Abstract
Ras proteins can activate at least three classes of downstream target proteins: Raf kinases, phosphatidylinositol-3 phosphate (PI3) kinase, and Ral-specific guanine nucleotide exchange factors (Ral-GEFs). In NIH 3T3 cells, activated Ral-GEFs contribute to Ras-induced cell proliferation and oncogenic transformation by complementing the activities of Raf and PI3 kinases. In PC12 cells, activated Raf and PI3 kinases mediate Ras-induced cell cycle arrest and differentiation into a neuronal phenotype. Here, we show that in PC12 cells, Ral-GEF activity acts opposite to other Ras effectors. Elevation of Ral-GEF activity induced by transfection of a mutant Ras protein that preferentially activates Ral-GEFs, or by transfection of the catalytic domain of the Ral-GEF Rgr, suppressed cell cycle arrest and neurite outgrowth induced by nerve growth factor (NGF) treatment. In addition, Rgr reduced neurite outgrowth induced by a mutant Ras protein that preferentially activates Raf kinases. Furthermore, inhibition of Ral-GEF activity by expression of a dominant negative Ral mutant accelerated cell cycle arrest and enhanced neurite outgrowth in response to NGF treatment. Ral-GEF activity may function, at least in part, through inhibition of the Rho family GTPases, CDC42 and Rac. In contrast to Ras, which was activated for hours by NGF treatment, Ral was activated for only ∼20 min. These findings suggest that one function of Ral-GEF signaling induced by NGF is to delay the onset of cell cycle arrest and neurite outgrowth induced by other Ras effectors. They also demonstrate that Ras has the potential to promote both antidifferentiation and prodifferentiation signaling pathways through activation of distinct effector proteins. Thus, in some cell types the ratio of activities among Ras effectors and their temporal regulation may be important determinants for cell fate decisions between proliferation and differentiation.
Ras proteins have the capacity to influence a wide variety of cellular processes, including cell cycle control, induction of differentiation, rearrangement of the actin cytoskeleton, apoptosis, and specific functions associated with fully differentiated cells (for reviews, see references 6 and 29). A growing body of evidence supports the idea that this is due, at least in part, to the ability of Ras proteins to influence multiple downstream target proteins. To date, the active GTP-bound form of Ras has been shown to bind to and activate three classes of proteins in cells: Raf protein kinases, phosphatidyl inositol-3 phosphate (PI3) kinase, and Ral-specific guanine nucleotide exchange factors (Ral-GEFs) (for a review, see reference 20).
Active Ras targets Raf kinases to the plasma membrane, where a secondary event, apparently phosphorylation, leads to kinase activation. Activation of Raf initiates a kinase cascade involving MEK and Erk proteins. Active Erk can alter cytoplasmic processes as well as influence events in the nucleus by phosphorylating transcription factors (for a review, see reference 31).
Active Ras also binds to and activates PI3 kinase, which can generate PtdIns-3,4-P2 and PtdIns-3,4-5-P3 (for a review, see reference 7). These signaling molecules have many functions in cells, including stimulation of signaling cascades that lead to Akt kinase, S6 kinase, and protein kinase C activation. PtdIns-3,4-5-P3 has also been shown to activate GEFs for Rac GTPases (13, 34), which can then promote a signaling cascade leading to Jun N-terminal kinase (JNK) activation. Active Rac proteins also have unique effects on the actin cytoskeleton (35).
More recently identified targets for Ras are a family of Ral-GEFs. These proteins promote the GTP-bound state of RalA and RalB, which comprise a distinct family of Ras-related GTPases (for a review, see reference 11). Four of these GEFs, Ral-GDS, RGL1 and RGL2, and Rlf, have domains that interact preferentially with active Ras-GTP. A fifth Ral-GEF, termed Rgr, was isolated as part of a fusion protein generated during transfection experiments (4). The fusion protein, termed Rsc, was cloned by its ability to confer tumor-forming activity on NIH 3T3 cells. The oncogenic activity derived from the exchange factor part of the fusion protein. Only a partial cDNA of Rgr has been cloned, so whether it is regulated by Ras binding or by some other upstream signal remains to be determined.
Ras binding activates Ral-GEFs (46, 50), at least in part, by targeting them to their substrates, Ral GTPases, which are present in the plasma membrane (24). All extracellular signals tested to date that activate Ras in cells also promote the GTP-bound state of Ral in a Ras-dependent manner (52). However, evidence suggests that Ral proteins are also activated by Ras-independent pathways that may be mediated by calcium (14, 46, 51). The functions of Ral proteins are only now beginning to be revealed. Recent experiments suggest they can influence at least two classes of signaling molecules. In the active GTP-bound state, Ral proteins can bind to RalBP1 (or RLIP or RLP), a GTPase-activating protein for CDC42 and Rac GTPases (3, 21, 37). Thus, one role for Ral may be to negatively regulate these Rho family GTPases. Ral proteins also appear to be associated constitutively with a phospholipase D1 (PLD1) (18, 27, 28). Although Ral binding itself does not activate PLD1, it does enhance PLD1 activation by the Arf GTPase (23). Thus, Ral may target PLD into a Ras-induced signaling complex, where the enzyme becomes activated to generate phosphatidic acid from phosphatidyl choline. Phosphatidic acid can then function on its own in cell signaling or be converted to diacylglycerol, an activator of a wide variety of signaling molecules (for a review, see reference 8).
Most studies of Ras signaling have focused on its ability to promote cell proliferation and oncogenesis. In these systems activation of the Raf kinase by Ras plays a major role. In many cases constitutive activation of this kinase can promote cell cycle progression and an oncogenic phenotype on its own, while inhibition of Raf-induced Erk activation suppresses transformation (43). Active PI3 kinases or Ral-GEFs have weaker oncogenic effects on their own (4, 19, 50), but they can clearly complement Raf effects in promoting cell proliferation and oncogenesis in immortalized fibroblast cell lines (39, 48). Furthermore, inhibition of Ral-GEF or PI3 kinase activities can suppress certain aspects of oncogenic transformation by Ras (22, 46).
However, in certain cell types, like primary fibroblasts and PC12 cells, Ras activity can produce the opposite effect, cell cycle arrest (12). In PC12 cells, this cell cycle arrest is associated with induction of cell differentiation into a neuronal phenotype. Activation of Ras by nerve growth factor (NGF) in PC12 cells is critical for neurotrophin-induced neurite outgrowth and cell cycle arrest (38, 53). Ras-activated Raf and PI3 kinases play important roles in mediating this effect. First, activated forms of Raf or PI3 kinases can produce, at least in part, effects similar to those of activated Ras (25, 53). Second, inhibition of the Ras-Raf-Erk kinase cascade (40) or PI3 kinase activity (16) prevents NGF-induced neurite outgrowth. The kinetics and magnitude of signaling pathway stimulation may also be important. Epidermal growth factor (EGF) may fail to promote differentiation and cell cycle exit because it activates Ras and the Raf-Erk pathway only transiently (5, 45).
In this study, we investigated the contribution of the Ral-GEF signaling pathway to NGF-induced differentiation of PC12 cells. We found that Ral-GEF signaling opposed the actions of Raf and PI3 kinase signaling. In particular, constitutive elevation of Ral-GEF activity suppressed neurite outgrowth and cell cycle arrest induced by NGF, whereas constitutive inhibition of Ral-GEF activity enhanced the rate of NGF-induced neurite outgrowth and cell cycle exit. However, NGF activated Ral only transiently, suggesting that Ral-GEF functions to delay the onset of cell cycle arrest and neurite outgrowth induced by NGF.
MATERIALS AND METHODS
Cell culture and transfections.
PC12 cells were cultured in Dulbecco’s modified Eagle’s medium plus 5% horse serum and 5% iron-enriched calf serum. For transfections, cells were plated on laminin–poly-l-lysine-coated coverslips at a density of 104 cells/coverslip (∼50% confluent). Eighteen hours later, DNA (total 2 μg) was added to 500 μl of OPTIMEM I containing 6 μl of Pfx-1 (Invitrogen). The cells were exposed to this solution for 4 h at 37°C. The solution was then replaced with complete medium. The next day, the medium was replaced with either complete medium or low-serum medium (containing 0.5% horse serum). In some cases, NGF (50 ng/ml) was added at this time. Transfection efficiency averaged ∼30%. For fos gene reporter assays, 100 ng of the complete fos promoter coupled to firefly luciferase (gifts from B. Cochran, Tufts Medical School) was transfected into 2 × 105 PC12 cells (in 35-mm-diameter dishes) by the procedure described above. The cells were then grown in low-serum medium for 2 days. A control plasmid, pRL-TK (Promega), containing the minimal herpes simplex virus thymidine kinase promoter coupled to the Renilla luciferase gene was cotransfected in each experiment for an internal control to compensate for differences in transfection efficiency. Cells were lysed and assayed for firefly and then Renilla luciferase activities on a luminometer, as described by the manufacturer.
