Abstract
Background: Intrahepatic cholangiocarcinoma (iCCA) accounts for about 10% of primary liver cancer. Surgery is the only potentially curative treatment. We report on our current series of 229 consecutive hepatic resections for iCCA, which is one of the largest Western single-center series published so far. Methods: Between January 2008 to December 2020, a total of 286 patients underwent 307 surgical explorations for intended liver resection of iCCA at our department. Data were analyzed with regard to (1) preoperative treatment of tumor, (2) operative details, (3) perioperative morbidity and mortality, (4) histopathology, (5) outcome measured by tumor recurrence, treatment of recurrence and survival and (6) prognostic factors for overall and disease-free survival. Results: the resectability rate was 74.6% (229/307). In total, 202 primary liver resections, 21 repeated, 5 re-repeated, and 1 re-re-repeated liver resections were performed. In primary liver resections there were 77% (155/202) major hepatectomies. In 39/202 (20%) of patients additional hepatic wedge resections and in 87/202 (43%) patients additional 119 other surgical procedures were performed next to hepatectomy. Surgical radicality in first liver resections was 166 R0-, 33 R1- and 1 R2-resection. Following the first liver resection, the calculated 1-, 3- and 5-year-survival is 80%, 39%, and 22% with a median survival of 25.8 months. Until the completion of data acquisition, tumors recurred in 123/202 (60.9%) patients after a median of 7.5 months (range 1–87.2 months) after resection. A multivariate cox regression revealed tumor size (p < 0.001), T stage (p < 0.001) and N stage (p = 0.003) as independent predictors for overall survival. N stage (p = 0.040), preoperative therapy (p = 0.005), T stage (p = 0.004), tumor size (p = 0.002) and M stage (p = 0.001) were independent predictors for recurrence-free survival. Conclusions: For complete surgical removal, often extended liver resection in combination with complex vascular or biliary reconstruction is required. However, despite aggressive surgery, tumor recurrence is frequent and long-term oncological results are poor. This indicated that surgery alone is unlikely to make great strides in improving prognosis of patients with iCCA, instead clearly suggesting that liver resection should be incorporated in multimodal treatment concepts.
Keywords: intrahepatic cholangiocarcinoma, cholangiocarcinoma, survival, liver resection, repeated liver resection
1. Introduction
Intrahepatic cholangiocarcinoma (iCCA), although less frequent than perihilar cholangiocarcinoma (pCCA) is the second most common primary liver tumor after hepatocellular carcinoma (HCC). It accounts for about 10% of primary liver malignancies but shows an increasing incidence in Western countries within the past decade [1]. Due to its intrahepatic and often peripheral localization, tumor related symptoms usually occur late in the course of the disease. Therefore, the majority of tumors are diagnosed in an already locally advanced or even metastatic stage when curative approaches are difficult. In general, the prognosis of patients with iCCA is poor with a reported median survival of about one year after diagnosis and a 5-year survival of about 10% only [2]. Liver resection is the standard of care if a potentially curative approach is intended [3,4,5,6,7]. However, due to the rarity of iCCA, data on liver resection are still limited as most series evaluating surgical therapy of iCCA are based on very small patient cohorts. In addition, in many series there is no clear differentiation between intra- and extra-hepatic cholangiocarcinoma, and outcome analysis is hampered by the fact that data have been collected over time periods exceeding one or even two decades. Due to ongoing progress in diagnostics, prognostication, and advances in liver surgery techniques as well as new multimodal treatment options, comparability of data is very limited.
Since 2008 we have adopted an aggressive surgical attitude in the treatment of iCCA. This study analyzes our temporary series of 229 consecutive resections for iCCA within the past thirteen years which is to the best of our knowledge one of the largest Western single-center series in the literature.
2. Materials and Methods
All patients undergoing surgical exploration and liver resections at our center are registered in a prospective institutional database. Patients who underwent liver surgery for intrahepatic cholangiocarcinoma (iCCA) from 2008 to 2020 were eligible for this analysis.
The diagnosis of iCCA was based on histology obtained either by pre- or intra-operative biopsy or by the resected specimen. Patients with hilar cholangiocarcinoma, gallbladder carcinoma, mixed hepatocellular/cholangiocarcinoma, or bile duct carcinoma not clearly attributable to the intrahepatic biliary tree as well as patients with severe parenchymal damage (cirrhosis, fibrosis > F2 or steatosis > 50%) were excluded from our analysis.
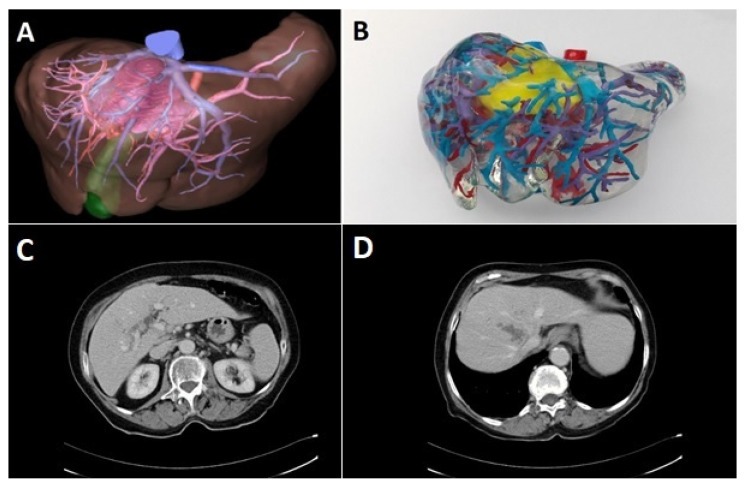
Preoperative diagnostic work-up included ultrasound of the abdomen and computed tomography (CT) of the abdomen and chest. Upper and lower gastrointestinal endoscopy was performed to exclude extrahepatic primary tumor in cases where the diagnosis of an iCCA was not made by biopsy. In selected cases, three-dimensional CT-scan of the liver including volumetry, virtual tumor resection, and computer-assisted risk analysis was performed prior to the resection. These were performed either by an external provider (MeVis Distant Services, MeVis AG, Bremen, Germany) or with a local reconstruction software (Synapse 3D, Fujifilm AG, Tokyo, Japan) by a trained surgical resident [8]. Since 2017, 3D-prints of the liver were performed on special request of the surgeon with non-flexible polyurethane rubber [9]. Especially in cases with anticipated complex vascular reconstructions, 3D-prints were ordered for preoperative planning (Figure 1).
Figure 1.
Preoperative 3D reconstruction as a PDF presentation (A) and 3D-print (B) with stained polyurethane rubber of the liver as well as CT-scan (C,D) in a 70-year-old patient with iCCA that infiltrated the right and middle hepatic vein and had contact to the left hepatic vein. Preoperative volumetric analysis of the segments 2/3 revealed a remnant volume of 563 mL. Resection was performed as an extended right-sided hemihepatectomy with hilar resection and reconstruction of the left portal vein and the medial of two branches of the left hepatic vein.
All surgical explorations and resections were performed by a team of experienced surgeons with special expertise in hepato-biliary surgery, and retrospectively classified according to the “New World” terminology [10]. Postoperatively, for at least two years, we conducted follow-up every three months; later on, the interval was increased to 6 months, if reasonable. Preferably, CT imaging was obtained at least every 6 months alternating with ultrasound examinations. For patients who were not able to undergo follow-up at our center, further information was retrieved from treating physicians.
The data collection was completed in February 2021. The data of the patients undergoing liver resection (n = 223) were further analyzed with regard to (1) preoperative treatment of tumor, (2) operative details, (3) perioperative morbidity and mortality, (4) pathologic findings, (5) outcome measured by tumor recurrence, treatment of recurrence and survival, and (6) prognostic factors for overall and disease-free survival. Surgical complications were assessed according to the Dindo–Clavien classification [11]. The TNM classification was performed according to the 8th edition of the classification of the Union for International Cancer Control (UICC) [12] (Table 1).
