Abstract
Aims
GLP-1RA has many beneficial properties, including anti-inflammatory, anti-obesogenic, pulmonary protective effects as well as beneficial impact on gut microbiome. However, the evidence regarding the benefit of GLP-1RA in Covid-19 patients with diabetes is still unclear. This study sought to analyze the benefit of pre-admission use of GLP-1RA in altering the mortality outcomes of coronavirus disease 2019 (Covid-19) patients with diabetes mellitus.
Methods
Using specific keywords, we comprehensively searched the potential articles on PubMed, Europe PMC, and medRxiv database until June 12th, 2021. All published studies on Covid-19 and GLP-1RA were retrieved. Statistical analysis was conducted using Review Manager 5.4 and Comprehensive Meta-Analysis version 3 software.
Results
A total of 9 studies with 19,660 diabetes mellitus patients who were infected by SARS-CoV-2 were included in the meta-analysis. Our data suggested that pre-admission use of GLP-1RA was associated with reduction in mortality rate from Covid-19 in patients with diabetes mellitus (OR 0.53; 95 %CI: 0.43–0.66, p < 0.00001, I2 = 0%, random-effect modelling). Further analysis showed that the associations were not influenced by age (p = 0.213), gender (p = 0.421), hypertension (p = 0.131), cardiovascular disease (p = 0.293), nor the use of metformin (p = 0.189) and insulin (p = 0.117).
Conclusions
Our study suggests that pre-admission use of GLP-1RA may offer beneficial effects on Covid-19 mortality in patients with diabetes mellitus. However, more randomized clinical trials are required to confirm this conclusion.
Keywords: Coronavirus disease 2019, Covid-19, Diabetes, GLP-1RA, Anti-diabetic
1. Introduction
The emergence of Covid-19 disease since the first case on December 2019 had caused up to 177,108,695 confirmed cases, including 3,840,223 deaths per June 2021 according to WHO.[1] Cases’ severity varied among all age populations depending on their comorbidities. Diabetes mellitus and obesity were found to be two of important risk factors which may contribute to the development of severe form of Covid-19, causing further inflammation and immune dysfunction that leads to the formation of cytokines storm which is life threatening.[2], [3], [4], [5], [6], [7] Several therapies had been proposed to control and manage these conditions.[8], [9], [10], [11], [12], [13], [14] Recently the use of GLP1-RA, as one alternative to treat DM patients, had shown promising effect to reduce excessive inflammation-induced acute lung injury and improving Covid-19 outcome.[15] In addition to stimulating postprandial insulin secretion, GLP1-RA also seems to have beneficial properties such anti-inflammatory, anti-obesogenic, pulmonary protective effects and gut microbiome modulating effects.[16] Furthermore, an experimental study on rats showed that the use of GLP1-RA (liraglutide) was capable to stimulate pulmonary ACE2 expression, an enzyme that has been demonstrated to oppose the pathway that is responsible for the progression of acute respiratory distress syndrome (ARDS), including the one caused by SARS-CoV-2 infection.[17], [18] However, the evidences regarding benefit of GLP-1RA in Covid-19 patients are still unclear. Therefore, the objective of this study is to evaluate and analyze the association between GLP1-RA and Covid-19 mortality.
2. Materials and methods
2.1. Eligibility criteria
The protocol of this study was registered in PROSPERO (CRD42021260638). The articles analyzed in this study has qualified the following entry criteria: fulfill the PICO framework (P: patients with diabetes mellitus who were infected with SARS-CoV-2; I: pre-admission use of GLP-1RA; C: the use of any other anti-diabetic drugs besides GLP-1RA prior to admission into the hospital; O: mortality from Covid-19), cohort, case-control, cross-sectional, and randomized or non-randomized clinical trial studies were included. All studies aside from original research articles (review articles, letter to editor or correspondence), case-series or case report studies, studies presented in any other language besides English, articles focusing on people below 18 years of age and pregnant women were excluded.
2.2. Search strategy and study selection
The articles from three databases (PubMed, Europe PMC, and medRxiv) were searched systemically. Search terms used include “GLP-1” OR “GLP-1RA” OR “glucagon-like peptide-1” OR “dulaglutide” OR “semaglutide” OR “exenatide” OR “liraglutide” AND “diabetes” OR “diabetes mellitus” AND “SARS-CoV-2”, OR “coronavirus disease 2019” OR “Covid-19” in a time range from 2019 until June 12, 2021 with English-language restriction. Our searching strategy details are listed in Table 1 . Initial screening of titles and abstracts was conducted to identify eligible articles. Searches of potential articles were also done by analyzing the list of references of eligible studies. The strategy for searching the article was presented in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram.
Table 1.
