Abstract
Activation of c-Jun N-terminal kinases (JNKs)/stress-activated protein kinases is an early response of cells upon exposure to DNA-damaging agents. JNK-mediated phosphorylation of c-Jun is currently understood to stimulate the transactivating potency of AP-1 (e.g., c-Jun/c-Fos; c-Jun/ATF-2), thereby increasing the expression of AP-1 target genes. Here we show that stimulation of JNK1 activity is not a general early response of cells exposed to genotoxic agents. Treatment of NIH 3T3 cells with UV light (UV-C) as well as with methyl methanesulfonate (MMS) caused activation of JNK1 and an increase in c-Jun protein and AP-1 binding activity, whereas antineoplastic drugs such as mafosfamide, mitomycin C, N-hydroxyethyl-N-chloroethylnitrosourea, and treosulfan did not elicit this response. The phosphatidylinositol 3-kinase inhibitor wortmannin specifically blocked the UV-stimulated activation of JNK1 but did not affect UV-driven activation of extracellular regulated kinase 2 (ERK2). To investigate the significance of JNK1 for transactivation of c-jun, we analyzed the effect of UV irradiation on c-jun expression under conditions of wortmannin-mediated inhibition of UV-induced stimulation of JNK1. Neither the UV-induced increase in c-jun mRNA, c-Jun protein, and AP-1 binding nor the activation of the collagenase and c-jun promoters was affected by wortmannin. In contrast, the mitogen-activated protein kinase/ERK kinase inhibitor PD98056, which blocked ERK2 but not JNK1 activation by UV irradiation, impaired UV-driven c-Jun protein induction and AP-1 binding. Based on the data, we suggest that JNK1 stimulation is not essential for transactivation of c-jun after UV exposure, whereas activation of ERK2 is required for UV-induced signaling leading to elevated c-jun expression.
Exposure of mammalian cells to DNA-damaging agents elicits a variety of responses including the rapid transcriptional activation of the so-called immediate-early inducible genes c-fos and c-jun. Dimerization of their gene products forms the transcription factor AP-1 (e.g., c-Jun/c-Fos or c-Jun/ATF-2), which gives rise to increased expression of AP-1 target genes such as c-jun itself (1, 47). Under conditions of c-Fos deficiency, cells are rendered hypersensitive to a broad spectrum of DNA-damaging agents, indicating that the expression of various c-Fos-regulated genes exerts a protective function (15, 21, 25, 42). As primary targets for UV-stimulated signaling, growth factor receptors such as the epidermal growth factor (EGF) receptor (26) as well as cytokine receptors (40) have been identified. Triggered by these receptors, UV irradiation activates a protein kinase cascade covering extracellular regulated kinases (ERKs) (37), c-Jun N-terminal kinases/stress-activated protein kinases (JNKs/SAPKs) (8), and p38 mitogen-activated protein kinases (37, 48). Phosphorylation of transcription factors by these kinases finally results in transcriptional activation of various target genes (4). In contrast to UV, genotoxic stress evoked by alkylating agents such as methyl methanesulfonate (MMS) fails to activate ERKs in human cells (49). Based on this observation and on the finding that suramin blocks only the UV-driven activation of mitogen-activated protein kinases and does not affect MMS-induced signaling (41, 49), it has been suggested that the primary cellular target of MMS-driven stimulation of signaling pathways is different from that of UV.
It has been shown previously that JNKs/SAPKs phosphorylate c-Jun on serines 63 and 73 (44, 45) and ATF-2 on threonines 69 and 73 (14, 28). This phosphorylation occurs while c-Jun is bound to its regulatory element in complex with ATF-2, whereby the complex formation is not affected by phosphorylation (18, 28, 46, 47). Exchange of the JNK-specific phosphate receptor amino acids of c-Jun as well as those of ATF-2 abolishes the transactivating capacity of these factors, thus preventing activation of c-jun expression (28, 38). Furthermore, phosphorylation of c-Jun by JNKs was reported to be required for activation of AP-1 and cellular transformation (44, 45). Overall, these reports indicate that phosphorylation by JNKs is very important for the physiological function of c-Jun/AP-1. However, to our best knowledge, it has not been shown that stimulation of JNK activity, for example, by overexpression of activated SAPK/ERK kinases (SEKs), leads to an increase in c-jun mRNA expression or c-jun promoter activity. Also, the effect of dominant-negative SEKs on stress-induced JNK activation and c-jun expression is largely unknown. Interestingly, embryonic stem cells lacking JNK upstream regulator SEK1/mitogen-activated protein kinase kinase 4 (MKK4) were not impaired in UV-stimulated activation of JNK (32, 50). One possible interpretation of this is that other MKKs such as the recently identified MKK7 might be of particular relevance for stress-induced JNK1 activation (10, 30). Because of the lack of suitable pharmacological JNK inhibitors, the effect of inhibition of stress-induced JNK1 activation on the expression of the endogenous c-jun gene has not been analyzed yet. Also, ionizing radiation and the anticancer drug cisplatin failed to stimulate JNK activity at physiologically relevant doses (27) but were able to activate c-jun and c-fos mRNA expression (9, 16, 20, 43). On the other hand, doxorubicin stimulated JNK activity (19, 35) but failed to increase AP-1 activity (7). In view of these divergent findings, it is rather unclear whether activation of JNK1 is an essential step in genotoxic stress-induced expression of c-jun.
We addressed the question of the physiological significance of JNK1, which has been reported previously to be a major UV-activated JNK isoform (14, 47), in the expression of c-jun by analyzing the consequences of pharmacological JNK1 blockage for UV-induced c-jun expression. As an inhibitor of genotoxic stress-induced JNK1 activation, we used wortmannin. Here, we demonstrate that wortmannin is highly efficient in blocking the UV-mediated activation of JNK1 but does not affect activation of ERK2. Under these conditions of wortmannin-blocked stimulation of UV-driven JNK1 activation, expression of c-jun was not impaired, indicating that JNK1 is not essentially required for transactivation of c-jun.
