Abstract
Diabetic kidney disease is the most common cause of end-stage kidney disease and poses a major global health problem. Finding new, safe, and effective strategies to halt this disease has proven to be challenging. In part that is because the underlying mechanisms are complex and not fully understood. However, in recent years evidence has accumulated suggesting that chronic hypoxia may be the primary pathophysiological pathway driving diabetic kidney disease, and chronic kidney disease of other etiologies, and was coined the ‘chronic hypoxia hypothesis’. Hypoxia is the result of a mismatch between oxygen delivery and oxygen demand. The primary determinant of oxygen delivery is renal perfusion (blood flow per tissue mass), whereas the main driver of oxygen demand is active sodium reabsorption. Diabetes mellitus is thought to compromise the oxygen balance by impairing oxygen delivery due to hyperglycemia-associated microvascular damage and exacerbate oxygen demand due to increased sodium reabsorption as a result of SGLT upregulation and glomerular hyperfiltration. The resultant hypoxic injury creates a vicious cycle of capillary damage, inflammation, deposition of extracellular matrix and, ultimately, fibrosis and nephron loss. This review will frame the role of chronic hypoxia in the pathogenesis of diabetic kidney disease and its prospect as a promising therapeutic target. We will outline the cellular mechanisms of hypoxia and evidence for renal hypoxia in animal and human studies. Additionally, we highlight the promise of newer imaging modalities including blood-oxygenation level dependent-MRI, and discuss salutary interventions such as sodium-glucose cotransporter-2 inhibition that (may) protect the kidney through amelioration of renal hypoxia.
Keywords: Diabetes Mellitus, Chronic Kidney Disease, Diabetic Kidney Disease, Renoprotection, Hypoxia, Chronic Hypoxia Hypothesis, BOLD-MRI, Sodium-glucose cotransporter 2 inhibition
Physiology of renal oxygen metabolism
The kidneys are highly metabolically active organs and second only to the heart with respect to oxygen consumption per tissue mass. Despite excessive oxygen delivery, owing to abundant perfusion, the kidneys are considerably susceptible to hypoxic injury 1. Oxygen deprivation is widely considered a key event in acute kidney injury (AKI) due to various causes including ischemia-reperfusion, sepsis, and exposure to nephrotoxins 2. Importantly, in recent years evidence has accumulated indicating that hypoxia plays an equally crucial role in the development and progression of chronic kidney disease (CKD) of various etiologies and could therefore represent a therapeutic target. The most common cause of CKD is diabetes which accounts for approximately 45% of the cases of end-stage kidney disease (ESKD) worldwide 3. This review will outline the role of chronic hypoxia in the pathogenesis of diabetic kidney disease (DKD) and will discuss promising interventions such as sodium-glucose cotransporter (SGLT)2 inhibition to mitigate kidney hypoxia.
Renal tissue oxygenation (PO₂) is the result of a delicate balance between oxygen delivery and oxygen demand (QO₂) 4. The principal determinant of oxygen demand is the formation of ATP required for the tubular reabsorption of filtered sodium. By secondary active transport several tubular transporters, including SGLT2, use the electrochemical gradient established by active extrusion of sodium by the Na+/K+ -ATPase (NKA) pump at the basolateral membrane to transport sodium along with water and other molecules. The production of ATP to drive the NKA pump is the primary source of QO2, as over 90% of ATP is generated through oxidative phosphorylation 5. Moreover, the proximal tubules, which constitute half of the kidney mass and are accountable for the majority of sodium reabsorption, almost exclusively use oxidative phosphorylation for ATP-production under normal conditions 6. The principal determinant of oxygen delivery on the other hand, is renal perfusion. In most tissues, such as the heart, an increase in QO2 is met by an increase in tissue perfusion. For the kidneys, however, the interplay between oxygenation and perfusion is more complicated. An increase in perfusion not only results in increased oxygen delivery, but likely also in an increase in glomerular filtration rate (GFR), filtered sodium, and, thus increased ATP demand and QO2. Moreover, renal perfusion needs to be regulated to maintain the kidneys primary function of excreting waste products and preserve extracellular fluid volume and electrolyte homeostasis 1. Therefore, additional mechanisms are necessary to preserve renal tissue oxygenation.
The renal cortex and medulla experience different levels of perfusion reflecting their different functions 7. The kidney cortex receives 20–25% of cardiac output in order to drive GFR. The amount of blood flow and oxygen delivery thereby exceeds the metabolic demand and could even potentially be perilous due to the formation of excessive reactive oxygen species (ROS). Diffusional shunting of oxygen from pre-glomerular arteries to post-glomerular veins has been argued to be an adaptive mechanism protecting the kidney from the harmful effects of hyperoxia and to maintain cortical tissue PO2 at 50–60 mmHg 5. In support of the hypothesis of diffusional oxygen shunting are findings that show that oxygen migrates through the renal vasculature faster than red blood cells do 8 and the PO2 of the renal vein exceeds the PO2 of both efferent arterioles and cortical nephron tissue 9. In addition, renal PO2 has been shown to remain stable when RBF is experimentally altered within the physiological range by ±30%, possibly explained by a corresponding change in oxygen shunting 10. The shunting of oxygen, however, does confer the risk of hypoxic injury, foremost in the medullary region.
In contrast to the well perfused cortex, the medulla receives a mere 10% of cortical blood flow, which is necessary to establish a hypertonic interstitium in the medulla as a prerequisite for urine concentration 7. Together with the high metabolic requirements of the medullary thick ascending limbs (mTAL), this result in a medullary tissue PO2 of 10–20 mmHg, which translates to a borderline hypoxic milieu 4. Medullary defenses against the worsening of hypoxia are posited to include a relative insensitivity to vasoactive substances in comparison to the cortical region, including angiotensin II, endothelin-1, and noradrenaline 7. Adenosine in particular has distinct and opposite effects on cortical and medullary blood flow, consistent with a primary role in metabolic control of renal function 11. In addition, the production of erythropoietin (EPO) is a hormonal response to hypoxic stimuli and increases hematocrit and the oxygen delivery capacity. These defenses however may not suffice to counteract progressive hypoxia. Furthermore, due to the close arrangement of the descending and ascending vasa recta, oxygen shunting is thought to also occur in the medulla, which endangers the inherently low PO2 to further aggravate the oxygen demand and supply imbalance, such as in a state of disease.
Chronic hypoxia hypothesis of diabetic kidney disease
It is this mismatch of oxygen delivery and oxygen demand that has been hypothesized to underlie CKD of various etiologies. The so called ‘chronic hypoxia hypothesis’ 12 proposes that glomerular disease, irrespective of the underlying primary pathoetiology, damages glomerular capillaries which leads to a restriction of glomerular and post-glomerular blood flow. Therefore, oxygen delivery is impaired and results in hypoxic injury of the downstream segments. The remaining unaffected glomeruli make up for the loss of tissue by increasing their single-nephron GFR. Following, their QO2 increases, which further reduces local PO2 and predisposes to a vicious cycle of hypoxic injury, nephron loss and progressive nephropathy 13. Diabetes in particular is thought to compromise the oxygen balance due to several mechanisms that increase ATP demand and therefore QO2, and at the same time possibly decrease ATP generation, as detailed below (figure 1).