Literature search strategy.
| Database | Keyword | Result |
|---|---|---|
| PubMed | (“glucagon-like peptide 1”[MeSH Terms] OR ”glucagon-like peptide 1”[All Fields] OR “glp 1”[All Fields]) OR GLP-1RA[All Fields] OR (”dulaglutide“[Supplementary Concept] OR ”dulaglutide“[All Fields]) OR (”semaglutide“[Supplementary Concept] OR ”semaglutide“[All Fields]) OR (”exenatide“[MeSH Terms] OR ”exenatide“[All Fields]) OR (”liraglutide“[MeSH Terms] OR ”liraglutide“[All Fields]) AND (”diabetes mellitus“[MeSH Terms] OR (”diabetes“[All Fields] AND ”mellitus“[All Fields]) OR ”diabetes mellitus“[All Fields] OR ”diabetes“[All Fields] OR ”diabetes insipidus“[MeSH Terms] OR (”diabetes“[All Fields] AND ”insipidus“[All Fields]) OR ”diabetes insipidus“[All Fields]) AND (”COVID-19”[All Fields] OR “COVID-19”[MeSH Terms] OR ”COVID-19 Vaccines“[All Fields] OR ”COVID-19 Vaccines“[MeSH Terms] OR ”COVID-19 serotherapy“[All Fields] OR ”COVID-19 Nucleic Acid Testing“[All Fields] OR ”covid-19 nucleic acid testing“[MeSH Terms] OR ”COVID-19 Serological Testing“[All Fields] OR ”covid-19 serological testing“[MeSH Terms] OR ”COVID-19 Testing“[All Fields] OR ”covid-19 testing“[MeSH Terms] OR ”SARS-CoV-2”[All Fields] OR “sars-cov-2”[MeSH Terms] OR ”Severe Acute Respiratory Syndrome Coronavirus 2”[All Fields] OR “NCOV”[All Fields] OR “2019 NCOV”[All Fields] OR ((“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields] OR “COV”[All Fields]) AND 2019/11/01[PubDate]: 3000/12/31[PubDate])) | 25 |
| Europe PMC | “GLP-1” OR “GLP-1RA” OR “glucagon-like peptide-1” OR “dulaglutide” OR “semaglutide” OR “exenatide” OR “liraglutide” AND “diabetes” OR “diabetes mellitus” AND “SARS-CoV-2”, OR “coronavirus disease 2019” OR “COVID-19” | 156 |
| medRxiv | “GLP-1” OR “GLP-1RA” OR “glucagon-like peptide-1” OR “dulaglutide” OR “semaglutide” OR “exenatide” OR “liraglutide” AND “diabetes” OR “diabetes mellitus” AND “SARS-CoV-2”, OR “coronavirus disease 2019” OR “COVID-19” | 19 |
2.3. Data extraction and quality assessment
Two researchers (TIH, DI) performed the data extraction. Every essential information about the study, including the characteristic of the population (age, gender, hypertension, cardiovascular disease, metformin and insulin usage/consumption), the number of patients who use GLP-1RA, as well as the mortality in Covid-19 patients were listed and arranged in one form.
Mortality from COVID-19, defined by the total number of patients who died during the follow-up period with positive Covid-19 status, will be the outcome of interest.
Two researchers (JEH, CP) assessed the quality of each study included in this study independently. The case-control and cohort studies’ quality were evaluated by using Newcastle–Ottawa Scale (NOS). The assessment reviews the selection, comparability, and outcome of each study, then each study was assigned a total score from zero to nine. A score of ≥7 is considered a good quality study.[19]
2.4. Statistical analysis
Review Manager 5.4 (Cochrane Collaboration) software and Comprehensive Meta-Analysis version 3 were used to perform the meta-analysis and meta-regression. Generic-Inverse Variance formula was utilized to calculate the odds ratio (OR) and its 95% confidence interval (95 %CI) for the mortality outcome. The heterogeneity was assessed by using the I2 statistic with a value of <25%, 26–50%, and >50% were considered as low, moderate, and high degrees of heterogeneity, respectively. Meta-regression with random-effects model was performed using a restricted-maximum likelihood for pre-specified variables including age, gender, hypertension, cardiovascular disease, and the use of metformin. Funnel plot analysis was used to evaluate the qualitative risk of publication bias, while the quantitative risk was assessed by using the Egger’s regression method.[20]
