Abstract
Cases of Pneumocystis jirovecii pneumonia (PCP) in patients suffering from COVID-19 were described in patients with various comorbidities and outcomes. The diagnosis of PCP in these patients is difficult due to clinical and radiological similarities. We carried out this study in order to better describe potentially at-risk patients and their outcomes. We retrospectively analyzed all patients with a P. jirovecii PCR performed in bronchoalveolar lavage fluid, tracheal aspirate, or sputum within a month after the COVID-19 diagnosis. Fifty-seven patients with COVID-19 infection were tested for P. jirovecii. Among 57 patients with COVID-19, four patients had a concomitant positive P. jirovecii PCR. These four patients were elderly with a mean age of 78. Two patients were immunocompromised, and the two others presented only diabetes mellitus. Three patients presented an ARDS requiring transfer to the ICU and mechanical ventilation. All patients presented lymphocytopenia. Three patients had probable PCP, and one had proven PCP. All patients died within two months after hospital admission. These co-infections are rare but severe, therefore, PCP should be considered in case of worsening of the condition of patients with severe COVID-19.
Keywords: pneumocystosis, Pneumocystis jirovecii, COVID-19, SARS-CoV-2, fungi
1. Introduction
COVID-19 causes a wide spectrum of symptoms ranging from asymptomatic disease to life-threatening severe acute respiratory distress syndrome (ARDS) and death [1]. This disease can be complicated with fungal superinfection, particularly aspergillosis [2]. Cases of Pneumocystis pneumonia (PCP) were reported in HIV-positive subjects and kidney transplant recipients as well as immunocompetent patients suffering from COVID-19 [3,4,5]. A study found that two among 145 patients (1.4%) with severe COVID-19 had a positive polymerase chain reaction (PCR) for Pneumocystis jirovecii [6]. Another one found that a P. jirovecii PCR was positive in 10 of 108 (9.3%) patients with severe SARS-CoV-2 infection [7]. The diagnosis of PCP during COVID-19 is challenging, and the need to treat patients with a positive P. jirovecii PCR in this context remains unclear. We analyzed all consecutive P. jirovecii PCR performed in COVID-19 patients in our center and described four cases of PCP, including one proven case and three probable ones following EORTC/MSG criteria [8].
2. Materials and Methods
Among adult patients with confirmed SARS-CoV-2 infection between 1 March 2020 and 1 February 2021, hospitalized in Strasbourg University Hospital, we retrospectively analyzed all patients with a P. jirovecii PCR performed in bronchoalveolar lavage (BAL) fluid, tracheal aspirate, or sputum. During the ongoing COVID-19 pandemic, no microscopic diagnosis was done.
All COVID-19 patients with a positive P. jirovecii PCR in a sample harvested within a month after the COVID-19 diagnosis were included. Patients who were not followed in our center were excluded. The medical records were analyzed to collect the medical history as well as demographic, clinical, biological, radiological, and microbiological data.
The study was approved by the Ethics Committee of the University Hospital of Strasbourg (No. CE-2020-51). Oral non-opposition to participation to the study was sought, and the patients who expressed opposition to participate were not included.
3. Results
Among patients with SARS-CoV-2 infection during the inclusion period, 57 had a concomitant investigation for P. jirovecii. Four patients (7.1%) had a positive P. jirovecii PCR and were diagnosed with PCP. Patients’ demographic data and underlying diseases are described in Table 1; microbiological, biological, and clinical data are described in Table 1.
Table 1.
