Abstract
Acupuncture is widely recognized as a potentially effective treatment for stroke rehabilitation. Researchers in this area are actively investigating its therapeutic mechanisms. Magnetic resonance imaging (MRI), as a noninvasive, high anatomical resolution technique, has been employed to investigate neuroplasticity on acupuncture in stroke patients from a system level. However, there is no review on the mechanism of acupuncture treatment for stroke based on MRI. Therefore, we aim to summarize the current evidence about this aspect and provide useful information for future research. After searching PubMed, Web of Science, and Embase databases, 24 human and five animal studies were identified. This review focuses on the evidence on the possible mechanisms underlying mechanisms of acupuncture therapy in treating stroke by regulating brain plasticity. We found that acupuncture reorganizes not only motor-related network, including primary motor cortex (M1), premotor cortex, supplementary motor area (SMA), frontoparietal network (LFPN and RFPN), and sensorimotor network (SMN), as well as default mode network (aDMN and pDMN), but also language-related brain areas including inferior frontal gyrus frontal, temporal, parietal, and occipital lobes, as well as cognition-related brain regions. In addition, acupuncture therapy can modulate the function and structural plasticity of post-stroke, which may be linked to the mechanism effect of acupuncture.
1. Introduction
Stroke is a common disease that affects one in four people during their lifetime [1], globally, and it continues to be a leading cause of death and long-term disability worldwide, imposing a significant financial burden on healthcare systems and families [2, 3]. Although stroke incidence and prevalence have declined worldwide, however, a recent national epidemiological survey [4, 5] indicated that China has an estimated 11 million prevalent cases of stroke, 2.4 million new cases of stroke, and 1.1 million stroke-related deaths. Hemiparesis and aphasia are two of the prominent impairments caused by a stroke that affect activities of daily living activities and quality of life [6–8]. More than 80% of poststroke patients experience upper or lower limb hemiplegia, severely disturbing their daily activities [9]. Some studies have found that almost 20%-40% of all stroke survivors have chronic aphasic symptoms [10, 11]. It is well known that returning to work and social activities is the key priority for stroke survivors. Therefore, it is critical to understand stroke pathogenesis and explore its appropriate treatment.
Previous studies [12, 13] have demonstrated that poststroke patients have structural and connectivity changes in their brains. Luckily, the brain's plasticity, a broad term for the proof the human brain to adapt to environmental pressure, experiences, and challenges including brain damage [14, 15], enables stroke rehabilitation. Although many patients experience some degree of spontaneous recovery, that is, a time-determined amount of improvement in physical function and activity [16], it is often incomplete and the recovery rates of neurological function vary. Therefore, external stimulus interventions are still needed. However, despite extensive research efforts on multiple treatment modalities, no single rehabilitation intervention has been demonstrated to be definitively beneficial for recovery [17]. Even the most commonly used repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) were not recommended for routine stroke treatment in two Cochrane reviews [18, 19]. Due to a lack of effective therapy, researchers considered alternative approaches that improve stroke recovery. As a relatively inexpensive and safe treatment, acupuncture has been widely employed to improve motor, sensation, and some neurological functions of stroke for thousands of years. Furthermore, several clinical [20–22] research and systematic reviews [17, 23, 24] revealed that acupuncture, as a promising intervention, could improve motor and language function and daily living activities. Numerous studies [25–27] have suggested that plasticity and reorganization contribute to the recovery.
However, the current understanding of neuroplasticity after stroke is primarily based on invasive methods, such as histology and immunohistochemistry, which do not allow for dynamic assessment of functional recovery and tissue remodeling [28]. In contrast, magnetic resonance imaging (MRI) can noninvasively monitor dynamic change after stroke and in vivo. Structural magnetic resonance imaging (sMRI) technique can provide a high anatomical resolution [29], whereas functional magnetic resonance imaging (fMRI) can reveal real-time brain activity by indirect measurement of regional blood flow [30]. Combined with sMRI and fMRI, the central nervous effect of acupuncture for stroke could be fully elucidated from an anatomical and functional perspective. Moreover, emerging clinical studies have demonstrated that acupuncture could reorganize motor-related networks and increase functional connectivity between premotor cortex (PM)/adjacent supplementary motor area (SMA) and supramarginal gyrus (SMG) [31–33]. In addition, acupuncture therapy has various properties, such as the choice of acupoints, whether deqi or not, which may be the influencing factors of acupuncture on the plasticity of stroke patients.
Nevertheless, the underlying neuroplasticity mechanisms on acupuncture for stroke have received little attention to date. Therefore, the review will mainly focus on the evidence to elucidate the possible mechanisms of acupuncture therapy in treating stroke through regulating brain plasticity based on MRI to better select and stratify patients for future appropriate treatment strategies that promote poststroke recovery. We firstly describe research characteristics of acupuncture for stroke based on MRI. Then, we discuss the neuroplasticity mechanism of acupuncture and its properties on stroke. Furthermore, we also review the limitations and prospects to be explored in the future.
2. Materials and Methods
We conducted a literature search for MRI studies on acupuncture for stroke published in PubMed, Web of Science, and Embase from inception to April 9, 2021. Database searches were conducted using the following keywords: (acupuncture OR electroacupuncture OR moxibustion) AND (stroke OR cerebral ischemia OR ischemic cerebrovascular disease OR hemiparesis or hemiplegia OR post-stroke) AND (MRI OR magnetic resonance imaging OR functional MRI and structural MRI OR BOLD OR ReHo OR ALFF OR fALFF OR white matter OR voxel-based analysis OR VBM OR voxel-based morphometry OR Freesurfer OR surface-based morphometry OR cortical thickness OR surface area OR cortical volume OR gray matter volume OR gray matter density OR DTI). Studies were eligible if they met the following inclusion criteria: (1) randomized controlled trials (RCTs) and nonrandomized studies (i.e., observational studies, case-control studies, and cohort studies); (2) patients met established diagnostic criteria of stroke; (3) subjects in the study at least underwent MRI of the brain on one occasion: under the acupuncture state or before and after acupuncture treatment. We excluded studies that met the following criteria: (1) protocol, case reports, or case series. (2) Other interventions that do not belong to traditional acupuncture, such as transcutaneous electrical nerve stimulation and transcutaneous vagus nerve stimulation. (3) Comorbid severe mental illness or neurological illness.
All identified studies were imported into EndNote; duplicate studies were removed first, and then after scanning titles and abstracts and reading the full text, eligible studies were decided whether they should be included in the review.
Two authors extracted the following data: publishing year, author, number of participants, type of ischemic stroke, intervention/control groups, needling details, types of acupuncture, acupuncture points, data analysis, and experimental design. Any inconsistencies were discussed and resolved with the third author until an agreement is reached. Twenty-four human studies and five animal studies were finally included (Figure 1).
Figure 1.
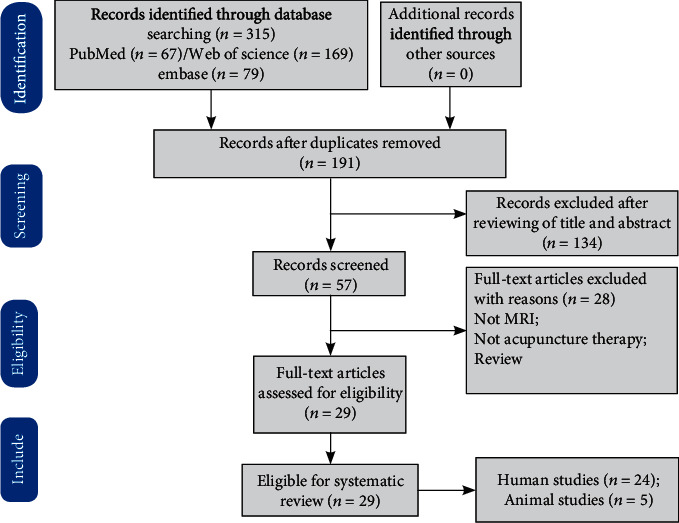
PRISMA flow diagram. Note: PRISMA: preferred reporting items for systematic reviews and meta-analyses.
