Rep. 2014;41:5563–5569. doi: 10.1007/s11033-014-3507-9. [DOI] [PubMed] [Google Scholar]
- 18.Hussain R.M., Ali M., Feng X., Li X. The Essence of NAC Gene Family to the Cultivation of Drought-Resistant Soybean (Glycine max L. Merr.) Cultivars. BMC Plant Biol. 2017;17:55. doi: 10.1186/s12870-017-1001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen K.H., Mostofa M.G., Li W., van Ha C., Watanabe Y., Le D.T., Thao N.P., Tran L.S.P. The Soybean Transcription Factor GmNAC085 Enhances Drought Tolerance in Arabidopsis. Environ. Exp. Bot. 2018;151:12–20. doi: 10.1016/j.envexpbot.2018.03.017. [DOI] [Google Scholar]
- 20.Nguyen K.H., Mostofa M.G., Watanabe Y., Tran C.D., Rahman M.M., Tran L.-S.P. Overexpression of GmNAC085 Enhances Drought Tolerance in Arabidopsis by Regulating Glutathione Biosynthesis, Redox Balance and Glutathione-Dependent Detoxification of Reactive Oxygen Species and Methylglyoxal. Environ. Exp. Bot. 2019;161:242–254. doi: 10.1016/j.envexpbot.2018.12.021. [DOI] [Google Scholar]
- 21.Hu H., Dai M., Yao J., Xiao B., Li X., Zhang Q., Xiong L. Overexpressing a NAM, ATAF, and CUC (NAC) Transcription Factor Enhances Drought Resistance and Salt Tolerance in Rice. Proc. Natl. Acad. Sci. USA. 2006;103:12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saad A.S.I., Li X., Li H.-P., Huang T., Gao C.-S., Guo M.-W., Cheng W., Zhao G.-Y., Liao Y.-C. A Rice Stress-Responsive NAC Gene Enhances Tolerance of Transgenic Wheat to Drought and Salt Stresses. Plant Sci. 2013;203:33–40. doi: 10.1016/j.plantsci.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Liu G., Li X., Jin S., Liu X., Zhu L., Nie Y., Zhang X. Overexpression of Rice NAC Gene SNAC1 Improves Drought and Salt Tolerance by Enhancing Root Development and Reducing Transpiration Rate in Transgenic Cotton. PLoS ONE. 2014;9:e86895. doi: 10.1371/journal.pone.0086895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An X., Liao Y., Zhang J., Dai L., Zhang N., Wang B., Liu L., Peng D. Overexpression of Rice NAC Gene SNAC1 in Ramie Improves Drought and Salt Tolerance. Plant Growth Reg. 2015;76:211–223. doi: 10.1007/s10725-014-9991-z. [DOI] [Google Scholar]
- 25.Uddin M.N., Hossain M.A., Burritt D.J. Salinity and drought stress: Similarities and differences in oxidative responses and cellular redox regulation. In: Ahmad P., editor. Water Stress and Crop Plants: A Sustainable Approach. John Wiley & Sons; Singapore: 2016. pp. 86–101. [Google Scholar]
- 26.Pinheiro D.T., Delazari F., Nick C., Mattiello E.M., dos Santos Dias D.C.F. Emergence and Vegetative Development of Melon in Function of the Soil Salinity. Aust. J. Crop Sci. 2019;13:458–464. doi: 10.21475/ajcs.19.13.03.p1551. [DOI] [Google Scholar]
- 27.Hoang X.L.T., Nguyen N.C., Nguyen Y.N.H., Watanabe Y., Tran L.S.P., Thao N.P. The Soybean GmNAC019 Transcription Factor Mediates Drought Tolerance in Arabidopsis in an Abscisic Acid-Dependent Manner. Int. J. Mol. Sci. 2020;21:286. doi: 10.3390/ijms21010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C.-T., Ru J.-N., Liu Y.-W., Li M., Zhao D., Yang J.-F., Fu J.-D., Xu Z.-S. Maize WRKY Transcription Factor ZmWRKY106 Confers Drought and Heat Tolerance in Transgenic Plants. Int. J. Mol. Sci. 2018;19:3046. doi: 10.3390/ijms19103046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H., Meng J., Peng X., Tang X., Zhou P., Xiang J., Deng X. Rice WRKY4 Acts as a Transcriptional Activator Mediating Defense Responses toward Rhizoctonia solani, the Causing Agent of Rice Sheath Blight. Plant Mol. Biol. 2015;89:157–171. doi: 10.1007/s11103-015-0360-8. [DOI] [PubMed] [Google Scholar]
