Abstract
BACKGROUND
The repeated waves of the COVID-19 pandemic have highlighted the necessity to optimize vaccine responses in immunocompromised populations. We investigated the safety and immunogenicity of a third, booster, dose of the Pfizer BNT162b2 vaccine in heart transplant (HT) patients.
METHODS
The cohort comprised 96 adult HT patients who received a third homologous dose of the BNT162b2 vaccine 168 days after the second dose. The vaccine-induced antibody responses of both receptor-binding domain (RBD) IgG and neutralizing antibodies were assessed in all patients, with a positive antibody response being defined as the presence of either IgG anti-RBD or neutralizing antibodies. For a subset of patients, T cell response was also studied.
RESULTS
The third dose was associated with a low rate of adverse events, mostly mild pain at the injection site. No serious adverse events were recorded, and there were no episodes of rejection. At 18 days following the third dose of the vaccine, the positive antibody response increased from 23% to 67%, with a corresponding increase in neutralizing capacity. The third dose elicited SARS-CoV-2 neutralization titers >9-fold and IgG anti-RBD antibodies >3-fold of the range achieved after the two primary doses. Mycophenolate use, lower eGFR and higher C-reactive protein were independently associated with a reduced likelihood of generating an immune response. Importantly, a specific T-cell response following the third dose was evident in the majority of transplant recipients.
CONCLUSIONS
An homologous third booster dose of the BNT162b2 vaccine gave overall consistent tolerability and a good safety profile, while eliciting humoral and cellular immune responses.
KEYWORDS: heart transplantation, COVID-19 pandemic, booster, BNT162b2 vaccine, IgG anti-RBD, neutralizing antibodies
Abbreviations: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ELISA, enzyme-linked immunosorbent assay; GMT, geometric mean titer; HT, heart transplantation; IFN-γ, interferon gamma; ISHLT, International Society for Heart and Lung Transplantation; PBMCs, peripheral blood mononuclear,r cells; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SOT, solid organ transplants
The repeated waves of the coronavirus disease 2019 (COVID-19) pandemic have highlighted the necessity to optimize vaccine responses in vulnerable immunocompromised populations, including the recipients of solid organ transplants (SOT). In the general population,1 the two-dose protocol for SARS-CoV-2 mRNA vaccines has been shown to elicit an excellent antibody response, but in SOT recipients antibody responses after 2 doses are markedly attenuated.2 , 3 This attenuated antibody response translates into a clinical picture that includes breakthrough infections in fully vaccinated (2 doses) transplant recipients,4 with the disease course being comparable to that in non-vaccinated SOT recipients.5
Following antigenic stimulation by vaccination or infection, the development of long-term protective immunity depends on a series of immunological events that include the activation, proliferation, differentiation and coordination of antigen-specific B and T cells. Accumulated knowledge on this cascade of events constitutes the basis for the development of antiviral – and other – vaccines, for monitoring the response to the vaccines, and for drug development. In the COVID-19 arena, knowledge about the T-cell response to SARS-CoV-2 infection and vaccination remains limited, and research progress is being impeded by the complexity of the T cell response and the attendant problem of quantifying it definitively.6
The COVID-19 vaccination immune paresis in heart transplant (HT) recipients,3 and the ongoing pandemic that is putting this vulnerable population at high risk, serve as a call to action. For most vaccines, repeat vaccinations are necessary to induce efficient and long-lasting protection from infection. Repeat immunogenic stimulations not only increase the intensity and durability of adaptive immunity, but also influence its quality. For SOT patients, early reports provide encouraging evidence regarding the immunogenicity and antibody response following a third (booster) vaccine dose.7, 8, 9, 10 However, the limitations of these studies are the small number of HT recipients, the lack of data on the cellular responses and neutralizing antibodies after the booster dose, and the lack of data on immunosuppression and clinical effectiveness.11 In July 2021, administration of a third dose of the Pfizer BNT162b2 COVID-19 vaccine was approved by the Israel Government for all SOT recipients and other immunocompromised patients, independently of serology evaluation. In compliance with the recommendations of the Joint Statement about Vaccine Efficacy in Organ Transplant Recipients,11 we promoted the third vaccination of the HT patients in our care, in the context of a clinical research study. Here, we describe our experience with an homologous third dose of the Pfizer BNT162b2 vaccine, with emphasis on identifying and characterizing the safety and immunogenicity of this booster dose.
Methods
Study population and surveillance
The cohort comprised 96 adult stable HT patients, who had previously received two doses of the BNT162b2 vaccine (Pfizer, New York, USA and BioNTech, Mainz, Germany) and were subsequently vaccinated with an homologous third dose of the BNT162b2 vaccine. None of the patients in the cohort were vaccinated during the three months after transplantation, and none were treated for rejection or with T-cell depleting agents or specific B-cell depletion agents during the 9 months prior to vaccination.12 , 13 Given the high levels of antibodies in our subpopulation of patients vaccinated before transplant, we excluded these patients from third dose vaccination (n=9). We also excluded patients who had recovered from COVID-19 disease and who exhibited high levels of neutralizing antibodies (n=15), given the association of neutralizing antibodies with attenuated disease severity,13 until further information and safety data are available. All patients underwent thorough clinical, laboratory and cardiac evaluation on day of vaccination (before the vaccination) and were prospectively followed at 2 to 3 weeks after the third vaccine with repeated clinical, laboratory, and cardiac evaluations. Laboratory evaluation included testing for antibodies and, for subset of patients, assessment of a SARS-Co-V-2 specific T cell response. The institutional protocol for post-transplant immunosuppression comprises a calcineurin inhibitor, a mycophenolate-based drug, and a corticosteroid. Conversion to everolimus is instituted per the patient's risk profile, as is steroid wean. Cardiac evaluation included noninvasive hemodynamic assessment; anamnestic and clinical examination by a transplant cardiologist; electrocardiography; and any additional intervention deemed necessary in accordance with the institutional follow-up protocol, which includes follow-up for rejection and cardiac allograft vasculopathy, as previously described.14 The study was approved by our institutional review board (8314-21-SMC).