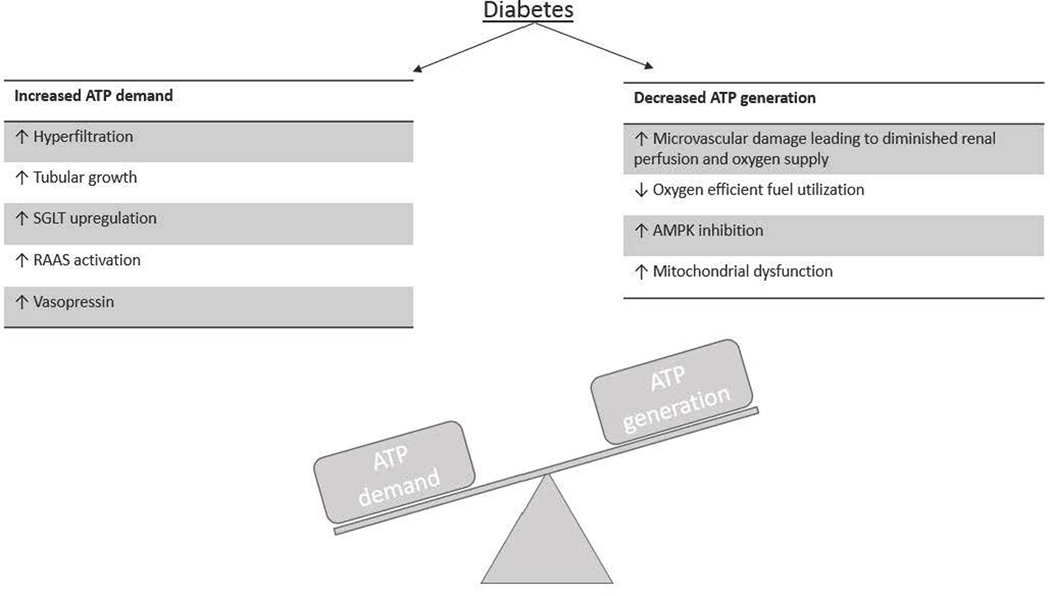
Figure 1. Diabetic kidneys are prone to develop an adenosine triphosphate (ATP) demand-generation imbalance.
Diabetes is affected by mechanisms that increase renal ATP consumption and decrease ATP generation. ATP demand is foremost dependent on sodium reabsorption, which is increased owing to (i) hyperglycemia-induced hyperfiltration, (ii) tubular growth, (iii) sodium–glucose cotransporter (SGLT) upregulation, (iv) renin-angiotensin-aldosterone system (RAAS) activation, and (v) vasopressin elevation. At the same time, ATP generation is decreased owing to (i) vascular dysfunction resulting in decreased oxygen delivery, (ii) a metabolic fuel shift in parts of the tubule from glucose oxidation to free fatty acid oxidation, which results in less ATP generation per oxygen molecule, (iii) by inhibition of adenosine monophosphate–activated protein kinase (AMPK), and (iv) owing to mitochondrial dysfunction.
i. Increased renal ATP demand:
As stated, the biggest contributor to ATP consumption is tubular sodium reabsorption. Diabetes and hyperglycemia increase renal sodium transport due to 1) tubular growth, which enhances the tubular transport machinery including an upregulation of sodium-glucose cotransporters (SGLTs) in order to reabsorb the enhanced amounts of filtered glucose, as well as 2) an increase in GFR due to the physiology of the tubuloglomerular feedback (TGF) mechanism. The TGF mechanism involves the macula densa, which are specialized tubular cells at the end of the thick ascending limb that sense the luminal sodium-chloride delivery and cause inverse changes in GFR, such that sodium-chloride delivery to the further distal tubule is stabilized. Tubular growth and enhanced sodium-glucose cotransport increase the proximal tubular reabsorption of sodium-chloride and fluid. As a result, the amount of sodium-chloride that reaches the macula densa is lowered and the physiology of the TGF mechanism increases GFR through changes in afferent and efferent arteriolar tone 11. Both experimental and observational studies report glomerular hyperfiltration in response to hyperglycemia as an early clinical entity of diabetes 14–16. In addition to an increased sodium reabsorption due to SGLT upregulation and hyperfiltration, the renin-angiotensin-aldosterone system (RAAS) can be activated in diabetes which stimulates sodium reabsorption through the epithelial sodium channel 17, 18, as well as the elevation of vasopressin levels induces antinatriuretic effects 19–24 by increasing the mRNA expression and subsequent protein abundance of the Na+/K+ ATPase 25, 26. Taking all these diabetes-induced alterations into account, mathematical modeling of the rat nephron has predicted an increase of sodium transport and sodium transport-dependent QO2 by ~50% and 100%, respectively 15.
ii. Decreased ATP generation:
Because of an increased renal ATP demand, efficient substrate utilization is required to generate enough ATP to sustain function. However, animal data show that the kidneys are unable to compensate for the increased ATP consumption due to the effects of diabetes on fuel generation 27–29. The less oxygen-efficient fuel profile observed in diabetes is thought to be secondary to insulin resistance (IR). IR shifts renal fuel utilization away from glucose, glutamine and citrate towards free fatty acids (FFA) oxidation, due to increased delivery of FFAs and activation of FFA metabolism 30–32, although this may differ between the various tubular segments. Compared to other substrates, FFAs are less energy efficient with a lower ATP yield per oxygen consumed 33, 34. Further, IR results in impaired ability to synthesize ATP by inhibition of adenosine monophosphate-activated protein kinase (AMPK) 35–37, mitochondrial dysfunction 38–40, and reduced electrolyte transport efficiency 41.
In conclusion, the net effect of increased ATP demand and decreased ATP generation is an ATP deficit and increased renal QO2. Together with a reduction in oxygen delivery, due to hyperglycemia-associated damage of the microvasculature, the diabetes disease state makes the kidneys more susceptible to the self-reinforcing process of hypoxic tissue damage (figure 2).
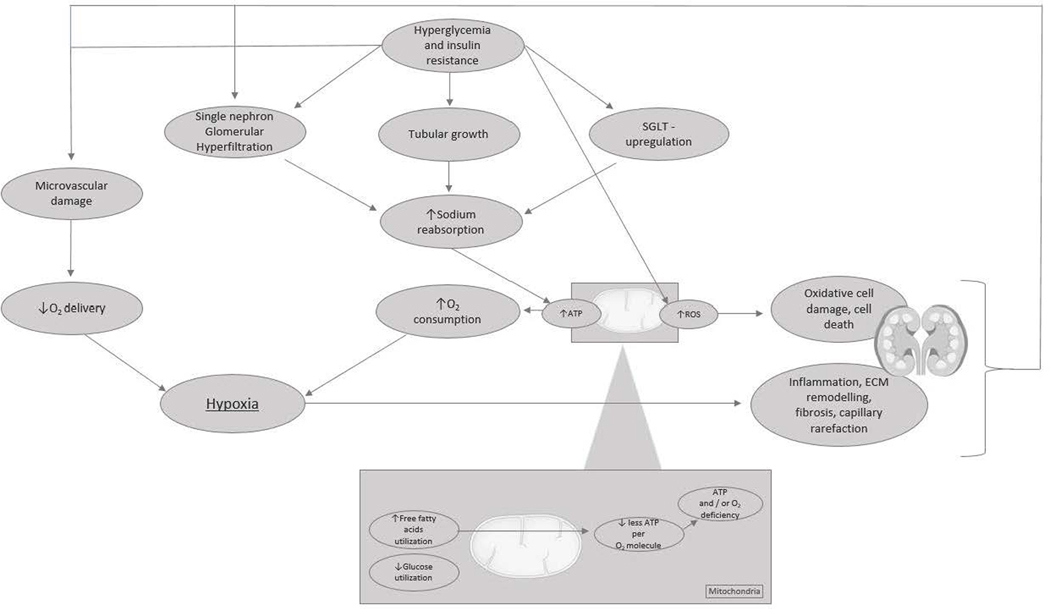
Figure 2. The vicious cycle of renal tissue deterioration after hyperglycemia-induced renal hypoxia.
Chronic hyperglycemia following insulin resistance is perceived to induce (single-nephron) hyperfiltration, tubular growth, and upregulation of sodium–glucose cotransporters. All adaptations lead to an increase in sodium reabsorption, which increases adenosine triphosphate (ATP) demand and oxygen (O2) consumption. Diversely, hyperglycemia causes microvascular damage, resulting in a decrease in renal perfusion and O2 delivery, and a metabolic shift in energy substrate use from glucose oxidation toward the less efficient oxidation of free fatty acids, which results in less generation of ATP per O2 molecule. The net effect of increased ATP demand and decreased ATP generation is an ATP deficit and renal tissue hypoxia. Hypoxia triggers inflammation, extracellular matrix (ECM) remodeling, capillary rarefaction, and fibrosis. In addition, hyperglycemia leads to oxidative cell damage directly because of the increased formation of reactive oxygen species (ROS) as a byproduct of the electron transport chain. The deterioration of renal tissue initiates a vicious cycle of hypoxic injury, glomerular loss, and progressive nephropathy.
