Abstract
Being overweight is associated with pregnancy-related disorders such as gestational diabetes mellitus (GDM), hypertensive disorders of pregnancy (HDP), and excessive maternal weight gain (MWG). Exercise and metformin reduce the risk of these disorders. This network meta-analysis (NMA) aims to compare the effect of metformin and different types of exercise (aerobic, resistance and combined) on the risk of GDM, HDP, and MWG among overweight/obese pregnant women. Medline, EMBASE, Web of Science and Cochrane Library were searched from inception to June 2021. Meta-analyses and NMAs were performed. Sixteen randomized controlled trials were included. In the NMA, aerobic exercise showed an effect on GDM (RR = 0.51, 95% CI = 0.26, 0.97), and metformin a reduction in MWG (MWG = −2.93 kg, 95% CI = −4.98, −0.87). No intervention showed any effect on the reduction of HDP. Our study suggests that aerobic exercise may have the greatest effect in reducing the risk of GDM, and perhaps, the MWG. Strategies should be developed to increase adherence to this type of intervention among overweight women without contraindications. Although metformin could reduce MWG, medicalization of pregnancy in healthy women is not justified with the present results. More research is needed on the effect of the intensity and frequency of exercise sessions and the length of interventions.
Keywords: gestational diabetes mellitus, exercise, metformin, pregnancy, overweight, obesity, systematic review, network meta-analysis
1. Introduction
Overweight and obesity are a major public health problem [1]. Obesity increases the risk of developing pregnancy complications, such as gestational diabetes mellitus (GDM), preeclampsia, or gestational hypertension [2]. GDM refers to any degree of carbohydrate intolerance with onset or first recognition during the second or third trimester of pregnancy [3], and has a prevalence about 17% [4,5]. GDM increases the risk of induced and caesarean deliveries and type 2 diabetes in women, as well as neonatal hypoglycemia, macrosomia, type 2 diabetes, obesity, and cardiovascular diseases in offspring [6,7,8,9,10].
It has been advocated that exercise could reduce the risk of GDM, excessive maternal weight gain (MWG) and hypertensive disorders of pregnancy (HDP) [11,12,13], an umbrella of disorders that includes the following clinical entities: pregnancy-induced hypertension (PIH), preeclampsia-eclampsia, chronic hypertension, and chronic hypertension with superimposed preeclampsia [14]. International recommendations suggest at least 30 min of moderate to vigorous physical activity, preferably aerobic or combined (resistance and aerobic exercise), per day, 3–4 times per week [15]. The effect of resistance exercise interventions on the health of pregnant women remains unclear [16]. However, the exercise recommendation for overweight/obese pregnant women remains a debatable issue [17].
Oral antidiabetics are another therapeutic strategy that has become widespread among women with polycystic ovary syndrome (PCOS) and obesity. Metformin reduces insulin resistance, endothelial dysfunction, and hyperglycemia, and it may prevent the development of HDP by reducing the secretion of soluble fms-like tyrosine kinase-1 (sFlt-1) [18,19,20,21,22]. However, a recent Cochrane review concluded that there is not enough evidence to support the use of metformin in women with obesity during pregnancy to improve maternal and infant outcomes [23].
A previous network meta-analysis (NMA) reported no effect of exercise or metformin on the risk of GDM and HDP (i.e., preeclampsia, PIH) among overweight/obese pregnant women, although it did show an effect of exercise on reducing MWG [24]. However, these two therapeutic options showed a clear trend towards a protective effect against GDM and HDP. Considering that the aforementioned NMA analyzed exercise as a single prescription, and that the results reported by the NMA were inconclusive but promising, we conducted this systematic review and NMA to comparatively synthesize data on the effect of metformin and different types of exercise interventions (i.e., aerobic, resistance, combined) on GDM, HDP and MWG among overweight/obese pregnant women.
2. Materials and Methods
This systematic review and NMA was conducted in accordance with the Preferred Reporting Items for Systematic Review incorporating NMA (PRISMA-NMA) guidelines and the Collaboration Handbook for Systematic Reviews of Interventions [25,26,27]. The study protocol was registered in PROSPERO (CDR:42019121715) and published elsewhere [28].
2.1. Search Strategy
Two reviewers (CP-M and IC-R) independently searched Medline, EMBASE, Web of Science and Cochrane Library databases from inception through June 2021. For unpublished trials, we searched through June 2021 clinicaltrials.gov, EudraCT and the grey literature, including Google Scholar, OPEN GRAY, Theseo and Networked digital library of theses and dissertations. We reviewed the reference list of articles included in this review and the list of previous systematic reviews. Relevant studies were identified using a combination of the following terms: (a) population; (b) interventions; and (c) clinical trials. The complete electronic search strategy is detailed in Appendix A.
2.2. Elegibility
Studies addressing the effect of exercise or metformin in pregnant women with overweight/obesity on the risk of GDM, HDP or MWG were included in the NMA. Inclusion criteria were as follows: (i) Type of study: Randomized controlled trials (RCTs), with no language restrictions; (ii) type of participants: Overweight or obese pregnant women; (iii) type of interventions: Metformin, structured exercise program; (iv) type of outcome assessment: Incidence of GDM, any HDP, and/or MWG (kg).
Exclusion criteria were as follows: (i) Single-arm pre-post studies, non-RCTs; (ii) studies whose target population was primarily women with PCOS, pregestational insulin resistance, or other diseases; (iii) dietary intervention as the primary co-intervention; (iv) nutraceutical interventions; or (v) unstructured physical activity/exercise intervention.
2.3. Data Extraction
Data from the included studies were extracted independently by two reviewers (CP-M and IC-R) according to the following predetermined information for each study: (i) Reference; (ii) study design; (iii) country; (iv) characteristics of intervention (type of intervention, length, intensity); (v) sample characteristics (type of population, sample size, mean age); and (vi) outcomes (risk of GDM, risk of HDP, MWG).
2.4. Categorization of Available Evidence
The classification of overweight or obesity was obtained from the classification made by the authors of the studies. When they did not report this classification, the internationally accepted values according to body mass index (BMI) were considered: overweight (BMI 25–30 kg/m2) and obese (BMI > 30 kg/m2) [1].
Exercise was defined as a subset of structured and repetitive physical activity with the objective of improving or maintaining physical fitness [29]. Exercise interventions were classified into three categories: (1) aerobic/endurance exercise; (2) strength/resistance exercise; and (3) combined exercise. Aerobic exercise included exercises such as swimming, cycling, jogging, running, and walking with the aim of increasing energy expenditure. Strength exercise included basic exercises with dumbbells and elastic bands aimed at increasing muscle strength. In combined interventions, aerobic exercise and strength exercises were alternated or combined.
The intensity of the exercise interventions was classified as light, light-moderate, moderate, moderate-vigorous, and vigorous, as reported by the authors. When intensity was not reported, criteria from the American College of Sports Medicine guidelines were used to estimate it [30,31,32].
2.5. Risk of Bias Assessment
Two researchers (CP-M and IC-R) independently conducted risk of bias assessment of included RCTs using the Cochrane Collaboration’s tool for assessing risk of bias (RoB2) [33]. The RoB2 evaluates risk of bias according to six domains: (1) Randomization process; (2) assignment to intervention; (3) adherence to intervention; (4) missing outcome data; (5) measurement of the outcome; and (6) selection of the reported result. Overall bias is considered “low risk of bias” if the study is classified as “low risk” in all domains, “some concerns” if there is at least one domain classified as “some concern”, and “high risk of bias” if there is at least one domain classified as “high risk” or several domains with “some concerns” that are considered critical to the validity of the results. Disagreements were resolved by discussion among the researchers, but if disagreements persisted, a third reviewer resolved the conflict (VM-V).
2.6. Grading of Quality of Evidence
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) tool was used to assess the quality of evidence and make recommendations [34,35]. Each outcome scored high, moderate, low, or very low evidence, according to study design, risk of bias, inconsistency, indirect evidence, imprecision, publication bias, large effect, possible confounding variables, and dose-response gradient.
2.7. Data Synthesis
The included RCTs were summarized qualitatively in an ad hoc table describing the types of direct and indirect comparisons. We conducted our NMAs in accordance with the PRISMA-NMA statement [26].
A network geometry graph was used to assess the robustness of the evidence in which the node size is proportional to the number of participants in the trial and the thickness of the continuous line connecting the nodes is proportional to the number of participants in the trials directly comparing the two treatments, and the dashed lines represent indirect comparisons [36,37].
We assessed consistency by testing whether the intervention effects estimated from direct comparisons were consistent with those estimated from indirect comparisons. Consequently, the Wald test was conducted, due to low statistical power, the side-splitting assessment was also used [38].
To compare the effects of different types of metformin/exercise interventions, a standard pairwise meta-analysis was performed for direct comparisons between interventions and controls/non-interventions. We used the DerSimonian–Laird method to perform all direct comparisons [39], and statistical heterogeneity was examined by calculating the I2 statistic, ranging from 0 to 100%. Heterogeneity was considered not important (<40%), moderate (30 to 60%), substantial (50 to 90%), or considerable (>75%) [27]. Additionally, the corresponding p values were considered. Finally, to determine the size and clinical relevance of the heterogeneity, the τ2 statistic was calculated. A τ2 estimate of 0.04 was interpreted as low, 0.14 as moderate and 0.40 as a substantial degree of clinical relevance of heterogeneity [40,41]. These results were displayed by creating both forest plots and a league table. For statistically significant results, we calculated the number needed to treat (NNT).
