Abstract
COVID-19 infection can cause inflammatory reactions that could involve several organs. In the pediatric population, Multi-System Inflammatory Syndrome in Children (MIS-C) has been reported as one of the consequences of COVID-19. We report a unique pediatric COVID-19 patient with MIS-C, associated with paralysis of the extremities. MRI showed abnormal signal in the cervical spinal cord compatible with transverse myelitis. Methylprednisolone and IVIG were administered, without significant symptom improvement. As a next step, Infliximab was tried for her, and she responded remarkably well to this treatment. Infliximab may be considered as a treatment option in COVID-19 patients with transverse myelitis.
Keywords: COVID-19, Multi-System Inflammatory Syndrome, Transverse myelitis, Pediatric, Infliximab, Immunotherapy
Abbreviation: SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2; COVID-19, Coronavirus disease 2019; Multi-System Inflammatory Syndrome in Children, MIS-C; TM, Transverse myelitis; LETM, longitudinally extensive transverse myelitis; IVIG, intravenous immunoglobulin; CMV, cytomegalovirus; TNF-α, tumor necrosis factor alpha
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged first in Wuhan, Hubei Province, China in late 2019 and has spread around the world, making it a global health concern (Moriguchi et al., 2020). Coronavirus disease 2019 (COVID-19) has been mostly recognized with its respiratory symptoms, such as cough and dyspnea (Zimmermann and Curtis, 2020). However, many other complications have been reported. These complications are the result of either direct viral infection or the host immune response (Azer, 2020; Chow et al., 2020). Among them, Guillain-Barré syndrome, stroke, and transverse myelitis are potential life-threatening neurological conditions (Chow et al., 2020; Whittaker et al., 2020).
Transverse myelitis (TM) is an acute or subacute inflammatory myelopathy, which can result in motor, sensory, and autonomic dysfunction (West, 2013). A few cases of TM as a post-COVID-19 complication have been reported, but details of treatment have not been described in the majority of the pediatric cases as the reports were predominantly focused on imaging (Baghbanian and Namazi, 2020; Chakraborty et al., 2020; Kaur et al., 2020; Lindan et al., 2021; Valiuddin et al., 2020). It is a potentially debilitating condition, which necessitates the importance of effective diagnosis and therapy. Nevertheless, no consensus has been established for the treatment of this complication yet and the treatments reported in previous cases have not had a good prognosis. In this article, we report a child with longitudinally extensive transverse myelitis (LETM) who had a favorable response to Infliximab therapy in addition to routine management.
2. Case presentation
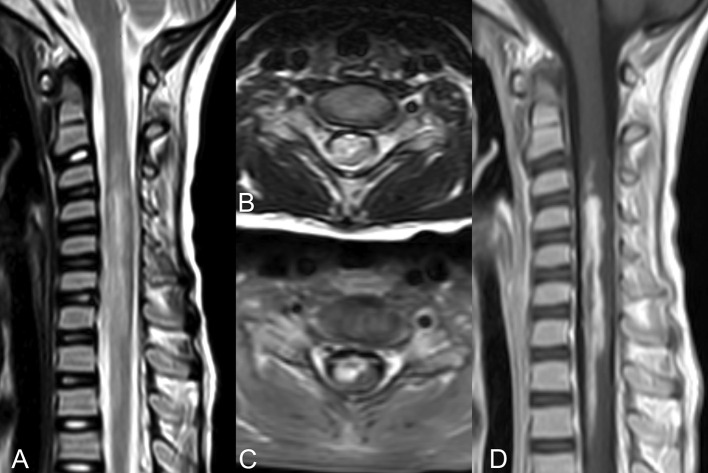
A 9-year-old female presented with fever and progressive paralysis of all four limbs after falling while playing. She was suspected to only have a mild trauma to the neck, and the timing of symptom onset and progression was not compatible with the traumatic accident (>24 h after trauma). She was admitted to the emergency department (ED) six days after symptoms initiation. Upon admission, a nasopharyngeal swab test was performed due to fever and SARS-CoV-2 was detected via PCR assay. In the ED, the patient and her parents did not