3. Results
3.1. Study selection and characteristics
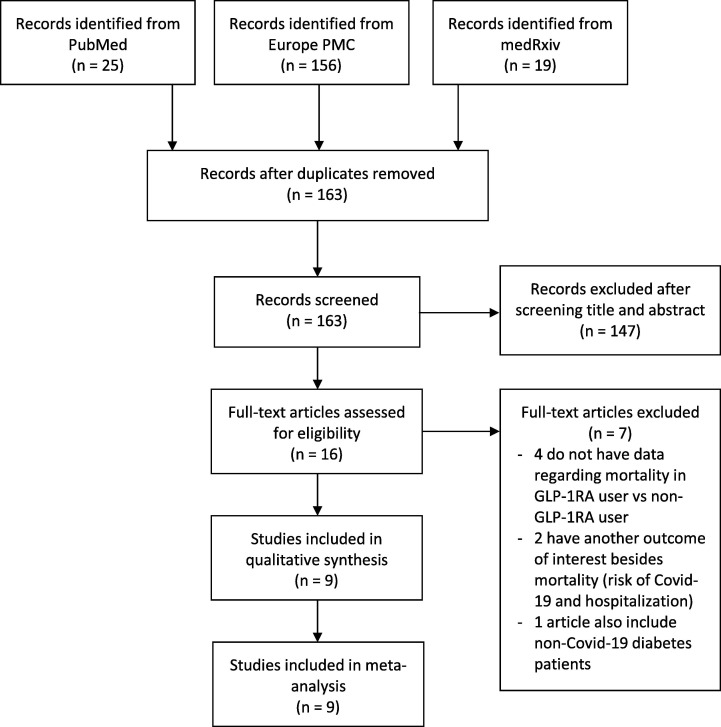
The searches on the databases yielded 195 studies. Through eliminating duplicates articles, a total of 163 records remained to be further assessed. Another 147 studies were removed after screening through the titles and abstracts while matching the inclusion and exclusion criteria. Further evaluation for its eligibility on the remaining 16 full-text articles was completed. A total of 7 articles were excluded in which four articles had no data regarding the mortality in GLP-1RA user compared with non GLP-1RA user, another two have another outcome of interest besides mortality (risk of Covid-19 and hospitalization), and lastly one article also include non-Covid-19 diabetes patients. The details regarding excluded articles can be seen in Appendix A. As a result, 9 studies were included in this meta-analysis[21], [22], [23], [24], [25], [26], [27], [28], [29] with an overall of 19,660 Covid-19 patients with diabetes mellitus (Fig. 1 ). All 9 studies were retrospective cohort studies, and the characteristics of each studies were shown in Table 2 .
Fig. 1.
PRISMA diagram of the detailed process of selection of studies for inclusion in the systematic review and meta-analysis.
Table 2.
Characteristics of included studies.
| Study | Sample size | Design | Country | Overall age mean ± SD | Male n (%) | Hypertension n (%) | Cardiovascular disease n (%) | Metformin use n (%) | Insulin use | GLP-1RA use n (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Death | Alive | ||||||||||
| Cariou B et al.[21] 2020 | 1317 | Retrospective cohort | France | 69.8 ± 13 | 855 (64.9%) | 1003 (77.2%) | 621 (47.1%) | 746 (56.6%) | 504 (38.3%) | 9 (6.4%) | 114 (9.7%) |
| Israelsen SB et al.[22] 2021 | 996 | Retrospective cohort | Denmark | 60 ± 14 | 521 (52.3%) | N/A | N/A | 645 (64.7%) | 224 (24.8%) | 14 (26.4%) | 356 (42%) |
| Izzi-Engbeaya C et al.[23] 2021 | 337 | Retrospective cohort | England | 65.8 ± 17.5 | 202 (60%) | 238 (70.6%) | 91 (27%) | 169 (50.1%) | 108 (31%) | No data on death/alive Total = 5 (1.4%) | |
| Nyland JE et al.[24] 2020 | 12,954 | Retrospective cohort | USA | 62.2 ± 15.2 | 6244 (48.2%) | 5881 (45.4%) | 3420 (26.4%) | 6192 (47.8%) | 7435 (57.4%) | 32 (2.9%) | 797 (6.7%) |
| Orioli L et al.[25] 2021 | 73 | Retrospective cohort | Belgium | 69 ± 14 | 35 (48%) | 59 (80.8%) | 32 (43.8%) | 45 (61.6%) | 31 (45.6%) | 0 (0%) | 5 (8.8%) |
| Ramos-Rincon JM et al.[26] 2021 | 790 | Retrospective cohort | Spain | 85.8 ± 4.5 | 418 (52.9%) | 666 (84.3%) | 625 (79.1%) | 420 (53.1%) | 211 (26.7%) | 11 (2.9%) | 13 (3.3%) |
| Silverii GA et al.[27] 2021 | 159 | Retrospective cohort | Italy | 73.3 ± 12.6 | 86 (54.1%) | N/A | N/A | 76 (47.8%) | 43 (27%) | 1 (1.7%) | 6 (6%) |
| Sourij H et al.[28] 2020 | 238 | Retrospective cohort | Austria | 71.1 ± 12.9 | 152 (63.9%) | 169 (71%) | 160 (67.2%) | 77 (32.3%) | 52 (21.9%) | 0 (0%) | 3 (1.7%) |
| Wargny M et al.[29] 2021 | 2796 | Retrospective cohort | France | 69.7 ± 13.2 | 1782 (63.7%) | 2126 (76.8%) | 302 (11.4%) | 1553 (55.6%) | 1039 (37.2%) | 33 (5.7%) | 221 (10%) |
3.2. Quality of study assessment
Quality assessment of cohort and case-control studies using NOS scale indicated all included studies had a good quality (Table 3 ). Altogether, all studies were acceptable to be further analyzed using meta-analysis.
Table 3.
