Abstract
We aimed to compare the mortality and comfort associated with high-flow nasal cannula oxygenation (HFNCO) and high-concentration mask (HCM) in older SARS-CoV-2 infected patients who were hospitalized in non-intensive care units. In this retrospective cohort study, we included all consecutive patients aged 75 years and older who were hospitalized for acute respiratory failure (ARF) in either an acute geriatric unit or an acute pulmonary care unit, and tested positive for SARS-CoV-2. We compared the in-hospital prognosis between patients treated with HFNCO and patients treated with HCM. To account for confounders, we created a propensity score for HFNCO, and stabilizing inverse probability of treatment weighting (SIPTW) was applied. From March 2020 to January 2021, 67 patients (median age 87 years, 41 men) were hospitalized with SARS-CoV-2-related ARF, of whom 41 (61%) received HFNCO and 26 (39%) did not. Age and comorbidities did not significantly differ in the two groups, whereas clinical presentation was more severe in the HFNCO group (NEW2 score: 8 (5–11) vs. 7 (5–8), p = 0.02, and Sp02/Fi02: 88 (98–120) vs. 117 (114–148), p = 0.03). Seven (17%) vs. two (5%) patients survived at 30 days in the HFNCO and HCM group, respectively. Overall, after SIPTW, HFNCO was significantly associated with greater survival (adjusted hazard ratio (AHR) 0.57, 95% CI 0.33–0.99; p = 0.04). HFNCO use was associated with a lower need for morphine (AHR 0.39, 95% CI 0.21–0.71; p = 0.005), but not for midazolam (AHR 0.66, 95% CI 0.37–1.19; p = 0.17). In conclusion, HFNCO use in non-intensive care units may reduce mortality and discomfort in older inpatients with SARS-CoV-2-related ARF.
Keywords: COVID-19, pneumonia, coronavirus, comfort, mortality
1. Introduction
SARS-CoV-2 has infected millions of individuals worldwide, but its burden has been particularly heavy in the older population. Nearly one-third of older patients hospitalized with SARS-CoV-2 pneumonia die in hospital [1,2]. Most deaths are the result of acute respiratory failure (ARF) linked to viral pneumonia, for which optimal therapeutic management is still a matter of debate. Many of these patients are admitted to intensive care units (ICUs) because they require mechanical ventilation. Older comorbid patients are disproportionately affected and at a much higher risk of death. However, they are frequently refused ICU access, especially in the current context of scarce resources [3]. Older patients with ARF are thus frequently hospitalized outside the ICU, requiring alternatives to tracheal intubation. In this context, aside from drug therapies, high-flow nasal cannula oxygenation (HFNCO) was suggested as a promising non-invasive tool for SARS-CoV-2-related ARF [4].
HFNCO, delivering up to 60 L/min of oxygen, is a well-documented device in the supportive care of hospitalized patients with ARF, improving pre-oxygenation when intubation is needed and reducing mortality [5,6]. However, side effects have been reported (nasal bridge ulceration, pneumothorax, epistaxis) [7], though they have only been partially assessed in frail older adults. These side effects are comparable with those under conventional oxygen devices: mask discomfort, nasal, and oral dryness, eye irritation, nasal and eye trauma, bronchoconstriction, and gastric distention [8]. Few studies have focused on the impact of HFNCO in older patients [9,10,11], and, to the best of our knowledge, only one of them studied the impact of HFNCO during SARS-CoV-2 ARF [10]. However, this study was observational with no comparison group.
The absence of evidence for SARS-CoV-2 ARF management in frail older populations and the disparities in available care resources worldwide have led to a disconcerting heterogeneity of practices. There is an urgent need for specific data in older patients with this life-threatening and now frequent condition.
Since March 2020, older patients admitted to our hospital in the acute geriatric unit (AGU) or the acute pulmonary care unit (APCU) for SARS-CoV-2-related ARF have been treated either with a high-concentration mask (HCM) or with HFNCO, depending on their oxygen needs and symptoms, as well as equipment availability.
In this study, we aimed to investigate the impact of HFNCO compared to high-concentration mask (HCM) oxygen therapy on the survival and comfort of patients hospitalized for SARS-CoV-2-related ARF outside the ICU.
