Abstract
Objective
The associations of Lewy bodies (LBs) with olfactory dysfunction, parkinsonism, and higher odds of dementia were assessed in Black and White community-dwelling elders and racial differences in these associations were tested.
Methods
Black decedents (n = 81) were matched 2-to-1 by age, sex, years of education, and follow-up time in the study with White decedents (n = 154) from 4 longitudinal studies of dementia and aging. Participants underwent uniform clinical examination and cognitive, motor, and olfactory testing. LBs were detected in 7 brain regions by α-synuclein immunohistochemistry and racial differences in their association with olfaction, parkinsonism, and odds of dementia were determined using regression analyses.
Results
The mean scores of the odor test, global parkinsonism signs, and global cognition were lower in Black than White decedents; the frequency of dementia was similar in both groups. The frequency of LBs was similar in Black and White decedents (∼25%), as was the frequency of LBs in individual brain regions, while the mean LB counts/mm2 were similar in all regions except the cingulate cortex, which showed higher mean LB counts in Black decedents. In regression analyses, LBs were associated with impaired olfaction (−2.23, 95% confidence interval [CI] −3.45 to −1.01) and higher odds of dementia (odds ratio 3.0, 95% CI 1.10–8.17) in both racial groups; an association with parkinsonism was stronger in Black than White decedents.
Conclusions
The frequency, distribution, and clinical manifestations of LBs are similar in Black and White elders.
The major Lewy body (LB) disorders include Parkinson disease (PD),1,2 dementia with LBs (DLB),3 and incidental LB disease, which may represent preclinical PD because clinical features of PD are absent.4 Biological risk factors such as the APOE ε4 allele have a high frequency in DLB and PD dementia.5 Much of the information on the association of LBs with age-related clinical characteristics such as olfactory dysfunction, parkinsonism, and dementia is derived from studies of community-dwelling mainly White elders without a clinical diagnosis of PD. Our prior studies have shown that LBs are associated with impaired olfaction, an association that is strongest in the late pathologic stage when LBs extend to the neocortex,6,7 and that nigral LBs are associated with parkinsonism.8 Neocortical LBs are also reported to be independently associated with higher odds of dementia in community-dwelling White elders.9-11 Racial differences exist in the prevalence of disease because African American (AA) people are twice as likely to have Alzheimer dementia12 and half as likely to be diagnosed with PD compared to non-Hispanic White people.13 Few clinical-pathologic studies include well-characterized Black decedents; thus the extent to which LBs associate with age-related clinical characteristics such as olfaction, parkinsonism, and dementia in older Black adults is largely unknown.
Our primary objective was to study racial differences in the frequency, regional distribution, and mean LB counts/mm2 in older Black vs White community-dwelling decedents and test for racial differences in the association of LBs with olfactory dysfunction, parkinsonism, and dementia in separate regression models because differences may have clinical, research, and health care policy implications. Decedents were from 1 of 4 harmonized longitudinal studies of aging and dementia.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The 4 longitudinal studies of aging and dementia included the Rush Memory and Aging Project (Rush MAP), the Religious Orders Study (ROS), the Minority Aging Research Study (MARS), and the AA Core, which were approved by the Institutional Review Board of Rush University Medical Center. Dementia was not present in Black or White decedents at enrollment when they signed informed consent for annual clinical evaluations and an Anatomical Gift Act form for brain donation. Data collection in MARS began in 2004; optional brain donation was added in 2010.
Participants and Clinical Evaluation
Race was determined by self-identification using the 1990 Census categories. By March 2020, 88 Black community-dwelling elders were autopsied; after exclusion of cases with missing clinical data, only 81 cases were available for analyses from MARS (n = 32), the AA Core (n = 12), Rush MAP (n = 14), and ROS (n = 23). Black decedents were matched 2-to-1 with White decedents (n = 154) by age at death, sex, years of education, and their follow-up time in the studies using Mahalanobis distance matching as described previously.14 The groups could not be matched by socioeconomic status because data on income were not available for many of the participants. The antemortem and postmortem data collections were similar in all the studies, allowing combined analyses of the cohorts.
Participants underwent uniform clinical evaluations at baseline and annually thereafter for odor testing, parkinsonism signs, and cognitive function. Since 2011, olfaction was assessed annually by the Brief Smell Identification Test (BSIT),15 which assessed the ability to recognize 12 familiar odors. The score for the test was the number of correct choices plus 0.25 assigned for each missing response to a maximum of 2 as described previously.16 Over a mean period of 5.5 (SD 3.2) years, each participant was tested 1–8 times and the last BSIT was done a mean of 2.94 (SD 2.93) years prior to death and this score was used in analyses. In prior research, the BSIT score correlated with the 40-item University of Pennsylvania Smell Identification Test from which it was derived17 and to have adequate short-term temporal stability.15
Motor evaluation included a modified version of the Unified Parkinson’s Disease Rating Scale (UPDRS) that included 26 items administered by nurse clinicians to assess 4 parkinsonism signs (gait disturbance, bradykinesia, rigidity, and tremor).18 These measures had high interrater reliability and short-term stability among nurses compared with a movement disorders specialist. The scores of the 4 signs were averaged to provide a summary global parkinsonism score as described previously.18 The last valid mean global parkinsonism score was obtained a mean of 2.0 (SD 2.4) years prior to death for all participants. The parkinsonism sign scores had a positively skewed distribution; therefore the summary global parkinsonism score was square root transformed and the transformed scores were used in all analyses.