Molecular mechanisms of hypoxia
Histological examination of chronically diseased kidneys, irrespective of the primary etiology, show characteristic changes suggestive of chronic tissue hypoxia 42. As such, common pathological findings include a loss of glomerular and peritubular microvasculature as well as the presence of tubulointerstitial fibrosis, extracellular matrix (ECM) accumulation, and inflammatory cell infiltration 43, 44. Even more, experimental studies support a causal role of hypoxia on a pro-fibrogenic renal tissue profile. In a series of experiments Norman et al. exposed in vitro human renal cells to hypoxia (1% O2) and observed hypoxia to increase ECM (collagen) production, to decrease ECM turnover, and to promote fibrogenesis 45, 46.
The hypoxia inducible factor (HIF) family is thought to stand at the center of the cellular response to hypoxia and targets hundreds of genes that are important for counteraction to hypoxia and tissue adaptation47. As such, HIF activation leads to an increase in oxygen supply by promoting angiogenesis through increased vascular endothelial growth factor (VEGF) expression and by upregulation of EPO production. Conversely, HIF activation leads to a decrease of the oxygen demand. Within minutes HIF induces metabolic suppression of ATP-consuming processes such as protein formation. Conjointly, HIF instigates a shift from aerobic ATP production through oxidative phosphorylation to anaerobic ATP production by glycolysis, termed the Pasteur effect 48. By reduction of mitochondrial leak respiration, which is a reaction to oxidative stress due to increased electrolyte transport, HIF further conserves oxygen use 49. Lastly, alongside the effect on tissue oxygenation, HIF plays a vital role in the repairment of cellular damage by recruitment of inflammatory cells and induction of matrix synthesis.
Taken together HIF orchestrates an array of presumably adaptive effects. However, the renoprotective role of HIF is controversial and is mainly supported in the event of acute kidney injury. Experimental evidence suggests that when HIF and its downstream pathways become permanently activated, its effects can become detrimental 50. In fact, chronic HIF activation may then promote inflammation, pathological ECM accumulation, and progressive fibrosis, all characteristics of chronic hypoxia and possibly contributors to permanent damage. Several excellent reviews have outlined these contradictory observations and conclude that future research is merited to obtain a deeper understanding of HIF and its potential 47, 50, 51.
Renal hypoxia and diabetic kidney disease – evidence from animal studies
With the employment of a variety of research techniques, cellular and tissue hypoxia have been common findings in animal models of DKD (table 1).
Table 1.
Evidence of renal hypoxia in experimental models of diabetes as shown by multiple measurement techniques
| Ref. | Animal | Method | Measurement | Outcome | Observations |
|---|---|---|---|---|---|
| 52 | Rat T1D | Sealed chamber + oxygen probe | Reduction of O2 percentage in a sealed chamber after a given time | Oxygen consumption (QO2) | QO2 of renal cells of diabetic rats is higher than that of the corresponding cells of nondiabetic controls |
| 53 | Rat T1D | ||||
|
| |||||
| 52 | Rat T1D | Ouabain - inhibition | Inhibition of sodium reabsorption by blockade of the basolateral sodium extrusion by the NKA-pump | NKA (in)dependent QO2 | NKA-dependent QO2 of renal cells of diabetic rats is higher than that of healthy controls |
| 53 | Rat T1D | ||||
|
| |||||
| 53 | Rat T1D | Micro-electrodes | Micro-electrodes placed at various depths of renal tissue | Tissue oxygen tension (PO2) | PO2 of the renal parenchyma of diabetic rats is decreased in comparison with healthy control parenchyma, predominantly in the medullar region |
| 54 | Rat T1D | ||||
| 55 | Rat T1D | ||||
| 49 | Rat T1D | ||||
| 56 | |||||
|
| |||||
| 54 | Rat T1D | BOLD-MRI | The level of deoxyhemoglobin, which affects the relaxation rate of water molecules in a magnetic field, as a measure of arterial oxygenation | Transverse relaxation rate (R2*) as an indicator of PO2, assuming equilibrium between renal tissue and arterial oxygenation | PO2 of the renal parenchyma of diabetic rats is decreased when compared with healthy controls, as early as 2 d after the induction of diabetes |
| 57 | |||||
|
| |||||
| 58 | Rat T1D | HIF- Immunostaining | Antibody binding to HIF-complexes | Histological visualization of the tissue reaction to (moderate) hypoxia | HIF immunostaining increases during 30 d after diabetes induction, but is no longer present after 90 d HIFs are present more strongly in the proximal tubules of diabetic mice than in healthy controls |
| 59 | Mouse T2D | ||||
|
| |||||
| 58 | Rat T1D | PIM- immunostaining | Pimonidazole binds to and undergoes reductions in hypoxic cells with PO2 < 10mmHg | Histological visualization of tissue experiencing severe hypoxia | PIM immunostaining increases during 30 d after diabetes induction, but is no longer present after 90 d |
BOLD-MRI: blood oxygen level dependent – magnetic resonance imaging, HIF: hypoxia-inducible facto, NKA: Na+-K+-ATPase, O2: oxygen, PIM: pimonidazole adduct, PO2: tissue partial pressure of oxygen, T1D: type 1 diabetes, T2D: type 2 diabetes, QO2 : oxygen consumption.
In 1994 Korner et al. were the first to show that the induction of T1D by streptozotocin (STZ) led to an increase of cellular QO2 in rat renal tubules 52. The increased QO2 mirrored an increase in tubular sodium transport, as the effect was abolished by administration of ouabain which blocks the NKA pump. Likewise, as measured with micro-electrodes, Palm and colleagues showed a marked decrease of PO2 throughout the entire renal parenchyma following the induction of diabetes, predominantly in the medullar region 53. The study also confirmed an increase in tubular QO2, again accountable to enhanced tubular activity of NKA as well as to ROS-dependent cellular oxygen consumption. Also Nordquist et al. measured a decrease of renal PO2 and increase of renal QO2 after the induction of diabetes, which was indicated to result from an increased oxygen utilization due to glomerular hyperfiltration and oxygen loss through mitochondrial leak respiration 49. Importantly, Franzén et al. showed intrarenal hypoxia to precede the development of albuminuria, indicating a causal role of hypoxia on the initiation of DKD 56.
Furthermore, various studies using different forms of immunohistochemical staining have provided insight into the distribution and extent of hypoxic tissue occurring during the development of DKD. By HIF staining, which visualizes the hypoxic response of regions with limited oxygenation, and pimonidazole adduct (PIM) staining, which identifies cells that experience severe hypoxia as the substance exponentially binds to tissue with a PO2 below 10 mmHg, hypoxia was shown to be present as early as 7 days after diabetes induction 58. Both HIF and PIM staining intensified during the first 30 days after induction, most markedly in the medulla region. Interestingly, the staining was no longer present at 90 days after diabetes induction, which can possibly be explained by declining tubular transport activity after a prolonged diabetes duration 60.
To further our understanding of short- and long-term hypoxia in vivo in both animals and humans, it has been essential to develop a non-invasive measurement method of oxygen metabolism. In this regard, the development of blood-oxygenation-level-dependent (BOLD)-MRI has been of considerable value. BOLD-MRI is a non-invasive measurement method, which measures deoxyhemoglobin as an endogenous contrast agent and as such does not require injection of any exogenous contrast agent. If one assumes that blood PO2 is in a dynamic equilibrium with the surrounding tissue PO2, then the BOLD-MRI measurements reflects tissue PO2. The outcomes measure is expressed as the apparent relaxation rate (R2*), which inversely relates to level of tissue oxygenation 61, and has been validated by comparing the outcomes with renal PO2 measurements by micro-electrodes in swine 62.
BOLD-MRI has been used to study the early stages of renal disease in several rat models of diabetic nephropathy. A study by dos Santos et al. showed a decrease of renal PO2 only two days after the induction of diabetes, which aggravated over the time course of 28 days, and was assessed by both BOLD-MRI and micro-electrode measurements 54. In addition, Ries et al. measured PO2 of the cortex and three medullar regions of different depths by BOLD-MRI 57. Their results indicated intrarenal oxygenation to be lower in all four regions of the kidney of diabetic animals as compared with healthy controls. Hypoxia was most pronounced in the outer strip of the outer medulla, which corresponds to the region with the highest metabolic burden.