We assessed the principle of transitivity, which means checking that the synthesis of direct comparisons of two treatments has been conducted in similar studies on the most important clinical and methodological characteristics; thus, we assume that the populations included in these studies were similar in terms of the baseline distribution of effect modifiers, namely, baseline age and baseline BMI [42].
To identify superiority, the probability that each intervention was the most effective relative to the others was presented graphically using rankograms [36]. Additionally, the surface under the cumulative ranking (SUCRA) was estimated for each intervention. The SUCRA consists of assigning a numerical value between 0 and 1 to simplify the ranking of each intervention in the rankogram. The best intervention would obtain a SUCRA value close to 1, and the worst intervention a value close to 0 [37].
As a sensitivity analysis, we conducted a subgroup analysis including only pregnant women with obesity in which a standard meta-analysis and a NMA procedures were used for each outcome. Additionally, a sensitivity re-analysis under a Bayesian perspective was conducted. Furthermore, random effects meta-regression models showed that the age of the population included in the study did not influence the effect of exercise or metformin in GDM, HDP or MWG.
Finally, the small study effect and publication bias were assessed visually using a network funnel plot [43]. All analyses were conducted in Stata 15.0 (Stata, College Station, TX, USA).
2.8. Modifications to the Initial Protocol
In the study protocol [28], the target population was initially delimited to all pregnant women, including healthy women, overweight/obese women, or women with PCOS. In conducting this study, it was decided to include only women with overweight and obesity, and to conduct a subgroup study in women with obesity, to improve the transitivity principle. It was also planned to include any clinical trial, but it was decided to include only RCTs to increase the quality of the final analyses. Finally, the protocol included the performance of Bayesian NMAs. Subsequently, it was decided to conduct frequentist methods for NMAs, and a sensitivity analysis with Bayesian methods.
3. Results
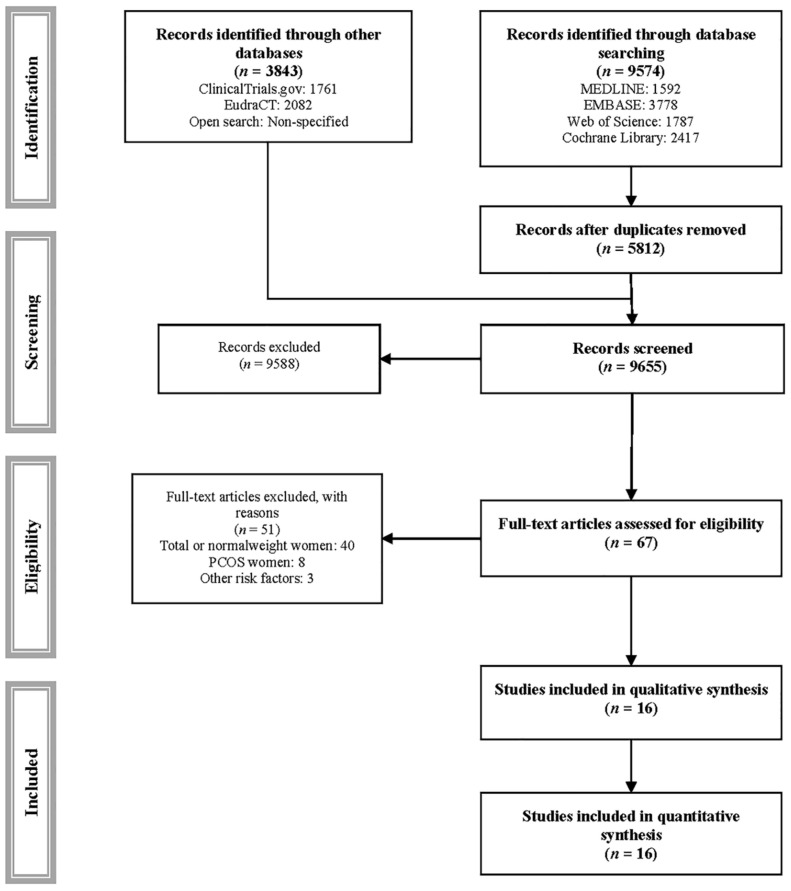
Sixteen RCTs [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59] were included in the analyses (Figure 1, Table 1, Table S1), and 51 were excluded (Table S2). Of the included studies, six included overweight pregnant women and 15 with obesity. The studies were conducted in 11 countries: Five in Europe (one in Ireland and one in Norway, two in the Netherlands, three in Spain and two in the United Kingdom), three in the Americas (one each in Canada and the United States and two in Brazil), one in Africa (Egypt), one in Asia (China) and one in Oceania (New Zealand). A total of 2903 pregnant women were included in the RCTs (412 in aerobic exercise, 925 in combined exercise, 40 in resistance exercise, and 1526 in metformin interventions). The interventions were generally performed before 20 weeks of gestation, generally at the end of the first trimester or at the beginning of the second trimester of gestation. The frequency of exercise sessions was two to five times per week, lasting 12 to 30 weeks. The dose of metformin was 1000 to 3000 mg daily, with a length of treatment of 20 to 25 weeks. Details of the interventions are described in Table S3.
Figure 1.
PRISMA flowchart of study selection.
Table 1.
Characteristics of included trials.
| Reference | Design | Country | Intervention | Target Women | Sample | Age | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | I | C | I | C | GDM | HDP | MWG | |||||
| Kong K et al. (2014)-1 [44] | RCT | United States | Aerobic exercise | Overweight | 19 | 9 | 9 | 26.2 ± 2.6 | 27.3 ± 3.6 | ✓ | ✓ | ✓ |
| Kong K et al. (2014)-2 [44] | RCT | United States | Aerobic exercise | Obese | 18 | 9 | 10 | 28.6 ± 5.3 | 25.7 ± 4.0 | ✓ | ✓ | ✓ |
| Seneviratne SN et al. (2016) [45] | RCT | New Zealand | Aerobic exercise | Obese | 75 | 38 | 37 | NA | NA | ✓ | ✓ | ✓ |
| Wang C et al. (2017) [46] | RCT | China | Aerobic exercise | Overweight | 300 | 150 | 150 | 32.1 ± 4.6 | 32.5 ± 4.9 | ✓ | ✓ | ✓ |
| Barakat R et al. (2016)-1 [47] | RCT | Spain | Combined exercise | Overweight | 164 | 90 | 78 | NA | NA | ✓ | ✓ | - |
| Barakat R et al. (2016)-2 [47] | RCT | Spain | Combined exercise | Obese | 54 | 25 | 29 | NA | NA | ✓ | ✓ | - |
| Bisson M et al. (2015) [48] | RCT | Canada | Combined exercise | Obese | 50 | 25 | 25 | 30.5 ± 3.7 | 31.0 ± 4.0 | ✓ | ✓ | ✓ |
| Daly N et al. (2017) [49] | RCT | Ireland | Combined exercise | Obese | 88 | 44 | 44 | 30.0 ± 5.1 | 29.4 ± 4.8 | ✓ | - | ✓ |
| Garnæs KK et al. (2016) [50] | RCT | Norway | Combined exercise | Obese | 91 | 46 | 45 | 31.3 ± 3.8 | 31.4 ± 4.7 | ✓ | ✓ | ✓ |
| Nascimento SL et al. (2011) [51] | RCT | Brazil | Combined exercise | Overweight and Obese | 82 | 40 | 42 | 29.7 ± 6.8 | 30.9 ± 5.9 | - | - | ✓ |
| Oostdam N et al. (2012) [52] | RCT | Netherlands | Combined exercise | Obese | 121 | 62 | 59 | 30.8 ± 5.2 | 30.1 ± 4.5 | ✓ | - | ✓ |
| Ruiz JR et al. (2013) [53] | RCT | Spain | Combined exercise | Overweight and Obese | 275 | 146 | 129 | NA | NA | ✓ | ✓ | ✓ |
| Barakat R et al. (2009)-1 [54] | RCT | Spain | Resistance exercise | Overweight | 28 | 14 | 14 | NA | NA | - | - | ✓ |
| Barakat R et al. (2009)-2 [54] | RCT | Spain | Resistance exercise | Obese | 12 | 9 | 3 | NA | NA | - | - | ✓ |
| Abd El Fattah EA et al. (2016) [55] | RCT | Egypt | Metformin | Obese | 200 | 100 | 100 | 26.9 ± 5.2 | 26.2 ± 5.5 | ✓ | ✓ | ✓ |
| Brink HS et al. (2018) [56] | RCT | Netherlands | Metformin | Obese | 49 | 24 | 25 | 29.3 ± 5.2 | 30.7 ± 5.2 | ✓ | ✓ | - |
| Chiswick C et al. (2015) [57] | RCT | UK | Metformin | Obese | 449 | 226 | 223 | 28.7 ± 5.8 | 28.9 ± 5.1 | ✓ | ✓ | ✓ |
| Nascimento IB et al. (2020) [58] | RCT | Brazil | Metformin | Obese | 378 | 189 | 189 | 28.6 ± 6.2 | 29.6 ± 6.1 | ✓ | ✓ | - |
| Syngelaki A et al. (2016) [59] | RCT | UK | Metformin | Obese | 450 | 225 | 225 | 32.9 | 30.8 | ✓ | ✓ | - |
T: Total; I: Intervention; C: Control; GDM: Gestational diabetes mellitus; HDP: Hypertensive disorders of pregnancy; MWG: Maternal weight gain.