In this review, 24 stroke patient studies were included that use MRI to investigate the mechanism of acupuncture for stroke. The results indicated that acupuncture could modulate brain plasticity in motor-related and language-related networks of stroke patients. For acupuncture modality, 20 studies applied manual acupuncture (MA), and two studies used electroacupuncture (EA). Stroke types were found to be related to ischemic stroke. The publication years ranged from 2006 to 2020, indicating that research on this aspect has gradually become a research hotspot over the last 15 years. The sample size of the study ranged from 7 to 43 (mean 24). The study design mainly has several kinds: before vs. after acupuncture, acupuncture vs. sham acupuncture (SA), acupuncture vs. waiting group, patients vs. healthy controls (HC), and acupuncture plus drugs/conventional therapy vs. drugs/conventional therapy. In addition, except for five studies [34–38] with resting-state (RS) and long-term effects, all other studies investigated task-stating and instant effects. The detailed characters of included studies are listed in Table 1. In addition, five animal experiments also were included. The years of publication ranged from 2011 to 2021, and the study design mainly includes middle cerebral artery occlusion (MCAO) vs. MCAO plus EA group vs. sham operation group and EA vs. non-EA group. The detailed characters of the included studies are listed in Table 2.
Table 1.
Characteristics of the 24 included stroke patients studies.
| N | Author (year) | Journal | Stroke information | MRI information | Acupuncture information | Data analysis | Experimental design | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | Affected side | Type of stroke, lesions (N) | Interval since stroke | Scanner | Intervention | Comparison | Acupoints | |||||
| 1 | Li et al. 2006 [39] | Journal of magnetic resonance imaging | 12 stroke | The left side somatosensory deficits | IS, right hemispheric striatocapsular infarction | More than 6 months | 1.5 T | MA | Stroke vs. HC | LI4 and LI11 | SPM | Block/R(45 s)-S(45 s), 3 times |
| 2 | Schaechter et al. 2007 [34] | The journal of alternative and complementary medicine | 7 stroke | NA | IS (5), HS (2), 5 left, and 2 right | 4.6 ± 3.2 years | 3 T | MA, SA (Streitberger needle, noninvasive control) | 4 VA vs. 3 SA | N | GLM | RS/twice weekly for 10 weeks. |
| 3 | Li and Yang 2010 [40] | Complementary therapies in medicine | 7 aphasia stroke/14 HC | The right side of the body | IS (6), HS (1); the occlusion of the middle cerebral artery, left hemisphere | More than 6 months | 1.5 T | EA | Stroke vs. HC | SJ8 | SPM | Block/R(45 s)-S(45 s), 3 times |
| 4 | Huang et al. 2011 [41] | NRR | 12 stroke | The left hemiataxia and sensory disturbance | IS, right hemisphere | 6.08 ± 6.40 months | 3 T | MA, SA (nonacupuncture points in close proximity to acupuncture points) | 6 VA vs. 6 SA | SJ5 | ReHo | Block/S(30 s)-R(30sn), 6 times |
| 5 | Shen et al. 2012 [37] | ECAM | 20 stroke | Basal ganglia, and completely or partially covered the internal capsules | IS | 10.70 ± 11.13 hours | 1.5 T | MA | 10 acupuncture plus conventional treatments vs. 10 only conventional treatments | Du23, Du 20, EX-HN3, PC 6, and Sp 6 | FA and ADC | RS/30 min, once a day, for 2 weeks |
| 6 | Cho et al. 2013 [42] | Chinese journal of integrative medicine | 11 stroke/10 HC | The left side of the body | IS, right hemisphere | 2-6 months | 3 T | MA | Stroke vs. HC | LI11 and ST36 | SPM | Block/R(30 s)-S(30 s), 3 times |
| 7 | Huang et al. 2013 [43] | Acupuncture in medicine | 10 stroke | The right hemiplegia | IS, left hemispheric | 1-12 months | 3 T | MA | Before vs. after | SJ5 | GLM | Tactile control (6 min)-R(5 min)-block/S(30 s)-R(30 s), 6 times |
| 8 | Chen et al. 2013 [44] | NRR | 10 stroke/6 HC | The right hemiataxia | IS, left basal ganglia | 5.30 ± 3.71 months | 3 T | MA | Stroke vs. HC | SJ5 | FC | Block/R(30s)-S(30s), 6 times |
| 9 | Bai et al. 2014 [31] | ECAM | 9 stroke/8 HC | The left side of the body | IS, right hemispheric striatocapsular | 2-12 weeks | 3 T | MA | Before vs. after | GB34 | FC | NRER/R(1 min)-S(1 min)-R(8 min) |
| 10 | Chen et al. 2014 [45] | PLoS one | 24 stroke | The right hemiplegia | IS, left basal ganglia | 1 month-1 year | 3 T | MA, SA (tactile control, a noninvasive control) | 12 VA vs. 12 SA | SJ5 | FC | Block/rested for 5 min-SA(6 min 30 s)-R(6 min 2 s)-VA(6 min 30 s). |
| 11 | Qi et al. 2014 [46] | NRR | 16 stroke | The right hemiataxia | IS, primarily in the left hemisphere | 4.63 ± 3.85 months/4.63 ± 4.41 months | 3 T | MA, SA (nonacupuncture points in close proximity to acupuncture points) | 8 VA vs. 8 SA | SJ5 | SPM | Block/R(30 s)-S(30 s), total 6 min 6 s |
| 12 | Xie et al. 2014 [32] | ECAM | 9 stroke/8 HC | The left side of the body | IS, unilateral right-sided striatocapsular lesions | 53.6 ± 41.6 days | 3 T | MA | Stroke vs. HC | GB34 | GLM and GCA | NRER/R(1 min)-S(1 min)-R(8 min) |
| 13 | Zhang et al. 2014 [47] | ECAM | 8 stroke/10 HC | The left side of the body | IS, right hemispheric corona radiate, internal capsule, or basal ganglia infarction | 2–12 weeks | 3 T | MA | Stroke vs. HC | GB34 | SPM | NRER/R(1 min)-S(1 min)-R(8 min) |
| 14 | Li et al. 2015 [48] | NRR | 12 stroke | The right hemiataxia | IS, left basal ganglia | 1 month-12 months | 3 T | MA | Before vs. after | SJ5 | SPM | Block/(30 s)-S(30 s), 6 times |
| 15 | Gao et al. 2015 [49] | Experimental and therapeutic medicine | 10 stroke/10 HC | NA | IS, right subcortical | At least 6 months | 3 T | MA | Stroke vs. HC | ST36 | GLM | Block/(30 s)-S(30 s), 6 times |
| 16 | Chang et al. 2017 [50] | Wiener klinische Wochenschrift | 43 poststroke motor aphasia | NA | Cerebral hemorrhage or cerebral infarction | 14 days to 2 years | 3 T | EA | 22 EA vs. 21 WT | HT5, GB39 | GLM | Block/R(30 s)-S(30 s), total 6 min 6 s |
| 17 | Fu et al. 2017 [51] | Medicine | 19 stroke/17 HC | The left hemiplegia | IS, internal capsule, and neighboring regions in the right hemisphere | 2 weeks to 6 months | 3 T | MA | Before vs. after | GB34 | ICA | RS/R(8 min 10 s)-needing in (1 min)-S(1 min)-R(8 min 10 s) |
| 18 | Ning et al. 2017 [52] | Frontiers in human neuroscience | 18 stroke/20 HC | The left motor hemiparesis | First-ever IS, right subcortical stroke | Within 6 months after the onset | 3 T | MA | Before vs. after | GB34 | GLM, FC | NRER/R(1 min)-S(1 min)-R(8 min) |
| 19 | Li et al. 2017 [35] | Neural plasticity | 17 stroke/14 HC | The right side of the body | IS, left basal ganglia, caudate nucleus, centrum semiovale, and lenticular nucleus | At least three weeks | 3 T | MA | 8 MA + drug vs. 9 drug | DU20, GB20, bilateral GB-39, LI-11, LI-4, ST-36, SP-6 | FC | RS/two hours a day for 5 days a week, one week a course, continuous four courses |