In conclusion, various animal studies employing various measurement techniques have observed an early decline in tissue oxygenation following the induction of DKD. Also of note but beyond the scope of this review, animal studies concerning the role of hypoxia in CKD due to etiologies other than diabetes, have largely reported similar findings 12. However, although animal models have greatly contributed to our understanding of hypoxia and the development of DKD, the renal physiology between murines and humans can differ as a consequence of interspecies differences, differences in the pathogenesis underlying CKD, and difference in disease duration. Animal data therefore might not fully translate to human clinic, which argues the need for human research.
Renal hypoxia and diabetic kidney disease – evidence from human studies
In human research, BOLD-MRI was first applied in the mid-1990s and much of the early literature focused on the medulla 63. Since this region is known to be at risk for ischemic injury, understanding the endogenous mechanisms that maintain oxygenation status and effects of exogenous maneuvers that can modulate the oxygenation status were the aim of early studies 63–68. Of these, the most interesting and widely used maneuver in this regard is the application of furosemide, which inhibits sodium-chloride reabsorption along the mTAL and hence reduces QO2 and increases PO2 69, 70. This intervention therefore allows for the estimation of QO2 determined by medullary tubular solute reabsorption.
Now, with the gaining interest in the chronic hypoxia hypothesis, multiple studies have evaluated renal oxygenation status in CKD of various etiologies 71–73. The initial human BOLD-MRI studies showed conflicting results 74. This may have been related to a lack of standardization of confounding factors such as water consumption or use of low field strength (1.5T).75 Since then, technical progress has been made and several studies indeed indicate renal function to relate to renal oxygenation. Pruijm et al. demonstrated that patients with CKD and high cortical R2* values were three times more likely to develop an adverse renal outcome 75. Moreover, various studies showed that cortical oxygenation independently predicts progression of CKD by annual GFR loss 75–77. In addition, BOLD-MRI measurement has been combined with arterial spin labeling (ASL) to quantify renal perfusion in patients with diabetes and stage 3 CKD78. The results indicated that cortical oxygenation in patients with mild to moderate CKD was reduced, although the measurement did not reach statistical significance. Importantly, cortical blood flow was reduced and the lower renal perfusion and lower tubular function, as evaluated by furosemide response, were associated with a faster deterioration of renal function.
Also in research concerning DKD in specific, BOLD-MRI measurements have assessed a lower PO2 of the renal cortex in patients with DKD compared with healthy controls 79. Paradoxically however, although cortical PO2 declined with progression of nephropathy, medullary hypoxia was alleviated. This could be the result of a deterioration of tubular function and therefore oxygen requirement.
Although of great merit to the research field, the use of BOLD-MRI currently has its limitations. A common finding in all the studies to-date in CKD is that cortical R2* is only minimally increased compared to controls. However, cortical perfusion has been shown to be substantially reduced in CKD with diabetes and strongly associated with eGFR 80, 81. It is not yet clear whether this anomalous finding of small increase in cortical R2* in CKD is a reflection of oxygenation being conserved in CKD due to a compensatory reduction in QO2 or a limitation of BOLD-MRI as implemented to-date. Furthermore, R2* is determined by deoxyhemoglobin content, which is inherently dependent on hematocrit, fractional blood volume and oxygen saturation of hemoglobin. While decreased oxygen saturation increases R2*, reductions in hematocrit and/or fractional blood volume will decrease R2*. Since this combination is expected in CKD, it is suspected that R2* may not be specific to oxygenation. Accordingly, direct estimation of oxygen saturation of hemoglobin 82 may be necessary to fully appreciate the extent of renal hypoxia in DKD by BOLD-MRI 83. This however necessitates additional measurement of fractional blood volume which is not readily available. It is inherently an imaging concept and requires blood pool contrast media 84 or hemoglobin-labeling to measure 85. Overall, BOLD MRI (as currently implemented) is more suitable in evaluating acute changes in intra-renal oxygen availability by physiologic or pharmacologic maneuvers than in comparing relative oxygen status between different kidneys. A study to investigate the effect of acute and chronic inhibition of SGLT2 on renal oxygenation as evaluated by BOLD MRI is currently underway 86.
Intervening with renal hypoxia - therapeutic targets
Given the presumed role for hypoxia as driver of progressive DKD, a logical step would be to develop strategies or drugs that target tissue hypoxia. In the next sections we will discuss several interventions that are speculated to have the ability to mitigate deterioration of PO2 and tissue integrity, of which SGLT2 inhibitors are the most prominent candidate.
SGLT2 inhibition – a paradigm shift in DKD treatment and prevention
The SGLT2 inhibitors are the most recent oral agents in the therapeutic armamentarium for the treatment of diabetes. They exert their glycemic control by induction of glucosuria through blockade of SGLT2, the main renal transporter of glucose. SGLT2 is expressed in the apical brush border of the proximal tubule and actively reabsorb glucose together with sodium in a 1:1 stoichiometry 87. As such, SGLT2 mediates all glucose reabsorption in the early proximal convoluted tubule and, in euglycemia, ~97% of glucose reabsorption on whole kidney level. The remaining ~3% of filtered glucose is reabsorbed by SGLT1 in the late proximal tubule. In addition to the glucose lowering properties, SGLT2 inhibition has systemic pleiotropic effects, which include a decrease of blood pressure, body weight, and uric acid 88, 89. In long-term cardiovascular outcome trials including EMPA-REG OUTCOME (empagliflozin), CANVAS (canagliflozin) and DECLARE-TIMI 58 (dapagliflozin), SGLT2 inhibitors improved renal outcomes including reduction in ESKD 90. This was confirmed in the dedicated kidney trial CREDENCE, which studied the effects of canagliflozin on renal outcomes in DKD patients, demonstrating a relative risk reduction of end-stage kidney disease of 32% 91.
Although these trials demonstrated exciting results, the mechanisms underlying the beneficial effects are yet to be determined. Both the glucose lowering as well as the small pleiotropic systemic effects individually cannot account for the renoprotective effect observed 92. Remarkable with SGLT2 inhibition is the acute drop in GFR, reversible after drug discontinuation, which is consistent with a functional GFR decline due to increased delivery of sodium to the macula densa and the physiology of TGF 88, 93, 94, but could also be secondary to an increment in proximal tubule hydrostatic pressure following a decrease in reabsorption of sodium and therefore water uptake 93, 95. This reduction in GFR will result in reduced tubular workload and QO2, thus has the potential to result in improved tissue oxygenation.
Current evidence of effects of SGLT2 inhibition on renal hypoxia
Using mathematical modeling Layton et al. predicted the effect of SGLT2 inhibition on QO2 of the various segments of the renal tubule of rats. The model predicted a reduction in GFR, and a concomitant reduction of cortical QO2 by 30% 15, 96. Further modelling of the nephron predicted that SGLT2 inhibition shifts tubular glucose and sodium load and transport work downstream to segments in the outer medulla, including the late proximal tubule, where SGLT1 partially compensates for SGLT2 blockade, as well as the mTAL of the loop of Henle 14–16. As a consequence, outer medullary PO2 is expected to be reduced by SGLT2 inhibition, although the reduction in GFR attenuates such an effect. By lowering medullary PO2, SGLT2 inhibition may render the outer medullary region more vulnerable to hypoxia and injury. On the other hand, the transport shift may mimic systemic hypoxia and induce HIF signaling in the cortex/outer medulla and enhance the expression of protective genes and stimulate EPO release with secondary improvement in renal and systemic oxygen supply 97, 98. Thus, SGLT2 inhibition may be a double-edge sword with regard to medullary oxygenation and it is yet unknown if the final outcome of medullary hypoxia is adverse or could be of benefit to overall kidney function due to counter responses.
In correspondence to the modeling studies, non-selective SGLT inhibition and selective SGLT2 inhibition in animal models of type 1 diabetes have indeed shown to improve renal oxygenation of primarily the cortical region. Korner et al. showed that SGLT inhibition by phlorizin lead to a rapid dissipation of hyperfiltration, a reduction of NKA-activity and sodium reabsorption, and a normalization of QO2 of renal tubular cells of diabetic rats 52.93 A rat model of T1D recently extended these results and showed SGLT inhibition to reduce glomerular hyperfiltration and reverse cortical hypoxia 55. However, the study also showed that sodium reabsorption was shifted from the proximal tubule to the distal segments of the nephron, at the cost of a deterioration of medullar tissue oxygenation. As indicated above, it is unclear whether medullary hypoxia is unbeneficial, or could contribute to preserve renal function due to secondary effects such as HIF-activation and EPO formation.
