Abstract
Metal cluster-based compounds have difficulty finishing the photocatalytic carbon dioxide reduction reaction (CO2RR) and water oxidation reaction (WOR) simultaneously because of the big challenge in realizing the coexistence of independently and synergistically reductive and oxidative active sites in one compound. Herein, we elaborately designed and synthesized one kind of crystalline reduction–oxidation (RO) cluster-based catalysts connecting reductive {M3L8(H2O)2} (M = Zn, Co, and Ni for RO-1, 2, 3 respectively) cluster and oxidative {PMo9V7O44} cluster through a single oxygen atom bridge to achieve artificial photosynthesis successfully. These clusters can all photocatalyze CO2-to-CO and H2O-to-O2 reactions simultaneously, of which the CO yield of RO-1 is 13.8 μmol/g·h, and the selectivity is nearly 100%. Density functional theory calculations reveal that the concomitantly catalytically reductive and oxidative active sites (for CO2RR and WOR, respectively) and the effective electron transfer between the sites in these RO photocatalysts are the key factors to complete the overall photosynthesis.
Keywords: polyoxometalates, crystalline materials, RO cluster-based photocatalysts, CO2 reduction reaction, artificial photosynthesis
Introduction
Effective coupling of photocatalytic carbon dioxide reduction reaction (CO2RR) and water oxidation reaction (WOR) to achieve artificial photosynthesis (with water as a hole scavenger) is the most important and green means to recycle atmospheric CO2.1−5 Both CO2RR and WOR are multielectron and multiproton transfer processes in which the photocatalysts are responsible for supplying multiple electrons, and water can serve as an abundant source of protons.6,7 Currently, photocatalysts mainly include enzymes,8,9 nanosemiconductors,10−15 organic semiconductors,16−18 and crystalline coordination compounds,19−21 of which metal cluster-based coordination compounds22−24 often show distinct advantages owing to their rich redox capabilities, tunable structures, and abilities to being multielectron donors or acceptors. In particular, their well-defined crystalline structural information is helpful to survey the photocatalytic reaction mechanism and structure–function relationship accurately.25−27 So far, however, a single metal cluster-based coordination compound has difficulty accomplishing CO2RR and WOR concurrently because of the challenges in realizing the coexistence of reductive and oxidative sites as well as the effective charge transfer between the catalytic sites. That is also the reason why most metal cluster-based catalysts could only achieve the half-reaction of artificial photosynthesis with the assistance of an additional sacrificial agent (triethylamine, triethanolamine for CO2RR or AgNO3 for WOR, etc.).28−30 If one designed and constructed a crystalline reduction–oxidation (RO) cluster-based coordination compound by connecting reductive clusters and oxidative clusters through effective linkers (bridging atoms or conductive ligands), it would strongly promote the charge transfer between the active sites of CO2RR and WOR and would be expected to accomplish artificial photosynthesis.
In view of the above design ideas, we think that such new RO cluster-based catalysts for artificial photosynthesis should fulfill at least two conditions as follows: (i) excellent light-absorption and explicit catalytic active centers with enough reductive and oxidative capabilities to complete the CO2RR and WOR half-reactions, respectively; (ii) suitable linkers between reductive and oxidative clusters for efficient electron transfer, etc. Furthermore, the synthesis conditions of RO cluster-based compounds should be adaptive for the growth of reductive and oxidative components and their further assembling. Polyoxometalates (POMs), known as “electronic sponges”, have the advantages of multielectron donors and structural stability, which have widespread applications due their active metal ions with high valence states.31−34 Therefore, POMs can be considered as the “oxidation” part in RO clusters. Meanwhile, because POM-based coordination polymers were usually synthesized by hydrothermal methods at high temperature,35,36 the growth conditions of the “reduction” part would better be similar. According to our survey, the classical linear trinuclear cluster {M3LxS12–x} (M = the first transition low-valent metal ions, L = azole ligands, S = solvent molecules, x = 6–8) are readily available and usually have good reducibility and stability.37−40 Importantly, the two terminal metal ions coordinated with two/three solvent molecules in {M3} can serve as efficient reductive active sites after linking with POMs.
Based on the above considerations, the linear trinuclear {M3L8(H2O)2}, M = Zn, Co, Ni, L = 1,4-di(4H-1,2,4-triazol-4-yl)benzene (p-tr2Ph), simplified as M3, as a reduction cluster and the phosphovanadomolybdate ({PMo9V7O44}) as an oxidation cluster were taken into account to construct three crystalline RO cluster-based photocatalysts, Zn3(H2O)2(p-tr2Ph)5PMo9V7O44 (RO-1), Co3(H2O)2(p-tr2Ph)5PMo9V7O44 (RO-2), and Ni3(H2O)2(p-tr2Ph)5PMo9V7O44 (RO-3). Compared with the traditional α-Keggin POM, the phosphovanadomolybdate {PMo9V7O44} has more advantages in oxidative catalytic reactions for its more positive redox potential.41,42 Ultraviolet–visible-near-infrared (UV–vis-NIR) spectra revealed that RO-1, 2, and 3 all had a broad range of light absorption in 250–2500 nm. As expected, on account of strong redox ability and suitable band structures for reducing CO2 to CO coupling with oxidizing H2O to O2, artificial photosynthetic overall reaction was successfully realized when RO-1, 2, and 3 were used as photocatalysts. Among them, RO-1 exhibits the highest CO yield of 138 μmol/g along with the O2 evolution in a gas–solid system among reported crystalline photocatalysts. Density functional theory (DFT) calculation uncovered that the photogenerated electrons were efficiently transferred from {PMo9V7O44} to {M3} under light irradiation, and CO2-to-CO and H2O-to-O2 reactions were finished by {M3} and {PMo9V7O44}, respectively, which were in line with our initial expectation and subsequent characterizations. More importantly, this work proposes a novel design strategy to establish crystalline RO cluster-based catalysts to achieve the artificial photosynthetic overall reaction.