Antibodies.
α-RalA antibodies were generated in rabbits with a glutathione S-transferase (GST)-RalA fusion as the antigen. Antibodies were affinity purified with RalA Sepharose. Mouse α-RalA monoclonal antibody, α-RalB polyclonal antibodies, and rabbit α-CDC42 and α-Rac antibodies were purchased from Transduction Laboratories. Mouse α-MYC epitope antibodies were isolated from culture media from the 9E10 hybridoma cell line. Mouse α-BrdU antibody was obtained from Boehringer Mannheim, while rabbit anti-β-galactosidase antibodies were a gift from Amy Yee, Tufts Medical School.
Immunofluorescence.
Cells were fixed in 4% formaldehyde–phosphate-buffered saline (PBS) for 15 min at room temperature. The cells were incubated in bovine serum albumin for 30 min. The primary antibody in 1% bovine serum albumin–0.5% Saponin–PBS was added for 30 min at 37°C. The secondary antibody (fluorescein isothiocyanate [FITC]-conjugated or tetramethyl rhodamine isothiocyanate [TRITC]-conjugated anti-mouse or anti-rabbit immunoglobulin G) was prepared and used in a similar manner. The sample was mounted in 2% n-propyl gallate–50% glycerol–50% PBS. For actin staining, the cells were processed as described above but exposed to TRITC-labeled phalloidin (Molecular Probes) (0.1 μg/ml) instead of primary antibodies.
BrdU labeling.
At various times after transfection, the cells were pulsed with BrdU (30 μM/ml) for 2 h at 37°C. The cells were then fixed in methanol-acetone for 15 min at −20°C. The primary antibody was either anti-Ral immunoglobulin G, anti-β-galactosidase polyclonal antibody (a gift from A. Yee), anti-BrdU, or anti-MYC epitope (9E10) monoclonal antibody. The secondary antibodies used were the same as those described above.
DNA constructs.
Many of the signaling molecules were subcloned into the vector pCAG(SB), which is driven by an actin promoter. Ral28N and Ral72L were excised from pBSK constructs (46) with BamHI and HindIII and cloned into these sites in pCAG(SB). The Ral exchange factor region, termed Rgr, of the oncogene product Rsc was isolated by PCR starting at codon 264 and ending at the translation stop codon, position 748. 5′ and 3′ BamHI cloning sites were included in the primers so that the PCR product could be cloned into pCAG(SB), which had been previously altered to contain DNA sequences encoding a MYC epitope followed by a BamHI cloning site. Ral-GDS, previously cloned into pJ4 (46), was also cloned into MYC-pCAGSB via BamHI cloning sites. Dominant negative and activated forms of CDC42 were isolated by PCR, using primers with BamHI sites, which also allowed their cloning into pCAG(SB) in frame with sequences containing a MYC epitope tag. Wild-type Ras and effector mutants 12V37G and 12V35S in pSRα were obtained from M. White, University of Texas, Southwestern Medical School.
Ral-GTP and Ras-GTP precipitation assays.
For quantitation of GTP-bound Ral proteins, amino acids 397 to 518 from RalBP1 were expressed as a GST fusion protein in Escherichia coli and affinity purified with glutathione agarose. Ten micrograms of immobilized fusion protein were incubated with 500 μl (5 mg of protein/ml) of lysates of PC12 cells (in 50 mM Tris-HCl [pH 7.5] plus 150 mM NaCl, 20 mM MgCl2, 1% Nonidet P-40, and protease inhibitors) for 2 h at 4°C. The beads were then washed in lysis buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting with antibodies to either RalA or RalB. For quantitation of GTP-bound Ras, the GST fusion construct of the Ras-binding domain of Raf was used in a similar fashion except that the immunoblots were probed with anti-Ras antibodies (Upstate Biotechnology).
RESULTS
Excess Ral-GEF activity suppresses NGF- and Ras-induced neurite formation.
A strong body of evidence supports the idea that Ras can induce Ral-GEF activation. Consequently, the influence of this putative Ras effector system on neurite outgrowth of PC12 cells was assessed by altering the activity of Ral-GEFs in these cells. To elevate Ral-GEF activity, we first expressed a Ras mutant (12V37G) that preferentially activates Ral-GEFs over Raf or PI3 kinases (49). The mutant was transiently transfected into PC12 cells, and 3 days later cells expressing the protein were identified by immunofluorescence with anti-Ras antibodies. Greater than 90% of cells expressing Ras12V37G failed to display neurites (Fig. 1A, left panel). In contrast, significant neurite outgrowth was observed in greater than 80% of cells expressing a mutant Ras12V35S, which is known to preferentially activate Raf kinases (48) (Fig. 1A, right panel). These results suggest that unlike Ras-induced Raf activation, Ras induced Ral-GEF activation is not sufficient to promote neurite outgrowth.
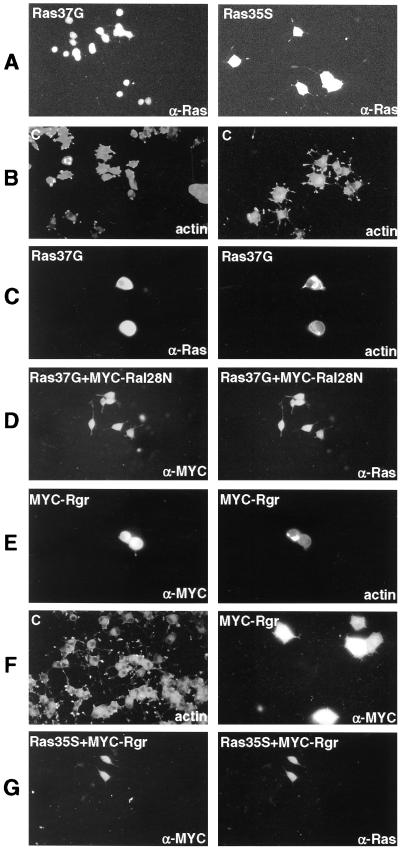
FIG. 1.
Effect of Ral-GEF activity on neurite outgrowth of PC12 cells. PC12 cells were transiently transfected with expression plasmids under various conditions, and 3 days later the cells expressing the appropriate protein were identified by immunofluorescence. (A) Cells transfected with Ras12V37G (left) or Ras12V35S (right) were identified with α-Ras antibodies and FITC-coupled secondary antibodies. Endogenous Ras could not be detected with this antibody. All cells shown expressed exogenous Ras. (B) Cells were either untreated (left) or treated with NGF (50 ng/ml) 1 day after transfection (right), and all cells in the population were stained 2 days later with TRITC-phalloidin to detect filamentous actin. (C) Cells were transfected with Ras37G and treated 24 h later with NGF (50 ng/ml). Two days later transfected cells in the population were detected with rabbit α-Ras antibodies plus FITC-labeled secondary antibodies (left). Cells were also stained for actin with TRITC-phalloidin (right). Again, all cells shown expressed exogenous protein. (D) Cells were transfected with Ras12V37G plus MYC-Ral28N and treated with NGF as for panel C. Cells expressing Ral28N were detected with α-MYC antibodies and FITC-labeled secondary antibody (left). Cells also expressing Ras12V37G were detected with α-Ras antibodies and TRITC-labeled secondary antibodies (right panel). (E) Cells were transfected with MYC-Rgr and treated with NGF as for panel C. Transfected cells were detected with α-MYC antibodies and FITC-labeled secondary antibodies (left panel). Cells in the population were also stained for actin (right panel) with TRITC-phalloidin. (F) Cells were treated with NGF every day for 4 days and stained for actin (left panel). In a separate transfection, cells were transfected with MYC-Rgr and treated with NGF (50 ng) every day for 4 days. Transfected cells were detected with α-MYC antibodies. (G) Cells were transfected with Ras12V35S plus MYC-Rgr and treated with NGF as for panel C. Cells were stained for Ras and Rgr as described above. The label at the top left of each panel indicates the transfected gene, while the label at the bottom right of each panel indicates the protein being detected by immunofluorescence microscopy. At least 50 transfected cells were inspected for each experiment.