Outcome measures
The main outcomes for this study were: 1) Tolerability and reactogenicity; 2) vaccine-induced antibody response of receptor-binding domain (RBD) IgG and neutralizing antibodies; and 3) ex-vivo interferon gamma (IFN-γ) T cell response.
Tolerability and reactogenicity
Patients were actively screened for 'solicited' adverse events,15 both local reactions (redness, swelling, pain at injection site) and systemic reactions (fever, fatigue, headache, chills, vomiting, diarrhea, new or worsened joint pain, use of antipyretic or pain medication) within the seven days after the third dose. Patients were also requested to report any suspected adverse event.
Antibody detection testing
Samples from HT patients were evaluated with an “in-house” enzyme-linked immunosorbent assay (ELISA) that detects IgG antibodies against the receptor- binding domain (RBD) of SARS-CoV-2.16 , 17 A SARS-CoV-2 pseudo-virus (psSARS-2) neutralization assay was performed to detect SARS-CoV-2 neutralizing antibodies using a green fluorescent protein (GFP) reporter-based pseudotyped virus with a vesicular stomatitis virus (VSV) backbone coated with the SARS-CoV-2 spike (S) protein, which was generously provided by Dr. Gert Zimmer (Institute of Virology and Immunology (IVI), Mittelhäusern, Switzerland). Following titration, 100 focus forming units (ffu) of pseudo SARS-2 were incubated with twofold serial dilutions of heat-inactivated (56°C for 30 min) sera. Thereafter, the virus/serum mixture was transferred to Vero E6 cells and incubated for 90 min at 37°C. Plates were incubated for 24 h, and 50% plaque reduction titer was calculated by counting green fluorescent foci using a fluorescence microscope (EVOS M5000, Invitrogen). Sera not capable of reducing viral replication by 50% at 1 to 8 dilution or below were considered non-neutralizing.18
Peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using UNI-SEP+ (Novamed). Plasma was collected and spun at 1000 × g for 20 min to remove platelets before collection of PBMCs. Following one wash with phosphate-buffered saline and one wash with 4Cell® Nutri-T Medium (Sartorius), cells were resuspended in 4Cell Nutri-T-Medium and counted using the Countess II Cell counter (Invitrogen).
IFN-γ ELISpot assay
Fresh PBMCs were used in all ELISpot assays using the Elispot IFN-γ kit [Autoimmun Diagnostika GmbH (AID)] according to the manufacturer's instructions. Briefly, fresh PBMCs were added to duplicate wells at 2 × 105 cells in 50 μL per well and stimulated with 50 μL of SARS-CoV-2 peptide pools (S-complete, Miltenyi Biotech) (2 μg/mL per peptide). 4Cell Nutri-T Medium was used as the negative control, and phytohemagglutinin (PHA), as the positive control. After 16–20 h at 37 °C, 5% CO2, 95% humidity, cells were removed, and secreted IFN-γ was detected by adding alkaline phosphatase conjugated secondary antibody for 2 h. The plates were developed using BCIP/NBT substrate. ELISpot plates were scanned on an AID ELISpot Reader. The unspecific background [mean spot forming units (SFU) from negative control wells] was subtracted from experimental readings.
Statistical analysis
HT recipients were grouped according to their antibody response to the third vaccine dose, with the groups being designated positive antibody response (patients exhibiting either IgG anti-RBD or neutralizing antibodies) and negative antibody response (neither IgG anti-RBD nor neutralizing antibodies). Continuous variables were tested for distribution by using the Shapiro-Wilk test, and results are presented as means ± standard deviation if normally distributed, and as median (interquartile range) if non-normally distributed. For comparison of categorical variables between the groups, a chi-square test was used, and categorical variables are given as frequencies and percentages. Student's t-test and the Mann-Whitney U test were performed for comparison of normally distributed continuous variables and for non-normal distribution, respectively. Multivariable logistic regression analysis was used to identify factors associated with vaccine-induced antibody response. Results are presented as odds ratios (OR), 95% confidence intervals (CI). All P values reflect the results of two-sided tests. Statistical analyses were conducted using R (version 4.0.3).
Results
Study population
The study cohort comprised 96 HT recipients, aged 61.0 [49.8, 68.0] years; 71% were male; the time from transplant to third vaccination was 6.3 [3.5, 13.6] years, given within 168 (±18) days from the second dose. Comorbidities were frequent, with hypertension (73%) and diabetes mellitus (44%) being the most common. Immunosuppression with a calcineurin inhibitor and mycophenolate was the most frequently followed protocol (75%); 79% patients were on >2 immunosuppressive agents at time of vaccination, and 20% had already been weaned off chronic steroids. On the day of the third vaccination, absolute lymphocyte count was 1.5 ( ± 0.65) K/μL; estimated glomerular filtration rate (eGFR) was 78 ( ± 33) mL/min/1.73 m2; and troponin I was within the normal range (Table 1 ).
Table 1.