Results and Discussion
Characterization of RO-1, 2, and 3 Photocatalysts
X-ray crystallography diffraction indicated that RO-1, 2, and 3 are isostructural and crystallize in the triclinic system with a P̅1 space group (Table S1). RO-1, 2, and 3 consist of {M3} (Zn3, Co3, and Ni3) as the reduction component and {PMo9V7O44} as the oxidation component, respectively (Figure 1a and b). The asymmetric unit of RO-1, 2, and 3 contains 1/2 of a {PMo9V7O44} node, 2 metal ions, 2.5 p-tr2Ph ligands, 1 coordinated H2O molecule, and several free H2O molecules (Figure S1). There are two kinds of metal coordinated environments in the {M3} cluster. The M1 ion is six-coordinated with four N atoms from ligands, one O1 atom from {PMo9V7O44} and one O1w atom from the coordinated H2O molecule. The M2 ion employs a common octahedron geometry surrounded with six N atoms from six p-tr2Ph ligands. There are three kinds of coordination environments of p-tr2Ph in the structure (Figure S2). {PMo9V7O44} can be considered as a heteroatom α-Keggin POM with four additional capping metal ions in the equatorial position (Figures 1b and S3). Sixteen metal ions are located at three layers from top to bottom in a “4 + 8 + 4” configuration, of which the first and third layers are all Mo ions. The second layer composes seven V ions and one Mo ion (the positions of two metal atoms are occupied by Mo atom and V atom in a disorderly fashion, and the chemical occupancy is close to 0.5). The {PMo9V7O44} node links with the {M3} cluster by V–O–M bonds forming a zigzag chain (Figure 1c). Each {M3} cluster links with four other {M3} clusters through ligands to constitute a 2D layer (Figure S4). In the cavity of layers, {PMo9V7O44}, as pillars, were encapsulated and connected with two {M3} clusters from adjacent layers to assemble into a 3D layer–pillar framework (Figures 1d and S5). Owing to the restriction of single-crystal X-ray diffraction (SCXRD) methods for distinguishing elements of V, Co, Ni, and Zn, X-ray photoelectron spectroscopy (XPS) and inductively coupled plasma (ICP) are measured to verify the existence and abundance ratios of Mo, V, Zn, Co, and Ni elements. XPS spectra confirmed the existence of Mo, V, Zn (RO-1), Co (RO-2), and Ni (RO-3) elements in the structures, respectively (Figure S6). ICP results further determined that the stoichiometric ratios of Mo/V/M (M = Zn, Co, Ni) in RO-1, 2, and 3 are all close to 9:7:3 (Table S2). Besides, high-resolution peaks of the Mo 3d spectra are located in the same positions in RO-1, 2, and 3 of Mo 3d5/2 (ca. 232.5 eV) and Mo 3d3/2 (ca. 235.5 eV), validating the presence of the MoV and MoVI in these three compounds (Figures S7 and S8). Likewise, the high-resolution V 2p peaks of RO-1, 2, and 3 reveal in the same region of V 2p (ca. 517.0 eV), demonstrating the single presence of VIV (Figure S9).
Figure 1.
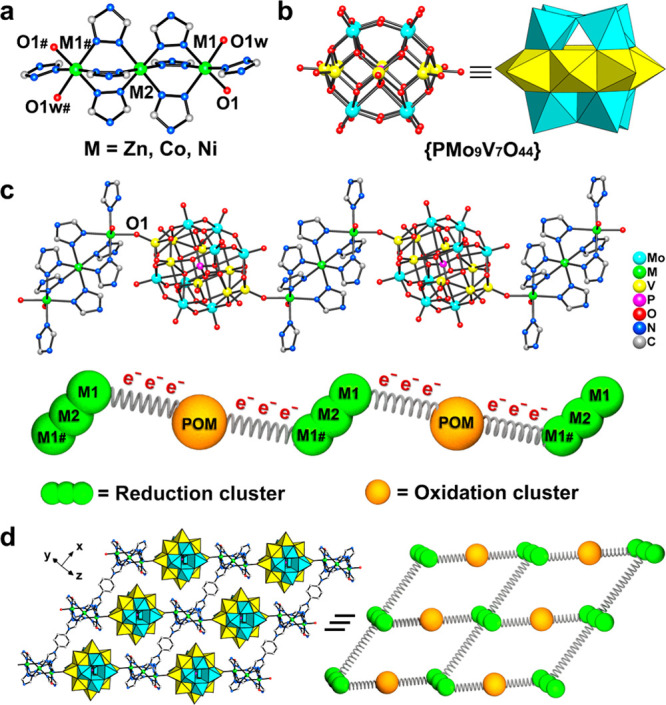
Summary of the structure of RO-1, 2, and 3. (a) Coordination environments of the {M3} node. (b) Structure of the {PMo9V7O44} node. (c) 1D chain constructed from reduction clusters ({M3}) and oxidation cluster ({PMo9V7O44}). (d) Spatial arrangement and corresponding to simplify the diagram. All hydrogen atoms are omitted for clarity. Mo, cyan; M, green; V, yellow; P, pink; O, red; N, blue; C, gray.
Powder X-ray diffraction (PXRD) patterns of the experimental RO-1, 2, and 3 were well-matched with the simulated curves from SCXRD, which proved the high purity of the samples (Figure S10). Furthermore, when RO-1, 2, and 3 were immersed into 1 M HCl and 0.01 M NaOH aqueous solution for 24 h at room temperature, no peaks changed in PXRD patterns, attesting that these RO cluster-based compounds have excellent chemical stability (Figure S11, S12). Thermogravimetric analysis (TGA) exhibited that RO-1, 2, and 3 have similar weight decreasing platforms and good thermostability below 300 °C (Figure S13).
The light absorption of photocatalysts was examined by UV–vis–NIR diffuse reflectance spectroscopy. It is noted that H3PMo12O40·xH2O has light response with wavelengths in the ranges 250–450 and 1400–2500 nm. In contrast, RO-1, 2, and 3 have an obviously spectral range across the entire absorption region (250–2500 nm), which can be attributed to the introduction of V ions in polyoxometalate and electronic transfer after assembling with the reduction cluster (Figures 2a and S14). Such wide ranges of light absorption cause RO-1, 2, and 3 all to appear black (Figures 2a inset, S15, and S16). The band gap energy of RO-1, 2, and 3 were further determined to be 1.80 (RO-1), 1.82 (RO-2), and 1.88 eV (RO-3) by Taus plots (Figures 2b, S17, and S18), demonstrating that they have semiconductor-like characteristics. To evaluate the oxidation ability of RO-1, 2, and 3, the highest occupied molecular orbital (HOMO) energy levels, which equal the ionization potential, were calculated to be 1.16 (RO-1), 1.22 (RO-2) and 1.23 V (RO-3) (vs normal hydrogen electrode (NHE), pH = 7) by ultraviolet photoelectron spectroscopy (UPS) (Figures 2c, S19, and S20).43 All the HOMO energy levels are more positive than the oxidation potential of O2/H2O (0.82 V vs NHE, pH = 7),44,45 which indicated that RO-1, 2, and 3 could achieve H2O-to-O2 catalysis thermodynamically. On this basis, the lowest unoccupied molecular orbital (LUMO) energy levels were evaluated to be −0.64, −0.60, and −0.65 V for RO-1, 2, and 3, respectively, suggesting that they are able to convert CO2 to most chemical fuels like CO (−0.51 V vs NHE), CH4 (−0.24 V vs NHE), and so on.46 In additional, to verify the LUMO energy levels accurately, Mott–Schottky plots were performed at frequencies of 1000, 1500, and 2000 Hz (Figures S21–S23). The values were calculated to be −0.64 (RO-1), −0.61 (RO-2), and −0.66 V (RO-3) vs NHE, which are consistent with those obtained from Eg and UPS results. According to the results of bandgap, UPS, and Mott–Schottky plots, the band structure diagrams of RO-1, 2, and 3 were obtained (Figure 2d), which indicated that RO-1, 2, and 3 can theoretically catalyze the artificial photosynthetic overall reaction.