A standardized battery of cognitive performance tests was performed,6,19 of which the Mini-Mental State Examination (MMSE) and Complex Ideational Material were used for descriptive and diagnostic purposes only. Of the remaining 19 tests, 17 in common across the 4 cohorts assessed function in 5 cognitive abilities including episodic, semantic, and working memory, perceptual speed, and visuospatial ability. The composite scores for each of these domains were obtained and the z scores of all 17 tests were averaged to derive the global cognition score.20 The last valid mean cognitive z scores computed for Black and White decedents prior to death were a mean of 0.9 (SD 0.8) and 1.0 (SD 1.1) years, respectively. Dementia and clinical Alzheimer disease (AD) were diagnosed using the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association.21 Dementia status was assigned after review of all clinical information by personnel blinded to the pathologic diagnoses.
APOE genotyping was done using DNA by Polymorphic DNA Technologies blinded to all clinical data. High throughput sequencing of codon 112 (position 3937) and codon 158 (position 4075) of exon 4 of the APOE gene on chromosome 19 was done as described previously.22
Neuropathology Evaluation
The mean postmortem interval was 11.4 hours (SD 10.9). Brain autopsy and fixation were performed following a standard protocol as described previously.20 Macroscopic examination of the whole brain documented the location and size of macroscopic infarcts and the degree of atherosclerosis in vessels at the brain base, which was assessed using a 6-tiered semiquantitative scale from 0 (none) to 6 (severe) as described previously.20 Eleven brain subregions from 1 hemisphere were dissected for microscopic evaluation. Paraffin-embedded sections (6 μm) stained with hematoxylin & eosin were used to detect microscopic infarcts and hippocampal sclerosis (HS) and assess the degree of arteriolosclerosis as described previously.20 Substantia nigra neuronal loss and arteriolosclerosis in basal ganglionic vessels were graded using a 4-tiered semiquantitative scale from 0 (none) to 4 (severe) as described previously.8 Cerebral amyloid angiopathy (CAA) was assessed in meningeal and intracortical vessels in sections from the midfrontal, midtemporal, inferior parietal, and occipital cortices, which were immunostained for β-amyloid and graded as described previously.23
Degenerative Brain Pathologies
LBs were localized in 7 brain regions (midfrontal, midtemporal, entorhinal, cingulate and inferior parietal cortices, amygdala, and substantia nigra) using an antiphosphorylated α-synuclein antibody and recorded as a dichotomous variable.6,8,24 LBs in these 7 brain subregions were assigned to 4 stages (amygdala predominant, nigral, limbic, and neocortical) using a modified version of the categories of Lewy-related pathology.3,24 In stage 1 (amygdala predominant), LBs were present in only the amygdala or entorhinal cortex, while in LB stage 2 (nigral), LBs were also present in the substantia nigra. In LB stage 3 (limbic), LBs were additionally present in the anterior cingulate or midtemporal cortices, while in LB stage 4 (neocortical), in addition to the previous regions, LBs were also present in the inferior parietal or midfrontal cortices. In addition, the total LBs/mm2 in neurons in the 7 subregions were quantitated at a 100× magnification, in a 1 mm2 area of greatest density. Pathologic diagnosis of PD included presence of LBs with moderate to severe nigral neuronal loss.8
Pathologic diagnosis of AD included intermediate and high likelihood cases as defined by the National Institute on Aging–Reagan criteria.25 Neuritic and diffuse plaques and neurofibrillary tangles in the midfrontal, midtemporal, inferior parietal, and entorhinal cortices and hippocampal CA1, having the highest density of these structures, were quantitated from sections stained by the modified Bielschowsky silver stain.20 Limbic-predominant age-related transactive response DNA-binding protein 43 (TDP-43) neuropathologic change (LATE-NC) was localized by immunohistochemistry in the amygdala, entorhinal cortex, hippocampus CA1 and subiculum, and dentate neurons and 4 neocortical areas (anterior temporal pole, midtemporal, orbital frontal, and midfrontal cortices). A semiquantitative estimate of phosphorylated TDP-43 cytoplasmic inclusions in neurons and glia was obtained as described previously.20 LATE-NC distribution was divided into 3 stages as described previously.26 In stage 1, LATE-NC was localized to the amygdala; in stage 2, there was further extension to the hippocampus or entorhinal cortex; in stage 3, there was additional involvement of neocortical areas. Due to the small number of decedents in both racial groups, LATE-NC distribution was dichotomized into none/mild (none and stage 1) and moderate/severe (stages 2–3) for descriptive and analytic purposes.26
Statistical Analyses
Demographics, clinical characteristics, and age-related pathologies, including LB stages, PD, AD, HS, macroscopic and microscopic infarcts, and vascular pathologies (arteriolosclerosis, atherosclerosis, and CAA), in all Black and White decedents and in those with and without LBs, were compared using the χ2 test, t statistics, or the Wilcoxon rank sum test as appropriate. The frequency of LBs in each brain region in Black and White decedents was compared using the χ2 test; the mean LB counts/mm2 in each region in both racial groups were compared using the t test.