Newcastle-Ottawa quality assessment of observational studies.
| First author, year | Study design | Selection | Comparability | Outcome | Total score | Result |
|---|---|---|---|---|---|---|
| Cariou B et al.[21] 2020 | Cohort | *** | ** | *** | 8 | Good |
| Israelsen SB et al.[22] 2021 | Cohort | *** | ** | ** | 7 | Good |
| Izzi-Engbeaya C et al.[23] 2021 | Cohort | *** | ** | *** | 8 | Good |
| Nyland JE et al.[24] 2020 | Cohort | ** | ** | *** | 7 | Good |
| Orioli L et al.[25] 2021 | Cohort | *** | ** | *** | 8 | Good |
| Ramos-Rincon JM et al.[26] 2021 | Cohort | *** | ** | *** | 8 | Good |
| Silverii GA et al.[27] 2021 | Cohort | ** | ** | *** | 7 | Good |
| Sourij H et al.[28] 2020 | Cohort | *** | ** | *** | 8 | Good |
| Wargny M et al.[29] 2021 | Cohort | *** | ** | *** | 8 | Good |
3.3. GLP-1RA and mortality outcome
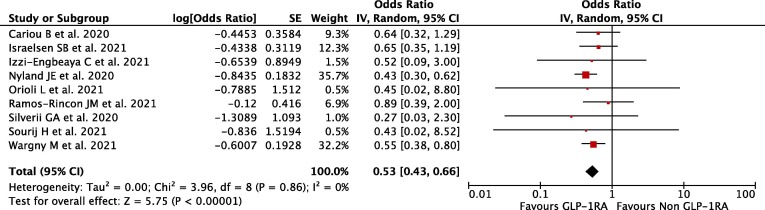
Our pooled analysis showed that pre-admission use of GLP-1RA in diabetes mellitus patients was associated with reduction of mortality rate from Covid-19, with no relevant heterogeneity (OR 0.53; 95 %CI: 0.43–0.66, p < 0.00001, I 2 = 0%, random-effect modelling) (Fig. 2 ).
Fig. 2.
Forest plot that demonstrates the association of pre-admission GLP-1RA use with mortality outcome.
3.4. Meta regression
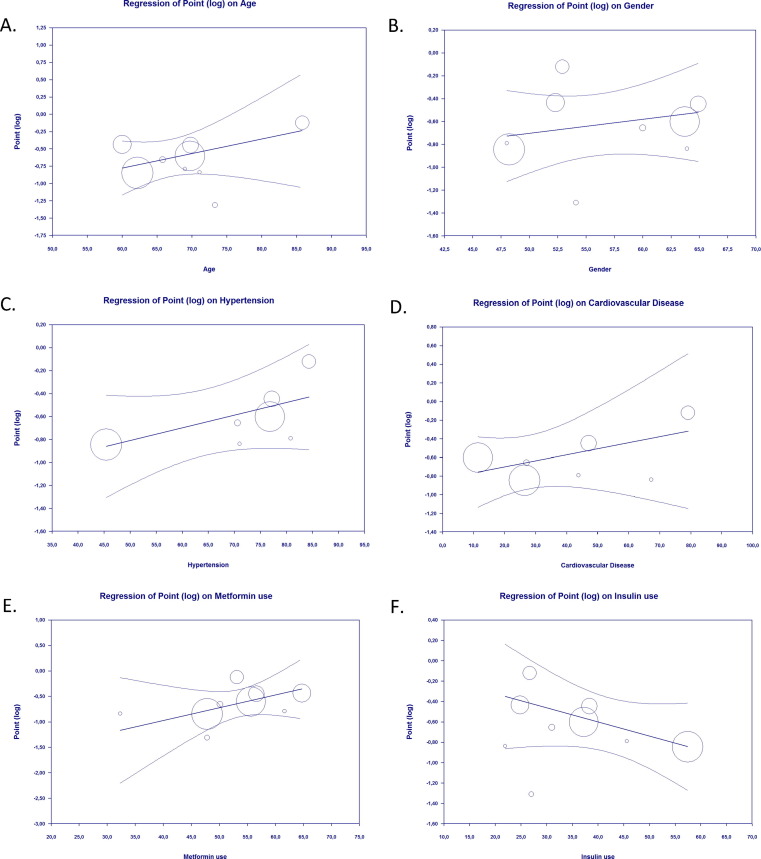
Our meta-regression suggested the association between pre-admission use of GLP-1RA and mortality from Covid-19 was not affected by age (p = 0.213) (Fig. 3 A), gender (p = 0.421) (Fig. 3B), hypertension (p = 0.131) (Fig. 3C), cardiovascular disease (p = 0.293) (Fig. 3D), nor the use of metformin (p = 0.189) (Fig. 3E) and the use of insulin (p = 0.117) (Fig. 3F).
Fig. 3.
Bubble-plot for Meta-regression. Meta-regression analysis showed that the association between pre-admission GLP-1RA use and mortality outcome was not affected by age (A), gender (B), hypertension (C), cardiovascular disease (D), the use of metformin (E), and the use of insulin (F).