Recipient Characteristics, Stratified by Antibody Response to the Third BNT162b2 Vaccination in Heart Transplant Recipients
| Variable | Total cohort n = 96 | Positive antibody response n = 64 | Negative antibody response n = 32 | p value |
|---|---|---|---|---|
| Recipient characteristics | ||||
| Age, years, median (IQR) | 61.0 [49.8, 68.0] | 58.0 [47.0, 68.0] | 65.00 [58.8, 70.3] | 0.012 |
| Female sex, n (%) | 28.0 (29.2) | 15.0 (23.4) | 13.0 (40.6) | 0.131 |
| BMI, kg/m2 (mean ± SD) | 26.8 (4.7) | 26.9 (4.0) | 26.8 (5.9) | 0.918 |
| Diabetes mellitus, n (%) | 42.0 (43.8) | 26.0 (40.6) | 16.0 (51.6) | 0.429 |
| Hypertension, n (%) | 70.0 (72.9) | 42.0 (65.6) | 28.0 (87.5) | 0.042 |
| Cardiac allograft vasculopathy, n (%) | 22.0 (22.9) | 15.0 (24.2) | 7.0 (22.6) | 1.000 |
| Immunosuppression dataa | ||||
| Mycophenolic acid therapy, n (%) | 75.0 (78.1) | 47.0 (73.4) | 28.0 (87.5) | 0.144 |
| Mycophenolate sodium, n (%) | 52.0 (54.2) | 30.0 (46.9) | 22.0 (68.8) | 0.070 |
| Mycophenolate mofetil, n (%) | 23.0 (24.0) | 17.0 (26.6) | 6.0 (18.8) | 0.554 |
| Mycophenolate sodium dose, mg (mean ± SD) | 1147.3 (378.1) | 1160.67 (398.7) | 1129.09 (356.5) | 0.769 |
| Mycophenolate mofetil dose, mg (mean ± SD) | 1347.8 (487.0) | 1352.9 (492.6) | 1333.3 (516.4) | 0.935 |
| Everolimus therapy, n (%) | 21.0 (22.1) | 18.0 (28.6) | 3.0 (9.4) | 0.062 |
| Immunosuppression protocol | 0.660 | |||
| Tacrolimus + mycophenolate + prednisone, n (%) | 51.0 (53.1) | 33.0 (51.6) | 18.0 (56.2) | |
| Cyclosporine + mycophenolate + prednisone n (%) | 7.0 (7.3) | 4.0 (6.2) | 3.0 (9.4) | |
| Tacrolimus + mycophenolate, n (%) | 13.0 (13.5) | 7.0 (10.9) | 6.0 (18.8) | |
| Cyclosporine + mycophenolate, n (%) | 1.0 (1.0) | 1.0 (1.6) | 0.0 (0.0) | |
| Cyclosporine + everolimus + prednisone, n (%) | 2.0 (2.1) | 2.0 (3.1) | 0.0 (0.0) | |
| Tacrolimus + everolimus + prednisone, n (%) | 12.0 (12.5) | 10.0 (15.6) | 2.0 (6.2) | |
| Mycophenolate + everolimus + prednisone, n (%) | 3.0 (3.1) | 2.0 (3.1) | 1.0 (3.1) | |
| Everolimus + cyclosporine, n (%) | 2.0 (2.1) | 2.0 (3.1) | 0.0 (0.0) | |
| Everolimus + tacrolimus, n (%) | 1.0 (1.0) | 1.0 (1.6) | 0.0 (0.0) | |
| Cyclosporine + prednisone, n (%) | 1.0 (1.0) | 0.0 (0.0) | 1.0 (3.1) | |
| Tacrolimus + prednisone, n (%) | 2.0 (2.1) | 1.0 (1.6) | 1.0 (3.1) | |
| Tacrolimus + everolimus + mycophenolate + prednisone, n (%) | 1.0 (1.0) | 1.0 (1.6) | 0.0 (0.0) | |
| Chronic prednisone, n (%) | 77.0 (80.2) | 52.0 (81.2) | 25.0 (78.1) | 0.928 |
| Prednisone dose, mg, median (IQR) | 2.5 [2.0, 2.5] | 2.5 [2.0, 2.5] | 2.5 [2.5, 3.0] | 0.139 |
| Tacrolimus trough level, μg/L, median (IQR)b | 9.8 [6.9, 12.0] | 9.5 [6.1, 11.1] | 10.6 [8.9, 13.1] | 0.033 |
| Cyclosporine trough level, μg/L, median (IQR)b | 128.0 [107.0, 138.0] | 132.0 [101.0, 138.0] | 118.0 [113.0, 143.0] | 0.796 |
| Laboratory dataa | ||||
| Lymphocyte absolute, K/μL, n (%) | 1.5 (0.7) | 1.6 (0.6) | 1.4 (0.7) | 0.292 |
| White blood cell, K/μL, n (%) | 7.0 (2.5) | 7.2 (2.2) | 6.6 (2.9) | 0.305 |
| Neutrophil absolute, K/μL, n (%) | 5.0 (2.0) | 5.1 (1.9) | 4.8 (2.2) | 0.535 |
| Neutrophil/lymphocyte ratio, n (%) | 3.8 (1.9) | 3.8 (1.9) | 3.9 (2.0) | 0.778 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 77.8 (33.0) | 85.8 (34.7) | 62.0 (21.4) | 0.001 |
| C-reactive protein, mg/L (mean ± SD) | 6.3 (8.1) | 5.2 (5.9) | 8.7 (11.0) | 0.043 |
| Low-density lipoprotein, mg/dL (mean ± SD) | 79.7 (34.7) | 81.9 (36.0) | 75.6 (32.5) | 0.445 |
| Triglycerides, mg/dL(mean ± SD) | 164.8 (81.2) | 160.1 (83.1) | 170.6 (78.5) | 0.583 |
| Troponin I HS, baseline, ng/L, median (IQR) | 4.1 [3.0, 6.7] | 4.0 [3.1, 6.9] | 4.2 [2.9, 6.5] | 0.759 |
| Troponin I HS, post third vaccine, ng/L, median (IQR) | 3.9 [2.6, 5.5] | 3.5 [2.4, 5.1] | 4.3 [3.2, 7.6] | 0.161 |
| ∆ troponin, ng/L, median (IQR) | -0.5 [-1.5, 0.2] | -0.6 [-1.6, 0.0] | -0.3 [-0.9, 0.9] | 0.141 |
| CPK baseline, ng/L, median (IQR) | 88.0 [62.0, 133.0] | 101.5 [74.0, 133.0] | 68.0 [46.0, 92.5] | 0.005 |
| CPK post 3rd vaccine dose, ng/L, median (IQR) | 77.0 [54.3, 130.3] | 88.0 [61.8, 131.5] | 59.5 [44.0, 98.5] | 0.043 |
| ∆ CPK, ng/L, median (IQR) | -6.0 [-21.0, 5.0] | -9.0 [-22.8, 3.8] | -1.0 [-10.5, 9.0] | 0.093 |
| Timetable | ||||
| HT to 1st vaccine, years, median (IQR) | 5.9 [2.9, 13.1] | 5.6 [3.3, 10.8] | 8.0 [2.5, 14.7] | 0.655 |
| Time of 2nd vaccine from 1st vaccine, days (mean ± SD) | 21.3 (3.1) | 21.4 (3.7) | 21.0 (1.5) | 0.518 |
| Time of 3rd vaccine from 2nd vaccine, days (mean ± SD) | 167.5 (18.0) | 163.9 (20.1) | 174.7 (9.6) | 0.005 |
| Time of 3rd vaccine to antibody testing, days (mean ± SD) | 17.5 (3.9) | 17.5 (4.3) | 17.6 (3.0) | 0.868 |
| Follow-up from 3rd vaccine, days (mean±SD) | 32.3 (2.4) | 32.5 (2.5) | 32.1 (2.3) | 0.494 |
On day of 3rd vaccine.