Multivariable linear regression models that included both Black and White decedents were used to determine the association of LBs separately with the outcome measures of the odor test, parkinsonism, global cognition, and cognitive domain scores. The association of LBs with dementia as a binary outcome and the association of APOE ε4 with LBs was tested by logistic regression analyses. In both the linear and logistic regression analyses, a term for an interaction between Black race and LBs was included as the primary predictor to test for racial differences in the association of LBs with the outcome measures. All models controlled for age, sex, education, and years from last test assessment to death and the other age-related pathologies listed above. LBs were analyzed as an ordinal variable based on the 4 LB stages described above. In addition, in separate analyses, LBs were treated as a dichotomous summary diagnosis of LB disease, present or absent.6,8 Results were presented as point estimates with 95% confidence intervals (CIs). All analyses were carried out using Statistical Analysis Software (SAS/STAT 14.1, SAS Institute Inc.). Model assumptions were examined graphically and analytically and were adequately met. A nominal threshold of p < 0.05 was used for statistical significance.
Data Availability
The data used in this study can be requested from the RADC Research Resource Sharing Hub (radc.rush.edu).
Results
Black decedents (n = 81) had a mean age at death of 84.7 years, their years of education was a mean of 15.2 years (table 1), and their mean follow-up time in the studies was 6.5 years; for matched White decedents (n = 154), these values were similar, being 85.3, 15.3, and 6.6 years, respectively. The ordinal staging LB variable and the dichotomous summary diagnosis of LB disease variable used in this study gave similar results (data not shown).
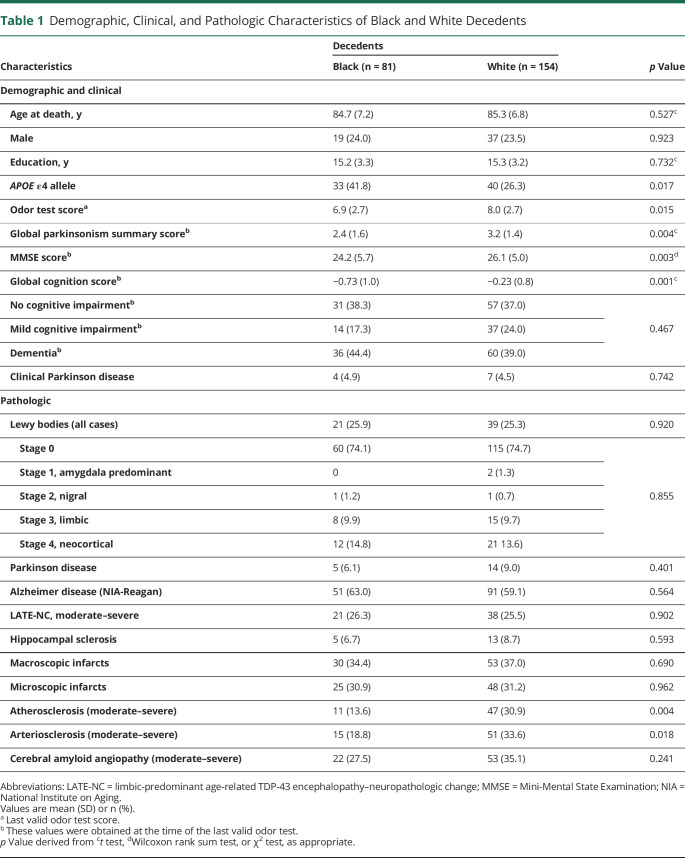
Table 1.
Demographic, Clinical, and Pathologic Characteristics of Black and White Decedents
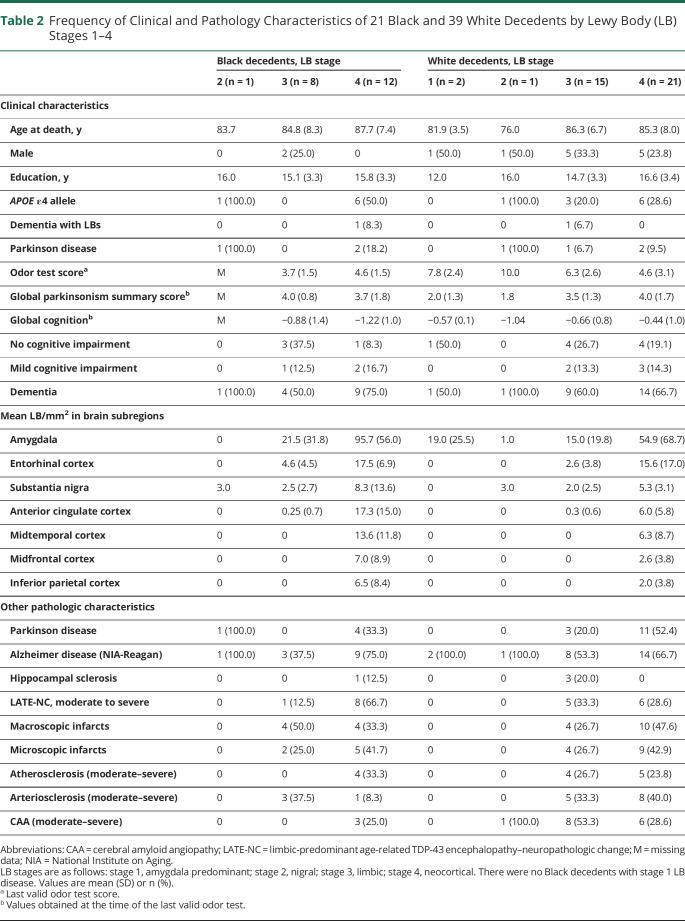
The most frequent clinical diagnosis in both racial groups was dementia, followed by no cognitive impairment and mild cognitive impairment (table 1). One Black and 1 White participant were diagnosed with DLB (table 2) and by pathology they were diagnosed with stage 4 and stage 3 LB disease, respectively. Diagnosis of clinical PD was low, being 4.9% in Black decedents and 4.5% in White decedents (table 1). There were no racial differences in these clinical diagnoses. The frequency of clinical and pathologic characteristics of Black and White decedents in the 4 LB stages is shown in table 2. The frequency of mixed pathologies is higher in LB stages 3 and 4 in both racial groups. The frequency of APOE ε4 allele was higher in Black than White decedents (41.8% vs 26.3%, p = 0.017; table 1). In logistic regression analyses that included both Black and White decedents, the APOE ε4 allele was not related to LBs (odds ratio [OR] 