Fourteen studies focused on the prevention of GDM (three with aerobic exercise, six with combined exercise and five with metformin interventions), eleven on the prevention of HDP (three with aerobic exercise, four with combined exercise and five with metformin interventions), and twelve on the reduction of MWG (three with aerobic exercise, six with combined exercise, one with resistance exercise, and two with metformin interventions). Details of the GDM diagnostic criteria and the type of HDP included are described in Table S4.
3.1. Gestational Diabetes Mellitus
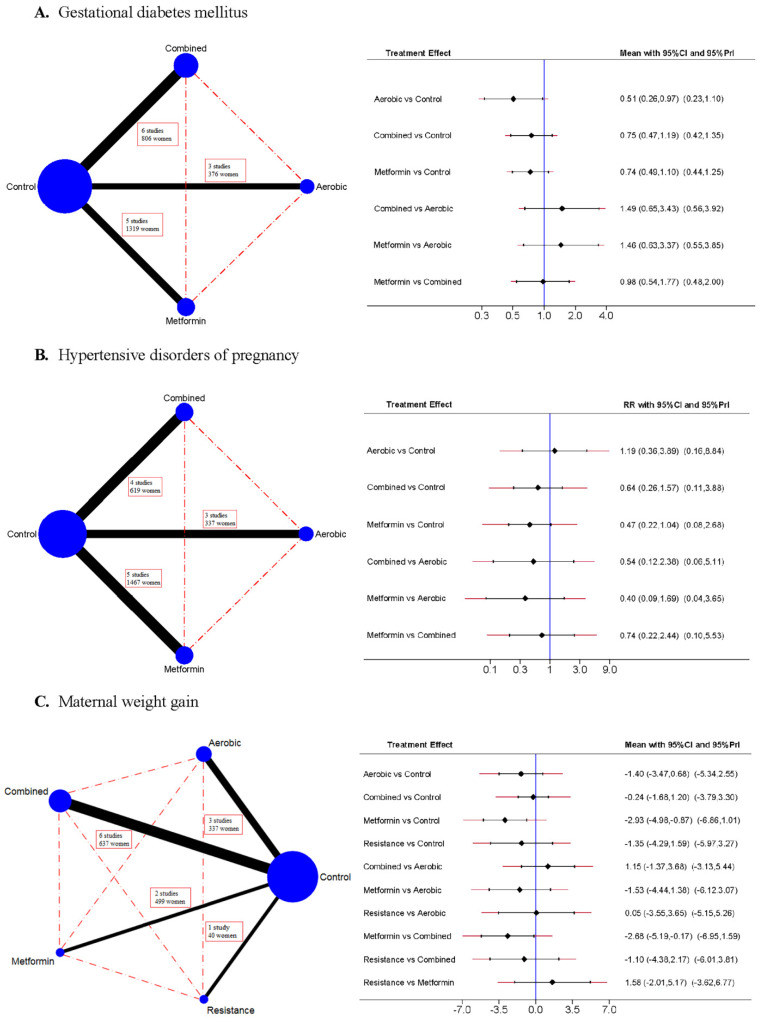
Table 2A, Figure 2A, and Figure S1A show the pairwise comparisons and NMA. Aerobic exercise showed a protective effect in the pairwise comparisons (upper diagonal) and in the NMA estimates (under diagonal) (RR = 0.59, 95% CI = 0.41, 0.85, and RR = 0.51, 95% CI = 0.26, 0.97, respectively). The NNT of aerobic exercise was 13 women to prevent one case.
Table 2.
Results for direct pairwise comparisons and network meta-analysis.
| A. Gestational Diabetes Mellitus | |||||
|---|---|---|---|---|---|
| Control | Aerobic | Combined | Resistance | Metformin | |
| Control | 0.59 * (0.41, 0.85) |
0.91 (0.67, 1.22) |
0.78 (0.59, 1.02) |
||
| Aerobic | 0.51 * (0.26, 0.97) |
- | - | ||
| Combined | 0.75 (0.47, 1.19) |
1.49 (0.65, 3.43) |
- | ||
| Resistance | |||||
| Metformin | 0.74 (0.49, 1.10) |
1.46 (0.63, 3.37) |
0.98 (0.54, 1.77) |
||
| B. Hypertensive disorders of pregnancy | |||||
| Control | 0.95 (0.56, 1.62) |
0.65 (0.36, 1.14) |
0.48 (0.19, 1.22) |
||
| Aerobic | 1.19 (0.36, 3.89) |
- | - | ||
| Combined | 0.64 (0.26, 1.57) |
0.54 (0.12, 2.38) |
- | ||
| Resistance | |||||
| Metformin | 0.47 (0.22, 1.04) |
0.40 (0.09, 1.69) |
0.74 (0.22, 2.44) |
||
| C. Maternal weight gain | |||||
| Control | −1.91 * (−2.74, −1.07) |
−0.31 (−1.06, 0.44) |
−1.35 (−3.57, 0.88) |
−2.82 (−7.26, 1.62) |
|
| Aerobic | −1.40 (−3.47, 0.68) |
- | - | - | |
| Combined | −0.24 (−1.68, 1.20) |
1.15 (−1.37, 3.68) |
- | - | |
| Resistance | −1.35 (−4.29, 1.59) |
0.05 (−3.55, 3.65) |
−1.10 (−4.38, 2.17) |
- | |
| Metformin | −2.93 * (−4.98, −0.87) |
−1.53 (−4.44, 1.38) |
−2.68 * (−5.19, −0.17) |
−1.58 (−5.17, 2.01) |
|
Light gray indicates lack of direct and indirect comparisons. Dark gray separates direct from indirect comparisons. Gestational diabetes mellitus and hypertensive disorders of pregnancy were measured as risk ratio (95% CI). Maternal weight gain was measured as mean difference (95% CI). Upper diagonal: Standard meta-analysis. Lower diagonal: Network meta-analysis estimates. * Statistical significance.
Figure 2.
Network mappings and network meta-analyses estimates for gestational diabetes mellitus (A), hypertensive disorders of pregnancy (B) and maternal weight gain (C). The network mappings (left) represent the number of participants in each intervention arm, and direct and indirect comparisons. The forest plot/interval plot (right) represent the effect size estimates in the network meta-analysis. The risk ratio (RR) was estimated for dichotomous variables, and the main difference (Mean) for continuous variables, with their 95% confidence intervals.
3.2. Hypertensive Disorders of Pregnancy
Table 2B, Figure 2B, and Figure S1B show that no intervention produced a significant effect on pairwise comparisons or NMA, although metformin almost reached statistical significance in the NMA (RR = 0.47, 95% CI = 0.22, 1.04).
3.3. Maternal Weight Gain
Table 2C, Figure 2C, and Figure S1C show that aerobic exercise showed a protective effect in the pairwise comparisons, and metformin in NMA estimates (MWG = −1.91 kg, 95% CI = −2.74, −1.07, and MWG = −2.93 kg, 95% CI = −4.98, −0.87, respectively).
3.4. Risk of Bias
According to the Cochrane Collaboration tool for assessing risk of bias (RoB2), 10 out of 16 studies (62.5%) showed a high risk of bias for overall bias, and five (31.3%) showed some concerns. By domains, 6.3% of studies showed high risk for the randomization process; 18.8% and 56.3% showed high risk and some concerns, respectively, for assignment to an intervention; 43.8% and 18.8% showed high risk and some concerns, respectively, for adhering to an intervention; 18.8% and 18.8% showed high risk and some concerns, respectively, for missing outcome data; and 62.5% showed some concerns for measurement of the outcome. No significant risk of bias was detected for the selection of the reported results. The total risk of bias is detailed in Figure S2.
3.5. Grades of Recommendation, Assessment, Development, and Evaluation
According to the GRADE tool, aerobic exercise for the prevention of GDM, combined exercise for the prevention of HDP, and resistance exercise for the reduction MWG were scored as having low certainty, while the remaining interventions were scored as having very low certainty. All or almost all interventions were at serious or very serious risk of bias, inconsistency, and indirectness, and some interventions also had imprecision and publication bias. The full assessment is detailed in Table S5.
3.6. Transitivity
There was no statistically significant difference in baseline age between the two interventions in any outcome. However, baseline BMI was higher in the metformin intervention compared with the exercise interventions. The transitivity study is detailed in Table S6.
3.7. Probabilities
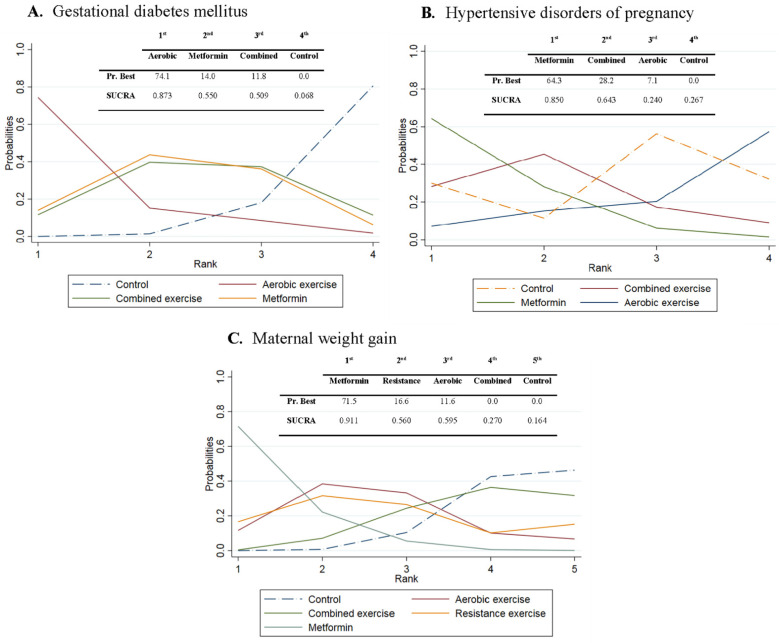
Aerobic exercise showed the highest probability of being the best intervention to prevent GDM (PrBest = 74.1%, SUCRA = 0.873), and metformin to prevent HDP and reduce MWG (PrBest = 64.3%, SUCRA = 0.850 and PrBest = 71.5%, SUCRA = 0.911, respectively) (Figure 3, Figure S3).
