Abstract
Objective
To assess the role of biomarkers of Alzheimer disease (AD), neurodegeneration, and small vessel disease (SVD) as mediators in the association between diabetes mellitus and cognition.
Methods
The study sample was derived from MEMENTO, a cohort of French adults recruited in memory clinics and screened for either isolated subjective cognitive complaints or mild cognitive impairment. Diabetes was defined based on blood glucose assessment, use of antidiabetic agent, or self-report. We used structural equation modeling to assess whether latent variables of AD pathology (PET mean amyloid uptake, Aβ42/Aβ40 ratio, and CSF phosphorylated tau), SVD (white matter hyperintensities volume and visual grading), and neurodegeneration (mean cortical thickness, brain parenchymal fraction, hippocampal volume, and mean fluorodeoxyglucose uptake) mediate the association between diabetes and a latent variable of cognition (5 neuropsychological tests), adjusting for potential confounders.
Results
There were 254 (11.1%) participants with diabetes among 2,288 participants (median age 71.6 years; 61.8% women). The association between diabetes and lower cognition was significantly mediated by higher neurodegeneration (standardized indirect effect: −0.061, 95% confidence interval: −0.089, −0.032), but not mediated by SVD and AD markers. Results were similar when considering latent variables of memory or executive functioning.
Conclusion
In a large clinical cohort in the elderly, diabetes is associated with lower cognition through neurodegeneration, independently of SVD and AD biomarkers.
Type 2 diabetes is a risk factor for cognitive decline and dementia.1,2 Several underlying mechanisms could be involved, such as chronic hyperglycemia leading to advanced glycation end products, atherosclerosis, and subsequent cerebrovascular lesions.3-5 Insulin dysregulation, including insulin resistance and insulin deficiency, may promote cerebral hypometabolism6 and amyloid and tau pathologies, hallmarks of Alzheimer disease (AD).7 Diabetes has also been associated with brain structural modifications such as cerebral atrophy and cerebrovascular lesions.8-10 Moreover, whereas diabetes is associated with cerebral hypometabolism,11,12 results are conflicting regarding its association with amyloid and tau pathology, whether measured in the brain (PET) or in CSF.11,13,14
Previous studies have suggested a mediating role of neurodegeneration and small vessel disease (SVD) biomarkers on the association between diabetes and cognition.15-17 However, the mediating role of AD-specific lesions (amyloid plaques and neurofibrillary tangles) and the correlation between those different brain features have not been considered so far.
We estimated the mediating effect of biomarkers of AD, neurodegeneration, and SVD in the association between diabetes and cognition in older adults without dementia recruited from French memory clinics.
Methods
The MEMENTO Cohort
The MEMENTO cohort is a clinic-based study of patients presenting with a large variety of cognitive symptoms or subjective cognitive complaints, who were enrolled between April 2011 and June 2014, within the French national network of university hospital–based memory clinics.18 At inclusion, participants presented with (1) mild cognitive impairment, performing 1 SD worse than the mean of the participant's own age, sex, and education-level group, in one or more cognitive domains, this deviation being identified for the first time through cognitive tests performed recently (less than 6 months preceding screening phase); or (2) isolated cognitive complaints, if participants had subjective cognitive complaint (assessed through visual analogic scale), without any objective cognitive deficit as defined previously, while 60 years or older. All participants had a Clinical Dementia Rating scale19 score ≤0.5. Main exclusion criteria have been described elsewhere.22 All examinations (including neuropsychological battery administration, clinical examinations, brain MRI, CSF samples, and fluorodeoxyglucose [FDG] and amyloid PET) followed standardized procedures.18
Among the 2,323 participants included in the MEMENTO cohort, 2,288 participants from 26 study centers were included in this analysis after exclusion of participants with missing data on diabetes status (n = 35).
Standard Protocol Approvals, Registrations, and Patient Consents
This study was performed in accordance with the Declaration of Helsinki. All participants provided written informed consent. The MEMENTO cohort protocol has been approved by the local ethics committee (Comité de Protection des Personnes Sud-Ouest et Outre Mer III; approval number 2010-A01394-35) and was registered in ClinicalTrials.gov (Identifier: NCT01926249).
Diabetes Definition
Participants were classified as having diabetes at baseline visit either in presence of fasting blood glucose ≥7 mmol/L (≥126 mg/dL) or nonfasting blood glucose ≥11.1 mmol/L (≥200 mg/dL) or antidiabetic drug intake (Anatomical Therapeutic Chemical classification system: code A10A “insulins and analogues” and code A10B “blood glucose lowering drugs, excl. insulins”) or self-reported history of diabetes.