Whole blood trough levels were measured on the day of vaccination (at least 4 half-lives on fixed-dose regimen).
Abbreviations: BMI, Body mass index; CPK, creatine phosphokinase; HT, heart transplantation; SD, standard deviation.
Tolerability and reactogenicity
Among the vaccine recipients, 67% reported at least one adverse event after the third dose. By age group, 78% in the younger group (aged 18 to 55 years) and 61% in the older group (aged >55 years) reported at least one adverse event after the third dose. With the exception of a patient reporting chills of moderate severity, adverse events were of mild severity, and no allergic reactions, emergency room visits or hospitalization for local or systemic adverse events were reported. There were no differences between patients with a positive vs. a negative antibody response in rates of local (62% vs. 56%, P=0.756, respectively) or systemic adverse events (19% vs. 22%, P=0.957, respectively).
Local reactions (Table 2 ). Among the vaccine recipients, 60% reported at least one local injection site reaction after the third dose. By age group, 67% in the younger group and 57% in the older group reported at least one local reaction after the third dose. Pain at the injection site was the most frequent solicited local reaction. All local reactions were mild in severity in both age groups.
Table 2.
Local and Systemic Reactions to the Third BNT162b2 Vaccination in Heart Transplant Recipients
| Reactiona | Total cohort n = 96 | Age <55 years n = 33 | Age ≥ 55 years n = 63 | p value |
|---|---|---|---|---|
| Local reactions | ||||
| Any local reaction, n (%) | 57.0 (60.0) | 22.0 (66.7) | 35.0 (56.5) | 0.455 |
| Pain at the injection site, n (%) | ||||
| Mild | 57.0 (60.0) | 22.0 (66.7) | 35.0 (56.5) | 0.455 |
| Redness, n (%) | ||||
| Mild | 1.0 (1.1) | 0 (0.0) | 1.0 (1.6) | 1.000 |
| Swelling, n (%) | ||||
| Mild | 1.0 (1.1) | 0 (0.0) | 1.0 (1.6) | 1.000 |
| Systemic reactions | ||||
| Any systemic reaction | 19.0 (20.0) | 13.0 (39.4) | 6.0 (9.7) | 0.001 |
| Fever, n (%) | ||||
| Any | 3.0 (3.2) | 0.0 (0.0) | 1.0 (1.6) | 0.573 |
| Fatigue, n (%) | ||||
| Mild | 16.0 (16.8) | 11.0 (33.3) | 5.0 (8.1) | 0.004 |
| Headache, n (%) | ||||
| Mild | 8.0 (8.4) | 6.0 (18.2) | 2.0 (3.2) | 0.035 |
| Chills, n (%) | ||||
| Moderate | 1 (1.1) | 0 (0.0) | 1 (1.6) | 1.000 |
| Vomiting, n (%) | ||||
| Mild | 1 (1.1) | 0 (0.0) | 1 (1.6) | 1.000 |
| Diarrhea, n (%) | None | |||
| New or worsening muscle or joint pain, n (%) | ||||
| Mild | 6 (6.3) | 5 (15.2) | 1 (1.6) | 0.032 |
| Use of antipyretic or pain medication, n (%) | 1 (1.1) | 0 (0.0) | 1 (1.6) | 1.000 |
Mild: no interference with activity; moderate: some interference with activity; severe: prevention of daily activity.
Systemic reactions (Table 2). Among the vaccine recipients 20% reported at least one systemic reaction after the third dose, mainly fatigue and headache. The frequency of systemic adverse events was higher in the younger (39%) than the older age group (10%). Most of the systemic events were mild in severity in both age groups.
At one month (mean 33 days) after the third dose, no clinical episodes of rejection, as suggested by a troponin leak or allograft dysfunction, had occurred, and, no breakthrough infections had been documented.
Immunogenicity of the third dose of BNT162b2 vaccine
Antibody response
Antibody responses before and after the third dose were assessed in all 96 participants. The time between transplantation and the third vaccination was 6.3 (3.5, 13.6) years. The first two doses were given 21 ± 3 days apart, and the third dose was administered 168 ± 18 days after the second dose (Table 1).
