Abstract
In this paper, we review our work in the last 10 years wherein we examined the sulfides in the acetone extracts of garlic (Allium sativum), onion (A. cepa), and Welsh onion (A. fistulosum), obtained and characterized the structures of new sulfides, three 3,4-dimethylthiolane-type sulfides from onion and Welsh onion, respectively, and four acyclic-type, nine 3,4-dimethyl- thiolane-type, four 2-methylthiolane (and thiane)-type, two 1,2-dithiolane-type, and two 2-oxothiolane-type sulfides, together with (E)-ajoene and one kujounin-type sulfide from garlic. During this process, structural corrections were made in onionin A group, garlicnin A, and garlicnin B group in some 3,4-dimethylthiolane-type sulfides. Next, hypothetical pathways for the production of the aforementioned sulfides were proposed. Furthermore, it was revealed that a typical 3,4-dimethylthiolane-type sulfide, onionin A1 obtained from onion, having the isomeric structure of garlicnin B1 obtained from garlic, decreased tumor proliferation and controlled tumor metastasis. These results showed that onionin A1 is an effective agent for controlling tumors, and that the antitumor effects observed in vivo are likely caused by reversing the antitumor immune system. Activation of the antitumor immune system by onionin A1 might be an effective adjuvant therapy for patients with osteosarcoma, ovarian cancer and other malignant tumors.
Keywords: Garlic; Onion; Welsh onion; 3,4-dimethylthiolane-type; Onionin A1; Garlicnin B1; Antitumor effect
Introduction
Garlic (Allium sativum L.) is ranked at the top of the list of designer foods showing anti-cancer effects by the National Cancer Institute [1]. Generally, the biological activity of garlic is distinguished in two categories: cardiovascular disease prevention and cancer prevention. Activities in the former category include the inhibition of cholesterol synthesis, platelet aggregation, and arterial smooth muscle cell proliferation, as well as anti-inflammatory, antioxidant, and hydrogen sulfide-mediated vasodilatory effects. The activities in the latter category include the effects on carcinogen metabolism, i.e., enhanced cellular glutathione synthesis that induces cell cycle arrest and apoptosis, and prevention of Helicobacter pylori infection, gastric cancer, and colorectal cancer [2–6].
The chemistry of Allium sulfides began with the discovery of allicin and alliin in 1944 [7] and 1951 [8], respectively, in garlic. In 1971, two types of vinyldithiin derivatives [9] were identified as thermally decomposed compounds by GC analysis of allicin. In 1984, Block and Ahmad determined the structure of ajoene in ether fraction [10]. It was also found that volatile garlic oils contained many sulfur compounds, such as diallylsulfide, (Z and E)-ajoene, 1,3-vinyldithiin, and 1,2-vinyldithiin, produced by the decomposition of thiosulfinates [11]. Unexpectedly, there were few clarified sulfides from garlic; in particular, cyclic sulfides before our study. Therefore, we had started the investigation for aiming at the isolation, structural characterization, and antitumor activity of the cyclic sulfides (sulfur-containing compounds including sulfoxides) from garlic, onion (A. cepa), and Welsh onion (A. fistulosum). The present review provides a brief description of the above-mentioned study.