Figure 2.
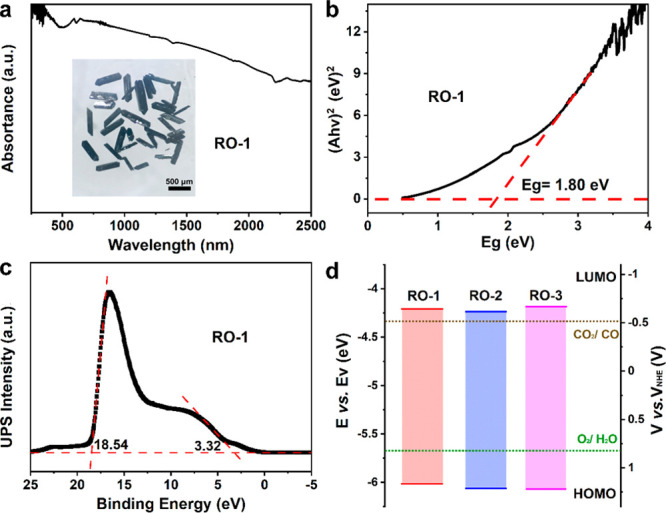
Optical characterizations of photocatalysts. (a) UV–vis–NIR absorption spectrum of RO-1. (inset) Photograph of RO-1 under an optical microscope. (b) Band gap energy (Eg) analysis of RO-1. The intersection of the baseline and the slant dashed line is the band gap. (c) UPS spectrum of RO-1. (d) Energy bandgap diagrams of RO-1, 2, and 3 with respect to the CO2RR and WOR potentials (pH = 7). The electron volts (eV, left y axis) are converted to electrochemical energy potentials in volts (V, right y axis) according to which 0 V vs RHE (reversible hydrogen electrode) equals −4.44 eV vs evac (vacuum level). Then, the RHE potentials are further converted to NHE (pH = 7) according to E (vs NHE, pH = 7) = E (vs RHE) – 0.0591 × 7.
Catalytic Performance of Artificial Photosynthesis
On account of the above-mentioned suitable band structure, we considered that these crystalline RO cluster-based compounds can be used as catalysts to conduct artificial photosynthesis. All the CO2RRs were investigated in the gas–solid system under light irradiation (λ = 300–1100 nm) without additional cocatalyst, photosensitizer, or sacrificial agent (experimental details are described in the Photocatalytic Experiments section). RO-1, 2, and 3 showed high performance and selectivity of CO2-to-CO conversion only with the participation of water vapor. By testing CO yields at 2 h intervals, roughly linear curves were obtained by gas chromatography (GC) analysis (Figure 3a, S24, and S25). When RO-1 was used as a photocatalyst, the amount of CO rose to 138 μmol/g after 10 h, which is higher than 122 μmol/g for RO-2 and more than three times higher for RO-3 (42 μmol/g). A trace amount of CH4 (<1 μmol/g) was detected in the gas phase. Additionally, H2 as a competitive product was not produced during the reaction (Figure S26). In order to detect the yield of O2 and compare it with that of CO, we performed photocatalytic tests in an online evaluation system. The results showed that the molar ratio of CO to O2 were all close to 2/1 along with RO-1, 2, and 3 as photocatalysts (Figure S27). To explore the reasons for the different photocatalytic properties of RO-1, 2, and 3, the charge separation efficiencies of RO-1, 2, and 3 were measured by transient photocurrent response under periodic light irradiation. As we can see in Figure S28, the photocurrent profiles showed RO-1 had unattenuated and more sharp current than RO-2 or RO-3, which reflected the higher separating and transfer efficiency of photoinduced electron–hole pairs in RO-1. The successively increasing resistance values of RO-1, 2, and 3 obtained from EIS Nyquist plots also indicated that the photogenerated electrons are easier to migrate in RO-1 (Figure S29). Besides, the lowest PL intensity of RO-1 revealed its better suppressed radiative electron–hole recombination than RO-2 and RO-3, which is consistent with the previous reports47,48 (Figure S30). The effects of different qualities of RO-1 on the photocatalytic CO2 reduction performance in 10 h were further examined (Figure 3b). The highest CO evolution for 2 mg catalysts was 15.5 μmol/g/h (correspond to reduction yield: 7.6 μL). When the quality of catalysts was added to 5 mg, the catalytic rate of CO was slightly reduced to 13.8 μmol/g/h (16.9 μL). When the catalysts continued increased to 10 and 20 mg, the catalytic rate decreased significantly to 8 and 4.1 μmol/g·h, respectively, which were mainly affected by the contact area of CO2 with a solid catalyst.
Figure 3.
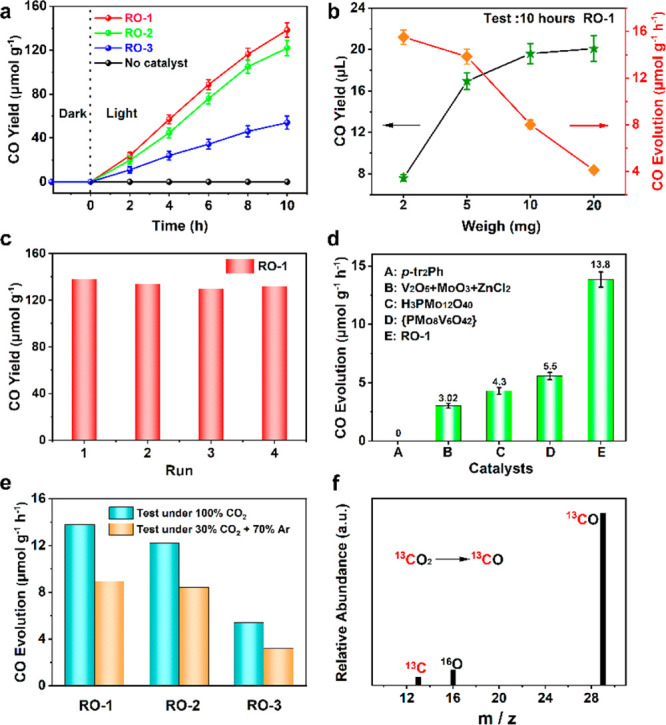
Photochemical performance of CO2RR by using different photocatalysts. (a) Time-dependent CO yield of RO-1, 2, and 3. (b) Relationship between yield and rate of CO generation in different quality of RO-1. (c) Yield of CO for RO-1 as a CO2RR photocatalyst in four continuous runs. (d) Yield of CO in a series of comparison tests. (e) Comparison of the CO generation of RO-1, 2, and 3 under atmospheres of 100% CO2 and 30% CO2 (+ 70% Ar). (f) Mass spectrum of generated 13CO under 13CO2 catalyzed by RO-1.
