Abstract
Background:
Biological and epidemiological evidence suggest that herpes simplex virus type 2 (HSV-2) elevates HIV acquisition and transmission risk. We improved previous estimates of the contribution of HSV-2 to HIV infections by using a dynamic-transmission model.
Setting:
WHO regions.
Methods:
We developed a mathematical model of HSV-2/HIV transmission among 15-49-year-old heterosexual, non-drug-injecting populations, calibrated using region-specific demographic and HSV-2/HIV epidemiological data. We derived global and regional estimates of the contribution of HSV-2 to HIV infection over ten years (the transmission population-attributable fraction, tPAF) under three additive scenarios, assuming: (1) HSV-2 only increases HIV acquisition (“conservative”); (2) HSV-2 also increases HIV transmission (“liberal”); (3) HIV/ART (antiretroviral therapy) also modifies HSV-2 transmission and HSV-2 decreases ART effect on HIV transmission (“fully liberal”).
Results:
Under the conservative scenario, the predicted tPAF was 37.3% (95% uncertainty interval 33.4-43.2%) and an estimated 5.6 (4.5-7.0) million incident heterosexual HIV infections were due to HSV-2 globally over 2009-2018. The contribution of HSV-2 to HIV infections was largest for the African region (tPAF=42.6% (38.0-51.2%)), and lowest for the European region (tPAF=11.2% (7.9-13.8%)). The tPAF was higher among female sex-workers, their clients, and older populations, reflecting their higher HSV-2 prevalence. The tPAF was ~50% and 1.3-2.4-fold higher for the liberal/fully liberal than the conservative scenario across regions.
Conclusion:
HSV-2 may have contributed to at least 37% of incident HIV infections in the last decade worldwide, and even more in Africa, and may continue to do so despite increased ART access unless future improved HSV-2 control measures, such as vaccines, become available.
Keywords: HIV, herpes simplex virus type 2 (HSV-2), modelling, population attributable fraction, Global Health, sexually transmitted infections
Introduction
An estimated 38.0 million people were living with HIV globally in 2019,1 while 491 million people aged 15-49 were estimated to have herpes simplex virus type 2 (HSV-2) infection in 2016.2 Both infections are primarily sexually transmitted with shared risk factors. Substantial evidence supports the existence of biological associations between these two infections through multiple pathways: HSV-2 infection increases HIV acquisition3 and transmission risk,4 while HIV infection increases HSV-2 transmission risk5 and disease severity,4 with anti-retroviral therapy (ART) attenuating this synergy.6–9
Understanding the burden of HIV that may be due to HSV-2 is important for targeting existing prevention measures against both infections,10 and for encouraging the development of better interventions against HSV-2, such as vaccines.11
One recent 2016 estimate of global and World Health Organisation (WHO) regional incident HIV infections attributable to HSV-2 infection suggested that approximately 30% (420,000 cases) of sexually transmitted incident HIV infections were attributable to HSV-2 among 15-49 year-olds, the majority in Africa.12 However, these estimates were based on the classical population-attributable fraction (cPAF) formula, which may not reflect the total contribution of HSV-2 to HIV13 due to three main limitations. First, the cPAF only accounts for one cofactor effect: the increased HIV acquisition risk due to HSV-2, when HSV-2 may affect HIV in other ways (and HIV/ART might also affect HSV-2). Second, as the cPAF only depends on the magnitude of the cofactor effect and HSV-2 prevalence, it only represents the direct contribution of HSV-2 to incident HIV infections and does not capture the secondary effects of HSV-2 on onward HIV transmissions, which for infectious diseases is best estimated with transmission dynamic models over long time periods.13–15 Third, cPAF calculations do not involve fitting a model to empirical data (e.g. HIV incidence, or HIV prevalence or incidence ratios by HSV-2 infection status), which may reduce its validity.
The association between HSV-2 and HIV acquisition has been well documented.3,16–18 The latest meta-analysis reported an almost three-fold increase in HIV acquisition risk among individuals infected with HSV-2 for more than one year (i.e. established HSV-2 infection) and a five-fold increase for those infected less than one year (i.e. recent HSV-2 infection).3 Recurrent HSV-2 reactivations lead to breaks in the genital mucosa and influxes of immune cells that are targets for HIV uptake and initial replication. Thus, the elevated risk of HIV during the first year of HSV-2 infection may be due to more frequent mucosal HSV-2 replication4,19,20 and ulceration, with associated increases in HIV target cells in the genital area. In co-infected individuals, HSV-2 infection can also increase HIV transmission risk by increasing HIV genital shedding.21–23 ART, which decreases HIV replication both in plasma and genital fluids, may somewhat modify HSV-2 cofactor effects on HIV transmission whereas HSV-2 may potentially modify the efficacy of ART for people living with HIV (PLHIV),7 but these are less precisely understood and quantified.6,9,24
Given the cPAF limitations, we aimed to validate and improve previous estimates of the contribution of HSV-2 to HIV. Using a dynamic model of HSV-2 and HIV transmission in a heterosexual population over time, calibrated to region-specific demographic, behavioural and HSV-2 and HIV epidemiological data, we derived global and WHO regional estimates of the population-level contribution of HSV-2 to HIV for 2009-2018 and 2019-2028. We evaluated this contribution in both relative (i.e. transmission PAF, tPAF) and absolute (i.e. number of incident HIV infections) terms, under different HSV-2/HIV/ART cofactor assumptions. We also examined the extent to which previous cPAF estimates, or short-term tPAF may have underestimated this contribution.
Methods
Compartmental model structure
The model represents an open and growing heterosexual population stratified by sex and sexual behaviour (lower/higher risk) r, age group a (“younger”: 15–24 years, “older”: 25–49 years), each with their specific numbers of sex acts and intervention (condom use, circumcision, ART) coverage (Figures S1-S2). Individuals join the sexually-active population (HSV-2- and HIV-uninfected) at age 15 and leave it through ageing, sex-specific background mortality,25 and AIDS death (Tables S1-S2). The modelled higher-risk groups are female sex workers (FSWs) and their male clients (CFSWs), who are assumed to engage in sex work for a fixed duration, which is varied.26 Individuals leaving higher-risk groups are replaced by lower-risk individuals (independently of HIV status) to maintain the proportion of higher-risk individuals constant over time. Men who have sex with men (MSM), and people who inject drugs (PWID) were not modelled due to the paucity of data on HSV-2 prevalence, their sexual contacts with female partners, or the coverage of ART among those HIV-infected in many regions.
Uninfected individuals get infected with HIV, HSV-2 or both via heterosexual transmission according to an age- and risk level-specific per-capita force of infection, which varies over time (Figure S1). Stages of HSV-2 infection, s, are uninfected, first year and >one year since infection. Stages of HIV infection, i, are uninfected, infected-untreated and infected-treated, with associated HIV-related death rates (Figure S2). After 1996, HIV-infected individuals can initiate ART (Table S3), which reduces their HIV transmission risk and HIV-related death rate; they may also withdraw from treatment and re-initiate later.