Figure 3.
Relative rankings of treatments for gestational diabetes mellitus (A), hypertensive disorders of pregnancy (B) and maternal weight gain (C). Graphs of the rankograms for each outcome. In each graph, the data of each intervention were detailed in a table for the probability of being the best intervention (Pr. Best) and its SUCRA.
3.8. Subgroup Analysis among Pregnant Women with Obesity
Subgroup analysis showed no effect of any intervention for GDM and HDP, but metformin significantly reduced MWG (MWG = −2.88 kg, 95% CI = −5.37, −0.40) (Table S7, Figure S4).
3.9. Sensitivity and Metarregresion Analyses
Analyses with Bayesian methods showed no statistically significant differences compared to frequentist analyses. Furthermore, random effects meta-regression showed no association between age and the effect of the interventions on the different outcomes.
3.10. Heterogeneity and Publication Bias
Aerobic and combined exercise showed no important heterogeneity and a low degree of clinical relevance of heterogeneity (I2 = 0.00% and τ2 = 0.00) for preventing GDM, HDP, and reducing MWG. Metformin and resistance exercise also showed no important heterogeneity and a low degree of clinical relevance of heterogeneity for preventing GDM and reducing MWG, respectively (I2 = 0.00% and τ2 = 0.00). Metformin showed considerable heterogeneity for preventing HDP and reducing MWG (I2 = 80.78% and I2 = 97.26%, respectively), and a substantial degree of clinical relevance of heterogeneity (τ2 = 0.76 and τ2 = 9.98, respectively) (Figure S1). Finally, there was evidence of publication bias only for MWG reduction (Figure S5).
4. Discussion
4.1. Main Findings
Our NMA shows that aerobic exercise reduces the risk of GDM by 49% in overweight/obese pregnant women, while metformin reduces MWG by 2.88–2.93 kg, depending on whether or not of women with overweight are included. Due to the effect size, the results also suggest that aerobic exercise may reduce MWG, and metformin the risk of HDP, which requires further research.
4.2. Interpretation
A previous meta-analysis reported a beneficial effect on GDM (RR = 0.71) [60] among overweight or obese women, but another meta-analysis found no effect [61]. In addition, the aforementioned NMA [24] also did not reach significance in its estimates and did not consider separately the effect of each type of exercise. Our data show that aerobic exercise is the recommended intervention to reduce the risk of GDM. The effect of exercise in preventing this pregnancy disorder could be due to the influence of exercise on improving glucose tolerance by increasing GLUT4 expression in muscle, skeletal muscle glycogen synthesis pathway activity, and TGF-β2 expression in fat tissue [62,63,64]. Moreover, aerobic exercise also improves pancreatic islet cell function, increases myonectin levels and decreases adipokine levels and oxidative stress [65,66]. Finally, it also increases caloric expenditure by increasing the number and size of mitochondria, carnitine transferase activity, and β-oxidation of fatty acids [63,65,66].
Previous meta-analyses showed an inconsistent effect of metformin on HDP. Thus, while one meta-analysis showed a protective effect (RR = 0.51) on the risk of preeclampsia [67], the others reported no effect [23,68,69]. Metformin had no effect on PIH [23,67,68,69]. Our results show no effect of metformin on HDP. However, it is difficult to rule out possible benefits of metformin on the risk of preeclampsia considering that metformin has been shown to improve endothelial dysfunction, poor vascularization, hypertension, and preeclampsia-related vasoconstriction by reducing soluble fms-like tyrosine kinase-1 and soluble endoglin levels; in addition, modulation of sirtuin 1 activity improves nitric oxide bioavailability through deacetylation of nitric oxide synthase. Moreover, closing the loop could also improve the activity of complex 1 of the mitochondrial electron transport chain, whose lower activity is associated with endothelial dysfunction [70,71].
Previous meta-analyses estimated [69,72] that metformin reduced MWG by 1.35 to 1.49 kg, whereas the previous NMA [24] found no effect. Moreover, regarding exercise, the previous meta-analysis and NMA showed reductions of 1.14 and 0.96 kg, respectively [24,60]. Our ranking of interventions shows that metformin is the most effective intervention to prevent excess MWG, followed by aerobic exercise. Aerobic exercise significantly reduces MWG, probably due to increased energy expenditure and fatty acid oxidation. Meanwhile, metformin reduces appetite by decreasing neuropeptide Y and Agouti-related protein and increases proopiomelanocortin and leptin and insulin sensitivity. In muscle, liver and adipose tissue, metformin increases fat oxidation and decreases hepatic glucose and fat synthesis by increasing AMPK activity [73,74,75].
Sensitivity analysis among pregnant women with obesity confirmed that metformin reduced the MWG. However, in women with obesity, aerobic exercise lost its effect on GDM. This was mainly due to the exclusion of the Wang C et al. study, as it had the one largest weight in the analyses, and the largest effect on GDM of all the aerobic exercise studies, which may be due, at least in part, to greater adherence and compliance of women to exercise sessions than in the other aerobic exercise studies.
Although our study has certain limitations, it is possible that increased adherence to exercise recommendations may reduce the risk of GDM and excess MWG in overweight/obese pregnant women. Although it is unclear what factors may increase adherence to physical activity recommendations, strategies to increase compliance such as increased health education in this group of women [76,77,78], should be implemented, and professionally supervised exercise, preferably aerobic exercise, and group exercise with other pregnant women could be recommended. Furthermore, metformin could reduce MWG, and perhaps, the risk of HDP. However, the potential benefits do not justify the medicalization of pregnancy, and the use of metformin should be carefully assessed in each woman, considering the specific benefits and risks.
4.3. Limitations
Some limitations must be acknowledged. First, the scarcity of studies could influence the publication bias analyzes, the assessment of transitivity requirement, and the statistical power of the effect estimates, especially in aerobic exercise, which included only three studies. Second, due to the scarcity of studies, the effect of length, frequency, intensity, or other covariates could not be analyzed, being a substantial source of heterogeneity among interventions. Third, the diagnostic criteria of GDM varied among the included studies. Fourth, the outcome HDP as a single clinical entity, including PIH and pre-eclampsia, two related entities but which might respond differently to each intervention. Fifth, we found no studies on resistance exercise for GDM and HDP that met our inclusion criteria. Sixth, moderate to high risk of bias was found in most studies, with especially important being whether the high risk was due to adhering to intervention or missing outcome data domains. Seventh, transitivity analysis showed a difference in BMI baseline in the exercise and metformin interventions. Eighth, although the meta-regression analyses did not find an association between age and effect, due to the low number of studies this association cannot be ruled out.
5. Conclusions
Active pregnancy is currently recommended to prevent some pregnancy-related complications among overweight/obese pregnant women, including the risk of GDM, HDP, and excessive MWG. One of the limitations of previous studies was to determine which type of exercise is most effective in preventing pregnancy-related complications. Our study suggests that aerobic exercise is the most effective in reducing the risk of GDM and, perhaps, MWG. Metformin showed an effect in reducing MWG, although the lack of effect on other outcomes, the low quality of evidence and, especially, the risks associated with its administration, do not justify the medicalization of pregnancy in healthy pregnant women with obesity. Finally, due to the limitations of our study, further research is needed on the effect of intensity and weekly frequency of exercise sessions and length of interventions, as well as further RCTs in this population group to increase the statistical power of the results.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10163490/s1, Table S1. Direct estimates obtained, Table S2. Excluded trials with reasons ([79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129]), Table S3. Characteristics of the interventions, Table S4. Measure of outcomes, Table S5. Grades of Recommendation, Assessment, Development, and Evaluation, Table S6. Transitivity analysis, Table S7. Results for direct pairwise comparisons and network meta-analysis among pregnant women with obesity, Figure S1. Direct pairwise comparisons by outcome, Figure S2. Risk of bias, Figure S3. Cumulative probabilities by outcome, Figure S4. Direct pairwise comparisons and network meta-analysis by outcome among pregnant women with obesity, Figure S5. Funnel plot by outcome.