| 20 | Wu et al. 2017 [38] | Journal of traditional Chinese medicine | 21 stroke | NA | IS | Less than six months | 3 T | MA | 11 MA plus CT vs. 10 CT | DU20, GB20, LI11, LI4, GB34, ST36, SP6, and GB39 | ReHo | RS/30 min, 2 times/week for 5 weeks |
| 21 | Wu et al. 2018 [36] | ECAM | 21 stroke | NA | IS | Less than six months | 3 T | MA | 11 MA plus CT vs. 10 CT | DU20, GB20, LI11, LI4, GB34, ST36, SP6, and GB39 | VBM | RS/30 min, 2 times/week for 5 weeks |
| 22 | Han et al. 2019 [33] | ECAM | 22 stroke/22 HC | The left side of the body | IS, right-hemispheric subcortical infarct | 41.68 ± 25.02 days | 3 T | MA | Stroke vs. HC | GB34 | FC | NRER/R(8 min 10 sec)-S(1 min)-R(8 min 10 sec) |
| 23 | Chen et al. 2020 [53] | Chinese journal of integrative medicine | 10 stroke | The left side of the body | IS, the vascular occlusion in the right basal ganglia | 1 month-3 years | 3 T | MA | Before vs. after | LI11 and ST36 | ReHo | RS/R(5 min)-S(15 min)-R(5 min) |
| 24 | Han et al. 2020 [54] | Neural plasticity | 26 stroke/21HC | The left side of the body | IS, right hemispheric subcortical infarct | 41.04 ± 29.71 days | 3 T | MA | Stroke vs. HC | GB34 | Graph theoretical network | RS/R(8 min 10 s)-S(60 s)-R(8 min 10 s) |
Note: ADC: apparent diffusion coefficient; BOLD: blood oxygen level-dependent; CT: conventional treatments; EA: electroacupuncture; ECAM: Evidence-Based Complementary and Alternative Medicine; FA: fractional anisotropy; FC: functional connectivity; GCA: granger causality analysis; GLM: general liner model; HC: health controls; HS: hemorrhagic stroke; IS: ischemic stroke; MA: manual acupuncture; N: number; NA: not applicable; NRER: nonrepeated event-related; RS: resting state; NRR: Neural Regeneration Research; ReHo: regional homogeneity; TBSS: tract-based spatial statistics; R: rest; s: seconds; SA: sham acupuncture; S: stimulation; VA: verum acupuncture; min: minutes; VBM: voxel-based morphometry; WT: waiting list; ICA: independent component analysis; Y: yes. A.
Table 2.
Characteristics of the five included animal studies.
| Study (years) | Journal | Stroke information | MRI information | Groups | Acupuncture information | Data analysis | |||
|---|---|---|---|---|---|---|---|---|---|
| Main symptoms | Species | The affected side | Magnet strength (T) | Treatment group | Acupoints | ||||
| Zhang et al. 2011 [55] | Brain injury | Middle cerebral artery occlusion (MCAO) model | SD rats, after 24 hours of the surgery | Left side | NA | (1) MCAO, n = 6 (2) MCAO + EA, n = 6 |
EA, 30 minutes | DU20 | DWI |
| Wu et al. 2012 [56] | Acupuncture in medicine | Transient middle cerebral artery occlusion (tMCAO) | SD rats, after 30 minutes of the surgery | Left side | 1.5-T | (1) SC, n = 12 (2) tMCAO, n = 12 (3) tMCAO + EA, n = 12 |
MA, 30 minutes for 28 days | DU20, DU14, LI10, and ST36 | ADC value and the FA |
| Liang et al. 2017 [57] | Journal of stroke and cerebrovascular diseases | Motor impairments/middle cerebral artery occlusion (MCAO) | SD rats, after 24 hours of the surgery | Left side | 7.0 T | (1) SC, n = 12 (2) MCAO, n = 9 (3)MCAO + EA, n = 9 |
EA, 30 minutes per day for 7 consecutive days | ST36 and LI11 | ReHo |
| Wen et al. 2018 [58] | Journal of stroke and cerebrovascular diseases | Middle cerebral artery occlusion induced cognitive deficit (MICD) | SD rats, after 24 hours of the surgery | Left side | 7.0 T | (1) SC, n = 12 (2) MICD, n = 12 (3) MICD + EA, n = 12 |
EA, 30 minutes per day for 14 consecutive days | DU20 and DU24 | ALFF |
| Li et al. 2021 [81] | Acupuncture in medicine | Motor impairments/middle cerebral artery occlusion (MCAO) | SD rats, after 24 hours of the surgery | Left side | 7.0 T | (1) SC, n = 12 (2) MCAO, n = 9 (3) MCAO + EA, n = 9 |
EA, 30 minutes per day for 14 consecutive days | LI11 and ST36 | FC, left motor cortex as the seed region |
Note: ALFF: amplitude of low-frequency fluctuations; DWI: diffusion-weighted imaging; SC: sham-operated control; SD: Sprague-Dawley rats.
3. Modulation of Brain Plasticity in Stroke
Stroke alters the landscape of the brain and impairs the function of various systems and structures [59]. One of the most striking features of the brain is its ability to adapt to external and internal stimuli. Indeed, several decades ago, Hebb [60] puts forward a theoretical framework that described the phenomenon of brain adaptation to the environment based on experience and development. The theories of neuroplasticity showed that thinking and learning change both the brain's physical structure and functional organization. Basic mechanisms that are involved in plasticity include neurogenesis, programmed cell death, and activity-dependent synaptic plasticity [61]. As the research progresses, neural plasticity is a general term that refers to functional and structural changes that occur in the brain during development, interaction with the environment, aging, learning, and in response to trauma [62, 63]. Adult brain plasticity following stroke is due to numerous diffuse and redundant connections in the central nervous system and the ability to form new structural and functional circuits through remappings between related cortical regions [64]. MRI has the advantage of providing repeated whole-brain measurements, making it ideal for longitudinal studies of network-level brain plasticity [62].
Brain plasticity occurs at many levels from molecules to cortical reorganization [27]. Advances in MRI technology have allowed system-level monitoring of brain structure and function in vivo. Functional plasticity can be detected through changes in the strength of functional interactions between brain regions, whereas structural changes can be identified in vivo indirectly and nonspecifically via sMRI measures [62].
Pathologically, damage to regions of the motor-related cerebral cortex, such as primary motor area (M1), premotor area (PMA), supplementary motor area (SMA), somatosensory area (S1), prefrontal cortex (PFC), and posterior parietal cortex (PPC) [65], results in hemiplegia. In contrast, damage to regions of the left perisylvian network, including inferior frontal gyrus (IFG), middle frontal gyrus (MFG), angular gyrus (AG), supramarginal gyrus (SMG), superior temporal gyrus (STG), middle temporal gyrus (MTG), inferior temporal gyrus (ITG), and supplementary motor area (SMA), leads to aphasia. Fortunately, a large body of research evidence indicates that the brain recovers rapidly and reorganizes its structure and function following a stroke. In other words, specific linguistic impairments caused by stroke showed substantial recovery in the first few months following a stroke [66], and hemispheric interactions have complex effects on the recovery of brain function after stroke [67, 68].