The force of HIV or HSV-2 infection depends on an annual number of sex acts, a sexual mixing matrix by age/risk groups, the prevalence of the infection among the pool of partners, a baseline infection-specific per-act transmission probability, the level of ART coverage and efficacy among PLHIV, and the different HSV-2 and HIV/ART cofactor effects due to the presence of the other infection in the susceptible or infected partner (depending on the scenario modelled). The force of infection also includes a meta-parameter reflecting the changes in the combined levels and efficacies of interventions (male circumcision and condom use) against HSV-2/HIV over time by risk group. This simple but unified approach was selected to tackle shortcomings in intervention data (especially condom use during non-commercial sex) in several regions. The meta-parameter takes the form of a sigmoidal curve function which increases over time, in agreement with a review of available data on the coverage of these interventions in each region (presented in Appendix Section 3).
The model accounts for five types of cofactor effects, each represented by a sex-, age-, and risk level-specific relative risk (RR) parameter applied to the per sex act HIV or HSV-2 transmission probability in the force of infection, as follows: 1) increased risk of HIV acquisition for individuals with recent () or established () HSV-2 infection (versus HSV-2-uninfected individuals), 2) increased HIV transmission probability () among HSV-2-HIV co-infected individuals (versus HSV-2-uninfected PLHIV), 3) increased HSV-2 transmission probability () among HSV-2-HIV co-infected individuals (versus HSV-2-infected, HIV-uninfected individuals), 4) mitigating effect of ART on HIV-associated increases in HSV-2 transmission probability (), and 5) modifying effect of HSV-2 on ART-related reductions in HIV transmission probability () (Table 1 and Appendix). These cofactors were combined in three main scenarios reflecting the strength of evidence on each: i) a “conservative” one that only accounted for cofactor 1, ii) a “liberal” one that accounted for cofactors 1 and 2, and iii) a “fully liberal” scenario accounting for cofactors 1-5 (Tables 1 and S4), which aimed at reflecting interactions with weak strength of evidence, or for which there is no direct evidence.
Table 1:
Modelled HIV/HSV-2 cofactors.
| Cofactor | Description | Prior distribution used in model |
Source (see appendix) | ||
|---|---|---|---|---|---|
| Conservative scenarioa | Liberal scenarioa | Fully liberal scenarioa | |||
| 1 | Per-act increased risk of HIV acquisition for HSV-2-infected individuals a) For recent HSV-2 infection b) For established HSV-2 infection |
All females: T (7.2, 1, 12)b All males: T (4.7, 1, 11) Lower-risk females: T (2.5, 1, 4) Lower-risk males: T (3.1, 1, 5) FSWsc: T (1.5, 1, 3) CFSWsd: T (1.8, 1, 3) |
Adapted from 3, See Table S4 and section 2.2 of appendix | ||
| 2 | Per-act increased risk of HIV transmission for HSV-2-infected individuals () | 1 | T (1.33,1,1.93) | See Table C1 and section C1.3 | |
| 3 | Per-act increased risk of HSV-2 transmission for HIV-infected individuals () | 1 | 1 | T (2.55,1.39,4.68) | See Table C1 and section C1.4 |
| 4 | Efficacy of ART in reducing the per-act increase in HSV-2 transmission risk due to HIV per sex act () | 1 | 1 | T (0.58,0.37,0.92) | See Table C1 and section C1.5 |
| 5 | Effect of HSV-2 on the efficacy of ART in reducing per-act HIV transmission risk () | 1 | 1 | U (0.95-1.00)e | See Table C1 and section C1.6 |
Description of per-act HIV/HSV-2 cofactor prior distributions used in the model under the different model scenarios.
Conservative scenario: HSV-2 only increases HIV acquisition risk; liberal scenario: HSV-2 increases HIV acquisition and transmission risk; fully liberal scenario: HSV-2 increases HIV acquisition and transmission risk, HIV increases HSV-2 transmission risk but this is reduced if the individual is on ART, and HSV-2 can slightly alter the effect of ART on HIV transmission risk.
T(m,a,b) – triangular distribution (m=mode, a=minimum, b=maximum).
CFSWs – clients of FSWs
FSW – female sex workers.
U(a,b) – uniform distribution (a=minimum, b=maximum).
Sex acts can occur between any age and sexual risk group, except between lower-risk males and FSWs. The model can represent a range of sexual mixing and the number of sex acts between groups are balanced for internal consistency. The model was coded in R and solved using the Runge-Kutta 4th order method.27 Further details on model assumptions and equations are presented in the Appendix.
Model parameterisation and fitting
Overview
For each WHO region, the model was separately parameterised and fitted using a Bayesian framework that accounted for parameter and empirical estimates of fitting outcomes uncertainty. Twenty million different parameter set combinations were generated from ranges of plausible parameter values, from which up to 100 sets that produced model outcomes consistent with each empirical estimate were selected (Tables S1-3). We used the fitted model to conduct three types of analysis: a) the main tPAF estimation, b) uncertainty analyses around tPAF estimates, c) comparison with previous cPAF estimates.
Plausible parameter value ranges and empirical estimates of the fitting outcomes were obtained from the published literature for each available WHO region,25,28 and sourced from other region data when not available. Where possible, we used the same HSV-2 prevalence, HIV incidence, and HSV-2/HIV cofactor data as in the analysis that recently produced global and WHO regional cPAF estimates.12
Model parameters
Demographic parameters (e.g. population growth rate, sex-specific age distribution, and mortality rates) were obtained from the United Nations Population Division for each WHO region.25 Published literature on key populations informed the region-specific distribution of the population by sexual risk groups, and duration of commercial sex activity for FSWs and CFSWs.29–32 Biological parameters for HIV and HSV-2 infection were sourced from published literature.3,18,33,34 The prior distributions of the five cofactors effects between HSV-2 and HIV/ART were elicited from the literature reviews (see Appendix). Sexual behaviours and mixing could vary between regions, but were sampled from the same prior distributions: the model assumed two weekly sex acts between lower-risk females and lower-risk males and between CFSWs and lower-risk females, while FSWs had between 200 and 1000 sex acts per year, based on a review of African studies of FSWs.32
Rates of ART initiation per WHO region were varied over time and fitted to sex-specific UN Joint Programme on HIV/AIDS (UNAIDS) coverage estimates. Annual ART drop-out rates were set to 8%.28,35 The proportion of PLHIV on ART that are virally suppressed was based on region-specific UNAIDS estimates28, with those achieving viral suppression (<1000 copies/ml) assumed to have a reduced per-act HIV transmission risk of 96%-100%.36,37 The parameters reflecting changes in HIV/HSV-2 interventions coverage over time was based on Demographic Health Surveys (DHS)38 and UNAIDS28 data in each WHO region (Figures S4-S22) and published literature about intervention efficacies,39,40 and assuming high uncertainty in estimates.
Model fitting outcomes and comparison with other data
For each region, the model was simultaneously fitted to the following demographic and epidemiological empirical data (Table S3): 1) Number of incident heterosexual HIV infections by sex/age among non-PWID in 201541,42 (Table S2) and HIV prevalence among FSW29,32 (and among CFSW in Africa); 2) HSV-2 prevalence by age/sex in 201243 and among FSW and CFSW where available;12 3) HIV incidence rate ratios (IRRs) by HSV-2 status and recency of HSV-2 infection (i.e. the ratio of the HIV incidence rate among individuals with <1 year of infection versus among those HSV-2-uninfected, and similarly for >1 year of infection versus uninfected), among females and males separately (Table 1),3 4) HIV prevalence ratio by HSV-2 status, and 5) ART coverage by region, over time. We also visually compared our predicted HSV-2 incidence and prevalence estimates with previously published estimates2,43,44 (see Appendix), whereas the HIV prevalence in Africa was compared to UNAIDS estimates (which included MSM and PWID).45
Main tPAF analysis
We estimated the fraction (i.e. tPAF) and numbers of incident HIV infections contributed by HSV-2 by WHO region and globally (summing over all regions) by comparing the cumulative number of infections between each main scenario and its counterfactual (which assumed no biological cofactor effects i.e. all values set to 1) over 2009-2018 and 2019-2028. For those WHO regions with the most HSV-2 prevalence data (i.e. Africa, Americas), the model was refitted for each of the three main scenarios. The remaining regions were fitted only to scenarios i) and ii).