report any recent upper respiratory infection (URI) symptoms. Her vital signs showed decreased blood pressure and bradycardia. However, the rest of the cardiovascular and pulmonary physical examinations were normal. On neurological exam, there was a conspicuous reduction in the strength of the left upper and right lower limbs to 1/5, and right upper and left lower limbs to 2/5. Plantar reflexes were present, and deep tendon reflexes were 1+. She had a normal gag reflex and no sensory levels. Fundoscopy was performed and showed no abnormal finding. Rectal examination demonstrated normal sphincter tone and anal wink was present. Her laboratory results showed elevated inflammatory markers such as C-reaxtive protein, ESR, lactate dehydrogenase, and hypocalcemia (Table 1 ). Lumbar puncture showed no red or white blood cells, protein of 15 mg/dL, glucose of 69 mg/dL, and IgG of 2.6 (normal up to 8) and no oligoclonal bands were detected. Neuromyelitis optica IgG and myelin oligodendrocyte glycoprotein antibodies were negative. CSF viral panel (HSV, EBV, enterovirus and CMV) and stool culture for polio were checked for the patient and the results were negative. She underwent brain CT and MRI as workup for her limb weakness, which did not show any abnormalities. However, spinal MRI showed abnormal spinal cord signal involving both grey and white matter and extending beyond three vertebral segments, predominantly in the cervical region (Fig. 1 ). There was no sign of vertebral or cervical ligamentous traumatic injury or disc annular fissure. She was initially treated with 10 mg/kg q12h calcium gluconate (on 1st and 2nd days of admission), 2 g/kg intravenous immunoglobulin (IVIG) (on 2nd and 4th days of admission), and 30 mg/kg IV methylprednisolone (on the 2nd, 4th, 6th, 8th and 10th days of admission). This treatment plan resulted in no significant improvement in limb strength.
Table 1.
Laboratory data during hospitalization.
| Markers | Day −1 | Day1 | Day3 | Day5 | Day 9 | Day11 | Day15 | Day21 |
|---|---|---|---|---|---|---|---|---|
| COVID-19 PCR | Positive | |||||||
| COVID-19 IgG, ratio | 0.1 | |||||||
| COVID-19 IgM, ratio | 0.43 | |||||||
| WBC, cells/μL | 5400 | 9700 | 7800 | 11,600 | 11,400 | 12,900 | 3900 | |
| Lymphocyte, percentage | 40.1% | 25.3% | 19.7% | 22% | 19.6% | 13.7% | 19% | |
| Absolute lymphocyte count | 2165.4 | 2454.1 | 1536.6 | 2552 | 2234.4 | 1767.3 | 741 | |
| Neutrophil, percentage | 49.7% | 63.6% | 70.7% | 70.4% | 69.7% | 79.4% | 74% | |
| Absolute neutrophil count | 2684 | 6169 | 5515 | 8166 | 7946 | 10,243 | 2886 | |
| Hb, g/dL | 11.2 | 11.5 | 9.4 | 10.2 | 9 | 8.2 | 8.2 | |
| Platelets, cells/mL | 238,000 | 262,000 | 298,000 | 361,000 | 287,000 | 174,000 | 111,000 | |
| ESR, mm/h | 49 | 81 | 82 | 81 | 115 | 85 | 25 | |
| CRP, mg/dl | 4 | 1 | 2 | 2 | 1 | 2 | 1 | |
| D-Dimer, ng/mL | 486 | 1100 | 990 | 3000 | 900 | 470 | 234 | |
| Ferritin, ng/mL | 53 | 346 | 131 | |||||
| Fibrinogen, mg/dL | 270 | 197 | ||||||
| LDH, U/L | 483 | 790 | 712 | 852 | 503 | 604 | 536 | |
| Troponin, ng/L | Nega | 10 | 11 | 20 | 30 | 19 | 4.3 | |
| ALT, U/L | 21 | 17 | 20 | 107b | 74 | 117 | 55 | |
| AST, U/L | 32 | 28 | 36 | 89b | 61 | 45 | 45 | |
| PT, sec | 11.7 | 12.5 | 12 | |||||
| PTT, sec | 28 | 30 | 29 | |||||
| INR | 1.1 | 1 | 1.1 | |||||
| NMO-IgG (Anti-aquaporin 2) | < 0.1 | |||||||
| Anti-MOG | < 0.1 |
Qualitative measurement.