In this regard, we use 5 mg RO-1 to evaluate the cyclic performance. In the cycle tests, we only refilled the saturated CO2 and replaced fresh water for another 10-h light irradiation. RO-1, 2, and 3 retain at least 90% of their original activity after four cycles (Figure 3c, S31, and S32). The photocatalytic durability was further evaluated by the long-time test. After 30 h of continuous exposure, the yields of CO were 398.7 (RO-1), 345.0 (RO-2), and 143.1 μmol/g (RO-3), manifesting that these RO cluster-based compounds have outstanding photocatalytic stability (Figure S33). What’s more, the PXRD patterns (Figure S34), Fourier transform infrared spectroscopy (FTIR) curves (Figures S35–S37), and XPS spectra (Figure S38) before and after cyclic experiments were not apparently changed, which proved the structural stability of RO-1, 2, and 3 during the photocatalytic process.
In order to prove the RO cluster-based photocatalysts did work during the artificial photosynthesis, a series of comparative experiments were carried out, and the results are presented in Figure 3d and Table S3. No product was detected when the system was placed in the dark, revealing that the reaction was a light-driven process. When the system was in the absence of H2O, only traces of CO were observed by GC, which can be explained by the participation of original water molecules in the skeletons. Besides, compared with {PMo8V6O42}49 (Figure S39) (5.5 μmol/g/h) or H3PMo12O40·xH2O (4.3 μmol/g/h), the structure coupling with reduction clusters had the higher performance of photocatalytic reaction. This suggests that efficient electron–hole separation may happen in RO photocatalysts under light irradiation to achieve a longer lifetime. The physical mixture of MoO3, V2O5, and MCl2 (ZnCl2/CoCl2/NiCl2) was also examined, and the CO evolution was less than RO-1, 2, and 3, revealing the superiority of the coordination network. Beyond that, compared with the condition of a pure CO2 atmosphere, experiments under low concentration CO2 (30% CO2 + 70% Ar) were also performed (Figure 3e) which has more practical significance. It can be found that the photocatalysts still had good activity under an atmosphere of 30% CO2 due to the adsorption capacity of CO2 on the catalyst surface.
To further confirm that the CO and O2 products were indeed converted from CO2 and H2O, 13CO2 and H218O were used for isotopic tracing experiments to search for the source of C in CO and O in O2 through gas chromatography mass spectrometry (GC-MS) analysis. Under the same test conditions, the signal at m/z 29 corresponding to 13CO was detected, which demonstrated that the production of CO originated from CO2 (Figure 3f). By comparing m/z of O2 using H216O and H218O as a reagent, the apparent peak at 36 matching to 18O2 suggested the produced O2 was converted from H218O (Figures S40 and S41). Considering the above-mentioned points, it is proven that three RO cluster-based catalysts possess the ability to catalyze the artificial photosynthetic overall reaction.
Photoexcitation Process and Reaction Mechanism
To get insight into the photoexcitation process of RO-1, 2, and 3 and further the intrinsic mechanism and the reaction pathways of artificial photosynthesis, the proposed catalytic processes were studied by TDDFT (cluster calculations) and free energy profiles (periodic calculations) (Figure S42). Figure 4a depicts the simulated absorption spectrum and the measured solid UV–vis absorption of RO-1, showing a broad absorption in the near-infrared region due to a small band gap of the system (Figure S43). Based on the orbital transition analysis, the two strongest transitions in Figure 4a are mainly contributed by the transitions from the {PMo9V7O44} fragment to the {M3} part (Figure 4b). Besides, internal transitions of {PMo9V7O44} and {M3} can also be determined (Figures S44 and S45), which explains the weak photocatalytic performance of POM itself. The electron–hole separation from {PMo9V7O44} to {M3} endows RO-1, 2, and 3 with higher catalytic activities. Next, to identify the reactive sites and deeply explore the difference of photoreaction properties among RO-1, 2, and 3, the Gibbs free energy (G) of the CO2RR and WOR paths are compared. The conversion of the CO2-to-CO reduction pathway contains three steps: CO2 activation and hydrogenation (CO2 → *COOH), dehydration (*COOH → *CO), and CO desorption (*CO → CO), where the first step has the highest positive energy difference acting as a rate-determining step (Figure 4c and Table S4). The energy change from CO2 to *COOH on the Zn site (RO-1, 0.91 eV) is lower than Mo on the {PMo8V6O42} fragment, the Co site (RO-2, 1.25 eV), and Ni site (RO-3, 1.89 eV), implying the high activity of RO-1 for CO2 photoreduction. In addition, the higher free energy change for the formation of *H than *COOH on the Zn site of RO-1 explains the high selectivity of CO production (Figure S46).
Figure 4.
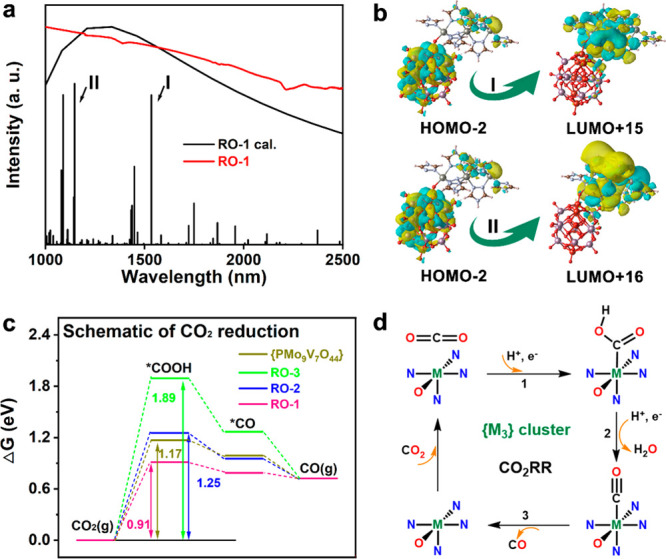
(a) Comparisons of experimental and calculated absorption spectra of RO-1. (b) Transitions from the {PMo9V7O44} fragment to the {M3} part. (c) Free-energy profile for the CO2RR pathway. (d) Proposed mechanism of CO2RR catalyzed by RO-1, 2, and 3 catalysts.