Table 1.
Definitions of the 8th edition of the UICC classification.
| T-Stage | N-Stage | UICC-Stage | |||
|---|---|---|---|---|---|
| T1a | Solitary tumor ≤5 cm without vascular invasion | N0 | No regional lymph node metastases | UICC Ia | T1a N0 M0 |
| T1b | Solitary tumor ≥5 cm without vascular invasion | UICC Ib | T1b N0 M0 | ||
| T2 | Solitary tumor with intrahepatic vascular invasion or multiple tumors, with or without vascular invasion | N1 | Regional lymph node metastasis present | UICC II | T2 N0 M0 |
| T3 | Tumor perforating the visceral peritoneum | Recommendation of harvesting of at least 6 lymph nodes | UICC IIIa | T3 N0 M0 | |
| T4 | Tumor with infiltration of local extrahepatic structures | UICC IIIb | T4 and/or N1, M0 | ||
| UICC IV | any T, any N, M1 | ||||
All patients signed an informed consent that allowed the data and follow-up to be collected anonymously and potentially used for scientific analysis. Abiding by the regulations of the federal state law (state hospital law §36 & §37) and according to the independent ethics committee of Rhineland-Palatinate, no ethical approval was necessary for this study.
Statistical Analyses
SPSS 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY, USA: IBM Corp.) was used to perform statistics. Categorical data was analyzed using the Chi2 test in cross tabulation. Survival analyses were conducted with the Kaplan Meier model and for comparison of factors influencing survival the log rank test was utilized. A p-value of <0.05 was considered significant. All analyses were an intention to treat, and no patients were excluded. Recurrence-free survival was defined according to Punt and colleagues [13].
3. Results
During the study period from January 2008 to December 2020, a total of 286 patients (137 female and 149 male) with a median age of 65 years (range: 28–84 years) underwent 307 surgical explorations for intended liver resection of iCCA at our department. This included 33 explorations for recurrent iCCA (parts of these data were already published in [14,15,16,17]).
3.1. Surgical Procedures and Intraoperative Data
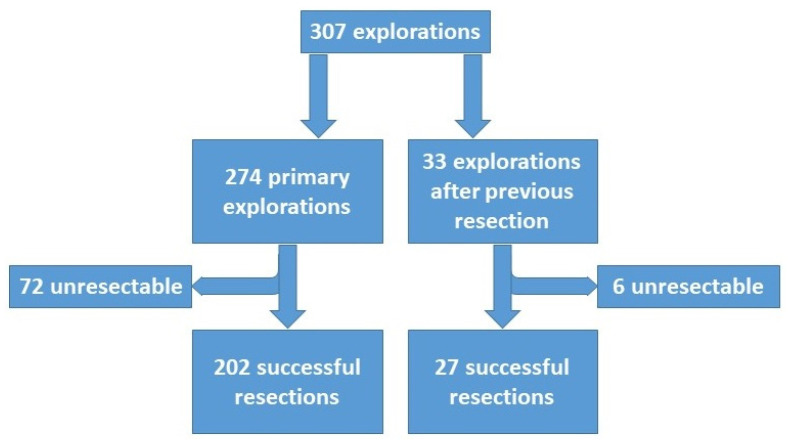
The overall resectability rate was 74.6% (229/307) for all explorations, 73.7% (202/274) in attempted first liver resection and 81.8% (27/33) in repeated resection (Figure 2). In total, 202 primary liver resections, 21 repeated, 5 re-repeated and 1 re-re-repeated liver resection were performed (Table 2). In primary liver resections, there were 77% (155/202) major hepatectomies (resection of three or more segments). In addition, the caudate lobe was removed in 53 patients, and in another 39 patients, additional atypical resections were performed to remove satellite lesions. In 87/202 (43%) patients, 119 additional surgical procedures were performed in addition to hepatectomy (Table 3; Figure 3e,f).
Figure 2.
Flowchart of all explorations. Further subdivision in primary and repeated explorations.
Table 2.
Operative Procedures in liver resections.
| Operative Procedures in 202 Primary Liver Resections | |||
| Primary Surgery | n = 202 | % | |
| Bisegmentectomy | 31 | 15.3 | |
| Monosegmentectomy | 14 | 6.9 | |
| Subsegmentectomy | 2 | 1 | |
| Resection of three liver segments | 13 | 6.4 | |
| Right hemihepatectomy | 27 | 13.4 | |
| Left hemihepatectomy | 31 | 15.3 | |
| Mesohepatectomy (≥three central segments) | 11 | 5.4 | |
| Right trisectionectomy | 33 | 16.3 | |
| Left trisectionectomy | 32 | 15.8 | |
| ALPPS | 8 | 4 | |
| Additional liver resections * | in 86 patients | ||
| Caudate lobectomy | 53 | ||
| Wedge resection | 39 | ||
| Operative Procedures in 27 Repeated Liver Resections | |||
| Repeated Resections | 1st Rep. Expl. | 2nd Rep. Expl. | 3rd Rep. Expl. |
| n = 21 | n = 5 | n = 1 | |
| Hemihepatectomy | 1 | - | - |
| Bisegmentectomy | 6 | 1 | - |
| Monosegmentectomy | 6 | 3 | - |
| Subsegmentectomy | 7 | 1 | 1 |
| Extrahepatic lymph node resection | 1 | - | - |
| Repeated exploration | 6 | - | - |
* Procedures performed in addition to the main surgical procedure. rep. expl. = repeated exploration.
Table 3.
Extensions of primary resection (n (%)).
| Visc/Vasc Extension | 87 (100) | |
| Visceral only | 43 (49.5) | |
| Vascular only | 27 (31) | |
| Both | 17 (19.5) | |
| Vascular Extension | Cases n = 44 * | Infiltration n (%) |
| Hepatic artery | 1 | 0 (-) |
| Portal vein | 16 | 6 (37.5) |
| Major hepatic vein | 22 | 4(18.2) |
| Vena cava inferior | 16 | 6 (37.5) |
| Visceral Extension | Cases n = 60 * | Infiltration n (%) |
| Diaphragm | 12 | 4 (33.3) |
| Adrenal gland | 5 | 2 (40) |
| Hilar bifurcation | 43 | 19 (44.2) |
| Pericardium | 1 | 1 (100) |
| Duodenum | 1 | 1 (100) |
| Colon | 1 | 1 (100) |
| Stomach | 1 | 1 (100) |
* Cases are the number of patients in which visceral or vascular extensions were performed. In some patients more than one extension was performed therefore the total number of extensions is unlike the number of cases.
Figure 3.