3.5. Publication bias
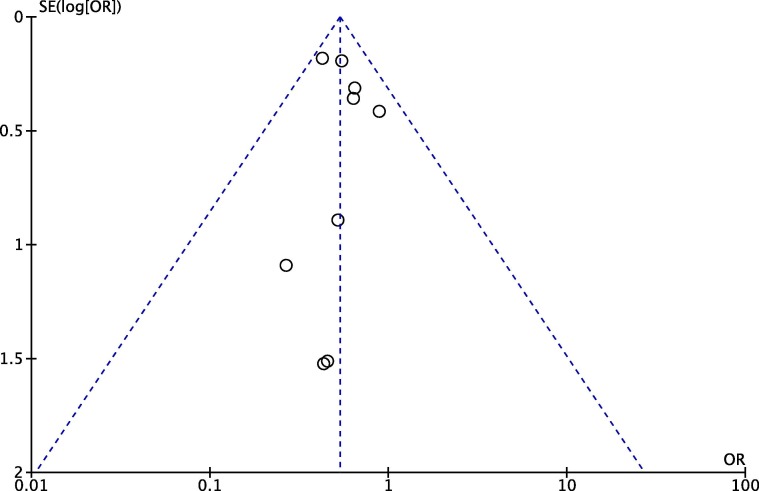
Funnel plot analysis showed a relatively symmetrical inverted-plot (Fig. 4 ), while Egger’s regression method also showed non-significant result (p = 0.737) for the association between pre-admission GLP-1RA use and mortality outcome, showing no indication of publication bias.
Fig. 4.
Funnel plot analysis for the association of pre-admission GLP-1RA use with mortality outcome.
4. Discussion
Our analysis shows that pre-admission use of GLP-1RA is associated with reduced mortality in COVID-19 patients with diabetes mellitus. This association was not affected by age, gender, hypertension, cardiovascular disease, nor the use of metformin and insulin.
There are some explanations how GLP-1RA could affect the prognosis of Covid-19 patients. Glucagon-like peptide-1 (GLP-1), an incretin hormone, is responsible for facilitating postprandial insulin secretion. GLP-1-based drugs work on GLP-1 receptors (GLP-1R) that are primarily located on epithelial of the lung and immune cells. In COVID-19 patients, with or without DM, GLP-1RA have been considered to have several mechanisms that may improve the outcome of the disease. Besides their glucose-lowering effects, they serve multiple benefits on controlling lung injury caused by inflammation through pulmonary protective effects, anti-obesogenic properties, and modulation of gut microbiota.[30]
Frequent fluctuations of uncontrolled blood glucose level may build up oxidative stress, causing further production of inflammatory cytokines and dysregulation of the innate and adaptive immunity.[31] Furthermore, there has been a speculation that SARS-CoV-2 viral replication will be promoted in the state of elevated glucose levels.[32], [33] Therefore, controlling blood glucose level is important as part of treatment strategy in COVID-19 patients. GLP1-RA is an excellent agent for non-severe COVID-19 (Insulin is still the treatment of choice for severe COVID-19 with type 2 Diabetes) because it has high efficacy in controlling blood glucose level with low risk of hypoglycemia[30] and there is no considerable disadvantage of GLP-1RA that may interfere metabolism in COVID‐19 patients with diabetes.[34]
Minor concern regarding GLP‐1RA effects on promoting the expression of angiotensin converting enzyme‐2 (ACE‐2) has also been proposed. To enter pulmonary epithelial cells, SARS-CoV2 attaches to ACE-2 receptors, hence any drug that upregulates the receptor may worsen the outcome of the infection. GLP-1RA may stimulate ACE2 expression, although it still remains unknown whether the drugs increase ACE2 expression in human population.[35], [36]
In addition to pancreas, GLP-1-based drugs show anti-inflammatory and immunoregulatory effect in multiple organs. GLP‐1R is abundantly expressed in multiple organs including brain, pancreas, kidney, stomach, heart, and predominantly in lung epithelia and immune cells.[37] GLP‐1RA mainly express its anti‐inflammatory properties through the suppression of cytokine and chemokine production, stimulation of eNOS/sGC/PKG signaling pathway, inactivation of the NF‐ĸB signaling, as well as attenuation of thioredoxin‐interacting protein levels.[35] Animal studies in mice with experimental lung injury also showed that GLP‐1RA reduce mucus secretion, and preserve lung function.[38], [39], [40], [41], [42] In addition, Rogliani et al.[43] reported that GLP‐1RAs improves forced expiratory volume in 1 s ‐ FEV1, forced vital capacity ‐ FVC, and maximal expiratory flow at 75% and 50% ‐ MEF75 and MEF50 in diabetes mellitus patients, regardless on their blood glucose levels.