Neuropsychological Evaluation
A full neuropsychological test battery was administered to participants.18 Global cognition was assessed by Mini-Mental State Examination (MMSE),20 long-term memory was assessed by Free and Cued Selective Reminding Test (FCSRT),21 semantic verbal fluency via animal words,22 visuo-spatial abilities by Rey-Osterrieth Complex Figure Test,23 and attention and executive functions by Trail Making Test (TMT) A and B.24
Biomarkers Assessment
MRI
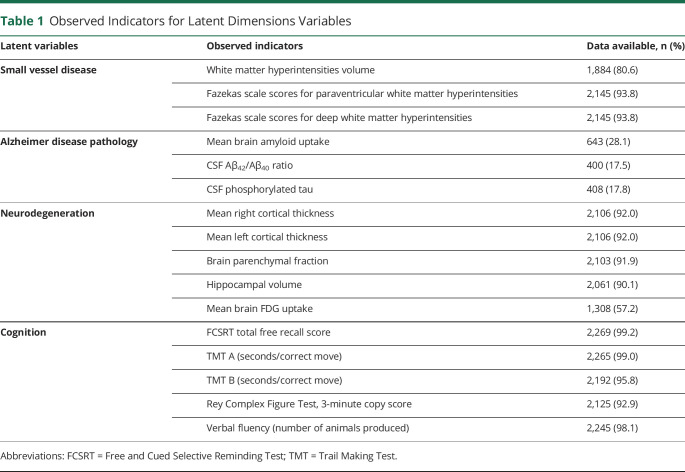
As part of the inclusion criteria, participants had to agree to undergo brain MRI. Brain magnetic resonance images were acquired after a standardization of the imaging processes and coordinated by CATI (cati-neuroimaging.com), a neuroimaging platform dedicated to multicenter studies.25 Full details are described elsewhere.18 Briefly, MRI machines of 1.5T and 3T were used across centers using harmonized protocols. All MRI scans acquired were then centralized, quality checked, and postprocessed to obtain standardized measurements for each participant. Whole-brain, gray matter, and white matter volumes were assessed with Statistical Parametric Mapping 8,26 hippocampal volumes with the SACHA software,27 and mean cortical thickness of each hemisphere with FreeSurfer 5.3 averaged in the region of interest (ROI) of the Desikan-Killiany atlas.28 White matter lesion volumetry was performed using WHASA software29 complemented by a centralized visual assessment by a trained rater using the Fazekas and Schmidt scale.30
FDG-PET
18F-FDG-PET was offered to all participants but was not mandatory. PET images were acquired after a standardization of the acquisition and reconstruction imaging parameters, coordinated by CATI.31 After a centralized quality check and postprocessing performed by CATI, the following measures were obtained: mean FDG-PET uptake for the ROIs of the Automated Anatomical Labeling atlas relative to the pons reference region,32 including partial volume correction, and mean FDG-PET uptake for a set of AD-specific ROIs inferred from the Alzheimer's Disease Neuroimaging Initiative database,33 expressed as standard uptake value ratios (SUVRs).
PET Amyloid Imaging
PET amyloid imaging was available for 643 participants of the analytical sample, using either 18F-florbetapir (Amyvid, Eli Lilly) (n = 437) or 18F-flutemetamol (Vizamyl, GE Healthcare) (n = 206) radioligands. Mean brain amyloid SUVR was computed, harmonized across the radioligands,34 and used for the current study.
CSF Sampling
Lumbar puncture was offered to all participants but was not mandatory, and CSF centralized measurements of β-amyloid 42 peptide (Aβ42), Aβ40, total tau, and phosphorylated tau levels were performed using the standardized INNOTEST sandwich ELISA (Fujirebio).
Potential Confounding Factors
Sociodemographic information recorded at baseline included age, sex, and education (low education defined as no or primary school, intermediate education defined as secondary school or high school, and high education defined as university). Lifestyle factors included smoking status (never, former, and current smoker) and current alcohol consumption (none, ≤1 drink/day, and >1 drink/day). Hypertension was defined as antihypertensive drug intake or mean of 3 blood pressure measurements either ≥140 mm Hg for systolic blood pressure or ≥90 mm Hg for diastolic blood pressure. Dyslipidemia was defined by plasma cholesterol >6.24 mmol/L or use of any lipid-lowering drugs. Body mass index was categorized as <20 kg/m2, 20–25 kg/m2, 25.1–29.9 kg/m2, and ≥30 kg/m2. History of cardiovascular disease was defined as a self-reported history of myocardial infarction, angina pectoris, coronary artery, or peripheral artery disease. History of stroke was self-reported. Depression was assessed with the Neuropsychiatric Inventory–Clinician.35 APOE ε2, ε3, or ε4 alleles were determined for all participants by KBiosciences (kbioscience.co.uk) as described elsewhere.18 APOE ε4 status was defined as presence of at least one ε4 allele.
Statistical Analyses
Baseline characteristics were compared according to baseline diabetic status for the analytical sample. We used χ2 test (or Fisher exact test when appropriate) and Student t test (or nonparametric Mann-Whitney-Wilcoxon test when appropriate) for categorical and continuous variables comparisons, respectively.
Brain parenchymal fraction was computed as the sum of gray matter and white matter volumes divided by total intracranial volume. Total hippocampal volume was computed as the sum of left and right hippocampal volumes. White matter hyperintensity (WMH) volume and hippocampal volume were adjusted for total intracranial volume using the residual approach.36 Mean FDG uptake across the brain was used.
Structural equation modeling37 was used to examine a potential mediating role of biomarkers respectively of AD, SVD, and neurodegeneration in the association between diabetes and cognition. Structural equation modeling was preferred over standard regression modeling for its ability to directly focus the mediation analysis on the dimensions of interest (here cognition, SVD, AD, and neurodegeneration), and to define each dimension from several noisy observed indicators. The observed indicators of the 4 latent variables of interest, namely AD pathology, SVD, neurodegeneration, and cognition, are listed in Table 1. They were determined from the literature and validated in preliminary separated structural equation modeling analyses. Correlated residuals were assumed between left and right cortical thicknesses and between TMT A and TMT B scores to account for a potential common source of measurement error. Mean brain amyloid SUVR was normalized using a logarithmic transformation and then standardized (z score) by radioligand. The relationships between diabetes, potential confounders, and latent variables of AD pathology, neurodegeneration, SVD, and cognition were modeled in the structural linear regressions. For ease of interpretation, the 4 latent variables were standardized (mean 0, variance 1) so that 1 unit corresponds to the SD of a given dimension. The indirect effects of diabetes on cognition through the latent dimensions were estimated with their 95% confidence interval (CI), using path analysis technique.37 All linear regressions of mediators and cognition were adjusted for the following potential confounding factors: age, sex, education (high education vs low and intermediate), smoking status (current smoker vs never or former smoker), alcohol consumption (>1 drink/day vs ≤1 drink/day), hypertension, dyslipidemia, obesity (≥30 kg/m2), and APOE genotype (ε4 carrier vs ε4 noncarrier). Missing values for observed indicators of latent variables and for confounding factors were handled using a full information maximum likelihood approach, assuming missingness at random. The multicentric nature of the data was accounted for and Huber-White robust standard errors were reported to correct for the potential intracenter correlation.38 The general goodness of fit was evaluated using robust Tucker-Lewis Index (TLI), robust Comparative Fit Index (CFI), robust root mean square error of approximation (RMSEA) and its 90% CI, p value for test of close fit (null hypothesis RMSEA <0.05), and standardized root mean square residual (SRMR) with cutoffs recommended in the literature.39
Table 1.