Appendix A
Appendix A.1. Medline, EMBASE, Web of Science, Cochrane Library
(“pregnant” OR “pregnancy”) AND (“physical activity” OR “physical exercise” OR “exercise” OR “metformin”) AND (“trial” OR “randomized control trial” OR “randomized controlled trial” OR “controlled pre-post study”)
Appendix A.2. NCT Trials
Condition or disease: pregnancy; Other terms: physical activity
Condition or disease: pregnancy; Other terms: physical exercise
Condition or disease: pregnancy; Other terms: metformin
Appendix A.3. EudraCT
(“pregnancy” OR “pregnant”) AND (“physical activity” OR “physical exercise” OR “exercise” OR “metformin”)
Appendix A.4. Other Databases
Open search (not specified)
Author Contributions
Conceptualization and methodology, C.P.-M. and I.C.-R.; resources and data curation, C.P.-M., C.Á.-B. and I.C.-R.; investigation, C.P.-M.; formal analysis, C.P.-M.; visualization and validation, C.P.-M., M.L.-L.-T., G.S.-M., R.P.-L. and B.R.-M.; writing—original draft preparation, C.P.-M.; writing—review and editing, all authors; supervision, I.C.-R., C.Á.-B. and V.M.-V.; project administration, V.M.-V.; funding acquisition, V.M.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Consejería de Educación, Cultura y Deportes—Junta de Comunidades de Castilla-La Mancha and FEDER funds (SBPLY/17/180501/000533). C.P.-M. is supported by a grant from the Universidad de Castilla-La Mancha (2018-CPUCLM-7939).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flegal K.M., Carroll D., Kit B.K., Ogden C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA J. Am. Med. Assoc. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Marchi J., Berg M., Dencker A., Olander E.K., Begley C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015;16:621–638. doi: 10.1111/obr.12288. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Classification and diagnosis of diabetes. Diabetes Care. 2015;38:S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 4.Guariguata L., Linnenkamp U., Beagley J., Whiting D.R., Cho N.H. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res. Clin. Pract. 2014;103:176–185. doi: 10.1016/j.diabres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Metzger B.E. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc10-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendland E.M., Torloni M.R., Falavigna M., Trujillo J., Dode M.A., Campos M.A., Duncan B.B., Schmidt M.I. Gestational diabetes and pregnancy outcomes—A systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12 doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellamy L., Casas J.P., Hingorani A.D., Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 8.Chodick G., Elchalal U., Sella T., Heymann A.D., Porath A., Kokia E., Shalev V. The risk of overt diabetes mellitus among women with gestational diabetes: A population-based study. Diabet. Med. 2010;27:779–785. doi: 10.1111/j.1464-5491.2010.02995.x. [DOI] [PubMed] [Google Scholar]
- 9.Coustan D.R. Gestational diabetes mellitus. Clin. Chem. 2013;59:1310–1321. doi: 10.1373/clinchem.2013.203331. [DOI] [PubMed] [Google Scholar]
- 10.Godfrey K.M., Lillycrop K.A., Burdge G.C., Gluckman P.D., Hanson M.A. Epigenetic Mechanisms and the Mismatch Concept of the Developmental Origins of Health and Disease. Pediatr. Res. 2007;61:5R–10R. doi: 10.1203/pdr.0b013e318045bedb. [DOI] [PubMed] [Google Scholar]
- 11.Davies G.A.L., Wolfe L.A., Mottola M.F., MacKinnon C., Arsenault M.Y., Bartellas E., Cargill Y., Gleason T., Iglesias S., Klein M., et al. Exercise in pregnancy and the postpartum period. J. Obstet. Gynaecol. Can. 2003;25:516–522. doi: 10.1016/S1701-2163(16)30304-8. [DOI] [PubMed] [Google Scholar]
- 12.Davenport M.H., Ruchat S.M., Poitras V.J., Jaramillo Garcia A., Gray C.E., Barrowman N., Skow R.J., Meah V.L., Riske L., Sobierajski F., et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 2018;52:1367–1375. doi: 10.1136/bjsports-2018-099355. [DOI] [PubMed] [Google Scholar]
- 13.Sanabria-Martínez G., García-Hermoso A., Poyatos-León R., Álvarez-Bueno C., Sánchez-López M., Martínez-Vizcaíno V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: A meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015;122:1167–1174. doi: 10.1111/1471-0528.13429. [DOI] [PubMed] [Google Scholar]
- 14.Wilkerson R.G., Ogunbodede A.C. Hypertensive Disorders of Pregnancy. Emerg. Med. Clin. N. Am. 2019;37:301–316. doi: 10.1016/j.emc.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 15.ACOG Physical Activity and Exercise during Pregnancy and the Postpartum Period: ACOG Committee Opinion Summary, Number 804. Obstet. Gynecol. 2020;135:991–993. doi: 10.1097/AOG.0000000000003773. [DOI] [PubMed] [Google Scholar]
- 16.Barakat R., Perales M. Resistance exercise in pregnancy and outcome. Clin. Obstet. Gynecol. 2016;59:591–599. doi: 10.1097/GRF.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 17.Davies G.A.L., Wolfe L.A., Mottola M.F., MacKinnon C. No. 129-Exercise in Pregnancy and the Postpartum Period. J. Obstet. Gynaecol. Can. 2018;40:e58–e65. doi: 10.1016/j.jogc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Chiefari E., Arcidiacono B., Foti D., Brunetti A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017;40:899–909. doi: 10.1007/s40618-016-0607-5. [DOI] [PubMed] [Google Scholar]
- 19.Skeffington K.L., Higgins J.S., Mahmoud A.D., Evans A.M., Sferruzzi-Perri A.N., Fowden A.L., Yung H.W., Burton G.J., Giussani D.A., Moore L.G. Hypoxia, AMPK activation and uterine artery vasoreactivity. J. Physiol. 2016;594:1357–1369. doi: 10.1113/JP270995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brownfoot F.C., Hastie R., Hannan N.J., Cannon P., Tuohey L., Parry L.J., Senadheera S., Illanes S.E., Kaitu’U-Lino T.J., Tong S. Metformin as a prevention and treatment for preeclampsia: Effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am. J. Obstet. Gynecol. 2016;214:356.e1–356.e15. doi: 10.1016/j.ajog.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Agatisa P.K., Ness R.B., Roberts J.M., Costantino J.P., Kuller L.H., McLaughlin M.K. Impairment of endothelial function in women with a history of preeclampsia: An indicator of cardiovascular risk. Am. J. Physiol. Heart Circ. Physiol. 2004;286 doi: 10.1152/ajpheart.00298.2003. [DOI] [PubMed] [Google Scholar]
- 22.Banek C.T., Bauer A.J., Needham K.M., Dreyer H.C., Gilbert J.S. AICAR (5-aminoimidazole-4-carboxamide-3-ribonucleoside) administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1159–H1165. doi: 10.1152/ajpheart.00903.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dodd J.M., Grivell R.M., Deussen A.R., Hague W.M. Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes. Cochrane Database Syst. Rev. 2018;2018:CD010564. doi: 10.1002/14651858.CD010564.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatzakis C., Goulis D.G., Mareti E., Eleftheriades M., Zavlanos A., Dinas K., Sotiriadis A. Prevention of gestational diabetes mellitus in overweight or obese pregnant women: A network meta-analysis. Diabetes Res. Clin. Pract. 2019;158 doi: 10.1016/j.diabres.2019.107924. [DOI] [PubMed] [Google Scholar]
- 25.Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G., Atkins D., Barbour V., Barrowman N., Berlin J.A., et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutton B., Catalá-López F., Moher D. La extensión de la declaración PRISMA para revisiones sistemáticas que incorporan metaanálisis en red: PRISMA-NMA. Med. Clin. 2016;147:262–266. doi: 10.1016/j.medcli.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J.P., Green S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. John Wiley and Sons; Hoboken, NJ, USA: 2008. [Google Scholar]
- 28.Pascual-Morena C., Martínez-Vizcaíno V., Álvarez-Bueno C., Pozuelo-Carrascosa D.P., Notario-Pacheco B., Saz-Lara A., Fernández-Rodriguez R., Cavero-Redondo I. Exercise vs. metformin for gestational diabetes mellitus: Protocol for a network meta-analysis. Medicine. 2019;98:e16038. doi: 10.1097/MD.0000000000016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caspersen C.J., Powell K.E., Christenson G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1902;100:126–131. doi: 10.1093/nq/s9-IX.228.365-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riebe D., Ehrman J., Liguori G., Magal M., Medicine A.C.S. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Kluwer, Wolters; Philadelphia, PA, USA: 2018. [Google Scholar]
- 31.Mcnair D., Lorr M., Droppleman L.F. Manual for the Profile of Mood States. Educational and Industrial Testing Services; San Diego, CA, USA: 1971. 27p [Google Scholar]
- 32.Borg G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982;14:377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Eldridge S., Campbell M.K., Campbell M.J., Drahota-Towns A., Giraudeau B., Higgins J.P.T., Reeves B.C., Siegfried N. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): Additional considerations for cluster-randomized trials. [(accessed on 6 August 2021)];Portsm. Res. Portal. 2016 Available online: https://www.semanticscholar.org/paper/Revised-Cochrane-risk-of-bias-tool-for-randomized-Eldridge-Campbell/d0b0ef43d768e6d3a574cc84d75e91722a2f7d4e#citing-papers. [Google Scholar]
