Abstract
We investigated mortality and predictors of mortality due to intensive care unit-associated candidemia (ICUAC) versus non-ICUAC by Candida species. This study included all candidemia cases in 11 hospitals from 2017 to 2018 in South Korea. The all-cause mortality rates in all 370 patients with ICUAC were approximately twofold higher than those in all 437 patients with non-ICUAC at 7 days (2.3-fold, 31.1%/13.3%), 30 days (1.9-fold, 49.5%/25.4%), and 90 days (1.9-fold, 57.8%/30.9%). Significant species-specific associations with 7- and 30-day ICUAC-associated mortality were not observed. Multivariate analysis revealed that ICU admission was an independent predictor of Candida glabrata (OR, 2.07–2.48) and Candida parapsilosis-associated mortality (OR, 6.06–11.54). Fluconazole resistance was a predictor of C. glabrata-associated mortality (OR, 2.80–5.14). Lack (less than 3 days) of antifungal therapy was the strongest predictor of 7-day mortality due to ICUAC caused by Candida albicans (OR, 18.33), Candida tropicalis (OR, 10.52), and C. glabrata (OR, 21.30) compared with 30- and 90-day mortality (OR, 2.72–6.90). C. glabrata ICUAC had a stronger association with lack of antifungal therapy (55.2%) than ICUAC caused by other species (30.6–36.7%, all p < 0.05). Most predictors of mortality associated with ICUAC were distinct from those associated with non-ICUAC and were mediated by Candida species.
Keywords: ICU, candidemia, mortality, Candida species, lack of antifungal therapy
1. Introduction
Candidemia is an opportunistic infection associated with high morbidity and mortality rates [1,2,3]. In recent decades, the epidemiology of candidemia has greatly changed globally [4]. Although Candida albicans is the most common species isolated, the proportions of candidemia due to non-albicans Candida species (NAC), such as Candida glabrata, Candida tropicalis, and Candida parapsilosis, continue to increase [5], and the emergence of antifungal resistance in NAC bloodstream isolates has become a matter of concern [6]. Candidemia in intensive care unit (ICU) patients (ICU-associated candidemia, hereafter ICUAC) is an important issue due to its increasing incidence and higher mortality rate than non-ICUAC [7,8,9,10]. Given that each Candida species has a unique virulence potential, antifungal susceptibility pattern, and clinical characteristics [5], the changing epidemiology of candidemia may have different implications for the management of—and mortality due to—ICUAC and non-ICUAC according to Candida species.
The data on 7, 30, and 90-day mortality rates and predictive factors of ICUAC-associated mortality due to different Candida species compared with those of non-ICUAC have not yet been elucidated in detail. In addition, despite recent advances in the antifungal management of ICU patients at high risk of invasive candidiasis [11,12], questions on the impact of lack of antifungal therapy on the Candida species-related mortality of ICUAC remain. Therefore, we performed a multicenter study of the epidemiology of candidemia and the antifungal resistance profiles of Candida bloodstream isolates at 11 hospitals in South Korea over a 2-year period. In this large population of patients with candidemia, we investigated the clinical features, therapeutic practices including lack of antifungal therapy, and all-cause mortality rates according to Candida species in ICUAC vs. non-ICUAC patients. We also assessed the clinical and microbiological variables associated with mortality due to ICUAC vs. non-ICUAC caused by four common Candida species. This is the first contemporary study to show significant differences in 7-, 30- and 90-day mortality rates and predictors of mortality between patients with ICUAC caused by four common Candida species and those with non-ICUAC in South Korea.
2. Materials and Methods
2.1. Candida Isolates and Clinical Data Collection
This multicentre study was designed to investigate all cases of Candida bloodstream infections (BSIs) occurring from January 2017 to December 2018 in 11 university hospitals that were participating in a national survey of antimicrobial resistance in South Korea [13,14,15]. The 11 hospitals are located in different districts throughout South Korea and have a total of 8762 beds, ranging from 705 to 1779 beds per hospital. The first Candida-positive blood samples per patient per species collected [13,14,15], and all Candida isolates, were submitted to Chonnam National University Hospital (Gwangju, Korea) for species identification and antifungal susceptibility testing. A total of 829 nonduplicated Candida BSI isolates were collected from 807 patients during the 2-year period. Among the 807 patients, 786 had positive blood cultures for a single Candida species (338 C. albicans, 149 C. tropicalis, 147 C. glabrata and 111 C. parapsilosis and 41 uncommon species), and more than two Candida species were isolated simultaneously from 21 patients (mixed candidemia). Information on patient demographics (age, sex, infection origin, admission types), comorbidities, severity of infection (age-adjusted Charlson comorbidity index, severe sepsis, bacteremia), clinical status at the time of positive culture (prior antifungal therapy before blood culture, total parenteral nutrition, surgery within 30 days, neutropenia (less than 500/µL), immunosuppressive therapy, urinary catheter, central venous catheter (CVC)), and therapeutic measures (antifungal therapy and CVC removal) was collected at each sentinel hospital [14,15,16].
2.2. Species Identification and Antifungal Susceptibility Testing
Candida species were identified based on matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (Biotyper; Bruker Daltonics, Billerica, MA, USA) with library version 4.0 or sequencing of the D1/D2 domains of the 26S rRNA gene [14,16,17]. In vitro antifungal tests for susceptibility to fluconazole, amphotericin B, and micafungin were performed using the CLSI M27-A3 broth microdilution method. The interpretative guideline in the CLSI document M60 ED1 was used to classify isolates using species-specific clinical break points (CBPs) [18]. If there was no CBP for an antifungal agent for the corresponding species, epidemiological cutoff values (ECVs) were used [19,20].
2.3. Definition
Candidemia was defined as the isolation of Candida from at least one blood culture [11]; cases of invasive candidiasis without candidemia or colonization were excluded. If the patient with candidemia was hospitalized in the ICU at the time of blood sampling, the candidemia was regarded as ICUAC. Antifungal therapy was defined as the administration of systemic antifungal agents for >72 h; lack of antifungal therapy was defined as no antifungal therapy, or treatment with antifungals for less than 3 days after the date of the first positive blood culture collection [17].
2.4. Statistical Analysis
The relationships between mortality and demographic, clinical, and microbiological variables were analyzed by multivariate analysis. Variables with a p-value < 0.1 on univariate analysis were included in multivariate analysis. Univariate analyses were based on the chi-squared or Fisher’s exact test, as appropriate, for discrete variables. To identify factors associated with 7-, 30-, and 90-day mortality, backward stepwise Cox regression models were used. Statistical analysis was performed using SPSS (version 26, IBM Corp., Armonk, NY, USA). Statistical significance was determined at a level of p < 0.05.