Observed Indicators for Latent Dimensions Variables
Several sensitivity analyses were performed. First, we used a different definition of “diabetes” by excluding a self-reported history of diabetes. Second, additional baseline characteristics associated with availability of MRI, FDG-TEP, amyloid-PET, and CSF data (living alone, Clinical Dementia Rating scale score, prevalent dementia, depression, stroke history, cardiovascular history, and physical activity expressed as metabolic equivalent of task minutes per week; Table 2) were used as auxiliary variables in the estimation process under FIML to strengthen the missing at random assumption. Third, as the mediation analysis framework makes the implicit assumption that mediators (i.e., AD pathology, SVD, and neurodegeneration) are anterior to the outcome (i.e., cognition), we tried to preserve this assumption by excluding biomarkers measurements performed more than 6 months after cognitive assessments. Fourth, as CSF biomarkers are prone to variability whereas brain biomarkers are indicators of accumulated burden of lesions,40 we performed a sensitivity analysis using only brain amyloid load as indicator of the latent variable for AD pathology. Finally, we also compared the results with those obtained when considering interactions between diabetes and each mediator in the main adjusted model, as recommended for mediation analysis.41
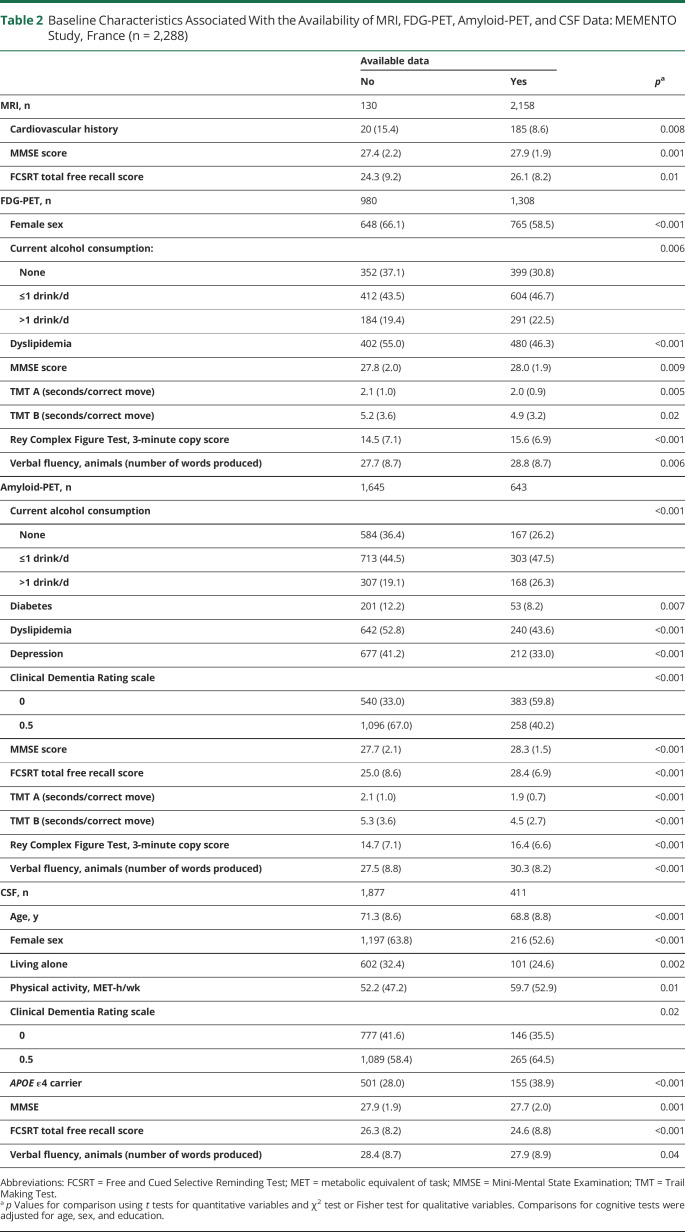
Table 2.
Baseline Characteristics Associated With the Availability of MRI, FDG-PET, Amyloid-PET, and CSF Data: MEMENTO Study, France (n = 2,288)
We also explored the mediating pathways in the association of diabetes with specific cognitive domains in separate models: a latent variable for memory (indicators: total free recall score and verbal fluency) and a latent variable for executive functioning (indicators: TMT A and TMT B scores).
Analyses were conducted using SAS v9.3 (SAS Institute Inc.), and R version 3.5.142 with the lavaan package for structural equation modeling analysis.38
Data Availability
Anonymized data will be shared by request from any qualified investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with EU legislation on the general data protection regulation.
Results
Baseline Description
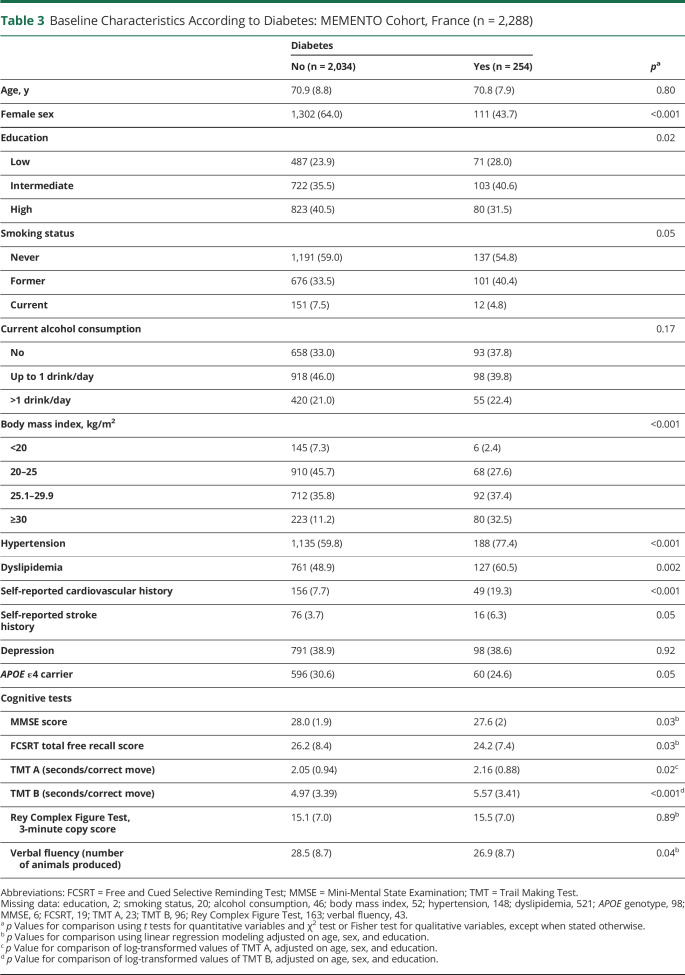
Compared to participants without diabetes at baseline, participants with diabetes (254 [11.1%]) were more likely to be men and to have lower education level. They were also more likely to have hypertension, dyslipidemia, obesity, and history of cardiovascular disease or stroke. Participants with diabetes had on average lower performances on executive functions and attention, memory, and semantic verbal fluency (Table 3).