0.79, 95% CI 0.41–1.54; p = 0.492) and this finding did not differ by race since in an additional model the term for an interaction between APOE ε4 allele and Black race was not significant. The mean global cognition score was lower in Black compared to White decedents (table 1). In linear regression analyses, LBs in both Black and White decedents were associated with global cognition (−0.13, 95% CI −0.25 to −0.002) and with semantic memory (−0.27, 95% CI −0.46 to −0.08).
Table 2.
Frequency of Clinical and Pathology Characteristics of 21 Black and 39 White Decedents by Lewy Body (LB) Stages 1–4
Pathologic Findings
The frequency of LBs was similar in Black and White decedents (25.9% and 25.3%, respectively) (table 1). Of the decedents with LBs, 5 of 21 Black and 14 of 39 White decedents also had moderate to severe nigral neuronal loss and were diagnosed with PD by neuropathology assessment (tables 1 and 2). The frequency of brain subregions having LBs was similar in both racial groups (table 3), with high involvement of the entorhinal cortex, amygdala, and substantia nigra in both groups. Mean LB counts/mm2 were highest in the amygdala in both Black and White decedents and there was no racial difference in the mean LB counts across brain regions except for the anterior cingulate cortex, in which the mean LB counts were higher in Black compared to White decedents (table 3). The frequency of age-related pathologies such as AD, LATE-NC, and HS or vascular pathologies such as infarcts and CAA were similar in Black and White decedents (table 1). The frequency of atherosclerosis and arteriolosclerosis was lower in Black than White decedents in this matched sample.
Table 3.
Frequency of Brain Subregions With Lewy Bodies (LBs) and Mean LB/mm2 in These Subregions in 21 Black and 39 White Decedents
LBs and Olfaction
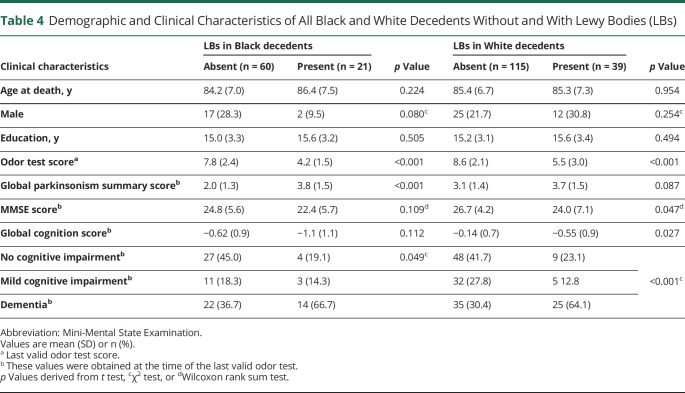
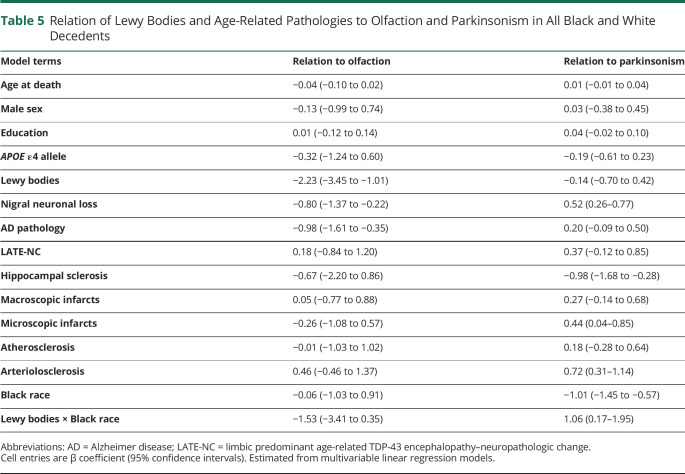
Overall, the last valid mean BSIT scores proximate to death were lower in Black than White decedents, being 6.9 (SD 2.7) and 8.0 (SD 2.7), respectively (table 1). In both races, the mean BSIT scores were lower in those with LBs compared to those without LBs (table 4). In a linear regression model that controlled for demographics and time from the last valid BSIT to death, LBs in both Black and White decedents were associated with lower BSIT scores (−3.27; 95% CI −4.10 to −2.45). This association persisted when terms for AD pathology and nigral neuronal loss were added to the model (−2.86; 95% CI −3.75 to −1.96). The association of LBs with lower BSIT scores persisted when other age-related degenerative and vascular pathologies were added to the model (table 5), while nigral neuronal loss and AD pathology were independently associated with impaired olfaction. In this model, a term for the interaction of LBs with race failed to reach significance (table 5), indicating that the association of LBs with impaired olfaction was similar in Black and White decedents.