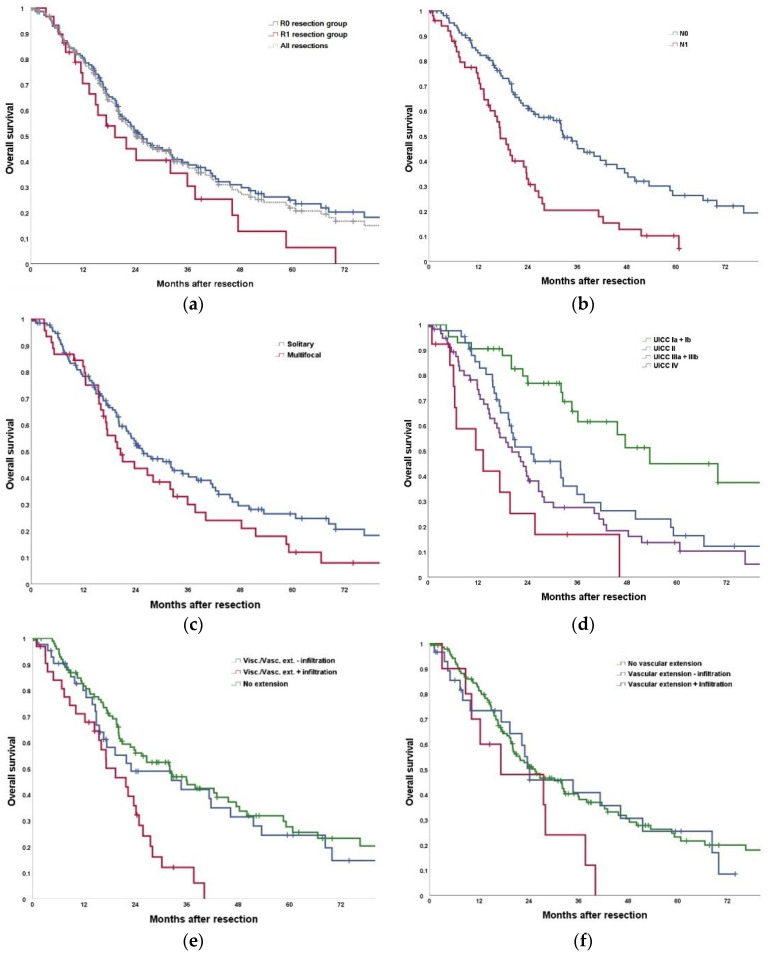
(a) Comparison of overall survival of patients with R0 versus R1 resection; p = 0.092. Additionally, combined depiction of the complete cohort. Perioperative deaths were excluded. (b) Comparison of overall survival of patients with N0 or N1 status; p < 0.001. Perioperative deaths were excluded. (c) Comparison of overall survival of patients with solitary versus multifocal iCCA; p = 0.144. Perioperative deaths were excluded. (d) Comparison of overall survival of different UICC groups; p < 0.001. Subgroup comparison: UICC I vs. UICC II p = 0.002; UICC I vs. UICC III p < 0.001; UICC I vs. UICC IV p < 0.001; UICC II vs. UICC III p = 0.252; UICC II vs. UICC IV p = 0.014; UICC III vs. UICC IV p = 101. Perioperative deaths and patients with Nx status were excluded. (e) Comparison of overall survival of patients with visceral and/or vascular extension (VVE) versus no extension; p < 0.001. Subgroup comparison: no extension vs. VVE − infiltration p = 0.465; no extension vs. VVE + infiltration p < 0.001; VVE − infiltration vs. VVE + infiltration p = 0.007. Perioperative deaths were excluded. (f) Comparison of overall survival of patients without vascular extension (VE), VE without (−) infiltration and VE with (+) infiltration; p = 0.163. Subgroup comparison: no VE vs. VE − infiltration p = 0.746; no VE vs. VE + infiltration p = 0.058; VE − infiltration vs. VE + infiltration p = 0.125. Perioperative deaths were excluded.
3.2. Preoperative Treatment
Preoperatively, endoscopic intraductal stents or percutaneous transhepatic cholangiodrainages to relieve jaundice had been placed in 10 and 3 patients, respectively. One patient underwent both procedures. Prior to the first liver resection, two patients had undergone loco-regional therapy for iCCA with chemoembolization (n = 1) or radiofrequency ablation (n = 1). In 18 patients with large iCCA considered to be irresectable, secondary resectability was achieved after chemotherapy and downsizing of tumors. Prior to repeated liver resection (n = 21), one patient was treated with chemotherapy. Thirteen patients had undergone their first liver resection in our department and eight patients in referring hospitals.
3.3. Morbidity and Mortality
After primary resection, a total of 131 complications (Clavien–Dindo grade I–IV) occurred in 80 of 202 patients (39.6%). The distribution of complications (highest complication only) is listed in Table 4. The overall 90-day-mortality rate was 7.9%.
Table 4.
Morbidity after primary resection.
| Morbidity | Primary Resection n = 202 |
|---|---|
| Bile leakage | 42 |
| Intraabdominal abscess | 20 |
| Portal vein thrombosis | 8 |
| Massive pleural effusion | 5 |
| Massive ascites | 10 |
| Wound infection | 17 |
| Pleural empyema | 1 |
| Ileus | 3 |
| Bile duct stenosis | 1 |
| Pneumonia | 11 |
| Bleeding | 9 |
| Cardial event | 5 |
| Highest Dindo–Clavien cl. | |
| No morbidity | 104 |
| Type I | 8 |
| Type II | 18 |
| Type IIIa | 35 |
| Type IIIb | 8 |
| Type IVa | 7 |
| Type IVb | 4 |
cl. = classification.
3.4. Postoperative Treatment
A total of 60 patients underwent adjuvant chemotherapy, most often with capecitabin (n = 44) followed by gemcitabine and cisplatin (n = 8). Another eight patients were included in the ACTICCA trial [18]. Two patients underwent postoperative radiation therapy.
3.5. Pathology
The median tumor diameter (in case of multifocal tumor the diameter of the largest nodule) was 7 cm (range 4–20 cm). Tumors were solitary in 150 patients (74.3%; Figure 3c). Overall, in the first liver resections, there were 166 R0 resections, 33 R1 resections and 1 R2 resection. In two cases, resection status was not determined (Rx) (Table 5; Figure 3a). Repeated resection resulted in 24 R0 resections and three R1 resections.
Table 5.
Histopathological staging after primary liver resection.
| n = 202 | |
|---|---|
| Solitary tumors | 150 |
| Multifocal tumors | 52 |
| Tumor size in cm * (median (range)) | 7 (4–20) |
| Lymph nodes harvested (median (range)) | 5 (0–31) |
| R-stage | |
| R0 | 166 |
| R1 | 33 |
| R2 | 1 |
| Rx | 2 |
| T-stage | |
| T1a | 34 |
| T1b | 51 |
| T2 | 76 |
| T3 | 15 |
| T4 | 26 |
| N-stage | |
| N0 | 123 |
| N1 | 58 |
| Nx | 21 |
| L-stage | |
| L0 | 168 |
| L1 | 34 |
| V-stage | |
| V0 | 155 |
| V1 | 43 |
| V2 | 4 |
| Pn-stage | |
| Pn0 | 144 |
| Pn1 | 58 |
| Grading | |
| G1 | 3 |
| G2 | 129 |
| G3 | 51 |
| G4 | 1 |
| No grading ** | 18 |
| Clinical M-stage | |
| cM0 | 188 |
| M1 | 14 |
| UICC-stage ‡ | |
| Ia | 23 |
| Ib | 31 |
| II | 47 |
| IIIa | 9 |
| IIIb | 58 |
| IV | 13 |
* Size of largest nodule, if multifocal; ** after application of preoperative chemotherapy; ‡ due to Nx status 21/202 patients were not classified.
Vascular infiltration was present in 16/202 patients and in 16/55 resected vessels, respectively. Infiltration of the vena cava and portal vein was found in 6/16 cases (37.5%) each, whereas there was infiltration of the vascular wall of only 4/22 (18%) of resected major liver veins. The only resected and reconstructed artery did not show tumor invasion (Table 3).
At primary liver resection, lymphadenectomy was performed in 89.6% (181/202) of cases. A median number of 5 lymph nodes was harvested (range 0–31). Lymph node metastasis were seen in 58/181 (32%) patients (Table 5; Figure 3b). In the repeated liver resection, no more lymphadenectomy was performed.