Table 3.
Baseline Characteristics According to Diabetes: MEMENTO Cohort, France (n = 2,288)
At baseline, 65.3% of participants with diabetes were taking antidiabetic medications (oral antidiabetic agents, 57.5%; insulin, 13.8%). Diabetes status was solely based on self-report in 60 (23.6%) of the diabetic participants. The median self-reported duration of diabetes was 10.0 years (interquartile range, 4.9–19.4 years).
Diabetes, Latent Biomarkers, and Latent Cognition
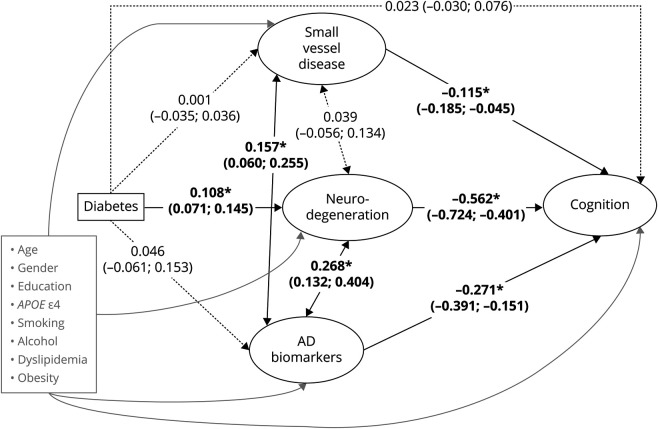
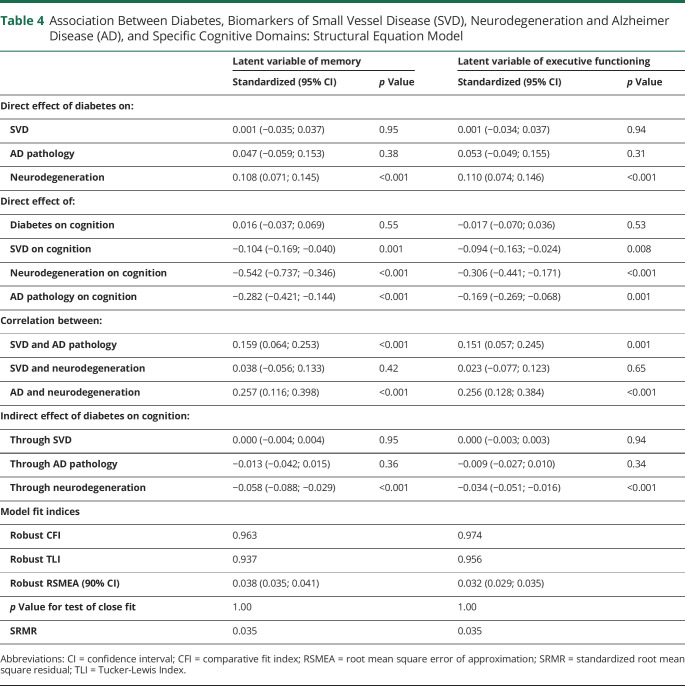
The model fit was adequate according to the recommended cutoffs: robust CFI = 0.951, robust TLI = 0.926, robust RMSEA = 0.040 (90% CI 0.037, 0.042), p value for test of close fit = 1.00, and SRMR = 0.038. Associations between diabetes, AD pathology, SVD, neurodegeneration and cognition are presented in the Figure. Presence of diabetes was significantly associated with higher neurodegeneration but was not significantly associated with AD pathology and SVD. Higher levels of SVD, neurodegeneration, and AD pathology were independently associated with lower cognition. Once adjusted for neurodegeneration, AD pathology, and SVD, there was no direct effect of diabetes on cognition (standardized β = 0.023; 95% CI −0.030, 0.076; p = 0.40). Association between diabetes and lower cognition was mainly mediated by higher neurodegeneration (standardized β = −0.061; 95% CI −0.089, −0.032; p < 0.001). The indirect effect of diabetes on cognition via SVD and AD pathology was not statistically significant (standardized β = 0.000; 95% CI −0.004, 0.004; p = 0.98 and standardized β = −0.013; 95% CI −0.040, 0.015; p = 0.38, respectively). In complementary analyses considering specific cognitive functions, associations between diabetes and lower memory or lower executive functioning were also mainly mediated by higher neurodegeneration (standardized β = −0.058; 95% CI −0.088, −0.029; p < 0.001 and standardized β = −0.034; 95% CI −0.051, −0.016; p < 0.001, respectively) (Table 4).
Figure. Structural Equation Model for the Association Between Diabetes, Small Vessel Disease, Neurodegeneration, Alzheimer Disease (AD) Biomarkers, and Cognition.