3. Results
3.1. Species Distribution and Antifungal Resistance
The species distributions and antifungal susceptibility profiles of 829 nonduplicate Candida bloodstream isolates from 807 patients are listed in Table 1. The four most common species were C. albicans (42.6%), C. glabrata (19.2%), C. tropicalis (18.8%), and C. parapsilosis (13.5%). When we compared the frequencies of Candida species between the ICUAC and non-ICUAC groups, C. tropicalis was more common in the ICUAC group (23.2% vs. 15.0%, p = 0.003). Resistance to fluconazole and micafungin was found in 4.2% (34/813) and 0.2% (2/813) of the Candida isolates, respectively. Fluconazole resistance was detected in 6.3% (ICUAC 9.2% vs. non-ICUAC 4.3%) of C. glabrata isolates, 1.3% (1.1% vs. 1.5%) of C. tropicalis isolates, 3.6% (8.2% vs. 0%, p = 0.034) of C. parapsilosis isolates, and only 0.3% (0% vs. 0.5%) of C. albicans isolates. Overall, fluconazole resistance was more frequent in ICU settings than in non-ICU settings (6.1% vs. 2.5%, p = 0.010). Resistance to amphotericin B (exceeding ECV = 2 mg/L) was detected in only one Candida haemulonii ICU isolate.
Table 1.
Species distribution and antifungal susceptibilities of 829 Candida bloodstream isolates from 370 ICU and 437 non-ICU patients in 11 hospitals over a two-year period.
| Species | No (%) of Isolates | No. (%) Fluconazole Resistance 1 | No. (%) Micafungin Resistance 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | ICU | Non-ICU | Total | ICU | Non-ICU | Total | ICU | Non-ICU | |
| C. albicans | 353 (42.6) | 156 (40.7) | 197 (44.2) | 1 (0.3) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C. glabrata | 159 (19.2) | 65 (17.0) | 94 (21.1) | 10 (6.3) | 6 (9.2) | 4 (4.3) | 1 (0.6) | 1 (1.5) | 0 (0.0) |
| C. tropicalis | 156 (18.8) | 89 (23.2) 2 | 67 (15.0) | 2 (1.3) | 1 (1.1) | 1 (1.5) | 1 (0.6) | 0 (0.0) | 1 (1.5) |
| C. parapsilosis | 112 (13.5) | 49 (12.8) | 63 (14.1) | 4 (3.6) | 4 (8.2) 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C. krusei | 15 (1.8) | 10 (2.6) | 5 (1.1) | 15 (100) | 10 (100) | 5 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C. lusitaniae | 9 (1.1) | 5 (1.3) | 4 (0.9) | 1 (11.1) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C. guilliermondii | 8 (1.0) | 1 (0.3) | 7 (1.6) | 1 (12.5) | 1 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C. dubliniensis | 1 (0.1) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | - |
| Others 3 | 16 (1.9) | 7 (1.8) | 9 (2.0) | NA | NA | NA | NA | NA | NA |
| Total | 829 (100) 4 | 383 (100) | 446 (100) | 34 (4.2) | 23 (6.1) 2 | 11 (2.5) | 2 (0.2) | 1 (0.3) | 1 (0.2) |
Abbreviations: ICU, intensive care unit; NA, non-applicable. 1 Total proportion of isolates for which the MICs exceed the current Clinical and Laboratory Standards Institute (CLSI) species-specific breakpoints or epidemiological cutoff values [18,19,20]. Total resistance to fluconazole was evident in 4.2% (34/813), 6.1% (23/376), and 2.5% (11/437) of the total, ICU, and non-ICU isolates, respectively; resistance to micafungin was evident in 0.2% (2/813), 0.3% (1/376), and 0.2% (1/437) of the total, ICU, and non-ICU isolates, respectively. 2 p-value < 0.05, ICU vs. non-ICU. 3 Other Candida species, for which CLSI breakpoints or epidemiological cutoff values are absent, include 7 isolates (2 C. haemulonii, 2 C. pelliculosa, 1 C. fabianii, 1 C. lipolytica, and 1 C. utilis) from ICU patients and 9 isolates (2 C. intermedia, 2 C. orthopsilosis, 1 C. haemulonii, 1 C. pelliculosa, 1 C. auris, 1 C. metapsilosis, and 1 C. norvegensis) from non-ICU patients. 4 A total of 829 non-duplicate Candida bloodstream isolates from 807 patients. Only one Candia species was recovered from 786 patients, but more than two different Candida species were recovered from 21 patients (9 C. albicans + C. glabrata, 3 C. albicans + C. tropicalis, 2 C. tropicalis + C. krusei, 1 C. albicans + C. guilliermondii, 1 C. albicans + C. lusitaniae + C. intermedia, 1 C. albicans + C. dublinensis, 1 C. tropicalis + C. glabrata, 1 C. tropicalis + C. lusitaniae, 1 C. glabrata + C. parapsilosis, and 1 C. glabrata + C. guilliermondii).
3.2. Baseline Characteristics
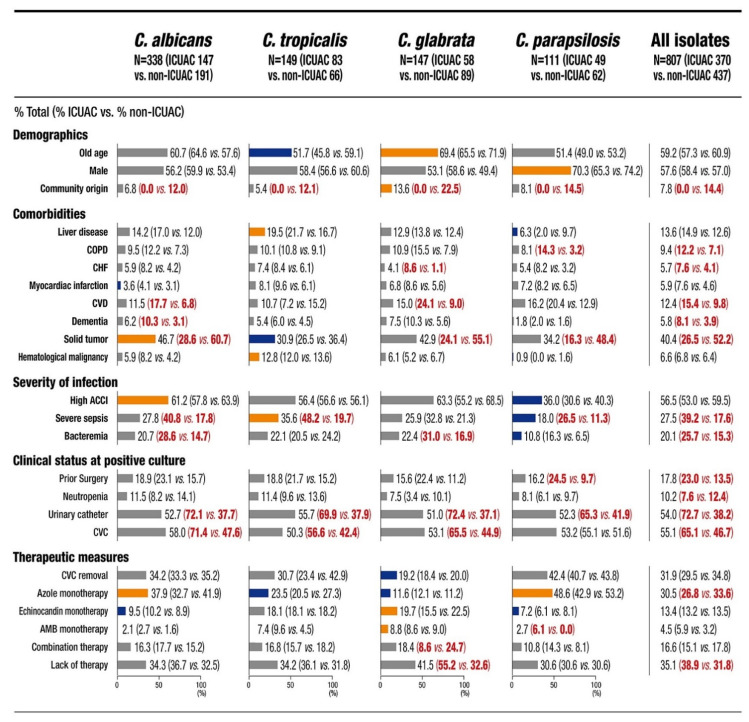
Among the 807 patients, 370 (45.8%) were classified as having ICUAC. Figure 1 shows the baseline characteristics of all 807 patients and the 745 (92.3%) patients with single-species candidemia attributable to the four common Candida species. Several characteristics were more frequently associated with a particular Candida species. When ICUAC was compared to non-ICUAC by species, severe sepsis (C. albicans, C. tropicalis and C. parapsilosis), bacteremia (C. albicans, and C. glabrata), prior surgery (C. parapsilosis), urinary catheter placement (all four species), and CVC placement (C. albicans, C. tropicalis and C. glabrata) were frequently associated with ICUAC, in addition to various comorbidities. Of note, ICUAC had a stronger association with lack of antifungal therapy than non-ICUAC (38.9% vs. 31.8%, p = 0.035), especially for C. glabrata candidemia (ICUAC 55.2% vs. non-ICUAC 32.6%, p = 0.007). Additionally, C. glabrata ICUAC had a stronger association with lack of antifungal therapy (55.2%) than ICUAC caused by other species (30.6–36.7%, all p < 0.05).