MATERIALS AND METHODS
Materials.
GST-Jun (1/166) was obtained from P. Angel (Heidelberg, Germany); Coll-CAT (−73/+63) and c-Jun-CAT (−196/+195) constructs as well as c-fos, c-jun, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) hybridization probes were provided by H. J. Rahmsdorf (Karlsruhe, Germany). rhoB cDNA was obtained from T. Hunter (San Diego, Calif.). The phosphatidylinositol (PI) 3-kinase inhibitor wortmannin, mitomycin C, and MMS were purchased from Sigma; the MEK inhibitor PD98059 was from Calbiochem. Treosulfan was provided by Medac (Hamburg, Germany), N-hydroxyethyl-N-chloroethylnitrosourea (HeCNU) was provided by G. Eisenbrand (Kaiserslautern, Germany), and mafosfamide was provided by J. Pohl (Asta Medica, Frankfurt, Germany). Antibodies were obtained from Santa Cruz (San Diego, Calif.).
Cell culture.
NIH 3T3 cells were routinely grown in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal calf serum. For UV irradiation, the medium was removed and added again after treatment. Treatment with MMS and cytostatic drugs was performed by putting the agents directly into the medium.
Kinase assays.
JNK1 activity was determined by immune complex kinase assay. After immunoprecipitation with JNK1-specific antibody (Santa Cruz, catalog no. sc-474), the immunoprecipitate was incubated for 30 min at 30°C in 40 μl of reaction buffer containing 25 mM HEPES (pH 7.6), 20 mM MgCl2, 20 mM β-glycerolphosphate, 0.1 mM sodium orthovanadate, 2 mM dithiothreitol, 25 μM ATP, and 1 μCi of [γ-32P]ATP. As substrate for JNK1, 1 μg of GST-Jun (1/166) was used. Reaction products were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and visualized by autoradiography. Additionally, SEK-mediated phosphorylation of JNK1 was analyzed after immunoprecipitation of JNK1 by Western blotting with phosphospecific JNK antibody (Santa Cruz, catalog no. sc-6254). ERK2 activation was analyzed by Western blotting with ERK2-specific antibody (Santa Cruz, catalog no. sc-154) as described elsewhere (39).
Band shift analysis.
For determination of AP-1-specific binding, band shift analysis with an AP-1-specific oligonucleotide derived from the mouse collagenase promoter was performed (5′-AGTGGTGACTCATCACT 3′). The oligonucleotide was 32P labeled by the use of T4 kinase and was incubated with extracts from treated or nontreated NIH 3T3 cells. Extracts for band shift analysis were prepared by high-salt extraction as described elsewhere (46). After determination of protein concentration (3), 2 to 5 μg of protein was incubated with 32P-labeled oligonucleotide for 30 min at room temperature. After the incubation period, reaction products were separated on nondenaturing 5% polyacrylamide gels. After the run, gels were dried and subjected to autoradiography.
Northern blot analysis.
Ten to twenty micrograms of total RNA, prepared according to the method of Chomczynski and Sacchi (5), was separated on an 0.8% agarose gel. RNA was transferred overnight onto Hybond N+ filters (Amersham) with 50 mM NaOH as transfer buffer. Prehybridization was performed in buffer containing 7% SDS, 1 mM EDTA, and 0.5 mM Na-phosphate (pH 7.4). Hybridization was done overnight in the same buffer additionally containing 1% bovine serum albumin and 32P-labeled probe. cDNA probes were 32P labeled by random priming (Stratagene). The filter was hybridized with c-jun-specific probe and subsequently rehybridized with c-fos, rhoB, and GAPDH cDNA probes.
Western blot analysis.
For immunological detection of c-Jun, 30 μg of protein from total extracts was separated by SDS gel electrophoresis and wet blotted to nitrocellulose with a Bio-Rad blotting chamber. The filter was blocked by overnight incubation with phosphate-buffered saline–0.2% Tween supplemented with 5% dry milk. Hybridization with c-Jun-specific antibody (1:1,000; Santa Cruz; catalog no. sc-45) was done for 2 h at room temperature in phosphate-buffered saline–0.2% Tween–5% dry milk. c-Jun proteins were detected after incubation with peroxidase-coupled anti-mouse immunoglobulin G by chemiluminescence (Amersham, ECL detection kit).
Promoter chloramphenicol acetyltransferase (CAT) analyses.
In order to analyze the effect of UV irradiation on the level of the promoter, expression analyses with Coll-CAT (−73/+63) as well as c-Jun-CAT (−196/+195) promoter constructs were performed. Five micrograms of the corresponding promoter constructs was transfected by the CaCl2 coprecipitation technique into logarithmically growing NIH 3T3 cells. At 24 h after transfection, cells were pretreated or not (control) with wortmannin (200 nM), and 30 min later, cells were UV irradiated (40 J/m2). After irradiation, cells were further incubated for 4 h in the presence of wortmannin (200 nM) before the medium was replaced. Twenty-four hours later, cells were harvested for determination of the amount of CAT protein by an enzyme-linked immunosorbent assay-based assay (Boehringer, Mannheim, Germany).