Latent variables of interest are indicated in ovals and observed variables in rectangles. Directed arrows represent linear regressions. Bidirectional arrows represent correlations. Standardized regression coefficient estimates are presented with their 95% confidence interval. Solid lines indicate statistically significant associations and correlations at the 5% level. Dotted lines indicate non–statistically significant associations and correlations at the 5% level. Adjustment covariates and their directed arrows to small vessel disease, neurodegeneration, AD biomarkers, and cognition are represented in gray. For readiness, the observed indicators defining each latent variable (listed in Table 1) and residual variances for all variables were omitted. *p < 0.001.
Table 4.
Association Between Diabetes, Biomarkers of Small Vessel Disease (SVD), Neurodegeneration and Alzheimer Disease (AD), and Specific Cognitive Domains: Structural Equation Model
Sensitivity Analyses
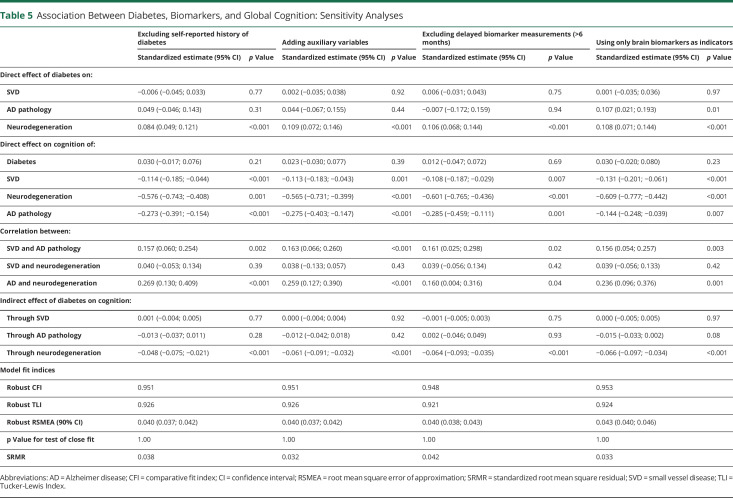
Results were similar when excluding self-reported history from the definition of diabetes, when adding auxiliary variables to the estimation process, or when excluding delayed measures of biomarkers (Table 5). When using only brain amyloid load as indicator of the latent variable for AD pathology, the indirect pathway linking diabetes to lower cognition through higher neurodegeneration was of similar magnitude (standardized β = −0.066; 95% CI −0.097, −0.034; p < 0.001). Diabetes was significantly associated with higher AD pathology (standardized β = 0.107; 95% CI 0.021, 0.193; p = 0.01), and higher AD pathology was significantly associated with lower cognition (standardized β = −0.144; 95% CI −0.248, −0.039; p = 0.007). The indirect pathway linking diabetes to lower cognition through AD pathology remained non-statistically significant (standardized β = −0.015; 95% CI −0.033, 0.002; p = 0.08). When considering interaction between diabetes and each intermediate latent variable, the indirect effects of diabetes on cognition via neurodegeneration (standardized β = −0.059; 95% CI −0.089, −0.030; p < 0.001), AD pathology (standardized β = −0.011; 95% CI −0.034, 0.012; p = 0.34), and SVD (standardized β = −0.001; 95% CI −0.006, 0.003; p = 0.54) remained virtually the same.
Table 5.
Association Between Diabetes, Biomarkers, and Global Cognition: Sensitivity Analyses
Discussion
In a cross-sectional analysis of a large clinical cohort of participants with either isolated cognitive complaints or mild cognitive impairment, we report that the deleterious effect of diabetes on cognitive performance is mainly mediated through markers of neurodegeneration whereas AD pathology (amyloid, p-tau) or SVD pathology do not seem to play a major role.
The association between diabetes and markers of neurodegeneration such as brain atrophy8,12,13,43 and brain hypometabolism11,12 has been consistently reported in cross-sectional studies. While diabetes is a risk factor for vascular disease and stroke, its association with subclinical cerebrovascular lesions (silent brain infarcts, WMH, cerebral microbleeds) is uncertain.44 In the present study, diabetes was not associated with SVD, even though participants with diabetes had more frequent self-reported history of stroke.
The mediating role of neurodegeneration and SVD in the association between diabetes and cognition has already been investigated in several studies. In a sample of 4,206 older adults of the Age, Gene/Environment Susceptibility–Reykjavik Study (mean age 76 years, 11% with diabetes), MRI markers of neurodegeneration (gray matter, normal white matter, and total brain tissue volumes) and SVD (cortical infarcts, subcortical infarcts, white matter lesions, and cerebral microbleeds) significantly mediated the cross-sectional association of diabetes with lower processing speed and executive function.15 In a longitudinal analysis on 817 participants from the Alzheimer's Disease Neuroimaging Initiative cohort (mean age 75 years, 15% with diabetes), the effect of diabetes on cognitive decline up to 60 months (mean follow-up time, 30 months) was significantly mediated by baseline cortical thickness.17 Similarly, in a sample of 448 older adults of the Swedish National Study on Aging and Care in Kungsholmen (mean age at baseline, 72 years), a higher cardiovascular burden, including diabetes as a component, was associated with a faster MMSE decline over 9 years, this effect being largely mediated by brain MRI markers of atrophy (volumes of total gray matter, ventricles, and hippocampus) and SVD (volume of WMHs).16 Nevertheless, none of those studies accounted for AD biomarkers, unlike the present study.
Insulin resistance and associated insulin signaling impairment promote Aβ accumulation and tau phosphorylation.7 However, no association between diabetes and amyloid and tau biomarkers was reported in previous studies.11,13,45 In the present study, diabetes was associated with higher brain amyloid load measured on PET imaging, but diabetes was not associated with the latent variable of AD pathology, which included CSF biomarkers of amyloid and tau. This discrepancy between brain and CSF biomarkers can partly be explained by the variability of CSF biomarkers, whereas brain biomarkers are indicators of accumulated lesions.