Double-checked.
Fig. 1.
Magnetic resonance imaging of the spine. A, B. T2-weighted images of the cervical spine show abnormal T2 hyperintensity involving a large cross section of the cervical spinal cord involving both grey and white matter, extending from the C2-3 to T2-T3 level. C, D. Postcontrast axial and sagittal T1-weighted images of the spine show heterogeneous, but avid contrast enhancement of the involved portions of the spinal cord. There was no sign of traumatic injury to the cervical spine.
Five days after admission, the patient started to have palmar rash, which was self-limited. During the seventh day of hospitalization, she experienced hypotension which was managed by norepinephrine. One day after receiving norepinephrine, she started having a severe headache. Brain CT was performed, which was normal. Her plasmapheresis plan also did not proceed due to hemodynamic instability. Laboratory data demonstrated elevated ferritin levels (Table 1). Two doses (5 mg/kg) of Infliximab were administered on the tenth and twenty-second days of admission, while calcium gluconate and norepinephrine were put on hold until day sixteen. Following these steps, her symptoms improved, with her right upper and left lower limb strength increasing to 3/5 and the left upper and right lower limb increasing to 4/5. Her blood pressure stabilized and gradually improved.
3. Discussion
COVID-19 neurological complications consist of a broad spectrum of conditions in all age groups (Asadi-Pooya and Simani, 2020). Transverse myelitis can have a wide range of etiologies, including systemic autoimmune disease, recurrent central nervous system autoimmune disease, and is also associated with various infectious agents (Lyons, 2015). Herpes simplex virus, Epstein-Barr virus, varicella zoster virus, and cytomegalovirus (CMV) are known common infectious associations of TM, while LETM is also related to flavivirus and enterovirus infections as well (Kincaid and Lipton, 2006). One of the hallmarks of TM is weakness in the upper and lower extremities, which was the neurological main presentations in our patient. Other potential presenting symptoms of TM are bilateral symmetric sensory changes, lower back pain, and bladder dysfunction (West, 2013). The diagnosis of TM is based on transverse myelitis consortium group criteria (Transverse Myelitis Consortium Working Group, 2002), which was met in our patient. Fulfilling these criteria, as well as neuroimaging findings in favor of myelitis, confirmed the diagnosis of TM. The patient also met the centers for disease control definition of MIS-C associated with SARS-Cov-2 infection as evidenced by her clinical and laboratory indicators of inflammation (Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C), 2021).
Considering the potential association of TM and COVID-19 in this child with MIS-C, corticosteroids and intravenous immunoglobulin were initiated, as previously suggested in some adult studies (Chakraborty et al., 2020; Sarma and Bilello, 2020; Valiuddin et al., 2020). Despite the administration of these medications, weakness in extremities was not improved in our patient. The next planned step was plasmapheresis (Baghbanian and Namazi, 2020), which was put on hold due to her autonomic instability. Autonomic instability has previously been reported in patients with COVID-19 (Amaratunga et al., 2020), but it could also be a sign of sympathetic hypoactivity as a result of myelitis as well (Profice et al., 2013). Monoclonal antibodies are less commonly used drugs in TM therapy and are often considered as one of the last treatment options. Our medical team, based on local availability, decided to prescribe Infliximab, a monoclonal antibody against tumor necrosis factor alpha (TNF-α) in two separate doses. In some of the previous studies on concomitant TM and COVID-19, patients have benefitted from Rituximab (a monoclonal antibody against CD20 protein) (Ghosh et al., 2020; Kaur et al., 2020). One of these studies showed a good response to Rituximab (Ghosh et al., 2020), while another study used Rituximab after remission of acute TM and did not report the outcome of Rituximab injection (Kaur et al., 2020). In addition, one study reported positive effects of Infliximab on radiotherapy-induced TM (Chang et al., 2018). To the best of our knowledge, this case study is the first pediatric case of concomitant TM and COVID-19, who responded well to empirical Infliximab therapy.