RESULTS
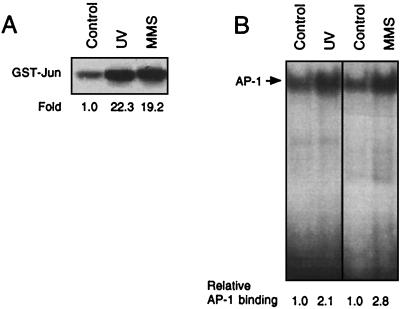
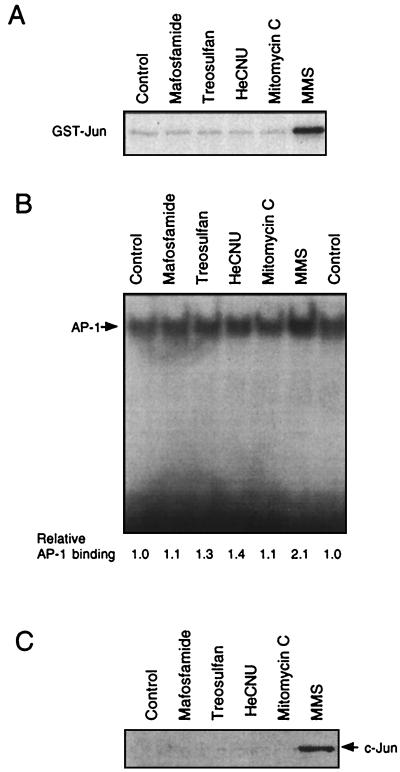
In this study, we asked the question of the physiological significance of JNKs for genotoxic stress-induced signaling. First, we analyzed whether activation of JNKs/SAPKs is a general early response of cells exposed to DNA-damaging agents. Therefore, we measured the activity of JNK1, which is known to be the major UV-stimulated JNK (14, 47), after exposure of cells to various kinds of DNA-damaging agents. In the next step, we analyzed whether drug-induced changes in JNK1 activity, via JNK-mediated activation of the transcription factor c-Jun/ATF-2 (28, 38, 47), are related to an increase in the expression of c-jun mRNA and c-Jun protein and changes in AP-1 binding activity. JNK1 activity was determined by the immune complex kinase assay with JNK1-specific antibody. UV irradiation of NIH 3T3 cells as well as treatment with the alkylating agent MMS caused a rapid and strong increase in JNK1 activity (Fig. 1A) which was accompanied by enhanced AP-1 binding (Fig. 1B). Interestingly, various antineoplastic drugs such as the cyclophosphamide analogue mafosfamide, as well as treosulfan, HeCNU, and mitomycin C, did not elicit JNK1 activation and also did not increase AP-1 binding activity (Fig. 2A and B). In contrast to MMS, the antineoplastic agents also failed to increase c-Jun protein level as determined 4 h after treatment (Fig. 2C) and to stimulate c-jun promoter activity (data not shown). We would like to note that the cytostatic drugs were used at concentrations exerting cytotoxic effects comparable to those of UV and MMS (<1% colony formation). Even at highly cytotoxic concentrations (up to 150 μM), mafosfamide did not affect AP-1 binding activity as analyzed up to 8 h after treatment (data not shown). Thus, for the genotoxic agents tested, activation of JNK1 and subsequent increase in c-Jun protein and AP-1 activity are not general phenomena but appear to be agent specific.
FIG. 1.
Stimulation of JNK1 activity and AP-1 binding by DNA-damaging treatments. Logarithmically growing NIH 3T3 cells were left untreated (Control) or were exposed to UV (40 J/m2) and MMS (1 mM). At 30 min (UV) and 1 h (MMS) after treatment, cells were harvested and analyzed for JNK1 activity (A). For analysis of AP-1 binding activity (B), cells were harvested 4 h after treatment. Determination of JNK1 activity and AP-1 binding activity was performed as described in Materials and Methods. Autoradiograms were densitometrically analyzed, and relative JNK1 activity and AP-1 binding, respectively, in the untreated control were set to 1.0.
FIG. 2.
Antineoplastic drugs stimulate neither JNK1 activity nor AP-1 binding and do not cause an increase in c-Jun protein level. (A and B) Logarithmically growing NIH 3T3 cells were treated with various cytostatic drugs (mafosfamide, 60 μM; treosulfan, 500 μM; HeCNU, 60 μM; mitomycin C, 0.5 μg/ml) and, as a control, MMS (2 mM). One and four hours after treatment, cells were harvested for determination of JNK1 activity (A) and AP-1 binding activity (B), respectively. (C) Four hours after exposure to the agents indicated, the amount of c-Jun protein was determined by Western blot analysis. Thirty micrograms of protein from total cell extracts was separated by SDS-polyacrylamide gel electrophoresis, and after blotting to nitrocellulose, c-Jun protein was detected with c-Jun-specific antibody (Santa Cruz).