Although it needs to be replicated in longitudinal studies, our finding that neurodegeneration mediates the association between diabetes and cognitive performance, independently of biomarkers of AD and SVD, supports the hypothesis of a direct role of diabetes-related insulin resistance in the development of cognitive impairment in older adults with diabetes. Indeed, insulin also plays an important role in neuronal synaptic plasticity and facilitates learning and memory in humans4 and, therefore, impaired insulin signaling could directly contribute to neuronal dysfunction and degeneration. As impaired insulin signaling has also been linked to promotion of amyloid-β accumulation and tau hyperphosphorylation,7 brain insulin resistance could be a therapeutic target in AD and related dementias. Several exploratory clinical trials have reported a beneficial effect on cognition of intranasal insulin for healthy participants and participants with diabetes, mild cognitive impairment, or AD,46 and longer-term trials are ongoing.
The MEMENTO study has several strengths to answer the current objectives. First, a wide range of biomarkers was acquired in a highly standardized setting on more than 2,000 participants allowing a multidimensional assessment of brain aging and pathology biomarkers. Indeed, we were able to include simultaneously brain MRI, brain FDG-PET, amyloid-PET, and CSF data in a mediation analysis of the diabetes–cognition association, offering a unique insight on underlying mechanisms. Second, we were able to model brain biomarkers as latent variables in a structural equation modeling framework, accounting for measurement error of the indicators, and we were able to estimate direct and indirect effects of diabetes on several domains of cognition. Third, results were robust to several sensitivity analyses. There are also some limitations. First, the temporal relationship between diabetes, biomarkers, and cognition is not ensured by the cross-sectional design, and causality cannot be claimed. Nevertheless, we can hypothesize that diabetes preceded biomarkers measures in most participants with diabetes (duration was 4.9 years or more in 75% of participants with diabetes). We also modeled correlations between neurodegeneration, AD pathology, and SVD instead of directed relationships because the causal interpretation of their interrelations requires longitudinal data. Second, no tau-PET data were available to assess tau pathology, and we had to use CSF phosphorylated tau as a proxy for cerebral tau accumulation, assuming a strong correlation between both, as suggested by existing evidence.40 Third, the analytical strategy relies on the assumption that data are missing at random. This assumption may be strong for CSF and PET-amyloid data, for which 70%–80% of data were missing. However, we used a broad range of baseline characteristics associated with availability of CSF and PET-amyloid data as auxiliary variables in the estimation process, thus making the missing-at-random assumption more plausible. We must also acknowledge the unavailability of data regarding past and current glucose control that prevented us from exploring whether diabetes control modified the explored relationships. Finally, the observed findings may not fully translate in the general older population, as participants in the MEMENTO study are adults with either isolated cognitive complaints or mild cognitive impairment who were seeking care in memory clinics.
The current results suggest that the detrimental effect of diabetes on cognition is mediated by neurodegeneration, independently of AD and SVD pathologies, in a population of older adults at risk for dementia. Longitudinal studies are needed to reinforce and confirm these findings.
Acknowledgment
The MEMENTO cohort is sponsored by Bordeaux University Hospital (coordination: CIC1401-EC, Bordeaux) and was funded through research grants from the Fondation Plan Alzheimer (Alzheimer Plan 2008–2012), the French Ministry of Research and Higher Education (Plan Malandies Neurodégénératives [2016–2020]). The MEMENTO cohort has received funding support from AVID, GE Healthcare, and FUJIREBIO through private–public partnerships. The Insight-PreAD substudy was promoted by INSERM in collaboration with the Institut du Cerveau et de la Moelle Épinière, Institut Hospitalo-Universitaire, and Pfizer and has received support within the “Investissement d'Avenir” (ANR-10-AIHU-06) program. Sponsor and funders were not involved in the study conduct, analysis, or interpretation of data.
Glossary
- Aβ42
β-amyloid 42 peptide
- AD
Alzheimer disease
- CFI
Comparative Fit Index
- CI
confidence interval
- FCSRT
Free and Cued Selective Reminding Test
- FDG
fluorodeoxyglucose
- MMSE
Mini-Mental State Examination
- RMSEA
robust root mean square error of approximation
- ROI
region of interest
- SRMR
standardized root mean square residual
- SUVR
standard uptake value ratio
- SVD
small vessel disease
- TLI
Tucker-Lewis Index
- TMT
Trail Making Test
- WMH
white matter hyperintensity
Appendix. Authors
Appendix 2. Coinvestigators

Contributor Information