In animal models of type 2 diabetes, in which a mutation of the gene encoding the leptin receptor induces a metabolic state of obesity and insulin resistance, renal benefits by selective SGLT2 inhibition have also been indicated. In a study by Tanaka and colleagues SGLT2 inhibition was shown to reduce GFR and concomitantly normalize tricarboxylic acid (TCA) cycle activity, which reduces oxygen consumption. In addition, SGLT2 inhibition led to less TCA-metabolite accumulation, less oxidative stress, and a decrease in albuminuria, although no effect on tubulointerstitial inflammation was observed 99. Gallo et al. demonstrated that treatment with SGLT2 inhibition attenuated the diabetes-induced upregulation of profibrotic genes in diabetic mice, although urinary markers of tubular damage exhibited no change 100. Lastly, following SGLT2 inhibition Bessho et al. observed a decrease of HIF-1α expression in the proximal tubular cells, suggesting an alleviation of tissue hypoxia, together with a decrease in tubular injury and interstitial fibronectin 59.
The effect of SGLT2 inhibition on human kidneys has thus far been limited to in vitro research. Although loss of SGLT2 expression under cultured conditions remains a risk, exposure of human proximal tubular cells to glucose infusion in the presence and absence of an SGLT2 inhibitor, has shown an increase of markers of renal inflammation following hyperglycemia, yet a decrease of inflammation after the initiation of SGLT2 inhibition 101. In addition, it has been demonstrated that the supplementation of SGLT2 inhibition to human renal tubular epithelial cells (HRTECs) that are exposed to hypoxia (1% oxygen), inhibits HIF-1α expression and the expression of specific target genes that contribute to tissue fibrosis 59. Moreover, SGLT2 inhibition decreased QO2 of the HRTECs and diminished regions positive for pimonidazole staining.
Summarizing, the performed studies to date provide encouraging evidence for a beneficial effect of SGLT2 inhibition on renal oxygenation and potential reactive tissue damage. In addition to the repeatedly observed decrease in renal oxygen demand, it is indicated that SGLT2 inhibition also has the ability to increase oxygen supply by attenuation of capillary rarefaction of peritubular capillaries following injury through a VEGF-dependent pathway 102. Future research in humans is imperative to further examine the advantages or disadvantages of selective SGLT2 inhibition on various regions the kidney and will elucidate the concordance between animal and human studies on renal physiology and tissue oxygenation.
Other interventions
Many chronic diseases, including diabetes, hypertension, and cardiovascular disease, are related to lifestyle and responsive to lifestyle modifications such as dietary intervention. Because sodium handling plays an essential role in the renal energy- and oxygen requirements, it has been thought that a change in sodium intake could have a beneficial influence on oxygenation status. Therefore, Pruijm. et al. requested normotensive and hypertensive non-diabetic young men to adhere to an one-week diet of high-sodium intake and an one-week diet of low-sodium intake 68. In these individuals, low sodium-diet did not improve cortical oxygenation but did show a significant benefit on medullary oxygenation. In contrast, an animal model of T1D showed that a low-sodium intake increased GFR, possibly due to activation of TGF as a result of a low filtered sodium concentration, called the sodium paradox 103. The added value of a low-salt diet on renal oxygenation and QO2 in patients with diabetes, particularly for people with T1D, therefore remains in question.
Other therapeutic interventions besides SGLT2 inhibition that have the potential to mitigate hypoxia risk, might be most effective if they address both sides of the metabolic mismatch equation, i.e. ATP consumption and ATP generation. A promising example includes combining agents that may lower renal ATP consumption (e.g. vasopressin receptor blockers 21, and dual SGLT1 and 2 inhibitors 4) with interventions to improve ATP generation (e.g. mitochondrial peptides and bioavailable-small molecule activators of AMPK and mTORC1 inhibitors 104–106, or even glucagon-like peptide (GLP)-1 receptors agonists 107. Even small enhancements in fuel utilization minute to minute may translate into large improvements in renal function and ultimately clinical outcomes, although data are currently limited.
In addition to the prevention of hypoxia, hypoxia-adaptability is a promising therapeutic target as well. As stated, the HIF system induces cell-type specific gene expression changes to promote cell survival in response to hypoxia including increased EPO production in the kidney. Prolyl hydroxylase (PH) inhibitors are novel drug agents, which are designed to increase EPO production by increasing expression of HIF-2α under normoxic conditions. Two recent phase three trials have shown the beneficial effect of Roxadustat on Hb levels in patient with CKD with and without dialyses treatment 108, 109. Data also suggest that EPO has direct renoprotective effects beyond improving hematocrit and oxygen carrying capacity. For example, EPO has been shown to prevent podocyte injury 110, 111, improve endothelial function and attenuate albuminuria 112. However, since chronic HIF stabilization may lead to ongoing inflammation and pathological tissue alterations, the safety profile and therapeutic effect are currently evaluated in ongoing trials.
Another line of investigational drugs with the ability to regulate the antioxidant defense is aimed at nuclear factor erythroid factor 2 (Nfr2) 113. Nrf2 is a transcription factor that controls the expression of several antioxidants which detoxify and eliminate ROS. Tolvaptan, a vasopressin type 2 antagonist which is currently indicated for the treatment of hyponatremia and polycystic kidney disease, has shown to upregulate the Nfr2 antioxidant pathway and subsequently reduce proteinuria and improve renal function in mice 114. In addition, the Nrf2 activator bardoxolone methyl (BARD) showed to improve renal function after 24 and 52 weeks of use in patients with CKD 115. A following trial however terminated early due to an excess in heart failure events 116, primarily occurring in the presence of baseline B-type ANP elevation and prior hospitalization for heart failure 117. Nevertheless, several global trials currently continue to research the effect of BARD on renal function in different populations 118. Both aforementioned therapeutic agents are only two highlights of the many antioxidant drugs under investigation, including N-acetyl cysteine, coenzyme Q10 and vitamin E 53, 119. Furthermore, an integrated approach by patient tailored micronutrient therapy with anti-oxidant properties, might have the ability to mitigate oxidative damage.
Conclusion and final notes
Data summarized in this review support the hypothesis that a potential mismatch between renal oxygen demand and oxygen delivery may underlie DKD, in large part driven by hyperglycemia, hyperfiltration, tubular growth, and altered substrate metabolism. As a result, chronic hypoxia is posited to drive a vicious cycle of inflammation, fibrosis, and nephron loss, ultimately resulting in a state of progressive DKD. To date, human research on renal hypoxia has been scarce due to the lack of non-invasive technologies. With the development of BOLD-MRI to measure tissue oxygenation, possibly combined with positron emission tomography tracers that can assess QO2, there now is a promising modality to further examine hypoxia-related pathophysiological mechanisms.
Assuming hypoxia is a major culprit in the pathogenesis of DKD, targeting the underlying risks factors early in the course of the disease holds promise as a major therapeutic avenue for treatment and prevention of DKD. Ideally therapeutic strategies should both attenuate inappropriate renal energy expenditure and improve substrate metabolism to normalize renal QO2, as well as to optimize renal oxygen delivery. SGLT2 inhibition has received considerable attention due to the observed clinical benefits and might modulate several factors related to renal oxygen homeostasis. Therefore, future studies addressing the underlying mechanisms of SGLT2 inhibition and other compounds in development, will be critical to delineate their therapeutic promise.
ACKNOWLEDGEMENTS
P.V.P. is supported in part by grant support from NIDDK (R01 DK106557). V.V. was supported by NIH grants R01DK112042, R01DK106102, R01HL139836, RF1AG061296, the UAB/UCSD O’Brien Center of Acute Kidney Injury NIH-P30DK079337, and the Department of Veterans Affairs, and by The University of Edinburgh College of Medicine & Veterinary Medicine PhD Studentship. P.B. receives salary and research support by NIH/NIDDK (K23 DK116720–01), in addition to research support by Thrasher Research Fund, Juvenile Diabetes Research Foundation (JDRF 2-SRA-2019–845-S-B, 2-SRA-2018–627-M-B), NIDDK/DiaComp, NIDDK/KPMP Opportunity Pool, International Society of Pediatric and Adolescent Diabetes (ISPAD), Colorado Clinical & Translational Sciences Institute (CCTSI) and Center for Women’s Health Research at University of Colorado. D.V.R. is supported by a fellowship of the Dutch Diabetes Foundation and EU Marie Curie Program.