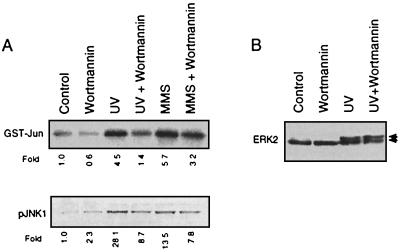
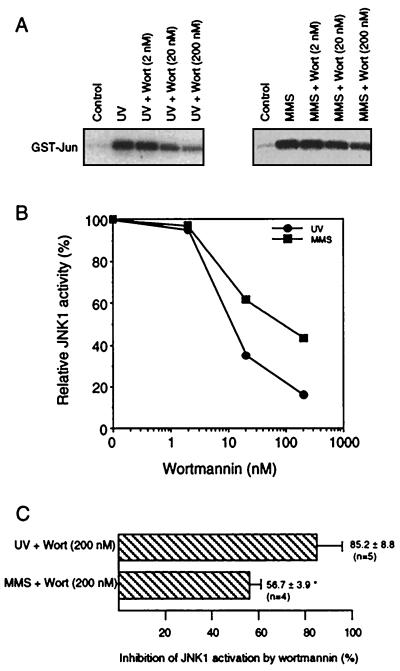
So far, stimulating effects of JNKs on transcription factors such as ATF-2 and c-Jun have been analyzed mainly by transient-transfection experiments (28, 38). One experimental approach to investigating whether JNK1 is an essential component in the transactivation of the endogenous c-jun gene is to analyze the effect of UV irradiation on c-jun expression under conditions of pharmacological inhibition of JNK1. This kind of analysis enables a valuation of the physiological significance of JNK1 for UV-driven c-jun expression within the natural cellular context. Since PI 3-kinase is assumed to be involved in the regulation of the small GTPase Rac by platelet-derived growth factor (17, 36) and Rac is known to play an important role in the UV-induced activation of JNKs, but not ERKS (6, 29), the question arose whether inhibition of PI 3-kinase by the specific inhibitor wortmannin might affect stimulation of JNKs by UV light. As shown in Fig. 3A (upper panel), wortmannin largely reduced UV-mediated activation of JNK1. Wortmannin also reduced the extent of UV-induced phosphorylation of JNK1 as analyzed by Western blotting with phosphospecific JNK antibody (Fig. 3A, lower panel). An inhibitory effect of wortmannin was not observed for UV-driven stimulation of ERK2 (Fig. 3B), which indicates the specificity of the effect evoked by wortmannin. To analyze whether differences do exist in the inhibitory capacity of wortmannin for UV- and MMS-induced JNK1 activation, dose-response analyses were performed (Fig. 4A and B). Since ∼10 nM wortmannin caused reduction of UV-stimulated JNK activation by 50%, we suggest that the inhibitory effect of wortmannin is due to a specific inhibition of PI 3-kinase. In the case of MMS-driven JNK1 stimulation, ∼100 nM wortmannin was required to reduce JNK1 activity by ∼50%. At higher concentrations of wortmannin (e.g., 200 nM), the UV-stimulated JNK1 activity was inhibited by 80 to 90% and MMS-driven JNK1 activation was inhibited by 50 to 60% (Fig. 4C). Thus, the most specific and efficient inhibitory effect of wortmannin was found for the stimulation of JNK1 by UV-C, indicating that PI 3-kinase-coupled receptors are important elements in UV-induced signaling to JNKs.
FIG. 3.
UV activation of JNK1 but not ERK2 is blocked by the PI 3-kinase inhibitor wortmannin. (A) Logarithmically growing NIH 3T3 cells were pretreated or not (Control) with 200 nM wortmannin. After an incubation period of 30 min, cells were UV irradiated (40 J/m2) or treated with MMS (2 mM). After a further incubation period of 30 min (for UV irradiation) and 60 min (for MMS) in the presence of the corresponding concentration of wortmannin, cells were harvested for determination of JNK1 activity (upper panel). An aliquot of the immunoprecipitated JNK1 was subjected to Western blot analysis with a phosphospecific JNK antibody (pJNK1 [lower panel]). Shown are the autoradiograms. Autoradiograms were densitometrically analyzed, and relative JNK1 activity (and the amount of pJNK1, respectively) in the untreated control was set to 1.0. (B) Logarithmically growing NIH 3T3 cells were pretreated or not (Control) with 200 nM wortmannin. After an incubation period of 30 min, cells were UV irradiated (40 J/m2). Ten minutes after irradiation, cells were harvested for determination of ERK2 activation by Western blot analysis. Arrows indicate the positions of the nonphosphorylated (lower band) and phosphorylated (activated) (upper band) ERK2 protein.
FIG. 4.
Wortmannin preferentially inhibits UV-driven activation of JNK1. (A) Logarithmically growing NIH 3T3 cells were not treated (Control) or pretreated for 30 min with the indicated concentration of wortmannin (Wort; 2 to 200 nM). Thereafter, cells were either UV irradiated (40 J/m2) or treated with MMS (1 mM). After a further incubation period of 30 min (for UV irradiation) and 60 min (for MMS) in the presence of the corresponding concentration of wortmannin, cells were harvested for determination of JNK1 activity. Shown are the autoradiograms. (B) Quantitative densitometric analysis of the autoradiograms shown in panel A. (C) Statistical analysis of the inhibitory effect of wortmannin on JNK1 activation by UV irradiation and MMS treatment, respectively. Data shown are mean values ± standard deviations from five (UV) and four (MMS) independent experiments, respectively. ∗, P < 0.01.
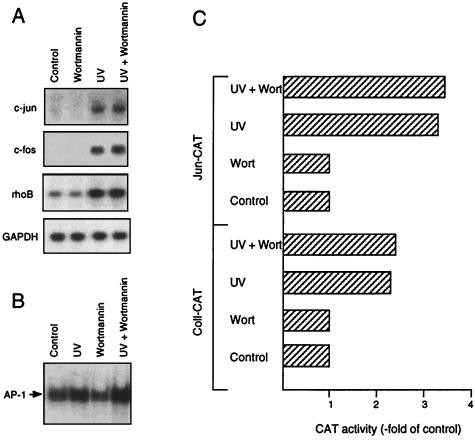
Next we analyzed whether the wortmannin-mediated reduction in the UV-driven activation of JNK1 affects the induction of c-Jun protein. Surprisingly, the increase in c-Jun protein after treatment of cells with both UV and MMS was not affected by pretreatment with wortmannin (Fig. 5), indicating that inhibition of JNK1 stimulation does not block c-jun expression. This was verified by Northern blot analysis showing that the UV-induced increase in c-jun mRNA level was not reduced by wortmannin (Fig. 6A). The same was true for other immediate-early inducible genes such as c-fos and rhoB (Fig. 6A) (13). In line with these data, the UV-induced rise in AP-1 binding activity was not inhibited by wortmannin (Fig. 6B). We should note that we determined in parallel the inhibitory effect of wortmannin on JNK1 stimulation, in order to ensure the effectiveness of treatment (data not shown). We also analyzed the effect of wortmannin on the UV-stimulated transactivation of the c-jun and collagenase promoters. Exposure to UV light resulted in a ∼3.5- and a ∼2.5-fold increase in the activity of the promoters of c-jun and collagenase, respectively. Pretreatment of cells with wortmannin did not inhibit the extent of activation of both promoters by UV (Fig. 6C). Based on the data, we conclude that the activation of JNK1 by UV is not decisive for the transcriptional activation of c-jun.