3.6. Survival, Recurrence, and Treatment of Recurrence
Following the first liver resection, the calculated 1-, 3- and 5-year-survival is 80%, 39%, and 22% with a median survival of 25.8 months (Figure 3a). Until the completion of data collection for this analysis, tumor recurred in 60.9% (123/202) of patients after a median of 7.5 months (range 1–87.2 months) after resection. The underlying tumor stages were UICC Ia (n = 6/22), Ib (n = 16/32), II (n = 36/47), IIIa (n = 4/9), IIIb (n = 40/58) and IV (n = 9/13); due to Nx UICC stage was not assessable in 12/21 patients (Figure 3d). The initial site of recurrence was intrahepatic only in 54 (44%), extrahepatic only in 28 (23%) and both intra- and extra-hepatic in 41 (33%) patients. Treatment of recurrence consisted of repeated hepatectomy (n = 13), resection of extrahepatic tumor (n = 1), chemotherapy (n = 78) and/or chemoembolization (n = 6), local ablation (n = 7), radiation/chemoradiation (n = 3) or best supportive (n = 15) only.
So far, 100 of the 123 patients with recurrent tumor have died after a median time of 19.9 months (range: 3.1–104.7 months) after resection and 12.8 months (range 0.5–54.7 months) after diagnosis of recurrence. A total of 23 patients (18.7%) are alive with a median survival of 29 months (range 7.6–130.4 months) after initial resection and 20.7 months (range 1–115.3 months) after diagnosis of recurrence.
3.7. Outcome after Associating Liver Partition and Portal Vein Ligation (ALPPS)
In total, eight ALPPS were performed for solitary (n = 3) and multifocal (n = 5) iCCA. There were eight R0 resections. In multifocal iCCA, all patients died within 22 months after liver resection. So far, the three patients with ALPPS for solitary iCCA are alive without evidence of recurrence 11.5 years, 3 years, and 5 months after ALPPS. The patient being alive 11.5 years after ALPPS had recurrent iCCA one year after initial resection. This patient underwent repeated resection (wedge resection) and is now alive without evidence of repeated recurrence 10.5 years after repeated resection and 11.5 years after ALPPS, respectively.
3.8. Exploration Group
Palliative chemotherapy was performed in 62 patients with various protocols most of them based on Gemcitabine and Cisplatin. Further treatments included transarterial chemoembolization (TACE, n = 3), selective internal radiotherapy (SIRT, n = 2), radiation (n = 2) or best supportive care (n = 4). So far, 45/62 patients have died after a median of 9.6 months (range: 0.6–59.8; IQR: 3.9–20.9), 17 are alive at a follow-up between 6 and 59.8 months.
3.9. Predictors of Survival and Recurrence-Free Survival
In univariate analysis the following factors had significant influence on overall survival: extended resection, visceral extension, vascular infiltration, visceral infiltration, tumor size, T-stage, N-stage, M-stage, Pn-stage, and UICC stage (Table 6). In a multivariate cox regression tumor size (p < 0.001), T stage (p < 0.001) and N stage (p = 0.003) were predictors for overall survival (Table 7).
Table 6.
Univariate survival analysis.
| Kaplan Meier | ||
|---|---|---|
| OS | RFS | |
| Age | 0.329 | 0.334 |
| Gender | 0.336 | 0.097 |
| ASA classification | 0.723 | 0.317 |
| Preoperative biopsy | 0.388 | 0.515 |
| Preoperative therapy | 0.886 | 0.053 |
| Major resection | 0.072 | 0.065 |
| Extended resection | 0.040 | 0.039 |
| Vascular extension | 0.290 | 0.326 |
| Visceral extension | 0.024 | 0.020 |
| Vascular infiltration | 0.018 | 0.538 |
| Visceral infiltration | 0.004 | 0.079 |
| Morbidity | 0.920 | 0.846 |
| Severe morbidity (≥Clavien-Dindo 3a) [11] | 0.822 | 0.764 |
| Tumor size (<5 vs. 5–10 vs. >10 cm) * | <0.001 | <0.001 |
| Solitary vs. multifocal tumors | 0.149 | 0.001 |
| T stage | <0.001 | <0.001 |
| N stage | <0.001 | 0.001 |
| M stage | 0.001 | <0.001 |
| L stage | 0.534 | 0.702 |
| V stage | 0.053 | 0.301 |
| Pn stage | 0.003 | 0.053 |
| R stage | 0.197 | 0.207 |
| Grading | 0.344 | 0.598 |
| UICC stage | <0.001 | <0.001 |
For survival analyses perioperative deaths (n = 18) were excluded; for multivariate analysis, p-values < 0.1 were further analyzed in Table 7 (parameters underlined); significant p-values <0.05 are bold; * size of the largest nodule obtained out of the histology report; UICC stage was not included in multivariate analysis; ASA = American Society of Anesthesiologists.
Table 7.
Predictors of survival.
| Overall Survival | Recurrence-Free Survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Gender | ‡ | ‡ | ‡ | |||
| Preoperative therapy | 1.975 | 1.223–3.191 | 0.005 | |||
| Major resection | ‡ | ‡ | ‡ | ‡ | ‡ | ‡ |
| Extended resection | ‡ | ‡ | ‡ | ‡ | ‡ | ‡ |
| Visceral extension | ‡ | ‡ | ‡ | ‡ | ‡ | ‡ |
| Vascular infiltration | ‡ | ‡ | ‡ | |||
| Visceral infiltration | ‡ | ‡ | ‡ | ‡ | ‡ | ‡ |
| Tumor size (<5 vs. 5–10 vs. >10 cm) * | 1.674 | 1.275–2.199 | <0.001 | 1.503 | 1.166–1.936 | 0.002 |
| Solitary vs. multifocal tumors | ‡ | ‡ | ‡ | |||
| T stage | 1.333 | 1.134–1.566 | <0.001 | 1.250 | 1.076–1.452 | 0.004 |
| N stage | 1.494 | 1.149–1.943 | 0.003 | 1.276 | 1.011–1.611 | 0.040 |
| M stage | ‡ | ‡ | ‡ | 2.812 | 1.507–5.247 | 0.001 |
| V stage | ‡ | ‡ | ‡ | |||
| Pn stage | ‡ | ‡ | ‡ | ‡ | ‡ | ‡ |
For survival analyses perioperative deaths (n = 18) were excluded; for multivariate analysis, p-values <0.1 were further analyzed using backward selection (see Table 6); * size of the largest nodule obtained out of the histology report; ‡ eliminated value in backward selection; UICC stage was not included in multivariate analysis due to the fact that multiple tested parameters are included within the UICC staging.
For recurrence-free survival upon univariate analysis the following parameters had significant influence: extended resection, visceral extension, tumor size, multifocality, T-stage, N-stage, M-stage and UICC stage (Table 6). In multivariate cox regression analysis N stage (p = 0.040), preoperative therapy (p = 0.005), T stage (p = 0.004), tumor size (p = 0.002) and M stage (p = 0.001) were independent predictors for recurrence-free survival (Table 7).
4. Discussion
Surgical treatment of iCCA is still one of the main challenges in hepatobiliary surgery. Most often, iCCAs are diagnosed late in the course of the disease when tumors are locally advanced or even in a metastatic stage. In our series, the vast majority of tumors required major hepatectomy (in almost 80%) and additional operative procedures such as complex vascular and biliary reconstructions in nearly 50% of our cases, exceeding in this regard other reports by far. Thus, the presented series is one of the largest in the Western world, not just because of the numbers but also with regard to complexity of procedures. Further, as a single center series pursuing the same aggressive surgical strategy over the entire inclusion period, the comparability of data is also relatively well in contrast to often inhomogeneous multicentric data.