2. Materials and Methods
2.1. Study Design
We performed an observational retrospective study using hospital records from the AGU and APCU of a French 1800-bed University Hospital. Patients were admitted during the first two waves of the pandemic from March 2020 to January 2021. Participants (n = 67) were categorized into two groups, either receiving HFNCO (n = 41) or receiving HCM (n = 26). In-hospital 30-day survival was the primary outcome.
To investigate the comfort of patients undergoing HFNCO, we considered several secondary outcomes. Morphine is used to relieve the symptoms of dyspnea and enhance comfort in ARF [12]; therefore, we aimed to evaluate the impact on dyspnea by comparing the morphine prescription between the two groups.
Midazolam is used in our units for sedation of terminally ill patients [13], as stated in French Law, but also and more frequently, at lower titration, to relieve the symptoms of anxiety [14], which are especially frequent in ARF patients. The association of HFNCO with anxiety and restlessness was thus evaluated by comparing the prescription of midazolam between the two groups.
2.2. Patients
We included all consecutive patients aged 75 years or older hospitalized for SARS-CoV-2 ARF in the AGU or the APCU of our hospital.
ARF was defined as having a respiratory rate superior to 30 breaths per minute, labored or paradoxical breathing, signs of hypercapnia, or difficulty talking [15]. SARS-CoV-2 infection confirmed by a positive real-time polymerase chain reaction (RT-PCR) was mandatory. Patients were excluded if invasive ventilation or exclusive palliative care were decided before HFNCO/HMC or if there was a contraindication to HFNCO (consciousness disorder, claustrophobia, upper airway obstruction, facial trauma or deformity, abundant sputum, or emesis) [16]. Patients who were admitted to the ICU during their hospital stay were also not included.
2.3. Data Collection
The data were collected through a manual review of each participant’s medical record. Demographic data, site of SARS-CoV-2 pneumonia acquisition (i.e., community acquired or nursing-home acquired pneumonia), medical history, clinical, biological features, and acute treatment at baseline (i.e., ARF onset) were extracted from electronic or handwritten medical records. The Charlson comorbidity index was computed retrospectively using patient medical history [17]. The NEW2 prognostic score [18], which was developed to predict in-hospital mortality using 5 clinical variables (respiration rate, oxygen saturation, systolic blood pressure, pulse rate, and level of consciousness or new confusion) and the WHO severity scale [19], specifically dedicated to COVID-19 (S1: no pneumonia; S2: pneumonia, with SpO2 ≥ 90% on room air; S3: severe pneumonia, with respiratory rate > 30 breaths/min or SpO2 < 90% on room air; and S4: critical disease, with acute respiratory distress syndrome) were also calculated. To appreciate hypoxemia, we used the SpO2/FiO2 ratio, which has been broadly used during the COVID-19 pandemic [20], and collected respiratory rate, heart rate, and temperature. The degree of radiological damage (in percentage) on thoracic CT was obtained from standardized radiologist reports. For analysis purposes, the WHO severity scale and radiological damage on thoracic CT scan were computed as binary covariates, respectively, <S3 or more and <50% of radiological damage or more.
This study was strictly retrospective and observational, and as such, it did not require approval by an Institutional Review Board, in accordance with Article L1121-1 of the French Public Health Code (Law n°2012-300, dated 5 March 2012).
2.4. Statistical Analysis
2.4.1. Missing Values
A manual review was performed for all missing data. There were no missing data for the main outcome variables: comorbidity, RT-PCR date, admission date, or first administration of HMC and HFNCO. We considered missing values as missing at random, and 10 datasets were imputed using multiple imputation with the predictive mean matching method [21].
2.4.2. Description of Covariates
Continuous variables are described as medians (interquartile range (IQR)) for the continuous variables and the numbers (percentage) for categorical variables. The Wilcoxon rank test, or Fisher’s exact test and the log-rank test were computed to compare continuous, dichotomized, and survival variables, respectively.
2.4.3. Propensity Score
Older patients who are put on HFNCO often have a more severe presentation than patients with HCM. To account for potential confounders relative to the association of HFNCO use and mortality, we created a propensity score for each patient and applied stabilized inverse probability weighting (SIPW) [22]. To do so, we first computed a non-parsimonious logistic regression where HFNCO was the explanatory variable. The covariates were selected based on their influence on HFNCO according to the literature and the differences in baseline characteristics [23]. The balance between the HCM and HFNCO groups were verified graphically for covariates.