Table 4.
Demographic and Clinical Characteristics of All Black and White Decedents Without and With Lewy Bodies (LBs)
Table 5.
Relation of Lewy Bodies and Age-Related Pathologies to Olfaction and Parkinsonism in All Black and White Decedents
LBs and Parkinsonism
Overall, the mean global parkinsonism score was lower in Black compared to White decedents (table 1). Black decedents with LBs (table 4) had a higher mean global parkinsonism score compared to those without LBs; in White decedents, parkinsonism scores were similar in those with and without LBs. In a linear regression model that controlled for demographics, LBs in both Black and White decedents were associated with the global parkinsonism score (0.62; 95% CI 0.20–1.05). However, when nigral neuronal loss and age-related pathologies were added to the model, the association of LBs with the parkinsonism score was no longer observed, while HS and arteriolosclerosis were independently associated with the global parkinsonism score (table 5). In the latter model, a term for the interaction of LBs with race (table 5) showed a race by LB interaction such that the LB association with the global parkinsonism score was greater in Black than White decedents.
LBs and Dementia
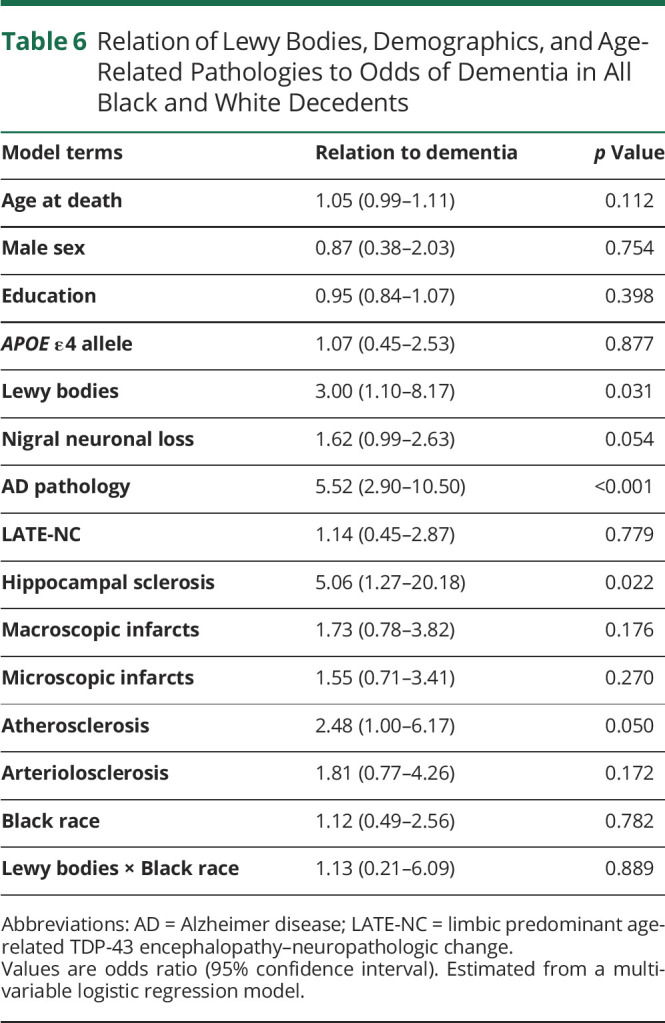
Overall, the frequency of dementia was similar in Black and White decedents (table 1). In both races, the frequency of dementia was higher in those with LBs as compared to those without LBs (table 4). In a logistic regression model that controlled for demographics, LBs in both Black and White decedents were associated with higher odds of dementia (OR 3.96, 95% CI 2.09–7.49). This association persisted when terms for AD pathology and nigral neuronal loss were added to the model (OR 3.12, 95% CI 2.59–8.76). The association of LBs with dementia persisted when age-related degenerative and vascular pathologies were also added to the model (table 6). In the latter model, a term for the interaction between LBs and race (table 6) was not significant, indicating no racial difference in the association of LBs with higher odds of dementia.
Table 6.
Relation of Lewy Bodies, Demographics, and Age-Related Pathologies to Odds of Dementia in All Black and White Decedents
Discussion
More than 200 elderly Black and White participants in clinical–pathologic studies underwent antemortem tests for olfaction, parkinsonism, and cognition and postmortem neuropathology evaluation to detect LBs in multiple brain regions. The overall frequency of LBs and their regional distribution were similar in Black and White decedents. LBs were associated with olfactory dysfunction, parkinsonism, and higher odds of dementia in both racial groups, suggesting that the pathologic and clinical manifestations of LB disease are similar in Black and White elders.