Figure 1.
Baseline characteristics of the patients with intensive care unit-associated candidemia (ICUAC) versus non-ICUAC by Candida species and all species. The proportions of each variable (%) are indicated in the bar chart, and the statistical significance between a specific Candida species and all other Candida species within a given category (p < 0.05) is represented by colored bars (orange, more frequent; blue, less frequent). The statistical significance (p < 0.05) of each variable between ICUAC versus non-ICUAC within a given category is represented by red numbers. Abbreviations: COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; CVD, cerebrovascular disease; ACCI, age-adjusted Charlson comorbidity index; CVC, central venous catheter; AMB, amphotericin B.
3.3. Mortality Rate
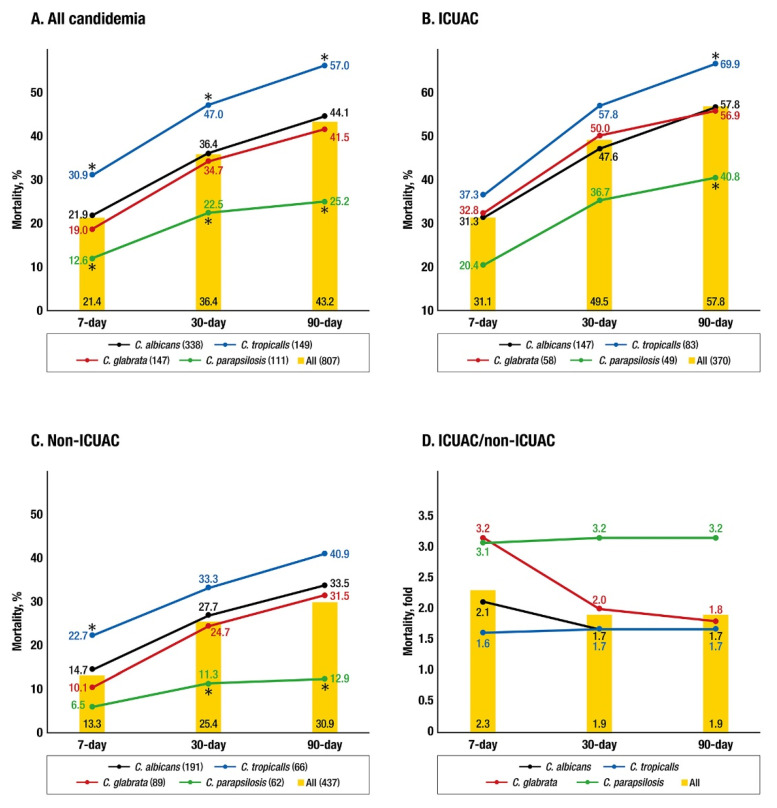
The cumulative all-cause mortality rates of 807 patients with candidemia at 7, 30, and 90 days were 21.4%, 36.4%, and 43.2%, respectively. The mortality rates in all 370 patients with ICUAC were approximately twofold higher than those in all 437 patients with non-ICUAC at 7 days (2.3 fold, 31.1%/13.3%), 30 days (1.9 fold, 49.5%/25.4%), and 90 days (1.9 fold, 57.8%/30.9%). Figure 2 shows the 7-, 30-, and 90-day mortality rates in patients with ICUAC and non-ICUAC due to the four most common species. Patients with candidemia due to C. tropicalis and C. parapsilosis showed significantly higher and lower mortality rates, respectively, than all other patients at all observation days (C. tropicalis, p = 0.002, 0.003, and <0.001; C. parapsilosis, p = 0.015, 0.001, and <0.001 at 7, 30, and 90 days, respectively, Figure 2A). For the ICUAC group, significantly higher (C. tropicalis) and lower (C. parapsilosis) species-specific mortality rates were found at only 90 days (p = 0.012 and 0.010, respectively); significant species-specific differences in mortality were not observed at 7 and 30 days. Additionally, mortality tended to be higher in patients with C. glabrata ICUAC than in those with C. albicans ICUAC at 7 and 30 days (Figure 2B). Significantly higher 7-day mortality was observed in patients with C. tropicalis non-ICUAC (p = 0.014), whereas lower 30- and 90-day mortality rates were found in patients with C. parapsilosis non-ICUAC (p = 0.006 and 0.001, respectively) (Figure 2C). When ICUAC-associated mortality due to a given Candida species was compared with non-ICUAC-associated mortality due to the same species on a given day, mortality was increased approximately twofold (1.7–2.1) in C. albicans and C. tropicalis candidemic patients at 7, 30, and 90 days and more than threefold in C. glabrata candidemic patients at 7 days (3.2 fold) and C. parapsilosis candidemic patients at 7, 30 and 90 days (3.1–3.2 fold) (Figure 2D).
Figure 2.
The cumulative 7-, 30-, and 90-day mortality rates in patients with all candidemia (338 C. albicans, 149 C. tropicalis, 147 C. glabrata, 111 C. parapsilosis, and 807 all) (A), intensive care unit-associated candidemia (ICUAC) (147 C. albicans, 83 C. tropicalis, 58 C. glabrata, 49 C. parapsilosis, and 370 all) (B), and non-ICUAC (191 C. albicans, 66 C. tropicalis, 89 C. glabrata, 62 C. parapsilosis, and 437 all) (C). Fold changes in the ICUAC mortality rates compared to the non-ICUAC mortality rates, stratified by four common Candida species and all candidemia (D). Mortality due to all candidemia within a given category is represented by yellow-colored bars. Asterisks indicate that the mortality rate in patients with candidemia due to a specific Candida species was significantly different to those in all other patients within a given category (p < 0.05).