As research on poststroke recovery increases, one meta-analysis [69] of motor-related neural activity after stroke included 36 studies and demonstrated that consistently activated regions include contralesional primary motor cortex (M1), bilateral ventral premotor cortex, and supplementary motor area (SMA) compared with healthy controls (HC). Interestingly, this is consistent with another meta-analysis [9], which investigated the modulation of interhemispheric activation balance (IHAB) in stroke patients with motor recovery and demonstrated that IHAB is upregulated in sensorimotor cortex(SMC) and premotor cortex (PMC), but not significantly changed in SMA and cerebellum (CB). In addition, several studies also investigated the underlying mechanism of language processing in aphasia and found that early stroke patients showed significantly decreased functional connectivity (FC) in the language network [70]. Rs-fMRI studies also revealed a significant correlation between disrupted functional connectivity and the severity of poststroke language impairment [71].
4. Brain Plasticity in Stroke with Acupuncture
Brain plasticity provides a critical theoretical basis for central nervous system therapy [72]. In this review, 24 human and five experimental studies investigated the neuroplasticity mechanism of acupuncture in treating poststroke motor impairment, motor aphasia, and cognitive impairment from different analytical methods, study, and experimental designs based on MRI. We summarized the findings based on analytic methods and different rehabilitation aspects.
4.1. Stroke Patients' Studies
FC provides one method based on a system-level approach to quantify the functional integration of various brain regions by correlating brain activity to detect neural interactions between regions, which are quite compelling [73]. Moreover, FC analyses can provide experience-dependent plasticity at the macro level of large-scale functional networks, which are foundational to remediation interventions that maximize function recovery [74]. FC of the three studies used M1 as the region of seed interest, and the results revealed that acupuncture increased FC between left primary motor cortex (M1) and right M1, premotor cortex, supplementary motor area (SMA), thalamus, and cerebellum.
The Granger causality analysis is used to analyze the flow of information between time series, which has been widely used in the field of neuroscience [75]. A study [32] used the multivariate Granger causal analysis method and found that acupuncture induced a concentrated and bidirectional enhancement in effective connectivity between cerebellum and primary sensorimotor cortex in stroke patients. In addition, acupuncture probably integrated the effective connectivity internetwork by modulating multiple networks and transferring information between left frontoparietal network (LFPN) and sensorimotor network (SMN) by default mode network (aDMN and pDMN) as the relay station [51].
Graph theoretical analysis provides an uncomplicated but powerful mathematical framework to describe topological properties of brain networks, such as modularity, efficiency, and hubs [76, 77]. A study [54] using this method found that acupuncture could modulate the disrupted patterns of the whole-brain network following stroke, elucidating the possible mechanisms underlying the functional reorganization of poststroke brain networks following acupuncture intervention from a large-scale perspective.
Regional homogeneity (ReHo) is used to evaluate signal synchronization by calculating the time-series similarity in BOLD signals within local brain regions [78]. Acupuncture was found to increase ReHo values in the right precentral gyrus and superior frontal gyrus while decreasing them in the right superior parietal lobule, left fusiform gyrus, and left supplementary motor area.
Apart from that, voxel-based-morphometry (VBM) and diffusion tensor imaging (DTI) are popular structural MRI technique to investigate regional differences in brain volume and microstructural integrity [79, 80]. According to some studies [36, 37], acupuncture could lead to pronounced structural reorganization in frontal areas and network of DMN areas and increase fractional anisotropy (FA) values.
In addition, the plasticity function of acupuncture on stroke is manifested not only in motor function but also in language. Acupuncture was found to activate language-related brain areas, including frontal, temporal, parietal, and occipital lobes, as well as insula, precuneus, and other wide range of brain function areas.
In order to determine the efficacy of acupuncture, the design of comparison mainly has three kinds: stroke vs. HC, VA vs. SA, and VA plus drug vs. drug. Results demonstrated that VA compared with SA, VA plus drug compared with drug, and acupuncture in patients compared with that in HC all have the characteristics of remodeling the brain structure and function of stroke patients.
4.2. Animal Studies
Each of the five animal studies investigated the mechanism of acupuncture for stroke; among them, four studies focus on poststroke motor impairments, while the fourth examines poststroke cognitive impairment.
Wen et al. [58] investigated the effect of EA for middle cerebral artery occlusion induced cognitive deficit (MICD) group and found that brain infarction volume was reduced and ALFF was decreased in auditory cortex, cingulate gyrus, lateral nucleus group of dorsal thalamus, and hippocampus after 14 days of treatment.
One study [55] using DWI (diffusion-weighted imaging) indicated that the mechanism by which EA can treat acute stroke may be by reducing cerebral edema. While Wu et al. [56] found that acupuncture improved motor function, brain microscopy using DTI technique.
A recent study [81] showed that EA could decrease the infarct volumes of MCAO rats, improve mNSS scores, and enhance FC between the left motor cortex and left cerebellum posterior lobe, right motor cortex, left striatum, and bilateral sensory cortex.
ReHo also was used to investigate the regional neural activity alterations of stroke, and the results showed that EA could increase ReHo in auditory and motor cortex, lateral nucleus group of dorsal thalamus, hippocampus, and others [57].
Briefly, the above studies demonstrated that acupuncture promotes stroke-related neural plasticity from structural and functional aspects. The rehabilitation mechanism of acupuncture on stroke patients may be linked to the remodeling of motor and cognitive brain regions such as motor cortex, bilateral striatum, and sensory cortex hippocampus.
4.3. Factors Associated with the Brain Plasticity of Acupuncture
In this review, the influencing factors of acupuncture on stroke plasticity based on MRI include the type of SA, deqi, different acupoints, and different pathological states.
Compared to SA, four studies [34, 37, 41, 45, 46] demonstrated that VA produces a greater maximum activation change in the motor-related area, improves blood flow to ischemic areas, and promotes stroke recovery. However, one study [46] discovered significant impact variations between VA and SA at TE5, but little difference between verum acupoint and nonacupoint, implying that different SA types also have distinct brain responses.
Eight studies [32, 33, 39, 40, 42, 44, 47, 49, 54] have compared the differences in brain plasticity between stroke patients and HC and found that the modulation effect of acupuncture on stroke patients was more specific and more obvious than that of HC in brain regions associated with disease.
In terms of the choice of acupoints, the most commonly used acupuncture points are mainly in the limbs, and the most frequently used acupoints are GB34 and SJ5.
One study [48] investigated the central mechanism of deqi of acupuncture in the treatment of ischemic stroke and found that compared with the non-deqi group, the deqi group produced marked activation of the right anterior lobe of the cerebellum and right limbic lobe.
5. Discussion
In this review, we included 24 human studies and 5 animal studies and found that that acupuncture reorganizes not only motor-related network, M1, SMA, sensorimotor network (SMN), FA, aDMN, and pDMN but also language-related brain areas include inferior frontal gyrus frontal, temporal, parietal and occipital lobes as well as cognition-related brain regions. In addition, the plasticity of acupuncture is influenced by deqi, acupoints, and physiological state.
5.1. Brain Plasticity of Acupuncture
Stroke causes not only local structural changes in the injured brain regions but also damage to neuronal networks, impairing sensation, movement, or cognition [64, 82]. Under physiological conditions, both hemispheres inhibit each other, and after a stroke, this balance of interaction/inhibition may be upset due to damage to one side of the brain. Moreover, recent studies [83, 84] have demonstrated that interhemispheric imbalance is closely related to the motor function of the affected hand in chronic stroke patients. Thus, several studies [31, 42, 43, 46] have indicated that acupuncture could inhibit contralesional brain activity while activating the ipsilesional motor cortex. Huang et al. [43] indicated that acupuncture results in lateralization in unilateral stroke patients. This lateralization may represent an enhancement of the compensatory process through acupuncture that redistributes function to the intact cortex, especially the unaffected hemisphere. In addition, studies [44, 52] also showed that acupuncture could stimulate bilateral regions, modulate whole-brain network, and enhance functional connectivity. This indicated that acupuncture could not only specifically regulate the bilateral dynamic balance of the brain but also modulate the whole brain network and functional connections as a whole.
