Abstract
Purpose
Approximately 1–2% of chronic myeloid leukemia (CML) patients harbor atypical BCR-ABL1 transcripts that cannot be monitored by real-time quantitative PCR (RT-qPCR) using standard methodologies. Within the European Treatment and Outcome Study (EUTOS) for CML we established and validated robust RT-qPCR methods for these patients.
Methods
BCR-ABL1 transcripts were amplified and sequenced to characterize the underlying fusion. Residual disease monitoring was carried out by RT-qPCR with specific primers and probes using serial dilutions of appropriate BCR-ABL1 and GUSB plasmid DNA calibrators. Results were expressed as log reduction of the BCR-ABL1/GUSB ratio relative to the patient-specific baseline value and evaluated as an individual molecular response (IMR).
Results
In total, 330 blood samples (2–34 per patient, median 8) from 33 CML patients (19 male, median age 62 years) were analyzed. Patients expressed seven different atypical BCR-ABL1 transcripts (e1a2, n = 6; e6a2, n = 1; e8a2, n = 2; e13a3, n = 4; e14a3, n = 6; e13a3/e14a3, n = 2; e19a2, n = 12). Most patients (61%) responded well to TKI therapy and achieved an IMR of at least one log reduction 3 months after diagnosis. Four patients relapsed with a significant increase of BCR-ABL1/GUSB ratios.
Conclusions
Characterization of atypical BCR-ABL1 transcripts is essential for adequate patient monitoring and to avoid false-negative results. The results cannot be expressed on the International Scale (IS) and thus the common molecular milestones and guidelines for treatment are difficult to apply. We, therefore, suggest reporting IMR levels in these cases as a time-dependent log reduction of BCR-ABL1 transcript levels compared to baseline prior to therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-021-03569-8.
Keywords: Chronic myeloid leukemia, CML, BCR-ABL1, Atypical transcripts, Molecular monitoring
Introduction
Chronic myeloid leukemia (CML) is characterized by the Philadelphia chromosome (Ph), produced by the balanced reciprocal translocation t(9;22)(q34;q11) leading to the breakpoint cluster region-Abelson (BCR-ABL1) fusion gene (Hehlmann et al. 2007). This oncogene is translated into the chimeric BCR-ABL1 protein with constitutive tyrosine kinase activity which results in reduced apoptosis, deregulated cell proliferation and decreased differentiation of hematopoietic progenitors (Quintás-Cardama and Cortes 2009).
More than 95% of Ph+ CML patients express the typical e13a2 (b2a2) and/or e14a2 (b3a2) BCR-ABL1 transcripts located within the major breakpoint cluster region (M-BCR) associated with a p210 fusion protein. Overall, three breakpoint regions are present in the BCR gene whereby the M-BCR spans from exon 12 to exon 16 (formerly named exons b1–b5). The most frequent breakpoint region of the ABL1 gene is located at a 200 kb segment upstream of exon 2 (a2) (Ross et al. 2013). Nevertheless, 1–2% of CML patients have rearrangements outside the M-BCR or with another ABL1 exon (Cayuela et al. 2005; Baccarani et al. 2019). For instance, the transcript e1a2 with the breakpoint in the minor breakpoint cluster region (m-BCR) producing a p190 protein was found initially in two-thirds of Ph+ acute lymphoblastic leukemia (ALL) patients. A breakpoint in the micro breakpoint cluster region (µ-BCR) leads to the largest chimeric BCR-ABL1 with an e19a2 junction translated into a p230 protein product (Melo 1997). Several other uncommon fusion transcripts in CML have been identified so far such as e6a2 (Hochhaus et al. 1996) or e8a2 (Branford et al. 2000) as well as transcripts missing the ABL1 exon a2 [e.g. e13a3 (b2a3) or e14a3 (b3a3)].
The characterization of the precise rearrangement at diagnosis with a qualitative multiplex PCR and sequencing (Cross et al. 1994) is of critical importance for subsequent monitoring of residual disease and assessment of treatment response (Hochhaus et al. 2020). Thus far, reference material only exists for common transcripts and therefore CML patients with atypical BCR-ABL1 subtypes remain non-standardised (Yu et al. 2017). Within the European Treatment and Outcome Study (EUTOS) for CML we sought to set up and validate robust qPCR methods for each of these atypical BCR-ABL1 transcripts. Furthermore, since the levels of disease for these cases cannot be expressed on the International Scale (IS), we suggest a new evaluation criterion for molecular monitoring of these patients—the individual molecular response (IMR) level based on a log reduction from pretreatment levels.