The reported survival after hepatectomy for iCCA ranges between 31% and 59% at 3 years and 21% to 45% at 5 years, depending on the selection criteria for surgery (Table 8). The herein presented results with a 3- and 5-year-survival of 35% and 22% are at the lower range of the reported data, but in our series the extent of liver resection and additional procedures such as vascular reconstructions exceeded those of most other reports by far, indicating more advanced tumors and more difficult resections [19,20]. Looking exclusively at the subgroup undergoing hepatectomy without operative extension, the results with 46% and 28% survival at 3- and 5-years are in accordance with most published data. These results are comparable to the results of surgical therapy in many other gastrointestinal malignancies, endorsing that therapeutic nihilism is not justified in iCCA. However, the presented data also clearly indicate that even with an aggressive surgical approach the chance of cure is still small in iCCA.
Table 8.
Review of the literature—survival after liver resection for iCCA.
| Author | Reference | Year | Period of Data Collection (Years) | Number of Resections | Survival (%) | Median Survival (Months) | ||
|---|---|---|---|---|---|---|---|---|
| 1-Year | 3-Years | 5-Years | ||||||
| Shimada | [21] | 2007 | 7 | 74 | 69 | 35 | 31 | 24 |
| Konstadoulakis | [22] | 2008 | 15 | 54 | 80 | 49 | 25 | 22 * |
| Lang | [23] | 2009 | 9.5 | 83 | 78 | 31 | 21 | 24 |
| Jonas | [24] | 2009 | 20 | 195 | 60.2 | - | 22.2 | - |
| de Jong | [25] | 2011 | 37 | 449 | 77.5 | 44.3 | 30.7 | 27.3 |
| Farges | [26] | 2011 | 11 | 116 | 92 | 69 | 45 | 58 * |
| Wang | [27] | 2013 | 6 | 367 | 61.9 | 40.8 | 35.2 | 21 |
| Bektas | [28] | 2015 | 16 | 221 † | 67 | 40 | 27 | 33 |
| Bergeat | [29] | 2015 | 16.7 | 107 | 79.8 | 49.4 | 34.6 | 32.8 |
| Doussot | [30] | 2015 | 20.3 | 188 | 91 | 59 | 45 | 48.7 |
| Souche | [31] | 2015 | 16 | 125 | 80 | 48 | 28 | 35 |
| Buettner | [32] | 2017 | 26.5 | 1057 | 78.9 | 51.4 | 39.2 | 37.4 |
| Conci | [33] | 2018 | 21 | 282 | - | - | 40.6 | 45.9 |
| Bartsch | [14] | 2018 | 8 | 102 | 72 | 32 | 21 | 21 |
| Schnitzbauer | [34] | 2020 | 10 | 488 | - | - | - | 32.2 |
| Lang | pres. | 2021 | 13 | 202 | 72 | 35 | 22 | 22.5 |
* R0 resections, † including explorations; pres. = present study.
In our series, there was resection/reconstruction of major hepatic vessels or the inferior vena cava in 22% of the cases. Notably, pathology confirmed tumor infiltration of major vessels in only 29% of the suspected cases. As infiltration is difficult to assess by preoperative or intraoperative imaging, our approach is to resect and reconstruct vessels whenever infiltration is suspected. This aggressive approach seems to be justified as this can be done with acceptable low morbidity and mortality.
The most aggressive surgical approach to iCCA is ALPPS [35]. Our results after ALPPS for iCCA are consistent with data from a multicentric analysis by Li et al. [36]. While there seems to be hardly any benefit of such an aggressive approach in multifocal tumors ALPPS seems to be justified in selected cases of solitary iCCA. The patient surviving 11.5 years is to the best of our knowledge the longest survivor worldwide after an ALPPS procedure at all.
Depending on the aggressiveness of the surgical approach and the quality of preoperative diagnostic and staging procedures reported resectability rates of iCCA show great variability ranging between 50% and 75% [14,23,37,38]. This is considerably lower than in most if not in all other hepatobiliary malignancies. Most often multinodular intrahepatic tumor spread or less often peritoneal seedings are the main causes of irresectability. In our series the resectability rate of almost 80% is high but this is at the expense of the need for often extensive resections with frequently complex resections and reconstruction of adjacent structures. We follow this aggressive approach as resection offers the only chance for cure.
It has already been shown that routine use of staging laparoscopy results in a reduction of about 20% of explorative laparotomies in biliary cancer [39,40]. Avoiding a large laparotomy can help to guide these patients towards an immediate palliative treatment. In addition, computer-assisted operation planning and 3D reconstruction of liver anatomy that may provide significant anatomical information. In recent years, we used 3D-prints of the liver for operation planning in selected cases [9]. Although very instructive, it needs to be evaluated whether these techniques can also contribute to a better assessment of resectability, to a reduction of perioperative morbidity and mortality and finally to an improvement of the oncological outcome.
One aim of our analysis was to identify prognostic markers and risk factors for tumor recurrence after hepatic resection. Like other studies, we could confirm extended resection, visceral extension, vascular infiltration, visceral infiltration, tumor size, T-stage, N-stage, M-stage, Pn-stage, and UICC stage to be significantly associated with tumor recurrence and poor survival after R0 resection. In multivariate analysis, tumor size, T stage and N stage were significantly associated with a worse overall survival, while N stage, preoperative therapy, T stage, tumor size and M stage were associated with worse recurrence-free survival. Therefore, based on the presented data, we do not consider the presence of any of these prognostic factors (with the exception of UICC stage IV) as a contraindication to liver resection. In particular, we do not consider the potential need for extension of resections a contraindication, the more as even modern imaging modalities do not reliably predict macro- or micro-vascular infiltration.
There is agreement that resection of stage IV iCCA should only be performed in selected cases in palliative intention where tumor associated symptoms cannot be controlled otherwise. However, there is still discussion about surgical therapy in the presence of lymph node involvement [41]. Up to now, there is no evidence whether lymphadenectomy is of prognostic value only or also beneficial for survival [42]. Our data with a median survival of 18 months and a 5-year-survival of 12% after R0 resections in the presence of lymph node metastases suggest a probable survival benefit of liver resection. However, in this patient group the need for effective perioperative therapy is highly evident.
The new UICC classification recommends the removal of at least 6 lymph nodes from the hilar region and along the lesser gastric curve in left-sided iCCA or retropancreatic in right-sided iCCA [12]. Hopefully, this will help to further improve both treatment stratification of iCCA and, with ongoing progress in adjuvant chemotherapy, also oncological outcome.
Tumor recurrence is by far the most frequent cause of death after resection of iCCA [43,44,45]. Our data reveal that the liver is the most frequent primary site of tumor recurrence with 50% of cases where the liver is the only initial site of recurrence. We performed repeated resection in 27 cases with a survival even longer than in the entire resection group [16]. In a recent German multicenter study with 113 repeated resections, mainly by minor hepatectomies or segmentectomies, a 3- and 5-year disease-free survival of 36% and 28% was reported [46]. These survival rates are even slightly better than after primary resection, suggesting that there might be a selection bias indicating a more favorable biology in those recurrent tumors remaining confined to the liver [47].
Due to the often-advanced disease at the time of diagnosis, there is a substantial number of cases where an R1 resection cannot be avoided. In particular, in large or centrally located tumors, sometimes there is no more margin to be left or final histology shows microscopic tumor invasion of the resection margin which at operation had been assumed to be tumor-free. Similarly, although in the current series all resections were performed with curative intention, we achieved an R1-resection rate of 16%. In former years, R1 resection was supposed to provide little survival benefit rather than to face the patients to the risks of major hepatic surgery [48,49,50,51]. However, with the availability of effective chemotherapy, the value of R1 resections probably needs to be re-evaluated, in particular in combination with neoadjuvant and downsizing therapy strategies [52].