V.V. has served as a consultant and received honoraria from Astra-Zeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceutical and Merck, and received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen Pharmaceutical over the past 36 months. G.D.L. has received lecture fees from Sanofi, Astra Zeneca, and Jansen, and has served as a consultant for Abbvie, Sanofi, Novo Nordisk, Astra Zeneca, Boehringer Ingelheim, and MSD. P.B. has acted as a consultant for Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Novo Nordisk, Sanofi and Horizon Pharma. PB serves on the advisory board of XORTX. D.V.R. has acted as a consultant and received honoraria from Boehringer Ingelheim and Lilly, Merck, Novo Nordisk, Sanofi, and AstraZeneca and has received research operating funds from Boehringer Ingelheim–Lilly Diabetes Alliance, AstraZeneca, Merck, and Novo Nordisk, with all honoraria paid to his employer (Amsterdam University Medical Center, location VUmc).
Footnotes
DISCLOSURES
The other authors report no conflicts.
References
- 1.Evans RG, Goddard D, Eppel GA, et al. Factors that render the kidney susceptible to tissue hypoxia in hypoxemia. Am J Physiol Regul Integr Comp Physiol 2011; 300: R931–R940. [DOI] [PubMed] [Google Scholar]
- 2.Evans RG, Ince C, Joles JA, et al. Haemodynamic influences on kidney oxygenation: Clinical implications of integrative physiology. Clin Exp Pharmacol Physiol 2013; 40: 106–122. [DOI] [PubMed] [Google Scholar]
- 3.Schiffer TA, Friederich-Persson M. Mitochondrial Reactive Oxygen Species and Kidney Hypoxia in the Development of Diabetic Nephropathy. Front Physiol 2017; 8: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansell P, Welch WJ, Blantz RC, et al. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol 2013; 40: 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nourbakhsh N, Singh P. Role of Renal Oxygenation and Mitochondrial Function in the Pathophysiology of Acute Kidney Injury. Nephron Clin Pract 2014; 127: 149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forbes JM. Mitochondria–Power Players in Kidney Function? Trends EndocrinolMetab 2016; 27: 441–442. [DOI] [PubMed] [Google Scholar]
- 7.Evans RG, Denton K, Eppel GA, et al. Possible mechanisms underlying the differential regulation of renal cortical and medullary blood flow vol. 22. J Hypertens, 2003. [Google Scholar]
- 8.Levy MN, Sauceda G. Diffusion of oxygen from arterial to venous segments of renal capillaires. Am J Physiol 1959; 196: 1336–1339. [DOI] [PubMed] [Google Scholar]
- 9.Welch WJ, Baumgärtl H, Lübbers D, et al. Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int 2001; 59: 230–237. [DOI] [PubMed] [Google Scholar]
- 10.Leong C-L, Anderson WP, O’Connor PM, et al. Evidence that renal arterial-venous oxygen shunting contributes to dynamic regulation of renal oxygenation. Am J Physiol Renal Physiol 2007; 292: F1726–F1733. [DOI] [PubMed] [Google Scholar]
- 11.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 2006; 86: 901–940. [DOI] [PubMed] [Google Scholar]
- 12.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int 2008; 74: 867–872. [DOI] [PubMed] [Google Scholar]
- 13.Fattah H, Layton A, Vallon V. How Do Kidneys Adapt to a Deficit or Loss in Nephron Number? Physiol 2019; 34: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherney DZ, Miller JA, Scholey JW, et al. Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in type 1 diabetes. Diabetes Care 2010; 33: 1344–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Layton AT, Vallon V, Edwards A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am j physiol Renal physiol 2016; 310: F1269–F1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonneijck L, Muskiet MHA, Smits MM, et al. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J Am Soc Nephrol 2017; 28: 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovshin JA, Boulet G, Lytvyn Y, et al. Renin-angiotensin-aldosterone system activation in long-standing type 1 diabetes. JCI Insight 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaika O, Mamenko M, Staruschenko A, et al. Direct activation of ENaC by angiotensin II: recent advances and new insights. Curr hypertens rep 2013; 15: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjornstad P, Johnson RJ, Snell-Bergeon JK, et al. Albuminuria is associated with greater copeptin concentrations in men with type 1 diabetes: A brief report from the T1D exchange Biobank. J Diabetes Complications 2017; 31: 387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjornstad P, Maahs DM, Jensen T, et al. Elevated copeptin is associated with atherosclerosis and diabetic kidney disease in adults with type 1 diabetes. J Diabetes Complications 2016; 30: 1093–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bragadottir G, Redfors B, Nygren A, et al. Low-dose vasopressin increases glomerular filtration rate, but impairs renal oxygenation in post-cardiac surgery patients. Acta anaesthesiol Scand 2009; 53: 1052–1059. [DOI] [PubMed] [Google Scholar]
- 22.Jensen T, Bjornstad P, Johnson RJ, et al. Copeptin and Estimated Insulin Sensitivity in Adults With and Without Type 1 Diabetes: The CACTI Study. Can j diabetes 2019; 43: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kortenoeven ML, Pedersen NB, Rosenbaek LL, et al. Vasopressin regulation of sodium transport in the distal nephron and collecting duct. Am J Physiol Renal Physiol 2015; 309: F280–299. [DOI] [PubMed] [Google Scholar]
- 24.Ricksten SE, Bragadottir G, Redfors B. Renal oxygenation in clinical acute kidney injury. Crit Care 2013; 17: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertuccio CA, Ibarra FR, Toledo JE, et al. Endogenous vasopressin regulates Na-K-ATPase and Na(+)-K(+)-Cl(−) cotransporter rbsc-1 in rat outer medulla. Am J Physiol Renal Physiol 2002; 282: F265–270. [DOI] [PubMed] [Google Scholar]
- 26.Blot-Chabaud M, Djelidi S, Courtois-Coutry N, et al. Coordinate control of Na,K-atpase mRNA expression by aldosterone, vasopressin and cell sodium delivery in the cortical collecting duct. Cell mol biol (Noisy-le-Grand, France) 2001; 47: 247–253. [PubMed] [Google Scholar]
- 27.Haase VH. The VHL/HIF oxygen-sensing pathway and its relevance to kidney disease. Kidney Int 2006; 69: 1302–1307. [DOI] [PubMed] [Google Scholar]
- 28.Nangaku M. Chronic Hypoxia and Tubulointerstitial Injury: A Final Common Pathway to End-Stage Renal Failure. J Am Soc Nephrol 2006; 17: 17. [DOI] [PubMed] [Google Scholar]
- 29.Singh DK, Winocour P, Farrington K. Mechanisms of Disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol 2008; 4: 216. [DOI] [PubMed] [Google Scholar]
- 30.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 2000; 49: 677–683. [DOI] [PubMed] [Google Scholar]
- 31.Murea M, Freedman BI, Parks JS, et al. Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin j Am Soc Nephrol : CJASN 2010; 5: 2373–2379. [DOI] [PubMed] [Google Scholar]
- 32.Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids health dis 2015; 14: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Fry BC, Layton AT. Modeling glucose metabolism and lactate production in the kidney. Math biosci 2017; 289: 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinkle PC. P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta 2005; 1706: 1–11. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Satriano J, Thomas JL, et al. Interactions between HIF-1alpha and AMPK in the regulation of cellular hypoxia adaptation in chronic kidney disease. Am J Physiol Renal Physiol 2015; 309: F414–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto S, Hsu CC, Hamm G, et al. Mass Spectrometry Imaging Reveals Elevated Glomerular ATP/AMP in Diabetes/obesity and Identifies Sphingomyelin as a Possible Mediator. EBioMedicine 2016; 7: 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrier RW, Harris DC, Chan L, et al. Tubular hypermetabolism as a factor in the progression of chronic renal failure. Am J Kidney Dis 1988; 12: 243–249. [DOI] [PubMed] [Google Scholar]