To adjust for confounders, we computed SIPW-adjusted Kaplan–Meier curves for in-hospital 30-day survival, and morphine and midazolam prescription. The Cox proportional hazard regression was then weighted on the SIPW to construct a marginal structural Cox model [24]. Statistical tests were two-tailed, and a p-value of <0.05 was considered significant.
The data management and statistical analyses were performed using Rstudio version 1.3.1073 (RStudio Team, PBC, Boston, MA, USA).
3. Results
3.1. Population
A total of 67 consecutive patients aged ≥75 years were included (37 in the AGU group and 30 in the APCU group). These patients were admitted to hospital for SARS-CoV-2-related ARF between 3 March 2020 and 27 January 2021.
The baseline characteristics are presented in Table 1. The median age was 86 years (range of 77–99 years; (IQR) 84–89 years). There was no significant difference in age between patients in the HCM and HFNCO groups.
Table 1.
Baseline characteristics (n (%) or median (interquartile range), unless stated otherwise).
| HCM n = 26 |
HFNCO n = 41 |
p | ||
|---|---|---|---|---|
| Demographics | ||||
| Acute geriatric unit | 23 (88) | 14 (34) | <0.001 | |
| Acute pulmonary care unit | 3 (12) | 27 (66) | ||
| Age (years) | 86 (84–89) | 87 (85–90) | 0.785 | |
| Men | 14 (54) | 27 (66) | 0.468 | |
| CAP | 13 (50) | 31(76) | 0.172 | |
| NHAP | 13 (50) | 10 (24) | ||
| Medical history | ||||
| High blood pressure | 20 (76.9) | 31 (75.6) | 1.000 | |
| Dyslipidemia | 7 (26.9) | 14 (34.1) | 0.726 | |
| Diabetes | 11 (42.3) | 14 (34.1) | 0.679 | |
| Atrial fibrillation | 10 (38.5) | 11 (26.9) | 0.465 | |
| Neurocognitive disorder | 14 (53.8) | 5 (12.2) | 0.001 | |
| Chronic kidney disease | 8 (30.8) | 11 (26.8) | 0.944 | |
| Chronic respiratory failure | 3 (11.5) | 3 (7.3) | 0.880 | |
| Charlson index (mean (SD)) | 2.81 (2.58) | 2.17 (2.01) | 0.262 | |
| Degree of radiological damage | ||||
| <10% | 1 (1.5) | 5 (7.4) | 0.884 | |
| 10–25% | 4 (5.9) | 7 (10.4) | ||
| 25–50% | 9 (13.4) | 12 (17.9) | ||
| 50–75% | 4 (5.9) | 9 (13.4) | ||
| >75% | 2 (2.9) | 3 (4.4) | ||
| Severity scores | ||||
| WHO severity score S1 | 1 (1.5) | 3 (4.4) | 0.650 | |
| WHO severity score S2 | 5 (7.4) | 4 (5.9) | ||
| WHO severity score S3 | 15 (22.4) | 24 (35.8) | ||
| WHO severity score S4 | 6 (8.9) | 9 (13.4) | ||
| NEW2 score | 8 (5.25–11) | 7 (5–8) | 0.016 | |
| Clinical examination | ||||
| Respiratory rate (bpm) | 31(24–35.75) | 26 (21.75–48) | 0.265 | |
| Heart rate (bpm) | 94.0 (73.25–100.0) | 86.5 (66.25–106.5) | 0.956 | |
| Temperature (°C) | 36.55 (36.08–37.65) | 36.95 (36.40–37.48) | 0.515 | |
| SpO2/FiO2 | 117 (114–148) | 88 (98–120) | 0.025 | |
| Biology | ||||
| Hemoglobin (g/dL) | 12.7 (11.4–13.7) | 12.2 (11.3–13.7) | 0.832 | |