Collaborators: on behalf of the MEMENTO Cohort Study Group, Michèle Allard, Sandrine Andrieu, Pierre Anthony, Christine Astier, Alexandre Augier, Nicolas Auguste, Sophie Auriacombe, John Avet, Olivier Bailon, Fabrice-Guy Barral, Jean Barré, Annick Barthelaix, Catherine Bayle, Olivier Beauchet, Samia Belkacem, Douraied Ben Salem, Karim Bennys, Géraldine Bera, Eric Berger, Marc G Berger, Emilie Bergouin, François Bertin-Hugault, Guillaume Bertrand, François-Xavier Bertrand, Catherine Beze, Valérie Boilet, Alain Bonafé, Yasmina Boudali, Hatem Bouhladour, Clémence Boully, Vincent Bouteloup, Claire Boutet, Serge Bracard, Antoine Brangier, Pierre-Yves Brillet, Laure Caillard, Fabienne Calvas, Agnès Camus, Vincent Camus, Sandrine Canaple, Antoine Carpentier, Pascaline Cassagnaud, Françoise Cattin, Ludivine Chamard, Stéphane Chanalet, Mathieu Chastan, Sophie Chauvelier, Valérie Chauvire, Samia Cheriet, Anthony Clotagatide, Emmanuel Cognat, Lora Cohen, Jean-Marc Constans, Marie-Hélène Coste, Jean-Philippe Cottier, François Cotton, Isabelle Couret, Olivier-François Couturier, Pascale Cowppli-Bony, Véronique Cressot, Benjamin Crétin, Keren Danaila, Jacques Darcourt, Jean-François Dartigues, Ana-Maria Dascalita, Renaud David, Xavier De Petigny, Delphine De Verbizier-Lonjon, Marielle Decousus, Isabelle Defouilloy, Christine Delmaire, Julien Delrieu, Catherine Demuyinck, Vincent Deramecourt, Hervé Deramond, Thomas Desmidt, Marie-Dominique Desruet, Julien Detour, Agnès Devendeville, Mira Didic, Maritchu Doireau, Antonio Dos Santos, Patrice Douillet, Foucaud Du Boisgueheneuc, Delphine Dubail, Laure Ducroq-Ducastaing, Julien Dumurgier, Diane Dupuy, Emmanuelle Duron, Inna Dygai-Cochet, Véronique Eder, Stéphane Epelbaum, Frédérique Etcharry-Bouyx, Daniel Fagret, Catherine Faisant, Karim Farid, Denis Fédérico, Olivier Felician, Philippe Fernandez, Pacôme Fosse, Alexandra Foubert-Samier, Isabelle Franck, Monique Galitzky, Céline Gallazzini-Crepin, Radka Gantchev, Laurence Garbarg-Chenon, Guillaume Gautier, Emmanuel Gerardin, Claire Gervais, Jean-Claude Getenet, Nadine Girard, Fabienne Giraud, Chantal Girtanner, Valérie Gissot, Caroline Grangeon, Daniel Grucker, Eric Guedj, Claude Gueriot, Yves Guilhermet, Rémy Guillevin, Sophie Haffen, Didier Hannequin, Sandrine Harston, Anne Hitzel, Caroline Hommet, Claude Hossein-Foucher, Fabrice Hubele, Agnès Jacquin-Piques, Betty Jean, Joanne Jenn, Laure Joly, Thérèse Jonveaux, Adrien Julian, Aurélie Kas, Anna Kearney-Schwartz, Alice Keles, Antony Kelly, Nathalie Keromnes, Lejla Koric, Alexandre Krainik, Stéphane Kremer, Florian Labourée, Franck Lacoeuille, Francoise Lala, Chantal Lamy, Jean-Louis Laplanche, Cyrille Launay, Stéphane Lehericy, Sylvain Lehmann, Hermine Lenoir, Marcel Levy, Stéphanie Libercier, Marie-Anne Mackowiak-Cordoliani, Eloi Magnin, Zaza Makaroff, Athina Marantidou, Isabelle Marcet, Cécilia Marelli, Sophie Marilier, Idalie Martin, Olivier Martinaud, Catherine Martin-Hunyadi, Aïcha Medjoul, Isabelle Merlet, Danielle Mestas, Marc-Etienne Meyer, Jean-Marc Michel, Agnès Michon, Isabelle Migeon-Duballet, Karl Mondon, Clément Morgat, Véronique Moullart, Christian Moussard, Aurélie Mouton, Izzie Jacques Namer, Georges Niewiadomski, Guillaume Nivaggioni, Marie Noblet, Michel Nonent, Fati Nourhashemi, Hélène Oesterle, Galdric Orvoen, Amandine Pallardy, Pierre-Yves Pare, Anne Pasco, Pierre Payoux, Cécile Pays, Isabelle Pellegrin, Rémy Perdrisot, Bertille Perin, Christine Perret-Guillaume, Grégory Petyt, Nathalie Philippi, Geneviève Pinganaud, Matthieu Plichart, Gabriel Pop, Michèle Puel, Mathieu Queneau, Solène Querellou, Muriel Quillard-Muraine, Valérie Quipourt, Chloé Rachez, Micheline Razzouk-Cadet, Anne-Sophie Rigaud, Hélène Robin-Ismer, Mathieu Rodallec, Yves Rolland, Adeline Rollin-Sillaire, Olivier Rouaud, Caroline Roubaud, Isabelle Rouch, Julie Roux, Guillaume Sacco, Pierre-Yves Salaun, François Salmon, Alicia Sanchez, Maria-Joao Santiago-Ribeiro, Alain Sarciron, Nathalie Sastre-Hengan, Christian Scheiber, Anne-Marie Schneider, Franck Semah, Amélie Serra, Marie-Laure Seux, Hélène Sordet-Guépet, Maria Eugenia Soto, Mathieu Tafani, Jean-Yves Tanguy, Michael Taroux, Marc Teichmann, Catherine Terrat, Jamila Thabet, Claire Thalamas, Catherine Thomas-Anterion, Anne-Cécile Troussière, Renata Ursu, Pierre Vera, Martine Vercelletto, Olivier Vercruysse, Antoine Verger, Philippe Viau, Marie-Neige Videau, Thierry Voisin, Nathalie Wagemann, Aziza Waissi-Sediq, Jing Xie, Nathanaëlle Yeni, Michel Zanca, and Jean Zinszner
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Chatterjee S, Peters SAE, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawlings AM, Sharrett AR, Albert MS, et al. The association of late-life diabetes status and hyperglycemia with incident mild cognitive impairment and dementia: the ARIC study. Diabetes Care. 2019;42(7):1248-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayeda ER, Whitmer RA, Yaffe K. Diabetes and cognition. Clin Geriatr Med. 2015;31(1):101-ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdile G, Fuller SJ, Martins RN. The role of type 2 diabetes in neurodegeneration. Neurobiol Dis. 2015;84:22-38. [DOI] [PubMed] [Google Scholar]
- 5.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willette AA, Bendlin BB, Starks EJ, et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol. 2015;72(9):1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharadwaj P, Wijesekara N, Liyanapathirana M, et al. The link between type 2 diabetes and neurodegeneration: roles for amyloid-β, amylin, and tau proteins. J Alzheimers Dis. 2017;59(2):421-432. [DOI] [PubMed] [Google Scholar]
- 8.Moran C, Phan TG, Chen J, et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013;36(12):4036-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider ALC, Selvin E, Sharrett AR, et al. Diabetes, prediabetes, and brain volumes and subclinical cerebrovascular disease on MRI: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Diabetes Care. 2017;40(11):1514-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marseglia A, Fratiglioni L, Kalpouzos G, Wang R, Bäckman L, Xu W. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: a population-based cohort study. Alzheimers Dement. 2019;15(1):25-33. [DOI] [PubMed] [Google Scholar]