The global 10-year tPAF overall and by population subgroups, is reported as the median and 95% uncertainty interval (UI, 2.5 and 97.5th percentiles46) derived from 50 randomly selected paired samples of baseline and counterfactual simulations summed over all regions.
Uncertainty analysis
We used univariate Spearman's rank correlation to identify the drivers of the uncertainty in tPAF2009-2018 estimates due to key uncertain model parameters and epidemiological outcomes in the conservative (all regions) and fully liberal (Africa and Americas region) scenarios.
Comparative analysis
We also derived and compared our model 10-year tPAF, 1-year tPAF for the year 2016, and cPAF (using the posterior model estimates of the HSV-2 prevalence and HIV IRR by HSV-2 status)12,14,47 with previously published cPAF.12
Results
Model fitting and comparison with other data
The model estimates of the annual number of incident HIV infections, HSV-2 and HIV prevalence, HIV incidence per HSV-2 status, and ART coverage among the modelled population reflected available empirical estimates used for fitting and those used only for comparison (Figures 1 and S23-30). HSV-2/HIV biological interactions were necessary to reproduce empirical estimates of HIV incidence by HSV-2 status for the African region, which has the most data on HIV incidence according to HSV-2 prevalence (Figure S31). Model fits to the demographic characteristics and available HSV-2 and HIV epidemiologic estimates for high-risk groups are shown in Figures S23-28. The model predicted a decline in incident HIV infections across regions and reflected the slight decline in empirical HSV-2 prevalence estimates over 2002-2016 across regions, but somewhat underestimated the speed of the decline for the Eastern Mediterranean region (Figures 1 and S24). The posterior distributions of the relative-risks of the increase in HIV acquisition risk due to HSV-2 (cofactor 1: ) were similar across WHO regions, except for Europe, where it was lower (Table S9). This was due to HSV-2 and HIV epidemics being extremely concentrated in that region, leading to high HIV IRRs by HSV-2 status even with a lower biological cofactor effect.
Figure 1: WHO-region-specific annual numbers of incident HIV infections and HSV-2 prevalence.
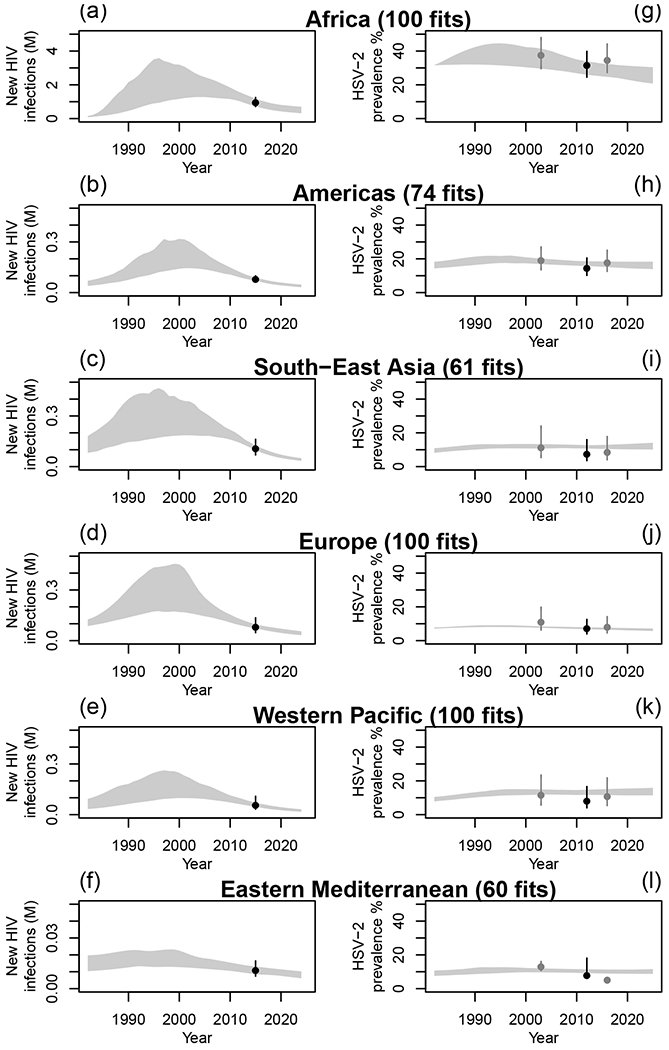
Model fits to available estimates of annual numbers of incident HIV infections (in millions (M)) from UNAIDS46,49 (panels a-f) and HSV-2 prevalence44 (panels g-l) among heterosexual non-PWID individuals, for each WHO region. Grey shaded areas represent the 95%UI of model predictions across all parameter sets. Dark dots and intervals represent empirical estimates used for fitting and grey dots and intervals represent empirical estimates only used for comparison (i.e. not used at the fitting stage).44
Our model was also in good agreement with other data only used for comparison. Our estimated HSV-2 incidence rates suggested the same decline over time across all regions as previous estimates (Figure S29), whereas HIV prevalence in the African region was consistent, although slightly lower, than UNAIDS estimates for the region (Figure S30). The posterior distributions of the changes in intervention (condoms and male circumcision) levels over time are shown in Figures S32-33.
tPAF estimates: conservative scenario
Figure 2 and Table 2 show the model estimates of tPAF2009-2018 and tPAF2019-2028 across WHO regions under the conservative scenario (i.e. HSV-2 only increases HIV acquisition risk). Results suggest that HSV-2 contributed to 37.3% (95%UI: 33.4-43.2) of all incident heterosexual HIV infections among 15-49-year-old globally between 2009-2018, corresponding to a total of 5.6 (4.5-7.0) million incident HIV infections (Table 2a-b). The contribution of HSV-2 to HIV predicted over 2019-2028 (33.8% (28.6-42.6)) was slightly lower than for 2009-2018 overall and across regions, while the number of incident HIV infections due to HSV-2 markedly decreased over time (2.2 million (1.5-3.6) over 2019-2028, Table 2a-b), reflecting the predicted decrease in the total number of incident HIV infections. For 2009-2018, the estimated fraction and number of incident HIV infections due to HSV-2 were largest for the WHO Africa region (42.6% (38.0-51.2); 4.8 million (3.6-6.5), respectively) and lowest for the Europe region (11.2% (7.9-13.8); 0.11 (0.07-0.15) million, respectively). A similar pattern was predicted for the 2019-2028 period (Figure 2a, Table 2a-b)
Figure 2: Contribution of HSV-2 to HIV incident infections by region.