As for the process of water oxidation reaction, comparing the Gibbs free energy of each step, the first step of the adsorption of *OH intermediate is considered to be the potential-determinant step (Figure S47 and Table S4), and the computed ΔG values are as follows: ΔGRO-1 (1.51 eV) < ΔGRO-2 (1.71 eV) < ΔGRO-3 (1.89 eV). Hence, the H2O-to-O2 process can be achieved easily along with RO-1 as photocatalyst. This is the reason why RO-1 is one of the most suitable candidates for the artificial photosynthetic overall reaction. According to the above analysis, a credible photocatalytic mechanism is proposed (Figure 4d). When light illuminated on the catalysts, the polyoxometalate serving as the light harvester produces photogenerated electron–hole pairs. Then the photoelectrons transfer to the trinuclear cluster where the coordinately unsaturated metal ions act as reductive active site to convert CO2 to CO. The photogenerated holes in the polyoxometalate are used to complete the water oxidation reaction.
Conclusion
In summary, we meticulously designed and synthesized three RO cluster-based catalysts (RO-1, 2, and 3), in which the independent and cooperative active clusters with reduction and oxidation can be used to complete the photocatalytic CO2RR and WOR, respectively. Moreover, due to the more efficient electron transfer between the reduction and oxidation clusters along with their strong redox capabilities, RO-1 exhibited the highest CO properties (138 μmol/g) in crystalline materials without any sacrificial agent, and the selectivity was close to 100%. Significantly, this work provides a feasible design strategy for constructing reduction–oxidation metal cluster-based compounds to be used as catalysts for artificial photosynthesis.
Experimental Section
Synthesis of RO-1 [Zn3(H2O)2(p-tr2Ph)5] [PMoV5MoVI4VIV7O44]·8H2O
A mixture of p-tr2Ph (15 mg, 0.07 mmol), NH4VO3 (15 mg, 0.13 mmol), ZnCl2 (30 mg, 0.22 mmol), Na2MoO4·2H2O (50 mg, 0.21 mmol), H3PO3 (10 mg, 0.12 mmol), and H2O (5 mL) was vigorous stirred for 30 min, transferred to a Teflon-lined stainless-steel reactor, and kept at 180 °C for 72 h. After 3 days, it was cooled down to 30 °C at a rate of 10 °C/h. The product was isolated by filtration, separated from an amorphous yellow powder and black block crystals of RO-1 by decantation, then washed with distilled water; it was collected in 70% yield based on p-tr2Ph. Anal. Calcd for C50H60Zn3Mo9N30O54PV7 (Mw 3394.42): C, 17.68%; H, 1.77%; N, 12.37%. Found: C, 17.51%; H, 1.84%; N, 12.26%.
Synthesis of RO-2 [Co3(H2O)2(p-tr2Ph)5] [PMoV5MoVI4VIV7O44]·10H2O
The preparation of RO-2 was like the method of RO-1. Instead of ZnCl2, CoCl2·6H2O (30 mg, 0.12 mmol) as a substitute was added in solution. Finally, black rhombus crystals were obtained (40% yield based on p-tr2Ph) and washed with distilled water. Anal. Calcd for C50H64Co3Mo9N30O56PV7 (Mw 3411.13): C, 17.59%; H, 1.88%; N, 12.32%. Found: C, 17.33%; H, 1.94%; N, 12.16%.
Synthesis of RO-3 [Ni3(H2O)2(p-tr2Ph)5] [PMoV5MoVI4VIV7O44]·6.5H2O
The preparation of RO-3 was similar to the method used for RO-1. Instead of ZnCl2, NiCl2·6H2O (30 mg, 0.12 mmol) as a substitute was added in solution. Finally, black rhombus crystals were obtained (60% yield based on p-tr2Ph) and washed with distilled water. Anal. Calcd for C50H57Ni3Mo9N30O52.5PV7 (Mw: 3341.36): C, 17.96%; H, 1.71%; N, 12.57%. Found: C, 17.75%; H, 1.86%; N, 12.44%.
Synthesis of {PMo8V6O42} [H2N(C2H4)2NH2]4(H3O)[PMoV2MoVI6VIV4O40(VIVO)2]·xH2O
{PMo8V6O42} is prepared based on previous reports with slight modifications. A mixture of NH4VO3 (73 mg, 0.625 mmol), Na2MoO4·2H2O (151 mg, 0.625 mmol), H3PO4 (72 μL, 1.25 mmol), MnCl2·4H2O (123 mg, 0.625 mmol), piperazine (107.5 mg, 1.25 mmol), and H2O (5 mL) was vigorously stirred for 30 min and transferred it to a Teflon-lined stainless-steel reactor and maintained at 150 °C for 144 h. After cooling to room temperature, black block crystals of {PMo8V6O42} were obtained (60% yield based on NH4VO3), washed with distilled water, and air-dried.
Photocatalytic Experiments
The crystals of RO-1, 2, and 3 were washed with distill water and air-dried to be spared. Before photocatalytic reaction, the catalysts were ground into powder and weight 5 mg (or 2/10/20 mg). Then the powder was spread on a round-bottomed quartz crucible with ϕ20 mm and the quartz dish is placed in a 50 mL top-irradiation photocatalytic quartz reactor, which adds 0.2 mL distilled water as a sacrificial agent in the reactor. The reactor was ventilated for 20 min at a flow rate of 0.1 L/min under the test gas (pure CO2, pure Ar, 30% CO2 + 70% Ar) to ensure that the air in the reaction system was completely exchanged. During the overall reaction process, water will form a saturated vapor pressure in the system when exposed on a 300 W xenon lamp (λ = 300–1100 nm, light intensity 200 mW cm–2); that is, the catalyst will only be in contact with water vapor and the test atmosphere. The experiments to evaluate the apparent quantum yield of CO of RO-1, 2, and 3 were measured under monochromatic light (380 nm).
Isotopic Labeling Control Experiments
In isotope experiments, 13CO2 was employed to confirm the carbon source of CO formed by photocatalytic CO2 reduction and H218O was employed to confirm the oxygen source of product O2. The 13CO and 18O2 were analyzed using gas chromatography mass spectrometry (GC-MS) (Figures 3f, S35, and S36).
Electrochemical Experiments
The electrochemical experiments (the Mott–Schottky plots and transient photocurrent response) are tested in a standard three-electrode system: indium–tin oxide conductive film glass (ITO glass, 1 cm × 2 cm) modified with catalyst samples, a carbon rod, and a Ag/AgCl electrode was employed as the working electrode, counter electrode, and the reference electrode, respectively. The Mott–Schottky plots were measured over an alternating current (AC) frequency of 1000, 1500, and 2000 Hz. The electrochemical impedance spectroscopy (EIS) plots were performed under similar conditions except using carbon cloth as the work electrode. The same bias voltage was set when EIS plots of RO-1, 2, and 3 were performed. All of the electrochemical tests were performed in a 0.2 M Na2SO4 aqueous solution. PL spectra and were recorded by a FluoroMax-4 spectrofluorometer (Horiba Scientific).