Patients characteristics.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Sex | F | M | M | M |
| Age | 80 | 70 | 83 | 80 |
| BMI | 36 | 35 | 28 | 28 |
| Underlying disease | Rheumatoid arthritis | Renal cell carcinoma in 2008, Autologous HSCT in 2013 for IgA myeloma complicated by amylosis, Ewing sarcoma with pulmonary metastases ongoing treatment, chronic kidney disease, pulmonary pneumocystosis six month ago |
Diabetes mellitus, chronic heart failure, chronic kidney disease, atrial fibrillation, asthma | Diabetes mellitus, pacemaker for atrioventricular block, hypertension, transitory ischemic attack |
| Immunosuppressive treatment | Prednisone, Celecoxib | Cyclophosphamide, Doxorubicine | No | No |
| Pneumocystosis prophylaxis | No | TMP-SMX | No | No |
| Microbiological diagnosis | ||||
| COVID-19 | PCR on NPS | PCR on NPS | PCR on NPS | PCR on NPS |
| P. jirovecii | PCR on BAL | PCR on tracheal aspirate | PCR and cytology on BAL | PCR on tracheal aspirate |
| Time between hospitalization and P. jirovecii PCR (days) | 1 | 1 | 40 | 25 |
| BDG (Fungitell Cape Cod.) | NR | NR | 123 pg/mL | 137 pg/mL |
| Biological data | ||||
| Lymphocytes count (cells/mmc) | 300 | 222 | 570 | 600 |
| LDH (IU/L) | NR | 811 | 437 | 267 |
| Clinical data | ||||
| Time from symptom onset to hospitalization | 5 days | 5 days | 5 days | 7 days |
| CT-scann | GGO >75%; AC | GGO > 75%; AC; nodules | GGO 50%; PE; crazy paving | GGO > 50%; AC; PE |
| ICU transfer * | Yes, day 1 | Yes, day 1 | No | Yes, day 30 |
| Ventilation | Mechanical | Mechanical | Low flow nasal oxygen | HFNC then mechanical |
| Steroid therapy | Yes | No | Yes | Yes |
| Pneumocystosis treatment | Yes, TMP-SMX | Yes, TMP-SMX | Yes, TMP-SMX | Yes, TMP-SMX, then atovaquone |
| Outcome | Death 18 days after admission | Death 3 days after admission | Death 44 days after admission | Death 44 days after admission |
F: female; M: male; BMI: body mass index; HSCT: hematopoietic stem cell transplantation; TMP-SFX: trimethoprime-sulfamethoxazole; PCR: polymerase chain reaction; NPS: nasopharyngeal swab; P. jirovecii: Pneumocystis jirovecii; BAL: bronchoalveolar lavage; BDG: serum (1,3)-β-D-glucan; NR: not recorded; LDH: lactate dehydrogenase; CT-scan: computed tomography scanner; GGO: Ground-glass opacities; AC: alveolar condensation; PE: pleural effusion; ICU: intensive care unit; HFNC: high-flow nasal oxygen with cannula. * ICU transfer after hospitalization.
The patients were elderly, with a mean age of 78 (range 70–83). Two patients were immunocompromised and at risk of PCP before the occurrence of COVID-19, one with rheumatoid arthritis treated by steroid therapy and one with history of hematopoietic stem cell transplantation and with an Ewing’s sarcoma metastasized to the lung and treated by cyclophosphamide (Sandoz, Levallois-Perret, France) and doxorubicine (Accord-healthcare, Lille, France). The other two patients only had diabetes mellitus. In addition, three patients were treated with systemic steroids for COVID-19. All these patients presented with lymphocytopenia at admission.