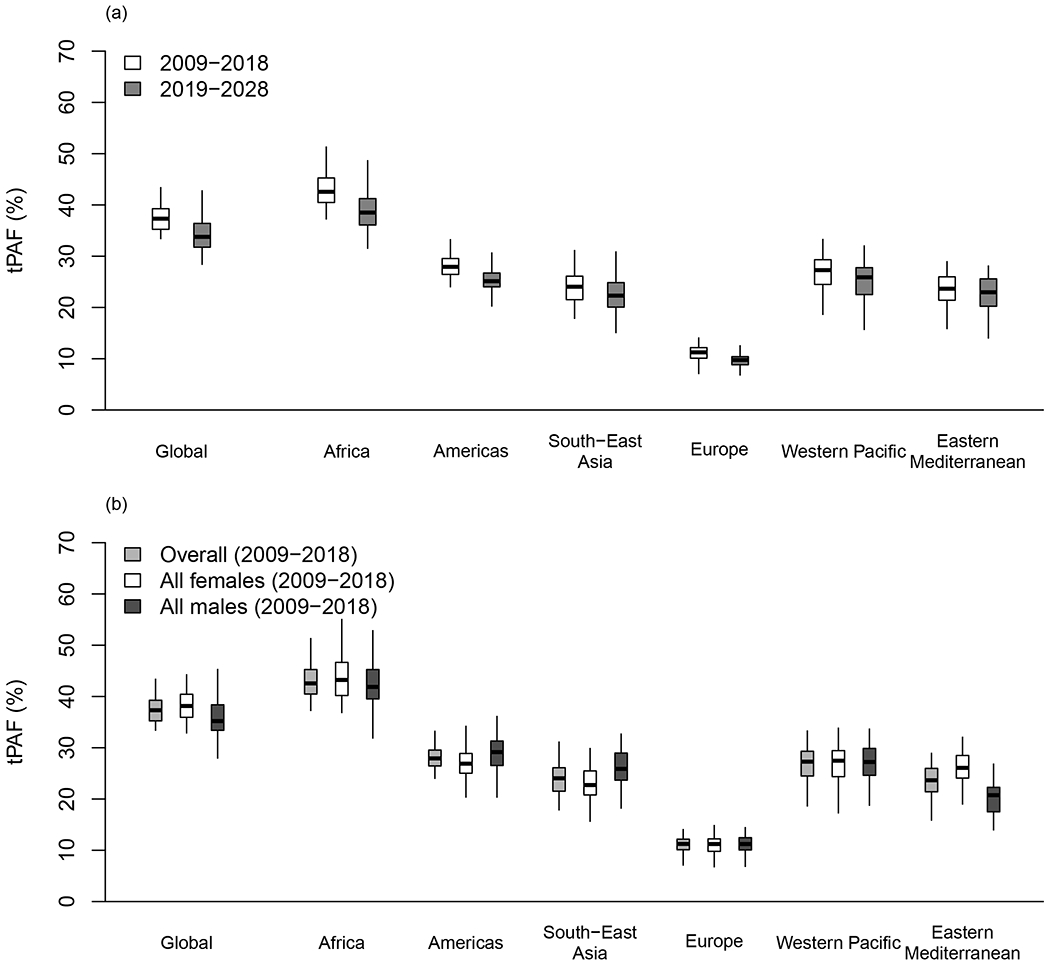
Contributions estimated under the conservative scenario (where HSV-2 only increases HIV acquisition). Boxplot shows the transmission population-attributable fraction (tPAF) among 15-49-year-old heterosexual, non-drug injecting populations across WHO regions: a) over 2009-2018 and 2019-2028, b) by sex over 2009-2018. Boxplots represent median, interquartile range and 95%UI.
Table 2:
Model estimates of the fraction (i.e. tPAF) (a,c), and number (b,d) of incident heterosexual HIV infections occurring among non-PWID populations (in thousands) attributable to HSV-2 over 2009-2018 and 2019-2028 by WHO region under the conservative (a-b) and liberal (c-d) scenarios, median (95% uncertainty interval).
| Global | Africa | Americas | South-East Asia | Europe | Western Pacific | Eastern Mediterranean | |
|---|---|---|---|---|---|---|---|
| a) tPAF: Fraction of incident HIV infections attributable to HSV-2 - Conservative scenario | |||||||
|
| |||||||
| 2009-2018 | |||||||
|
| |||||||
| Overall | 37.3% (33.4-43.2) |
42.6% (38.0-51.2) |
27.9% (23.6-33.6) |
24.0% (18.6-29.7) |
11.2% (7.9-13.8) |
27.3% (20.0-32.1) |
23.7% (17.8-27.9) |
| Females | 38.1% (33.0-44.1) |
43.2% (37.2-51.2) |
26.9% (22.8-34.3) |
22.7% (15.9-28.9) |
11.2% (7.2-14.4) |
27.5% (18.0-32.7) |
26.1% (19.1-31.2) |
| Males | 35.2% (29.8-43.5) |
41.9% (33.8-51.8) |
29.2% (22.4-35.3) |
25.9% (18.6-31.6) |
11.2% (7.8-13.8) |
27.2% (20.2-32.9) |
20.8% (14.2-26.4) |
|
| |||||||
| 2019-2028 | |||||||
|
| |||||||
| Overall | 33.8% (28.6-42.6) |
38.5% (32.4-47.6) |
25.1% (20.9-30.8) |
22.3% (16.7-29.4) |
9.8% (6.5-12.1) |
25.9% (18.5-30.8) |
23.0% (17.4-27.7) |
| Females | 34.9% (28.6-44.0) |
39.6% (32.0-49.1) |
24.0% (19.7-31.3) |
21.3% (14.0-28.6) |
9.5% (6.0-12.6) |
26.1% (16.1-30.8) |
25.5% (18.4-30.5) |
| Males | 31.7% (25.5-41.5) |
37.1% (28.7-48.2) |
26.5% (20.7-32.5) |
23.8% (15.7-30.7) |
10.0% (6.7-12.3) |
25.0% (18.2-31.8) |
19.6% (13.5-25.6) |
|
| |||||||
| b) Number of incident HIV infections attributable to HSV-2 - Conservative scenario | |||||||
|
| |||||||
| 2009-2018 | |||||||
|
| |||||||
| Overall | 5595 (4497-6953) |
4843 (3577-6563) |
265 (203-324) |
325 (261-405) |
110 (73-146) |
180 (129-236) |
27 (18-36) |
| Females | 3477 (2768-4610) |
3194 (2328-4252) |
141 (115-182) |
177 (118-229) |
56 (34-73) |
103 (63-129) |
17 (11-23) |
| Males | 1983 (1547-2723) |
1675 (1108-2332) |
120 (86-160) |
150 (99-208) |
54 (38-77) |
78 (54-121) |
9 (6-15) |
|
| |||||||
| 2019-2028 | |||||||
|
| |||||||
| Overall | 2191 (1513-3646) |
1898 (1206-2984) |
107 (81-140) |
101 (70-144) |
45 (28-63) |
65 (42-92) |
18 (12-26) |
| Females | 1399 (980-2366) |
1234 (798-1972) |
57 (45-78) |
54 (30-82) |
23 (12-33) |
36 21-53) | 12 (7-18) |
| Males | 741 (474-1333) |
648 (365-1123) |
48 (33-69) |
44 (27-71) |
21 (14-32) |
27 (18-44) |
6 (4-11) |
|
| |||||||
| c) tPAF: Fraction of incident HIV infections attributable to HSV-2 - Liberal scenario | |||||||
|
| |||||||
| 2009-2018 | |||||||
|
| |||||||
| Overall | 51.0% (42.7-58.2) |
56.5% (46.7-65.2) |
43.2% (31.8-53.3) |
36.5% (25.0-47.2) |
26.8% (16.2-35.8) |
39.2% (28.0-49.1) |
37.3% (29.6-45.0) |
| Females | 51.0% (42.6-59.0) |
56.1% (45.8-65.2) |
41.0% (29.9-51.6) |
34.5% (23.4-45.1) |
24.2% (15.7-31.5) |
37.0% (27.9-46.9) |
36.6% (29.1-44.3) |
| Males | 51.6% (42.4-58.2) |
56.6% (45.3-66.4) |
46.3% (34.5-56.3) |
39.1% (25.9-49.9) |
29.3% (17.0-39.8) |
40.9% (27.8-52.6) |
38.2% (27.9-47.3) |
|
| |||||||
| 2019-2028 | |||||||
|
| |||||||
| Overall | 47.6% (39.7-55.3) |
52.3% (42.1-61.7) |
40.1% (28.4-50.1) |
33.8% (22.3-44.4) |