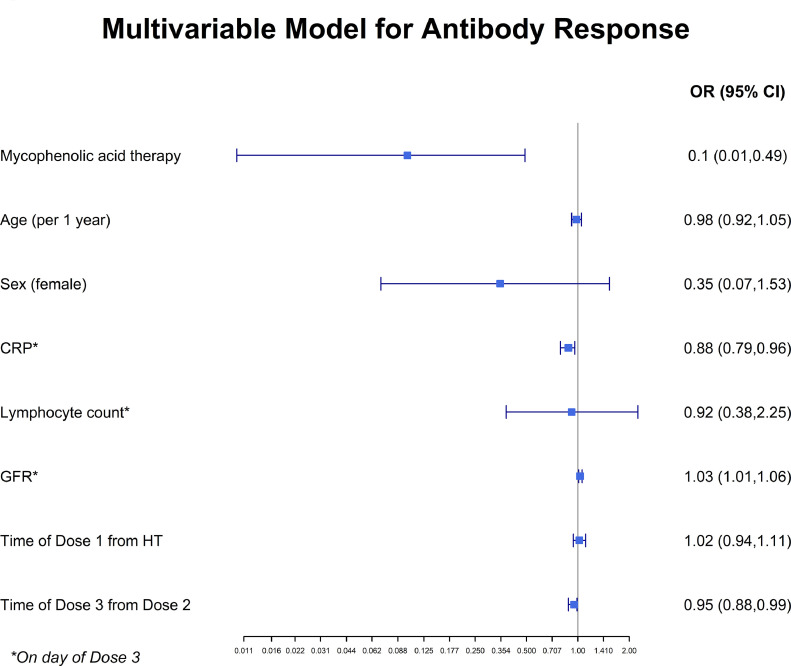
Immediately before the third dose, a positive antibody response was seen in 26 (23%) of the HT recipients. At 18 days following the third dose, a positive antibody response was detected in 64 (67%) of the HT recipients. The geometric mean titer (GMT) for neutralizing antibodies was 3.05 (95% CI, 2.05 to 4.55) before the third dose and 27.25 (95% CI, 15.70 to 47.30) after the third dose, and the GMT for IgG anti-RBD antibodies was 0.49 (95% CI 0.39 to 0.62) and 1.58 (95% CI 1.24 to 2.00), before and after the third dose, respectively (Figure 1 ). The third, booster, dose of the homologous BNT162b2 vaccine elicited SARS-CoV-2 neutralization titers > 9-fold and IgG anti-RBD antibodies > 3-fold of the range achieved after the two primary doses of the vaccine. The immunosuppression characteristics of the patients, by antibody responses, are presented in Table 1. Values for lymphocytes, white blood cells, and the neutrophil/lymphocyte ratio were similar for patients with a positive antibody response vs. those with a negative antibody response. Younger age, higher eGFR and lower C-reactive protein (CRP) values were observed for the positive antibody response group. In an adjusted multivariable logistic regression analysis, mycophenolate use was independently associated with a reduced likelihood of achieving a positive antibody response (OR=0.1, 95% CI 0.01-0.49, P=0.01). Higher eGFR and lower CRP were independently associated with an increased likelihood of achieving a positive antibody response (Figure 2 ).
Figure 1.
Quantitation of receptor-binding domain (RBD) IgG (A) and neutralizing (B) antibodies before and after a third, booster, BNT162b2 dose. Solid lines and numbers indicate the geometric mean titer. In each panel, the horizontal bars indicate the mean geometric titers and the I bars indicate 95% confidence intervals. Dashed line indicates the limit level of positive antibodies.
Figure 2.
Multivariable logistic regression analysis. OR for positive antibody response with 95% CI. Mycophenolate use was independently associated with a reduced likelihood of achieving a positive antibody response. CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HT, heart transplantation; OR, odds ratio.
T-cell response
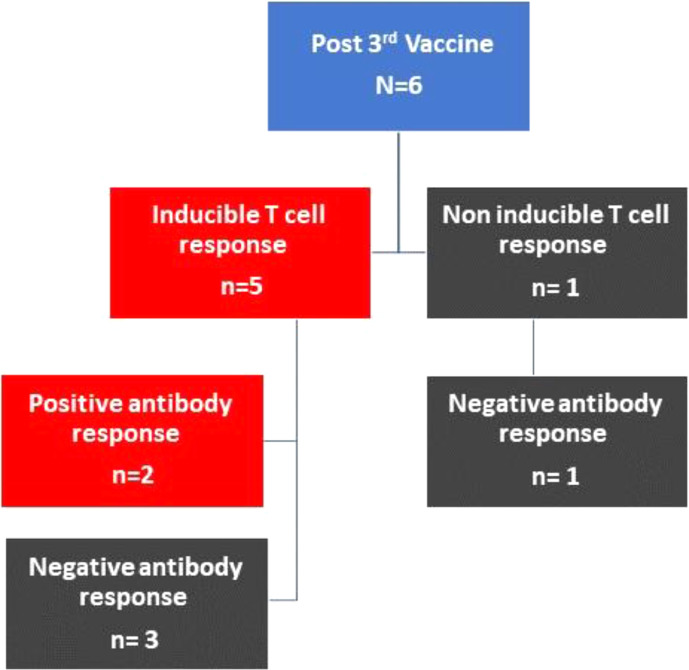
COVID-19 specific cellular T-cell immune responses were assessed in a total of 15 patients (Figure 3 ). Inducible T-cell immunity was demonstrated for 12 (80%) patients after the third dose.
Figure 3.
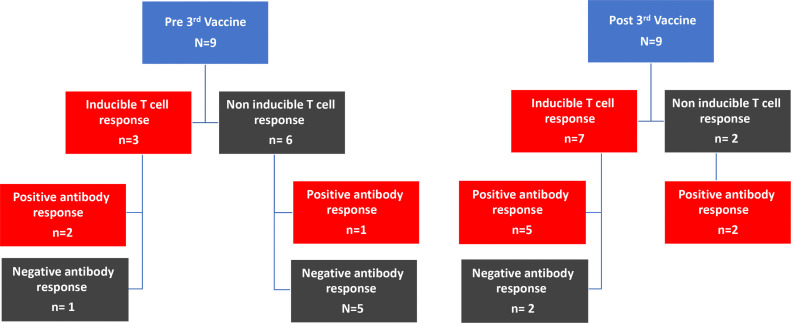
T-cell response COVID-19 specific cellular T cell immune responses assessed in a total of 15 patients. In 9 of these patients (A) the T-cell immune response was measured immediately before giving the third dose and at 19 days following the third dose. For 6 patients not showing an antibody response after 2 doses of vaccine, T cell response was evaluated only after the third dose (B).
In 9 of these patients, the T-cell immune response was measured immediately before giving the third dose and at 19 days following the third dose (Figure 3A). Inducible T-cell immunity was demonstrated for 2 (22%) of the 9 patients before the third dose, but for a higher number, 7 (78%), at 19 days after the third dose. One of the 9 patients did not have specific SAR-CoV-2 antibodies but did show an inducible T-cell immunity response before the third dose; for this patient the inducible T-cell immunity response strengthened after the third dose, but antibodies were still not detected. In 4 patients not demonstrating a T-cell immune response or an antibody response after two doses of the BNT162b2 vaccine, the third dose elicited a strong inducible T-cell immune response in all 4 and a positive antibody response in 3 of the 4 patients. T'wo patients did not demonstrate any induced T-cell response before or after third dose.