Ras12V37G expression was then tested for its effects on NGF-induced neurite outgrowth. As expected, the addition of NGF to control PC12 cells (grown in low-serum medium to enhance the effect of NGF) promoted the initiation of neurite outgrowth when examined 2 days later (Fig. 1B, compare left [no NGF] and right [plus NGF] panels). On the other hand, initiation of neurite outgrowth by NGF was suppressed in cells transiently transfected with Ras12V37G (Fig. 1C). Greater than 90% of cells expressing the mutant Ras protein had no detectable neurites after NGF treatment. Although it is known that Ras12V37G activates Ral-GEFs more efficiently than Raf or PI3 kinase in cells, it may also activate additional undefined Ras effector proteins. To confirm that the observed inhibitory effect on neurite outgrowth was due, at least in part, to activation of the Ral-GEF signaling pathway, the cells were cotransfected with a dominant negative Ral28N mutant. Coexpression of this mutant partially restored the ability of NGF to initiate neurite outgrowth in Ras12V37G-expressing cells (Fig. 1D). Greater than 80% of cells expressing both Ras12V37G and Ral28N initiated neurite outgrowth. Ral28N is analogous to Ras17N and thus cannot bind to downstream targets even when it is in the GTP-bound state (3, 9). Therefore, it is thought to suppress the activity of Ral-specific GEFs in cells by binding to their catalytic domains and forming dead-end complexes (41). This finding argues that Ras12V37G inhibits neurite outgrowth at least in part by promoting Ral-GEF activity.
To gain additional support for this idea, the isolated catalytic domain of a Ral-GEF, Rgr (4), was transiently transfected into PC12 cells. Expression of Rgr also suppressed the initiation of neurite outgrowth after NGF treatment in greater than 80% of transfected cells (Fig. 1E). This inhibition was detectable even when cells were given repeated doses of NGF, which yielded more extensive neurites in control cells (compare left and right panels of Fig. 1F). Rgr expression also suppressed neurite outgrowth in greater than 80% of PC12 cells induced by the Raf-activating Ras12V35S mutant (compare Fig. 1G, left and right panels, to Fig. 1A, right panel).
Rgr was also transiently transfected into PC12 cells after they had already been induced to differentiate by repeated NGF treatment. In this case, neurites were still present (data not shown), arguing that excessive Ral-GEF activity prevents NGF-induced neurite outgrowth but does not cause the retraction of existing neurites.
Inhibition of Ral-GEF activity enhances NGF-induced neurite outgrowth.
Since elevated Ral-GEF activity was associated with the suppression of neurite outgrowth, we investigated the effect of inhibiting endogenous Ral-GEF activity. This was accomplished by transfection of the dominant inhibitory Ral28N mutant described above. After transfection, PC12 cells were treated with NGF, again under low-serum conditions. Two days later the morphology of transfected cells (detected with a Ral-specific antibody) was compared to that of nontransfected cells. As expected, NGF promoted modest neurite outgrowth in most of the nontransfected cells (Fig. 2A; note the cells labeled in the right panel but not the left panel). Strikingly, cells expressing inhibitory Ral28N displayed dramatically enhanced neurite outgrowth (Fig. 2A). Greater than 90% of NGF-stimulated PC12 cells expressing Ral28N had neurites that were greater than three times the length of their cell bodies. Less than 1% of nontransfected cells displayed neurites of similar size. The effects of Ral28N expression required NGF stimulation, since <5% of unstimulated cells or cells treated with EGF had neurites longer than those of nontransfected cells (data not shown). Similar results were observed when the experiments described above were performed in high-serum medium.
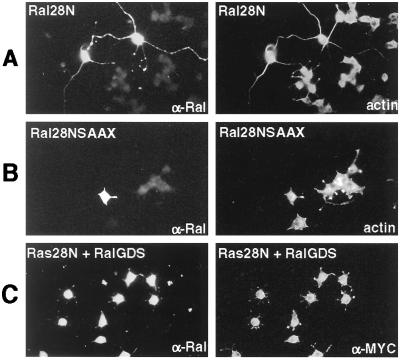
FIG. 2.
Effect of Ral-GEF inhibition on neurite outgrowth of PC12 cells. PC12 cells were transiently transfected as described in the legend to Fig. 1. (A) Cells were transfected with Ral28N and treated 24 h later with NGF (50 ng/ml). Two days later the transfected cells were detected with α-Ral antibodies (left). All cells in the population were stained for F-actin with TRITC-phalloidin (right). (B) Cells were transfected with Ral28NSAAX and treated as described for panel A. (C) Cells were cotransfected with Ral28N and MYC-Ral-GDS. Twenty-four hours later the cells were treated with NGF. Two days later the cells were stained with both rabbit α-Ral antibodies and mouse α-MYC antibodies. Ral28N expression was detected with FITC-labeled anti-rabbit secondary antibodies (left), and MYC-Ral-GDS was detected with TRITC-labeled anti-mouse secondary antibodies (right).
To test for the specificity of this effect, a dominant negative Ral mutant with a mutation in its lipid modification site (cysteine to serine 208) was used. It is known that this defect inactivates dominant negative GTPases (10). Expression of this mutant (Ral28NSAAX) in PC12 cells had no detectable effect on NGF neurite outgrowth (Fig. 2B). Neurites that were greater than three times the width of the cell body were found in less than 5% of transfected cells. In addition, while transfection of MYC-tagged Ral-GDS did not have any effect on NGF-induced neurite outgrowth (data not shown), it did prevent the long neurites normally associated with Ral28N expression (Fig. 2C). This finding is consistent with the proposed mode of Ral28N action.
These findings, along with those generated with Ras12V37G and an active Ral-GEF, show that constitutive activation of the Ral-GEF signaling pathway suppresses neurite outgrowth and inhibition of this pathway enhances neurite outgrowth induced by NGF. Thus, Ras-induced Ral-GEF activation opposes the neurite-stimulatory actions of the other Ras target proteins, Raf and PI3 kinases.
Changes in Ral-GEF activity alter cell cycle regulation.
As part of its differentiation-inducing effect, NGF treatment causes PC12 cells to exit from the cell cycle. Therefore, one possible mechanism participating in Ral-GEF-induced changes in differentiation potential was a disturbance in cell cycle regulation. To test this hypothesis, the proportion of PC12 cells progressing through the S phase of the cell cycle under various conditions was evaluated by BrdU labeling. PC12 cells were transiently transfected with a β-galactosidase reporter clone to allow detection of transfected cells. At various times, the cells were then exposed to BrdU for 2 h and then stained for BrdU uptake with mouse α-BrdU monoclonal antibodies (Fig. 3). As has been shown previously (47), treatment of cells grown in high-serum medium with NGF for 4 days suppressed BrdU labeling, such that only ∼7% of transfected cells were labeled compared to ∼47% for untreated control cells (Fig. 3A). In contrast, cells cotransfected with Ras37G maintained ∼25% BrdU labeling after NGF treatment. This value is likely an underestimate because not all of the cells expressing β-galactosidase also expressed Ras12V37G. The effect of Ras37G on the cell cycle was mediated at least in part by Ral-GEF activation, since coexpression of Ral28N reversed the effect of the Ras mutant. Importantly, transfection of Rgr also suppressed NGF-induced cell cycle exit, as evidenced by BrdU labeling of 20% of the cells (Fig. 3A). Thus, one mechanism of Ral-GEF-induced inhibition of PC12 cell differentiation may be preventing NGF-induced cell cycle arrest.
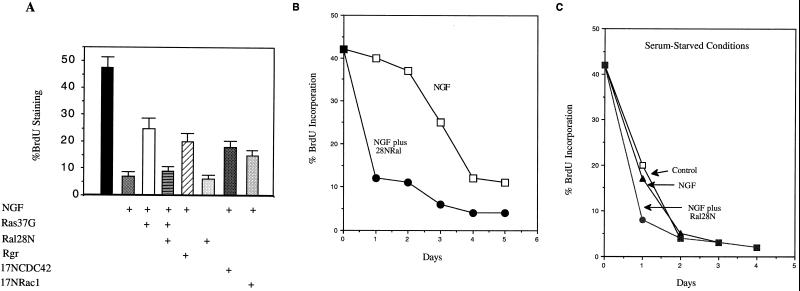
FIG. 3.
Effect of Ral-GEF activity on cell cycle progression of PC12 cells. (A) PC12 cells were transiently transfected with an empty expression vector or vectors encoding various signaling molecules. Twenty-four hours later, the cells were treated daily with NGF or buffer for 4 days. The cells were then exposed to 30 μg of BrdU/ml for 2 h. To identify transfected cells, a vector expressing β-galactosidase was included in all transfections. The cells were then fixed and stained with rabbit β-galactosidase antibodies to detect transfected cells and mouse anti-BrdU monoclonal antibodies to detect cells that had passed through the S phase of the cell cycle. The data are expressed as the proportion of transfected cells incorporating detectable amounts of BrdU; a representative sample of three experiments, each performed in triplicate, is shown (± standard deviations). (B) PC12 cells were grown in serum-containing medium and transiently transfected with empty vector (□) or Ral28N (●) and processed as described for panel A. The cells were treated with NGF (50 ng/ml) each day after transfection and processed at daily intervals afterward. The data represent the average of duplicate determinations. (C) Experiment performed as for panel B except that cells were grown in low-serum (0.5%) medium once NGF was added. □, Empty vector, low serum only; ▴, empty vector, low serum plus NGF; ●, Ral28N, low serum plus NGF.