Materials and methods
Patients and samples
Peripheral blood samples were obtained from 33 patients (19 male, median age 62 years) with atypical BCR-ABL1 fusions from nine prospective studies and outside of clinical trials after written informed consent (Table 1 and supplemental table S1). A total of 330 samples (2–34 per patient; median, 8) of different time points were analyzed. Patients expressed seven different atypical BCR-ABL1 transcripts (e1a2, n = 6; e6a2, n = 1; e8a2, n = 2; e13a3, n = 4; e14a3, n = 6; e13a3 and e14a3, n = 2; e19a2, n = 12).
Table 1.
Patients’ characteristics
| Total | (n = 33) | |
|---|---|---|
| Gender, n (%) | ||
| Male | 19 | (57.6) |
| Female | 9 | (27.3) |
| Unknown | 5 | (15.2) |
| Age (years), median (range) | 62 | (28–78) |
| BCR-ABL1 transcript type, n (%) | ||
| e19a2 | 12 | (36.4) |
| e1a2 | 6 | (18.2) |
| e14a3 | 6 | (18.2) |
| e13a3 | 4 | (12.1) |
| e13a3/e14a3 | 2 | (6.1) |
| e8a2 | 2 | (6.1) |
| e6a2 | 1 | (3.0) |
RNA extraction and cDNA synthesis
Freshly isolated leukocytes (1 × 107 cells) from peripheral EDTA blood samples were lysed in 1 mL TRIzol® (Invitrogen GmbH, Karlsruhe, Germany) and stored at −20 °C until RNA extraction. Frozen samples were allowed to thaw at room temperature and RNA was isolated using the acid guanidinium thiocyanate-phenol–chloroform extraction method. Reverse transcription of 7.7 µL total RNA (maximum 4 µg of RNA) was performed immediately after isolation with random hexamer primer and M-MLV reverse transcriptase (Invitrogen GmbH) for 60 min at 37 °C according to the manufacturer’s recommendations.
Multiplex PCR
For identification of atypical BCR-ABL1 fusion genes multiplex PCR was performed as designed by Cross et al. (1994) which uses four oligonucleotide primers (BCR-C: ACCGCATGTTCCGGGACAAAAG, B2B: ACAGAATTCCGCTGACCATCAATAAG, C5e−: ATAGGATCCTTTGCAACCGGGTCTGAA, CA3−: TGTTGACTGGCGTGATGTAGTTGCTTGG). PCR was carried out with a master mix containing: 1.2 × Taq polymerase buffer with MgCl2 (Qiagen, Hilden, Germany), 0.24 mM desoxynucleoside triphosphate (dNTPs) mix (ThermoFisher Scientific, Darmstadt, Germany), 0.6 µM of each primer (Eurofins Genomics GmbH, Ebersberg, Germany), 0.2 mM MgCl2 (Qiagen) and 0.03 U/µL Taq Polymerase (Jena Bioscience, Jena, Germany). After adding 1 µL of cDNA to 19 µL PCR mix, the reaction was performed using a thermocycler (Eppendorf, Hamburg, Germany) with the following conditions: initial denaturation at 96 °C for 2 min, 35 cycles of 96 °C for 45 s, 60 °C for 30 s, 72 °C for 50 s and final extension at 72 °C for 10 min. Finally, reaction products were electrophoresed on a 3% agarose gel for detection of the BCR-ABL1 transcript. As controls for multiplex PCR the cell lines SD1 (e1a2 BCR-ABL1+ ALL cell line), K562 (e14a2 BCR-ABL1+ CML blast crisis cell line) and BV173 (e13a2 BCR-ABL1+ CML blast crisis cell line) were used.
Direct sequencing
All patient samples with an unusual PCR band on multiplex PCR analysis were characterized by Sanger sequencing and confirmed to have atypical BCR-ABL1 fusions. Therefore, cDNA was amplified with oligonucleotides for the respective atypical BCR-ABL1 transcript (Table 2). All PCRs were performed using AmpliTaqGold (Life Technologies, Darmstadt, Germany) with the following conditions: initial denaturation for 10 min at 94 °C, 45 cycles of denaturation for 60 s at 94 °C, annealing for 60 s at 60 °C and extension for 60 s at 72 °C followed by the final elongation for 10 min at 72 °C. Purified amplicons were sequenced bidirectionally by standard Sanger sequencing on a 3500 Genetic Analyzer (Life Technologies). Purification was performed as previously described (Rinke et al. 2013) and subsequent sequencing reactions were carried out using the BigDye Terminator Cycle Sequencing Kit v1.1 (Life Technologies) according to the manufacturer’s recommendations. The sequence data analysis was performed using the Mutation Surveyor software (SoftGenetics, State College, PA, USA).
Table 2.