Sample Preparation of ITO Glass
The as-synthesized crystal (2 mg) was ground to a powder and then dispersed in 1 mL of solvent (990 μL ethanol and 10 μL 0.5% Nafion) by ultrasonication to form a homogeneous ink. Subsequently, 200 μL of the ink was dropped onto both sides of the ITO glass and dried at room temperature.
Density Functional Theory (DFT)
Periodic Calculations
The structure optimization and free energy calculations were performed within the framework of DFT as implemented in the Vienna Ab initio software package (VASP 5.3.5) code within the Perdew–Burke–Ernzerhof (PBE) generalized gradient approximation and the projected augmented wave (PAW) method.
Model Cluster Calculations
To perform time-dependent density-functional theory (TDDFT), a model cluster is constructed based on the optimized structure from periodic calculations. The cluster calculations are done by the ORCA package employing the resolution of identity approximation.
For detailed settings and results, please see the Supporting Information.
Acknowledgments
This work was financially supported by the NSFC (nos. 21622104, 92061101, 21871141, 21871142, and 21901122); the Natural Science Research of Jiangsu Higher Education Institutions of China (19KJB150011), and a project funded by China Postdoctoral Science Foundation (nos. 2018M630572 and 2019M651873); Priority Academic Program Development of Jiangsu Higher Education Institutions and the Foundation of Jiangsu Collaborative Innovation Center of Biomedical Functional Materials; The East-West Cooperation Project of Ningxia Key R & D Plan (no. 2019BFH02014); and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX20_1171).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.1c00186.
Author Contributions
⊥ X.-X.L. and L.Z. contributed equally to this work. Y.-Q.L., J.L., K.H., and X.-X.L. conceived and designed the idea. X.-X.L. synthesized the photocatalysts. L.Z. and X.-X.L. conducted the characterizations and designed the photocatalytic related experiments. L.Y., T.W., and J.-Y.W. assisted with the characterizations, designed the CO2RR related experiments, collected the data. L.Z. and X.-X.L. analyzed the data. L.-Z.D. assisted with dealing with the data of SCXRD. Y.-Q.L., J.L., and X.-X.L. discussed the results and prepared the manuscript. All the authors reviewed and contributed to this paper.
The authors declare no competing financial interest.
Supplementary Material
References
- Zhang T.; Lin W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. 10.1039/C4CS00103F. [DOI] [PubMed] [Google Scholar]
- Fang X.; Kalathil S.; Reisner E. Semi-biological approaches to solar-to-chemical conversion. Chem. Soc. Rev. 2020, 49, 4926–4952. 10.1039/C9CS00496C. [DOI] [PubMed] [Google Scholar]
- Yu L.; Ba X.; Qiu M.; Li Y.; Shuai L.; Zhang W.; Ren Z.; Yu Y. Visible-light driven CO2 reduction coupled with water oxidation on Cl-doped Cu2O nanorods. Nano Energy 2019, 60, 576–582. 10.1016/j.nanoen.2019.03.083. [DOI] [Google Scholar]
- Zhang M.; Lu M.; Lang Z.-L.; Liu J.; Liu M.; Chang J.-N.; Li L.-Y.; Shang L.-J.; Wang M.; Li S.-L.; Lan Y.-Q. Semiconductor/Covalent-Organic-Framework Z-Scheme Heterojunctions for Artificial Photosynthesis. Angew. Chem., Int. Ed. 2020, 59, 6500–6506. 10.1002/anie.202000929. [DOI] [PubMed] [Google Scholar]
- Kim W.; McClure B. A.; Edri E.; Frei H. Coupling carbon dioxide reduction with water oxidation in nanoscale photocatalytic assemblies. Chem. Soc. Rev. 2016, 45, 3221–3243. 10.1039/C6CS00062B. [DOI] [PubMed] [Google Scholar]
- Hunter B. M.; Gray H. B.; Müller A. M. Earth-Abundant Heterogeneous Water Oxidation Catalysts. Chem. Rev. 2016, 116, 14120–14136. 10.1021/acs.chemrev.6b00398. [DOI] [PubMed] [Google Scholar]
- Zhao G.; Huang X.; Wang X.; Wang X. Progress in catalyst exploration for heterogeneous CO2 reduction and utilization: a critical review. J. Mater. Chem. A 2017, 5, 21625–21649. 10.1039/C7TA07290B. [DOI] [Google Scholar]
- Sakimoto K. K.; Wong A. B.; Yang P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 2016, 351, 74–77. 10.1126/science.aad3317. [DOI] [PubMed] [Google Scholar]
- Woolerton T.; Sheard S.; Pierce E.; Ragsdale S.; Armstrong F. CO2 photoreduction at enzyme-modified metal oxide nanoparticles. Energy Environ. Sci. 2011, 4, 2393–2399. 10.1039/c0ee00780c. [DOI] [Google Scholar]
- Wang Y.; Zhang Z.; Zhang L.; Luo Z.; Shen J.; Lin H.; Long J.; Wu J. C. S.; Fu X.; Wang X.; Li C. Visible-Light Driven Overall Conversion of CO2 and H2O to CH4 and O2 on 3D-SiC@2D-MoS2 Heterostructure. J. Am. Chem. Soc. 2018, 140, 14595–14598. 10.1021/jacs.8b09344. [DOI] [PubMed] [Google Scholar]
- Sasan K.; Lin Q.; Mao C.; Feng P. Open framework metal chalcogenides as efficient photocatalysts for reduction of CO2 into renewable hydrocarbon fuel. Nanoscale 2016, 8, 10913–10916. 10.1039/C6NR02525K. [DOI] [PubMed] [Google Scholar]
- Xiong X.; Mao C.; Yang Z.; Zhang Q.; Waterhouse G. I. N.; Gu L.; Zhang T. Photocatalytic CO2 Reduction to CO over Ni Single Atoms Supported on Defect-Rich Zirconia. Adv. Energy Mater. 2020, 10, 2002928. 10.1002/aenm.202002928. [DOI] [Google Scholar]