| Leucocyte (/mm3) | 7.4 (5.8–12.6) | 8.0 (6.0–9.0) | 0.061 | |
| Neutrophils (/mm3) | 5.4 (4.4–10.4) | 6.0 (4.6–9.1) | 0.944 | |
| Lymphocyte (/mm3) | 0.71 (0.49–1.27) | 0.72 (0.44–1.06) | 0.152 | |
| Fibrinogen (g/L) | 6.2 (4.8–6.8) | 7.1 (5.2–7.9) | 0.316 | |
| Creatinine (µmol/L) | 108.5 (82.0–140.0) | 94 (77.0–144.0) | 0.276 | |
| C reactive protein (mg/L) | 118.0 (70.9–153.5) | 108.5 (64.4–190.5) | 0.813 | |
| Procalcitonin (µg/L) | 0.37 (0.16–8.66) | 0.59 (0.21–1.51) | 0.053 | |
| Lactate (g/L) | 2.2 (1.3–3.1) | 1.9 (1.4–2.1) | 0.302 | |
| Albumin (mg/L) | 27 (23–33) | 24 (22–27) | 0.049 | |
| Acute management | ||||
| Corticosteroids | 15 (60) | 37 (90) | 0.009 | |
| Antibiotics | 23 (92) | 38 (93) | 1.000 | |
HCM, high-concentration mask; HFNCO, high-flow nasal cannula oxygenation; CAP, community-acquired pneumonia; NHAP, nursing-home-acquired pneumonia; WHO severity index, world health organization severity index [19]; ARF, acute respiratory failure; NEWS2, National Early Warning score [18]; SD, standard deviation.
The type of hospital unit differed significantly between the two groups (27 patients (66%) received HFNCO in the APCU vs. 14 (34%) in the AGU p < 0.001). When compared to AGU patients, APCU patients were more frequently men (23 (77%) vs. 18 patients (46%), p = 0.037) and lived less frequently in nursing homes (5 (17%) vs. 19 patients (51%), p = 0.025). The corticosteroids were more often administered at baseline in the APCU (29 patients (96%) vs. 23 (64%) p = 0.003) and there was a trend toward better in-hospital survival (seven patients (33%) in APCU vs. two patients (5%) in AGU, p = 0.08).
A comparison of HCM and HFNCO patients showed that there was no significant difference in comorbidities except for neurocognitive disorders, which were less frequent in the HFNCO group (5 patients (12%) vs. 14 patients (54%) in the HCM group, p < 0.001).
Although the median NEW2 score was lower in the HFNCO group (8 (5–11) vs. 7 (5–8) in the HCM group p = 0.016), the WHO severity score did not differ significantly between the groups: 34 patients (83%) in the HFNCO group had a WHO severity score vs. 14 (76%) patients in the HCM group, p = 0.7.
The baseline respiratory rate was elevated in both groups (26 (21–35) per minute vs. 31 (24–36) per minute, p = 0.3). However, the median SpO2/FiO2 was lower in the HFNCO group (88 (98–120) vs. 117 (114–148) in the HCM group, p = 0.03).
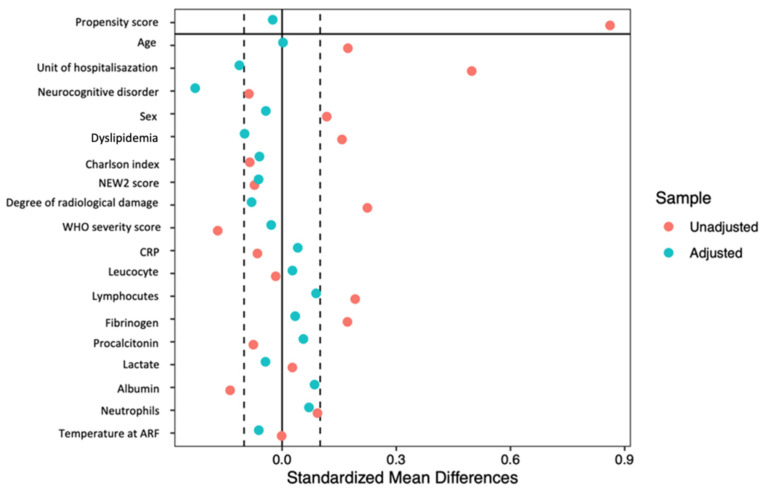
After SIPTW, the differences in the baseline covariates that were apparent in the overall sample (including unit of hospitalization) were no longer present (Figure 1).
Figure 1.
Unadjusted and stabilized inverse probability weighting-adjusted standard mean difference of covariates at baseline.