Oligonucleotides used for Sanger sequencing and RT-qPCR for the specific atypical BCR-ABL1 transcripts
| Name | Primer sequence 5′ to 3’ | Location | Transcript | Product size (bp) |
|---|---|---|---|---|
| E6F2 | CAAAGATGCCAAGGATCCAACGACCAAG | BCR exon 6 | e6a2 | 534 |
| E19F1 | GGAGGAGGTGGGCATCTACCG | BCR exon 19 | e19a2 | 567 |
| E8F2 | ACGGCAGTCCATGACGGTGAAGAAG | BCR exon 8 | e8a2 | 524 |
| B2A | TTCAGAAGCTTCTCCCTGACAT | BCR exon b2 | e13a3/e14a3 | 422/497 |
| BCR12 | CAGATCTGGCCCAACGATGG | BCR exon 1 | e1a2 | 539 |
| NA4- | CGGCTCTCGGAGGAGACGTAGA | ABL exon 4 | ABL | |
| GUS10-lc | AGAAACGATTGCAGGGTTTCAC | GUS exon 10 | GUS | 205 |
| ENR1162 | CCGAGTGAAGATCCCCTTTTTA | GUS exon 12 | GUS |
Cloning of quantification standards
PCR products for the e6a2, e8a2, e14a3, and e19a2 transcripts were generated from patient cDNA samples that expressed the relevant fusions using the oligonucleotides shown in Table 3. The PCR products were cloned into the plasmid pCR2.1 vector using the TA Cloning® Kit (Thermo Fisher) following the manufacturer’s instructions to generate pCR2.1_e6a2, pCR2.1_e8a2, pCR2.1_e14a3 and pPCR2.1_e19a2, respectively. Furthermore, fragments of the BCR, GUSB, and ABL1 transcripts, 963, 813, and 1803 bp in size, respectively, were generated from K562-derived cDNA and ligated into the vector pCR2.1. Following digestion using EcoR I for pCR2.1_BCR, Xba I/Kpn I for pCR2.1_GUSB and Spe I/Xba I for pCR2.1_ABL1 the resulting fragments were subcloned into pUC18 vector to create pUC18_BCR_GUSB (4556 bp) and pUC18 BCR_GUSB_ABL1 (6275 bp) backbone plasmids (supplemental figures S1 and S2).
Table 3.
Oligonucleotides for the generation of atypical BCR-ABL1 transcripts inserts for cloning
| Oligoname | Primer sequence 5′ to 3’ | Transcript | Product size (bp) |
|---|---|---|---|
| BCRex6F | CAAAGATGCCAAGGATCCAACGACCAAG | e6a2 | 1478 |
| ABLex10R | CTTCGTTCTGAGATACTGGATTCCT | ||
| BCRex7-8F | GTCCTCCATGACTTGCTGAAGCACACT | e8a2 | 973 |
| ABLex5R | TCTTCCACCTCCATGGTGTC | ||
| BCRex13F | TTCAGAAGCTTCTCCCTGACAT | e14a3 | 1463 |
| ABLex10R | CTTCGTTCTGAGATACTGGATTCCT | ||
| BCRex18F | GGAGGAGGTGGGCATCTACCG | e19a2 | 1646 |
| ABLex10R | CTTCGTTCTGAGATACTGGATTCCT |
To allow amplification of the target and control gene from the same construct, the transcript fragments were subcloned into the respective control gene backbone plasmids. Plasmids pUC18 BCR_GUS_e6a2, pUC18 BCR_GUS_e19a2 and pUC18 BCR_GUS_ABL_e14a3 were generated by digestion of pCR2.1 constructs with their specific enzymes (Hind III for e14a2, Hind III/Xba I for e6a2 and e19a2, see supplemental figures S3, S5 and S6), while plasmid pUC18_BCR_GUSB_e8a2 was created by amplification of e8a2 from pCR2.1_e8a2 with Sal I tagged primers (tagged sequence in bold and underlined with the enzyme cutting site indicated by the /):
BCRex7-8F: CGAGAG/TCGACGTCCTCCATGACTTGCTGAAGCACACT, ABLex5R: CGAGAG/TCGACTCTTCCACCTCCATGGTGTC.
The resulting e8a2 PCR product was digested with Sal I and cloned into pUC18_BCR_GUSB digested with Sal I to create pUC18_BCR_GUSB_e8a2 (supplemental figure S4).
Standard plasmids used for e1a2 measurement were generated as previously described (Müller et al. 2008). Briefly, e1a2 amplicons were generated from SD1 cell line-derived cDNA and cloned into pCR2.1 vector using the TOPO™ TA Cloning Kit. GUSB fragments were generated from K562-derived cDNA using oligonucleotides with an Xba I restriction site. Following Xba I digestion products were ligated into the pCR2.1 plasmid using T4 ligase generating plasmid pME3 (supplemental figure S7).
To enable the use as qPCR standards the plasmids were linearised, quantified and serially diluted. Restriction enzymes and molecular weights are shown in Table 4.
Table 4.