FIG. 5.
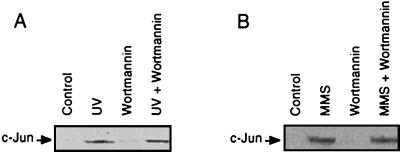
UV- and MMS-stimulated induction of c-Jun protein is not impaired by wortmannin. Cells pretreated or not (Control) with wortmannin (200 nM, 30 min) were irradiated with 40 J/m2 (A) or treated with 1 mM MMS (B). Four hours after exposure, the amount of c-Jun protein was analyzed by Western blotting with c-Jun-specific antibody.
FIG. 6.
Wortmannin does not inhibit UV-stimulated expression of c-jun. (A) NIH 3T3 cells were pretreated or not (Control) with wortmannin (200 nM). Thirty minutes later, cells were UV irradiated (40 J/m2), and after a further incubation period of 30 min in the presence of wortmannin, total RNA was isolated and subjected to Northern blot analysis with c-jun, c-fos, and rhoB-specific hybridization probes. As a control for the amount of RNA loaded onto the filter, rehybridization was done with a GAPDH probe. Shown are the autoradiograms. (B) Wortmannin treatment and UV irradiation were performed as described for panel A. Four hours after irradiation, cells were harvested for determination of AP-1 binding activity. The autoradiogram is shown. (C) NIH 3T3 cells were transfected with 5 μg of collagenase-CAT construct (Coll-CAT) and c-jun-CAT construct (Jun-CAT), by the calcium phosphate coprecipitation technique. Twenty-four hours after transfection, cells were pretreated or not (Control) with wortmannin (Wort; 200 nM) for 30 min. Subsequently, cells were irradiated (40 J/m2) or not (Control) and further incubated for 4 h in the presence of wortmannin (200 nM). Thereafter, medium was replaced by fresh medium. After an incubation period of 24 h, cells were harvested for determination of the amount of CAT protein by an enzyme-linked immunosorbent assay-based assay system (Boehringer).
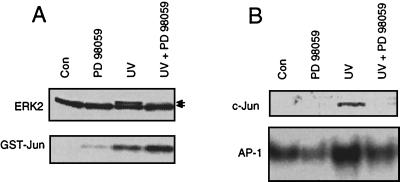
As we have shown above in Fig. 3B, wortmannin did not affect the activation of ERK2 by UV. In view of this, we asked whether stimulation of ERK2 might be sufficient for induction of c-jun by UV irradiation. To address this question, we used the MEK inhibitor PD98059, which specifically blocked UV activation of ERK2 without inhibiting JNK1 stimulation (Fig. 7A). As shown in Fig. 7B, inhibition of ERK2 activation was accompanied by obstruction of the UV-stimulated increase in c-Jun protein level and AP-1 binding. Overall, these data indicate that ERK2 activation is essential for the UV-driven increase in c-Jun protein level and AP-1 binding activity whereas JNK1 stimulation is not essentially required for transactivation of c-jun by UV light.
FIG. 7.
MEK inhibitor PD98059 blocks UV-stimulated ERK2 activation and impairs induction of c-Jun and AP-1. (A) Logarithmically growing NIH 3T3 cells were pretreated or not (control [Con]) with 50 μM MEK inhibitor PD98059. After an incubation period of 30 min, cells were UV irradiated (40 J/m2). Ten minutes after irradiation, cells were harvested for determination of activation of ERK2 and JNK1, as described in Materials and Methods. Shown are the autoradiograms. (B) Pretreatment with PD98059 and subsequent UV irradiation of cells were performed as described for panel A. After an incubation period of 4 h in the presence of PD98059 (50 μM), cells were harvested for Western blotting (c-Jun) and AP-1 binding analysis (AP-1). The autoradiograms are shown.
DISCUSSION
JNK1 is known to be a major JNK/SAPK which is stimulated after UV irradiation of cells (14, 47). This work was performed to elucidate whether activation of JNK1 is an essential component in the induction of endogenous c-jun RNA and c-Jun protein and the rise in AP-1 binding activity. In particular, we wished to address the question of the physiological significance of JNK1 stimulation by UV-C for transactivation of c-jun. To this end, we investigated the effect of UV irradiation on the expression of the endogenous c-jun gene under conditions of JNK1 inhibition. Furthermore, we analyzed the effects of different types of DNA-damaging agents on JNK1 activity, on the level of c-Jun protein, and on AP-1 binding activity.