3.2. Outcomes
Overall, seven (17%) patients in the HFNCO were alive at 30 days vs. only two (5%) patients in the HCM group. The in-hospital mortality was related to COVID-19 respiratory failure in all cases. During the hospital stay, 49 (73%) patients received morphine (26 (68%) in the HFNCO group vs. 23 (92%) in the HCM group), with a median time before introduction of 4 (1–31) days, and 46 (69%) received midazolam (26 (67%) in the HFNCO group vs. 20 (80%) in the HCM group), with a median time to introduction of 6 (2–31) days.
The associations between HFNCO and the main and secondary outcomes, before and after SIPW, are presented in Table 2. The in-hospital 30-day survival (HR 0.57 (95% CI 0.33–0.99), p = 0.04) and time to morphine introduction (HR 0.39 (95% CI 0.21–0.71), p = 0.002) were significantly associated with HFNCO after SIPW. The time to midazolam introduction (HR 0.66 (95% CI 0.37–1.19), p = 0.17) did not significantly differ between the two groups. After stratification by hospital unit, the weighted HRs for in-hospital mortality were similar in the AGU (HR 0.62 (95% CI 0.25–1.10), p = 0.09) and APCU (HR 0.43 (95% CI 0.13–1.37), p = 0.15).
Table 2.
Association between high-flow nasal cannula oxygenation (HFNCO) and in-hospital outcome in SARS-CoV-2-related acute respiratory failure, before and after adjustment for confounders by stabilized inverse probability weighting.
| Crude Hazard Ratio | Weighted Hazard Ratio | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p | HR (95% CI) | p |
| Survival at 30 days | 0.58 (0.34–0.99) | 0.04 | 0.57 (0.33–0.99) | 0.04 |
| Morphine introduction | 0.40 (0.23–0.70) | 0.002 | 0.39 (0.21–0.71) | 0.002 |
| Midazolam introduction | 0.71 (0.40–1.28) | 0.26 | 0.66 (0.37–1.19) | 0.17 |
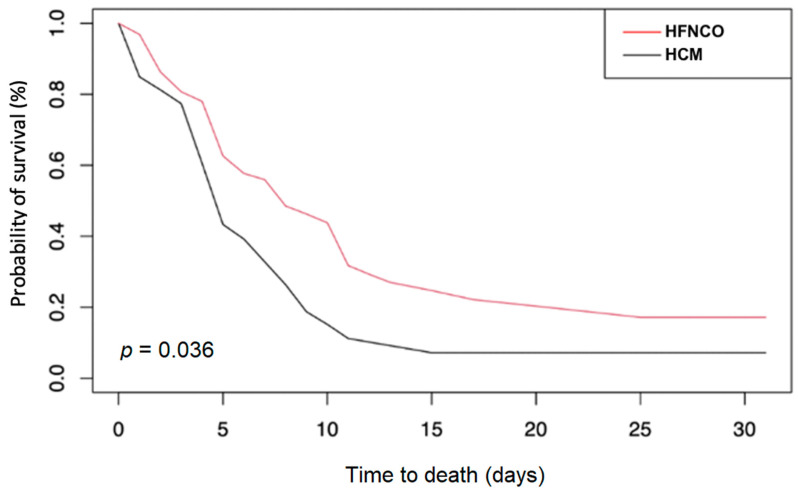
The SIPW-adjusted Kaplan–Meier curve for in-hospital survival is presented in Figure 2. Although a majority of patients died within 10 days in both groups, HFNCO was associated with higher 30-day survival (p = 0.036).
Figure 2.
SIPW-adjusted Kaplan–Meier curves of 30 day mortality in older patients with high-flow nasal cannula oxygenation (HFNCO) versus high-concentration mask (HCM).
4. Discussion
In this study of older patients hospitalized outside the ICU for SARS-CoV-2-related ARF, only 13% survived at 30 days, with most of the deaths occurring within the 10 days following the first positive SARS-CoV-2 RT-PCR result. In the majority of patients, respiratory discomfort during the hospital stay required morphine introduction, and midazolam was administered for sedation/anxiolysis. Our observational data suggest that HFNCO may improve the prognosis as mortality was reduced by nearly half.