Generated plasmids and their respective restriction enzymes used for linearisation
| Plasmid | Inserts | Backbone | Size (bp) | Linearisation |
|---|---|---|---|---|
| pME3 | e1a2, GUSB | pCR2.1 | 5288 | Not I |
| pUCe6a2 | e6a2, BCR, GUSB | pUC18 | 6034 | Hind III |
| pUCe8a2 | e8a2, BCR, GUSB | pUC18 | 5529 | EcoR V |
| pUCe19a2 | e19a2, BCR, GUSB | pUC18 | 6202 | Hind III |
| pUCe14a3 | e14a3, BCR, ABL1, GUSB | pUC18 | 7738 | Sal I |
Real-time quantitative polymerase chain reaction (RT-qPCR)
Expression analysis of atypical BCR-ABL1 transcripts was performed using the LightCycler instrument 1.5 (Roche Diagnostics, Mannheim, Germany). Each 20 µL reaction mix contained 4 µL LightCycler-FastStart DNA MasterPLUS HybProbe master mix (Roche Diagnostics), 2 µL cDNA template or plasmid dilution, 0.5 µM forward primer (specific for atypical BCR-ABL1 transcript; Table 2), 0.5 µM reverse primer (ABL1 primer NA4-), 0.25 μM of each hybridization probe listed in Table 5 (TIB Molbiol, Berlin, Germany) and 1 µL Uracil-DNA-Glycosidase (UDG; New England Biolabs GmbH, Frankfurt/Main, Germany). Cycler conditions were: 2 min UDG activation at 50 °C, 10 min denaturation at 95 °C, 45 cycles of 60 s at 95 °C, 10 s at 60 °C and 26 s at 72 °C. For quantification, a 5 log series of plasmid dilutions for every atypical BCR-ABL1 transcript was amplified within the PCR. Beta-glucuronidase (GUSB) transcripts were measured as an internal control using the same standard plasmids as for the BCR-ABL1 measurement.
Table 5.
Hybridization probes for monitoring atypical BCR-ABL1 transcripts
| Name | Hybridization probe sequence 5′ to 3’ | Location |
|---|---|---|
| a3-3´HP | LC Red640-AATGGGGAATGGTGTGAAGCCCAAA-phosphate | ABL exon 3 |
| a3-5´HP | TGAAAAGCTCCGGGTCTTAGGCTATAATCA-fluorescine | ABL exon 3 |
| GUS-F | TGATCCAGACCCAGATGGTACTGCT-fluorescine | GUSB exon 11 |
| GUS-LC | LC Red640-TAGCAGACTTTTCTGGTACTCTTCAGTGAACA-phosphate | GUSB exon 11 |
Every CML patient had his individual baseline value at diagnosis which was calculated as the ratio BCR-ABL1/GUSB. This baseline value was set as 1 and log-reduction was calculated for every time of investigation so that one log-reduction means IMR1, two log-reductions IMR2 and so on.
Results
Detection of atypical BCR-ABL1 transcripts by multiplex PCR
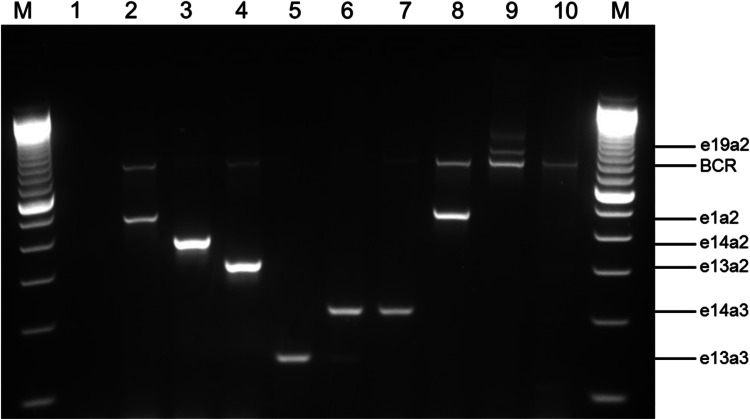
Atypical BCR-ABL1 fusion genes were determined by multiplex PCR whereby the size of the resulting PCR product depended on the breakpoint in the BCR and ABL1 gene. Figure 1 represents eight different BCR-ABL1 transcripts detected in patients and cell lines. The typical BCR-ABL1 transcripts e13a2 and e14a2 are shown by the CML cell lines BV173 and K562 with a PCR product size of 310 bp (lane 4) and 385 bp (lane 3), respectively. The small fusion transcript e13a3 with a product size of 128 bp is found in lane 5 of the agarose gel and one sample with the BCR-ABL1 double transcript e13a3 and e14a3 (product size 128 and 203 bp) is shown in lane 6. A much greater DNA fragment of 481 bp presents the e1a2 fusion gene found in the ALL cell line SD1 (lane 2) and rarely in CML patients (lane 8). The largest currently known BCR-ABL1 transcript in CML patients is the e19a2 transcript with a PCR product size of 925 bp (lane 9) which is located above the BCR control band. This BCR fragment with a size of 808 bp serves as an internal control for the RNA quality and was visible for patients without a BCR-ABL1 fusion gene (lane 10). All atypical transcripts were verified by Sanger sequencing.