The effect of inhibiting Ral-GEF activity on cell cycle regulation was analyzed in a similar way. Ral28N expression caused cell cycle arrest in cells grown in high-serum medium such that the BrdU-labeling efficiency of transfected cells was similar to that of NGF-treated control cells (Fig. 3A). Ral 28N expression also accelerated cell cycle arrest induced by NGF, such that maximal inhibition of BrdU uptake occurred after only 2 days of NGF treatment versus 4 days for control NGF-treated cells (Fig. 3B). These findings suggested that at least part of the mechanism underlying Ral28N enhancement of neurite outgrowth was acceleration of cell cycle exit. However, we observed that serum starvation also accelerated cell cycle exit in NGF-treated cells and that this rate was augmented only marginally in cells expressing Ral28N (Fig. 3C). Nevertheless, Ral28N expression dramatically enhanced neurite outgrowth even when PC12 cells were serum starved along with NGF treatment (Fig. 2A). These findings argue that an additional mechanism besides accelerated cell cycle exit is involved in enhanced neurite outgrowth induced by Ral28N expression.
Connection between Ral and Rho family GTPases.
Although the functions of Ral GTPases in cells are poorly understood, recent findings suggest that they may be connected to the functions of the Rho family of GTPases. For example, a putative downstream target protein that binds to Ral-GTP, termed RalBP1 (or RLIP or RIP) (3, 21, 37), is a GTPase-activating protein for CDC42 and Rac. In addition, Ral is constitutively associated with a PLD isoform in cells (18) that may be activated by Rho family proteins (1, 27). Interestingly, like Ral-GEF, elevation of Rho activity also inhibits neurite outgrowth, while suppression of Rho activity promotes neurite outgrowth (17, 26). Thus, it is possible that Ral and Rho influence neurite outgrowth cooperatively through PLD regulation. We have previously shown that PLD binding to Ral requires the N terminus of the GTPase. Moreover, expression of a Ral mutant lacking this region (ΔN11Ral) acts as a dominant negative molecule that blocks src-induced PLD activation as efficiently as expression of Ral28N (18). Thus, if Ral28N expression promotes neurite outgrowth mainly by suppressing PLD activation, expression of ΔN11Ral should produce a similar phenotype. However, Fig. 4A (left panel) shows that this was not the case. Expression of the deletion mutant had no detectable effect on NGF-induced neurite outgrowth in PC12 cells, suggesting some other function of Ral is involved.
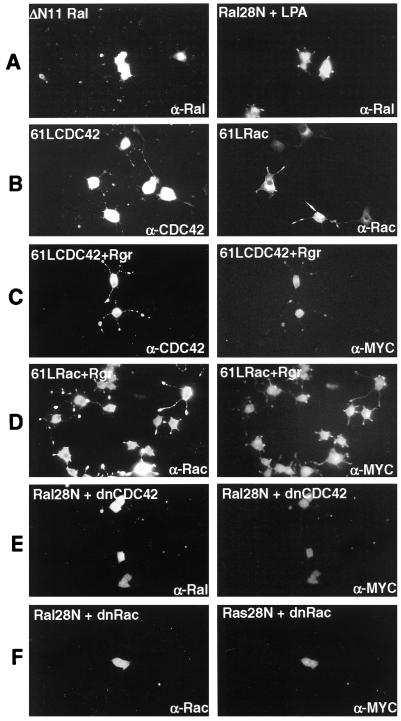
FIG. 4.
Involvement of Rho family proteins in Ral signaling in PC12 cells. PC12 cells were transiently transfected with various expression constructs, and 24 h later the cells were treated with NGF. The cells were then processed as described in the legend to Fig. 1. (A) The cells were transfected with ΔN11Ral, and transfected cells were detected with α-Ral antibodies (left), or cells were transfected with Ral28N, and 3 days later the cells were treated with lysophosphatidic acid (2.4 μg/ml) for 90 min (right). (B) Cells were transfected with 61LCDC42 (left) or 61LRac1 (right). Three days later, the cells expressing GTPases were detected with either rabbit α-CDC42 or α-Rac1 antibody. (C) Cells were transfected with MYC-Rgr and activated 61LCDC42. Three days later, the cells were fixed and labeled with both rabbit α-CDC42 antibodies and mouse α-MYC monoclonal antibodies. CDC42 was detected with FITC-labeled anti-rabbit secondary antibodies (left), and Rgr was detected with TRITC-labeled anti-mouse secondary antibodies (right). (D) Cells were transfected with Rgr and 61LRac1 and processed as for panel B except that 61LRac-expressing cells were detected with rabbit α-Rac antibodies. (E) Cells were transfected with Ral28N plus MYC-17NCDC42. Three days later transfected cells were stained with both rabbit α-Ral antibodies and mouse anti-MYC antibodies. Ral28N expression was detected with FITC-labeled anti-rabbit secondary antibodies (left), and Rgr was detected with TRITC-labeled anti-mouse secondary antibodies (right). (F) Cells were transfected with Ral28N plus MYC-17NRac1 and processed as for panel E.
If enhanced Rho activity promotes neurite retraction by activating a PLD that requires proper localization by active Ral, then neurites expressing Ral28N should be resistant to the effects of Rho activation. It has been shown that lysophosphatidic acid (LPA) can activate Rho to induce neurite retraction in PC12 cells (17). Neurites induced by Ral28N expression still retracted in response to the addition of LPA (Fig. 4A, right panel), suggesting that Ral and Rho influence neurites by distinct mechanisms.
In contrast to Rho, enhanced CDC42 and Rac1 activities have been reported to promote neurite outgrowth in PC12 cells (26). We found that under the conditions we employed, expression of GTPase-deficient 61LCDC42 or 61LRac1 did not promote neurite outgrowth on their own (data not shown). However, 61LCDC42 did clearly enhance neurite outgrowth induced by NGF treatment (Fig. 4B) while 61LRac1 had a less pronounced effect. Since the Ral target, RalBP1, is a GTPase-activating protein (GAP) for CDC42 and Rac, Rgr expression might inhibit CDC42 and/or Rac by activating RalBP1. If this were the case, inhibition of neurite outgrowth by Rgr expression might be suppressed by expression of a GAP-resistant, constitutively activated form of CDC42 and/or Rac. Consequently, 61LCDC42 and 61LRac1 were transfected into PC12 cells along with MYC-Rgr. Figure 4C and D shows that, as predicted, most cells expressing both Rgr and 61LCDC42 or 61LRac grew neurites after NGF treatment to a degree similar to that found in cells expressing only the activated GTPases (Fig. 4C and D).
These results added support to the idea that Rgr may function by blocking CDC42 and/or Rac activity. If this is correct, inhibition of these GTPases by expression of dominant negative Rac or CDC42 in PC12 cells would be expected to mimic the effects of Rgr expression. Microinjection of 17NCDC42 or 17NRac1 have already been shown to block NGF-induced neurite outgrowth (26), and we obtained similar results by transient transfection (data not shown). Moreover, like Rgr, expression of 17NRac1 or 17NCDC42 also partially suppressed NGF-induced cell cycle arrest (Fig. 3A).
According to this model, suppression of Ral activation by Ral28N expression might inhibit RalBP1 activity. This would allow more efficient activation of CDC42 and Rac in NGF-stimulated PC12 cells. If so, Ral28N induction of neurite formation should be dependent upon CDC42 and/or Rac activation. To test this hypothesis, PC12 cells were cotransfected with Ral28N and MYC epitope-tagged dominant negative 17NRac1 or dominant negative 17NCDC42. As predicted, coexpression of either of these inhibitory GTPases blocked neurite induction by Ral28N (Fig. 4E and F) in >90% of cells expressing both proteins. These findings, using both dominant negative and activated Rho family GTPases, support the idea that Ral-GEFs function in this system, at least in part, through inhibition of CDC42 and Rac.
Ral-GEF and fos promoter regulation.
In fibroblasts, activation of Ral-GEF signaling can stimulate the regulatory elements of the fos gene promoter, as detected in fos reporter assays (33, 36, 50). It was proposed that this activity contributes to Ral-GEF’s ability to complement Raf and PI3 kinase in cellular transformation. We therefore investigated fos gene regulation by Ral-GEF signaling in PC12 cells.