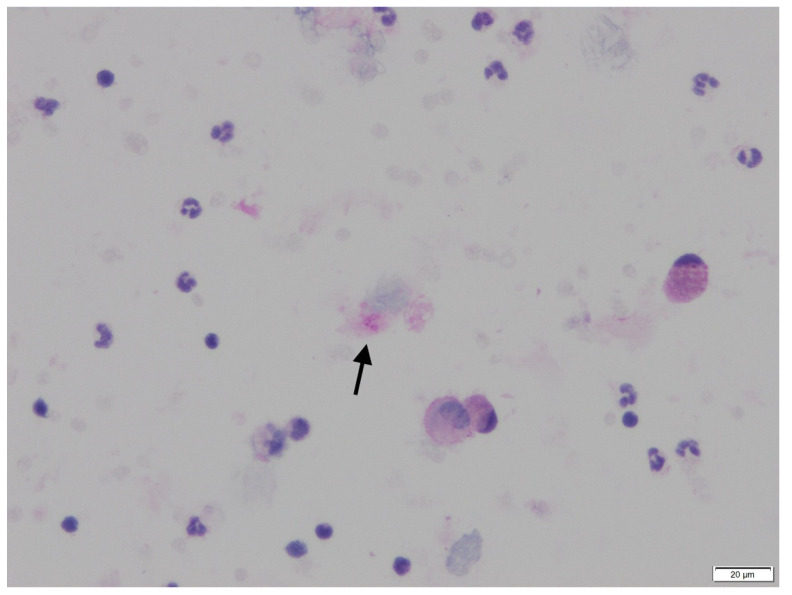
Three of four patients presented an ARDS requiring a stay in the ICU and mechanical ventilation. All of them had significant CT scan lesions with ground-glass opacities affecting more than 50% of the lung. P. jirovecii isolation was performed by PCR on tracheal aspirate for two patients and on BAL for the others. The BAL cytology showed round microorganisms suggestive of Pneumocystis cysts in one patient (Figure 1). Serum (1,3)-β-D-glucan (Fungitell cape Cod, East Falmouth, MA, USA) performed in two patients was positive.
Figure 1.
PAS (Periodic Acid Schiff) staining highlights the round cysts with a small central condensation (arrow) corresponding to the organisms of Pneumocystis, isolated on BAL cytology of patient 3.
All patients were treated by trimethoprim-sulfamethoxazole (TMP-SMX, Roche, Boulogne-Billancourt, France) for PCP. For one of them, TMP-SMX was stopped quickly after a skin rash and switched with atovaquone. All the patients also received broad-spectrum antimicrobial therapies for bacterial or viral superinfection.
All four patients died within two months (range 3–50 days) after hospital admission. Three of them died in the ICU. The last patient (patient 3) died suddenly four days after TMP-SMX introduction for proven PCP in rehabilitation service; his death was possibly attributed to PCP. The diagnosis was made by positive P. jirovecii PCR and cytology from BAL (Figure 1) after pulmonary worsening 40 days after hospital admission.
4. Discussion
Here, we identified four cases of PCP among 57 COVID-19 patients screened for P. jirovecii using PCR in BAL, tracheal aspirate, or sputum. Three were classified as probable and one as proven following EORTC/MSG criteria due to a positive BAL cytology [8].
Regarding risk factors, two patients had previous immunocompromised condition and three received steroids for COVID-19 therapy. None of the patients were treated with any other anti-inflammatory drug such as tocilizumab. The prognosis was poor for all patients.
The prevalence of positive P. jirovecii PCR and PCP in COVID-19 patients is unknown. Blaize et al. found positive P. jirovecii PCR in two of 145 (1.4%) SARS-CoV-2 infected patients in ICU [6]. Another study found 9.3% (10/108) of positive P. jirovecii PCR in COVID-19 patients presenting with ARDS [7].
Several cases of co-infections of SARS-CoV-2 with P. jirovecii were also described in patients with known risk factors for PCP. A case was described in a newly diagnosed HIV patient with COVID-19 who presented a severe depletion of CD4 T cells, fine reticular changes in CT scan, and an elevated level of lactate dehydrogenase (LDH), successfully treated with TMP-SMX [3]. Another case involved a renal transplant recipient with lymphocytopenia treated with tacrolimus, mycophenolate mofetil, and methylprednisolone [4,9]. He died despite a treatment by TMP-SMX. Interestingly, a case in an 84-year-old patient without history of immunosuppression was described [5]. She presented with severe COVID-19 and was hospitalized in the ICU. The diagnosis was made on lymphocytopenia, elevated serum (1,3)-β-D-glucan level, positive P. jirovecii PCR on a tracheal aspirate, cystic changes on the CT scan, and after a favorable outcome under treatment by TMP-SMX [5]. In severe COVID-19 cases, CD4+ T and CD8+ T cell levels could be remarkably low [1]. This lymphocytopenia was suggested as a risk factor for PCP, but the study by Blaize et al. did not find any link between the lymphocytopenia encountered in severe COVID-19 cases and the occurrence of PCP [6].