Fig. 1.
Multiplex PCR for BCR-ABL1 and BCR transcripts. Lane 1, PCR negative control; lane 2, SD1 cell line (e1a2 BCR-ABL1, 481 bp); lane 3, K562 cell line (e14a2 BCR-ABL1, 385 bp); lane 4, BV173 cell line (e13a2 BCR-ABL1, 310 bp); lane 5, e13a3 BCR-ABL1 CML patient (128 bp); lane 6, e13a3 and e14a3 BCR-ABL1 CML patient (128 and 203 bp); lane 7, e14a3 BCR-ABL1 CML patient (203 bp); lane 8, e1a2 BCR-ABL1 CML patient (481 bp); lane 9, e19a2 BCR-ABL1 CML patient (925 bp); lane 10, BCR-ABL1 negative patient; lane M, 100 bp marker. BCR bands (808 bp) are an internal positive control for all cell lines and patients
Monitoring of response to therapy in CML patients with atypical BCR-ABL1 transcripts by RT-qPCR
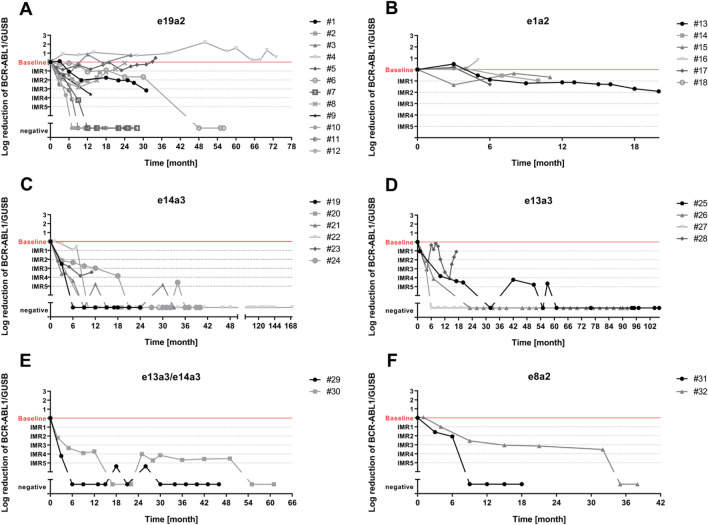
Molecular monitoring of 33 CML patients with atypical BCR-ABL1 transcripts was performed over time periods ranging from 3 months to a maximum of 14 years (median follow-up 16 months) by qPCR (Fig. 2). A total of 330 samples (2–34 per patient, median 8) were analyzed. Most of the patients carried the e19a2 BCR-ABL1 transcript (n = 12) followed by the fusion gene e1a2 found in six patients. Fusion of another ABL1 exon could be found in six patients harboring the e14a3 BCR-ABL1 transcript and four patients with the e13a3 fusion gene. Two patients expressed both e13a3 and e14a3. The rare atypical BCR-ABL1 transcript e8a2 was detected in two patients and monitored in median for 2 years (range 18–38 months). We also analyzed two follow-up samples of one patient with the e6a2 fusion gene (data not shown).
Fig. 2.
Monitoring of CML patients with atypical BCR-ABL1 transcripts treated with tyrosine kinase inhibitors by RT-qPCR as log reduction in relation to the individual baseline value. The molecular response to therapy was evaluated as individual molecular response (IMR) levels. a Patients with e19a2 BCR-ABL1 transcript (n = 12) were monitored in the median for 17 months (range 9–73 months), whereby three patients relapsed (#2, #3, #8) and two patients (#4, #5) did not respond to therapy. b Monitoring of six CML patients with the atypical BCR-ABL1 transcript e1a2 was performed for a median time period of 8 months (range 5–20 months). All patients showed an unsatisfied molecular response to therapy. c Patients (n = 6) with the rarely found e14a3 BCR-ABL1 transcript could be monitored in the median for 24 months (range 11–170 months) and reached deep molecular remission. d Four patients harbored the e13a3 BCR-ABL1 transcript and could be monitored in the median for 19 months (range 17–106 months). After 17 months patient #28 relapsed, but all other remained in deep molecular remission. e The double transcript e13a3/e14a3 could be found in two CML patients with a very good response to therapy. The monitoring could be performed over a median time period of 21 months (range 46–61 months). f Patients with e8a2 BCR-ABL1 transcript (n = 2) were monitored in the median for 11 months (range 18–38 months) and reached a deep molecular remission
The individual molecular response (IMR) level
For patients with atypical BCR-ABL1 transcripts the application of the international scale is not applicable for the assessment of treatment response due to the use of different PCR primers. Therefore, individual molecular response (IMR) level for CML patients with atypical BCR-ABL1 transcript were applied. With the IMR, the molecular response to therapy is assessed based on the individual baseline of every patient at diagnosis, i.e. IMR1 means a 1 log reduction from the baseline diagnostic sample for that individual, IMR2 a 2 log reduction, etc.