- 30.Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Two Transcription Factors, DREB1 and DREB2, with an EREBP/AP2 DNA Binding Domain Separate Two Cellular Signal Transduction Pathways in Drought-and Low-Temperature-Responsive Gene Expression, Respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taji T., Ohsumi C., Iuchi S., Seki M., Kasuga M., Kobayashi M., Yamaguchi-Shinozaki K., Shinozaki K. Important Roles of Drought-and Cold-inducible Genes for Galactinol Synthase in Stress Tolerance in Arabidopsis thaliana. Plant J. 2002;29:417–426. doi: 10.1046/j.0960-7412.2001.01227.x. [DOI] [PubMed] [Google Scholar]
- 32.Jeong J.S., Kim Y.S., Baek K.H., Jung H., Ha S.-H., do Choi Y., Kim M., Reuzeau C., Kim J.-K. Root-Specific Expression of OsNAC10 Improves Drought Tolerance and Grain Yield in Rice under Field Drought Conditions. Plant Physiol. 2010;153:185–197. doi: 10.1104/pp.110.154773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinozaki K., Yamaguchi-Shinozaki K. Molecular Responses to Dehydration and Low Temperature: Differences and Cross-Talk between Two Stress Signaling Pathways. Curr. Opin. Plant Biol. 2000;3:217–223. doi: 10.1016/S1369-5266(00)80068-0. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi-Shinozaki K., Shinozaki K. Organization of cis-Acting Regulatory Elements in Osmotic- and Cold-Stress-Responsive Promoters. Trends Plant Sci. 2005;10:88–94. doi: 10.1016/j.tplants.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Shukla P.S., Agarwal P., Gupta K., Agarwal P.K. Molecular Characterization of an MYB Transcription Factor from a Succulent Halophyte Involved in Stress Tolerance. AoB Plants. 2015;7:plv054. doi: 10.1093/aobpla/plv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinde H., Dudhate A., Tsugama D., Gupta S.K., Liu S., Takano T. Pearl Millet Stress-Responsive NAC Transcription Factor PgNAC21 Enhances Salinity Stress Tolerance in Arabidopsis. Plant Physiol. Biochem. 2019;135:546–553. doi: 10.1016/j.plaphy.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Nuruzzaman M., Manimekalai R., Sharoni A.M., Satoh K., Kondoh H., Ooka H., Kikuchi S. Genome-Wide Analysis of NAC Transcription Factor Family in Rice. Gene. 2010;465:30–44. doi: 10.1016/j.gene.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Liao X., Guo X., Wang Q., Wang Y., Zhao D., Yao L., Wang S., Liu G., Li T. Overexpression of MsDREB6.2 Results in Cytokinin-deficient Developmental Phenotypes and Enhances Drought Tolerance in Transgenic Apple Plants. Plant J. 2017;89:510–526. doi: 10.1111/tpj.13401. [DOI] [PubMed] [Google Scholar]
- 39.Du X., Li W., Sheng L., Deng Y., Wang Y., Zhang W., Yu K., Jiang J., Fang W., Guan Z. Over-Expression of Chrysanthemum CmDREB6 Enhanced Tolerance of Chrysanthemum to Heat Stress. BMC Plant Biol. 2018;18:178. doi: 10.1186/s12870-018-1400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan Q., Liao X., He M., Li X., Wang Z., Ma H., Yu S., Liu S. Tolerance Analysis of Chloroplast OsCu/Zn-SOD Overexpressing Rice under NaCl and NaHCO3 Stress. PLoS ONE. 2017;12:e0186052. doi: 10.1371/journal.pone.0186052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin X., Cui Y., Wang M., Xia X. Overexpression of a Novel MYB-Related Transcription Factor, OsMYBR1, Confers Improved Drought Tolerance and Decreased ABA Sensitivity in Rice. Biochem. Biophys. Res. Commun. 2017;490:1355–1361. doi: 10.1016/j.bbrc.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 42.Kong X., Zhou S., Yin S., Zhao Z., Han Y., Wang W. Stress-Inducible Expression of an F-Box Gene TaFBA1 from Wheat Enhanced the Drought Tolerance in Transgenic Tobacco Plants without Impacting Growth and Development. Front. Plant Sci. 2016;7:1295. doi: 10.3389/fpls.2016.