Although significantly better than the data of non-resected tumors, the achieved long-term results even after extensive resections with complex reconstructions are poor. This indicates that there is a limit to the contribution made by radical resections alone. Hence, since the first results of the BILCAP trial (adjuvant capecitabine versus observation following R0/R1 resection of bile duct cancer) became available, adjuvant chemotherapy with capecitabine is currently the standard of care after resection of iCCA in our institution [53].
In our series, we had 18 resections following neoadjuvant CTx for down-sizing. The survival data in these patients are at least comparable with those of patients undergoing upfront surgery without chemotherapy. Assuming that the tumors in the downsizing group were more advanced, these results suggest the effectiveness of CTx. Consistently, in a multivariate analysis, preoperative chemotherapy was one prognostic factor significantly associated with improved recurrence-free survival. Certainly, our series is too small to draw any valid conclusion, but our data are in line with some recent reports from the literature. In a French study neoadjuvant chemotherapy led to a secondary resectability in 39/74 (53%) patients with initially borderline resectable or irresectable iCCA [54]. These patients had survival rates similar to the group with initially resectable iCCA. Similarly, in a recent propensity-matched analysis (203 versus 487 patients with iCCA) based on data from the National Cancer Database (NCDB, USA), neoadjuvant treatment was associated with a significantly higher R0 resection rate and better survival compared to adjuvant therapy (median OS: 40.3 vs 32.8 months; p = 0.01) [55]. This suggests the potential effectiveness of neoadjuvant (downsizing) treatment for iCCA and justifies further evaluation of this concept [56,57].
Further, cholangiocarcinoma has become a hallmark of modern precision medicine. With the development of next-generation sequencing (NGS) targeted therapies become increasingly available. In the palliative setting, first promising results have been reported after treatment with fibroblast growth factor receptor (FGFR) and isocitrate dehydrogenase (IDH) inhibitors [58]. In future it is possible that these targeted therapies may also play a role in preoperative neoadjuvant or downsizing therapy [59,60].
5. Conclusions
In conclusion, our results suggest that complete surgical resection may provide prolonged survival even in locally advanced but not metastatic iCCA. As iCCA is usually diagnosed late in the course of disease, often extended liver resection in combination with complex vascular or biliary reconstruction is necessary to achieve complete tumor removal. Improved surgical techniques, in particular those derived from liver transplant surgery, have continuously pushed the frontiers of liver surgery. However, as is the case for iCCA, the limits of what is technically feasible are often reached. Nevertheless, even when pulling out all stops of surgical skills, the long-term oncological results have remained poor, indicating that surgery alone is unlikely to make significant strides in improving the prognosis of iCCA. This strongly suggests that surgery of iCCA should be integrated into multimodal perioperative treatment concepts. Recent results have shown the efficacy of adjuvant chemotherapy, and there are also some promising results with neoadjuvant therapy, in particular regarding downsizing therapy to achieve secondary resectability. Further studies are needed to better evaluate treatment options in order to shed further light on this topic.
Acknowledgments
Tiemo S. Gerber is supported by the Clinician Scientist Fellowship “Else Kröner Research College: 2018_Kolleg.05”.
Author Contributions
Conceptualization, H.L. and F.B.; methodology, H.L. and F.B.; software, F.B.; validation, H.L. and F.B.; formal analysis, F.B.; investigation, H.L. and F.B.; resources, H.L.; data curation, J.B. and F.B.; writing—original draft preparation, H.L. and F.B.; writing—review and editing, H.L., J.B., S.H., T.H., L.-K.H., R.M., J.M., F.H., T.S.G., F.F., A.W., J.U.M., R.K., B.K.S. and F.B.; visualization, T.H. and F.B.; supervision, H.L.; project administration, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All patients signed informed consent that data and follow-up will be collected anonymously and potentially used for scientific analysis. Abiding by the regulations of the federal state law (state hospital law §36 & §37) and according to the independent ethics committee of Rhineland-Palatinate, no ethical approval was necessary for this study.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rizvi S., Khan S.A., Hallemeier C.L., Kelley R.K., Gores G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertuccio P., Malvezzi M., Carioli G., Hashim D., Boffetta P., El-Serag H.B., La Vecchia C., Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019;71:104–114. doi: 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Weber S.M., Ribero D., O’Reilly E.M., Kokudo N., Miyazaki M., Pawlik T.M. Intrahepatic cholangiocarcinoma: Expert consensus statement. HPB. 2015;17:669–680. doi: 10.1111/hpb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ejaz A., Cloyd J.M., Pawlik T.M. Advances in the diagnosis and treatment of patients with intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2020;27:552–560. doi: 10.1245/s10434-019-07873-z. [DOI] [PubMed] [Google Scholar]
- 5.Cloyd J.M., Ejaz A., Pawlik T.M. The Landmark series: Intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2020;27:2859–2865. doi: 10.1245/s10434-020-08621-4. [DOI] [PubMed] [Google Scholar]
- 6.Bridgewater J., Galle P.R., Khan S.A., Llovet J.M., Park J.W., Patel T., Pawlik T.M., Gores G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Beal E.W., Cloyd J.M., Pawlik T.M. Surgical treatment of intrahepatic cholangiocarcinoma: Current and emerging principles. J. Clin. Med. 2020;10:104. doi: 10.3390/jcm10010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paschold M., Huettl F., Kneist W., Boedecker C., Poplawski A., Huber T., Lang H. Local, semi-automatic, three-dimensional liver reconstruction or external provider? An analysis of performance and time expense. Langenbecks Arch. Surg. 2020;405:173–179. doi: 10.1007/s00423-020-01862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber T., Huettl F., Tripke V., Baumgart J., Lang H. Experiences with three-dimensional printing in complex liver surgery. Ann. Surg. 2021;273:e26–e27. doi: 10.1097/SLA.0000000000004348. [DOI] [PubMed] [Google Scholar]
- 10.Nagino M., DeMatteo R., Lang H., Cherqui D., Malago M., Kawakatsu S., DeOliveira M.L., Adam R., Aldrighetti L., Boudjema K., et al. Proposal of a new comprehensive notation for hepatectomy: The “New World” Terminology. Ann. Surg. 2021;274:1–3. doi: 10.1097/SLA.0000000000004808. [DOI] [PubMed] [Google Scholar]
- 11.Clavien P.A., Barkun J., de Oliveira M.L., Vauthey J.N., Dindo D., Schulick R.D., de Santibanes E., Pekolj J., Slankamenac K., Bassi C., et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 12.Brierley J., Gospodarowicz M., Wittekind C. International Union against Cancer. TNM Classification of Malignant Tumours. 8th ed. Wiley Blackwell; Oxford, UK: 2017. [Google Scholar]