- 34.Neumann I., Pantoja T., Peñaloza B., Cifuentes L., Rada G. El sistema GRADE: Un cambio en la forma de evaluar la calidad de la evidencia y la fuerza de recomendaciones. Rev. Med. Chil. 2014;142:630–635. doi: 10.4067/S0034-98872014000500012. [DOI] [PubMed] [Google Scholar]
- 35.Salanti G., Del Giovane C., Chaimani A., Caldwell D.M., Higgins J.P.T. Evaluating the Quality of Evidence from a Network Meta-Analysis. PLoS ONE. 2014;9:e99682. doi: 10.1371/journal.pone.0099682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaimani A., Higgins J.P.T., Mavridis D., Spyridonos P., Salanti G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salanti G., Ades A.E., Ioannidis J.P.A. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Veroniki A.A., Vasiliadis H.S., Higgins J.P., Salanti G. Evaluation of inconsistency in networks of interventions. Int. J. Epidemiol. 2013;42:332–345. doi: 10.1093/ije/dys222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Der Simonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 40.Spiegelhalter D.J., Abrams K.R., Myles J.P. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. John Wiley & Sons, Ltd.; Chichester, UK: 2003. [DOI] [Google Scholar]
- 41.Stettler C., Allemann S., Wandel S., Kastrati A., Morice M.C., Schömig A., Pfisterer M.E., Stone G.W., Leon M.B., de Lezo J.S., et al. Drug eluting and bare metal stents in people with and without diabetes: Collaborative network meta-analysis. BMJ. 2008;337 doi: 10.1136/bmj.a1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cipriani A., Higgins J.P.T., Geddes J.R., Salanti G. Conceptual and technical challenges in network meta-analysis. Ann. Int. Med. 2013;159:130–137. doi: 10.7326/0003-4819-159-2-201307160-00008. [DOI] [PubMed] [Google Scholar]
- 43.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test measures of funnel plot asymmetry. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong K.L., Campbell C.G., Foster R.C., Peterson A.D., Lanningham-Foster L. A pilot walking program promotes moderate-intensity physical activity during pregnancy. Med. Sci. Sports Exerc. 2014;46:462–471. doi: 10.1249/MSS.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 45.Seneviratne S.N., Jiang Y., Derraik J.G.B., McCowan L.M.E., Parry G.K., Biggs J.B., Craigie S., Gusso S., Peres G., Rodrigues R.O., et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: A randomised controlled trial. BJOG. 2016;123:588–597. doi: 10.1111/1471-0528.13738. [DOI] [PubMed] [Google Scholar]
- 46.Wang C., Wei Y., Zhang X., Zhang Y., Xu Q., Sun Y., Su S., Zhang L., Liu C., Feng Y., et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017;216:340–351. doi: 10.1016/j.ajog.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 47.Barakat R., Pelaez M., Cordero Y., Perales M., Lopez C., Coteron J., Mottola M.F. Exercise during pregnancy protects against hypertension and macrosomia: Randomized clinical trial. Am. J. Obstet. Gynecol. 2016;214:649.e1–649.e8. doi: 10.1016/j.ajog.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 48.Bisson M., Almeras N., Dufresne S.S., Robitaille J., Rheaume C., Bujold E., Frenette J., Tremblay A., Marc I. A 12-Week Exercise Program for Pregnant Women with Obesity to Improve Physical Activity Levels: An Open Randomised Preliminary Study. PLoS ONE. 2015;10:e0137742. doi: 10.1371/journal.pone.0137742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daly N., Farren M., McKeating A., O’Kelly R., Stapleton M., Turner M.J. A Medically Supervised Pregnancy Exercise Intervention in Obese Women: A Randomized Controlled Trial. Obstet. Gynecol. 2017;130:1001–1010. doi: 10.1097/AOG.0000000000002267. [DOI] [PubMed] [Google Scholar]
- 50.Garnæs K.K., Mørkved S., Salvesen Ø., Moholdt T. Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial) PLoS Med. 2016;13:e1002079. doi: 10.1371/journal.pmed.1002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nascimento S.L., Surita F.G., Parpinelli M.A., Siani S., Pinto e Silva J.L. The effect of an antenatal physical exercise programme on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women: A randomised clinical trial. BJOG. 2011;118:1455–1463. doi: 10.1111/j.1471-0528.2011.03084.x. [DOI] [PubMed] [Google Scholar]
- 52.Oostdam N., Van Poppel M.N.M., Wouters M.G.A.J., Eekhoff E.M.W., Bekedam D.J., Kuchenbecker W.K.H., Quartero H.W.P., Heres M.H.B., Van Mechelen W. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: Results of a randomised controlled trial. BJOG. 2012;119:1098–1107. doi: 10.1111/j.1471-0528.2012.03366.x. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz J.R., Perales M., Pelaez M., Lopez C., Lucia A., Barakat R. Supervised exercise-based intervention to prevent excessive gestational weight gain: A randomized controlled trial. Mayo Clin. Proc. 2013;88:1388–1397. doi: 10.1016/j.mayocp.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 54.Barakat R., Lucia A., Ruiz J.R. Resistance exercise training during pregnancy and newborn’s birth size: A randomised controlled trial. Int. J. Obes. 2009;33:1048–1057. doi: 10.1038/ijo.2009.150. [DOI] [PubMed] [Google Scholar]
- 55.Fattah E. Can metformin limit weight gain in the obese with pregnancy? Int. J. Reprod. Contracept. Obstet. Gynecol. 2016;5:818–825. doi: 10.18203/2320-1770.ijrcog20160591. [DOI] [Google Scholar]
- 56.Brink H.S., Alkemade M., van der Lely A.J., van der Linden J. Metformin in women at high risk of gestational diabetes mellitus. Diabetes Metab. 2018;44:300–302. doi: 10.1016/j.diabet.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Chiswick C., Reynolds R.M., Denison F., Drake A.J., Forbes S., Newby D.E., Walker B.R., Quenby S., Wray S., Weeks A., et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2015;3:778–786. doi: 10.1016/S2213-8587(15)00219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.do Nascimento I.B., Sales W.B., Dienstmann G., de Souza M.L.R., Fleig R., Silva J.C. Metformin for prevention of cesarean delivery and large-for-gestational-age newborns in non-diabetic obese pregnant women: A randomized clinical trial. Arch. Endocrinol. Metab. 2020;64:290–297. doi: 10.20945/2359-3997000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Syngelaki A., Nicolaides K.H., Balani J., Hyer S., Akolekar R., Kotecha R., Pastides A., Shehata H. Metformin versus Placebo in Obese Pregnant Women without Diabetes Mellitus. N. Engl. J. Med. 2016;374:434–443. doi: 10.1056/NEJMoa1509819. [DOI] [PubMed] [Google Scholar]
- 60.Du M.C., Ouyang Y.Q., Nie X.F., Huang Y., Redding S.R. Effects of physical exercise during pregnancy on maternal and infant outcomes in overweight and obese pregnant women: A meta-analysis. Birth. 2019;46:211–221. doi: 10.1111/birt.12396. [DOI] [PubMed] [Google Scholar]
- 61.Nasiri-Amiri F., Sepidarkish M., Shirvani M.A., Habibipour P., Tabari N.S.M. The effect of exercise on the prevention of gestational diabetes in obese and overweight pregnant women: A systematic review and meta-Analysis. Diabetol. Metab. Syndr. 2019;11 doi: 10.1186/s13098-019-0470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Consitt L.A., Dudley C., Saxena G. Impact of endurance and resistance training on skeletal muscle glucose metabolism in older adults. Nutrients. 2019;11:2636. doi: 10.3390/nu11112636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moghetti P., Bacchi E., Brangani C., Donà S., Negri C. Metabolic Effects of Exercise. Front. Horm. Res. 2016;47:44–57. doi: 10.1159/000445156. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi H., Alves C.R.R., Stanford K.I., Middelbeek R.J.W., Nigro P., Ryan R.E., Xue R., Sakaguchi M., Lynes M.D., So K., et al. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 2019;1:291–303. doi: 10.1038/s42255-018-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yaribeygi H., Atkin S.L., Simental-Mendía L.E., Sahebkar A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. J. Cell. Physiol. 2019;234:12385–12392. doi: 10.1002/jcp.28066. [DOI] [PubMed] [Google Scholar]
- 66.Pourranjbar M., Arabnejad N., Naderipour K., Rafie F. Effects of Aerobic Exercises on Serum Levels of Myonectin and Insulin Resistance in Obese and Overweight Women. J. Med. Life. 2018;11:381–386. doi: 10.25122/jml-2018-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nascimento I.B.D., Dienstmann G., De Souza M.L.R., Fleig R., Hoffmann C.B.P.C., Silva J.C. Evaluation of Preeclampsia Results after Use of Metformin in Gestation: Systematic Review and Meta-analysis. Rev. Bras. Ginecol. Obstet. 2018;40:713–721. doi: 10.1055/s-0038-1675214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalafat E., Sukur Y.E., Abdi A., Thilaganathan B., Khalil A. Metformin for prevention of hypertensive disorders of pregnancy in women with gestational diabetes or obesity: Systematic review and meta-analysis of randomized trials. Ultrasound Obstet. Gynecol. 2018;52:706–714. doi: 10.1002/uog.19084. [DOI] [PubMed] [Google Scholar]