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen N.C., Hoang X.L.T., Nguyen Q.T., Binh N.X., Watanabe Y., Thao N.P., Tran L.S.P. Ectopic Expression of Glycine max GmNAC109 Enhances Drought Tolerance and ABA Sensitivity in Arabidopsis. Biomolecules. 2019;9:714. doi: 10.3390/biom9110714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamayun M., Hussain A., Khan S.A., Irshad M., Khan A.L., Waqas M., Shahzad R., Iqbal A., Ullah N., Rehman G. Kinetin Modulates Physio-Hormonal Attributes and Isoflavone Contents of Soybean Grown under Salinity Stress. Front. Plant Sci. 2015;6:377. doi: 10.3389/fpls.2015.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajabi Dehnavi A., Zahedi M., Ludwiczak A., Cardenas Perez S., Piernik A. Effect of Salinity on Seed Germination and Seedling Development of Sorghum (Sorghum Bicolor (L.) Moench) Genotypes. Agronomy. 2020;10:859. doi: 10.3390/agronomy10060859. [DOI] [Google Scholar]
- 46.Ma Q., Kang J., Long R., Zhang T., Xiong J., Zhang K., Wang T., Yang Q., Sun Y. Comparative Proteomic Analysis of Alfalfa Revealed New Salt and Drought Stress-Related Factors Involved in Seed Germination. Mol. Biol. Rep. 2017;44:261–272. doi: 10.1007/s11033-017-4104-5. [DOI] [PubMed] [Google Scholar]
- 47.Mittler R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 48.Mittler R. ROS Are Good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Sadak M.S., Abd El-Hameid A.R., Zaki F.S.A., Dawood M.G., El-Awadi M.E. Physiological and Biochemical Responses of Soybean (Glycine max L.) to Cysteine Application under Sea Salt Stress. Bull. Natl. Res. Cent. 2020;44:1–10. doi: 10.1186/s42269-019-0259-7. [DOI] [Google Scholar]
- 50.Ibrahim M.F.M., Faisal A., Shehata S.A. Calcium Chloride Alleviates Water Stress in Sunflower Plants through Modifying Some Physio-Biochemical Parameters. Am. Eurasian J. Agric. Environ. Sci. 2016;16:677–693. [Google Scholar]
- 51.Kaur H., Bhardwaj R.D., Grewal S.K. Mitigation of Salinity-Induced Oxidative Damage in Wheat (Triticum aestivum L.) Seedlings by Exogenous Application of Phenolic Acids. Acta Physiol. Plant. 2017;39:221. doi: 10.1007/s11738-017-2521-7. [DOI] [Google Scholar]
- 52.Prasad T.K., Anderson M.D., Martin B.A., Stewart C.R. Evidence for Chilling-Induced Oxidative Stress in Maize Seedlings and a Regulatory Role for Hydrogen Peroxide. Plant Cell. 1994;6:65–74. doi: 10.2307/3869675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Breusegem F., Vranová E., Dat J.F., Inzé D. The Role of Active Oxygen Species in Plant Signal Transduction. Plant Sci. 2001;161:405–414. doi: 10.1016/S0168-9452(01)00452-6. [DOI] [Google Scholar]
- 54.Neill S.J., Desikan R., Clarke A., Hurst R.D., Hancock J.T. Hydrogen Peroxide and Nitric Oxide as Signalling Molecules in Plants. J. Exp. Bot. 2002;53:1237–1247. doi: 10.1093/jexbot/53.372.1237. [DOI] [PubMed] [Google Scholar]
- 55.Vandenabeele S., van der Kelen K., Dat J., Gadjev I., Boonefaes T., Morsa S., Rottiers P., Slooten L., van Montagu M., Zabeau M. A Comprehensive Analysis of Hydrogen Peroxide-Induced Gene Expression in Tobacco. Proc. Natl. Acad. Sci. USA. 2003;100:16113–16118. doi: 10.1073/pnas.2136610100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foreman J., Demidchik V., Bothwell J.H.F., Mylona P., Miedema H., Torres M.A., Linstead P., Costa S., Brownlee C., Jones J.D.G. Reactive Oxygen Species Produced by NADPH Oxidase Regulate Plant Cell Growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 57.