A recent large clinical study of LB diseases reported that cognitively impaired AA and Hispanic patients were more likely to be female and have less educational attainment compared to White participants.27 This could not be confirmed in the current study, as Black and White decedents were matched on sex and educational attainment in an attempt to remove confounds associated with race. As observed in prior studies, APOE ε4 allele frequency was higher in Black than White decedents.26,28 This finding did not affect outcomes; in regression models that controlled for APOE ε4, the association of race with olfaction, parkinsonism, and dementia persisted. The lack of association between the APOE ε4 allele and LBs in Black or White decedents observed in logistic regression analyses may be attributed to small sample size, since a prior study of 652 mainly White decedents reported an association between APOE ε4 and the severity of LB pathology that was independent of AD pathology.5
The frequency of LBs in Black and White decedents in the present study was similar, contrasting the finding of another larger study of unmatched 110 Black and 2,500 White decedents with dementia in whom LB pathology was more common in Black than White decedents.29 The frequency of LBs in Black and White decedents in the current study was about 25% which is similar to previous studies of mainly White community-dwelling elders, in whom the frequency was about 18%,11 and in a general population study of Japanese elders, in whom the frequency was 22.5%.30 More extensive LB data collection in 13 brain regions including the spinal cord reported a LB frequency of 34%.31 In the current study, LB counts/mm2 in 6 brain regions were similar in Black and White decedents, with the highest counts documented in the amygdala, since both racial groups had a high frequency of neocortical LBs in which the amygdala is affected early in the course of LB disease. The mean LB counts in the anterior cingulate cortex that were greater in Black than White decedents is possibly related to chance as decedents in both groups were matched.
The prevalence of olfactory impairment was reported to be high among White elders and increased with age.32,33 The current finding that the BSIT scores were significantly lower in Black compared to White decedents has been reported previously.33,34 This racial difference in the odor test scores was not explained by differences in sex, education, cognition, physical or mental health, or health behaviors.33 Our prior studies6,7 of mainly White decedents provided a pathologic correlate for impaired olfaction that was associated with the presence of neocortical LBs. In the current study, linear regression analyses showed a strong association between LBs and BSIT scores in both Black and White decedents and this may be due to the similar frequency of neocortical LBs in both groups. AD is known to be independently associated with olfactory dysfunction in White decedents6,35 and similar findings are observed in the current study. Olfactory dysfunction is an early change since olfactory dysfunction in persons without dementia is associated with increased risk of mild parkinsonism signs36 and dementia.34 Impaired olfaction is also reported to predict faster cognitive decline and may indicate neurodegeneration in the brain of dementia-free older adults.37
Parkinsonism is common in older White adults, affecting 50% or more by 85 years.38 Our prior studies of community-dwelling White elders without PD reported that nigral LBs were associated with parkinsonism8 and that LBs in the spinal cord were associated with higher odds of clinical parkinsonism as compared to those with LBs only in cerebrum and midbrain.31 In the present study, the association between LBs and parkinsonism was stronger in Black than White decedents, possibly related to the higher mean parkinsonism scores in Black decedents with LBs as compared to White decedents. Although parkinsonism signs are reported to be higher in Black than White clinic cases,39 a recent clinical study found no racial differences in the degree of motor findings assessed by the UPDRS in Black compared to White participants.27 In our prior study, macroscopic and microscopic infarcts were reported to be associated with mild parkinsonism, especially parkinsonian gait.40 In the present study, the frequency of infarcts was similar in both racial groups. An unexpected finding was the higher frequency of arteriolosclerosis and atherosclerosis in White compared to Black decedents, which is the reverse of the findings of our prior study,41 in which a higher frequency of these vascular pathologies was present in Black compared to White decedents. However, the prior study included decedents from a Memory Clinic who had dementia at enrollment, while the current study included community-dwelling participants who were dementia-free at enrollment and at death about 60% of Black and White decedents were still dementia-free. In the current study, although the frequency of HS cases was small, this pathology was independently associated with parkinsonism. This finding could be attributed to chance because our decedents are matched and this observation was not reported in a larger study having consecutive samples.42
Data from the Aging, Demographics and Memory Study, which included participants from across the United States, reported that older age was consistently associated with greater risk of dementia (p < 0.0001) and that Black people were at greater risk for dementia than White people (p < 0.008).43 The frequency of dementia in the present study was similar in Black and White decedents and this may be related to the finding that frequencies of the major pathologies associated with dementia, namely AD, LATE-NC, LBs, and HS, were similar in the racial groups. Other studies have also reported no differences in the frequency of AD neuropathology among Black and White decedents,44,45 contrasting the clinical46 and pathology studies29 that reported that AD was more common in Black than White decedents. After AD, the second most common cause of degenerative dementia in patients >65 years are the LB dementias, which include DLB and dementia, which develops in 80% of patients with PD.2,47 In the current study, logistic regression analyses showed an association between LBs and higher odds of dementia in both Black and White decedents, which may be related to the finding that most cases in both racial groups had neocortical LBs. Prior studies of mainly White decedents have reported that neocortical LBs are an independent predictor of higher odds of dementia in LB diseases.9-11 Further analyses in the current study showed no racial difference in the association of LBs with higher odds of dementia, contrasting the findings of a prior pathology study of decedents with dementia,29 in whom LB dementia was more common in Black than White decedents, while another smaller pathology study of decedents with dementia reported that LB dementia was more common in White than Black decedents.48
There are several strengths in this study. Uniform clinical evaluation was performed in all 4 longitudinal studies, which included the BSIT, detailed assessment of parkinsonism signs, and cognition performance tests. There were high rates of clinical follow-up and all clinical examiners were blinded to previous evaluations and the results of brain autopsy findings. Neuropathology evaluations were uniform across the studies and performed blinded to the clinical data. α-Synuclein–immunostained sections were evaluated in all brain regions, whether or not substantia nigra LBs were observed in hematoxylin & eosin–stained sections. This study also used a sophisticated technique to match Black with White decedents by age at death, sex, years of education, and their follow-up time in the studies to reduce confounding associated with race and overcome the statistical challenges inherent to small sample sizes.