- 38.Cree-Green M, Gupta A, Coe GV, et al. Insulin resistance in type 2 diabetes youth relates to serum free fatty acids and muscle mitochondrial dysfunction. J Diabetes Complications 2017; 31: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindblom R, Higgins G, Coughlan M, et al. Targeting Mitochondria and Reactive Oxygen Species-Driven Pathogenesis in Diabetic Nephropathy. Rev diab stud : RDS 2015; 12: 134–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choo HJ, Kim JH, Kwon OB, et al. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 2006; 49: 784–791. [DOI] [PubMed] [Google Scholar]
- 41.Friederich M, Fasching A, Hansell P, et al. Diabetes-induced up-regulation of uncoupling protein-2 results in increased mitochondrial uncoupling in kidney proximal tubular cells. Biochim Biophys Acta - Bioenergetics 2008; 1777: 935–940. [DOI] [PubMed] [Google Scholar]
- 42.Schainuck LI, Striker GE, Cutler RE, et al. Structural-functional correlations in renal disease: Part II: The Correlations. Hum Pathol 1970; 1: 631–641. [DOI] [PubMed] [Google Scholar]
- 43.Norman JT, Fine LG. INTRARENAL OXYGENATION IN CHRONIC RENAL FAILURE. Clin Exp Pharmacol Physiol 2006; 33: 989–996. [DOI] [PubMed] [Google Scholar]
- 44.Manotham K, Tanaka T, Matsumoto M, et al. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int 2004; 65: 871–880. [DOI] [PubMed] [Google Scholar]
- 45.Norman JT, Orphanides C, Garcia P, et al. Hypoxia-induced changes in extracellular matrix metabolism in renal cells. Exp nephrol 1999; 7: 463–469. [DOI] [PubMed] [Google Scholar]
- 46.Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int 2000; 58: 2351–2366. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Wei Q, Guo C, et al. Hypoxia, HIF, and Associated Signaling Networks in Chronic Kidney Disease. Int J Mol 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Physiological Michiels C. and Pathological Responses to Hypoxia. Am j pathol 2004; 164: 1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordquist L, Friederich-Persson M, Fasching A, et al. Activation of Hypoxia-Inducible Factors Prevents Diabetic Nephropathy. J Am Soc Nephrol 2015; 26: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JW, Ko J, Ju C, et al. Hypoxia signaling in human diseases and therapeutic targets. Exp Mol Med 2019; 51: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka S, Tanaka T, Nangaku M. Hypoxia and hypoxia-inducible factors in chronic kidney disease. Ren Replace Ther 2016; 2: 25. [Google Scholar]
- 52.Körner A, Eklöf A-C, Celsi G, et al. Increased Renal Metabolism in Diabetes: Mechanism and Functional Implications. Diabetes 1994; 43: 629. [DOI] [PubMed] [Google Scholar]
- 53.Palm F, Cederberg J, Hansell P, et al. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 2003; 46: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 54.dos Santos EA, Li L-P, Ji L, et al. Early changes with diabetes in renal medullary hemodynamics as evaluated by fiberoptic probes and BOLD magnetic resonance imaging. Invest radiol 2007; 42: 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Neill J, Fasching A, Pihl L, et al. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol 2015; 309: F227–F234. [DOI] [PubMed] [Google Scholar]
- 56.Franzén S, Pihl L, Khan N, et al. Pronounced kidney hypoxia precedes albuminuria in type 1 diabetic mice. Am J Physiol Renal Physiol 2016; 310: F807–F809. [DOI] [PubMed] [Google Scholar]
- 57.Ries M, Basseau F, Tyndal B, et al. Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging 2003; 17: 104–113. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberger C, Khamaisi M, Abassi Z, et al. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int 2008; 73: 34–42. [DOI] [PubMed] [Google Scholar]
- 59.Bessho R, Takiyama Y, Takiyama T, et al. Hypoxia-inducible factor-1alpha is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep 2019; 9: 14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wald H, Scherzer P, Rasch R, et al. Renal tubular Na(+)-K(+)-ATPase in diabetes mellitus: relationship to metabolic abnormality. Am J Physiol 1993; 265: E96–101. [DOI] [PubMed] [Google Scholar]
- 61.Chen F, Li S, Sun D. Methods of Blood Oxygen Level-Dependent Magnetic Resonance Imaging Analysis for Evaluating Renal Oxygenation. Kidney Blood Pres Res 2018; 43: 378–388. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen M, Dissing TH, Merkenborg JAN, et al. Validation of quantitative BOLD MRI measurements in kidney: Application to unilateral ureteral obstruction. Kidney Int 2005; 67: 2305–2312. [DOI] [PubMed] [Google Scholar]
- 63.Prasad Pottumarthi V, Edelman Robert R, Epstein Franklin H. Noninvasive Evaluation of Intrarenal Oxygenation With BOLD MRI. Circulation 1996; 94: 3271–3275. [DOI] [PubMed] [Google Scholar]
- 64.Epstein FH, Prasad P. Effects of furosemide on medullary oxygenation in younger and older subjects. Kidney Int 2000; 57: 2080–2083. [DOI] [PubMed] [Google Scholar]
- 65.Hofmann L, Simon-Zoula S, Nowak A, et al. BOLD-MRI for the assessment of renal oxygenation in humans: acute effect of nephrotoxic xenobiotics. Kidney Int 2006; 70: 144–150. [DOI] [PubMed] [Google Scholar]
- 66.Li LP, Ji L, Santos E, et al. Effect of nitric oxide synthase inhibition on intrarenal oxygenation as evaluated by blood oxygenation level-dependent magnetic resonance imaging. Invest Radiol 2009; 44: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manotham K, Ongvilawan B, Urusopone P, et al. Angiotensin II receptor blocker partially ameliorated intrarenal hypoxia in chronic kidney disease patients: a pre-/post-study. Intern Med J 2012; 42: e33–37. [DOI] [PubMed] [Google Scholar]
- 68.Pruijm M, Hofmann L, Maillard M, et al. Effect of Sodium Loading/Depletion on Renal Oxygenation in Young Normotensive and Hypertensive Men. Hypertension 2010; 55: 1116–1122. [DOI] [PubMed] [Google Scholar]
- 69.Haddock B, Larsson HBW, Francis S, et al. Human renal response to furosemide: Simultaneous oxygenation and perfusion measurements in cortex and medulla. Acta physiol(Oxf) 2019; 227: e13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pruijm M HL, Piskunowicz M, Muller M, Zweiacker C,Bassi I, Vogt B, Stuber M, Burnier M. Determinants of Renal Tissue Oxygenation as Measured with BOLD-MRI in Chronic Kidney Disease and Hypertension in Humans. PLoSOne 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inoue T, Kozawa E, Okada H, et al. Noninvasive Evaluation of Kidney Hypoxia and Fibrosis Using Magnetic Resonance Imaging. J Am Soc Nephrol 2011; 22: 1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Milani B, Ansaloni A, Sousa-Guimaraes S, et al. Reduction of cortical oxygenation in chronic kidney disease: evidence obtained with a new analysis method of blood oxygenation level-dependent magnetic resonance imaging. Nephrol Dial Transpl 2017; 32: 2097–2105. [DOI] [PubMed] [Google Scholar]
- 73.Prasad PV, Thacker J, Li LP, et al. Multi-Parametric Evaluation of Chronic Kidney Disease by MRI: A Preliminary Cross-Sectional Study. PLoS One 2015; 10: e0139661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pruijm M, Mendichovszky IA, Liss P, et al. Renal blood oxygenation level-dependent magnetic resonance imaging to measure renal tissue oxygenation: a statement paper and systematic review. Nephrol Dial Transpl 2018; 33: ii22–ii28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pruijm M, Milani B, Pivin E, et al. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int 2018; 93: 932–940. [DOI] [PubMed] [Google Scholar]
- 76.Sugiyama K, Inoue T, Kozawa E, et al. Reduced oxygenation but not fibrosis defined by functional magnetic resonance imaging predicts the long-term progression of chronic kidney disease. Nephrol, dial, transpl 2018. [DOI] [PubMed] [Google Scholar]