The diagnosis of PCP in COVID-19 patients and the distinction between infection and colonization are very challenging [6]. Patients hospitalized in the ICU for COVID-19 might be at risk of PCP due to mechanical ventilation, use of corticosteroid therapy, or the existence of a cytokine storm leading to marked alveolar-interstitial pulmonary tissue [1]. However, there are radiological similarities between the two infections, the presence of cysts or fine reticular changes on CT scan being in favor of pneumocystosis, but similarities are not constant and are sometimes difficult to see [3,5,10]. The high sensitivity of PCR can lead to overdiagnosis of P. jirovecii infection in colonized patients, and the distinction between colonization and PCP can be difficult, especially in immunocompetent patients [5,6,7,10]. Direct examination is usually not performed in COVID-19 patients. The use of a staining methodology (Giemsa, Gomori-methenamine-silver stain, toluidine blue O, calcofluor white, or immunofluorescent stains via monoclonal antibodies) is very helpful to visualize cysts or trophozoite forms and therefore to differentiate infection from colonization, but this was not done in our center due to the estimated risk of aerosolization during COVID-19. As we have now a better knowledge on this viral pandemic, the use of these staining methods might be discussed, at least in some cases, using all necessary and extra precautions, in particular using a class II bio safety. This might be decisive in order to properly diagnose these co-infections. Serum (1,3)-β-D-glucan assay might be used for its negative predictive value to rule out the diagnosis of infection [7]. Finally, the diagnosis of infection must therefore be based, in addition to the mycological criteria, on a set of arguments including clinical worsening, immunosuppression, deep lymphocytopenia, serum (1,3)-β-D-glucan, and LDH assays and response to treatment [10].
The decision to treat or not treat these patients is also a matter of debate. In the series from Blaize et al., the two patients improved without specific treatment, which was in favor of the diagnosis of colonization by P. jirovecii [6]. In the study from Alanio et al., four of the 10 patients received TMP-SMX as prophylaxis and six were not treated, among whom four rapidly improved. Three patients died, including one treated and two non-treated patients [7]. In the present study, all patients with COVID-19 and a positive P. jirovecii PCR were treated with curative dosage. For three of them (patients 1, 2, and 4), the decision was based on the presence of underlying diseases and mechanical ventilation, clinical worsening, positive serum (1,3)-β-D-glucan (in one patient), elevated LDH (in two patients), and compatible chest CT scan, which were suggestive of PCP infection. The last patient without mechanical ventilation had proven PCP diagnosed on the cytology from BAL fluid. The high mortality of the patients from the current series might encourage curative—at least prophylactic—treatment in severe patients with COVID-19. Finally, the decision to introduce curative treatment, prophylaxis, or no treatment is challenging and should take into account all the arguments described before to distinguish colonization and infection, but larger studies are mandatory to conclude on this question.
Our retrospective case-series study has some limitations. First, only 57 samples from COVID-19 patients were tested for P. jirovecii superinfection, and some cases were possibly missed. On the contrary, the diagnosis of PCP in COVID-19 is difficult, as mentioned before, especially since direct examination was not performed in COVID-19 patients, and some patients were possibly excessively treated only based on the single positivity of the P. jirovecii PCR. Finally, the prognosis of the patients included in this study was very poor, but whether this high mortality is due to pneumocystosis or if the occurrence of pneumocystosis is merely a marker of very severe COVID-19 and immunosuppression remains unknown.