Eight patients (67%) with the atypical BCR-ABL1 transcript e19a2 reached an IMR1 3 months after diagnosis (Fig. 2a). During further monitoring six patients achieved a deep molecular remission, which we defined as a ≥ 4 log reduction from baseline, with undetectable BCR-ABL1. Only two CML patients (17%) with the e19a2 transcript had high BCR-ABL1 levels and did not reach any IMR level.
Patients with the e1a2 BCR-ABL1 transcript (n = 6) reached no better than IMR1 (50%) or failed to reach IMR1 (50%; Fig. 2b).
Patients with the atypical transcript e14a3 showed a good response and four of six patients (67%) already reached IMR2 or better 3 months after diagnosis (Fig. 2c). After 12 months BCR-ABL1 was not measurable in all patients.
All four CML patients with the e13a3 transcript showed a rapid decrease of their BCR-ABL1 levels and two of them (50%) had undetectable BCR-ABL1 2 years after diagnosis (Fig. 2d).
The double BCR-ABL1 transcript e13a3/e14a3 was found in two patients which showed rapidly declining BCR-ABL1 levels with an IMR4 (patient #29) and IMR2 (patient #30) level after 3 months, respectively (Fig. 2e).
The analyzed patients with the e8a2 BCR-ABL1 transcript showed a good response and achieved fast IMR1 or better (Fig. 2f).
Discussion
The hallmark of CML is the presence of the Philadelphia chromosome (Ph) with the associated BCR-ABL1 fusion gene. Depending on the breakpoints in the two involved genes, different transcripts are generated. Most CML patients express the typical e13a2 (b2a2) or e14a2 (b3a2) BCR-ABL1 transcripts corresponding to the major breakpoint cluster region (M-BCR). Alternate breakpoints in either of both genes generate other rare transcripts. These atypical fusion transcripts are only seen in 1–2% of CML patients (Baccarani et al. 2019) but require particular attention regarding molecular monitoring since they are not covered by routine RT-qPCR methods and might generate false-negative results.
The present study established oligonucleotides and plasmid standards for monitoring atypical BCR-ABL1 transcripts by RT-qPCR to assess treatment response and residual disease. At first, it is essential to determine the precise rearrangement by multiplex PCR at CML diagnosis prior to the start of TKI treatment (Cross et al. 1994). Otherwise false negative values could be measured and loss of response to therapy could not be detected as recently reported (Sharplin et al. 2019).
Concerning the clinical and hematologic features of e6a2 CML patients it was hypothesized that this CML type represents a different biological entity associated with a worse prognosis (Colla et al. 2004). We measured one patient with the atypical BCR-ABL1 transcript e6a2 3.7 and 4.2 years after diagnosis and found high BCR-ABL1/GUSB ratios of 14.2 and 63.4% respectively, which clearly indicates an inadequate molecular response. Even after allogeneic stem cell transplantation high BCR-ABL1 levels were detected.
The BCR-ABL1 transcript e1a2, typically seen in Ph+ ALL, is found in approximately 1% of CML patients and has also been associated with poor prognosis. Most of these patients do not achieve a molecular response with tyrosine kinase inhibitor (TKI) therapy and are candidates for stem cell transplantation (Verma et al. 2009). All six patients in our study cohort showed high BCR-ABL1 levels supporting the evidence of the inferior outcome of e1a2 CML patients described in the literature (Awad et al. 2019).
The fusion of BCR with exon a3 of ABL1 is extremely rare and is found in 0.9% of BCR-ABL1-positive patients (Baccarani et al. 2019; Snyder et al. 2004). This fusion transcript lacks part of the SH3 domain of ABL1 which contributes to leukemogenesis by negatively regulating kinase domain SH1 and activating the STAT5 signaling pathway. Because of an alteration of the tertiary structure of BCR-ABL1, the TKI response mechanism is different, but CML patients show a very good response to therapy and have a good prognosis (Duan et al. 2017). In total 36% (12 of 33 patients) of analyzed patients harbored the rearrangement involving a3 either with BCR exon 13 (e13a3) or exon 14 (e14a3). Two CML patients showed a double transcript e13a3/e14a3. Almost all patients (83%) achieved deep molecular remission with BCR-ABL1 values below the detection limit. Only patient #28 with the atypical transcript e13a3 showed increasing BCR-ABL1 levels corresponding to IMR1 16 month after the diagnosis after the previous achievement of IMR4.