- 77.Zhou H, Yang M, Jiang Z, et al. Renal Hypoxia: An Important Prognostic Marker in Patients with Chronic Kidney Disease. Am J Nephrol 2018; 48: 46–55. [DOI] [PubMed] [Google Scholar]
- 78.Prasad PV, Li LP, Thacker JM, et al. Cortical Perfusion and Tubular Function as Evaluated by Magnetic Resonance Imaging Correlates with Annual Loss in Renal Function in Moderate Chronic Kidney Disease. Am J Nephrol 2019; 49: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yin W-J, Liu F, Li X-M, et al. Noninvasive evaluation of renal oxygenation in diabetic nephropathy by BOLD-MRI. Eu J Radiol 2012; 81: 1426–1431. [DOI] [PubMed] [Google Scholar]
- 80.Li LP, Tan H, Thacker JM, et al. Evaluation of Renal Blood Flow in Chronic Kidney Disease Using Arterial Spin Labeling Perfusion Magnetic Resonance Imaging. Kidney Int Rep 2017; 2: 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mora-Gutiérrez JM, Garcia-Fernandez N, Roblero MFS, et al. Arterial spin labeling MRI is able to detect early hemodynamic changes in diabetic nephropathy. J Mag Reson Imaging 2017; 46: 1810–1817. [DOI] [PubMed] [Google Scholar]
- 82.Zhang JL, Morrell G, Rusinek H, et al. Measurement of renal tissue oxygenation with blood oxygen level-dependent MRI and oxygen transit modeling. Am J Physiol Renal Physiol 2014; 306: F579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prasad PV. Update on renal blood oxygenation level–dependent MRI to assess intrarenal oxygenation in chronic kidney disease. Kidney Int 2018; 93: 778–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pohlmann A, Cantow K, Huelnhagen T, et al. Experimental MRI Monitoring of Renal Blood Volume Fraction Variations En Route to Renal Magnetic Resonance Oximetry. Tomography (Ann Arbor, Mich) 2017; 3: 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rasmussen SN. Intrarenal red cell and plasma volumes in the non-diuretic rat. Determination by means of 51Cr labelled red cells and 125I-gamma-M-immunoglobulin. Pflugers Archiv : Eur j physiol 1973; 342: 61–72. [DOI] [PubMed] [Google Scholar]
- 86.Muller ME, Pruijm M, Bonny O, et al. Effects of the SGLT-2 Inhibitor Empagliflozin on Renal Tissue Oxygenation in Non-Diabetic Subjects: A Randomized, Double-Blind, Placebo-Controlled Study Protocol. Adv Ther 2018; 35: 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 2017; 60: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Bommel EJM, Muskiet MHA, Tonneijck L, et al. SGLT2 Inhibition in the Diabetic Kidney—From Mechanisms to Clinical Outcome. Clin J Am Soc Nephrol 2017; 12: 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2018; 41. [DOI] [PubMed] [Google Scholar]
- 90.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. The Lancet 2019; 393: 31–39. [DOI] [PubMed] [Google Scholar]
- 91.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 92.Muskiet MHA, van Raalte DH, van Bommel EJM, et al. Understanding EMPA-REG OUTCOME. Lancet Diabetes Endocrinol 2015; 3: 928–929. [DOI] [PubMed] [Google Scholar]
- 93.Vallon V, Richter K, Blantz RC, et al. Glomerular Hyperfiltration in Experimental Diabetes Mellitus. J Am SocNephrol 1999; 10: 2569. [DOI] [PubMed] [Google Scholar]
- 94.Cherney David ZI, Perkins Bruce A, Soleymanlou N, et al. Renal Hemodynamic Effect of Sodium-Glucose Cotransporter 2 Inhibition in Patients With Type 1 Diabetes Mellitus. Circulation 2014; 129: 587–597. [DOI] [PubMed] [Google Scholar]
- 95.Sällström J, Eriksson T, Fredholm BB, et al. Inhibition of sodium-linked glucose reabsorption normalizes diabetes-induced glomerular hyperfiltration in conscious adenosine A1-receptor deficient mice. Acta Physiol 2014; 210: 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol 2015; 308: F1343–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Layton AT, Vallon V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am J Physiol Renal Physiol 2018; 314: F969–f984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin sci 2018; 132: 1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanaka S, Sugiura Y, Saito H, et al. Sodium-glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int 2018; 94: 912–925. [DOI] [PubMed] [Google Scholar]
- 100.Gallo LA, Ward MS, Fotheringham AK, et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep 2016; 6: 26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Panchapakesan U, Pegg K, Gross S, et al. Effects of SGLT2 Inhibition in Human Kidney Proximal Tubular Cells-Renoprotection in Diabetic Nephropathy? PloS one 2013; 8: e54442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y, Nakano D, Guan Y, et al. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor–dependent pathway after renal injury in mice. Kidney Int 2018; 94: 524–535. [DOI] [PubMed] [Google Scholar]
- 103.Vallon V, Wead LM, Blantz RC. Renal hemodynamics and plasma and kidney angiotensin II in established diabetes mellitus in rats: effect of sodium and salt restriction. J Am Soc Nephrol 1995; 5: 1761. [DOI] [PubMed] [Google Scholar]
- 104.Szeto HH, Liu S, Soong Y, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol : JASN 2011; 22: 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Birk AV, Chao WM, Bracken C, et al. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 2014; 171: 2017–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morita M, Prudent J, Basu K, et al. mTOR Controls Mitochondrial Dynamics and Cell Survival via MTFP1. Mol Cell 2017; 67: 922–935.e925. [DOI] [PubMed] [Google Scholar]
- 107.Wang C, Li L, Liu S, et al. GLP-1 receptor agonist ameliorates obesity-induced chronic kidney injury via restoring renal metabolism homeostasis. PLoS One 2018; 13: e0193473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen N, Hao C, Liu BC, et al. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med 2019; 381: 1011–1022. [DOI] [PubMed] [Google Scholar]
- 109.Chen N, Hao C, Peng X, et al. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med 2019; 381: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 110.Eto N, Wada T, Inagi R, et al. Podocyte protection by darbepoetin: preservation of the cytoskeleton and nephrin expression. Kidney Int 2007; 72: 455–463. [DOI] [PubMed] [Google Scholar]
- 111.Aizawa K, Takeda S, Tashiro Y, et al. Renoprotection by continuous erythropoietin receptor activator in puromycin aminonucleoside-induced nephrotic syndrome. Am J Nephrol 2012; 36: 419–426. [DOI] [PubMed] [Google Scholar]
- 112.Serizawa K, Yogo K, Tashiro Y, et al. Epoetin beta pegol prevents endothelial dysfunction as evaluated by flow-mediated dilation in chronic kidney disease rats. Eur j pharmacol 2015; 767: 10–16. [DOI] [PubMed] [Google Scholar]
- 113.Yamawaki K, Kanda H, Shimazaki R. Nrf2 activator for the treatment of kidney diseases. Toxicol Appl Pharmacol 2018; 360: 30–37. [DOI] [PubMed] [Google Scholar]
- 114.Fujiki T, Ando F, Murakami K, et al. Tolvaptan activates the Nrf2/HO-1 antioxidant pathway through PERK phosphorylation. Sci Rep 2019; 9: 9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 2011; 365: 327–336. [DOI] [PubMed] [Google Scholar]
- 116.de Zeeuw D, Akizawa T, Audhya P, et al. Bardoxolone Methyl in Type 2 Diabetes and Stage 4 Chronic Kidney Disease. N Engl J Med 2013; 369: 2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chin MP, Wrolstad D, Bakris GL, et al. Risk Factors for Heart Failure in Patients With Type 2 Diabetes Mellitus and Stage 4 Chronic Kidney Disease Treated With Bardoxolone Methyl. J Card Fail 2014; 20: 953–958. [DOI] [PubMed] [Google Scholar]
- 118.Chin MP, Bakris GL, Block GA, et al. Bardoxolone Methyl Improves Kidney Function in Patients with Chronic Kidney Disease Stage 4 and Type 2 Diabetes: Post-Hoc Analyses from Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Study. Am J Nephrol 2018; 47: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sedeek M, Gutsol A, Montezano AC, et al. Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clin sci 2013; 124: 191–202. [DOI] [PubMed] [Google Scholar]