We demonstrate that treatment of cells with UV and the alkylating agent MMS results in activation of JNK1, stimulation of the c-jun promoter, an increase in the amount of c-Jun protein, and stimulation of AP-1 binding activity. Under identical experimental conditions (e.g., equitoxic doses), various cytostatic drugs, which are frequently used in cancer therapy, neither evoked stimulation of JNK1 activity nor increased the c-Jun protein level and AP-1 binding. Therefore, we suppose that rapid activation of JNK1 and the subsequent increase in c-jun expression and AP-1 activity are not general early responses of cells to genotoxic stress. Obviously, the stimulation of JNKs and c-jun expression depends on specific properties of the genotoxic agent to which the cells are exposed. This conclusion is in line with data recently reported by Liu et al. (27). The clinically relevant antineoplastic agents used in our study induce DNA cross-links which are major cytotoxic lesions (11, 12). Therefore, a low yield of DNA damage induced by these agents may exert a high level of cytotoxicity, compared to MMS or UV, whose cytotoxicity is due to lesions other than DNA interstrand cross-links (11, 24). Thus, it is possible that the critical dose required for stimulation of JNK signaling cannot be achieved with DNA cross-linking cytostatic drugs, if applied at equitoxic doses compared with methylating agents or UV light. Overall, for the DNA-damaging agents tested, the potency in activating JNK1 was related to their ability to increase c-Jun protein level and AP-1 binding activity. On the other hand, the antineoplastic agent doxorubicin was previously reported to stimulate JNK activity (19, 35) but failed to increase the AP-1 level (7) and c-jun expression (1a). This shows that JNK1 activation is not necessarily accompanied by c-jun induction. In line with this are our observations that the cytokine interleukin 1β (IL-1β) stimulates JNK1 activity without affecting c-Jun level and AP-1 binding and that overexpression of JNK1 does not stimulate c-jun promoter activity (13a). Furthermore, treatment of cells with cisplatin lacked significant JNK activation (9, 27) but clearly induced c-jun mRNA expression (9).
The question arising from these data is whether JNK1 activation is absolutely required for UV-induced transactivation of c-jun. An experimental approach which may be useful to address this question is based on the analysis of the UV-stimulated c-jun expression under conditions of JNK1 inhibition. As a potent pharmacological inhibitor of JNK activation, we identified the PI 3-kinase inhibitor wortmannin. Wortmannin largely blocked stimulation of JNK1 activity by UV and MMS but did not affect UV activation of ERK2, indicating the specificity of inhibition. As deduced from the concentration of wortmannin which is required to inhibit JNK1 activation by 50%, PI 3-kinase appears to be specifically involved in UV-induced signaling to JNKs. The maximum inhibition of UV-driven JNK1 activation, as obtained with 200 nM wortmannin, was 80 to 90%. This is in the same range as that observed for other PI 3-kinase-regulated physiological functions (2, 31, 33, 34). The data strongly indicate that PI 3-kinase-coupled receptors (such as the platelet-derived growth factor receptor and cytokine receptors) are involved in UV-driven signaling to JNKs. This is in agreement with recent data showing the interference of multiple growth factor and cytokine receptors in the JNK signaling cascade (40). It was proposed elsewhere that the EGF receptor predominantly participates in activation of ERKs (26). However, UV stimulation of ERKs was still observed in EGF receptor-deficient cells (22). Thus, overall it remains unclear whether the EGF receptor is a dominant element in initiating UV signaling to ERKs. Since we observed an inhibitory effect of wortmannin specifically on UV-induced activation of JNK1 but not on the UV stimulation of ERKs, we suggest that different types of growth factor receptors are involved in UV-induced signaling: PI 3-kinase-coupled receptors which trigger the activation of the JNK cascade and PI 3-kinase-independent receptors interfering mainly with the stimulation of ERKs.
The availability of wortmannin as a specific inhibitor of the UV-induced activation of JNK1 enabled us to analyze the physiological relevance of JNK1 activation to the induction of the endogenous c-jun gene by UV light. Surprisingly, under conditions of strong inhibition of UV-stimulated JNK1 activation, we observed neither a reduction of the UV-stimulated c-jun mRNA expression nor an effect on c-Jun protein level, AP-1 binding activity, and activation of the c-jun and collagenase promoters. Based on this, we suggest that, although predominantly activated by UV irradiation (14, 47), UV-driven JNK1 stimulation is not essential for transactivation of c-jun expression. The hypothesis of JNK1-independent, genotoxic stress-induced expression of c-jun is in agreement with the finding that ionizing radiation (doses up to 200 Gy) does not stimulate JNK activity (27), although it evokes both c-jun and c-fos induction (16, 20, 43). Identical results have been obtained with the antineoplastic drug cisplatin (9). Furthermore, very recently it was shown that UV-mediated AP-1 activation can be blocked without inhibiting JNK activity (23). Taken together, there are different lines of evidence which contradict the prevailing view of a general, major role of JNKs (in particular, JNK1) in genotoxic stress-induced signaling leading to gene expression. It remains possible that yet insufficiently characterized JNK isoforms, which are different from JNK1 and are not predominantly activated by UV, might be decisive for UV-induced signaling to c-Jun/ATF-2 and concomitant transactivation of c-jun. Based on our finding that inhibition of ERK2 activation by the MEK inhibitor PD98059 blocked the UV-driven increase in c-Jun expression and AP-1 binding activity, we hypothesize that stimulation of ERK2 activity after UV exposure is probably physiologically more relevant for the induction of c-jun than is the activation of JNK1.
In summary, we demonstrate that (i) the early activation of JNK1 and the subsequent increase in c-Jun protein and AP-1 binding are not general responses of cells to DNA-damaging treatments; (ii) the PI 3-kinase inhibitor wortmannin specifically blocks the UV-mediated activation of JNK1 but does not affect stimulation of ERK2; (iii) wortmannin-mediated blockage of UV-stimulated JNK1 activation does not inhibit the UV-driven increase in c-jun mRNA, c-Jun protein, AP-1 binding, and c-jun promoter activity; and (iv) inhibition of UV-mediated ERK2 activation by PD98059 is accompanied by inhibition of c-Jun induction and AP-1 activation. Based on the data, we suggest that PI 3-kinase-coupled growth factor receptors are important upstream elements in UV-induced signaling to JNKs. Since c-jun expression was not altered under conditions of JNK1 inhibition but was impaired by inhibition of ERK2, we further suggest that stimulation of JNK1 activity is not essential for transcriptional activation of the endogenous c-jun gene, whereas ERK2 stimulation is required.