- 13.Punt C.J., Buyse M., Kohne C.H., Hohenberger P., Labianca R., Schmoll H.J., Pahlman L., Sobrero A., Douillard J.Y. Endpoints in adjuvant treatment trials: A systematic review of the literature in colon cancer and proposed definitions for future trials. J. Natl. Cancer Inst. 2007;99:998–1003. doi: 10.1093/jnci/djm024. [DOI] [PubMed] [Google Scholar]
- 14.Bartsch F., Baumgart J., Hoppe-Lotichius M., Schmidtmann I., Heinrich S., Lang H. Visceral infiltration of intrahepatic cholangiocarcinoma is most prognostic after curative resection—Retrospective cohort study of 102 consecutive liver resections from a single center. Int. J. Surg. 2018;55:193–200. doi: 10.1016/j.ijsu.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Bartsch F., Baumgart J., Hoppe-Lotichius M., Straub B.K., Heinrich S., Lang H. Intrahepatic cholangiocarcinoma—Influence of resection margin and tumor distance to the liver capsule on survival. BMC Surg. 2020;20:61. doi: 10.1186/s12893-020-00718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartsch F., Paschold M., Baumgart J., Hoppe-Lotichius M., Heinrich S., Lang H. Surgical resection for recurrent intrahepatic cholangiocarcinoma. World J. Surg. 2019;43:1105–1116. doi: 10.1007/s00268-018-04876-x. [DOI] [PubMed] [Google Scholar]
- 17.Bartsch F., Tripke V., Baumgart J., Hoppe-Lotichius M., Heinrich S., Lang H. Extended resection of intrahepatic cholangiocarcinoma: A retrospective single-center cohort study. Int. J. Surg. 2019;67:62–69. doi: 10.1016/j.ijsu.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Stein A., Arnold D., Bridgewater J., Goldstein D., Jensen L.H., Klumpen H.J., Lohse A.W., Nashan B., Primrose J., Schrum S., et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial)—A randomized, multidisciplinary, multinational phase III trial. BMC Cancer. 2015;15:564. doi: 10.1186/s12885-015-1498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conci S., Vigano L., Ercolani G., Gonzalez E., Ruzzenente A., Isa G., Salaris C., Fontana A., Bagante F., Pedrazzani C., et al. Outcomes of vascular resection associated with curative intent hepatectomy for intrahepatic cholangiocarcinoma. Eur. J. Surg. Oncol. 2020;46:1727–1733. doi: 10.1016/j.ejso.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Ali S.M., Clark C.J., Zaydfudim V.M., Que F.G., Nagorney D.M. Role of major vascular resection in patients with intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2013;20:2023–2028. doi: 10.1245/s10434-012-2808-2. [DOI] [PubMed] [Google Scholar]
- 21.Shimada K., Sano T., Sakamoto Y., Esaki M., Kosuge T., Ojima H. Surgical outcomes of the mass-forming plus periductal infiltrating types of intrahepatic cholangiocarcinoma: A comparative study with the typical mass-forming type of intrahepatic cholangiocarcinoma. World J. Surg. 2007;31:2016–2022. doi: 10.1007/s00268-007-9194-0. [DOI] [PubMed] [Google Scholar]
- 22.Konstadoulakis M.M., Roayaie S., Gomatos I.P., Labow D., Fiel M.I., Miller C.M., Schwartz M.E. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: Operative results and long-term outcome. Surgery. 2008;143:366–374. doi: 10.1016/j.surg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Lang H., Sotiropoulos G.C., Sgourakis G., Schmitz K.J., Paul A., Hilgard P., Zopf T., Trarbach T., Malago M., Baba H.A., et al. Operations for intrahepatic cholangiocarcinoma: Single-institution experience of 158 patients. J. Am. Coll. Surg. 2009;208:218–228. doi: 10.1016/j.jamcollsurg.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Jonas S., Thelen A., Benckert C., Biskup W., Neumann U., Rudolph B., Lopez-Haanninen E., Neuhaus P. Extended liver resection for intrahepatic cholangiocarcinoma: A comparison of the prognostic accuracy of the fifth and sixth editions of the TNM classification. Ann. Surg. 2009;249:303–309. doi: 10.1097/SLA.0b013e318195e164. [DOI] [PubMed] [Google Scholar]
- 25.De Jong M.C., Nathan H., Sotiropoulos G.C., Paul A., Alexandrescu S., Marques H., Pulitano C., Barroso E., Clary B.M., Aldrighetti L., et al. Intrahepatic cholangiocarcinoma: An international multi-institutional analysis of prognostic factors and lymph node assessment. J. Clin. Oncol. 2011;29:3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 26.Farges O., Fuks D., Boleslawski E., Le Treut Y.P., Castaing D., Laurent A., Ducerf C., Rivoire M., Bachellier P., Chiche L., et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: A multicenter study by the AFC-IHCC-2009 study group. Ann. Surg. 2011;254:824–829. doi: 10.1097/SLA.0b013e318236c21d. discussion 30. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Li J., Xia Y., Gong R., Wang K., Yan Z., Wan X., Liu G., Wu D., Shi L., et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J. Clin. Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 28.Bektas H., Yeyrek C., Kleine M., Vondran F.W., Timrott K., Schweitzer N., Vogel A., Jager M.D., Schrem H., Klempnauer J., et al. Surgical treatment for intrahepatic cholangiocarcinoma in Europe: A single center experience. J. Hepatobiliary Pancreat. Sci. 2015;22:131–137. doi: 10.1002/jhbp.158. [DOI] [PubMed] [Google Scholar]
- 29.Bergeat D., Sulpice L., Rayar M., Edeline J., Merdignac A., Meunier B., Boucher E., Boudjema K. Extended liver resections for intrahepatic cholangiocarcinoma: Friend or foe? Surgery. 2015;157:656–665. doi: 10.1016/j.surg.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Doussot A., Groot-Koerkamp B., Wiggers J.K., Chou J., Gonen M., DeMatteo R.P., Allen P.J., Kingham T.P., D’Angelica M.I., Jarnagin W.R. Outcomes after resection of intrahepatic cholangiocarcinoma: External validation and comparison of prognostic models. J. Am. Coll. Surg. 2015;221:452–461. doi: 10.1016/j.jamcollsurg.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souche R., Addeo P., Oussoultzoglou E., Herrero A., Rosso E., Navarro F., Fabre J.M., Bachellier P. First and repeat liver resection for primary and recurrent intrahepatic cholangiocarcinoma. Am. J. Surg. 2015;212:221–229. doi: 10.1016/j.amjsurg.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Buettner S., Koerkamp B.G., Ejaz A., Buisman F.E., Kim Y., Margonis G.A., Alexandrescu S., Marques H.P., Lamelas J., Aldrighetti L., et al. The effect of preoperative chemotherapy treatment in surgically treated intrahepatic cholangiocarcinoma patients-A multi-institutional analysis. J. Surg. Oncol. 2017;115:312–318. doi: 10.1002/jso.24524. [DOI] [PubMed] [Google Scholar]
- 33.Conci S., Ruzzenente A., Vigano L., Ercolani G., Fontana A., Bagante F., Bertuzzo F., Dore A., Pinna A.D., Torzilli G., et al. Patterns of distribution of hepatic nodules (single, satellites or multifocal) in intrahepatic cholangiocarcinoma: Prognostic impact after surgery. Ann. Surg. Oncol. 2018;25:3719–3727. doi: 10.1245/s10434-018-6669-1. [DOI] [PubMed] [Google Scholar]
- 34.Schnitzbauer A.A., Eberhard J., Bartsch F., Brunner S.M., Ceyhan G.O., Walter D., Fries H., Hannes S., Hecker A., Li J., et al. The MEGNA Score and Preoperative Anemia are Major Prognostic Factors After Resection in the German Intrahepatic Cholangiocarcinoma Cohort. Ann. Surg. Oncol. 2020;27:1147–1155. doi: 10.1245/s10434-019-07968-7. [DOI] [PubMed] [Google Scholar]