Although several authors have already described the poor prognosis of older patients with SARS-CoV-2 ARF [2,25], to the best of our knowledge, this is the first study focusing on the use of HFNCO in older adults. These findings are of particular importance for patients refused ICU admission, an ethically problematic situation that occurred frequently during the pandemic due to ICU congestion following the extreme variations in incidence rates [3].
In situations with such a poor prognosis, acute care should focus at least as much on patient quality of life as on survival. Therefore, we aimed not only to assess whether HFNCO improved mortality but also if it was associated with reduced respiratory discomfort. Of note, mortality was related to COVID-19 respiratory failure in all cases. No side effects of oxygen device were reported in either group. Our results suggest that HFNCO tends to reduce the morphine requirement, a surrogate marker of discomfort in this study. Several studies evaluating the impact of HFNCO on comfort [26,27,28], comparing dryness of the ENT area [26] or visual analogic scale ratings [27], obtained contradictory results, and none of them focused on older patients. To the best of our knowledge, this study is also the first to investigate the impact of HFNCO on COVID-19-infected patients.
Midazolam is frequently used for anxiety or sedation purposes in older patients with ARF; however, its use did not significantly differ between the HFNCO and HCM groups. The high level of technical care, lack of family presence and isolation, and fear of death in those conditions lead to extreme levels of anxiety in our patients [29]. Anxiety cannot be curbed using ventilation alone, and treatments such as midazolam are often required. Moreover, midazolam is used at higher doses for another purpose in our units, namely sedation until death in terminally ill patients in cases of care-resistant distress [30], as specified by French Law.
Most of the reports concerning SARS-CoV-2 ARF are obtained from ICUs, but older patients were often denied access to such settings. One of the strengths of this study is the use of non-ICU data, so these results can be extrapolated to most medical units admitting older patients with severe COVID-19.
We acknowledge that there are some limitations to our work. Although this study included all consecutive patients suffering from SARS-CoV-2-related ARF, we were able to include only 67 patients aged ≥75 years. Nevertheless, this limited number of patients was sufficient to highlight a significant difference in both mortality and morphine consumption. Second, the patients tended to have a more severe presentation in the APCU than in the AGU. However, the propensity score method enabled us to take into account the main confounders, including neurocognitive disorders and hospitalization unit, the two main features that differed between the groups. Despite an adjustment for confounders, we cannot exclude that patients from the two groups had different prognoses at the baseline, given the observational design. However, an observational and pragmatic approach was preferred to a randomized trial, which would have raised serious feasibility and ethical issues. Given the retrospective design and the limited size, these preliminary results need to be confirmed.
5. Conclusions
Ensuring patient comfort and weighing the benefit–risk balance are major parts of a clinician’s work when faced with SARS-CoV-2-related ARF in older patients who are not able to access an ICU. High-flow nasal cannula oxygenation can help decrease mortality in older patients suffering from this dramatic clinical situation, while reducing discomfort with a lower morphine requirement. Further studies need to be performed on a larger scale to confirm these results.
Acknowledgments
We thank Suzanne Rankin for reviewing the English in the manuscript.
Author Contributions
Conceptualization, A.P. and A.H.; methodology, A.P. and A.H.; software, A.H.; validation, A.P., P.M., M.G. and P.B.; formal analysis, A.H.; investigation, A.H., M.P. and G.B.; resources, A.H. and M.P.; data curation, G.B.; writing—original draft preparation, A.H.; writing—review and editing, A.P., P.M., M.P., G.B., P.B. and M.G.; visualization, A.P.; supervision, A.P.; project administration, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. This study was strictly retrospective and observational; as such, it did not required approval by an Institutional Review Board, in accordance with Article L1121-1 of the French Public Health Code (Law no. 2012-300, dated 5 March 2012).