- Liang L.; Li X.; Sun Y.; Tan Y.; Jiao X.; Ju H.; Qi Z.; Zhu J.; Xie Y. Infrared Light-Driven CO2 Overall Splitting at Room Temperature. Joule 2018, 2, 1004–1016. 10.1016/j.joule.2018.02.019. [DOI] [Google Scholar]
- Jiang H.; Katsumata K.-i.; Hong J.; Yamaguchi A.; Nakata K.; Terashima C.; Matsushita N.; Miyauchi M.; Fujishima A. Photocatalytic reduction of CO2 on Cu2O-loaded Zn-Cr layered double hydroxides. Appl. Catal., B 2018, 224, 783–790. 10.1016/j.apcatb.2017.11.011. [DOI] [Google Scholar]
- Yu F.; Wang C.; Li Y.; Ma H.; Wang R.; Liu Y.; Suzuki N.; Terashima C.; Ohtani B.; Ochiai T.; Fujishima A.; Zhang X. Photothermal Catalysis: Enhanced Solar Photothermal Catalysis over Solution Plasma Activated TiO2. Adv. Sci. 2020, 7, 2070092. 10.1002/advs.202070092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.; Li D.; Han Y.; Jiang H.-L. Photocatalytic Molecular Oxygen Activation by Regulating Excitonic Effects in Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 20763–20771. 10.1021/jacs.0c09727. [DOI] [PubMed] [Google Scholar]
- Zhong W.; Sa R.; Li L.; He Y.; Li L.; Bi J.; Zhuang Z.; Yu Y.; Zou Z. A Covalent Organic Framework Bearing Single Ni Sites as a Synergistic Photocatalyst for Selective Photoreduction of CO2 to CO. J. Am. Chem. Soc. 2019, 141, 7615–7621. 10.1021/jacs.9b02997. [DOI] [PubMed] [Google Scholar]
- Yang S.; Hu W.; Zhang X.; He P.; Pattengale B.; Liu C.; Cendejas M.; Hermans I.; Zhang X.; Zhang J.; Huang J. 2D Covalent Organic Frameworks as Intrinsic Photocatalysts for Visible Light-Driven CO2 Reduction. J. Am. Chem. Soc. 2018, 140, 14614–14618. 10.1021/jacs.8b09705. [DOI] [PubMed] [Google Scholar]
- Fang Z.-B.; Liu T.-T.; Liu J.; Jin S.; Wu X.-P.; Gong X.-Q.; Wang K.; Yin Q.; Liu T.-F.; Cao R.; Zhou H.-C. Boosting Interfacial Charge-Transfer Kinetics for Efficient Overall CO2 Photoreduction via Rational Design of Coordination Spheres on Metal–Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 12515–12523. 10.1021/jacs.0c05530. [DOI] [PubMed] [Google Scholar]
- Jiang Z.; Xu X.; Ma Y.; Cho H. S.; Ding D.; Wang C.; Wu J.; Oleynikov P.; Jia M.; Cheng J.; Zhou Y.; Terasaki O.; Peng T.; Zan L.; Deng H. Filling metal–organic framework mesopores with TiO2 for CO2 photoreduction. Nature 2020, 586, 549–554. 10.1038/s41586-020-2738-2. [DOI] [PubMed] [Google Scholar]
- Rao H.; Schmidt L. C.; Bonin J.; Robert M. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nature 2017, 548, 74–77. 10.1038/nature23016. [DOI] [PubMed] [Google Scholar]
- Huang Y.-B.; Liang J.; Wang X.-S.; Cao R. Multifunctional metal–organic framework catalysts: synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. 10.1039/C6CS00250A. [DOI] [PubMed] [Google Scholar]
- Zhang W.-X.; Liao P.-Q.; Lin R.-B.; Wei Y.-S.; Zeng M.-H.; Chen X.-M. Metal cluster-based functional porous coordination polymers. Coord. Chem. Rev. 2015, 293–294, 263–278. 10.1016/j.ccr.2014.12.009. [DOI] [Google Scholar]
- Zeng L.; Wang Z.; Wang Y.; Wang J.; Guo Y.; Hu H.; He X.; Wang C.; Lin W. Photoactivation of Cu Centers in Metal–Organic Frameworks for Selective CO2 Conversion to Ethanol. J. Am. Chem. Soc. 2020, 142, 75–79. 10.1021/jacs.9b11443. [DOI] [PubMed] [Google Scholar]
- Dong L.-Z.; Zhang L.; Liu J.; Huang Q.; Lu M.; Ji W.-X.; Lan Y.-Q. Stable Heterometallic Cluster-Based Organic Framework Catalysts for Artificial Photosynthesis. Angew. Chem., Int. Ed. 2020, 59, 2659–2663. 10.1002/anie.201913284. [DOI] [PubMed] [Google Scholar]
- Li N.; Liu J.; Liu J.-J.; Dong L.-Z.; Li S.-L.; Dong B.-X.; Kan Y.-H.; Lan Y.-Q. Self-Assembly of a Phosphate-Centered Polyoxo-Titanium Cluster: Discovery of the Heteroatom Keggin Family. Angew. Chem., Int. Ed. 2019, 58, 17260–17264. 10.1002/anie.201910491. [DOI] [PubMed] [Google Scholar]
- Benseghir Y.; Lemarchand A.; Duguet M.; Mialane P.; Gomez-Mingot M.; Roch-Marchal C.; Pino T.; Ha-Thi M.-H.; Haouas M.; Fontecave M.; Dolbecq A.; Sassoye C.; Mellot-Draznieks C. Co-immobilization of a Rh Catalyst and a Keggin Polyoxometalate in the UiO-67 Zr-Based Metal–Organic Framework: In Depth Structural Characterization and Photocatalytic Properties for CO2 Reduction. J. Am. Chem. Soc. 2020, 142, 9428–9438. 10.1021/jacs.0c02425. [DOI] [PubMed] [Google Scholar]
- Saito D.; Yamazaki Y.; Tamaki Y.; Ishitani O. Photocatalysis of a Dinuclear Ru(II)–Re(I) Complex for CO2 Reduction on a Solid Surface. J. Am. Chem. Soc. 2020, 142, 19249–19258. 10.1021/jacs.0c09170. [DOI] [PubMed] [Google Scholar]
- Zhang H.-X.; Hong Q.-L.; Li J.; Wang F.; Huang X.; Chen S.; Tu W.; Yu D.; Xu R.; Zhou T.; Zhang J. Isolated Square-Planar Copper Center in Boron Imidazolate Nanocages for Photocatalytic Reduction of CO2 to CO. Angew. Chem., Int. Ed. 2019, 58, 11752–11756. 10.1002/anie.201905869. [DOI] [PubMed] [Google Scholar]