GLP-1R expressed copiously in lungs. In an animal study conducted at rats, GLP-1 improved pulmonary physiology and surfactant production.[18] GLP-1 owns anti-inflammatory and anti-atherogenic properties. Several inflammatory markers and cardiovascular markers (e.g. C-reactive protein) are reduced in Type 2 diabetes patients treated with GLP-1RA.[44] GLP-1 inhibited IL-1β production, cytokine secretion, interfere nuclear factor-kB pathway, and promoted survival after lipopolysaccharide induced systemic inflammation in rats. The fact that GLP-1 displays anti-inflammatory effects and GLP-1R presence in the lung further support that GLP-1RA might attenuate acute lung disease.[37], [38], [45], [46]
GLP‐1RA also seem to affect the activity of neural pathways involved in food intake, reward, and energy expenditure and thus have the ability to decrease body weight.[47] Long‐acting GLP‐1RA usage consistently results in significant weight loss.[48], [49], [50] Furthermore, liraglutide is also used for treating obesity/overweight patients with hypertension, diabetes, and dyslipidemia as a supporting drug to diet and exercise due to clinically significant weight loss, reduction in blood glucose level variability and multiple cardiometabolic risk factors. Weight reduction properties of GLP-1RA is favorable in overweight/obese COVID-19 patients because high BMI is associated with multiple drawbacks that increase susceptibility to COVID-19 such as chronic inflammation, high ACE-2 expression, and decreased vitamin D level, thus, decreasing fat mass opposes those risk factors.[48], [49], [50]
In an animal study GLP1-RA seems to be capable of reversing gut microbiota dysbiosis (Low Bacteroidetes/Firmicutes ratio) caused by type 2 diabetes mellitus/obesity. It is proposed that GLP-1 modulates gut microbiota by delaying gastric emptying time and gut transit time (thus affecting intraluminal environment), binding to neurons within hypothalamus (interfering gut-brain axis), and modulating appetite, weight loss, and glucose homeostasis.[51] Since dysbiosis causes increased intestinal permeability, endotoxemia, and activation of NF‐ĸB and other inflammatory pathways, any infectious diseases including COVID-19 might progress into a more severe form. Hence, the use of GLP1-RA might prevent Covid-19 progression by controlling gut microbiota dysbiosis which was precipitated by diabetes mellitus and/or obesity.[51], [52]
We are aware of some limitations in our study. First, our study is only based from observational studies which are not free from the potential influence of confounding factors. Second, most of the included studies do not include information regarding the dosage and the duration of GLP-1RA use, therefore we are unable to analyze and provide drug dose and duration recommendation. Finally, the result from our analysis was dominantly affected by 2 studies which have large weight during the meta-analysis calculation, probably because of large sample sizes, therefore the results from our study should be interpreted with caution.
5. Conclusion
Our meta-analysis indicates that pre-admission use of GLP-1RA have an association with favorable mortality outcomes of Covid-19 in patients with diabetes mellitus. This study suggests that GLP-1RA have the potency to be the drug of choice for the management of type 2 diabetes mellitus patients during COVID-19 pandemic to improve patients' outcome, especially those who have cardiovascular risk factors. Importantly, we believe further randomized clinical trial studies are worthwhile to confirm our study. Finally, GLP-1RA should be put into consideration as an important agent in COVID-19 treatment plan.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data analyzed in this study were a re-analysis of existing data, which are openly available at locations cited in the reference section.
References
- 1.World Health Organization. Coronavirus disease (COVID-19): situation report. Accessed June 18, 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---15-june-2021
- 2.Wu Z.H., Tang Y., Cheng Q. Diabetes increases the mortality of patients with COVID-19: a meta-analysis. Acta Diabetol. 2021 Feb;58(2):139–144. doi: 10.1007/s00592-020-01546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y., Lu Y., Huang Y.M., Wang M., Ling W., Sui Y., et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020 Dec;113 doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hariyanto T.I., Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(5):1463–1465. doi: 10.1016/j.dsx.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariyanto T.I., Kurniawan A. Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Sleep Med. 2021 Jun;82:47–53. doi: 10.1016/j.sleep.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putri C., Hariyanto T.I., Hananto J.E., Christian K., Situmeang R.F.V., Kurniawan A. Parkinson's disease may worsen outcomes from coronavirus disease 2019 (COVID-19) pneumonia in hospitalized patients: A systematic review, meta-analysis, and meta-regression. Parkinsonism Relat Disord. 2021 doi: 10.1016/j.parkreldis.2021.04.019. S1353-8020(21)00152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hariyanto T.I., Rosalind J., Christian K., Kurniawan A. Human immunodeficiency virus and mortality from coronavirus disease 2019: A systematic review and meta-analysis. South Afr J HIV Med. 2021 Apr 15;22(1):1220. doi: 10.4102/sajhivmed.v22i1.