There are also several limitations to this study. Social determinants of health, the broader social context in which people live and age, including the physical, economic, and social environment, differ between Black and White people in the United States and can challenge race comparisons. Black and White decedents were matched on factors such as age and sex; however, systemic social inequities exist that can affect our outcomes but cannot be captured because they are not fully measurable. Effects of age, sex, and years of education on racial differences in LBs could not be ascertained because the 2 racial groups were matched by these clinical features. Matching of the 2 racial groups could not be done by socioeconomic status as data on income were not available for many of the participants. The 2 groups were matched by years of education; while this does not fully capture other important social determinants that differ by race,49 it serves as a proxy for socioeconomic status and is more closely related to the long-term economic position of participants. Although participants in both racial groups in the current study are community-dwelling older adults, they are volunteers who are highly educated and may not represent the general population of older adults. Also, individuals agreed to participate in longitudinal studies with autopsy/brain donation, further limiting our ability to generalize to the broader population. However, achieving high numbers of Black participants who agree to autopsy is challenging in population-based studies, making it difficult to address questions of racial differences in underlying pathology. The percentage of LB cases in both racial groups may be an underestimate as only 1 section per brain subregion was used for detection of LBs; therefore, those with mild LB pathology may have been missed. The sample size of both Black and White decedents with LBs is low; therefore, LB associations will have to be confirmed with larger sample sizes of both Black and White decedents having LBs.
These results underscore the importance of including diverse populations in clinical studies. Although major differences in LB frequency, distribution, and clinical correlations were not detected in Black and White decedents, our results do not preclude the possibility of subtle differences in racial groups in future large-scale clinical studies.
Acknowledgment
The authors thank the participants of the Minority Aging Research Study, the African American Core, the Rush Memory and Aging Project, and the Religious Orders Study; and the staff of Rush Alzheimer’s Disease Center, including Woojeong Bang, Dominika Burba, and Shengying Chen.
Glossary
- AA
African American
- AD
Alzheimer disease
- BSIT
Brief Smell Identification Test
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- DLB
dementia with Lewy bodies
- HS
hippocampal sclerosis
- LATE-NC
limbic-predominant age-related TDP-43 encephalopathy–neuropathologic change
- LB
Lewy body
- MAP
Memory and Aging Project
- MARS
Minority Aging Research Study
- MMSE
Mini-Mental State Examination
- OR
odds ratio
- PD
Parkinson disease
- ROS
Religious Orders Study
- TDP-43
transactive response DNA-binding protein 43
- UPDRS
Unified Parkinson’s Disease Rating Scale
Appendix. Authors

Study Funding
This work was supported by National Institute on Aging grants P30AG10161, R01AG15819, R01AG17917, R01AG22018, R01AG56352, and R01NS78009.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211. [DOI] [PubMed] [Google Scholar]
- 2.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(1):1689-1837. [DOI] [PubMed] [Google Scholar]
- 3.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson DW, Fujishiro H, DelleDonne A, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol. 2008;115(4):437-444. [DOI] [PubMed] [Google Scholar]
- 5.Dickson DW, Heckman MG, Murray ME, et al. . APOE epsilon4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology. 2018;91(12):e1182-e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nag S, Yu L, VanderHorst VG, et al. Neocortical Lewy bodies are associated with impaired odor identification in community-dwelling elders without clinical PD. J Neurol. 2019;266(12):3108-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA. Lewy bodies and olfactory dysfunction in old age. Chem Senses. 2011;36(4):367-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchman AS, Shulman JM, Nag S, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012;71(2):258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurtig HI, Trojanowski JQ, Galvin J, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 2000;54(10):1916-1921. [DOI] [PubMed] [Google Scholar]
- 10.Ruffmann C, Calboli FC, Bravi I, et al. . Cortical Lewy bodies and Abeta burden are associated with prevalence and timing of dementia in Lewy body diseases. Neuropathol Appl Neurobiol. 2016;42(5):436-450. [DOI] [PubMed] [Google Scholar]
- 11.Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain. 2012;135(pt 10):3005-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steenland K, Goldstein FC, Levey A, Wharton W. A meta-analysis of Alzheimer’s disease incidence and prevalence comparing African-Americans and Caucasians. J Alzheimers Dis. 2016;50(1):71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahodwala N, Siderowf A, Xie M, Noll E, Stern M, Mandell DS. Racial differences in the diagnosis of Parkinson’s disease. Mov Disord. 2009;24(8):1200-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han SD, Barnes LL, Leurgans S, Yu L, Bennett DA, Boyle PA. Literacy mediates racial differences in financial and healthcare decision making in older adults. J Am Geriatr Soc. 2020;68(6):1279-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doty RL, Frye RE, Agrawal U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys. 1989;45(5):381-384. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64(7):802-808. [DOI] [PubMed] [Google Scholar]