23.4% (14.0-32.1) |
37.3% (25.2-47.9) |
36.5% (27.2-44.0) |
| Females | 47.7% (38.9-55.8) |
53.1% (42.8-62.1) |
37.7% (26.7-48.9) |
31.5% (20.5-44.0) |
20.7% (13.2-27.4) |
35.3% (25.3-45.0) |
36.0% (26.4-43.2) |
| Males | 47.5% (37.8-55.5) |
51.7% (39.6-62.7) |
43.0% (30.9-53.1) |
36.4% (22.5-47.2) |
25.8% (14.9-36.4) |
39.2% (25.9-50.9) |
37.4% (26.1-45.9) |
|
| |||||||
| d) Number of incident HIV infections attributable to HSV-2 - Liberal scenario | |||||||
|
| |||||||
| 2009-2018 | |||||||
|
| |||||||
| Overall | 7993 (6148-9813) |
6,365 (4609-8,637) |
420 (304-528) |
483 (334-678) |
266 (169-379) |
284 (176-377) |
41 (28-57) |
| Females | 4953 (3540-6397) |
4162 (2827-5732) |
213 (158-267) |
251 (169-337) |
127 (77-160) |
139 (93-187) |
24 (17-33) |
| Males | 3019 (2260-3975) |
2158 (1580-3348) |
209 (129-266) |
223 (136-352) |
143 (92-218) |
134 (78-205) |
17 (12-27) |
|
| |||||||
| 2019-2028 | |||||||
|
| |||||||
| Overall | 3232 (2301-4181) |
2542 (1661-3702) |
174 (113-240) |
147 (85-221) |
106 (66-159) |
102 (53-154) |
28 (18-41) |
| Females | 2044 (1367-2504) |
1670 (1101-2236) |
87 (61-119) |
77 (47-117) |
48 (31-64) |
50 (29-79) |
16 (10-23) |
| Males | 1187 (828-1673) |
879 (565-1437) |
85 (50-123) |
67 (36-111) |
56 (34-94) |
48 (25-81) |
12 (7-19) |
The tPAF did not vary by sex (Figure 2b, Table 2a), as the higher female HSV-2 prevalence counterbalanced the lower pooled estimates of the cofactor effect of established HSV-2 on HIV acquisition () among females in the literature. However, the number of incident HIV infections attributable to HSV-2 infection remained significantly higher among women than men (up to 1.7-fold higher in the Africa region), except in the Europe region where it was the same (Table 2b), reflecting sex-differences in incident heterosexual HIV infections among non-PWID within regions.
Due to higher HSV-2 prevalence, the tPAF2009-2018 was 1.4-1.9-fold higher for FSWs compared to lower-risk females across regions (1.4-fold higher in Africa), and 0.9-1.6-fold higher for CFSWs compared to lower-risk males, across all WHO regions (1.3-fold higher in Africa) (Figure S34). The tPAF2009-2018 estimates for WHO Africa region were 57% (45-64%) for FSWs and 50% (37-60%) for CFSWs, corresponding to 0.61 (0.24-1.15) and 0.53 (0.10-1.12) million infections over the decade, respectively. Most tPAF2009-2018 estimates were approximatively 1.6-fold higher among older than younger individuals for each sex (but 1.3-fold higher in the Africa region), given increasing HSV-2 prevalence with age (Figure S24).
tPAF estimates: liberal and fully liberal scenarios
tPAF2009-2018 estimates for the liberal scenario (i.e. HSV-2 elevates both HIV acquisition and transmission risk) were about 1.5-fold higher than under the conservative scenario for most WHO regions (Table 2, Figures S34-35). The estimated number of incident HIV infections due to HSV-2 increased from 5.6 (4.5-7.0) million (conservative scenario) to 8.0 (6.1-9.8) million (liberal scenario) globally over 2009-2018 (Table 2).
Figure 3 and S10 Table show the tPAF2009-2018 of each independent cofactor effect under the different scenarios. Except for Europe, separate tPAFs of the cofactor effect of HSV-2 on HIV transmission risk were generally lower (median: 18-25% across all regions) and more uncertain than tPAFs of the effect of HSV-2 on HIV acquisition risk (median: 25-44% and 12% in Europe). The latter tPAF being lower in Europe due to the lower posterior distribution of the cofactor reflecting this effect.
Figure 3: Contribution of each cofactor effect to HIV incident infections over 2009-2018.