Černý M., Habánová H., Berka M., Luklová M., Brzobohatý B. Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. Int. J. Mol. Sci. 2018;19:2812. doi: 10.3390/ijms19092812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wrzaczek M., Brosché M., Kangasjärvi J. ROS Signaling Loops—Production, Perception, Regulation. Curr. Opin. Plant Biol. 2013;16:575–582. doi: 10.1016/j.pbi.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Polidoros A.N., Mylona P.v., Scandalios J.G. Transgenic Tobacco Plants Expressing the Maize Cat2 Gene Have Altered Catalase Levels That Affect Plant-Pathogen Interactions and Resistance to Oxidative Stress. Transgenic Res. 2001;10:555–569. doi: 10.1023/A:1013027920444. [DOI] [PubMed] [Google Scholar]
- 60.Wang H., Yang J., Zhang Y., Hu Y., Wang A., Zhu J., Shen H. Drought Resistance of Cotton with Escherichia coli Catalase Gene KatE. Acta Bot. Boreal. Occid. Sin. 2014;34:2034–2040. [Google Scholar]
- 61.Matsumura T., Tabayashi N., Kamagata Y., Souma C., Saruyama H. Wheat Catalase Expressed in Transgenic Rice Can Improve Tolerance against Low Temperature Stress. Physiol. Plant. 2002;116:317–327. doi: 10.1034/j.1399-3054.2002.1160306.x. [DOI] [Google Scholar]
- 62.Gondim F.A., Gomes-Filho E., Costa J.H., Alencar N.L.M., Prisco J.T. Catalase Plays a Key Role in Salt Stress Acclimation Induced by Hydrogen Peroxide Pretreatment in Maize. Plant Physiol. Biochem. 2012;56:62–71. doi: 10.1016/j.plaphy.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 63.Zhao M.-X., Wen J.-L., Wang L., Wang X.-P., Chen T.-S. Intracellular Catalase Activity Instead of Glutathione Level Dominates the Resistance of Cells to Reactive Oxygen Species. Cell Stress Chaperones. 2019;24:609–619. doi: 10.1007/s12192-019-00993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pandey S., Fartyal D., Agarwal A., Shukla T., James D., Kaul T., Negi Y.K., Arora S., Reddy M.K. Abiotic Stress Tolerance in Plants: Myriad Roles of Ascorbate Peroxidase. Front. Plant Sci. 2017;8:581. doi: 10.3389/fpls.2017.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramana Gopavajhula V., Viswanatha Chaitanya K., Akbar Ali Khan P., Shaik J.P., Narasimha Reddy P., Alanazi M. Modeling and Analysis of Soybean (Glycine max. L) Cu/Zn, Mn and Fe Superoxide Dismutases. Genet. Mol. Biol. 2013;36:225–236. doi: 10.1590/S1415-47572013005000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu W., Duanmu H., Qiao Y., Jin X., Yu Y., Yu L., Chen C. Genome-Wide Identification and Characterization of the Soybean SOD Family during Alkaline Stress. PeerJ. 2020;8:e8457. doi: 10.7717/peerj.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corpas F.J., Fernández-Ocaña A., Carreras A., Valderrama R., Luque F., Esteban F.J., Rodríguez-Serrano M., Chaki M., Pedrajas J.R., Sandalio L.M. The Expression of Different Superoxide Dismutase Forms Is Cell-Type Dependent in Olive (Olea europaea L.) Leaves. Plant Cell Physiol. 2006;47:984–994. doi: 10.1093/pcp/pcj071. [DOI] [PubMed] [Google Scholar]
- 68.Xu J., Yang J., Duan X., Jiang Y., Zhang P. Increased Expression of Native Cytosolic Cu/Zn Superoxide Dismutase and Ascorbate Peroxidase Improves Tolerance to Oxidative and Chilling Stresses in Cassava (Manihot esculenta Crantz) BMC Plant Biol. 2014;14:208. doi: 10.1186/s12870-014-0208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y.C., Qu G.Z., Li H.Y., Wu Y.J., Wang C., Liu G.F., Yang C.P. Enhanced Salt Tolerance of Transgenic Poplar Plants Expressing a Manganese Superoxide Dismutase from Tamarix androssowii. Mol. Biol. Rep. 2010;37:1119. doi: 10.1007/s11033-009-9884-9. [DOI] [PubMed] [Google Scholar]