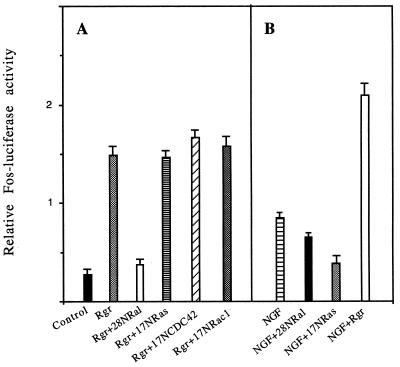
PC12 cells were transiently transfected with a fos promoter-luciferase reporter construct containing the complete 5′ upstream regulatory sequence of c-fos, and luciferase activity was measured 2 days later. Transfection of the catalytic domain of the Ral-GEF Rgr, which prevents neurite outgrowth in PC12 cells, enhanced Fos-luciferase activity approximately fivefold (Fig. 5A). This effect was due to the protein’s nucleotide exchange domain, since activity was suppressed by cotransfection of dominant negative Ral28N mutants but not dominant negative Ras17N mutants (Fig. 5A). Similar results were obtained upon transfection of Ras12V37G (data not shown).
FIG. 5.
Influence of Ral-GEF activity on fos gene regulation in PC12 cells. Cells were transfected with a Fos-luciferase reporter construct along with a minimal thymidine kinase promoter-Renilla reporter construct (as an internal control for transfection efficiency) and various expression plasmids. In some instances, NGF (50 ng/ml) was added to the cells 24 h later. After 2 days, firefly luciferase and Renilla luciferase activities were measured in cell lysates. The data are reported as the ratio of firefly-luciferase to Renilla luciferase activities and represents the average (± standard deviation) of at least two experiments, each performed in duplicate.
fos promoter activation was also observed upon transfection of Ras12V35S (data not shown), which enhances neurite outgrowth (Fig. 2A). Thus, fos promoter activation occurs regardless of whether a growth-promoting or cell cycle exit signal is generated, suggesting that fos promoter regulation by Rgr is not a major determinant of its effects on neurite outgrowth.
In addition, although transfection of Rgr led to fos promoter activation, inhibition of endogenous Ral-GEF activity by expression of dominant negative Ral28N mutants had little, if any, effect on NGF-induced Fos-luciferase activation (Fig. 5B). Under these conditions, neurite outgrowth was enhanced dramatically (Fig. 2A). Thus, although the Ral-GEF pathway has the capacity to activate the fos promoter in PC12 cells, Ral-GEF activation makes only a small contribution to NGF regulation of the fos promoter.
Finally, fos promoter activation by Rgr expression was enhanced even further by NGF treatment (Fig. 5A and B), consistent with the idea that NGF uses additional pathways (i.e., via Raf) to activate fos. These data also indicate that although NGF can no longer promote neurite outgrowth in Rgr-expressing cells, it is still capable of signaling from the cell surface to regulatory elements in the c-fos gene.
We were interested in determining if Ral-GEF was signaling to fos functions through CDC42 or Rac GTPases. These GTPases seem to be involved in Ral-GEF effects on neurite outgrowth, and they are capable of stimulating the fos promoter when activated. However, expression of dominant negative CDC42 or Rac had little, if any, effect on Rgr-induced fos promoter activity, indicating that some other signaling pathway from Ral-GEFs is involved in fos promoter activation (Fig. 5A).
NGF induces transient activation of Ral in PC12 cells.
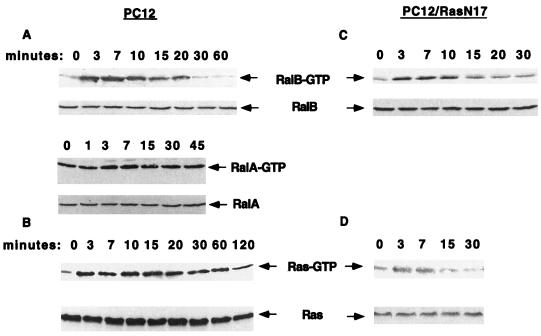
The results described above argue that signals emanating from Ral-GEFs have the potential to inhibit the differentiation of PC12 cells by antagonizing the actions of the Ras effectors, Raf and PI3 kinase. Yet NGF leads to neurite outgrowth in PC12 cells. Thus, we attempted to determine whether NGF does, in fact, stimulate a signaling pathway initiated by Ral-GEFs by measuring the activation state of Ral-GEF substrates RalA and RalB. A fragment of RalBP1 known to bind preferentially to GTP-Ral (3) was used as an affinity reagent to isolate and quantitate active Ral proteins from stimulated cells. This approach had already been used successfully to measure active Ral in platelets and fibroblasts (51, 52). Lysates of PC12 cells that had previously been stimulated for various times with NGF were exposed to a fusion protein of GST–Ral-binding domain of RalBP1 immobilized on glutathione agarose. After being washed, the bound proteins were immunoblotted with anti-RalA or -RalB antibodies. NGF treatment led to a rapid increase in the content of GTP-RalB, which peaked at ∼7 min, was maintained for ∼20 min, and then returned to baseline by 30 min (Fig. 6A). Total levels of RalB in cell lysates remained constant (Fig. 6A). NGF-induced changes in GTP-RalA were more modest and difficult to document convincingly, possibly because the basal level of GTP-RalA bound to RalBP1 beads was higher (Fig. 6A). The GTP-bound state of Ral proteins could have been affected by changes in the activity of signaling molecules other than Ral-GEFs, such as Ral-GAPs. Nevertheless, these results show that a signaling pathway that can be initiated by Ral-GEFs is activated upon NGF stimulation, although only transiently. Similar results were obtained when cells were treated with EGF (data not shown). A similar analysis was performed for Ras, using the Ras-binding domain of Raf and antibodies that recognize RasH, RasN, and RasK. As has been shown previously, NGF treatment rapidly activated Ras, but unlike Ral, Ras-GTP levels persisted for at least 2 h (Fig. 6B).
FIG. 6.
Effect of NGF on Ral-GTP levels in PC12 cells. PC12 cells or PC12-Ras17N cells were treated with NGF (50 ng/ml) for various times. The cells were lysed, and half of the lysates were incubated with glutathione agarose bound to the Ral-binding domain of RalBP1 expressed as a fusion protein with GST. The beads were washed and immunoblotted with anti-RalA or -RalB polyclonal antibodies. The other half of the cell lysates were incubated with glutathione beads bound to the Ras-binding domain of Raf expressed as a fusion protein with GST. The beads were washed and immunoblotted with anti-Ras antibody. Cell lysates from each time point were also directly immunoblotted with either anti-Ral or anti-Ras antibody to document that equal amounts of total GTPase were present in each sample. The data are representative of at least three experiments.
We attempted to determine whether NGF activation of RalB was dependent upon Ras activation by studying a commonly used PC12 cell line that constitutively expresses dominant negative Ras17N. This cell line fails to promote neurites in response to NGF treatment (44). Figure 6C shows that RalB still became activated by NGF treatment, although to a slightly lesser degree. In addition, the duration of RalB activation was reduced from 20 min in control cells to ∼10 min in Ras17N cells.
Because Ras17N expression had a partial effect on RalB activation, we investigated the extent of Ras inhibition in this cell line by measuring the content of Ras-GTP upon NGF stimulation, as described above. Figure 6D shows that the rise and duration of elevated Ras-GTP levels were clearly less than in control PC12 cells. However, Ras activation was not completely blocked in that a small but reproducible increase in Ras-GTP levels was observed for ∼7 min. Similar conclusions were reached when Erk activation by NGF treatment was assessed (data not shown). Thus, it is not certain that the elevation of GTP-Ral levels detected in Ras17N cells was truly Ras independent.
Nevertheless, it is clear from experiments on normal PC12 cells that Ral activity was not coupled to Ras activity during later periods (20 min to 2 h). This uncoupling of Ral from Ras may be necessary for NGF-induced neurite outgrowth, since we showed in Fig. 1 that constitutively elevated Ral-GEF activity suppressed NGF-induced differentiation of PC12 cells.
DISCUSSION
In PC12 cells, constitutive Ras activity promotes cell cycle arrest and differentiation into a neuronal phenotype. This phenotype is mediated in part by Ras-induced Raf activation, since constitutively activated versions of Raf can mimic, at least to some extent, the effects of activated Ras (53). Moreover, inhibition of the Ras-Raf-Erk pathway blocks stimulus-induced neurite outgrowth (40). Similar conclusions have been reached for Ras-induced PI3 kinase activity (16, 25). Remarkably, we have shown here that a third class of downstream Ras target, Ral-specific exchange factors, promotes the opposite effect. In particular, transfection of either a mutant Ras12V37G that preferentially activates Ral-GEFs over Raf or PI3 kinase, or an isolated catalytic domain of a Ral-GEF (Rgr), suppressed neurite outgrowth induced by NGF. Importantly, not all NGF signaling was blocked, since NGF treatment still enhanced fos promoter activation in Rgr-expressing cells. Rgr expression also suppressed neurite outgrowth promoted by a Ras mutant (12V35S) that preferentially activates Raf, documenting that Ral-GEF signaling antagonizes the action of other Ras effector pathways in this system.