For 6 patients not showing an antibody response after 2 doses of the vaccine, the T-cell response was evaluated only at 19 days after the third dose (Figure 3B). Among these 6 patients, 5 (83%) demonstrated an inducible T-cell response at 19 days after the third dose, and of the 5, only 2 demonstrated a positive antibody response to the third dose. One patient did not elicit either a T-cell or an antibody response after the third dose.
Discussion
In this study, a third booster dose of the Pfizer BNT162b2 COVID-19 vaccine was evaluated in 96 HT recipients originally vaccinated with 2 doses of the vaccine, approximately 6 months previously.
In our cohort, the third dose of the BNT162b2 vaccine was associated with a low rate of adverse events, characterized mostly by mild pain at the injection site. No serious adverse events were recorded, and there were no clinical episodes of rejection, as suggested by a troponin leak or allograft dysfunction. At 18 days following the third dose of the vaccine, the positive antibody response increased from 23% to 67%, with a corresponding increase in neutralizing capacity. We found that the third, booster, dose elicited SARS-CoV-2 neutralization titers > 9-fold and IgG anti-RBD > 3-fold of the range achieved after the primary doses of the vaccine. Mycophenolate use, lower eGFR and higher CRP were independently associated with a reduced likelihood of generating an immune response to the vaccine. Importantly, a specific T-cell response following the third, booster, dose was evident in the majority of transplant recipients. Cellular responses were evident in the absence of measurable antibodies, suggesting a cellular benefit, even when there did not appear to be an antibody response.
The first two reports of the administration of a third, booster, dose of mRNA COVID vaccines to SOT recipients have recently appeared in the literature.7 , 8 However, in these two studies only a minority of patients were cardiothoracic transplant recipients, namely, 8/101 and 3/30. In the study of Kamar et al. 7 of 101 SOT recipients, administration of a third booster dose of the BNT162b2 COVID-19 vaccine 2 months after the second dose increased detectable anti-spike antibody levels from 40% to 68% at one month after third dose, and boosted titers, and no serious adverse events were noted. In the report of Werbel et al. 8 that included 30 SOT recipients, antibody titers increased after the third dose (24-101 days after dose 2) in one third of the patients (n=6) who had negative antibody titers after the first 2 doses (n=24) and in all the patients who had low-positive antibody titers (n=6). One HT recipient developed rejection within a week following the booster dose. Nevertheless, while anti-RBD antibody titer does not allow firm conclusions to be drawn about their role in neutralizing infectivity, neutralizing antibody levels have been shown to be highly predictive of immune protection from symptomatic SARS-CoV-2 infection.13 , 19 We therefore tested the quality of induced anti-RBD antibodies and quantified both the magnitude of anti-RBD IgG response and the percentage neutralization of spike-receptor binding—as an in vitro surrogate for protection following vaccination—following the third booster dose. We demonstrated that the booster dose elicited a 9-fold increase in SARS-CoV-2 neutralization titers. Our data is consistent with a recently published randomized trial of a third dose of the mRNA1273 vaccine (Moderna) given to 120 organ transplant recipients, including 18 HT recipients, 3 months after the second dose10; 33 of 60 patients (55%) who received the mRNA-1273 vaccine and 10 of 57 patients (18%) who were given a placebo exhibited an anti-RBD antibody level above the threshold level. There was a minimal polyfunctional CD8+ T-cell response in both groups. Our data for a booster dose of the mRNA BNT162b2 vaccine thus complements these results.
It was recently reported that for a non-compromised population of young healthy adults, a three-dose administration of ZF2001 (a protein subunit vaccine targeting the RBD of the SARS-CoV-2 S protein) enhanced antibody responses compared with a two-dose protocol and showed seroconversion rates of neutralizing antibodies of between 92% and 97%.20 Similarly, a third dose of the ChAdOx1 nCoV-19 vaccine (a chimpanzee adenovirus-vectored vaccine), also known as AZD1222, given to non-immunocompromised patients, induced a strong boost to immune responses to the SARS-CoV-2 spike protein and higher neutralizing antibody titers and enhanced activity against variants.21 In addition – also for the non-compromised population – an ongoing clinical trial is assessing boostability with a third dose of BNT162b2 at 30 µg or at lower dose (5 or 10 µg) or a third and potentially a fourth dose of a prototype vaccine based upon the South African variant at 30 µg (ClinicalTrials.gov Identifier: NCT04368728).
In the realm of research currently being devoted to understanding all aspects of the SARS-CoV-2, further work is warranted to better understand the vaccine-induced immune response. Findings of particular interest that emerged from our study were that patients with no detectable humoral response, even after the third dose, nevertheless exhibited a specific T-cell response. The detection of specific T-cell responses in individuals lacking detectable circulating antibodies has also been described in vaccinated SOT recipients and convalescents of COVID-19 infections.22 Although the first line of defence against reinfection comprises pre-existing antibodies, vaccine-induced protection is not necessarily paralleled by the priming vaccine induced antibody response or by the presence of serum antibodies,23 but rather it may be attributed to effective long-term memory cells. B-cell activation through interaction with an antigen may result in differentiation either into activated effector B cells – ultimately becoming antibody-secreting plasma cells – or memory B cells. Plasma cells have a short life span, whereas memory cells with somatically mutated antigen receptors are believed to be long lived.24 , 25 In terms of the cellular response, immunization with a primary single dose of vaccine might result in a poor effector cell response (and ultimately a poor secreting response) but in the efficient development of memory B cells.26 Thus, a stepwise elevation of antibody production by repeated injections may not be mandatory for the development of long-term memory cells. Also, cross-reactivity with a former corona virus infection cannot be ruled out as a possible explanation for the presence of a specific T-cell response with no detectable humoral response.27 , 28
As the pandemic proceeds, concerns are being raised that emergence of SARS-CoV-2 variants might erode the effectiveness of natural and vaccine-elicited immunity. Indeed, the B.1.617.2 (Delta) variant of SARS-CoV-2 is the strain behind of the recent surge of cases worldwide. In Israel, 'the fourth wave' is threatening the success of the vaccination program against COVID-19. In deciding whether to embark on a program of a third vaccination for our HT patients, we had to take into consideration whether the currently available vaccine would be effective against the Delta strain. In this regard, we note that a recent study evaluating the effectiveness of the BNT162b2 and ChAdOx1 nCoV-19 vaccines against the Delta variant demonstrated only modest differences in vaccine effectiveness for the Delta variant as compared with the Alpha variant after two vaccine doses. For the BNT162b2 vaccine, the effectiveness of two doses was 93.7% (95% CI, 91.6 to 95.3) in people with the Alpha variant and 88.0% (95% CI, 85.3 to 90.1) in those with the Delta variant.29 Importantly, a study assessing neutralizing-antibody responses against the original virus first identified in Wuhan (WA1/2020) and the B.1.617.2 (Delta) variant showed that the B.1.617.2 variant was 2.9 times less susceptible to neutralization by serum from vaccinated persons than the WA1/2020 variant. Nonetheless, all serum samples from vaccinated persons still had detectable neutralizing activity above the threshold of detection against the Delta variant.30
Our finding of an association of immune paresis with the use of mycophenolate expend on the previously reported correlations following the second dose,2 , 3 but should be carefully interpreted before any clinical actions carried out, given the potential hazardous implications associated with withdrawal of antimetabolite therapy.3 The enhanced immune response demonstrated in our study following the third booster dose and the association with lower mycophenolate use might shed light on the previously observed more robust immune response in lung transplant patients after COVID-19 in contrast to the minimal response following vaccination. Our findings reinforce the previously suggested explanation3 that transplant patients might require a higher antigen load, as is achieved in natural infection, to overcome, at least partially, the immune paresis, perhaps promoted by the use of antimetabolite therapy.