3.4. Predictors of Mortality
Table 2 summarizes the results of a multivariate analysis of independent predictors of 7-, 30-, and 90-day mortality in all candidemic patients and patients with candidemia due to the four most common species. C. tropicalis as a causative agent was an independent predictor of higher mortality in all candidemic patients (odds ratios (ORs), 1.82 at 7 days, 1.45 at 30 days, and 1.43 at 90 days), whereas C. parapsilosis was a predictor of lower mortality (OR, 0.62 at 90 days). Several clinical variables were also independent predictors of mortality in all candidemic patients. Among those variables, lack of antifungal therapy was the only predictive factor related to 7-, 30-, and 90-day mortality in patients with candidemia due to all four Candida species; the ORs for 7-day mortality due to each type of candidemia were higher than those for 30- or 90-day mortality, especially for candidemia caused by C. glabrata (ORs, 45.86 at 7 days, 3.26 at 30 days, and 2.40 at 90 days, respectively). ICU admission was an independent predictor of mortality in candidemia caused by C. glabrata (OR, 2.48 at 7 days, 2.15 at 30 days, and 2.07 at 90 days; all p < 0.05) and C. parapsilosis (OR, 11.54 at 7 days, 6.93 at 30 days, and 6.06 at 90 days; all p < 0.05). Of note, fluconazole resistance was an independent predictor of 30- and 90-day mortality due to only C. glabrata candidemia (OR, 2.80 at 30 days and 2.84 at 90 days; all p < 0.05). Azole monotherapy was protective against mortality in candidemia due to C. albicans, C. tropicalis, and C. parapsilosis.
Table 2.
Multivariate analysis of predictive factors related to 7-, 30- and 90-day mortalities of all candidemia patients according to the Candida species.
| Species (No. of Isolates) and Variables 1 | 7-Day | 30-Day | 90-Day | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| All (807) | ||||||
| Lack of antifungal therapy | 14.58 (9.77–21.76) | <0.001 | 4.71 (3.71–5.98) | <0.001 | 3.74 (3.00–4.65) | <0.001 |
| CVC placement | 2.03 (1.40–2.95) | <0.001 | 1.59 (1.21–2.08) | 0.001 | 1.54 (1.20–1.98) | 0.001 |
| Urinary catheter placement | 1.87 (1.29–2.70) | 0.001 | 1.97 (1.47–2.63) | <0.001 | 2.10 (1.61–2.74) | <0.001 |
| Candidemia due to C. tropicalis | 1.82 (1.28–2.57) | 0.001 | 1.45 (1.10–1.91) | 0.008 | 1.43 (1.11–1.84) | 0.006 |
| Severe sepsis | 1.78 (1.30–2.45) | <0.001 | 1.92 (1.49–2.46) | <0.001 | 2.04 (1.62–2.57) | <0.001 |
| Prior surgery | 0.55 (0.36–0.86) | 0.009 | - | - | - | - |
| Azole monotherapy | 0.17 (0.10–0.30) | <0.001 | 0.45 (0.33–0.60) | <0.001 | 0.50 (0.39–0.66) | <0.001 |
| Hematologic malignancies | - | - | 1.52 (1.01–2.29) | 0.04 | - | - |
| ICU admission | - | - | 1.43 (1.09–1.86) | 0.009 | 1.39 (1.09–1.76) | 0.008 |
| CVC removal | - | - | 0.64 (0.45–0.92) | 0.01 | 0.58 (0.41–0.80) | 0.001 |
| Candidemia due to C. parapsilosis | - | - | - | - | 0.62 (0.42–0.93) | 0.01 |
| C. albicans (338) | ||||||
| Lack of antifungal therapy | 17.82 (9.32–34.08) | <0.001 | 5.87 (4.02–8.56) | <0.001 | 4.76 (3.37–6.70) | <0.001 |
| CVC placement | 2.44 (1.38–4.34) | 0.002 | 2.14 (1.39–3.29) | 0.001 | 2.22 (1.50–3.28) | <0.001 |
| Urine catheter use | 1.94 (1.13–3.33) | 0.01 | 2.26 (1.48–3.44) | <0.001 | 2.45 (1.66–3.60) | <0.001 |
| Severe sepsis | - | - | 1.80 (1.22–2.67) | 0.003 | 1.95 (1.36–2.78) | <0.001 |
| Prior surgery | 0.39 (0.18–0.85) | 0.01 | - | - | - | - |
| Azole monotherapy | 0.13 (0.06–0.29) | <0.001 | 0.35 (0.23–0.55) | <0.001 | 0.42 (0.29–0.60) | <0.001 |
| CVC removal | - | - | 0.55 (0.33–0.92) | 0.02 | 0.49 (0.30–0.81) | 0.006 |
| C. tropicalis (149) | ||||||
| Lack of antifungal therapy | 8.64 (4.52–16.53) | <0.001 | 4.19 (2.54–6.89) | <0.001 | 3.65 (2.32–5.75) | <0.001 |
| CVC placement | 2.29 (1.10–4.75) | 0.02 | - | - | - | - |
| Urinary catheter placement | 2.21 (1.02–4.81) | 0.04 | 2.59 (1.45–4.61) | 0.001 | 2.95 (1.73–5.03) | <0.001 |
| Severe sepsis | - | - | 2.18 (1.29–3.67) | 0.003 | 2.39 (1.48–3.85) | <0.001 |
| Azole monotherapy | 0.35 (0.14–0.90) | 0.02 | - | - | 0.48 (0.27–0.86) | 0.01 |
| Congestive heart failure | - | - | 2.48 (1.20–5.12) | 0.01 | 2.10 (1.03–4.28) | 0.04 |
| Neutropenia | - | - | 2.17 (1.10–4.26) | 0.02 | 2.22 (1.17–4.23) | 0.01 |
| C. glabrata (147) | ||||||
| Lack of antifungal therapy | 45.86 (6.19–339.57) | <0.001 | 3.26 (1.84–5.80) | <0.001 | 2.40 (1.43–4.01) | 0.001 |
| Severe sepsis | - | - | 1.93 (1.08–3.43) | 0.02 | 2.30 (1.37–3.86) | 0.002 |
| ICU admission | 2.48 (1.11–5.51) | 0.02 | 2.15 (1.21–3.83) | 0.009 | 2.07 (1.22–3.49) | 0.007 |
| Fluconazole resistance | - | - | 2.80 (1.17–6.66) | 0.02 | 2.84 (1.28–6.33) | 0.01 |
| C. parapsilosis (111) | ||||||
| Lack of antifungal therapy | 36.30 (4.28–307.86) | 0.001 | 9.18 (2.75–30.61) | <0.001 | 4.72 (1.51–14.76) | 0.008 |
| Urinary catheter placement | - | - | - | - | 4.35 (1.13–16.71) | 0.03 |
| Azole monotherapy | 0.05 (0.01–0.43) | 0.006 | 0.28 (0.10–0.84) | 0.02 | - | - |
| ICU admission | 11.54 (1.38–96.67) | 0.02 | 6.93 (1.84–26.09) | 0.004 | 6.06 (1.92–19.17) | 0.002 |
| CVC removal | - | - | - | - | 0.27 (0.08–0.88) | 0.02 |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval; ICU, intensive care unit; CVC, central venous catheter. 1 Only the variables that were statistically significant (p < 0.05) are listed. The results of univariate analysis are listed in Supplementary Table S1 (A–E).