- 17.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489-502. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, Shannon KM, Beckett LA, Goetz CG, Wilson RS. Metric properties of nurses’ ratings of Parkinsonian signs with a modified Unified Parkinson’s Disease Rating Scale. Neurology. 1997;49:1580-1587. [DOI] [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837-1844. [DOI] [PubMed] [Google Scholar]
- 20.Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun. 2018;6(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology. 1984;34(7):939-944. [DOI] [PubMed] [Google Scholar]
- 22.Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord. 2009;23(1):63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu L, Boyle PA, Nag S, et al. APOE and cerebral amyloid angiopathy in community-dwelling older persons. Neurobiol Aging. 2015;36(11):2946-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attems J, Toledo JB, Walker L, et al. Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathol. 2021;141(2):159-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute working group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(10):1095-1097. [DOI] [PubMed] [Google Scholar]
- 26.Nag S, Barnes LL, Yu L, Wilson RS, Bennett DA, Schneider JA. Limbic-predominant age-related TDP-43 encephalopathy in Black and White decedents. Neurology. 2020;95(15):e2056-e2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurasz AM, Smith GE, McFarland MG, Armstrong MJ. Ethnoracial differences in Lewy body diseases with cognitive impairment. J Alzheimers Dis. 2020;77(1):165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes LL, Arvanitakis Z, Yu L, Kelly J, De Jager PL, Bennett DA. Apolipoprotein E and change in episodic memory in blacks and whites. Neuroepidemiology. 2013;40(3):211-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graff-Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW. Neuropathologic differences by race from the National Alzheimer’s Coordinating Center. Alzheimers Dement. 2016;12(6):669-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T. Age-associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol. 2003;106(4):374-382. [DOI] [PubMed] [Google Scholar]
- 31.Buchman AS, Nag S, Leurgans SE, et al. Spinal Lewy body pathology in older adults without an antemortem diagnosis of Parkinson's disease. Brain Pathol. 2018;28(4):560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307-2312. [DOI] [PubMed] [Google Scholar]
- 33.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 2014;9(10):e107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaffe K, Freimer D, Chen H, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology. 2017;88(5):456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007;78(1):30-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louis ED, Marder K, Tabert MH, Devanand DP. Mild Parkinsonian signs are associated with lower olfactory test scores in the community-dwelling elderly. Mov Disord. 2008;23(4):524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dintica CS, Marseglia A, Rizzuto D, et al. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology. 2019;92(7):e700-e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louis ED, Tang MX, Schupf N, Mayeux R. Functional correlates and prevalence of mild parkinsonian signs in a community population of older people. Arch Neurol. 2005;62(2):297-302. [DOI] [PubMed] [Google Scholar]
- 39.Hemming JP, Gruber-Baldini AL, Anderson KE, et al. Racial and socioeconomic disparities in parkinsonism. Arch Neurol. 2011;68(4):498-503. [DOI] [PubMed] [Google Scholar]
- 40.Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA. Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke. 2011;42(11):3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchman AS, Yu L, Oveisgharan S, Farfel JM, Schneider JA, Bennett DA. Person-specific contributions of brain pathologies to progressive parkinsonism in older adults. J Gerontol A Biol Sci Med Sci. 2020;20(4):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the Aging, Demographics, and Memory study. Neuroepidemiology. 2007;29(1-2):125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riudavets MA, Rubio A, Cox C, Rudow G, Fowler D, Troncoso JC. The prevalence of Alzheimer neuropathologic lesions is similar in blacks and whites. J Neuropathol Exp Neurol. 2006;65(12):1143-1148. [DOI] [PubMed] [Google Scholar]
- 45.Wilkins CH, Grant EA, Schmitt SE, McKeel DW, Morris JC. The neuropathology of Alzheimer disease in African American and white individuals. Arch Neurol. 2006;63(1):87-90. [DOI] [PubMed] [Google Scholar]
- 46.Green RC, Cupples LA, Go R, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329-336. [DOI] [PubMed] [Google Scholar]
- 47.Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16(4):203-212. [DOI] [PubMed] [Google Scholar]
- 48.De la Monte SM, Hutchins GM, Moore GW. Racial differences in the etiology of dementia and frequency of Alzheimer lesions in the brain. J Natl Med Assoc. 1989;81(6):644-652. [PMC free article] [PubMed] [Google Scholar]
- 49.Barnes LL, Wilson RS, Hebert LE, Scherr PA, Evans DA, Mendes de Leon CF. Racial differences in the association of education with physical and cognitive function in older blacks and whites. J Gerontol B Psychol Sci Soc Sci. 2011;66(3):354-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study can be requested from the RADC Research Resource Sharing Hub (radc.rush.edu).