Informed Consent Statement
Patient consent was waived due to the retrospective design of this study and the use of anonymized data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zerah L., Baudouin É., Pépin M., Mary M., Krypciak S., Bianco C., Roux S., Gross A., Toméo C., Lemarié N., et al. Clinical Characteristics and Outcomes of 821 Older Patients with SARS-Cov-2 Infection Admitted to Acute Care Geriatric Wards. J. Gerontol. Ser. A. 2021;76:e4–e12. doi: 10.1093/gerona/glaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendes A., Serratrice C., Herrmann F.R., Genton L., Périvier S., Scheffler M., Fassier T., Huber P., Jacques M.-C., Prendki V., et al. Predictors of In-Hospital Mortality in Older Patients with COVID-19: The COVIDAge Study. J. Am. Med. Dir. Assoc. 2020;21:1546–1554. doi: 10.1016/j.jamda.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrell T.W., Ferrante L.E., Brown T., Francis L., Widera E., Rhodes R., Rosen T., Hwang U., Witt L.J., Thothala N., et al. AGS Position Statement: Resource Allocation Strategies and Age-Related Considerations in the COVID-19 Era and Beyond. J. Am. Geriatr. Soc. 2020;68:1136–1142. doi: 10.1111/jgs.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco C., Facciolongo N., Tonelli R., Dongilli R., Vianello A., Pisani L., Scala R., Malerba M., Carlucci A., Negri E.A., et al. Feasibility and Clinical Impact of Out-of-ICU Noninvasive Respiratory Support in Patients with COVID-19-Related Pneumonia. Eur. Respir. J. 2020;56:5. doi: 10.1183/13993003.02130-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papazian L., Corley A., Hess D., Fraser J.F., Frat J.-P., Guitton C., Jaber S., Maggiore S.M., Nava S., Rello J., et al. Use of High-Flow Nasal Cannula Oxygenation in ICU Adults: A Narrative Review. Intensive Care Med. 2016;42:1336–1349. doi: 10.1007/s00134-016-4277-8. [DOI] [PubMed] [Google Scholar]
- 6.Mellado-Artigas R., Ferreyro B.L., Angriman F., Hernández-Sanz M., Arruti E., Torres A., Villar J., Brochard L., Ferrando C. High-Flow Nasal Oxygen in Patients with COVID-19-Associated Acute Respiratory Failure. Crit. Care. 2021;25:58. doi: 10.1186/s13054-021-03469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalewajska M., Feret W., Wojczyński Ł., Witkiewicz W., Wiśniewska M., Kotfis K. Spontaneous Pneumothorax in COVID-19 Patients Treated with High-Flow Nasal Cannula Outside the ICU: A Case Series. Int. J. Environ. Res. Public Health. 2021;18:2191. doi: 10.3390/ijerph18042191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura M. High-Flow Nasal Cannula Oxygen Therapy in Adults: Physiological Benefits, Indication, Clinical Benefits, and Adverse Effects. Respir. Care. 2016;61:529–541. doi: 10.4187/respcare.04577. [DOI] [PubMed] [Google Scholar]
- 9.Xu J., Yang X., Huang C., Zou X., Zhou T., Pan S., Yang L., Wu Y., Ouyang Y., Wang Y., et al. A Novel Risk-Stratification Models of the High-Flow Nasal Cannula Therapy in COVID-19 Patients with Hypoxemic Respiratory Failure. Front. Med. 2020;7:607821. doi: 10.3389/fmed.2020.607821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagier J.-C., Amrane S., Mailhe M., Gainnier M., Arlotto S., Gentile S., Raoult D. High-Flow Oxygen Therapy in Elderly Patients Infected with SARS-CoV2 with a Contraindication for Transfer to an Intensive Care Unit: A Preliminary Report. Int. J. Infect. Dis. 2021;108:1–3. doi: 10.1016/j.ijid.2021.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.Y., Kim H.A., Huh K., Hyun M., Rhee J.Y., Jang S., Kim J.Y., Peck K.R., Chang H.H. Risk Factors for Mortality and Respiratory Support in Elderly Patients Hospitalized with COVID-19 in Korea. J. Korean Med. Sci. 2020;35:e223. doi: 10.3346/jkms.2020.35.e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soffler M.I., Rose A., Hayes M.M., Banzett R., Schwartzstein R.M. Treatment of Acute Dyspnea with Morphine to Avert Respiratory Failure. Ann. Am. Thorac. Soc. 2017;14:584–588. doi: 10.1513/AnnalsATS.201611-922CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prommer E. Midazolam: An Essential Palliative Care Drug. Palliat. Care Soc. Pract. 2020;14:5527. doi: 10.1177/2632352419895527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young C.C., Prielipp R.C. Benzodiazepines in the Intensive Care Unit. Crit. Care Clin. 2001;17:843–862. doi: 10.1016/S0749-0704(05)70183-4. [DOI] [PubMed] [Google Scholar]
- 15.Hakim R., Watanabe-Tejada L., Sukhal S., Tulaimat A. Acute Respiratory Failure in Randomized Trials of Noninvasive Respiratory Support: A Systematic Review of Definitions, Patient Characteristics, and Criteria for Intubation. J. Crit. Care. 2020;57:141–147. doi: 10.1016/j.jcrc.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura M. High-Flow Nasal Cannula Oxygen Therapy in Adults. J. Intensive Care. 2015;3:15. doi: 10.1186/s40560-015-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic. Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Smith G.B., Prytherch D.R., Meredith P., Schmidt P.E., Featherstone P.I. The Ability of the National Early Warning Score (NEWS) to Discriminate Patients at Risk of Early Cardiac Arrest, Unanticipated Intensive Care Unit Admission, and Death. Resuscitation. 2013;84:465–470. doi: 10.1016/j.resuscitation.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Clinical Management of COVID-19. [(accessed on 6 November 2020)]; Available online: https://www.who.int/publications-detail-redirect/clinical-management-of-covid-19.