Most patients of the analyzed cohort expressed e19a2 BCR-ABL1 transcripts (n = 12) whereby two patients never achieved IMR1 and three patients lost previously achieved IMR2 or IMR1. Arun et al. (2017) observed rapid disease progression, imatinib resistance and blast transformation in CML patients with e19a2 rearrangement.
Whether the fusion gene influences the clinical parameters and outcomes of the CML patients is still a point of discussion (Baccarani et al. 2019; Melo 1996). The study presented here confirms an inadequate molecular response to TKI therapy in patients with e1a2 BCR-ABL1 transcripts which might therefore be considered as a high-risk group (Awad et al. 2019).
In conclusion, although few patients are diagnosed with atypical BCR-ABL1 transcripts, their characterization is crucial for proper assessment of treatment response and to avoid false-negative results. We established several RT-qPCR protocols for monitoring all known unusual fusion transcripts, whereby the residual disease of these patients is assessed primarily by considering a decrease of BCR-ABL1/GUSB ratios over time compared to the initial patient-specific pretreatment sample. qPCR results of patients with atypical BCR-ABL1 transcripts cannot be reported on the International Scale (IS) and thus the common molecular milestones and guidelines for treatment discontinuation are difficult to apply. We recommend the evaluation of CML patients with atypical BCR-ABL1 transcripts using the "individual molecular response” (IMR) level presented here.
EUTOS recommendations
At diagnosis, multiplex PCR or equivalent should be performed to detect the underlying BCR-ABL1 transcript type in all patients with suspected CML.
For cases with unusual BCR-ABL1 transcript sizes direct sequencing should be performed to identify the precise fusion.
In the pretherapeutic sample, BCR-ABL1 should ideally be quantified using GUSB as an independent control gene.
Molecular monitoring should be performed at the intervals recommended for the specific treatment situation.
BCR-ABL1/GUSB ratios should be compared with the result from the pretherapeutic sample and expressed as individual molecular response (IMR).
In the case of undetectable BCR-ABL1 IMR response levels are scored based on GUSB transcript numbers and similar criteria as for MR scoring on the International Scale (Cross et al. 2015) is applied.
Calculation of a result expressed on the International Scale (IS) is not feasible and therefore not recommended.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The excellent technical assistance of Ms. Anja Waldau and Ms. Carolin Tröster is gratefully acknowledged.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by VS, HEW, GG, SM and TE. The first draft of the manuscript was written by VS and TE and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was supported by Novartis through the European Treatment and Outcome Study (EUTOS) for CML.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all patients included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arun AK, Senthamizhselvi A, Mani S, Vinodhini K, Janet NB, Lakshmi KM, Abraham A, George B, Srivastava A, Srivastava VM, Mathews V, Balasubramanian P (2017) Frequency of rare BCR-ABL1 fusion transcripts in chronic myeloid leukemia patients. Int J Lab Hematol 39(3):235–242 [DOI] [PubMed] [Google Scholar]
- Awad SA, Hohtari H, Javarappa KK, Brandstoetter T, Kim D, Potdar S, Heckman CA, Kytölä S, Porkka K, Doma E, Sexl V, Kankainen M, Mustjoki S (2019) BCR-ABL1 p190 in CML: a minor breakpoint with a major impact. Blood 134(Supplement_1):190–190 [Google Scholar]
- Baccarani M, Castagnetti F, Gugliotta G, Rosti G, Soverini S, Albeer A, Pfirrmann M (2019) The proportion of different BCR-ABL1 transcript types in chronic myeloid leukemia. An international overview. Leukemia 33(5):1173–1183 [DOI] [PubMed] [Google Scholar]
- Branford S, Rudzki Z, Hughes TP (2000) A novel BCR-ABL transcript (e8a2) with the insertion of an inverted sequence of ABL intron 1b in a patient with Philadelphia-positive chronic myeloid leukaemia. Br J Haematol 109(3):635–637 [DOI] [PubMed] [Google Scholar]
- Cayuela JM, Rousselot P, Nicolini F, Espinouse D, Ollagnier C, Bui-Thi MH, Chabane K, Raffoux E, Callet-Bauchu E, Tigaud I, Magaud JP, Hayette S (2005) Identification of a rare e8a2 BCR-ABL fusion gene in three novel chronic myeloid leukemia patients treated with imatinib. Leukemia 19(12):2334–2336 [DOI] [PubMed] [Google Scholar]