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hariyanto T.I., Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. 2020 Sep;19 doi: 10.1016/j.obmed.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hariyanto T.I., Kurniawan A. Dipeptidyl peptidase 4 (DPP4) inhibitor and outcome from coronavirus disease 2019 (COVID-19) in diabetic patients: a systematic review, meta-analysis, and meta-regression. J Diabetes Metab Disord. 2021 Mar;27:1–8. doi: 10.1007/s40200-021-00777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hariyanto T.I., Kurniawan A. Statin and outcomes of coronavirus disease 2019 (COVID-19): A systematic review, meta-analysis, and meta-regression. Nutr Metab Cardiovasc Dis. 2021 Jun 7;31(6):1662–1670. doi: 10.1016/j.numecd.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivan Hariyanto T., Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J Med Virol. 2021 Mar;93(3):1832–1836. doi: 10.1002/jmv.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hariyanto T.I., Hardyson W., Kurniawan A. Efficacy and Safety of Tocilizumab for Coronavirus Disease 2019 (Covid-19) Patients: A Systematic Review and Meta-analysis. Drug Res (Stuttg) 2021 May;71(5):265–274. doi: 10.1055/a-1336-2371. [DOI] [PubMed] [Google Scholar]
- 13.Hariyanto T.I., Halim D.A., Jodhinata C., Yanto T.A., Kurniawan A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2021 Jun;48(6):823–830. doi: 10.1111/1440-1681.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hariyanto T.I., Halim D.A., Rosalind J., Gunawan C., Kurniawan A. Ivermectin and outcomes from Covid-19 pneumonia: A systematic review and meta-analysis of randomized clinical trial studies. Rev Med Virol. 2021 Jun;6 doi: 10.1002/rmv.2265. [DOI] [Google Scholar]
- 15.Pang J., Liu M., Ling W., Jin T. Friend or foe? ACE2 inhibitors and GLP-1R agonists in COVID-19 treatment. Obes Med. 2021 Mar;22 doi: 10.1016/j.obmed.2020.100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belančić A., Kresović A., Troskot D.M. Glucagon-like peptide-1 receptor agonists in the era of COVID-19: Friend or foe? Clin Obes. 2021 Apr;11(2) doi: 10.1111/cob.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuba K., Imai Y., Rao S., Jiang C., Penninger J.M. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J Mol Med (Berl) 2006 Oct;84(10):814–820. doi: 10.1007/s00109-006-0094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romaní-Pérez M., Outeiriño-Iglesias V., Moya C.M., Santisteban P., González-Matías L.C., Vigo E., et al. Activation of the GLP-1 Receptor by Liraglutide Increases ACE2 Expression, Reversing Right Ventricle Hypertrophy, and Improving the Production of SP-A and SP-B in the Lungs of Type 1 Diabetes Rats. Endocrinology. 2015 Oct;156(10):3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- 19.Margulis A.V., Pladevall M., Riera-Guardia N., Varas-Lorenzo C., Hazell L., Berkman N.D., et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. 2014 Oct;10(6):359–368. doi: 10.2147/CLEP.S66677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020 Aug;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Israelsen S.B., Pottegård A., Sandholdt H., Madsbad S., Thomsen R.W., Benfield T. Comparable COVID-19 outcomes with current use of GLP-1 receptor agonists, DPP-4 inhibitors or SGLT-2 inhibitors among patients with diabetes who tested positive for SARS-CoV-2. Diabetes Obes Metab. 2021 Jun;23(6):1397–1401. doi: 10.1111/dom.14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izzi-Engbeaya C., Distaso W., Amin A., Yang W., Idowu O., Kenkre J.S., et al. Adverse outcomes in COVID-19 and diabetes: a retrospective cohort study from three London teaching hospitals. BMJ Open Diabetes Res Care. 2021 Jan;9(1) doi: 10.1136/bmjdrc-2020-001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyland J.E., Raja-Khan N.T., Bettermann K., Haouzi P.A., Kraschnewski J.L., Parent L.J., et al. Diabetes, Drug Treatment and Mortality in COVID-19: A Multinational Retrospective Cohort Study. SSRN Electron J. 2020 doi: 10.2139/ssrn.3725612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orioli L., Servais T., Belkhir L., Laterre P.F., Thissen J.P., Vandeleene B., et al. Clinical characteristics and short-term prognosis of in-patients with diabetes and COVID-19: A retrospective study from an academic center in Belgium. Diabetes Metab Syndr. 2021;15(1):149–157. doi: 10.1016/j.dsx.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos-Rincón J.M., Pérez-Belmonte L.M., Carrasco-Sánchez F.J., Jansen-Chaparro S., De-Sousa-Baena M., Bueno-Fonseca J., et al. Cardiometabolic therapy and mortality in very old patients with diabetes hospitalized due to COVID-19. J Gerontol A Biol Sci Med Sci. 2021 doi: 10.1093/gerona/glab124. May 4:glab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverii G.A., Monami M., Cernigliaro A., Vigneri E., Guarnotta V., Scondotto S., et al. Are diabetes and its medications risk factors for the development of COVID-19? Data from a population-based study in Sicily. Nutr Metab Cardiovasc Dis. 2021 Feb 8;31(2):396–398. doi: 10.1016/j.numecd.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sourij H., Aziz F., Bräuer A., Ciardi C., Clodi M., Fasching P., et al. COVID-19 fatality prediction in people with diabetes and prediabetes using a simple score upon hospital admission. Diabetes Obes Metab. 2021 Feb;23(2):589–598. doi: 10.1111/dom.14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wargny M., Potier L., Gourdy P., Pichelin M., Amadou C., Benhamou P.Y., et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia. 2021 Apr;64(4):778–794. doi: 10.1007/s00125-020-05351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aroda V.R. A review of GLP-1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018 Feb;20(Suppl 1):22–33. doi: 10.1111/dom.13162. [DOI] [PubMed] [Google Scholar]