The pathogenesis of stroke is complex, the time since stroke, lesion size, location, and other biological factors (such as age and sex) all contribute to the differences between individuals. Therefore, in clinical practice, individualized treatment is based on TCM syndrome differentiation theory, the theory of constitution, and characteristics of patient, season, and locality. However, among the included studies, the reason the current study did not apply individualized therapy is that it requires big data to explore the impact of patients, doctors, and acupuncture on efficacy. In the future, the use of artificial intelligence coupled with continuous monitoring should enable greater individualization and improve outcomes. Importantly, although different acupoints were used across studies, they all could remodel brain areas associated with stroke lesions. For instance, several studies [31, 33, 51, 52] have demonstrated that acupuncture could enhance FC of between bilateral M1s, between the cerebellum and primary sensorimotor cortex, which indicated that acupuncture has not only specific but also common effects on the disease.
Additionally, as described above, although acupuncture therapy can modulate the function and structural plasticity of poststroke in this review. Indeed, structural plasticity has been explored in only one study for the following reasons: on the one hand, the study did not detect structural changes in stroke patients; on the other hand, there was a change in structural plasticity following acupuncture, but the small changes were difficult to identify due to lacking of subdivision of brain regions.
5.2. Factors Associated with the Brain Plasticity of Acupuncture
This review found that different SA types also have distinct brain responses. The overlapping dermatomes between nonacupoints and verum acupoints may explain this phenomenon, as the segmental structure of the body and its interconnected reflex system offers neurophysiological effects [85]. Therefore, the selection of proper SA is critical in determining the efficacy of acupuncture.
When it comes to acupoint selection, the most frequently used acupoints are GB34 and SJ5. GB34 is located on the lateral aspect of the posterior knee, which is the most often used acupoints to generally improve symptoms in motor impairment patients. GB34 belongs to the sea point of gallbladder meridian of foot Shaoyang. SJ5 is found in the dorsal wrist lines on two inches between the ulna and radius. SJ5, belonging to Sanjiao Meridian of Hand Shaoyang, is one of “Ba-mai Jiao-hui point.” The two acupoints have been widely used to alleviate symptoms in motor impairment patients. Although the two acupoints were located in the upper and lower extremities, the activated brain regions included bilateral brain, such as somatosensory cortex and primary sensorimotor. This indicated that various acupoints treat the same disease in a convergent manner.
For decades, it was believed that the deqi of acupuncture is associated with its clinical efficacy. Using fMRI techniques in acupuncture research, several studies [86, 87] have found that acupuncture with deqi can stimulate significant brain activity compared to acupuncture without deqi. In this review, although the deqi group exhibited significant activation of the right anterior lobe of the cerebellum and right limbic lobe (BA30), larger sample sizes are still required for further validation.
Regarding different states and based on TCM theory, this review found that acupuncture showed specific modulations of a motor-related network in stroke patients relative to HC. This phenomenon is in line with the TCM theory that acupuncture can regulate the disorder of the body in dual-direction regulation. This indicated that acupuncture in patients mainly regulate the brain regions associated with the disease, while acupuncture in HC mainly activated brain areas directly associated with the main treatment effects.
In the aspect of different states, based on TCM theory, acupuncture can regulate the disorder of the body in dual-direction regulation. Acupuncture exhibits distinct regulatory effects on the body under physiological and pathological conditions. This indicated that acupuncture showed specific modulations of a motor-related network in stroke patients relative to HC.
In summary, the brain plasticity of acupuncture on stroke is influenced by many factors, such as deqi, acupoints, pathological state, and SA type. As a result, it is important to continue exploring the most effective strategies for treating stroke with acupuncture in the future.
5.3. Prospects for Brain Plasticity of Acupuncture
Numerous clinical and experimental researches revealed that the brain was plastic and could be remodeled by the environment and experience [88, 89]. Currently, the National Institutes of Health in the United States has recently adopted acupuncture as a treatment for poststroke rehabilitation, demonstrating that acupuncture is widely accepted for such therapy [90]. From a systematic level, this study found that the effective mechanism is that acupuncture can reorganize the brain structure and functional connections in stroke patients.
Indeed, acupuncture's function to reshape the brain is not limited to stroke, as a recent review [91] of brain plasticity in animals revealed that acupuncture could modulate the plasticity of various central nervous systems, such as depression, neuropathic pain, Alzheimer's disease, and cerebral vascular disorders. More research in humans still requires further verification.
In addition, this review stated that acupuncture's plasticity on stroke was affected by several factors, such as deqi, acupoints, pathological state, and type of sham needle. Exploring the impact of these influencing factors on the efficacy and constructing a pathway connecting “acupoint-brain” is also critical for future individualized therapy. Moreover, predicting the efficacy of individual patients receiving acupuncture treatment for stroke to achieve precision treatment impact is a problem that requires future research. The recent integration of machine learning (ML) and neuroimaging techniques provides a promising approach to understanding how acupuncture facilitates neuroplasticity at the individual level. This approach enables us to investigate not only the effect of acupuncture influencing factors on brain plasticity prediction but also the impact of specific brain plasticity on acupuncture efficacy prediction.
Interestingly, a recent review [92] examined the neuroplasticity of acupuncture using machine learning and neuroimaging techniques and found that brain functional plasticity is affected by different acupoints and acupuncture manipulations and that specific structural and functional neuroplasticity characteristics at baseline could accurately predict the improvement of symptoms after acupuncture treatment. This review summarizes two commonly used methods for predicting the efficacy of acupuncture. One method is to adopt the classification algorithms to predict patients' responses to acupuncture treatment. The other method is to construct the regression models to predict the continuous improvement in symptoms after acupuncture treatment. Currently, this is the mainly used medication for pain and functional dyspepsia. Since research in this field remains in its infancy and faces many challenges, many efforts remain to be done in the future.
Apart from that, although several reviews [93, 94] have been published on acupuncture on stroke in animals at the molecular level, there have been a few animal studies on MRI-based acupuncture in stroke treatment. One possible reason is that the MRI mechanism is easy to manipulate in humans, unlike studies at the cellular and molecular levels. The other reason is that experimental stroke models do not fit perfectly into clinical situations, influencing the extrapolation of results. However, animal research also exhibits several advantages, including low costs, small variations, controllable factors, and high reproducibility. As a result, additional experimental studies may be required to elucidate the mechanism of influencing acupuncture factors on stroke.
5.4. Limitations
Although this review comprehensively summarizes the evidence from extensive MRI-based literature on acupuncture for stroke, several limitations remain. First, the main criticism is of this review is that study design, experimental design, and analytic methods may influence the extrapolation of conclusions, which also makes it difficult to do a meta-analysis. More relatively consistent designs and methods are required to conduct a quantitative meta-analysis determining the brain region of plasticity on acupuncture for stroke in order to provide comprehensive evidence. Because systematic reviews and meta-analyses are important tools for summarizing specific topics that inform evidence-based practice in healthcare, guidelines, and policies in a comprehensive, meaningful manner [95]. Another drawback is the absence of SA as a control group, making it difficult to draw reliable conclusions and confirm acupuncture specificity. Third, the limited number of articles and small sample sizes limit the stability and reliability of this review; a large sample size and additional research are required. In addition, we include only peer-reviewed studies conducted in English, which could introduce some selection bias. Finally, since many studies did not perform correlation analyses between brain imaging and behavioral characteristics before and after treatment, it is difficult to clarify that changes in brain imaging are objective evidence of improvement in symptoms. Accordingly, future researchers should pay much attention to this research area. Therefore, to overcome the previously mentioned limitations, extensive research efforts need to be conducted in the future.