We also demonstrated higher CRP to be inversely related to vaccine responsiveness. This finding is in keeping with a recent report of the association of proinflammatory markers with negative vaccine responsiveness. It was suggested that the mechanism of this phenomenon may be related to an inflammation-dependent expansion of immunosuppressive regulatory T cells, previously reported to limit vaccine responsiveness, partly through the suppression of memory T-cell differentiation. 31
The strength of our study lies in three directions: 1) Neutralizing antibodies were assessed. The importance of neutralization assays is emphasized by data derived from trials in the general population, demonstrating a correlation between the level of neutralizing antibodies to the SARS-CoV-2 spike protein and symptomatic disease.13 2) We have provided evidence of cellular responses in the absence of measurable antibodies, emphasizing the importance of implementing cellular response assessment in surveillance and in the development of boosting strategies.32 3) By reporting in detail our findings for immunosuppression and our laboratory data, we have created a basis for assessment of the need for adjustment of immunosuppression in SOT recipients in anticipation of booster vaccination.
What, now, requires further study with the aim to enhance the protective vaccine-based immune responses to SARS-CoV-2? Among the open questions that remain to be addressed is the need to clarify the efficacy of generating high levels of memory T cells33, 34, 35, 36 by sequential administration of a different vaccine for repeat vaccination (heterologous prime/boost) vs. a strategy of using the same vaccine (homologous prime/boost). Questions also remain about the interval between doses. For example, for ChAdOx1 nCoV-19, a long extension of the dose interval (up to 45 weeks) between the first and second doses enhanced the immune response to the second dose vs. shorter dose intervals.21
Several potential limitations of the current study should be highlighted. While this study suggests a favorable safety profile, it was not designed to establish the vaccine clinical efficacy or the long-term effects of vaccination in HT patients. There is not yet an established threshold for vaccine-induced immune responses and protection from SARS-CoV-2 infection; thus, we have not confirmed clinical immunity beyond demonstrating that the third dose did indeed boost antibody responses. In-vitro virus neutralization studies using postimmunization serum have an inherent limitation as a substitute for clinical evidence of vaccine-mediated protection or escape from that protection. The continuous emergence of new SARS-CoV-2 variants necessitates real-time dynamic evaluation and adjustment considerations for both diagnostic and therapeutic approaches. Thus, identification of appropriate molecular targets across viral variants and understanding the binding modalities between these viral variants and the host cell receptor are important for the development and adjustment of diagnostic assays, vaccines and neutralizing antibodies. Finally, rare cases of myocarditis associated with COVID-19 mRNA vaccines and a single case of an early rejection episode have been reported, and thus, despite our reassuring data, larger prospective randomized studies and registry data are required.
In conclusion, an homologous third booster dose of the BNT162b2 vaccine gave overall consistent tolerability and a good safety profile, while eliciting humoral and cellular immune responses. There is an urgent need to optimize vaccination strategies, particularly for the immune compromised population, and our study opens the way towards achieving this goal, fostering hope for the protection against infection.
Disclosure statement
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
Acknowledgment
The authors gratefully acknowledge the invaluable contribution of Ms. Hana Algazi-Patal, the coordinator of heart transplants at the Sheba Medical Center, Ms. Sarit Skiano, Ms. Michal Kelishek, Ms. Merav Moreno, Ms. Tal Aharon Ms. Eitana Mor, Ms. Ravid Amitai and Mr. Aharon Greitzer, of the Heart Transplant Unit, Sheba Medical Center, for organizing the vaccination effort for our cohort. We thank our editor, Ms. Inez Mureinik, for critical reading of the manuscript.