Table 3 shows the independent predictors of 7-, 30-, and 90-day mortality due to ICUAC and non-ICUAC; these factors varied according to species and observation time. Lack of antifungal therapy was predictive of mortality due to ICUAC and non-ICUAC caused by the four species, except for non-ICUAC due to C. parapsilosis. Notably, lack of antifungal therapy was predictive of mortality (7-, 30-, and 90 day) due to ICUAC caused by C. albicans, C. tropicalis, and C. glabrata, and the ORs for 7-day mortality due to ICUAC caused by C. albicans (OR 18.33), C. tropicalis (OR 10.52) and C. glabrata (OR 21.30) were higher than those for 30- and 90-day mortality (ORs, 2.72–6.90). Azole monotherapy was an independent factor protective against 30- and 90-day mortality due to ICUAC caused by C. albicans, C. tropicalis, and C. parapsilosis but not ICUAC caused by C. glabrata. Fluconazole resistance was a predictor of 90-day mortality due to C. glabrata ICUAC (OR, 5.14, p = 0.002). Other than the antifungal therapy variables, most risk or protective factors for mortality due to ICUAC caused by C. tropicalis, C. glabrata, and C. parapsilosis were not predictive of mortality due to non-ICUAC caused by the same species. By contrast, most predictors of mortality due to C. albicans ICUAC (severe sepsis, urinary catheter placement, CVC placement and CVC removal) were also predictive of mortality due to C. albicans non-ICUAC.
Table 3.
Multivariate analysis of predictive factors related to 7-, 30- and 90-day mortalities of patients with ICUAC and non-ICUAC by the four common Candida species.
| Species (No. of Isolates) and Variables 1 | Setting | 7-Day | 30-Day | 90-Day | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| C. albicans (ICU 147; non-ICU 191) | |||||||
| Lack of antifungal therapy | ICUAC | 18.33 (7.65–43.92) | <0.001 | 6.90 (4.12–11.57) | <0.001 | 5.47 (3.42–8.75) | <0.001 |
| Non-ICUAC 2 | 34.64 (7.86–152.68) | <0.001 | 10.49 (4.68–23.51) | <0.001 | 3.97 (2.32–6.79) | <0.001 | |
| CVC placement | ICUAC | - | - | - | - | 1.86 (1.01–3.43) | 0.04 |
| Non-ICUAC | - | - | - | - | 2.05 (1.20–3.51) | 0.009 | |
| Urinary catheter placement | ICUAC | - | - | 2.56 (1.25–5.24) | 0.01 | 3.28 (1.65–6.51) | 0.001 |
| Non-ICUAC | - | - | 2.71 (1.25–5.84) | 0.03 | 1.79 (1.07–2.99) | 0.02 | |
| Severe sepsis | ICUAC | - | - | 1.69 (1.04–2.75) | 0.03 | 1.62 (1.03–2.56) | 0.03 |
| Non-ICUAC | - | - | - | - | 2.57 (1.49–4.43) | 0.001 | |
| Prior surgery | ICUAC | 0.40 (0.17–0.96) | 0.04 | - | - | - | - |
| Azole monotherapy | ICUAC | 0.11 (0.03–0.35) | <0.001 | 0.27 (0.14–0.52) | <0.001 | 0.33 (0.19–0.59) | <0.001 |
| Non-ICUAC | 0.06 (0.01–0.44) | 0.006 | 0.26 (0.11–0.62) | 0.002 | 0.46 (0.27–0.77) | 0.004 | |
| CVC removal | ICUAC | - | - | - | - | 0.49 (0.27–0.88) | 0.01 |
| Non-ICUAC | - | - | - | - | 0.34 (0.15–0.80) | 0.01 | |
| C. tropicalis (ICU 83; non-ICU 66) | |||||||
| Lack of antifungal therapy | ICUAC | 10.52 (3.91–28.25) | <0.001 | 4.01 (2.15–7.47) | <0.001 | 3.79 (2.14–6.74) | <0.001 |
| Non-ICUAC | 7.74 (2.59–23.17) | <0.001 | 4.54 (1.87–11.00) | 0.001 | 6.02 (2.45–14.81) | <0.001 | |
| Severe sepsis | ICUAC | - | - | 2.71 (1.42–5.19) | 0.004 | 2.16 (1.14–4.11) | 0.002 |
| Congestive heart failure | ICUAC | 3.93 (1.17–13.15) | 0.02 | 3.53 (1.43–8.69) | 0.006 | 3.16 (1.31–7.63) | 0.01 |
| Neutropenia | ICUAC | - | - | 2.50 (1.06–5.88) | 0.03 | 2.50 (1.09–5.73) | 0.01 |
| Azole monotherapy | ICUAC | - | - | 0.21 (0.08–0.59) | 0.003 | 0.20 (0.08–0.46) | 0.03 |
| C. glabrata (ICU 58; non-ICU 89) | |||||||
| Lack of antifungal therapy | ICUAC | 21.30 (2.79–162.83) | 0.003 | 2.72 (1.12–6.62) | 0.02 | 3.41 (1.59–7.32) | 0.002 |
| Non-ICUAC | - | - | 5.02 (1.82–13.88) | 0.002 | - | - | |
| Urinary catheter placement | ICUAC | - | - | 6.81 (1.71–27.06) | 0.006 | - | - |
| Solid tumor | ICUAC | 3.64 (1.38–9.57) | 0.009 | 2.86 (1.23–6.70) | 0.01 | - | - |
| Chronic kidney disease | ICUAC | - | - | 4.05 (1.45–11.31) | 0.007 | - | - |
| Fluconazole resistance | ICUAC | - | - | - | - | 5.14 (1.80–14.65) | 0.002 |
| C. parapsilosis (ICU 49; non-ICU 62) | |||||||
| Lack of antifungal therapy | ICUAC | - | - | 22.77 (4.17–124.26) | <0.001 | 12.90 (4.16–39.98) | <0.001 |
| Urinary catheter placement | ICUAC | - | - | - | - | 4.81 (1.25–18.48) | 0.02 |
| Azole monotherapy | ICUAC | - | - | 0.13 (0.03–0.50) | 0.003 | 0.19 (0.06–0.55) | 0.002 |
| Male | ICUAC | 0.26 (0.07–0.95) | 0.04 | - | - | - | - |
| Old age (≥65 yrs) | ICUAC | 9.96 (1.25–79.60) | 0.03 | - | - | - | - |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval; ICUAC, intensive care unit-associated candidemia; CVC, central venous catheter. 1 Only the variables that were statistically significant (p < 0.05) are listed. The results of univariate analysis are listed in Supplementary Table S2 (A–D). 2 Only mortality-predictive factors of non-ICUAC that were commonly found in both ICUAC and non-ICUAC are listed here. All independent predictive factors related to 7-, 30-, and 90-day mortalities of non-ICUAC are listed in Supplementary Table S3.