Importantly, transfection of a dominant negative Ral28N mutant, which is thought to prevent the catalytic domain of all Ral-GEF family members from activating endogenous Ral proteins, enhanced NGF induction of neurite outgrowth. Again, this is the opposite of what is observed when Ras-induced Raf or PI3 kinase activities are blocked in PC12 cells. Moreover, not all signaling via NGF receptors was altered, because NGF induction of fos gene activation was affected little if at all in cells expressing Ral28N. Together, these findings show that one potential function of Ral-GEF signaling in PC12 cells is to suppress neurite outgrowth.
Since NGF induction of neurite outgrowth is associated with cell cycle arrest, a possible mechanism involved in Ral-GEF action could be the prevention of cell cycle exit. Alternatively, Ral-GEF activity could allow cell cycle arrest but inhibit cellular machinery involved in neurite outgrowth, such as the actin cytoskeleton. The latter mechanism is plausible because a putative Ral target protein, RalBP1, is a GAP for the actin-regulating CDC42 and Rac GTPases. Support for the first model came from the observation that changes in Ral-GEF activity dramatically affected the cell cycle in a manner consistent with observed effects on neurite outgrowth. For example, expression of Ras37G or the Ral-GEF Rgr suppressed cell cycle exit upon NGF stimulation. In some experiments, NGF stimulation was performed during serum starvation, so that Ral-GEF activity overcame two growth-suppressing signals. When Rgr was expressed in differentiated cells, existing neurites remained intact, suggesting that the primary effect of the Ral pathway in this system is to influence the formation of neurites rather than their maintenance. However, it is likely that Ral-GEF proteins play an additional role in fully differentiated neurons, since Ral GTPases are expressed at high levels at nerve endings (2).
Inhibition of endogenous Ral-GEFs by expression of dominant negative Ral28N had the opposite effect. It promoted cell cycle exit in growing populations of PC12 cells as efficiently as NGF treatment did. However, unlike NGF treatment, expression of Ral28N was not sufficient to promote neurite outgrowth. Ral28N expression also accelerated cell cycle exit induced by NGF treatment of cells growing in serum, which is consistent with its ability to enhance NGF-induced neurite outgrowth under these conditions. Serum starvation also accelerated cell cycle exit. Like Ral28N, serum starvation also was not sufficient to promote neurite outgrowth. This suggested that Ral28N expression and serum starvation might both work solely by enhancing cell cycle exit. However, Ral28N expression in serum-starved cells had little additive effect on cell cycle exit, yet it still dramatically enhanced neurite outgrowth induced by NGF (Fig. 2). Apparently, inhibition of Ral-GEF promotes neurite outgrowth by an additional mechanism.
To enhance Ral-GEF activity in cells, we transfected either a Ras mutant shown to preferentially activate Ral-GEFs or an isolated catalytic domain of the Ral-GEF Rgr (4). The only known function of this fragment of Rgr is Ral activation, so it is likely that some function of this GTPase is responsible for suppressing cell cycle arrest induced by NGF. However, we did not observe comparable inhibition (or enhancement) of NGF-induced neurite outgrowth by expression of constitutively activated Ral mutants (data not shown). The fact that a Ral-GEF displays stronger biological activity than a GTPase-deficient version of its target protein has also been observed in fibroblasts (36, 49, 50). In that system, Ral-GEF activity complements fos gene activation and growth-promoting functions of Raf more efficiently than activated Ral. The reasons for this unusual phenotype are not yet known. Thus, we cannot completely exclude the possibility that the catalytic domain of the Ral-GEF used here, and Ral-GDS, RGL, and Rlf used in the experiments on fibroblasts, actually activate a GTPase different from Ral.
Ral GTPases are capable of interacting with at least two classes of signal transduction molecules that could potentially account for the effects on PC12 cells observed here. One is a PLD, and the other is a GAP for Rac and CDC42 GTPase. Ral proteins can bind to PLD1 and augment its activation by the Arf GTPase (18, 27, 28). Another protein capable of activating PLD1 is the Rho GTPase (1, 30). Interestingly, like that of Ral-GEFs, Rho activity also inhibits neurite outgrowth, and suppression of Rho activity enhances neurite outgrowth. Thus, we investigated whether cooperative regulation of PLD1 by Rho and Ral might account for the similar phenotypes of the two GTPase pathways. However, our data do not support this model. First, transfection of a mutant Ral lacking the segment required for PLD binding did not enhance neurite outgrowth. This mutant has been shown to block tyrosine kinase activation of PLD in fibroblasts and would be expected to mimic the effects of Ral28N expression if PLD activation was involved (18). Second, neurites induced by expression of Ral28N were still sensitive to neurite retraction induced by LPA, which functions through Rho activation. Ral28N-induced neurites would be resistant to LPA if active Ral and Rho were both required to inhibit neurite outgrowth through activation of a common PLD. Finally, activation of Rho causes retraction of fully formed neurites, whereas expression of Rgr did not.
We did find evidence supporting a role for CDC42 and Rac in Ral-GEF action. These GTPases have been implicated in Ral function by the observation that the Ral target, RalBP1, is a CDC42 and Rac GAP and thus has the potential to negatively regulate these proteins. A simple hypothesis is that Ral-GEFs activate Ral and then Ral activates the GAP activity of RalBP1, possibly by targeting it to Ral-containing membranes, where a fraction of active CDC42 and Rac might exist (Fig. 7). This could explain, at least in part, the neurite-inhibitory effects of Ral-GEF overexpression, since suppression of CDC42 or Rac activity (by expression of dominant inhibitory mutants of these GTPases) has been shown previously (and here) to inhibit neurite outgrowth (26). In addition, we found that expression of these dominant negative mutants mimicked the action of Rgr by suppressing NGF-induced cell cycle exit. We also found that expression of constitutively activated CDC42 or Rac mutants, which are insensitive to GAP proteins, at least partially reversed Rgr action.
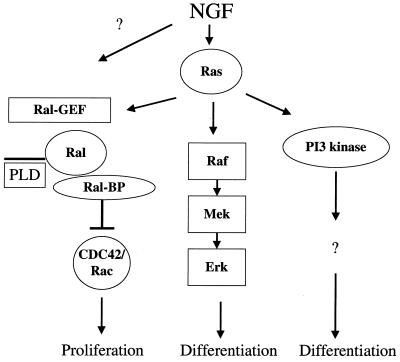
FIG. 7.
Ras effector pathways in NGF stimulated PC12 cells. Constitutive activation of Raf or PI3 kinase promotes cell cycle arrest and differentiation of PC12 cells into a neuronal phenotype. In contrast, constitutive activation of Ral-GEF promotes proliferation and blocks differentiation upon NGF stimulation. Of the two signaling molecules thought to be influenced by Ral-GEF, PLD and RalBP1, the latter appears to play a more important role in influencing neurite outgrowth in PC12 cells. NGF activates Ras and all three known Ras effector pathways. Ras and the Raf pathway are activated for hours, and this chronic activation is required for NGF-induced neurite outgrowth. In contrast, the Ral-GEF pathway (as assessed by the content of GTP-Ral) is activated by NGF for only ∼20 min. Thus, Ral-GEF signaling may function to delay the onset of cell cycle arrest and neurite outgrowth upon NGF stimulation. Dissociation of the Ral-GEF pathway from Ras activation may be necessary to allow NGF-induced differentiation of PC12 cells to occur.
Similarly, inhibition of Ral-GEF activity by Ral28N expression would be expected to potentiate CDC42 and Rac activity by suppressing RalBP1 activity (Fig. 7). This might explain some of the neurite-enhancing effects of Ral28N expression, since constitutively activated CDC42 and Rac have been shown here and previously by others to potentiate NGF-induced neurite outgrowth (26). Our observation that dominant negative CDC42 or Rac suppressed the effects of Ral28N on neurite outgrowth is also consistent with this model. Since RalBP1 has the capacity to bind to two highly related Eps homology domain proteins, Reps1 and POB (15, 55), these proteins may also be involved in regulating neurite outgrowth in PC12 cells.