ACKNOWLEDGMENTS
We thank H. J. Rahmsdorf (Karlsruhe, Germany) for providing c-jun, c-fos, and GAPDH hybridization probes as well as the promoter CAT constructs used. Furthermore, we thank C. Kost for technical assistance and D. Wilhelm as well as P. Angel (DKFZ, Heidelberg, Germany) for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (Fr 1241/1-1 and SFB 519, B4).
REFERENCES
- 1.Angel P, Bhattari K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product Jun/AP-1. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 1a.Angel, P. Personal communication.
- 2.Berger J, Hayes N, Szalkowski D M, Zhang B. PI 3-kinase activation is required for insulin stimulation of glucose transport into L6 myotubes. Biochem Biophys Res Commun. 1994;205:570–576. doi: 10.1006/bbrc.1994.2703. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Canman C E, Kastan M B. Three paths to stress relief. Nature. 1996;384:213–214. doi: 10.1038/384213a0. [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Coso A A, Chiariello M, Yu J-C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 7.Das K C, White C W. Activation of NF-kappaB by antineoplastic agents. Role of protein kinase C. J Biol Chem. 1997;272:14914–14920. doi: 10.1074/jbc.272.23.14914. [DOI] [PubMed] [Google Scholar]
- 8.Derijard B, Hibi M, Wu I H, Barrett T, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV-light and Ha-ras that binds and phosphorylates the c-jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 9.Fokstuen T, Rabo Y B, Zhou J N, Karlson J, Platz A, Shoshan M C, Hansson J, Linder S. The Ras farnesylation inhibitor BZA-5B increases the resistance to cisplatin in a human melanoma cell line. Anticancer Res. 1997;17:2347–2352. [PubMed] [Google Scholar]
- 10.Foltz I N, Gerl R E, Wieler J S, Luckach M, Salmon R A, Schrader J W. Human mitogen-activated protein kinase kinase 7 (MKK7) is a highly conserved c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activated by environmental stresses and physiological stimuli. J Biol Chem. 1998;273:9344–9351. doi: 10.1074/jbc.273.15.9344. [DOI] [PubMed] [Google Scholar]
- 11.Friedberg E C, Wallner G C, Siede W. DNA repair and mutagenesis. Washington, D.C: ASM Press; 1995. [Google Scholar]
- 12.Fritz G, Hengstler J G, Kaina B. High-dose selection with mafosfamide results in sensitivity to DNA cross-linking agents: characterization of hypersensitive cell lines. Cancer Res. 1997;57:454–460. [PubMed] [Google Scholar]
- 13.Fritz G, Kaina B, Aktories K. The Ras-related small GTP-binding protein RhoB is immediate-early inducible by DNA-damaging treatments. J Biol Chem. 1995;270:25172–25177. doi: 10.1074/jbc.270.42.25172. [DOI] [PubMed] [Google Scholar]
- 13a.Fritz, G., and B. Kaina. Unpublished data.
- 14.Gupta S, Campbell D, Derijard B, Davis R J. Transcription factor ATF-2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 15.Haas S, Kaina B. c-Fos is involved in the cellular defense against the genotoxic effect of UV radiation. Carcinogenesis. 1995;16:985–991. doi: 10.1093/carcin/16.5.985. [DOI] [PubMed] [Google Scholar]
- 16.Hallahan D E, Sukhatme V P, Sherman M L, Virudachalam S, Kufe D, Weichselbaum R R. Protein kinase C mediates X-ray inducibility of nuclear signal transducers EGR1 and Jun. Proc Natl Acad Sci USA. 1991;88:2156–2160. doi: 10.1073/pnas.88.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins P T, Eguinoa A, Qiu R G, Stokoe D, Cooke F T, Walters R, Wennstrom S, Claesson W L, Evans T, Symons M, et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 18.Herr I, van Dam G, Angel P. Binding of promoter-associated AP-1 is not altered during induction and subsequent repression of the c-jun promoter by TPA and UV-irradiation. Carcinogenesis. 1994;15:1105–1113. doi: 10.1093/carcin/15.6.1105. [DOI] [PubMed] [Google Scholar]
- 19.Herr I, Wilhelm D, Böhler T, Angel P, Debatin K-M. Activation of CD95 (APO-1/Fas) signaling by ceramide mediated cancer-therapy induced apoptosis. EMBO J. 1997;16:6200–6208. doi: 10.1093/emboj/16.20.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herskind C, Flentje M, Peschke P, Lorenz W J, Hahn E W. Radiation-induced expression of the c-fos proto-oncogene in normal and tumour-derived cell lines. In: Chapman J D, Dewey W C, Whitmore G F, editors. Radiation research: a twentieth century perspective. Vol. 1. San Diego, Calif: Academic Press, Inc.; 1991. [Google Scholar]
- 21.Hilberg F, Wagner E F. Embryonic stem (ES) cells lacking functional c-jun: consequences for growth and differentiation, AP-1 activity and tumorigenicity. Oncogene. 1992;7:2371–2380. [PubMed] [Google Scholar]
- 22.Huang C, Ma W, Bowden G T, Dong Z. Ultraviolet B-induced activator protein-1 activation does not require epidermal growth factor receptor but is blocked by a dominant negative PKClambda/iota. J Biol Chem. 1996;271:31262–31268. doi: 10.1074/jbc.271.49.31262. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Ma W-Y, Ryan C A, Dong Z. Proteinase inhibitors I and II from potatoes specifically block UV-induced activator protein-1 activation through a pathway that is independent of extracellular signal-regulated kinase, c-Jun N-terminal kinases and p38 kinase. Proc Natl Acad Sci USA. 1997;94:11957–11962. doi: 10.1073/pnas.94.22.11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaina B, Fritz G, Coquerelle T. Contribution of O6-alkylguanine and N-alkylpurines to the formation of sister chromatid exchanges, chromosomal aberrations, and gene mutations: new insights gained from studies of genetically engineered mammalian cell lines. Environ Mol Mutagen. 1993;22:283–292. doi: 10.1002/em.2850220418. [DOI] [PubMed] [Google Scholar]