5.5. Conclusions
In summary, the cumulative evidences demonstrated that acupuncture could modulate neural plasticity of stroke, activating not only motor-related brain but also language-related and cognitive-related brain regions. Consequently, acupuncture therapy can enhance clinical recovery following a stroke. However, additional research is necessary to validate the results due to the scarcity of data.
Acknowledgments
This work was supported by the National Key R&D Program of China (2019YFC1712200), International standards research on clinical research and service of Acupuncture-Moxibustion (2019YFC1712205), and Shenzhen's Sanming Project (SZSM201612001).
Data Availability
Our data are from the published literature.
Conflicts of Interest
The authors declare no competing financial interest.
Authors' Contributions
Z.J.H and L.C.J designed the whole study, analyzed the data, and wrote the manuscript. W.X.X and N.D.H searched and selected the studies. Z.J.H participated in the interpretation of data. Y.H.B offered good suggestions. All authors read and approved the final manuscript. Jinhuan Zhang and Chunjian Lu contributed equally to this work.
References
- 1.GBD 2016 Lifetime Risk of Stroke Collaborators, Feigin V. L., Nguyen G., et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. The New England Journal of Medicine. 2018;379(25):2429–2437. doi: 10.1056/NEJMoa1804492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology. 2019;18(5):439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stinear C. M., Lang C. E., Zeiler S., Byblow W. D. Advances and challenges in stroke rehabilitation. Lancet Neurology. 2020;19(4):348–360. doi: 10.1016/S1474-4422(19)30415-6. [DOI] [PubMed] [Google Scholar]
- 4.Wu S., Wu B., Liu M., et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurology. 2019;18(4):394–405. doi: 10.1016/S1474-4422(18)30500-3. [DOI] [PubMed] [Google Scholar]
- 5.Wang W., Jiang B., Sun H., et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 6.Urban P. P., Wolf T., Uebele M., et al. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke. 2010;41(9):2016–2020. doi: 10.1161/STROKEAHA.110.581991. [DOI] [PubMed] [Google Scholar]
- 7.Moulton E., Magno S., Valabregue R., et al. Acute diffusivity biomarkers for prediction of motor and language outcome in mild-to-severe stroke patients. Stroke. 2019;50(8):2050–2056. doi: 10.1161/STROKEAHA.119.024946. [DOI] [PubMed] [Google Scholar]
- 8.Sebastian R., Tsapkini K., Tippett D. C. Transcranial direct current stimulation in post stroke aphasia and primary progressive aphasia: current knowledge and future clinical applications. NeuroRehabilitation. 2016;39(1):141–152. doi: 10.3233/NRE-161346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Q., Li G., Liu T., et al. Modulation of interhemispheric activation balance in motor-related areas of stroke patients with motor recovery: systematic review and meta-analysis of fMRI studies. Neuroscience & Biobehavioral Reviews. 2015;57:392–400. doi: 10.1016/j.neubiorev.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen P. M., Stig Jørgensen H., Nakayama H., Raaschou H. O., Olsen T. S. Aphasia in acute stroke: incidence, determinants, and recovery. Annals of Neurology. 1995;38(4):659–666. doi: 10.1002/ana.410380416. [DOI] [PubMed] [Google Scholar]
- 11.Stark B. C., Warburton E. A. Improved language in chronic aphasia after self-delivered iPad speech therapy. Neuropsychological Rehabilitation. 2018;28(5):818–831. doi: 10.1080/09602011.2016.1146150. [DOI] [PubMed] [Google Scholar]
- 12.Griffis J. C., Metcalf N. V., Corbetta M., Shulman G. L. Structural disconnections explain brain network dysfunction after stroke. Cell Reports. 2019;28(10):2527–2540.e9. doi: 10.1016/j.celrep.2019.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J. H., Kyeong S., Kang H., Kim D. H. Structural and functional connectivity correlates with motor impairment in chronic supratentorial stroke: a multimodal magnetic resonance imaging study. Neuroreport. 2019;30(7):526–531. doi: 10.1097/WNR.0000000000001247. [DOI] [PubMed] [Google Scholar]
- 14.Johansson B. B. Brain plasticity and stroke rehabilitation. The Willis lecture. Stroke. 2000;31(1):223–230. doi: 10.1161/01.str.31.1.223. [DOI] [PubMed] [Google Scholar]
- 15.Pascual-Leone A., Amedi A., Fregni F., Merabet L. B. The plastic human brain cortex. Annual Review of Neuroscience. 2005;28(1):377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 16.Kwakkel G., Kollen B. J., van der Grond J., Prevo A. J. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34(9):2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- 17.Yang A., Wu H. M., Tang J. L., et al. Acupuncture for stroke rehabilitation. Cochrane Database of Systematic Reviews. 2016;D4131 doi: 10.1002/14651858.cd004131.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao Z., Wang D., Zeng Y., Liu M., Cochrane Stroke Group Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database of Systematic Reviews. 2013;D8862 doi: 10.1002/14651858.cd008862.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsner B., Kwakkel G., Kugler J., Mehrholz J. Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: a network meta-analysis of randomised controlled trials. Journal of Neuroengineering and Rehabilitation. 2017;14(1):p. 95. doi: 10.1186/s12984-017-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu P., Mills E., Moher D., Seely D. Acupuncture in poststroke rehabilitation: a systematic review and meta-analysis of randomized trials. Stroke. 2010;41(4):e171–e179. doi: 10.1161/STROKEAHA.109.573576. [DOI] [PubMed] [Google Scholar]
- 21.Rabinstein A. A., Shulman L. M. Acupuncture in clinical neurology. The Neurologist. 2003;9(3):137–148. doi: 10.1097/00127893-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y., Xue S. A., Zuo Z. Acupuncture therapy on apoplectic aphasia rehabilitation. Journal of Traditional Chinese Medicine. 2012;32(3):314–321. doi: 10.1016/S0254-6272(13)60031-X. [DOI] [PubMed] [Google Scholar]
- 23.Yang L., Tan J. Y., Ma H., et al. Warm-needle moxibustion for spasticity after stroke: a systematic review of randomized controlled trials. International Journal of Nursing Studies. 2018;82:129–138. doi: 10.1016/j.ijnurstu.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Zhang B., Han Y., Huang X., et al. Acupuncture is effective in improving functional communication in post-stroke aphasia. Wiener Klinische Wochenschrift. 2019;131(9-10):221–232. doi: 10.1007/s00508-019-1478-5. [DOI] [PubMed] [Google Scholar]
- 25.Cirillo C., Brihmat N., Castel-Lacanal E., et al. Post-stroke remodeling processes in animal models and humans. Journal of Cerebral Blood Flow and Metabolism. 2020;40(1):3–22. doi: 10.1177/0271678X19882788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeiler S. R. Should we care about early post-stroke rehabilitation? Not yet, but soon. Current Neurology and Neuroscience Reports. 2019;19(3) doi: 10.1007/s11910-019-0927-x. [DOI] [PubMed] [Google Scholar]
- 27.Johansson B. B. Current trends in stroke rehabilitation. A review with focus on brain plasticity. Acta Neurologica Scandinavica. 2011;123(3):147–159. doi: 10.1111/j.1600-0404.2010.01417.x. [DOI] [PubMed] [Google Scholar]
- 28.Diamond M. C., Krech D., Rosenzweig M. R. The effects of an enriched environment on the histology of the rat cerebral cortex. The Journal of Comparative Neurology. 1964;123:111–120. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
- 29.Erhart S. M., Young A. S., Marder S. R., Mintz J. Clinical utility of magnetic resonance imaging radiographs for suspected organic syndromes in adult psychiatry. The Journal of Clinical Psychiatry. 2005;66(8):968–973. doi: 10.4088/JCP.v66n0802. [DOI] [PubMed] [Google Scholar]