- Li N.; Liu J.-J.; Sun J.-W.; Dong B.-X.; Dong L.-Z.; Yao S.-J.; Xin Z.; Li S.-L.; Lan Y.-Q. Calix[8]arene-constructed stable polyoxo-titanium clusters for efficient CO2 photoreduction. Green Chem. 2020, 22, 5325–5332. 10.1039/D0GC01497D. [DOI] [Google Scholar]
- Martin-Sabi M.; Soriano-López J.; Winter R. S.; Chen J.-J.; Vilà-Nadal L.; Long D.-L.; Galán-Mascarós J. R.; Cronin L. Redox tuning the Weakley-type polyoxometalate archetype for the oxygen evolution reaction. Nat. Catal. 2018, 1, 208–213. 10.1038/s41929-018-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H.; Geletii Y. V.; Zhao C.; Vickers J. W.; Zhu G.; Luo Z.; Song J.; Lian T.; Musaev D. G.; Hill C. L. Polyoxometalate water oxidation catalysts and the production of green fuel. Chem. Soc. Rev. 2012, 41, 7572–7589. 10.1039/c2cs35292c. [DOI] [PubMed] [Google Scholar]
- Wang S.-S.; Yang G.-Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. 10.1021/cr500390v. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Liu J.; Li S.-L.; Su Z.-M.; Lan Y.-Q. Polyoxometalate-based materials for sustainable and clean energy conversion and storage. EnergyChem. 2019, 1, 100021. 10.1016/j.enchem.2019.100021. [DOI] [Google Scholar]
- Jin L.; Li X. X.; Qi Y. J.; Niu P. P.; Zheng S. T. Giant Hollow Heterometallic Polyoxoniobates with Sodalite-Type Lanthanide-Tungsten-Oxide Cages: Discrete Nanoclusters and Extended Frameworks. Angew. Chem., Int. Ed. 2016, 55, 13793–13797. 10.1002/anie.201608113. [DOI] [PubMed] [Google Scholar]
- Xie S. L.; Liu J.; Dong L. Z.; Li S. L.; Lan Y. Q.; Su Z. M. Hetero-metallic active sites coupled with strongly reductive polyoxometalate for selective photocatalytic CO2-to-CH4 conversion in water. Chem. Sci. 2019, 10, 185–190. 10.1039/C8SC03471K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-B.; Leng J.-D.; Wang Z.-Z.; Chen Y.-C.; Miao Y.; Tong M.-L.; Dong W. Reversible crystal-to-crystal transformation from a trinuclear cluster to a 1D chain and the corresponding spin crossover (SCO) behaviour change. Chem. Commun. 2017, 53, 7820–7823. 10.1039/C7CC04036A. [DOI] [PubMed] [Google Scholar]
- Ding B.; Yang E.-C.; Zhao X.-J.; Wang X. G. A linear trinuclear cobalt(II) complex with 4-(2-pyridine)-1,2,4-triazole: synthesis, structure and characterization. J. Coord. Chem. 2008, 61, 3793–3799. 10.1080/00958970802120184. [DOI] [Google Scholar]
- Gómez V.; Sáenz de Pipaón C.; Maldonado-Illescas P.; Waerenborgh J. C.; Martin E.; Benet-Buchholz J.; Galán-Mascarós J. R. Easy Excited-State Trapping and Record High TTIESST in a Spin-Crossover Polyanionic FeII Trimer. J. Am. Chem. Soc. 2015, 137, 11924–11927. 10.1021/jacs.5b07879. [DOI] [PubMed] [Google Scholar]
- Li X.-M.; Dong L.-Z.; Liu J.; Ji W.-X.; Li S.-L.; Lan Y.-Q. Intermediate-Temperature Anhydrous High Proton Conductivity Triggered by Dynamic Molecular Migration in Trinuclear Cluster Lattice. Chem. 2020, 6, 2272–2282. 10.1016/j.chempr.2020.06.007. [DOI] [Google Scholar]
- Yang C.; Zhu Q.; Sadakane M.; Zhang Z.; Li Y.; Ueda W. Vanadium-Enhanced Intramolecular Redox Property of a Transition-Metal Oxide Molecular Wire. Inorg. Chem. 2020, 59, 16557–16566. 10.1021/acs.inorgchem.0c02485. [DOI] [PubMed] [Google Scholar]
- Weinstock I. A.; Schreiber R. E.; Neumann R. Dioxygen in Polyoxometalate Mediated Reactions. Chem. Rev. 2018, 118, 2680–2717. 10.1021/acs.chemrev.7b00444. [DOI] [PubMed] [Google Scholar]
- Liu J.; Liu Y.; Liu N.; Han Y.; Zhang X.; Huang H.; Lifshitz Y.; Lee S.-T.; Zhong J.; Kang Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. 10.1126/science.aaa3145. [DOI] [PubMed] [Google Scholar]
- Hou W.; Hung W. H.; Pavaskar P.; Goeppert A.; Aykol M.; Cronin S. B. Photocatalytic Conversion of CO2 to Hydrocarbon Fuels via Plasmon-Enhanced Absorption and Metallic Interband Transitions. ACS Catal. 2011, 1, 929–936. 10.1021/cs2001434. [DOI] [Google Scholar]
- Xiang Q.; Cheng B.; Yu J. Graphene-Based Photocatalysts for Solar-Fuel Generation. Angew. Chem., Int. Ed. 2015, 54, 11350–11366. 10.1002/anie.201411096. [DOI] [PubMed] [Google Scholar]
- Li R.; Zhang W.; Zhou K. Metal–Organic-Framework-Based Catalysts for Photoreduction of CO2. Adv. Mater. 2018, 30, 1705512. 10.1002/adma.201705512. [DOI] [PubMed] [Google Scholar]
- Men Y.-L.; You Y.; Pan Y.-X.; Gao H.; Xia Y.; Cheng D.-G.; Song J.; Cui D.-X.; Wu N.; Li Y.; Xin S.; Goodenough J. B. Selective CO Evolution from Photoreduction of CO2 on a Metal-Carbide-Based Composite Catalyst. J. Am. Chem. Soc. 2018, 140, 13071–13077. 10.1021/jacs.8b08552. [DOI] [PubMed] [Google Scholar]
- Xu H.-Q.; Hu J.; Wang D.; Li Z.; Zhang Q.; Luo Y.; Yu S.-H.; Jiang H.-L. Visible-Light Photoreduction of CO2 in a Metal–Organic Framework: Boosting Electron–Hole Separation via Electron Trap States. J. Am. Chem. Soc. 2015, 137, 13440–13443. 10.1021/jacs.5b08773. [DOI] [PubMed] [Google Scholar]
- Li Y.; Wang E.; Wang S.; Duan Y.; Hu C.; Hu N.; Jia H. A highly reduced polyoxoanion with phosphorus-centered alternate layers of Mo/V oxides, [PMo2VMo6VIV4IVO40(VIVO)2]9–. J. Mol. Struct. 2002, 611, 185–191. 10.1016/S0022-2860(02)00072-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