We have shown that Ras12V37G and Rgr expression can transactivate a reporter construct containing the regulatory sequences of the c-fos gene. A similar result was observed previously in fibroblasts by transfection of Ras37G (50) or the Ral-GEFs Ral-GDS, RGL, and Rlf (33, 36, 50). Therefore, it is likely that Ral-GEF activity regulates PC12 cell function, at least in part, by altering gene expression patterns. However, discovery of the activation of the fos gene promoter in PC12 is not particularly revealing, since expression of Ras12V35S, which produces an effect which is the opposite of that of Rgr, also activates the fos promoter under the same conditions. Presumably, Ral-GEFs and Raf activate the fos promoter by different mechanisms and have the capacity to activate or inhibit different subsets of genes. Identification of Ral-GEF-specific alterations in gene expression may help reveal how these two signaling pathways induce opposing effects in PC12 cells.
Much attention has been focused on the Ras-induced Raf-Erk kinase cascade as a dominant growth-promoting branch of the Ras effector signaling system. However, the results reported here in PC12 cells, along with previous results in fibroblasts and thyrocytes, highlight novel features of the growth-promoting activity of the Ras-induced Ral-GEF signaling cascade. First, the Ral-GEF we used in these studies, Rgr, was isolated as an oncogene whose protein product is capable of causing 3T3 cells to form tumors in animals, despite their lack of focus-forming activity in tissue culture (4). Second, expression of a constitutively activated Rlf led to enhanced growth rates of 3T3 cells in culture (50). Third, thyroid-stimulating hormone promoted cell proliferation in thyrocytes through a Ras- but not Raf-dependent signaling pathway. Expression of Ras37G mimicked this effect, and Ral28N expression blocked it, suggesting that Ras-induced activation of Ral-GEFs was responsible (32). Finally, we show here that constitutive Ral-GEF activity promotes cell proliferation in NGF-treated PC12 cells while constitutive Raf and PI3 kinase activities promote cell cycle arrest and differentiation. Thus, elevated Ral-GEF activity can clearly promote cell proliferation. In fact, in the last two cell systems described above, cell proliferation is more tightly coupled to Ral-GEF activation than to Raf activation.
Recent studies document that Ral proteins can be activated by both Ras-dependent and Ras-independent pathways. Ras-dependent pathways are thought to be mediated by a family of Ral-GEFs that can bind to and be activated by Ras-GTP (11). The mechanism underlying Ras-independent Ral activation is not well understood beyond the fact that it can be initiated by elevated levels of calcium (14). We have shown here that NGF can lead to elevated levels of RalB and, to a lesser extent, RalA. We attempted to determine the Ras dependence of this event by using a PC12 cell line whose endogenous Ras exchange factors are blocked by expression of the dominant negative Ras17N mutant. However, we detected residual Ras activation upon NGF stimulation, which made firm conclusions difficult. Nevertheless, the data suggest that Ras-dependent and Ras-independent mechanisms exist. Ras dependence was suggested by the fact that the extent and duration of Ral activation was reduced in the mutant cell line. Ras-independent Ral activation was suggested by the fact that a large decrease in Ras activation led to only a small decrease in Ral activation. Clearly, additional studies will have to be employed to dissect what may be a complicated mechanism of Ral regulation.
An important conclusion from this study is that the ratio of Ral-GEF, Raf, and PI3 kinase activities can determine whether PC12 cells proliferate or differentiate, with Ral-GEF promoting the former and Raf and PI3 kinase promoting the latter. The Raf-Erk signaling pathway can also promote cell cycle arrest in other cell types, such as primary fibroblasts (42, 54), suggesting that this concept may be valid in other cell systems. The results reported here also show that the ratio of signals emanating from Raf and Ral-GEFs changes during PC12 cell stimulation. In particular, NGF activates both Erk and RalB acutely. However, Erk activation persists for hours, while Ral-GTP levels subside after ∼20 min. Thus, Ral activation likely contributes primarily to the early effects of NGF on PC12 cells, where it serves to delay the onset of cell cycle arrest and differentiation induced by NGF. The rapid inactivation of the Ral signaling pathway appears to be necessary to permit subsequent NGF-induced cell cycle arrest and differentiation. Consistent with this notion is our observation that EGF, which can enhance the proliferation of PC12 cells, also promotes Ral activation transiently.
Why Ral is unresponsive to Ras after ∼20 min of NGF stimulation remains to be determined. It is intriguing that recent results suggest that Raf also becomes uncoupled from Ras after acute activation in these cells (56). Sustained activation of Erk in response to NGF was reported to be due to NGF activation of the Ras-related GTPase Rap1, which in turn was shown to activate B-Raf. Interestingly, existing evidence indicates that Rap does not activate Ral-GEFs in most cell types (46, 52). It is tempting to speculate that Rap replaces Ras in activating Erk in the late stage of NGF signaling in PC12 cells, at least in part, to avoid sustained Ral-GEF activation.
ACKNOWLEDGMENTS
We thank A. Pellicer (N.Y.U. Medical School) for the Rgr clone, M. White (University of Texas, Southwestern Medical School) for the Ras effector mutants, and G. Cooper (Boston University) for the PC12-Ras17N cells. We also thank A. Polzin for helpful suggestions in preparing the manuscript.
The work was funded by a grant from NIGMS (no. GM47707).
REFERENCES
- 1.Bae C D, Min D S, Fleming I N, Exton J H. Determination of interaction sites on the small G protein RhoA for phospholipase D. J Biol Chem. 1998;273:11596–11604. doi: 10.1074/jbc.273.19.11596. [DOI] [PubMed] [Google Scholar]
- 2.Bielinski D F, Pyun N Y, Linko-Stentz K, Macara I, Fine R E. Ral and Rab3a are major GTP binding proteins of axonal rapid transport vesicles of synaptic vesicles and do not redistribute following depolarization stimulated synaptosomal exocytosis. Biochem Biophys Acta. 1993;1151:246–256. doi: 10.1016/0005-2736(93)90109-d. [DOI] [PubMed] [Google Scholar]
- 3.Cantor S, Urano T, Feig L A. Identification and characterization of RalBP1, a potential downstream target of Ral GTPases. Mol Cell Biol. 1995;15:4578–4584. doi: 10.1128/mcb.15.8.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Adamo D R, Novick S, Kahn J M, Leonardi P, Pellicer A. rsc: a novel oncogene with structural and functional homology with the gene family of exchange factors for Ral. Oncogene. 1997;14:1295–1305. doi: 10.1038/sj.onc.1200950. [DOI] [PubMed] [Google Scholar]
- 5.Dikic I, Schlessinger J, Lax I. PC12 cells overexpressing the insulin receptor undergo insulin-dependent neuronal differentiation. Curr Biol. 1994;4:702–708. doi: 10.1016/s0960-9822(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 6.Downward J. Ras signalling and apoptosis. Curr Opin Genet Dev. 1998;8:49–54. doi: 10.1016/s0959-437x(98)80061-0. [DOI] [PubMed] [Google Scholar]
- 7.Downward J. Role of phosphoinositide-3-OH kinase in Ras signaling. Adv Second Messenger Phosphoprotein Res. 1997;31:1–10. doi: 10.1016/s1040-7952(97)80004-3. [DOI] [PubMed] [Google Scholar]
- 8.Exton J H. New developments in phospholipase D. J Biol Chem. 1997;272:15579–15582. doi: 10.1074/jbc.272.25.15579. [DOI] [PubMed] [Google Scholar]
- 9.Farnsworth C L, Feig L A. Dominant inhibitory mutations in the Mg2+ binding site of Ras blocks its activation by GTP. Mol Cell Biol. 1991;11:4822–4829. doi: 10.1128/mcb.11.10.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feig L A, Cooper G M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feig L A, Urano T, Cantor S. Evidence for a Ras/Ral signaling cascade. Trends Biochem Sci. 1996;21:438–441. doi: 10.1016/s0968-0004(96)10058-x. [DOI] [PubMed] [Google Scholar]