- 69.Elmaraezy A., Abushouk A.I., Emara A., Elshahat O., Ahmed H., Mostafa M.I. Effect of metformin on maternal and neonatal outcomes in pregnant obese non-diabetic women: A meta-analysis. Int. J. Reprod. Biomed. 2017;15:461–470. doi: 10.29252/ijrm.15.8.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romero R., Erez O., Huttemann M., Maymon E., Panaitescu B., Conde-Agudelo A., Pacora P., Yoon B.H., Grossman L.I. Metformin, the aspirin of the 21st century: Its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am. J. Obstet. Gynecol. 2017;217:282–302. doi: 10.1016/j.ajog.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soobryan N., Murugesan S., Pandiyan A., Moodley J., Mackraj I. Angiogenic Dysregulation in Pregnancy-Related Hypertension—A Role for Metformin. Reprod. Sci. 2018;25:1531–1539. doi: 10.1177/1933719118773484. [DOI] [PubMed] [Google Scholar]
- 72.D’Ambrosio V., Brunelli R., Vena F., Di Mascio D., Marchetti C., Boccherini C., Piccioni M.G., Benedetti Panici P., Giancotti A. Metformin reduces maternal weight gain in obese pregnant women: A systematic review and meta-analysis of two randomized controlled trials. Diabetes Metab. Res. Rev. 2019;35 doi: 10.1002/dmrr.3164. [DOI] [PubMed] [Google Scholar]
- 73.Nicodemus N.A., Jr. Prevention of Excessive Gestational Weight Gain and Postpartum Weight Retention. Curr. Obes. Rep. 2018;7:105–111. doi: 10.1007/s13679-018-0312-0. [DOI] [PubMed] [Google Scholar]
- 74.Yerevanian A., Soukas A.A. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr. Obes. Rep. 2019;8:156–164. doi: 10.1007/s13679-019-00335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malin S.K., Kashyap S.R. Effects of metformin on weight loss: Potential mechanisms. Curr. Opin. Endocrinol. Diabetes Obes. 2014;21:323–329. doi: 10.1097/MED.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 76.Broberg L., Ersbøll A.S., Backhausen M.G., Damm P., Tabor A., Hegaard H.K. Compliance with national recommendations for exercise during early pregnancy in a Danish cohort. BMC Pregnancy Childbirth. 2015;15:317. doi: 10.1186/s12884-015-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amezcua-Prieto C., Lardelli-Claret P., Olmedo-Requena R., Mozas-Moreno J., Bueno-Cavanillas A., Jiménez-Moleón J.J. Compliance with leisure-time physical activity recommendations in pregnant women. Acta Obstet. Gynecol. Scand. 2011;90:245–252. doi: 10.1111/j.1600-0412.2010.01050.x. [DOI] [PubMed] [Google Scholar]
- 78.Martin-Arias A., Brik M., Vargas-Terrones M., Barakat R., Santacruz B. Predictive factors of compliance with a program of supervised exercise during pregnancy. Acta Obstet. Gynecol. Scand. 2019;98:807–808. doi: 10.1111/aogs.13527. [DOI] [PubMed] [Google Scholar]
- 79.Brislane Á., Jones H., Holder S.M., Low D.A., Hopkins N.D. The Effect of Exercise during Pregnancy on Maternal and Offspring Vascular Outcomes: A Pilot Study. Reprod. Sci. 2021;28:510–523. doi: 10.1007/s43032-020-00302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cavalcante S.R., Cecatti J.G., Pereira R.I., Baciuk E.P., Bernardo A.L., Silveira C. Water aerobics II: Maternal body composition and perinatal outcomes after a program for low risk pregnant women. Reprod. Health. 2009;6:1. doi: 10.1186/1742-4755-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clapp J.F., 3rd, Kim H., Burciu B., Lopez B. Beginning regular exercise in early pregnancy: Effect on fetoplacental growth. Am. J. Obstet. Gynecol. 2000;183:1484–1488. doi: 10.1067/mob.2000.107096. [DOI] [PubMed] [Google Scholar]
- 82.de Oliveria Melo A.S., Silva J.L.P., Tavares J.S., Barros V.O., Leite D.F.B., Amorim M.M.R. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: A randomized controlled trial. Obstet. Gynecol. 2012;120:302–310. doi: 10.1097/AOG.0b013e31825de592. [DOI] [PubMed] [Google Scholar]
- 83.Ghodsi Z., Asltoghiri M. Effects of aerobic exercise training on maternal and neonatal outcome: A randomized controlled trial on pregnant women in Iran. J. Pak. Med. Assoc. 2014;64:1053–1056. [PubMed] [Google Scholar]
- 84.Guelfi K.J., Ong M.J., Crisp N.A., Fournier P.A., Wallman K.E., Grove J.R., Doherty D.A., Newnham J.P. Regular Exercise to Prevent the Recurrence of Gestational Diabetes Mellitus: A Randomized Controlled Trial. Obstet. Gynecol. 2016;128:819–827. doi: 10.1097/AOG.0000000000001632. [DOI] [PubMed] [Google Scholar]
- 85.Hopkins S.A., Baldi J.C., Cutfield W.S., McCowan L., Hofman P.L. Effects of exercise training on maternal hormonal changes in pregnancy. Clin. Endocrinol. 2011;74:495–500. doi: 10.1111/j.1365-2265.2010.03964.x. [DOI] [PubMed] [Google Scholar]
- 86.Kasawara K.T., Burgos C.S.G., do Nascimento S.L., Ferreira N.O., Surita F.G., Pinto E., Silva J.L. Maternal and perinatal outcomes of exercise in pregnant women with chronic hypertension and/or previous preeclampsia: A randomized controlled trial. ISRN Obstet. Gynecol. 2013;2013:857047. doi: 10.1155/2013/857047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khoram S., Loripoor M., Pirhadi M., Beigi M. The effect of walking on pregnancy blood pressure disorders in women susceptible to pregnancy hypertension: A randomized clinical trial. J. Educ. Health Promot. 2019;8:95. doi: 10.4103/jehp.jehp_378_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kihlstrand M., Stenman B., Nilsson S., Axelsson O. Water-gymnastics reduced the intensity of back/low back pain in pregnant women. Acta Obstet. Gynecol. Scand. 1999;78:180–185. [PubMed] [Google Scholar]
- 89.Ko C.W., Napolitano P.G., Lee S.P., Schulte S.D., Ciol M.A., Beresford S.A.A. Physical activity, maternal metabolic measures, and the incidence of gallbladder sludge or stones during pregnancy: A randomized trial. Am. J. Perinatol. 2014;31:39–48. doi: 10.1055/s-0033-1334455. [DOI] [PubMed] [Google Scholar]
- 90.Labonte-Lemoyne E., Curnier D., Ellemberg D. Exercise during pregnancy enhances cerebral maturation in the newborn: A randomized controlled trial. J. Clin. Exp. Neuropsychol. 2017;39:347–354. doi: 10.1080/13803395.2016.1227427. [DOI] [PubMed] [Google Scholar]
- 91.Marquez-Sterling S., Perry A.C., Kaplan T.A., Halberstein R.A., Signorile J.F. Physical and psychological changes with vigorous exercise in sedentary primigravidae. Med. Sci. Sports Exerc. 2000;32:58–62. doi: 10.1097/00005768-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 92.McDonald S.M., Newton E., Strickland D., Isler C., Haven K., Kelley G., Chasan-Taber L., Kuehn D., May L.E. Influence of Prenatal Aerobic Exercise on Fetal Morphometry. Matern. Child Health J. 2020;24:1367–1375. doi: 10.1007/s10995-020-03000-7. [DOI] [PubMed] [Google Scholar]
- 93.Navas A., Carrascosa M.D.C., Artigues C., Ortas S., Portells E., Soler A., Yañez A.M., Bennasar-Veny M., Leiva A. Effectiveness of Moderate-Intensity Aerobic Water Exercise during Pregnancy on Quality of Life and Postpartum Depression: A Multi-Center, Randomized Controlled Trial. J. Clin. Med. 2021;10:2432. doi: 10.3390/jcm10112432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sedaghati P., Ziaee V., Ardjmand A. The effect of an ergometric training program on pregnants’ weight gain and low back pain. Gazz. Med. Ital. 2007;166:209–213. [Google Scholar]
- 95.Taniguchi C., Sato C. Home-based walking during pregnancy affects mood and birth outcomes among sedentary women: A randomized controlled trial. Int. J. Nurs. Pract. 2016;22:420–426. doi: 10.1111/ijn.12453. [DOI] [PubMed] [Google Scholar]
- 96.Tomić V., Sporiš G., Tomić J., Milanović Z., Zigmundovac-Klaić D., Pantelić S. The effect of maternal exercise during pregnancy on abnormal fetal growth. Croat. Med. J. 2013;54:362–368. doi: 10.3325/cmj.2013.54.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shen W.-C., Chen C.H. Effects of non-supervised aerobic exercise on sleep quality and maternal-fetal attachment in pregnant women: A randomized controlled trial. Complement. Ther. Med. 2021;57:102671. doi: 10.1016/j.ctim.2021.102671. [DOI] [PubMed] [Google Scholar]
- 98.Rakhshani A., Nagarathna R., Mhaskar R., Mhaskar A., Thomas A., Gunasheela S. The effects of yoga in prevention of pregnancy complications in high-risk pregnancies: A randomized controlled trial. Prev. Med. 2012;55:333–340. doi: 10.1016/j.ypmed.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 99.Sonmezer E., Özköslü M.A., Yosmaoğlu H.B. The effects of clinical pilates exercises on functional disability, pain, quality of life and lumbopelvic stabilization in pregnant women with low back pain: A randomized controlled study. J. Back Musculoskelet. Rehabil. 2021;34:69–76. doi: 10.3233/BMR-191810. [DOI] [PubMed] [Google Scholar]