- 70.Yarra R., Wei W. The NAC-Type Transcription Factor GmNAC20 Improves Cold, Salinity Tolerance, and Lateral Root Formation in Transgenic Rice Plants. Funct. Integr. Genomics. 2021;21:473–487. doi: 10.1007/s10142-021-00790-z. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X., Chen L., Shi Q., Ren Z. SlMYB102, an R2R3-Type MYB Gene, Confers Salt Tolerance in Transgenic Tomato. Plant Sci. 2020;291:110356. doi: 10.1016/j.plantsci.2019.110356. [DOI] [PubMed] [Google Scholar]
- 72.Mushke R., Yarra R., Kirti P.B. Improved Salinity Tolerance and Growth Performance in Transgenic Sunflower Plants via Ectopic Expression of a Wheat Antiporter Gene (TaNHX2) Mol. Biol. Rep. 2019;46:5941–5953. doi: 10.1007/s11033-019-05028-7. [DOI] [PubMed] [Google Scholar]
- 73.Sahoo D.P., Kumar S., Mishra S., Kobayashi Y., Panda S.K., Sahoo L. Enhanced Salinity Tolerance in Transgenic Mungbean Overexpressing Arabidopsis Antiporter (NHX1) Gene. Mol. Breed. 2016;36:144. doi: 10.1007/s11032-016-0564-x. [DOI] [Google Scholar]
- 74.Zhao X., Wei P., Liu Z., Yu B., Shi H. Soybean Na+/H+ Antiporter GmsSOS1 Enhances Antioxidant Enzyme Activity and Reduces Na+ Accumulation in Arabidopsis and Yeast Cells under Salt Stress. Acta Physiol. Plant. 2017;39:19. doi: 10.1007/s11738-016-2323-3. [DOI] [Google Scholar]
- 75.Amini S., Ghobadi C., Yamchi A. Proline Accumulation and Osmotic Stress: An Overview of P5CS Gene in Plants. J. Plant Mol. Breed. 2015;3:44–55. [Google Scholar]
- 76.Li Y., Chen Q., Nan H., Li X., Lu S., Zhao X., Liu B., Guo C., Kong F., Cao D. Overexpression of GmFDL19 Enhances Tolerance to Drought and Salt Stresses in Soybean. PLoS ONE. 2017;12:e0179554. doi: 10.1371/journal.pone.0179554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Y., Yu T.F., Ma J., Chen J., Zhou Y.b., Chen M., Ma Y.Z., Wei W.L., Xu Z.S. The Soybean BZIP Transcription Factor Gene GmbZIP2 Confers Drought and Salt Resistances in Transgenic Plants. Int. J. Mol. Sci. 2020;21:670. doi: 10.3390/ijms21020670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patade V.Y., Bhargava S., Suprasanna P. Salt and Drought Tolerance of Sugarcane under iso-Osmotic Salt and Water Stress: Growth, Osmolytes Accumulation, and Antioxidant Defense. J. Plant Interact. 2011;6:275–282. doi: 10.1080/17429145.2011.557513. [DOI] [Google Scholar]
- 79.Barak S., Farrant J.M. Extremophyte Adaptations to Salt and Water Deficit Stress. Funct. Plant Biol. 2016;43:v-x. doi: 10.1071/FPv43n7_FO. [DOI] [PubMed] [Google Scholar]
- 80.Dar M.I., Naikoo M.I., Rehman F., Naushin F., Khan F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In: Iqbal N., Nazar R., Khan N., editors. Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies. Springer; Delhi, India: 2016. pp. 155–166. [Google Scholar]
- 81.Yu Z., Wang X., Zhang L. Structural and Functional Dynamics of Dehydrins: A Plant Protector Protein under Abiotic Stress. Int. J. Mol. Sci. 2018;19:3420. doi: 10.3390/ijms19113420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi H., Ishitani M., Kim C., Zhu J.-K. The Arabidopsis thaliana Salt Tolerance Gene SOS1 Encodes a Putative Na+/H+ Antiporter. Proc. Natl. Acad. Sci. USA. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Apse M.P., Sottosanto J.B., Blumwald E. Vacuolar Cation/H+ Exchange, Ion Homeostasis, and Leaf Development Are Altered in a T-DNA Insertional Mutant of AtNHX1, the Arabidopsis Vacuolar Na+/H+ Antiporter. Plant J. 2003;36:229–239. doi: 10.1046/j.1365-313X.2003.01871.x. [DOI] [PubMed] [Google Scholar]