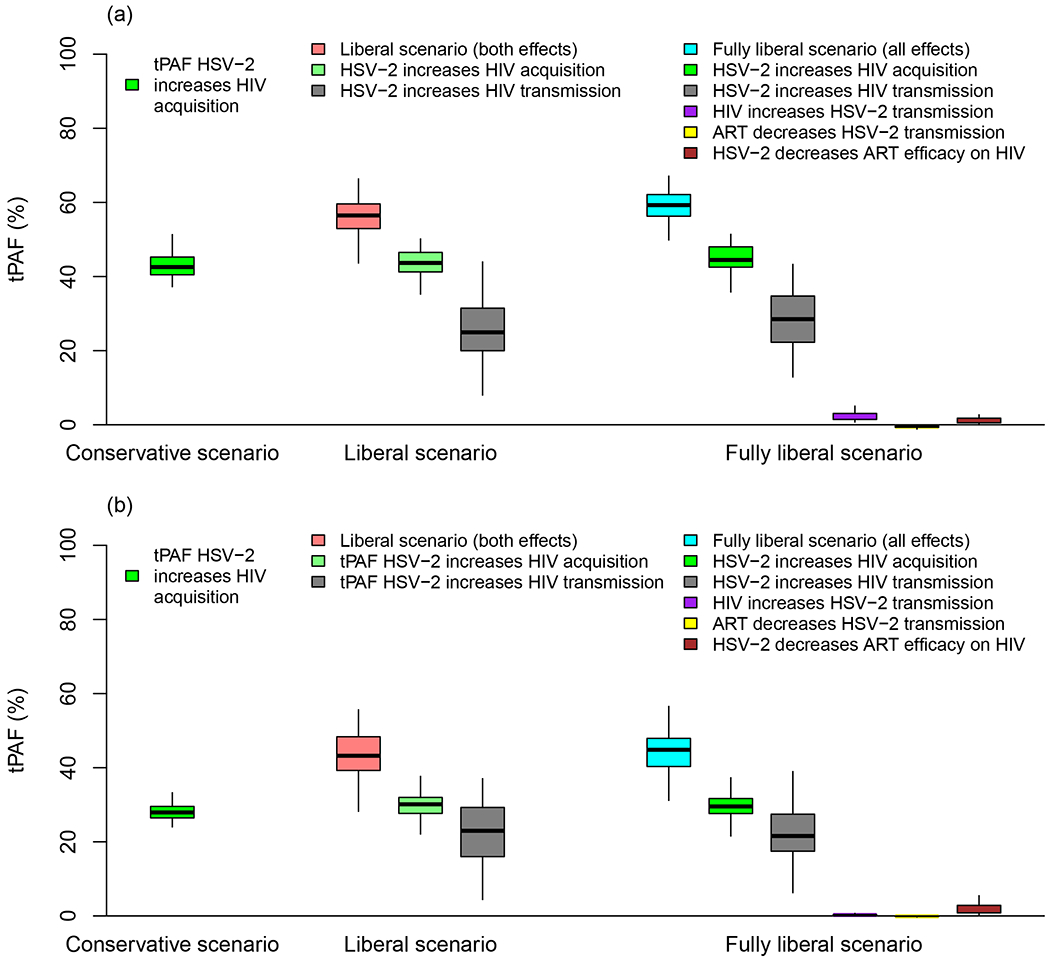
Boxplot shows the transmission population-attributable fraction (tPAF) of each cofactor effect included in each of the three modelled scenarios in WHO a) Africa and b) Americas region. Boxplots represent median, interquartile range, and 95% UI.
Interestingly, tPAF2009-2018 estimates under the liberal and fully liberal scenarios (which accounted for the modifying effect of ART of the HSV-2 cofactors effects on HIV) were very similar. Each of the three additional cofactors considered in the fully liberal scenario contributed to <2% incident HIV infections (Figure 3).
Uncertainty analysis
Under the conservative scenario, the two parameters that most influenced tPAF estimates were the effect of established HSV-2 infection on HIV acquisition (compared to HSV-2-uninfected), especially for lower-risk females, and HSV-2 prevalence (Figure S36). Parameters such as the increases in HIV acquisition according to HSV-2 disease stage or characteristics of higher-risk populations were less influential. In the fully liberal scenarios, the cofactor effect of HSV-2 on HIV transmission risk influenced tPAF2009-2018 estimates more than all the other parameters (Figure S37).
Comparison of tPAF and cPAF estimates
Comparison of modelled one-year and ten-year tPAF estimates under the conservative scenario and cPAF estimates (from our model and previous published estimates12) for the WHO Africa region, shows that: 1) the 10-year tPAF2009-2018 was 1.3-fold higher than the 1-year tPAF2016 for 2016; 2) the 1-year tPAF2016 and model cPAF2016 were similar; and 3) that the model cPAF2016 and previous recent cPAF estimates3 were similar for 2016 (Figure S38).
Discussion
Our analysis suggests that HSV-2 infection may have contributed to over a third of incident heterosexual HIV infections among non-PWID individuals over the past ten years, even when conservatively assuming that HSV-2 only increases HIV acquisition risk. The estimated contribution was greatest in Africa, with 43% (38-51%) of incident heterosexual HIV infections potentially attributable to HSV-2 infection over 2009-2018. We also predict that although the overall number of incident HIV infections is declining due to scale-up of interventions including ART, HSV-2 will continue to contribute to approximately one-third of new HIV infections over the next ten years.
Our main estimates (and those previously issued by the cPAF12) were likely to be conservative, as assuming that HSV-2 also increased HIV transmission risk elevated the estimated contribution of HSV-2 to HIV to 51% (43-58). Adding other possible interactions between HSV-2 and HIV/ART had a negligible additional impact on incident HIV infections. Importantly, we also showed that the classical PAF formula (or 1-year tPAFs) were likely to moderately underestimate the contribution of HSV-2 to HIV epidemics, compared to long-term tPAF, due to secondary transmissions not being accounted for.13 Our long-term conservative tPAF estimates were about 1.3-fold higher than cPAF estimates recently published for each WHO region12 (37% vs 30%).
This study has several strengths. By using a dynamic transmission model to quantify the tPAF of HSV-2 on HIV, we accounted for the direct effect of HSV-2/HIV associations as well as the indirect effect on onward transmission.13 By considering three different scenarios based on the strength of evidence from reviews of the different plausible associations between HSV-2 and HIV, we produced tPAF estimates that reflected the minimum expected association (conservative scenario), while exploring the maximal potential contribution (liberal scenarios), and considering effects with weak strength of evidence (fully liberal scenario). When fitting our model to adjusted IRR estimates, we allowed the prior distribution of the cofactor reflecting increases in HIV acquisition due to HSV-2 () to include the value 1 (no interaction) to acknowledge potential residual confounding biases by sexual activity in empirical pooled adjusted IRR estimates. The model also represented two stages of HSV-2 infection (first year, >1 year) that matched the time frames of the empirical RR estimates. As we constrained the model to fit HIV incidence by HSV-2 status (IRR), we needed to specify prior RR distributions that varied across gender and risk groups. However, this is not likely to have influenced our tPAF estimates since the posterior distributions of these cofactor effects did not significantly differ by sex and by region (except for Europe, where lower posterior parameter values were estimated (Table S9)). The model enabled a direct comparison between long-term and short-term tPAF as well as cPAF estimates used in previous studies. The HSV-2 prevalence data on which our estimates rely was pooled from more than 100 studies, all adjusted for test sensitivity and specificity43 to increase the robustness of our results.
Our study has some limitations mainly due to (lack of) data availability. Our modelled population is stratified by age and engagement in sex work but did not explicitly reflect more populations with specific HSV-2/HIV risks, such as PWID, MSM, 48,49 or individuals having multiple partners, given the current lack of data to parameterise the model for these populations. For example, there was no estimate of HSV-2 prevalence among MSM in Africa (where most HIV infections occur). Due to the absence of HIV prevalence data among heterosexual non-PWID nor among heterosexual groups with different levels of sexual activity at the region levels, the model was fitted to the number of incident HIV infections in 2015, the only year with an estimated breakdown of the total incident HIV infection among MSM and PWID available.48 It is unlikely that our models underestimate HSV-2 prevalence as the data used reflected the overall population, which included individuals from all risk groups. Not explicitly representing a more heterogeneous population is likely to have decreased the heterogeneity in HSV-2 and HIV risk in the model, especially within regions with low prevalence or concentrated epidemics. It may have also increased the level of biological interactions needed to fit the observed adjusted IRRs (which were adjusted for sexual behaviours such as the number of partners), leading to potentially higher tPAF estimates. The lower posterior distributions of the parameter reflecting increased HIV acquisition risk due to HSV-2, and its resulting lower tPAF estimates for the European region, suggest that our model partially captured this effect. All the underlying empirical adjusted IRR estimates (n=28, see table S5) were collected in sub-Saharan Africa (almost all in Southern Africa), thus there was no data to allow fitting our model to WHO-region-specific adjusted IRR estimates. The published adjusted estimates from non-African settings only measured HIV incidence among high-risk populations, which may partly explain why they were substantially lower than the estimates from African settings.