- 100.Sun Y.-C., Hung Y.-C., Chang Y., Kuo S.-C. Effects of a prenatal yoga programme on the discomforts of pregnancy and maternal childbirth self-efficacy in Taiwan. Midwifery. 2010;26:e31–e36. doi: 10.1016/j.midw.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 101.Bacchi M., Mottola M.F., Perales M., Refoyo I., Barakat R. Aquatic Activities during Pregnancy Prevent Excessive Maternal Weight Gain and Preserve Birth Weight: A Randomized Clinical Trial. Am. J. Health Promot. 2018;32:729–735. doi: 10.1177/0890117117697520. [DOI] [PubMed] [Google Scholar]
- 102.Backhausen M.G., Tabor A., Albert H., Rosthøj S., Damm P., Hegaard H.K. The effects of an unsupervised water exercise program on low back pain and sick leave among healthy pregnant women—A randomised controlled trial. PLoS ONE. 2017;12:e0182114. doi: 10.1371/journal.pone.0182114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barakat R., Pelaez M., Montejo R., Luaces M., Zakynthinaki M. Exercise during pregnancy improves maternal health perception: A randomized controlled trial. Am. J. Obstet. Gynecol. 2011;204:402.e1–402.e7. doi: 10.1016/j.ajog.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 104.Barakat R., Pelaez M., Lopez C., Montejo R., Coteron J. Exercise during pregnancy reduces the rate of cesarean and instrumental deliveries: Results of a randomized controlled trial. J. Matern. Fetal. Neonatal Med. 2012;25:2372–2376. doi: 10.3109/14767058.2012.696165. [DOI] [PubMed] [Google Scholar]
- 105.Barakat R., Pelaez M., Lopez C., Lucia A., Ruiz J.R. Exercise during pregnancy and gestational diabetes-related adverse effects: A randomised controlled trial. Br. J. Sports Med. 2013;47:630–636. doi: 10.1136/bjsports-2012-091788. [DOI] [PubMed] [Google Scholar]
- 106.Barakat R., Vargas M., Brik M., Fernandez I., Gil J., Coteron J., Santacruz B. Does Exercise During Pregnancy Affect Placental Weight? A Randomized Clinical Trial. Eval. Health Prof. 2018;41:400–414. doi: 10.1177/0163278717706235. [DOI] [PubMed] [Google Scholar]
- 107.Barakat R., Refoyo I., Coteron J., Franco E. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial. Braz. J. Phys. Ther. 2019;23:148–155. doi: 10.1016/j.bjpt.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brik M., Fernandez-Buhigas I., Martin-Arias A., Vargas-Terrones M., Barakat R., Santacruz B. Does exercise during pregnancy impact on maternal weight gain and fetal cardiac function? A randomized controlled trial. Ultrasound Obstet. Gynecol. 2019;53:583–589. doi: 10.1002/uog.20147. [DOI] [PubMed] [Google Scholar]
- 109.Broberg L., Tabor A., Rosthøj S., Backhausen M., Frokjaer V.G., Damm P., Hegaard H.K. Effect of supervised group exercise on psychological well-being among pregnant women with or at high risk of depression (the EWE Study): A randomized controlled trial. Acta Obstet. Gynecol. Scand. 2021;100:129–138. doi: 10.1111/aogs.13982. [DOI] [PubMed] [Google Scholar]
- 110.Cordero Y., Peláez M., De Miguel M., Perales M., Barakat Carballo R. Can moderate physical exercise during pregnancy act as a factor in preventing gestational diabetes? [Puede el ejercicio físico moderado durante el embarazo actuar como un factor de prevención de la diabetes gestacional?] RICYDE Rev. Int. Cienc. Deport. 2012;8:3–19. doi: 10.5232/ricyde2012.02701. [DOI] [Google Scholar]
- 111.Cordero Y., Mottola M.F., Vargas J., Blanco M., Barakat R. Exercise Is Associated with a Reduction in Gestational Diabetes Mellitus. Med. Sci. Sports Exerc. 2015;47:1328–1333. doi: 10.1249/MSS.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 112.da Silva S.G., Hallal P.C., Domingues M.R., Bertoldi A.D.A.D., da Silveira M.F., Bassani D., da Silva I.C.M.I., da Silva B.G.C., de Coll C.V.N., Evenson K. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: Results from the PAMELA study. Int. J. Behav. Nutr. Phys. Act. 2017;14:175. doi: 10.1186/s12966-017-0632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fernandez-Buhigas I., Brik M., Martin-Arias A., Vargas-Terrones M., Varillas D., Barakat R., Santacruz B. Maternal physiological changes at rest induced by exercise during pregnancy: A randomized controlled trial. Physiol. Behav. 2020:112863. doi: 10.1016/j.physbeh.2020.112863. [DOI] [PubMed] [Google Scholar]
- 114.Haakstad L.A.H., Bo K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: A randomised controlled trial. Eur. J. Contracept. Reprod. Health Care. 2011;16:116–125. doi: 10.3109/13625187.2011.560307. [DOI] [PubMed] [Google Scholar]
- 115.Pelaez M., Gonzalez-Cerron S., Montejo R., Barakat R. Protective Effect of Exercise in Pregnant Women Including Those Who Exceed Weight Gain Recommendations: A Randomized Controlled Trial. Mayo Clin. Proc. 2019;94:1951–1959. doi: 10.1016/j.mayocp.2019.01.050. [DOI] [PubMed] [Google Scholar]
- 116.Perales M., Calabria I., Lopez C., Franco E., Coteron J., Barakat R. Regular Exercise Throughout Pregnancy Is Associated With a Shorter First Stage of Labor. Am. J. Health Promot. 2016;30:149–154. doi: 10.4278/ajhp.140221-QUAN-79. [DOI] [PubMed] [Google Scholar]
- 117.Price B.B., Amini S.B., Kappeler K. Exercise in pregnancy: Effect on fitness and obstetric outcomes-a randomized trial. Med. Sci. Sports Exerc. 2012;44:2263–2269. doi: 10.1249/MSS.0b013e318267ad67. [DOI] [PubMed] [Google Scholar]
- 118.Ramírez-Vélez R., Lobelo F., Aguilar-de Plata A.C., Izquierdo M., García-Hermoso A. Exercise during pregnancy on maternal lipids: A secondary analysis of randomized controlled trial. BMC Pregnancy Childbirth. 2017;17:396. doi: 10.1186/s12884-017-1571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rodríguez-Blanque R., Sánchez-García J.C., Sánchez-López A.M., Mur-Villar N., Fernández-Castillo R., Aguilar-Cordero M.J. Influence of physical exercise during pregnancy on newborn weight: A randomized clinical trial [Influencia del ejercicio físico durante el embarazo sobre el peso del recién nacido: Un ensayo clínico aleatorizado] Nutr. Hosp. 2017;34:834–840. doi: 10.20960/nh.1095. [DOI] [PubMed] [Google Scholar]
- 120.Stafne S.N., Salvesen K.Å., Romundstad P.R., Eggebø T.M., Carlsen S.M., Mørkved S. Regular exercise during pregnancy to prevent gestational diabetes: A randomized controlled trial. Obstet. Gynecol. 2012;119:29–36. doi: 10.1097/AOG.0b013e3182393f86. [DOI] [PubMed] [Google Scholar]
- 121.Abd El Hameed A.A., Shreif H.E., Mowafy H.E. The role of continuing metformin therapy during pregnancy in the reduction of gestational diabetes and improving pregnancy outcomes in women with polycystic ovary syndrome. Middle East Fertil. Soc. J. 2011;16:204–208. doi: 10.1016/j.mefs.2011.04.002. [DOI] [Google Scholar]
- 122.Begum M.R., Khanam N.N., Quadir E., Ferdous J., Begum M.S., Khan F., Begum A. Prevention of gestational diabetes mellitus by continuing metformin therapy throughout pregnancy in women with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2009;35:282–286. doi: 10.1111/j.1447-0756.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- 123.Glueck C.J., Wang P., Kobayashi S., Phillips H., Sieve-Smith L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil. Steril. 2002;77:520–525. doi: 10.1016/S0015-0282(01)03202-2. [DOI] [PubMed] [Google Scholar]
- 124.Jamal A., Milani F., Al-Yasin A. Evaluation of the effect of metformin and aspirin on utero placental circulation of pregnant women with PCOS. Iran. J. Reprod. Med. 2012;10:265–270. [PMC free article] [PubMed] [Google Scholar]
- 125.Khattab S., Mohsen I.A., Aboul Foutouh I., Ashmawi H.S., Mohsen M.N., Van Wely M., Van Der Veen F., Youssef M.A. Can metformin reduce the incidence of gestational diabetes mellitus in pregnant women with polycystic ovary syndrome? Prospective cohort study. Gynecol. Endocrinol. 2011;27:789–793. doi: 10.3109/09513590.2010.540600. [DOI] [PubMed] [Google Scholar]
- 126.Lovvik T.S., Carlsen S.M., Salvesen O., Steffensen B., Bixo M., Gomez-Real F., Lonnebotn M., Hestvold K.V., Zabielska R., Hirschberg A.L., et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:256–266. doi: 10.1016/S2213-8587(19)30002-6. [DOI] [PubMed] [Google Scholar]
- 127.Valdés E., Sepúlveda-Martínez A., Candia P., Abusada N., Orellana R., Manukian B.B.B., Cuellar E. Metformin as a prophylactic treatment of gestational diabetes in pregnant patients with pregestational insulin resistance: A randomized study. J. Obstet. Gynaecol. Res. 2018;44:81–86. doi: 10.1111/jog.13477. [DOI] [PubMed] [Google Scholar]
- 128.Vanky E., Salvesen K.A., Heimstad R., Fougner K.J., Romundstad P., Carlsen S.M. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: Results of a randomized study. Hum. Reprod. 2004;19:1734–1740. doi: 10.1093/humrep/deh347. [DOI] [PubMed] [Google Scholar]
- 129.Vanky E., Stridsklev S., Heimstad R., Romundstad P., Skogøy K., Kleggetveit O., Hjelle S., Von Brandis P., Eikeland T., Flo K., et al. Metformin Versus placebo from first trimester to delivery in polycystic ovary syndrome: A randomized, controlled multicenter study. J. Clin. Endocrinol. Metab. 2010;95:E448–E455. doi: 10.1210/jc.2010-0853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed are available from the corresponding author.