- 84.Apse M.P., Aharon G.S., Snedden W.A., Blumwald E. Salt Tolerance Conferred by Overexpression of a Vacuolar Na+/H+ Antiport in Arabidopsis. Science. 1999;285:1256–1258. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- 85.Blumwald E. Sodium Transport and Salt Tolerance in Plants. Curr. Opin. Cell Biol. 2000;12:431–434. doi: 10.1016/S0955-0674(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 86.Hasegawa P.M., Bressan R.A., Zhu J.-K., Bohnert H.J. Plant Cellular and Molecular Responses to High Salinity. Annu. Rev. Plant Biol. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- 87.Adabnejad H., Kavousi H.R., Hamidi H., Tavassolian I. Assessment of the Vacuolar Na+/H+ Antiporter (NHX1) Transcriptional Changes in Leptochloa fusca L. in Response to Salt and Cadmium Stresses. Mol. Biol. Res. Commun. 2015;4:133. [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang H.-X., Blumwald E. Transgenic Salt-Tolerant Tomato Plants Accumulate Salt in Foliage but Not in Fruit. Nat. Biotechnol. 2001;19:765–768. doi: 10.1038/90824. [DOI] [PubMed] [Google Scholar]
- 89.Zhao F.-Y., Zhang X.-J., Li P.-H., Zhao Y.-X., Zhang H. Co-Expression of the Suaeda salsa SsNHX1 and Arabidopsis AVP1 Confer Greater Salt Tolerance to Transgenic Rice than the Single SsNHX1. Mol. Breed. 2006;17:341–353. doi: 10.1007/s11032-006-9005-6. [DOI] [Google Scholar]
- 90.Chen H., An R., Tang J.-H., Cui X.-H., Hao F.-S., Chen J., Wang X.-C. Over-Expression of a Vacuolar Na+/H+ Antiporter Gene Improves Salt Tolerance in an Upland Rice. Mol. Breed. 2007;19:215–225. doi: 10.1007/s11032-006-9048-8. [DOI] [Google Scholar]
- 91.Verma D., Singla-Pareek S.L., Rajagopal D., Reddy M.K., Sopory S.K. Functional Validation of a Novel Isoform of Na+/H+ Antiporter from Pennisetum glaucum for Enhancing Salinity Tolerance in Rice. J. Biosci. 2007;32:621–628. doi: 10.1007/s12038-007-0061-9. [DOI] [PubMed] [Google Scholar]
- 92.Xue Z.-Y., Zhi D.-Y., Xue G.-P., Zhang H., Zhao Y.-X., Xia G.-M. Enhanced Salt Tolerance of Transgenic Wheat (Tritivum aestivum L.) Expressing a Vacuolar Na+/H+ Antiporter Gene with Improved Grain Yields in Saline Soils in the Field and a Reduced Level of Leaf Na+ Plant Sci. 2004;167:849–859. doi: 10.1016/j.plantsci.2004.05.034. [DOI] [Google Scholar]
- 93.Fukuda A., Nakamura A., Tagiri A., Tanaka H., Miyao A., Hirochika H., Tanaka Y. Function, Intracellular Localization and the Importance in Salt Tolerance of a Vacuolar Na+/H+ Antiporter from Rice. Plant Cell Physiol. 2004;45:146–159. doi: 10.1093/pcp/pch014. [DOI] [PubMed] [Google Scholar]
- 94.Mishra S., Alavilli H., Lee B., Panda S.K., Sahoo L. Cloning and Characterization of a Novel Vacuolar Na+/H+ Antiporter Gene (VuNHX1) from Drought Hardy Legume, Cowpea for Salt Tolerance. Plant Cell Tissue Organ Cult. 2015;120:19–33. doi: 10.1007/s11240-014-0572-7. [DOI] [Google Scholar]
- 95.Qin F., Sakuma Y., Tran L.-S.P., Maruyama K., Kidokoro S., Fujita Y., Fujita M., Umezawa T., Sawano Y., Miyazono K. Arabidopsis DREB2A-Interacting Proteins Function as RING E3 Ligases and Negatively Regulate Plant Drought Stress–Responsive Gene Expression. Plant Cell. 2008;20:1693–1707. doi: 10.1105/tpc.107.057380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tizaoui K., Kchouk M.E. Genetic Approaches for Studying Transgene Inheritance and Genetic Recombination in Three Successive Generations of Transformed Tobacco. Genet. Mol. Biol. 2012;35:640–649. doi: 10.1590/S1415-47572012000400015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chuong N.N., Hoang X.L.T., Nghia D.H.T., Nguyen N.C., Thao D.T.T., Tran T.B., Ngoc T.T.M., Thu N.B.A., Nguyen Q.T., Thao N.P. Ectopic Expression of GmHP08 Enhances Resistance of Transgenic Arabidopsis toward Drought Stress. Plant Cell Rep. 2021;40:819–834. doi: 10.1007/s00299-021-02677-6. [DOI] [PubMed] [Google Scholar]