4. Discussion
Mortality from candidemia is reportedly associated with several clinical, microbiological, and host-related factors [10,21,22,23,24,25]. Although patient-related variables have consistently been reported as mortality predictors in candidemic patients, not all Candida species-related variables have been identified [10,25]. Here, we showed that mortality and the predictors of mortality associated with ICUAC are distinct from those associated with non-ICUAC and are mediated by Candida species and observation time (7, 30, and 90 days). Lack of antifungal therapy was the strongest predictor of 7-day mortality due to ICUAC caused by C. albicans, C. tropicalis, and C. glabrata compared with 30- and 90-day mortality. Our data show for the first time that ICU admission and fluconazole resistance are independent predictors of mortality due to C. glabrata candidemia, the most common NAC candidemia in many geographic areas [4]. More than half of the patients with C. glabrata ICUAC did not receive antifungal therapy for more than 3 days, highlighting the need for early diagnosis of ICUAC.
Few studies have compared the species distribution and resistance profiles of bloodstream Candida isolates from ICUAC versus non-ICUAC patients [12,26]. The most common Candida species recovered from 11 hospitals over the 2-year period was C. albicans (42.6%), followed by C. glabrata (19.2%), C. tropicalis (18.8%), and C. parapsilosis (13.5%), reflecting the recent trends of an increase in the number of C. glabrata candidemia cases and a decrease in the number of C. parapsilosis candidemia cases in South Korean hospitals [27]. In line with the results of a previous large study using SENTRY Antimicrobial Surveillance Program (2008–2009) data [26], we found that almost half of the candidemia cases developed in ICU patients, and C. tropicalis candidemia was more common in ICUAC than non-ICUAC patients. In contrast to the previously reported SENTRY data [26], we found that ICUAC isolates exhibited more resistance to fluconazole than non-ICUAC isolates, possibly because of higher fluconazole resistance in C. parapsilosis (ICUAC vs. non-ICUAC, 8.2% vs. 0.0%) and C. glabrata (9.2% vs. 4.3%) isolates from ICU patients than in non-ICU-related isolates.
C. tropicalis candidemia is associated with a worse outcome than C. albicans candidemia [10,21,23,28], whereas C. parapsilosis and C. glabrata candidemia are associated with better outcomes [10,29,30]. C. tropicalis candidemia is often found in ICU patients, especially in those with malignancies [22,29], and C. tropicalis might be related to the potential of virulence factors exhibited by this species, such as adhesion to different host surfaces, biofilm formation, infection and dissemination, and enzyme secretion [31]. By contrast, the source of C. parapsilosis candidemia was more likely related to removable focuses, such as the CVCs or other intravascular devices, and the lower virulence of C. parapsilosis compared to other Candida species may be a reason for the lower mortality rates observed [29]. Our data are in agreement with this notion, showing that C. tropicalis candidemia was an independent predictive factor for mortality at all time points (7, 30, and 90 days), but C. parapsilosis candidemia was associated with a lower 90-day mortality rate. In the ICU setting, significant species-specific differences in 7- and 30-day mortality were not found, but the mortality rate of C. glabrata ICUAC tended to be higher than that of C. albicans ICUAC at 7 and 30 days. The reason is unclear, but it could be, in part, explained by the fact that the predictors of mortality due to NAC ICUAC were different from those of mortality due to non-ICUAC caused by the same species, unlike those of C. albicans candidemia, which may contribute to increased mortality due to ICUAC compared with mortality due to non-ICUAC caused by NAC. Previous reports also showed that the 30-day mortality rates in patients with C. glabrata candidemia are 21.3–48.6%, but they can reach 50–60% among ICU patients [10,15]. This study showed that ICU admission was independently associated with mortality due to candidemia caused by C. glabrata and C. parapsilosis but not C. albicans or C. tropicalis. The mortality rates of C. albicans and C. tropicalis ICUAC were approximately twofold higher (1.7–2.1) than those of non-ICUAC on all observation days, whereas the mortality rates of C. glabrata (7 days) and C. parapsilosis (7, 30, and 90 days) ICUAC were more than threefold higher than those of non-ICUAC. Collectively, our data suggest that mortality due to candidemia and predictors of mortality are affected not only by the NAC species but also by the ICU setting.
Although there is broad consensus that patients who do not receive antifungal therapy have a higher risk of mortality due to candidemia [10,29,32], it is unclear which Candida species contribute to this increased mortality due to the lack of antifungal therapy for ICUAC or non-ICUAC. Our findings showed that lack (less than 3 days) of antifungal therapy was the only predictor of mortality in patients with ICUAC and non-ICUAC caused by four common Candida species, except for C. parapsilosis in non-ICUAC. In particular, it was the strongest predictor of 7-day mortality due to ICUAC caused by C. albicans (OR, 18.33), C. tropicalis (OR, 10.52), and C. glabrata (OR, 21.30) compared with 30- and 90-day mortality (OR, 2.72–6.90). We found that lack of antifungal therapy was more common in ICUAC (38.9%) than in non-ICUAC (31.8%) patients, which may, in part, be a result of earlier death of critically ill ICU patients. Interestingly, lack of antifungal therapy was more frequently found in patients with C. glabrata ICUAC (55.2%) than in those with ICUAC caused by other species (30.6–36.7%). The reason for this is unknown; however, previous studies have shown a significantly longer time-to-blood culture positivity for C. glabrata (e.g., 61.3 h for C. glabrata vs. 25.6 h for the other Candida species; p < 0.001) [33,34]. Given that non-culture-based diagnostic methods are seldom used in ICU patients in South Korea, this may lead to a delay in initiating antifungal treatment. A longer time-to-positivity duration in candidemic patients may have an impact on mortality [34,35,36]. The recognition of C. glabrata candidemia is frequently delayed, resulting in dramatic clinical deterioration and death of critically ill ICU patients [37], which might explain the higher 7-day mortality rate of C. glabrata ICUAC. Therefore, these results highlight the importance of the exploration studies of early risk management of ICUAC, such as prophylactic antifungal therapy, biomarker-based preemptive therapy and risk-based empirical therapy in patients with ICUAC, especially C. glabrata ICUAC [38,39].
In this study, C. glabrata was the only species for which fluconazole resistance was independently related to 30- and 90-day mortality due to candidemia (also 90-day mortality due to ICUAC). This finding is supported by a recent multicenter Korean study that showed that candidemic patients with fluconazole-resistant C. glabrata had higher mortality rates [15]. In that study, the cumulative mortality rates of candidemia caused by fluconazole-resistant C. glabrata isolates increased over time (day 30 (60.9%) and day 90 (78.1%)); these rates were significantly higher than those in patients with fluconazole-susceptible dose-dependent isolates (36.4% and 43.8%, respectively). Additionally, in that study, appropriate antifungal therapy was the only factor independently associated with favorable outcomes [15]. Our findings also confirmed that azole monotherapy may not promote favorable outcomes of C. glabrata candidemia, in contrast to the other three common species. Collectively, these results highlight that continued epidemiological surveillance is important, and future efforts should be directed toward developing new rapid diagnostic techniques—including techniques for candidemia detection, species identification, and antifungal susceptibility testing—to enable the timely administration of appropriate antifungal therapy.