- 30.Glover G. H. Overview of functional magnetic resonance imaging. Neurosurgery Clinics of North America. 2011;22(2):133–139. doi: 10.1016/j.nec.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai L., Tao Y., Wang D., et al. Acupuncture induces time-dependent remodelling brain network on the stable somatosensory first-ever stroke patients: combining diffusion tensor and functional MR imaging. Evidence-based Complementary and Alternative Medicine. 2014;2014:7. doi: 10.1155/2014/740480.740480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z., Cui F., Zou Y., Bai L. Acupuncture enhances effective connectivity between cerebellum and primary sensorimotor cortex in patients with stable recovery stroke. Evidence-Based Complementary and Alternative Medicine. 2014;2014:9. doi: 10.1155/2014/603909.603909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han X., Bai L., Sun C., et al. Acupuncture enhances communication between cortices with damaged white matters in poststroke motor impairment. Evidence-Based Complementary and Alternative Medicine. 2019;2019:11. doi: 10.1155/2019/4245753.4245753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaechter J. D., Connell B. D., Stason W. B., et al. Correlated change in upper limb function and motor cortex activation after verum and sham acupuncture in patients with chronic stroke. The Journal of Alternative and Complementary Medicine. 2007;13(5):527–532. doi: 10.1089/acm.2007.6316. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Wang Y., Liao C., Huang W., Wu P. Longitudinal Brain Functional Connectivity Changes of the Cortical Motor- Related Network in Subcortical Stroke Patients with Acupuncture Treatment. Neural Plasticity. 2017;2017:9. doi: 10.1155/2017/5816263.5816263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu P., Zhou Y., Liao C., et al. Structural changes induced by acupuncture in the recovering brain after ischemic stroke. Evidence-Based Complementary and Alternative Medicine. 2018;2018:8. doi: 10.1155/2018/5179689.5179689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen Y., Li M., Wei R., Lou M. Effect of acupuncture therapy for postponing Wallerian degeneration of cerebral infarction as shown by diffusion tensor imaging. The Journal of Alternative and Complementary Medicine. 2012;18(12):1154–1160. doi: 10.1089/acm.2011.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu P., Zeng F., Yin C., et al. Effect of acupuncture plus conventional treatment on brain activity in ischemic stroke patients: a regional homogeneity analysis. Journal of Traditional Chinese Medicine. 2017;37(5):650–658. [PubMed] [Google Scholar]
- 39.Li G., Jack C. R., Yang E. S. An fMRI study of somatosensory-implicated acupuncture points in stable somatosensory stroke patients. Journal of Magnetic Resonance Imaging. 2006;24(5):1018–1024. doi: 10.1002/jmri.20702. [DOI] [PubMed] [Google Scholar]
- 40.Li G., Yang E. S. An fMRI study of acupuncture-induced brain activation of aphasia stroke patients. Complementary Therapies in Medicine. 2011;19:S49–S59. doi: 10.1016/j.ctim.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y., Xiao H., Chen J., et al. Needling at the Waiguan (SJ5) in healthy limbs deactivated functional brain areas in ischemic stroke patients a functional magnetic resonance imaging study. Neural Regeneration Research. 2011;6:2829–2833. [Google Scholar]
- 42.Cho S., Kim M., Sun J. J., et al. A comparison of brain activity between healthy subjects and stroke patients on fMRI by acupuncture stimulation. Chinese Journal of Integrative Medicine. 2013;19(4):269–276. doi: 10.1007/s11655-013-1436-4. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y., Chen J., Lai X., et al. Lateralisation of cerebral response to active acupuncture in patients with unilateral ischaemic stroke: an fmri study. Acupuncture in Medicine. 2013;31(3):290–296. doi: 10.1136/acupmed-2012-010299. [DOI] [PubMed] [Google Scholar]
- 44.Chen J., Huang Y., Lai X., et al. Acupuncture at Waiguan (TE5) influences activation/deactivation of functional brain areas in ischemic stroke patients and healthy people: a functional MRI study. Neural Regeneration Research. 2013;8(3):226–232. doi: 10.3969/j.issn.1673-5374.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J., Wang J., Huang Y., et al. Modulatory effect of acupuncture at Waiguan (TE5) on the functional connectivity of the central nervous system of patients with ischemic stroke in the left basal ganglia. PLoS One. 2014;9(6, article e96777) doi: 10.1371/journal.pone.0096777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi J., Chen J., Huang Y., et al. Acupuncture at Waiguan (SJ5) and sham points influences activation of functional brain areas of ischemic stroke patients: a functional magnetic resonance imaging study. Neural Regeneration Research. 2014;9(3):293–300. doi: 10.4103/1673-5374.128227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Li K., Ren Y., et al. Acupuncture modulates the functional connectivity of the default mode network in stroke patients. Evidence-Based Complementary and Alternative Medicine. 2014;2014:7. doi: 10.1155/2014/765413.765413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M. K., Li Y. J., Zhang G. F., et al. Acupuncture for ischemic stroke: cerebellar activation may be a central mechanism following Deqi. Neural Regeneration Research. 2015;10(12):1997–2003. doi: 10.4103/1673-5374.172318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.GAO Y., LIN Z., TAO J., et al. Evidence of timing effects on acupuncture: a functional magnetic resonance imaging study. Experimental and Therapeutic Medicine. 2015;9(1):59–64. doi: 10.3892/etm.2014.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang J., Zhang H., Tan Z., Xiao J., Li S., Gao Y. Effect of electroacupuncture in patients with post-stroke motor aphasia. Wiener Klinische Wochenschrift. 2017;129(3-4):102–109. doi: 10.1007/s00508-016-1070-1. [DOI] [PubMed] [Google Scholar]
- 51.Fu C., Li K., Ning Y., et al. Altered effective connectivity of resting state networks by acupuncture stimulation in stroke patients with left hemiplegia. Medicine. 2017;96(47, article e8897) doi: 10.1097/MD.0000000000008897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ning Y., Li K., Fu C., et al. Enhanced functional connectivity between the bilateral primary motor cortices after acupuncture at Yanglingquan (GB34) in right-hemispheric subcortical stroke patients: a resting-state fMRI study. Frontiers in Human Neuroscience. 2017;11 doi: 10.3389/fnhum.2017.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen S., Cai D., Chen J., Yang H., Liu L. Altered brain regional homogeneity following contralateral acupuncture at Quchi (LI 11) and Zusanli (ST 36) in ischemic stroke patients with left hemiplegia: an fMRI study. Chinese Journal of Integrative Medicine. 2020;26(1):20–25. doi: 10.1007/s11655-019-3079-6. [DOI] [PubMed] [Google Scholar]
- 54.Han X., Jin H., Li K., et al. Acupuncture modulates disrupted whole-brain network after ischemic stroke: evidence based on graph theory analysis. Neural Plasticity. 2020;2020:10. doi: 10.1155/2020/8838498.8838498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang F., Wu Y., Jia J. Electro-acupuncture can alleviate the cerebral oedema of rat after ischemia. Brain Injury. 2011;25(9):895–900. doi: 10.3109/02699052.2011.581639. [DOI] [PubMed] [Google Scholar]
- 56.Wu Z., Hu J., du F., Zhou X., Xiang Q., Miao F. Long-term changes of diffusion tensor imaging and behavioural status after acupuncture treatment in rats with transient focal cerebral ischaemia. Acupuncture in Medicine. 2012;30(4):331–338. doi: 10.1136/acupmed-2012-010172. [DOI] [PubMed] [Google Scholar]
- 57.Liang S., Lin Y., Lin B., et al. Resting-state functional magnetic resonance imaging analysis of brain functional activity in rats with ischemic stroke treated by electro- acupuncture. Journal of Stroke and Cerebrovascular Diseases. 2017;26(9):1953–1959. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 58.Wen T., Zhang X., Liang S., et al. Electroacupuncture Ameliorates Cognitive Impairment and Spontaneous Low- Frequency Brain Activity in Rats with Ischemic Stroke. Journal of Stroke and Cerebrovascular Diseases. 2018;27(10):2596–2605. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 59.Cassidy J. M., Cramer S. C. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Translational Stroke Research. 2017;8(1):33–46. doi: 10.1007/s12975-016-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hebb D. The Organization of Behavior: A Neuropsychological Theory. New York: John Wiley & Sons; 1949. [Google Scholar]