- 35.Schnitzbauer A.A., Lang S.A., Goessmann H., Nadalin S., Baumgart J., Farkas S.A., Fichtner-Feigl S., Lorf T., Goralcyk A., Horbelt R., et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 36.Li J., Moustafa M., Linecker M., Lurje G., Capobianco I., Baumgart J., Ratti F., Rauchfuss F., Balci D., Fernandes E., et al. ALPPS for locally advanced intrahepatic cholangiocarcinoma: Did aggressive surgery lead to the oncological benefit? An international multi-center study. Ann. Surg. Oncol. 2020;27:1372–1384. doi: 10.1245/s10434-019-08192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhanasekaran R., Hemming A.W., Zendejas I., George T., Nelson D.R., Soldevila-Pico C., Firpi R.J., Morelli G., Clark V., Cabrera R. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol. Rep. 2013;29:1259–1267. doi: 10.3892/or.2013.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guglielmi A., Ruzzenente A., Campagnaro T., Pachera S., Valdegamberi A., Nicoli P., Cappellani A., Malfermoni G., Iacono C. Intrahepatic cholangiocarcinoma: Prognostic factors after surgical resection. World J. Surg. 2009;33:1247–1254. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]
- 39.D’Angelica M., Fong Y., Weber S., Gonen M., DeMatteo R.P., Conlon K., Blumgart L.H., Jarnagin W.R. The role of staging laparoscopy in hepatobiliary malignancy: Prospective analysis of 401 cases. Ann. Surg. Oncol. 2003;10:183–189. doi: 10.1245/ASO.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 40.Franken L.C., Coelen R.J.S., Roos E., Verheij J., Phoa S.S., Besselink M.G., Busch O.R.C., van Gulik T.M. Staging laparoscopy in patients with intrahepatic cholangiocarcinoma: Is it still useful? Visc. Med. 2020;36:501–505. doi: 10.1159/000506297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagante F., Spolverato G., Weiss M., Alexandrescu S., Marques H.P., Aldrighetti L., Maithel S.K., Pulitano C., Bauer T.W., Shen F., et al. Assessment of the lymph node status in patients undergoing liver resection for intrahepatic cholangiocarcinoma: The new eighth edition AJCC staging system. J. Gastrointest. Surg. 2018;22:52–59. doi: 10.1007/s11605-017-3426-x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X.-F., Xue F., Dong D.-H., Weiss M., Popescu I., Marques H.P., Aldrighetti L., Maithel S.K., Pulitano C., Bauer T.W., et al. Number and station of lymph node metastasis after curative-intent resection of intrahepatic cholangiocarcinoma impact prognosis. Ann. Surg. 2020 doi: 10.1097/SLA.0000000000004137. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X.F., Beal E.W., Bagante F., Chakedis J., Weiss M., Popescu I., Marques H.P., Aldrighetti L., Maithel S.K., Pulitano C., et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br. J. Surg. 2018;105:848–856. doi: 10.1002/bjs.10676. [DOI] [PubMed] [Google Scholar]
- 44.Hu L.S., Zhang X.F., Weiss M., Popescu I., Marques H.P., Aldrighetti L., Maithel S.K., Pulitano C., Bauer T.W., Shen F., et al. Recurrence patterns and timing courses following curative-intent resection for intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2019;26:2549–2557. doi: 10.1245/s10434-019-07353-4. [DOI] [PubMed] [Google Scholar]
- 45.Yoh T., Hatano E., Seo S., Okuda Y., Fuji H., Ikeno Y., Taura K., Yasuchika K., Okajima H., Kaido T., et al. Long-term survival of recurrent intrahepatic cholangiocarcinoma: The impact and selection of repeat surgery. World J. Surg. 2018;42:1848–1856. doi: 10.1007/s00268-017-4387-7. [DOI] [PubMed] [Google Scholar]
- 46.Bartsch F., Eberhard J., Ruckert F., Schmelzle M., Lehwald-Tywuschik N., Fichtner-Feigl S., Gaedcke J., Oldhafer K.J., Oldhafer F., Diener M., et al. Repeated resection for recurrent intrahepatic cholangiocarcinoma: A retrospective German multicentre study. Liver Int. 2021;41:180–191. doi: 10.1111/liv.14682. [DOI] [PubMed] [Google Scholar]
- 47.Spolverato G., Kim Y., Alexandrescu S., Marques H.P., Lamelas J., Aldrighetti L., Clark Gamblin T., Maithel S.K., Pulitano C., Bauer T.W., et al. Management and outcomes of patients with recurrent intrahepatic cholangiocarcinoma following previous curative-intent surgical resection. Ann. Surg. Oncol. 2016;23:235–243. doi: 10.1245/s10434-015-4642-9. [DOI] [PubMed] [Google Scholar]
- 48.Li M.X., Bi X.Y., Li Z.Y., Huang Z., Han Y., Zhao J.J., Zhao H., Cai J.Q. Impaction of surgical margin status on the survival outcome after surgical resection of intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Surg. Res. 2016;203:163–173. doi: 10.1016/j.jss.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Tsilimigras D.I., Sahara K., Wu L., Moris D., Bagante F., Guglielmi A., Aldrighetti L., Weiss M., Bauer T.W., Alexandrescu S., et al. Very early recurrence after liver resection for intrahepatic cholangiocarcinoma: Considering alternative treatment approaches. JAMA Surg. 2020;155:823–831. doi: 10.1001/jamasurg.2020.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spolverato G., Yakoob M.Y., Kim Y., Alexandrescu S., Marques H.P., Lamelas J., Aldrighetti L., Gamblin T.C., Maithel S.K., Pulitano C., et al. The impact of surgical margin status on long-term outcome after resection for intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2015;22:4020–4028. doi: 10.1245/s10434-015-4472-9. [DOI] [PubMed] [Google Scholar]
- 51.Spolverato G., Yakoob M.Y., Kim Y., Alexandrescu S., Marques H.P., Lamelas J., Aldrighetti L., Gamblin T.C., Maithel S.K., Pulitano C., et al. Facility type is associated with margin status and overall survival of patients with resected intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2019;26:4091–4099. doi: 10.1245/s10434-019-07657-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lang H. Should all intrahepatic cholangiocarcinomas receive neoadjuvant chemotherapy before resection? Br. J. Surg. 2021;108:598–599. doi: 10.1093/bjs/znab077. [DOI] [PubMed] [Google Scholar]
- 53.Primrose J.N., Fox R.P., Palmer D.H., Malik H.Z., Prasad R., Mirza D., Anthony A., Corrie P., Falk S., Finch-Jones M., et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 54.Le Roy B., Gelli M., Pittau G., Allard M.A., Pereira B., Serji B., Vibert E., Castaing D., Adam R., Cherqui D., et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br. J. Surg. 2017;105:839–847. doi: 10.1002/bjs.10641. [DOI] [PubMed] [Google Scholar]
- 55.Yadav S., Xie H., Bin-Riaz I., Sharma P., Durani U., Goyal G., Borah B., Borad M.J., Smoot R.L., Roberts L.R., et al. Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: A propensity score matched analysis. Eur. J. Surg. Oncol. 2019;45:1432–1438. doi: 10.1016/j.ejso.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 56.Akateh C., Ejaz A.M., Pawlik T.M., Cloyd J.M. Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma. World J. Hepatol. 2020;12:693–708. doi: 10.4254/wjh.v12.i10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riby D., Mazzotta A.D., Bergeat D., Verdure L., Sulpice L., Bourien H., Lievre A., Rolland Y., Garin E., Boudjema K., et al. Downstaging with radioembolization or chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2020;27:3729–3737. doi: 10.1245/s10434-020-08486-7. [DOI] [PubMed] [Google Scholar]
- 58.Zhang W., Zhou H., Wang Y., Zhang Z., Cao G., Song T., Zhang T., Li Q. Systemic treatment of advanced or recurrent biliary tract cancer. Biosci. Trends. 2020;14:328–341. doi: 10.5582/bst.2020.03240. [DOI] [PubMed] [Google Scholar]
- 59.Abou-Alfa G.K., Sahai V., Hollebecque A., Vaccaro G., Melisi D., Al-Rajabi R., Paulson A.S., Borad M.J., Gallinson D., Murphy A.G., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abou-Alfa G.K., Macarulla T., Javle M.M., Kelley R.K., Lubner S.J., Adeva J., Cleary J.M., Catenacci D.V., Borad M.J., Bridgewater J., et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.