Our analysis necessarily assumed that the associations between HSV-2 and HIV are causal, which is biologically plausible and likely not solely due to sexual confounding for several reasons.50 Evidence of HSV-2 infection increasing HIV acquisition risk comes from a systematic review of 55 longitudinal studies, restricted to adjusted IRR estimates with confirmed sequence of the two infections, which did not find any evidence that adjustment (or lack thereof) affected the observed association.3 These IRRs could not be reproduced in our model without assuming that HSV-2 increases HIV acquisition risk in the Africa region (Figure S31). Parameterising the transmission dynamic model for all WHO regions was challenging due to scarce data especially for higher-risk populations (e.g. size, turnover, HIV prevalence). We addressed this problem by making assumptions based on data from other regions and systematically evaluating the impact of these assumptions on our tPAF estimates in our uncertainty analysis, which showed that it did not substantially alter results. The impact of male circumcision and condom use in altering the per-act risk of HSV-2 and HIV transmission over time was modelled using a simple generic function due to data gaps for several regions. However, the general increasing shape of this function over time was qualitatively consistent with data across regions, suggesting that it was unlikely to influence results, especially given that the model was calibrated to reflect available data on time trends in HSV-2 prevalence by sex, risk groups and regions. However, our tPAF estimates for the next decade should be interpreted more cautiously than those for 2009-2018, as future trends in HIV and HSV-2 incidence are uncertain. Importantly, our model predicts a larger decrease in the number of incident HIV infections over 2009-2018 compared to the UNAIDS Spectrum model,51 due to only one data point of HIV incidence being available. This discrepancy is not likely to affect the tPAF estimates which are relative quantities but suggests that our analysis may underestimate the absolute number of incident HIV infections attributable to HSV-2 over 2019-2028.
Given differences across model assumptions and population characteristics, our results are broadly consistent with previous transmission dynamic model-based estimates, which typically reported short-term tPAFs (from instantaneous10 to 2-years52-54, Table S11). For example, tPAF estimates for the four cities of Kisumu (Kenya), Ndola (Zambia), Cotonou (Benin), and Yaoundé (Cameroon) over 1997-1998 ranged between 36% and 48% in Freeman et al.52 when HSV-2 increased HIV acquisition and transmission risk during clinical episodes only, but were around 50% when subclinical HSV-2 also increased HIV transmission risks. All previous models estimating tPAFs assumed that HSV-2 elevated HIV acquisition and transmission risk.
In conclusion, we estimated that HSV-2 potentially contributes a substantial number of incident HIV infections worldwide, and importantly, its relative impact may only slightly decrease over the next decade, despite ART scale-up. Our estimates for Africa highlight the importance of seeking new interventions to control HSV-2 infection in this region. This may not only improve the lives of millions of people affected by genital herpes but might also help to reduce HIV incidence. Potential interventions include vaccines to prevent HSV-2 infection or decrease HSV-2 shedding, which may in turn decrease its association with HIV. We estimated that perhaps 18% of incident HIV infections occurring over 2009-2018 across WHO regions may be linked to an increase in HIV transmission risk among HSV-2/HIV co-infected individuals, which means that a therapeutic vaccine against HSV-2 shedding or symptoms given to PLHIV may also have an impact on the epidemiology of HIV in addition to ART. Most published models consistently estimate that at least a third of HIV infections are likely attributable to HSV-2, independent of model assumptions. Although there are uncertainties around the magnitude of the interactions between HSV-2 and HIV, our model supported the presence of biological interactions since empirical incidence rate ratios could not be reproduced without assuming such biological effects, especially in Africa. Herpes likely has and will continue to play a key role in HIV epidemics; efforts for its prevention and control should remain a priority on the international agenda.
Supplementary Material
Acknowledgements
We thank Joshua Schiffer from the Fred Hutchinson Cancer Research Center and Anna Wald from the University of Washington for commenting on the HSV-2/HIV cofactors and HSV-2 transmission probabilities. We also thank Keith Sabin, Kimberley Marsh, and Juliana Daher from UNAIDS for providing HIV and ART estimates, and Charlotte James from Bristol University for extracting HSV-2 prevalence data.
Competing interests and sources of funding
KJL and KMET report a grant from GSK outside the submitted work. All other authors declare no competing interests.
This work was funded by the World Health Organization, through the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction, a cosponsored programme executed by the WHO, and via support from the US National Institute of Allergy and Infectious Diseases, part of the US National Institutes of Health (NIH) (NIH U01 AI108543). This work was partly supported by the HPTN Modelling Centre, which is funded by the NIH (NIH UM1 AI068617) through the HPTN Statistical and Data Management Center. RS, HLC, RFB, JS, LS, and MCB acknowledges funding from the MRC Centre for Global Infectious Disease Analysis (reference MR/R015600/1), jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the European Union. SG acknowledges additional funding support from NIH (U01AI139547). KJL and KMET thank the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Evaluation of Interventions at the University of Bristol, in partnership with Public Health England (PHE), for research support. RH receives funding from the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement, which is also part of the EDCTP2 programme supported by the European Union. Grant Ref: MR/R010161/1. RFB is supported by a Wellcome Trust Institutional Strategic Support Fund Fellowship (204801/Z/16/Z). PV also acknowledges support from the NIHR Health Protection Research Unit in Behavioural Science and Evaluation at University of Bristol.
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated, WHO, NHS, NIHR, the Department of Health and Social Care or Public Health England. The funding source had no role in writing the manuscript or the decision to submit it for publication.