- 31.Erener S. Diabetes, infection risk and COVID-19. Mol Metab. 2020 Sep;39 doi: 10.1016/j.molmet.2020.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Codo A.C., Davanzo G.G., Monteiro L.B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020 Sep 1;32(3):437–446.e5. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim S., Bae J.H., Kwon H.S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021 Jan;17(1):11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020 Jun;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin T., Liu M. Letter to the editor: Comment on GLP-1-based drugs and COVID-19 treatment. Acta Pharm Sin B. 2020 Jul;10(7):1249–1250. doi: 10.1016/j.apsb.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monda V.M., Porcellati F., Strollo F., Gentile S. ACE2 and SARS-CoV-2 infection: might GLP-1 receptor agonists play a role? Diabetes Ther. 2020;11(9):1909–1914. doi: 10.1007/s13300-020-00898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y.S., Jun H.S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediators Inflamm. 2016;2016:3094642. doi: 10.1155/2016/3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drucker DJ. Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications. Endocr Rev. 2020 Jun 1;41(3):bnaa011. https://doi.org/10.1210/endrev/bnaa011 [DOI] [PMC free article] [PubMed]
- 39.Viby N.E., Isidor M.S., Buggeskov K.B., Poulsen S.S., Hansen J.B., Kissow H. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 2013 Dec;154(12):4503–4511. doi: 10.1210/en.2013-1666. [DOI] [PubMed] [Google Scholar]
- 40.Toki S., Goleniewska K., Reiss S., Zhang J., Bloodworth M.H., Stier M.T., et al. Glucagon-like peptide 1 signaling inhibits allergen-induced lung IL-33 release and reduces group 2 innate lymphoid cell cytokine production in vivo. J Allergy Clin Immunol. 2018 Nov;142(5):1515–1528.e8. doi: 10.1016/j.jaci.2017.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou F., Zhang Y., Chen J., Hu X., Xu Y. Liraglutide attenuates lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2016 Nov;15(791):735–740. doi: 10.1016/j.ejphar.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Zhu T., Wu X.L., Zhang W., Xiao M. Glucagon Like Peptide-1 (GLP-1) Modulates OVA-Induced Airway Inflammation and Mucus Secretion Involving a Protein Kinase A (PKA)-Dependent Nuclear Factor-κB (NF-κB) Signaling Pathway in Mice. Int J Mol Sci. 2015 Aug 26;16(9):20195–20211. doi: 10.3390/ijms160920195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogliani P., Matera M.G., Calzetta L., Hanania N.A., Page C., Rossi I., et al. Long-term observational study on the impact of GLP-1R agonists on lung function in diabetic patients. Respir Med. 2019;154:86–92. doi: 10.1016/j.rmed.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Haffner S.M. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006 Jan 16;97(2A):3A–11A. doi: 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Ku H.C., Chen W.P., Su M.J. GLP-1 signaling preserves cardiac function in endotoxemic Fischer 344 and DPP4-deficient rats. Naunyn Schmiedebergs Arch Pharmacol. 2010 Dec;382(5–6):463–474. doi: 10.1007/s00210-010-0559-9. [DOI] [PubMed] [Google Scholar]
- 46.Mirabelli M., Chiefari E., Puccio L., Foti D.P., Brunetti A. Potential Benefits and Harms of Novel Antidiabetic Drugs During COVID-19 Crisis. Int J Environ Res Public Health. 2020 May 22;17(10):3664. doi: 10.3390/ijerph17103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabery S., Salinas C.G., Paulsen S.J., Ahnfelt-Rønne J., Alanentalo T., Baquero A.F., et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020 Mar 26;5(6) doi: 10.1172/jci.insight.133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies M.J., Bergenstal R., Bode B., Kushner R.F., Lewin A., Skjøth T.V., et al. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA. 2015 Aug 18;314(7):687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 49.Blackman A., Foster G.D., Zammit G., Rosenberg R., Aronne L., Wadden T., et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond) 2016 Aug;40(8):1310–1319. doi: 10.1038/ijo.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015 Jul 2;373(1):11-22. https://doi.org/10.1056/NEJMoa1411892 [DOI] [PubMed]
- 51.Belančić A. Gut microbiome dysbiosis and endotoxemia - Additional pathophysiological explanation for increased COVID-19 severity in obesity. Obes Med. 2020 Dec;20 doi: 10.1016/j.obmed.2020.100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao L., Chen Y., Xia F., Abudukerimu B., Zhang W., Guo Y., et al. A Glucagon-Like Peptide-1 Receptor Agonist Lowers Weight by Modulating the Structure of Gut Microbiota. Front Endocrinol (Lausanne) 2018 May;17(9):233. doi: 10.3389/fendo.2018.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed in this study were a re-analysis of existing data, which are openly available at locations cited in the reference section.