- Colla S, Sammarelli G, Voltolini S, Crugnola M, Sebastio P, Giuliani N (2004) e6a2 BCR-ABL transcript in chronic myeloid leukemia: is it associated with aggressive disease? Haematologica 89(5):611–613 [PubMed] [Google Scholar]
- Cross NC, Melo JV, Feng L, Goldman JM (1994) An optimized multiplex polymerase chain reaction (PCR) for detection of BCR-ABL fusion mRNAs in haematological disorders. Leukemia 8(1):186–189 [PubMed] [Google Scholar]
- Cross NC, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E, Lange T, Lion T, Polakova KM, Dulucq S, Martinelli G, Leibundgut EO, Pallisgaard N, Barbany G, Sacha T, Talmaci R, Izzo B, Saglio G, Pane F, Müller MC, Hochhaus A (2015) Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia 29(5):999–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan MH, Li H, Cai H (2017) A rare e13a3 (b2a3) BCR-ABL1 fusion transcript with normal karyotype in chronic myeloid leukemia: the challenges in diagnosis and monitoring minimal residual disease (MRD). Leuk Res 59:8–11 [DOI] [PubMed] [Google Scholar]
- Hehlmann R, Hochhaus A, Baccarani M (2007) Chronic myeloid leukaemia. Lancet 370(9584):342–350 [DOI] [PubMed] [Google Scholar]
- Hochhaus A, Reiter A, Skladny H, Melo JV, Sick C, Berger U, Guo JQ, Arlinghaus RB, Hehlmann R, Goldman JM, Cross NC (1996) A novel BCR-ABL fusion gene (e6a2) in a patient with Philadelphia chromosome-negative chronic myelogenous leukemia. Blood 88(6):2236–2240 [PubMed] [Google Scholar]
- Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, Clark RE, Cortes JE, Deininger MW, Guilhot F, Hjorth-Hansen H, Hughes TP, Janssen J, Kantarjian HM, Kim DW, Larson RA, Lipton JH, Mahon FX, Mayer J, Nicolini F, Niederwieser D, Pane F, Radich JP, Rea D, Richter J, Rosti G, Rousselot P, Saglio G, Saußele S, Soverini S, Steegmann JL, Turkina A, Zaritskey A, Hehlmann R (2020) European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 34(4):966–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo JV (1996) The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood 88(7):2375–2384 [PubMed] [Google Scholar]
- Melo JV (1997) BCR-ABL gene variants. Baillieres Clin Haematol 10(2):203–222 [DOI] [PubMed] [Google Scholar]
- Müller MC, Erben P, Saglio G, Gottardi E, Nyvold CG, Schenk T, Ernst T, Lauber S, Kruth J, Hehlmann R, Hochhaus A, European LeukemiaNet (2008) Harmonization of BCR-ABL mRNA quantification using a uniform multifunctional control plasmid in 37 international laboratories. Leukemia 22(1):96–102 [DOI] [PubMed] [Google Scholar]
- Quintás-Cardama A, Cortes J (2009) Molecular biology of BCR-ABL1-positive chronic myeloid leukemia. Blood 113(8):1619–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke J, Schäfer V, Schmidt M, Ziermann J, Kohlmann A, Hochhaus A, Ernst T (2013) Genotyping of 25 leukemia-associated genes in a single work flow by next-generation sequencing technology with low amounts of input template DNA. Clin Chem 59(8):1238–1250 [DOI] [PubMed] [Google Scholar]
- Ross DM, O’Hely M, Bartley PA, Dang P, Score J, Goyne JM, Sobrinho-Simoes M, Cross NC, Melo JV, Speed TP, Hughes TP, Morley AA (2013) Distribution of genomic breakpoints in chronic myeloid leukemia: analysis of 308 patients. Leukemia 27(10):2105–2107 [DOI] [PubMed] [Google Scholar]
- Sharplin K, Altamura H, Taylor K, Wellwood J, Taylor D, Branford S (2019) Chronic myeloid leukaemia: the dangers of not knowing your BCR-ABL1 transcript. Leuk Res 87:106231 [DOI] [PubMed] [Google Scholar]
- Snyder DS, McMahon R, Cohen SR, Slovak ML (2004) Chronic myeloid leukemia with an e13a3 BCR-ABL fusion: benign course responsive to imatinib with an RT-PCR advisory. Am J Hematol 75(2):92–95 [DOI] [PubMed] [Google Scholar]
- Verma D, Kantarjian HM, Jones D, Luthra R, Borthakur G, Verstovsek S, Rios MB, Cortes J (2009) Chronic myeloid leukemia (CML) with P190 BCR-ABL: analysis of characteristics, outcomes, and prognostic significance. Blood 114(11):2232–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Cui M, He X, Jing R, Wang H (2017) A review of the challenge in measuring and standardizing BCR-ABL1. Clin Chem Lab Med 55(10):1465–1473 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Not applicable.