References
- 1.Jackson LA, Anderson EJ, Rouphael NG, et al. mRNA-1273 Study Group. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [PMID: 32663912] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peled Y, Ram E, Lavee J, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40:759–762. doi: 10.1016/j.healun.2021.04.003. Epub 2021 Apr 21. PMID: 34034958; PMCID: PMC8058049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslam S, Danziger-Isakov L, Mehra MR. COVID-19 vaccination immune paresis in heart and lung transplantation. J Heart Lung Transplant. 2021;40:763–766. doi: 10.1016/j.healun.2021.04.018. Epub 2021 May 13. PMID: 34144891; PMCID: PMC8116313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali NM, Alnazari N, Mehta SA, et al. Development of COVID-19 infection in transplant recipients after SARS-CoV-2 vaccination. Transplantation. 2021;105:e104–e106. doi: 10.1097/TP.0000000000003836. [PMID: 34049360] [DOI] [PubMed] [Google Scholar]
- 5.Wadei HM, Gonwa TA, Leoni JC, et al. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. Published online April 23, 2021 doi: 10.1111/ajt.16618. 10.1111/ajt.16618Epub ahead of print. PMID: 33890410; PMCID: PMC8251487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minervina AA, Pogorelyy MV, Kirk AM, et al. Convergent epitope-specific T cell responses after SARS-CoV-2 infection and vaccination. posted medRxiv. 2021 doi: 10.1101/2021.07.12.2126022.7. [DOI] [Google Scholar]
- 7.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 Vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. Published online June 15, 2021 doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. Published online July 23, 2021 doi: 10.1001/jama.2021.12339. Published online July 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. Published online August 11, 2021 doi: 10.1056/NEJMc2111462. Epub ahead of print. PMID: 34379917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Available at SSRN: https://ishlt.org/ishlt/media/documents/ISHLT-AST_SARS-CoV-2-Vaccination_7-16-21.pdf
- 12.Danziger-Isakov L, Kumar D, AST ID Community of Practice Vaccination of solid organ transplant candidates and recipients: guidelines from the American Society of Transplantation Infectious Diseases community of practice. Clin Transplant. 2019;33:e13563. doi: 10.1111/ctr.13563. Epub 2019 Jun 5. Erratum in: Clin Transplant. 2020 Mar;34(3):e13806. PMID: 31002409. [DOI] [PubMed] [Google Scholar]
- 13.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. Epub 2021 May 17. PMID: 34002089. [DOI] [PubMed] [Google Scholar]
- 14.Peled Y, Lavee J, Raichlin E, et al. Metformin therapy reduces the risk of malignancy after heart transplantation. J Heart Lung Transplant. 2017;36:1350–1357. doi: 10.1016/j.healun.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Available at SSRN:https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine.
- 16.Oved K, Olmer L, Shemer-Avni Y, et al. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indenbaum V, Koren R, Katz-Likvornik S, et al. Testing IgG antibodies against the RBD of SARS-CoV-2 is sufficient and necessary for COVID-19 diagnosis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieterle ME, Haslwanter D, Bortz RH, 3rd, et al. A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition. Cell Host Microbe. 2020;28 doi: 10.1016/j.chom.2020.06.020. 486-496.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dispinseri S, Secchi M, Pirillo MF, et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun. 2021;12:2670. doi: 10.1038/s41467-021-22958-8. PMID: 33976165; PMCID: PMC8113594.s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S, Li Y, Dai L, Wang J, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21:1107–1119. doi: 10.1016/S1473-3099(21)00127-4. Mar 24Epub ahead of print. PMID: 33773111; PMCID: PMC7990482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flaxman A, Marchevsky Jenkin D, et al. The Oxford COVID vaccine, tolerability and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 (AZD1222). Accessed September 16, 2021. https://ssrn.com/abstract=3873839
- 22.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. doi: 10.1016/j.cell.2020.08.017. Oct 1158-168.e14Epub 2020 Aug 14. PMID: 32979941; PMCID: PMC7427556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West DJ, Calandra GB. Vaccine induced immunologic memory for hepatitis B surface antigen: implications for policy on booster vaccination. Vaccine. 1996;14 doi: 10.1016/0264-410x(96)00062-x. 1019-27. [DOI] [PubMed] [Google Scholar]
- 24.Ho F, Lortan JE, MacLennan IC, et al. Distinct short-lived and long-lived antibody-producing cell populations. Eur J Immunol. 1986;16:1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- 25.Schittek B, Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990;346:749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 26.Wiström J, Ahlm C, Lundberg S, Settergren B, et al. Booster vaccination with recombinant hepatitis B vaccine four years after priming with one single dose. Vaccine. 1999;17:2162–2165. doi: 10.1016/s0264-410x(99)00012-2. PMID: 10367949. [DOI] [PubMed] [Google Scholar]
- 27.Petrone L, Petruccioli E, Vanini V, et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin Microbiol Infect. 2021;27:286.e7–286.e13. doi: 10.1016/j.cmi.2020.09.051. Epub 2020 Oct 10. PMID: 33045370; PMCID: PMC7547312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mateus J, Dan JM, Zhang Z, et al. Low dose mRNA-1273 COVID-19 vaccine generates durable T cell memory and antibodies enhanced by pre-existing crossreactive T cell memory. medRxiv 2021.06.30.21259787; https://doi.org/10.1101/2021.06.30.21259787 [DOI] [PMC free article] [PubMed]
- 29.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. Epub ahead of print. PMID: 34289274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edara VV, Pinsky BA, Suthar MS, et al. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med. 2021;385:664–666. doi: 10.1056/NEJMc2107799. Epub 2021 Jul 7. PMID: 34233096; PMCID: PMC8279090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verschoor CP, Lelic A, Parsons R, et al. Serum C-reactive protein and congestive heart failure as significant predictors of herpes zoster vaccine response in elderly nursing home residents. J Infect Dis. 2017;216:191–197. doi: 10.1093/infdis/jix257. PMID: 28838148; PMCID: PMC5853411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Havlin J, Svorcova M, Dvorackova E, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40:754–758. doi: 10.1016/j.healun.2021.05.004. Epub 2021 May 21. PMID: 34120839; PMCID: PMC8139179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palgen JL, Feraoun Y, Dzangué-Tchoupou G, et al. Optimize prime/boost vaccine strategies: Trained immunity as a new player in the game. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.612747. PMID: 33763063; PMCID: PMC7982481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Normark J, Vikström L, Gwon YD, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N Engl J Med. 2021;385:1049–1051. doi: 10.1056/NEJMc2110716. Epub ahead of print. PMID: 34260850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt T, Klemis V, Schub D, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. Epub ahead of print. PMID: 34312554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, He Q, An C, et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerg Microbes Infect. 2021;10:1598–1608. doi: 10.1080/22221751.2021.1957401. Epub ahead of print. PMID: 34278956. [DOI] [PMC free article] [PubMed] [Google Scholar]