- 98.Thu N.B.A., Nguyen Q.T., Hoang X.L.T., Thao N.P., Tran L.S.P. Evaluation of Drought Tolerance of the Vietnamese Soybean Cultivars Provides Potential Resources for Soybean Production and Genetic Engineering. Biomed Res. Int. 2014;2014:809736. doi: 10.1155/2014/809736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shu K., Qi Y., Chen F., Meng Y., Luo X., Shuai H., Zhou W., Ding J., Du J., Liu J. Salt Stress Represses Soybean Seed Germination by Negatively Regulating GA Biosynthesis While Positively Mediating ABA Biosynthesis. Front. Plant Sci. 2017;8:1372. doi: 10.3389/fpls.2017.01372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y., Jiang L., Chen J., Tao L., An Y., Cai H., Guo C. Overexpression of the Alfalfa WRKY11 Gene Enhances Salt Tolerance in Soybean. PLoS ONE. 2018;13:e0192382. doi: 10.1371/journal.pone.0192382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wijewardana C., Raja Reddy K., Jason Krutz L., Gao W., Bellaloui N. Drought Stress Has Transgenerational Effects on Soybean Seed Germination and Seedling Vigor. PLoS ONE. 2019;14:e0214977. doi: 10.1371/journal.pone.0214977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patterson B.D., MacRae E.A., Ferguson I.B. Estimation of Hydrogen Peroxide in Plant Extracts Using Titanium(IV) Anal. Biochem. 1984;139:487–492. doi: 10.1016/0003-2697(84)90039-3. [DOI] [PubMed] [Google Scholar]
- 103.Bradford M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 104.Wang C.J., Yang W., Wang C., Gu C., Niu D.D., Liu H.X., Wang Y.P., Guo J.H. Induction of Drought Tolerance in Cucumber Plants by a Consortium of Three Plant Growth-Promoting Rhizobacterium Strains. PLoS ONE. 2012;7:e52565. doi: 10.1371/journal.pone.0052565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giannopolitis C.N., Ries S.K. Superoxide Dismutases: II. Purification and Quantitative Relationship with Water-Soluble Protein in Seedlings. Plant Physiol. 1977;59:315–318. doi: 10.1104/pp.59.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodríguez Y., Pérez E., Solórzano E., Meneses A.R., Fernández F. Peroxidase and Polyphenoloxidase Activities in Tomato Roots Inoculated with Glomus clarum or Glomus fasciculatum. Cult. Trop. 2001;22:11–16. [Google Scholar]
- 107.Le D.T., Aldrich D.L., Valliyodan B., Watanabe Y., Ha C.v., Nishiyama R., Guttikonda S.K., Quach T.N., Gutierrez-Gonzalez J.J., Tran L.-S.P. Evaluation of Candidate Reference Genes for Normalization of Quantitative RT-PCR in Soybean Tissues under Various Abiotic Stress Conditions. PLoS ONE. 2012;7:e46487. doi: 10.1371/annotation/6a5108f5-50f8-418e-854d-8f3eb94e6fc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Livak K.J., Schmittgen T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 109.Jiao C., Yang R., Zhou Y., Gu Z. Nitric Oxide Mediates Isoflavone Accumulation and the Antioxidant System Enhancement in Soybean Sprouts. Food Chem. 2016;204:373–380. doi: 10.1016/j.foodchem.2016.02.147. [DOI] [PubMed] [Google Scholar]
- 110.Stolf-Moreira R., Medri M.E., Neumaier N., Lemos N.G., Pimenta J.A., Tobita S., Brogin R.L., Marcelino-Guimarães F.C., Oliveira M.C.N., Farias J.R.B. Soybean Physiology and Gene Expression during Drought. Genet. Mol. Res. 2010;9:1946–1956. doi: 10.4238/vol9-4gmr851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.