- 20.Rice T.W., Wheeler A.P., Bernard G.R., Hayden D.L., Schoenfeld D.A., Ware L.B. Comparison of the Sp o 2 /F Io 2 Ratio and the Pa o 2/F Io 2 Ratio in Patients with Acute Lung Injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 21.Rezvan P.H., Lee K.J., Simpson J.A. The Rise of Multiple Imputation: A Review of the Reporting and Implementation of the Method in Medical Research. BMC Med. Res. Methodol. 2015;15:30. doi: 10.1186/s12874-015-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole S.R., Hernán M.A. Adjusted Survival Curves with Inverse Probability Weights. Comput. Methods Programs Biomed. 2004;75:45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J.J.Y., Lee K.S., Ang L.W., Leo Y.S., Young B.E. Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected with COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Clin. Infect. Dis. 2020;71:2199–2206. doi: 10.1093/cid/ciaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S., Ross C., Raebel M.A., Shetterly S., Blanchette C., Smith D. Use of Stabilized Inverse Propensity Scores as Weights to Directly Estimate Relative Risk and Its Confidence Intervals. Value Health. 2010;13:273–277. doi: 10.1111/j.1524-4733.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L., He W., Yu X., Hu D., Bao M., Liu H., Zhou J., Jiang H. Coronavirus Disease 2019 in Elderly Patients: Characteristics and Prognostic Factors Based on 4-Week Follow-Up. J. Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuquemelle E., Pham T., Papon J.-F., Louis B., Danin P.-E., Brochard L. Heated and Humidified High-Flow Oxygen Therapy Reduces Discomfort during Hypoxemic Respiratory Failure. Respire Care. 2012;57:1571–1577. doi: 10.4187/respcare.01681. [DOI] [PubMed] [Google Scholar]
- 27.Frat J.-P., Thille A.W., Mercat A., Girault C., Ragot S., Perbet S., Prat G., Boulain T., Morawiec E., Cottereau A., et al. High-Flow Oxygen through Nasal Cannula in Acute Hypoxemic Respiratory Failure. N. Engl. J. Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 28.Lewis S.R., Baker P.E., Parker R., Smith A.F. High-Flow Nasal Cannulae for Respiratory Support in Adult Intensive Care Patients. Cochrane Database Syst. Rev. 2021;3:CD010172. doi: 10.1002/14651858.CD010172.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muntsant A., Giménez-Llort L. Impact of Social Isolation on the Behavioral, Functional Profiles, and Hippocampal Atrophy Asymmetry in Dementia in Times of Coronavirus Pandemic (COVID-19): A Translational Neuroscience Approach. Front. Psychiatry. 2020;11:572583. doi: 10.3389/fpsyt.2020.572583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaporowska-Stachowiak I., Szymański K., Oduah M.-T., Stachowiak-Szymczak K., Łuczak J., Sopata M. Midazolam: Safety of Use in Palliative Care: A Systematic Critical Review. Biomed. Pharm. 2019;114:108838. doi: 10.1016/j.biopha.2019.108838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available.