- 12.Franza B R, Jr, Maruyama K, Garrels J I, Ruley H E. In vitro establishment is not a sufficient prerequisite for transformation by activated ras oncogenes. Cell. 1986;44:409–418. doi: 10.1016/0092-8674(86)90462-9. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller R D, Krishna U M, Falck J R, White M A, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 14.Hofer F, Berdeaux R, Martin G S. Ras-independent activation of Ral by a Ca(2+)-dependent pathway. Curr Biol. 1998;14:839–842. doi: 10.1016/s0960-9822(98)70327-6. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda M, Ishida O, Hinoi T, Kishida S, Kikuchi A. Identification and characterization of a novel protein interacting with Ral-binding protein 1, a putative effector protein of Ral. J Biol Chem. 1998;273:814–821. doi: 10.1074/jbc.273.2.814. [DOI] [PubMed] [Google Scholar]
- 16.Jackson T R, Blader I J, Hammonds-Odie L P, Burga C R, Cooke F, Hawkins P T, Wolf A G, Heldman K A, Gheibert A B. Initiation of NGF-stimulated neurite outgrowth requires activation of a phosphoinositide 3-kinase. J Cell Sci. 1996;109:289–300. doi: 10.1242/jcs.109.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalink K, van Corven E J, Hengeveld T, Morii N, Narumiya S, Moolenaar W H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang H, Luo J-Q, Urano T, Lu Z, Foster D A, Feig L A. Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature. 1995;378:409–412. doi: 10.1038/378409a0. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez C, et al. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. EMBO J. 1998;17:743–753. doi: 10.1093/emboj/17.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med. 1997;75:587–593. doi: 10.1007/s001090050143. [DOI] [PubMed] [Google Scholar]
- 21.Jullien-Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis J H. Bridging Ral GTPase to Rho pathways. J Biol Chem. 1995;270:22473–22477. doi: 10.1074/jbc.270.38.22473. [DOI] [PubMed] [Google Scholar]
- 22.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J H, Lee S D, Han J M, Lee T G, Kim Y, Park J B, Lambeth J D, Suh P G, Ryu S H. Activation of phospholipase D1 by direct interaction with ADP-ribosylation factor 1 and RalA. FEBS Lett. 1998;430:231–235. doi: 10.1016/s0014-5793(98)00661-9. [DOI] [PubMed] [Google Scholar]
- 24.Kishida S, Koyama S, Matsubara K, Kishida M, Matsuura Y, Kikuchi A. Colocalization of Ras and Ral on the membrane is required for Ras-dependent Ral activation through Ral GDP dissociation stimulator. Oncogene. 1997;15:2899–2907. doi: 10.1038/sj.onc.1201473. [DOI] [PubMed] [Google Scholar]
- 25.Kita Y, et al. Microinjection of activated phosphatidylinositol-3 kinase induces process outgrowth in rat PC12 cells through the rac-JNK signal transduction pathway. J Cell Sci. 1998;111:907–915. doi: 10.1242/jcs.111.7.907. [DOI] [PubMed] [Google Scholar]
- 26.Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J-Q, Liu X, Hammond S M, Colley W C, Feig L A, Frohman M A, Morris A J, Foster D A. RalA interacts directly with the Arf-responsive, PIP2 dependent phospholipase D1. Biochem Biophys Res Commun. 1997;235:854–859. doi: 10.1006/bbrc.1997.6793. [DOI] [PubMed] [Google Scholar]
- 28.Luo J Q, Liu X, Frankel P, Rotunda T, Ramos M, Flom J, Jiang H, Feig L A, Morris A J, Kahn R A, Foster D A. Functional association between Arf and RalA in active phospholipase D complex. Proc Natl Acad Sci USA. 1998;95:3632–3637. doi: 10.1073/pnas.95.7.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macara I G, Lounsbury K M, Richards S A, McKiernan C, Bar-Sagi D. The Ras superfamily of GTPases. FASEB J. 1996;10:625–630. doi: 10.1096/fasebj.10.5.8621061. [DOI] [PubMed] [Google Scholar]
- 30.Malcolm K C, Elliott C M, Exton J H. Evidence for Rho-mediated agonist stimulation of phospholipase D in rat1 fibroblasts. Effects of Clostridium botulinum C3 exoenzyme. J Biol Chem. 1996;271:13135–13139. doi: 10.1074/jbc.271.22.13135. [DOI] [PubMed] [Google Scholar]
- 31.Marais R, Marshall C J. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- 32.Miller M J, Prigent S, Kupperman E, Rioux L, Park S H, Feramisco J R, White M A, Rutkowski J L, Meinkoth J L. RalGDS functions in Ras- and cAMP-mediated growth stimulation. J Biol Chem. 1997;272:5600–5605. doi: 10.1074/jbc.272.9.5600. [DOI] [PubMed] [Google Scholar]
- 33.Murai H, Ikeda M, Kishida S, Ishida O, Okazaki-Kishida M, Matsuura Y, Kikuchi A. Characterization of Ral GDP dissociation stimulator-like (RGL) activities to regulate c-fos promoter and the GDP/GTP exchange of Ral. J Biol Chem. 1997;272:10483–10490. doi: 10.1074/jbc.272.16.10483. [DOI] [PubMed] [Google Scholar]
- 34.Nimnual A S, Yatsula B A, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 35.Nobes C D, Hall A. Rho, rac and cdc42 GTPases: regulators of actin structures, cell adhesion and motility. Biochem Soc Trans. 1995;23:456–459. doi: 10.1042/bst0230456. [DOI] [PubMed] [Google Scholar]
- 36.Okazaki M, Kishida S, Hinoi T, Hasegawa T, Tamada M, Kataoka T, Kikuchi A. Synergistic activation of c-fos promoter activity by Raf and Ral-GDP dissociation stimulator. Oncogene. 1997;14:515–521. doi: 10.1038/sj.onc.1200860. [DOI] [PubMed] [Google Scholar]
- 37.Park S H, Weinberg R A. A putative effector of Ral has homology to Rho/Rac GTPase activating proteins. Oncogene. 1995;11:2349–2355. [PubMed] [Google Scholar]
- 38.Robbins D J, Cheng M, Zhen E, Vanderbilt C A, Feig L A, Cobb M H. Evidence for a Ras-dependent extracellular signal-regulated protein kinase (ERK) cascade. Proc Natl Acad Sci USA. 1992;89:6924–6928. doi: 10.1073/pnas.89.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Viciana P, Warne P H, Khwaua A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 40.Sano M, Kitajima S. Activation of mitogen-activated protein kinases is not required for the extension of neurites from PC12D cells triggered by nerve growth factor. Brain Res. 1998;785:299–308. doi: 10.1016/s0006-8993(97)01403-0. [DOI] [PubMed] [Google Scholar]
- 41.Schweighoffer F, Cai H, Chevallier-Multon M C, Fath I, Cooper G M, Tocque B. The Saccharomyces cerevisiae SDC25 C-domain gene product overcomes the dominant inhibitory activity of Ha-Ras Asn-17. Mol Cell Biol. 1993;13:39–43. doi: 10.1128/mcb.13.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sewing A, Wiseman B, Lloyd A C, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5588–5597. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun H, Tonks N K, Bar-Sagi D. Inhibition of Ras-induced DNA synthesis by expression of the phosphatase MKP-1. Science. 1994;266:285–288. doi: 10.1126/science.7939666. [DOI] [PubMed] [Google Scholar]
- 44.Szeberenyi J, Cai H, Cooper G M. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990;10:5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traverse S, Seedorf K, Paterson H, Marshall C J, Cohen P, Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994;4:694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 46.Urano T, Emkey R, Feig L A. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;16:810–816. [PMC free article] [PubMed] [Google Scholar]
- 47.van Grunsven L A, Thomas A, Urdiales J L, Machenaud S, Choler P, Durand I, Rudkin B B. Nerve growth factor-induced accumulation of PC12 cells expressing cyclin D1: evidence for a G1 phase block. Oncogene. 1996;12:855–862. [PubMed] [Google Scholar]
- 48.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 49.White M A, Vale T, Camonis J H, Schaefer E, Wigler M H. A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J Biol Chem. 1996;271:16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- 50.Wolthuis R M, de Ruiter N D, Cool R H, Bos J L. Stimulation of gene induction and cell growth by the Ras effector Rlf. EMBO J. 1997;16:6748–6761. doi: 10.1093/emboj/16.22.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolthuis R M, Franke B, van Triest M, Bauer B, Cool R H, Camonis J H, Akkerman J W, Bos J L. Activation of the small GTPase Ral in platelets. Mol Cell Biol. 1998;18:2486–2491. doi: 10.1128/mcb.18.5.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolthuis R M, Zwartkruis F, Moen T C, Bos J L. Ras-dependent activation of the small GTPase Ral. Curr Biol. 1998;8:471–474. doi: 10.1016/s0960-9822(98)70183-6. [DOI] [PubMed] [Google Scholar]
- 53.Wood K W, Sarnecki C, Roberts T M, Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992;68:1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 54.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi A, Urano T, Goi T, Feig L A. An Eps homology (EH) domain protein that binds to the Ral-GTPase target, RalBP1. J Biol Chem. 1997;272:31230–31234. doi: 10.1074/jbc.272.50.31230. [DOI] [PubMed] [Google Scholar]
- 56.York R D, Yao H, Dillon T, Ellig C L, Eckert S P, McCleskey E W, Stork P J. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]