Our study provides new data regarding P. jirovecii infections in patients with COVID-19. These co-infections are rare but severe, therefore, PCP should be considered in case of worsening of the condition of patients with severe COVID-19. Additional studies are needed to clarify the epidemiology of P. jirovecii in COVID-19 patients, the diagnosis, and the treatments.
Author Contributions
V.G. (Victor Gerber) and F.D. designed the study. V.G. (Victor Gerber), Y.R., F.D., T.-N.C.-T., W.O., F.S., V.L., J.D. and J.B. enrolled the participants. F.D. supervised the study. V.G. (Victor Gerber), F.D., Y.R., V.G. (Valentin Greigert) and T.-N.C.-T. wrote the manuscript. W.O., F.S., V.L., J.D. and J.B., contributed to critical revision of the report. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University Hospital of Strasbourg (No. CE-2020-51).
Informed Consent Statement
Oral non-opposition to participation to the study was sought.
Conflicts of Interest
F.D. declares personal fees from Gilead outside the submitted work. Other authors have no conflict to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gangneux J.-P., Bougnoux M.-E., Dannaoui E., Cornet M., Zahar J.R. Invasive fungal diseases during COVID-19: We should be prepared. J. Mycol. Med. 2020;30:100971. doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baddley J.W. COVID-19 Associated Pulmonary Aspergillosis: Do We Have the CAPAcity to Improve Outcomes? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021:ciab259. doi: 10.1093/cid/ciab259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mang S., Kaddu-Mulindwa D., Metz C., Becker A., Seiler F., Smola S., Maßmann A., Becker S.L., Papan C., Bals R., et al. Pneumocystis jirovecii Pneumonia and SARS-CoV-2 Co-Infection in newly diagnosed HIV-1 infection. Clin. Infect. Dis. 2020;72:1487–1489. doi: 10.1093/cid/ciaa906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Francesco M.A., Alberici F., Bossini N., Scolari F., Pascucci F., Tomasoni G., Caruso A. Pneumocystis jirevocii and SARS-CoV-2 Co-Infection: A Common Feature in Transplant Recipients? Vaccines. 2020;8:544. doi: 10.3390/vaccines8030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon A.A., Berg D.D., Brea E.J., Deutsch A.J., Kidia K.K., Thurber E.G., Polsky S.B., Yeh T., Duskin J.A., Holliday A.M., et al. A Case of COVID-19 and Pneumocystis jirovecii Coinfection. Am. J. Respir. Crit. Care Med. 2020;202:136–138. doi: 10.1164/rccm.202003-0766LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaize M., Mayaux J., Luyt C.-E., Lampros A., Fekkar A. COVID-19-related Respiratory Failure and Lymphopenia Do Not Seem Associated with Pneumocystosis. Am. J. Respir. Crit. Care Med. 2020;202:1734–1736. doi: 10.1164/rccm.202007-2938LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alanio A., Dellière S., Voicu S., Bretagne S., Mégarbane B. The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J. Infect. 2020;82:84–123. doi: 10.1016/j.jinf.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., Clancy C.J., Wingard J.R., Lockhart S.R., Groll A.H., et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Permpalung N., Kittipibul V., Mekraksakit P., Rattanawong P., Nematollahi S., Zhang S.X., Mehta Steinke S. A Comprehensive evaluation of risk factors for Pneumocystis Jirovecii Pneumonia in adult solid organ transplant recipient. Transplantation. 2020 doi: 10.1097/TP.0000000000003576. [DOI] [PubMed] [Google Scholar]
- 10.Menon A.A., Berg D.D., Gay E.B. Reply to Blaize et al.: COVID-19–related Respiratory Failure and Lymphopenia Do Not Seem Associated with Pneumocystosis. Am. J. Respir. Crit. Care Med. 2020;202:1736–1737. doi: 10.1164/rccm.202008-3174LE. [DOI] [PMC free article] [PubMed] [Google Scholar]