References
- 1.UN Joint Programme on HIV/AIDS (UNAIDS). UNAIDS Data 2018. Geneva. 2018. [Google Scholar]
- 2.James C, Harfouche M, Welton NJ, et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98(5):315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17(12):1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van de Perre P, Segondy M, Foulongne V, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect Dis. 2008;8(8):490–497. [DOI] [PubMed] [Google Scholar]
- 5.Phipps W, Nakku-Joloba E, Krantz EM, et al. Genital Herpes Simplex Virus Type 2 Shedding Among Adults With and Without HIV Infection in Uganda. Journal of Infectious Diseases. 2016;213(3):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Low AJ, Konate I, Nagot N, et al. Cervicovaginal HIV-1 Shedding in Women Taking Antiretroviral Therapy in Burkina Faso: A Longitudinal Study. Jaids-J Acq Imm Def. 2014;65(2):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayaud P, Nagot N, Konate I, et al. Effect of HIV-1 and antiretroviral therapy on herpes simplex virus type 2: a prospective study in African women. Sexually Transmitted Infections. 2008;84(5):332–337. [DOI] [PubMed] [Google Scholar]
- 8.Tan DH, Murphy K, Shah P, Walmsley SL. Herpes simplex virus type 2 and HIV disease progression: a systematic review of observational studies. BMC Infect Dis. 2013;13:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford ES, Magaret AS, Spak CW, et al. Increase in HSV shedding at initiation of antiretroviral therapy and decrease in shedding over time on antiretroviral therapy in HIV and HSV-2 infected persons. Aids. 2018;32(17):2525–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital Herpes Has Played a More Important Role than Any Other Sexually Transmitted Infection in Driving HIV Prevalence in Africa. Plos One. 2008;3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston C, Gottlieb SL, Wald A. Status of vaccine research and development of vaccines for herpes simplex virus. Vaccine. 2016;34(26):2948–2952. [DOI] [PubMed] [Google Scholar]
- 12.Looker KJ, Welton NJ, Sabin KM, et al. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: a population attributable fraction analysis using published epidemiological data. Lancet Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra S, Baral SD. Rethinking the population attributable fraction for infectious diseases. Lancet Infect Dis. 2019. [DOI] [PubMed] [Google Scholar]
- 14.Rowe AK, Powell KE, Flanders WD. Why population attributable fractions can sum to more than one. Am J Prev Med. 2004;26(3):243–249. [DOI] [PubMed] [Google Scholar]
- 15.Mishra S, Pickles M, Blanchard JF, Moses S, Shubber Z, Boily MC. Validation of the Modes of Transmission Model as a Tool to Prioritize HIV Prevention Targets: A Comparative Modelling Analysis. Plos One. 2014;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. [DOI] [PubMed] [Google Scholar]
- 17.Glynn JR, Biraro S, Weiss HA. Herpes simplex virus type 2: a key role in HIV incidence. Aids. 2009;23(12):1595–1598. [DOI] [PubMed] [Google Scholar]
- 18.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. Journal of Infectious Diseases. 2002;185(1):45–52. [DOI] [PubMed] [Google Scholar]
- 19.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127–2137. [DOI] [PubMed] [Google Scholar]
- 20.Benedetti JK, Zeh J, Corey L. Clinical reactivation of genital herpes simplex virus infection decreases in frequency over time. Ann Intern Med. 1999;131(1):14–20. [DOI] [PubMed] [Google Scholar]
- 21.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–1153. [DOI] [PubMed] [Google Scholar]
- 22.Latif AS, Katzenstein DA, Bassett MT, Houston S, Emmanuel JC, Marowa E. Genital Ulcers and Transmission of Hiv among Couples in Zimbabwe. Aids. 1989;3(8):519–523. [DOI] [PubMed] [Google Scholar]
- 23.Barnabas RV, Celum C. Infectious Co-Factors in HIV-1 Transmission Herpes Simplex Virus Type-2 and HIV-1: New Insights and Interventions. Curr Hiv Res. 2012;10(3):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan DH, Raboud JM, Kaul R, Walmsley SL. Antiretroviral therapy is not associated with reduced herpes simplex virus shedding in HIV coinfected adults: an observational cohort study. BMJ Open. 2014;4(1):e004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United Nations, Department of Economic and Social Affairs, Population Division. United Nations World Population Prospects: The 2017 Revision. https://esa.un.org/unpd/wpp/. 2017. Accessed 01 February 2018.
- 26.Knight J, Baral SD, Schwartz S, et al. Contribution of high risk groups’ unmet needs may be underestimated in epidemic models without risk turnover: A mechanistic modelling analysis. Infectious Disease Modelling. 2020;5:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soetaert K, Petzoldt T, Setzer RW. Solving Differential Equations in R: Package deSolve. J Stat Softw. 2010;33(9):1–25.20808728 [Google Scholar]
- 28.UNAIDS. AIDSInfo. http://aidsinfo.unaids.org/.Published2018. Accessed 14/03/2018.
- 29.UNAIDS. Quick Start Guide for Spectrum 2018. 2018.
- 30.Fazito E, Cuchi P, Mahy M, Brown T. Analysis of duration of risk behaviour for key populations: a literature review. Sex Transm Infect. 2012;88Suppl 2:i24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baral S, Beyrer C, Muessig K, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(7):538–549. [DOI] [PubMed] [Google Scholar]
- 32.Mishra S Using mathematical models to characterize HIV epidemics for the design of HIV prevention strategies. School of Public Health: Department of Infectious Disease Epidemiology, Imperial College London; 2014. [Google Scholar]
- 33.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9(2):118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phipps W, Saracino M, Magaret A, et al. Persistent Genital Herpes Simplex Virus-2 Shedding Years Following the First Clinical Episode. Journal of Infectious Diseases. 2011;203(2):180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabillard D, Lewden C, Ndoye I, et al. Mortality, AIDS-morbidity, and loss to follow-up by current CD4 cell count among HIV-1-infected adults receiving antiretroviral therapy in Africa and Asia: data from the ANRS 12222 collaboration. J Acquir Immune Defic Syndr. 2013;62(5):555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodger AJ, Cambiano V, Bruun T, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. Jama. 2016;316(2):171–181. [DOI] [PubMed] [Google Scholar]
- 38.ICF. The DHS Program website. Funded by USAID. https://dhsprogram.com/publications/index.cfm. Published2018. Accessed 15/10/2018.
- 39.Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev. 2001(3):CD003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2009(2):CD003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reniers G, Slaymaker E, Nakiyingi-Miiro J, et al. Mortality trends in the era of antiretroviral therapy: evidence from the Network for Analysing Longitudinal Population based HIV/AIDS data on Africa (ALPHA). AIDS. 2014;28Suppl 4:S533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):E1192–E1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Looker KJ, Magaret AS, Turner KME, Vickerman P, Gottlieb SL, Newman LM. Global Estimates of Prevalent and Incident Herpes Simplex Virus Type 2 Infections in 2012. Plos One. 2015;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Looker KJ, Gamett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. B World Health Organ. 2008;86(10):805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.UNAIDS. Estimates of the number of incident HIV cases and prevalence by gender/age group over time within each WHO region (personal communication). 2018. [Google Scholar]
- 46.Hyndman RJ, Fan YN. Sample quantiles in statistical packages. Am Stat. 1996;50(4):361–365. [Google Scholar]
- 47.Mansournia MA, Altman DG. STATISTICS NOTES Population attributable fraction. Bmj-Brit Med J. 2018;360. [DOI] [PubMed] [Google Scholar]
- 48.UNAIDS. Estimated fraction of incident infection in 2015 by key populations within each WHO region (personal communication). 2018. [Google Scholar]
- 49.Beyrer C, Baral SD, van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omori R, Nagelkerke N, Abu-Raddad LJ. HIV and herpes simplex virus type 2 epidemiological synergy: misguided observational evidence? A modelling study. Sex Transm Infect. 2018;94(5):372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahy M, Marsh K, Sabin K, Wanyeki I, Daher J, Ghys PD. HIV estimates through 2018: data for decision-making. AIDS (London, England). 2019;33Suppl 3(Suppl 3):S203–S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman EE, Orroth KK, White RG, et al. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sexually Transmitted Infections. 2007;83:I17–I24. [DOI] [PubMed] [Google Scholar]
- 53.Foss AM, Vickerman PT, Mayaud P, et al. Modelling the interactions between herpes simplex virus type 2 and HIV: implications for the HIV epidemic in southern India. Sexually Transmitted Infections. 2011;87(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orroth KK, White RG, Korenromp EL, et al. Empirical observations underestimate the proportion of human immunodeficiency virus infections attributable to sexually transmitted diseases in the Mwanza and Rakai sexually transmitted disease treatment trials: Simulation results. Sex Transm Dis. 2006;33(9):536–544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