- 61.Galvan A. Neural plasticity of development and learning. Human Brain Mapping. 2010;31(6):879–890. doi: 10.1002/hbm.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sampaio-Baptista C., Sanders Z. B., Johansen-Berg H. Structural plasticity in adulthood with motor learning and stroke rehabilitation. Annual Review of Neuroscience. 2018;41(1):25–40. doi: 10.1146/annurev-neuro-080317-062015. [DOI] [PubMed] [Google Scholar]
- 63.Carey L., Walsh A., Adikari A., et al. Finding the intersection of neuroplasticity, stroke recovery, and learning: scope and contributions to stroke rehabilitation. Neural Plasticity. 2019;2019:15. doi: 10.1155/2019/5232374.5232374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy T. H., Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nature Reviews. Neuroscience. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 65.Bear M. F., Connors B. W., Paradiso M. A. Neuroscience: Exploring the Brain. 3rd. Lippincott Williams & Wilkins Publishers; 2007. [Google Scholar]
- 66.Kiran S. What is the nature of poststroke language recovery and reorganization? ISRN Neurology. 2012;2012:13. doi: 10.5402/2012/786872.786872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grefkes C., Fink G. R. Connectivity-based approaches in stroke and recovery of function. Lancet Neurology. 2014;13(2):206–216. doi: 10.1016/S1474-4422(13)70264-3. [DOI] [PubMed] [Google Scholar]
- 68.Silasi G., Murphy T. H. Stroke and the connectome: how connectivity guides therapeutic intervention. Neuron. 2014;83(6):1354–1368. doi: 10.1016/j.neuron.2014.08.052. [DOI] [PubMed] [Google Scholar]
- 69.Rehme A. K., Eickhoff S. B., Rottschy C., Fink G. R., Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage. 2012;59(3):2771–2782. doi: 10.1016/j.neuroimage.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 70.Nair V. A., Young B. M., la C., et al. Functional connectivity changes in the language network during stroke recovery. Annals of Clinical Translational Neurology. 2015;2(2):185–195. doi: 10.1002/acn3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu D., Chang J., Freeman S., et al. Changes of functional connectivity in the left frontoparietal network following aphasic stroke. Frontiers in Behavioral Neuroscience. 2014;8:p. 167. doi: 10.3389/fnbeh.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Green J. B. Brain reorganization after stroke. Topics in Stroke Rehabilitation. 2003;10(3):1–20. doi: 10.1310/H65X-23HW-QL1G-KTNQ. [DOI] [PubMed] [Google Scholar]
- 73.Maximo J. O., Cadena E. J., Kana R. K. The implications of brain connectivity in the neuropsychology of autism. Neuropsychology Review. 2014;24(1):16–31. doi: 10.1007/s11065-014-9250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kelly C., Castellanos F. X. Strengthening connections: functional connectivity and brain plasticity. Neuropsychology Review. 2014;24(1):63–76. doi: 10.1007/s11065-014-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stokes P. A., Purdon P. L. A study of problems encountered in Granger causality analysis from a neuroscience perspective. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(34):E7063–E7072. doi: 10.1073/pnas.1704663114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews. Neuroscience. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 77.Bassett D. S., Bullmore E. T. Human brain networks in health and disease. Current Opinion in Neurology. 2009;22(4):340–347. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zang Y., Jiang T., Lu Y., He Y., Tian L. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22(1):394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 79.Good C. D., Johnsrude I. S., Ashburner J., Henson R. N. A., Friston K. J., Frackowiak R. S. J. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14(1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y., Burock M. A. Diffusion tensor imaging in Parkinson's disease and Parkinsonian syndrome: a systematic review. Frontiers in Neurology. 2020;11:p. 531993. doi: 10.3389/fneur.2020.531993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Z., Yang M., Lin Y., et al. Electroacupuncture promotes motor function and functional connectivity in rats with ischemic stroke: an animal resting-state functional magnetic resonance imaging study. Acupuncture in Medicine. 2021;39(2):146–155. doi: 10.1177/0964528420920297. [DOI] [PubMed] [Google Scholar]
- 82.Liang S., Jiang X., Zhang Q., et al. Abnormal metabolic connectivity in rats at the acute stage of ischemic stroke. Neuroscience Bulletin. 2018;34(5):715–724. doi: 10.1007/s12264-018-0266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nowak D. A., Grefkes C., Ameli M., Fink G. R. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabilitation and Neural Repair. 2009;23(7):641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- 84.Takechi U., Matsunaga K., Nakanishi R., et al. Longitudinal changes of motor cortical excitability and transcallosal inhibition after subcortical stroke. Clinical Neurophysiology. 2014;125(10):2055–2069. doi: 10.1016/j.clinph.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 85.Ots T., Kandirian A., Szilagyi I., DiGiacomo S. M., Sandner-Kiesling A. The selection of dermatomes for sham (placebo) acupuncture points is relevant for the outcome of acupuncture studies: a systematic review of sham (placebo)-controlled randomized acupuncture trials. Acupuncture in Medicine. 2020;38(4):211–226. doi: 10.1177/0964528419889636. [DOI] [PubMed] [Google Scholar]
- 86.Asghar A. U., Green G., Lythgoe M. F., Lewith G., MacPherson H. Acupuncture needling sensation: The neural correlates of _deqi_ using fMRI. Brain Research. 2010;1315:111–118. doi: 10.1016/j.brainres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 87.Hui K. K., Marina O., Liu J., Rosen B. R., Kwong K. K. Acupuncture, the limbic system, and the anticorrelated networks of the brain. Autonomic Neuroscience. 2010;157(1-2):81–90. doi: 10.1016/j.autneu.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mandolesi L., Gelfo F., Serra L., et al. Environmental factors promoting neural plasticity: insights from animal and human studies. Neural Plasticity. 2017;2017:10. doi: 10.1155/2017/7219461.7219461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang C. H., Ma Z. Z., Huo B. B., et al. Diffusional plasticity induced by electroacupuncture intervention in rat model of peripheral nerve injury. Journal of Clinical Neuroscience. 2019;69:250–256. doi: 10.1016/j.jocn.2019.08.088. [DOI] [PubMed] [Google Scholar]
- 90.NIH Consensus Conference. Acupuncture. JAMA. 1998;280:1518–1524. [PubMed] [Google Scholar]
- 91.Xiao L. Y., Wang X. R., Yang Y., et al. Applications of acupuncture therapy in modulating plasticity of central nervous system. Neuromodulation. 2018;21(8):762–776. doi: 10.1111/ner.12724. [DOI] [PubMed] [Google Scholar]
- 92.Yin T., Ma P., Tian Z., et al. Machine learning in neuroimaging: a new approach to understand acupuncture for neuroplasticity. Neural Plasticity. 2020;2020:14. doi: 10.1155/2020/8871712.8871712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chavez L. M., Huang S. S., MacDonald I., Lin J. G., Lee Y. C., Chen Y. H. Mechanisms of acupuncture therapy in ischemic stroke rehabilitation: a literature review of basic studies. International Journal of Molecular Sciences. 2017;18(11):p. 2270. doi: 10.3390/ijms18112270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao B. Q., Tan F., Zhan J., Lai P. H. Mechanism underlying treatment of ischemic stroke using acupuncture: transmission and regulation. Neural Regeneration Research. 2021;16(5):944–954. doi: 10.4103/1673-5374.297061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muka T., Glisic M., Milic J., et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. European Journal of Epidemiology. 2020;35(1):49–60. doi: 10.1007/s10654-019-00576-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our data are from the published literature.