5. Conclusions
This is, to the best of our knowledge, the first multicenter study to reveal dynamics in mortalities and mortality-predictors of candidemia according to different Candida species and ICU admission. Above all, we highlight that the lack of antifungal therapy is the strong 7-day mortality-predictor of ICUAC, and it is more frequently found in patients with C. glabrata ICUAC than in those with ICUAC caused by other species, underscoring the importance of early etiologic diagnosis. Given that the Candida species-related mortality rates and mortality predictors of ICUAC are quite distinct from those of non-ICUAC, continued epidemiological surveillance is needed to identify possible changes in the species distribution and antifungal resistance patterns of Candida bloodstream isolates from ICU patients.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jof7080597/s1, Table S1 (A–E): Univariate analysis of predictive factors related to 7-, 30- and 90-day mortalities of candidemia patients, Table S2 (A–D): Univariate analysis of predictive factors related to 7-, 30- and 90-day mortalities of ICUAC and non-ICUAC, Table S3: Multivariate analysis of predictive factors related to 7-, 30- and 90-day mortalities of non-ICUAC by the four common Candida species.
Author Contributions
Conceptualization, J.H.S. (Jong Hee Shin); Funding acquisition, J.H.S. (Jong Hee Shin); Formal analysis and investigation, E.J.W. and Y.J.K.; Writing—original draft preparation, Y.J.K., E.J.W. and J.H.S. (Jong Hee Shin); Writing—review and editing: E.J.W. and J.H.S. (Jong Hee Shin); Resources, S.H.J., K.S.S., J.H.S. (Jeong Hwan Shin), Y.R.K., H.S.K., Y.A.K., Y.U., T.S.K., J.H.P., J.L. and S.H.K.; Material preparation and data collection, M.J.C. and S.A.B. All authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program, through the National Research Foundation of Korea (NRF), via funding from the Ministry of Education (NRF-2019R1A2C1004644), and by the Research Program funded by the Korea Centers for Disease Control and Prevention (grant 2019E540600).
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Chonnam National University Hospital (CNUH-2017-119).
Informed Consent Statement
Written informed consent was waived due to the nature of this study.
Data Availability Statement
All data generated or analyzed in this study are included in this published article, and the datasets are available from the corresponding author within the limits imposed by ethical and legal dispositions.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gudlaugsson O., Gillespie S., Lee K., Vande Berg J., Hu J., Messer S., Herwaldt L., Pfaller M., Diekema D. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 2003;37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 2.Koehler P., Stecher M., Cornely O.A., Koehler D., Vehreschild M., Bohlius J., Wisplinghoff H., Vehreschild J.J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019;25:1200–1212. doi: 10.1016/j.cmi.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Pappas P.G., Rex J.H., Lee J., Hamill R.J., Larsen R.A., Powderly W., Kauffman C.A., Hyslop N., Mangino J.E., Chapman S., et al. A prospective observational study of candidemia: Epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 2003;37:634–643. doi: 10.1086/376906. [DOI] [PubMed] [Google Scholar]
- 4.Lamoth F., Lockhart S.R., Berkow E.L., Calandra T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018;73:i4–i13. doi: 10.1093/jac/dkx444. [DOI] [PubMed] [Google Scholar]
- 5.Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014;20:5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 6.Wiederhold N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug. Resist. 2017;10:249–259. doi: 10.2147/IDR.S124918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassetti M., Garnacho-Montero J., Calandra T., Kullberg B., Dimopoulos G., Azoulay E., Chakrabarti A., Kett D., Leon C., Ostrosky-Zeichner L., et al. Intensive care medicine research agenda on invasive fungal infection in critically ill patients. Intensive Care Med. 2017;43:1225–1238. doi: 10.1007/s00134-017-4731-2. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti A., Sood P., Rudramurthy S.M., Chen S., Kaur H., Capoor M., Chhina D., Rao R., Eshwara V.K., Xess I., et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015;41:285–295. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 9.Colombo A.L., Guimaraes T., Sukienik T., Pasqualotto A.C., Andreotti R., Queiroz-Telles F., Nouer S.A., Nucci M. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: An analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Med. 2014;40:1489–1498. doi: 10.1007/s00134-014-3400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lortholary O., Renaudat C., Sitbon K., Madec Y., Denoeud-Ndam L., Wolff M., Fontanet A., Bretagne S., Dromer F., The French Mycosis Study Group Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010) Intensive Care Med. 2014;40:1303–1312. doi: 10.1007/s00134-014-3408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byun S.A., Won E.J., Kim M.N., Lee W.G., Lee K., Lee H.S., Uh Y., Healey K.R., Perlin D.S., Choi M.J., et al. Multilocus sequence typing (MLST) genotypes of Candida glabrata bloodstream isolates in Korea: Association with antifungal resistance, mutations in mismatch repair gene (Msh2), and clinical outcomes. Front. Microbiol. 2018;9:1523. doi: 10.3389/fmicb.2018.01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Rosa F.G., Trecarichi E.M., Montrucchio C., Losito A.R., Raviolo S., Posteraro B., Corcione S., Di Giambenedetto S., Fossati L., Sanguinetti M., et al. Mortality in patients with early- or late-onset candidaemia. J. Antimicrob. Chemother. 2013;68:927–935. doi: 10.1093/jac/dks480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H., Yoon E.J., Kim D., Jeong S.H., Shin J.H., Shin J.H., Shin K.S., Kim Y.A., Uh Y., Park C., et al. Establishment of the South Korean national antimicrobial resistance surveillance system, Kor-GLASS, in 2016. Eurosurveillance. 2018;23:1700734. doi: 10.2807/1560-7917.ES.2018.23.42.1700734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H., Yoon E.J., Kim D., Jeong S.H., Won E.J., Shin J.H., Kim S.H., Shin J.H., Shin K.S., Kim Y.A., et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: First one-year report from Kor-GLASS. Eurosurveillance. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.42.1800047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Won E.J., Choi M.J., Kim M.N., Yong D., Lee W.G., Uh Y., Kim T.S., Byeon S.A., Lee S.Y., Kim S.H., et al. Fluconazole-Resistant Candida glabrata bloodstream isolates, South Korea, 2008–2018. Emerg. Infect. Dis. 2021;27:779–788. doi: 10.3201/eid2703.203482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller M.A., Diekema D.J. Progress in antifungal susceptibility testing of Candida spp. by use of clinical and laboratory standards institute broth microdilution methods, 2010 to 2012. J. Clin. Microbiol. 2012;50:2846–2856. doi: 10.1128/JCM.00937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappas P.G., Kauffman C.A., Andes D.R., Clancy C.J., Marr K.A., Ostrosky-Zeichner L., Reboli A.C., Schuster M.G., Vazquez J.A., Walsh T.J., et al. Executive summary: Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016;62:409–417. doi: 10.1093/cid/civ1194. [DOI] [PubMed] [Google Scholar]