- 11.Roberts RO, Knopman DS, Cha RH, et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med. 2014;55(5):759-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Risacher SL, Huang E, Saykin AJ; Alzheimer's Disease Neuroimaging Initiative. Type 2 diabetes mellitus is associated with brain atrophy and hypometabolism in the ADNI cohort. Neurology. 2016;87(6):595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran C, Beare R, Phan TG, et al. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology. 2015;85(13):1123-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Risacher SL, Gao S, Boehm SL, Elmendorf JS, Saykin AJ. Type 2 diabetes mellitus and cerebrospinal fluid Alzheimer's disease biomarker amyloid β1-42 in Alzheimer's Disease Neuroimaging Initiative participants. Alzheimers Dement. 2018;10:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu C, Sigurdsson S, Zhang Q, et al. Diabetes, markers of brain pathology and cognitive function: the Age, Gene/Environment Susceptibility-Reykjavik Study. Ann Neurol. 2014;75(1):138-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Fratiglioni L, Kalpouzos G, et al. Mixed brain lesions mediate the association between cardiovascular risk burden and cognitive decline in old age: a population-based study. Alzheimers Dement. 2017;13(3):247-256. [DOI] [PubMed] [Google Scholar]
- 17.Moran C, Beare R, Wang W, Callisaya M, Srikanth V; Alzheimer's Disease Neuroimaging Initiative (ADNI). Type 2 diabetes mellitus, brain atrophy, and cognitive decline. Neurology. 2019;92(8):e823-e830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dufouil C, Dubois B, Vellas B, et al. Cognitive and imaging markers in non-demented subjects attending a memory clinic: study design and baseline findings of the MEMENTO cohort. Alzheimers Res Ther. 2017;9(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. [DOI] [PubMed] [Google Scholar]
- 20.Hugonot-Diner L. MMS version consensuelle GRECO. In: La Consultation en Gériatrie. Masson; 2001:13-20. [Google Scholar]
- 21.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38(6):900-903. [DOI] [PubMed] [Google Scholar]
- 22.Thurstone LL. Psychophysical analysis: by L.L. Thurstone, 1927. Am J Psychol. 1987;100(3-4):587-609. [PubMed] [Google Scholar]
- 23.Benton AL, Varney NR, Hamsher KD. Visuospatial judgment: a clinical test. Arch Neurol. 1978;35(6):364-367. [DOI] [PubMed] [Google Scholar]
- 24.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203-214. [DOI] [PubMed] [Google Scholar]
- 25.Operto G, Chupin M, Batrancourt B, et al. CATI: a large distributed infrastructure for the neuroimaging of cohorts. Neuroinformatics. 2016;14(3):253-264. [DOI] [PubMed] [Google Scholar]
- 26.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839-851. [DOI] [PubMed] [Google Scholar]
- 27.Chupin M, Hammers A, Liu RSN, et al. Automatic segmentation of the hippocampus and the amygdala driven by hybrid constraints: method and validation. NeuroImage. 2009;46(3):749-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968-980. [DOI] [PubMed] [Google Scholar]
- 29.Samaille T, Fillon L, Cuingnet R, et al. Contrast-based fully automatic segmentation of white matter hyperintensities: method and validation. PLoS One. 2012;7(11):e48953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fazekas F, Barkhof F, Wahlund LO, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2002;13(suppl 2):31-36. [DOI] [PubMed] [Google Scholar]
- 31.Habert M-O, Marie S, Bertin H, et al. Optimization of brain PET imaging for a multicentre trial: the French CATI experience. EJNMMI Phys. 2016;3(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchert R, Wilke F, Chakrabarti B, et al. Adjusted scaling of FDG positron emission tomography images for statistical evaluation in patients with suspected Alzheimer's disease. J Neuroimaging. 2005;15(4):348-355. [DOI] [PubMed] [Google Scholar]
- 33.Toussaint P-J, Perlbarg V, Bellec P, et al. Resting state FDG-PET functional connectivity as an early biomarker of Alzheimer's disease using conjoint univariate and independent component analyses. NeuroImage. 2012;63(2):936-946. [DOI] [PubMed] [Google Scholar]
- 34.Habert M-O, Bertin H, Labit M, et al. Evaluation of amyloid status in a cohort of elderly individuals with memory complaints: validation of the method of quantification and determination of positivity thresholds. Ann Nucl Med. 2018;32(2):75-86. [DOI] [PubMed] [Google Scholar]
- 35.de Medeiros K, Robert P, Gauthier S, et al. The Neuropsychiatric Inventory–Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr. 2010;22(6):984-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanfilipo MP, Benedict RHB, Zivadinov R, Bakshi R. Correction for intracranial volume in analysis of whole brain atrophy in multiple sclerosis: the proportion vs. residual method. NeuroImage. 2004;22(4):1732-1743. [DOI] [PubMed] [Google Scholar]
- 37.Bollen KA. Structural Equations with Latent Variables. John Wiley & Sons; 2014:474. [Google Scholar]
- 38.Rosseel Y. Lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48(1):1-36. [Google Scholar]
- 39.Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Methods Psychol Res. 2003;8(2):23-74. [Google Scholar]
- 40.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanderWeele TJ. Mediation analysis: a practitioner's guide. Annu Rev Public Health. 2016;37:17-32. [DOI] [PubMed] [Google Scholar]
- 42.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2018. R-project.org/. [Google Scholar]
- 43.Espeland MA, Bryan RN, Goveas JS, et al. Influence of type 2 diabetes on brain volumes and changes in brain volumes: results from the Women's Health Initiative Magnetic Resonance Imaging studies. Diabetes Care. 2013;36(1):90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moran C, Beare R, Phan T, et al. Neuroimaging and its relevance to understanding pathways linking diabetes and cognitive dysfunction. J Alzheimers Dis. 2017;59(2):405-419. [DOI] [PubMed] [Google Scholar]
- 45.Thambisetty M, Jeffrey Metter E, Yang A, et al. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2013;70(9):1167-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benedict C, Grillo CA. Insulin resistance as a therapeutic target in the treatment of Alzheimer's disease: a state-of-the-art review. Front Neurosci. 2018;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with EU legislation on the general data protection regulation.