4. Conclusion
Complications of COVID-19 infection are a result of direct infection or autoimmune diseases as a consequence of the host immune response. Transverse myelitis is one of the possible associated autoimmune diseases with COVID-19. TM management is crucial due to its high morbidity and potential mortality. A promising empirical response to Infliximab was seen in our patient. If confirmed in other patients, this can be a turning point in the treatment of this complex complication of COVID-19, but would need to be replicated in future studies.
Declaration of Competing Interest
None.
References
- Amaratunga E.A., Corwin D.S., Moran L., Snyder R. Bradycardia in patients with COVID-19: a calm before the storm? Cureus. 2020;12 doi: 10.7759/cureus.8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: a systematic review. J. Neurol. Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azer S.A. COVID-19: pathophysiology, diagnosis, complications and investigational therapeutics. New Microb. New Infect. 2020;37:100738. doi: 10.1016/j.nmni.2020.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghbanian S.M., Namazi F. Post COVID-19 longitudinally extensive transverse myelitis (LETM)-a case report. Acta Neurol. Belg. 2020 doi: 10.1007/s13760-020-01497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty U., Chandra A., Ray A.K., Biswas P. COVID-19-associated acute transverse myelitis: a rare entity. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-238668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang V.A., Simpson D.R., Daniels G.A., Piccioni D.E. Infliximab for treatment-refractory transverse myelitis following immune therapy and radiation. J. Immunother. Cancer. 2018;6:153. doi: 10.1186/s40425-018-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C.C.N., Magnussen J., Ip J., Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., De K., Roy D., Mandal A., Biswas Subrata, Biswas Subhrajyoti, Sengupta S., Naga D., Ghosh M., Benito-León J. A case of area postrema variant of neuromyelitis optica spectrum disorder following SARS-CoV-2 infection. J. Neuroimmunol. 2020;350:577439. doi: 10.1016/j.jneuroim.2020.577439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C) CDC; 2021. https://www.cdc.gov/mis-c/hcp/ [WWW Document], n.d. URL. (accessed 3.26.21) [Google Scholar]
- Kaur H., Mason J.A., Bajracharya M., McGee J., Gunderson M.D., Hart B.L., Dehority W., Link N., Moore B., Phillips J.P., Rogers D. Transverse myelitis in a child with COVID-19. Pediatr. Neurol. 2020;112:5–6. doi: 10.1016/j.pediatrneurol.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid O., Lipton H.L. Viral myelitis: an update. Curr. Neurol. Neurosci. Rep. 2006;6:469–474. doi: 10.1007/s11910-006-0048-1. [DOI] [PubMed] [Google Scholar]
- Lindan C.E., Mankad K., Ram D., Kociolek L.K., Silvera V.M., Boddaert N., Stivaros S.M., Palasis S., ASPNR PECOBIG Collaborator Group Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc. Health. 2021;5:167–177. doi: 10.1016/S2352-4642(20)30362-X. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J.L. Myelopathy associated with microorganisms. Continuum (Minneap. Minn.) 2015;21:100–120. doi: 10.1212/01.CON.0000461087.56371.e8. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profice P., Renna R., Pilato F., Sestito A., Infusino F., Bruno I., Pravatà E., Di Lazzaro V. Cardiovascular impairment in a patient with acute myelitis. Spinal Cord. 2013;51:511–513. doi: 10.1038/sc.2013.30. [DOI] [PubMed] [Google Scholar]
- Sarma D., Bilello L.A. A case report of acute transverse myelitis following novel coronavirus infection. CPCEM. 2020;4:321–323. doi: 10.5811/cpcem.2020.5.47937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transverse Myelitis Consortium Working Group Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505. doi: 10.1212/wnl.59.4.499. [DOI] [PubMed] [Google Scholar]
- Valiuddin H., Skwirsk B., Paz-Arabo P. Acute transverse myelitis associated with SARS-CoV-2: a case-report. Brain Behav. Immun. Health. 2020;5:100091. doi: 10.1016/j.bbih.2020.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West T.W. Transverse myelitis--a review of the presentation, diagnosis, and initial management. Discov. Med. 2013;16:167–177. [PubMed] [Google Scholar]
- Whittaker A., Anson M., Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol. Scand. 2020;142:14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr. Infect. Dis. J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]