- 25.Kaina B, Haas S, Kappes H. A general role for c-Fos in cellular protection against DNA-damaging carcinogens and cytostatic drugs. Cancer Res. 1997;57:2721–2731. [PubMed] [Google Scholar]
- 26.Knebel A, Rahmsdorf H J, Ullrich A, Herrlich P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 1996;15:5314–5325. [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z-G, Baskaran R, Lea-Chou E T, Wood L D, Chen Y, Karin M, Wang J Y J. Three distinct signaling responses by murine fibroblasts to genotoxic stress. Nature. 1996;384:273–276. doi: 10.1038/384273a0. [DOI] [PubMed] [Google Scholar]
- 28.Livingstone C, Patel G, Jones N. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 1995;14:1785–1797. doi: 10.1002/j.1460-2075.1995.tb07167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minden A, Lin A, Claret F-X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 30.Moriguchi T, Toyoshima F, Masuyama M, Hanafusa H, Gotoh Y, Nishida E. A novel SAPK/JNK kinase, MKK7, stimulated by TNFα and cellular stresses. EMBO J. 1997;16:7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura M, Nakashima S, Katagiri Y, Nozawa Y. Effect of wortmannin and 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) on N-formyl-methyionyl-leucyl-phenylanine-induced phospholipase D activation in differentiated HL60 cells: possible involvement of phosphatidyl 3-kinase in phospholipase D activation. Biochem Pharmacol. 1997;53:1929–1936. doi: 10.1016/s0006-2952(97)00169-x. [DOI] [PubMed] [Google Scholar]
- 32.Nishina H, Fischer K D, Radvanyi L, Shahinian A, Hakem R, Rubie E A, Bernstein A, Mak T W, Woodgett J R, Penninger J M. Stress-signaling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature. 1997;385:350–353. doi: 10.1038/385350a0. [DOI] [PubMed] [Google Scholar]
- 33.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 34.Okada T, Sakuma L, Fukui Y, Hazeki O, Ui M. Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:3536–3567. [PubMed] [Google Scholar]
- 35.Osborne M T, Chambers T C. Role of the stress-activated c-Jun NH2-terminal protein kinase pathway in the cellular response to adriamycin and other chemotherapeutic drugs. J Biol Chem. 1996;271:30950–30955. doi: 10.1074/jbc.271.48.30950. [DOI] [PubMed] [Google Scholar]
- 36.Parker P J. PI 3-kinase puts GTP on the Rac. Curr Biol. 1995;5:577–579. doi: 10.1016/s0960-9822(95)00113-8. [DOI] [PubMed] [Google Scholar]
- 37.Price M A, Cruzalegui F H, Treisman R. The p38 and ERK MAP kinase pathways cooperate to activate ternary complex factors and c-fos transcription in response to UV light. EMBO J. 1996;23:6552–6563. [PMC free article] [PubMed] [Google Scholar]
- 38.Pulverer B J, Kyriakis J M, Avruch J, Nikolakaki E, Woodgett J R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 39.Radler-Pohl A, Sachsenmaier C, Gebel S, Auer H-P, Bruder J T, Rapp U, Angel P, Rahmsdorf H J, Herrlich P. UV-induced activation of AP-1 involves obligatory extranuclear steps including Raf-1 kinase. EMBO J. 1993;12:1005–1012. doi: 10.1002/j.1460-2075.1993.tb05741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 41.Sachsenmaier C, Radler-Pohl A, Zinck R, Nordheim A, Herrlich P, Rahmsdorf H J. Involvement of growth factor receptors in the mammalian UVC response. Cell. 1994;78:963–972. doi: 10.1016/0092-8674(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber M, Baumann B, Cotton M, Angel P, Wagner E F. Fos is an essential component of the mammalian UV response. EMBO J. 1995;14:5338–5349. doi: 10.1002/j.1460-2075.1995.tb00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherman M L, Datta R, Hallahan D E, Weichselbaum R R, Kufe D W. Ionizing radiation regulates expression of the c-jun protooncogene. Proc Natl Acad Sci USA. 1990;87:5663–5666. doi: 10.1073/pnas.87.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smeal T, Binetruy B, Mercola D, Grover-Bardwick A, Heidecker G, Rapp U R, Karin M. Oncoprotein-mediated signaling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol Cell Biol. 1992;12:3507–3513. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smeal T, Binetruy B, Mercola D A, Birrer M, Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991;354:494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- 46.van Dam H, Duyndam M, Rottier R, Bosch A, de Vries-Smits L, Herrlich P, Zantema A, Angel P, van-der-Eb A J. Heterodimer formation of c-Jun and ATF-2 is responsible for induction of c-jun by the 243 amino acid adenovirus E1A protein. EMBO J. 1993;12:479–483. doi: 10.1002/j.1460-2075.1993.tb05680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic stress. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X Z, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science. 1996;272:1347–1349. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- 49.Wilhelm D, Bender K, Knebel A, Angel P. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol. 1997;17:4792–4800. doi: 10.1128/mcb.17.8.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang D, Tournier C, Wysk M, Lu H-T, Xu J, Davies R J, Flavell R A. Targeted disruption of the MKK4 gene causes embryonic death, inhibition of c-Jun NH2-terminal kinase activation and defects in AP-1 transcriptional activity. Proc Natl Acad Sci USA. 1997;94:3004–3009. doi: 10.1073/pnas.94.7.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]