- 18.CLSI . Performance Standards for Antifungal Susceptibility Testing of Yeast. 2nd ed. CLSI M60; Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2017. [Google Scholar]
- 19.CLSI . Epidemiological Cutoff Values for Antifungal Susceptibility Testing. 2nd ed. CLSI Suppplement M59; Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2018. [Google Scholar]
- 20.Almirante B., Rodriguez D., Park B.J., Cuenca-Estrella M., Planes A.M., Almela M., Mensa J., Sanchez F., Ayats J., Gimenez M., et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: Results from population-based surveillance, barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 2005;43:1829–1835. doi: 10.1128/JCM.43.4.1829-1835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghrenassia E., Mokart D., Mayaux J., Demoule A., Rezine I., Kerhuel L., Calvet L., De Jong A., Azoulay E., Darmon M. Candidemia in critically ill immunocompromised patients: Report of a retrospective multicenter cohort study. Ann. Intensive Care. 2019;9:62. doi: 10.1186/s13613-019-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munoz P., Giannella M., Fanciulli C., Guinea J., Valerio M., Rojas L., Rodriguez-Creixems M., Bouza E. Candida tropicalis fungaemia: Incidence, risk factors and mortality in a general hospital. Clin. Microbiol. Infect. 2011;17:1538–1545. doi: 10.1111/j.1469-0691.2010.03338.x. [DOI] [PubMed] [Google Scholar]
- 23.Paiva J.A., Pereira J.M., Tabah A., Mikstacki A., de Carvalho F.B., Koulenti D., Ruckly S., Cakar N., Misset B., Dimopoulos G., et al. Characteristics and risk factors for 28-day mortality of hospital acquired fungemias in ICUs: Data from the EUROBACT study. Crit. Care. 2016;20:53. doi: 10.1186/s13054-016-1229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poissy J., Damonti L., Bignon A., Khanna N., Von Kietzell M., Boggian K., Neofytos D., Vuotto F., Coiteux V., Artru F., et al. Risk factors for candidemia: A prospective matched case-control study. Crit. Care. 2020;24:109. doi: 10.1186/s13054-020-2766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C., Wang H., Yin M., Han H., Yue J.F., Zhang F., Shan T.C., Guo H.P., Wu D.W. The differences in the epidemiology and predictors of death between candidemia acquired in intensive care units and other hospital settings. Intern. Med. 2015;54:3009–3016. doi: 10.2169/internalmedicine.54.3744. [DOI] [PubMed] [Google Scholar]
- 26.Pfaller M.A., Messer S.A., Moet G.J., Jones R.N., Castanheira M. Candida bloodstream infections: Comparison of species distribution and resistance to echinocandin and azole antifungal agents in Intensive Care Unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009) Int. J. Antimicrob. Agents. 2011;38:65–69. doi: 10.1016/j.ijantimicag.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Ko J.H., Jung D.S., Lee J.Y., Kim H.A., Ryu S.Y., Jung S.I., Joo E.J., Cheon S., Kim Y.S., Kim S.W., et al. Changing epidemiology of non-albicans candidemia in Korea. J. Infect. Chemother. 2019;25:388–391. doi: 10.1016/j.jiac.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Ko J.H., Jung D.S., Lee J.Y., Kim H.A., Ryu S.Y., Jung S.I., Joo E.J., Cheon S., Kim Y.S., Kim S.W., et al. Poor prognosis of Candida tropicalis among non-albicans candidemia: A retrospective multicenter cohort study, Korea. Diagn. Microbiol. Infect. Dis. 2019;95:195–200. doi: 10.1016/j.diagmicrobio.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Lortholary O., Renaudat C., Sitbon K., Desnos-Ollivier M., Bretagne S., Dromer F., The French Mycoses Study The risk and clinical outcome of candidemia depending on underlying malignancy. Intensive Care Med. 2017;43:652–662. doi: 10.1007/s00134-017-4743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andes D.R., Safdar N., Baddley J.W., Playford G., Reboli A.C., Rex J.H., Sobel J.D., Pappas P.G., Kullberg B.J., Mycoses Study G. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin. Infect. Dis. 2012;54:1110–1122. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 31.Negri M., Silva S., Henriques M., Oliveira R. Insights into Candida tropicalis nosocomial infections and virulence factors. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:1399–1412. doi: 10.1007/s10096-011-1455-z. [DOI] [PubMed] [Google Scholar]
- 32.Tang H.J., Liu W.L., Lin H.L., Lai C.C. Epidemiology and prognostic factors of candidemia in cancer patients. PLoS ONE. 2014;9:e99103. doi: 10.1371/journal.pone.0099103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobos-Trigueros N., Kaasch A.J., Soriano A., Torres J.L., Vergara A., Morata L., Zboromyrska Y., De La Calle C., Alejo I., Hernandez C., et al. Time to positivity and detection of growth in anaerobic blood culture vials predict the presence of Candida glabrata in candidemia: A two-center European cohort study. J. Clin. Microbiol. 2014;52:3082–3084. doi: 10.1128/JCM.01198-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Ami R., Weinberger M., Orni-Wasserlauff R., Schwartz D., Itzhaki A., Lazarovitch T., Bash E., Aharoni Y., Moroz I., Giladi M. Time to blood culture positivity as a marker for catheter-related candidemia. J. Clin. Microbiol. 2008;46:2222–2226. doi: 10.1128/JCM.00214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunes C.Z., Marra A.R., Edmond M.B., da Silva Victor E., Pereira C.A. Time to blood culture positivity as a predictor of clinical outcome in patients with Candida albicans bloodstream infection. BMC Infect. Dis. 2013;13:486. doi: 10.1186/1471-2334-13-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garey K.W., Rege M., Pai M.P., Mingo D.E., Suda K.J., Turpin R.S., Bearden D.T. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin. Infect. Dis. 2006;43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 37.Epelbaum O., Chasan R. Candidemia in the intensive care unit. Clin. Chest. Med. 2017;38:493–509. doi: 10.1016/j.ccm.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Paiva J.A., Charles P.E. Biomarker-guided antifungal therapy in patients with suspected invasive candidiasis: Ready for prime time? Intensive Care Med. 2017;43:1889–1891. doi: 10.1007/s00134-017-4990-y. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Loeches I., Antonelli M., Cuenca-Estrella M., Dimopoulos G., Einav S., De Waele J.J., Garnacho-Montero J., Kanj S.S., Machado F.R., Montravers P., et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019;45:789–805. doi: 10.1007/s00134-019-05599-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study are included in this published article, and the datasets are available from the corresponding author within the limits imposed by ethical and legal dispositions.