Extraction and separation of garlic
Acetone was selected as the extracting solvent because it was expected to prolong the lifetime of allyl (or 1-propenyl) sulfenic acid and allyl thiosulfenic acid, which are derived easily by the decomposition of allicin. The acyclic and cyclic sulfides are stabilized by the electron-inductive interaction between acetone and sulfenic acids, and between acetone and cyclic sulfide. Chinese garlic was used, which is the same as Japanese garlic, because it was readily available and the occurrence of various sulfides, due to long drying storage, was expected. Chinese garlic (1.0 kg) was chopped and blended with acetone in a mixer. The mixtures were then soaked in acetone for 3 days at room temperature. During this time, sulfenic acid analogs might undergo chemical changes, such as cyclization and artificial reactions, to produce new sulfides. In particular, we intended to obtain stable cyclic sulfides possessing antitumor activity. Next, the filtrate was concentrated at 40 °C in vacuum to obtain the extract in a small volume that was partitioned between ethyl acetate and water. The ethyl acetate extractive (5.9 g) was separated by column chromatography on silica gel eluting with n-hexane: acetone (from 6: 1 to 2: 1) to yield 21 new sulfides named garlicnins A (48.2 mg) [12], B1 (242.0 mg), B2 (47.2 mg), B3 (29.8 mg), B4 (19.3 mg), C1 (26.4 mg), C2 (23.4 mg), C3 (14.6 mg) [13, 14], G (17.2 mg), I1 (17.4 mg) [15], I2 (15.6 mg) [16], J1 (17.4 mg) [15], J2 (19.4 mg) [17], L-1 (47.2 mg), L-2 (19.8 mg), L-3 (19.3 mg), L-4 (23.4 mg) [18], M (21.1 mg) [16], P (18.4 mg) [17], and onionins B1 (27.4 mg), and B2 (26.2 mg) [19], together with the known sulfide, (E)-ajoene (279.7 mg) [10], and kujounin A1 derivative (22.1 mg), which related to kujounin A1 obtained from Allium fistulosum by Matsuda et al. [20]. The structures of the obtained sulfides were characterized using high-resolution fast atom bombardment mass spectroscopy (HR-FABMS), 1H-NMR, 13C-NMR, 1H-1H NMR correlation spectroscopy (COSY), 1H-detected heteronuclear correlation through multiplet quantum coherence (HMQC), heteronuclear multiple bond correlation (HMBC) and nuclear Overhauser effect spectroscopy (NOESY). To determine the relative steric configuration of the cyclic sulfides, aromatic solvent-induced NMR shifts were applied [21, 22].
Extraction and separation of onion and Welsh onion
Similarly, the extraction and separation of onion (A. cepa) and Welsh onion (A. fistulosum) were performed. From onion bulbs (640 g), onionin A1 (42.2 mg) [23], onionin A2 (23.5 mg), onionin A3 (16.2 mg) [24], onionin B1 (16.4 mg), and B2 (20.5 mg) [19], were obtained, and from Welsh onion leaves (1.1 kg), onionin A1 (34.2 mg), onionin A2 (22.1 mg) and onionin A3 (16.4 mg) [24] were obtained.
Structures of isolated sulfides from garlic, onion, and Welsh onion
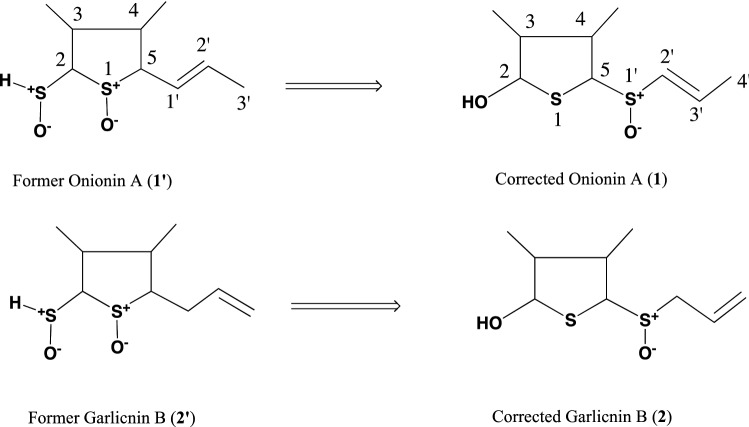
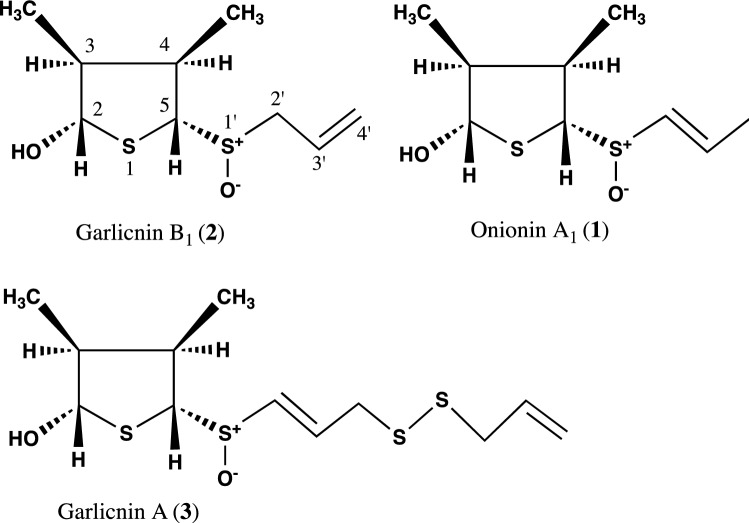
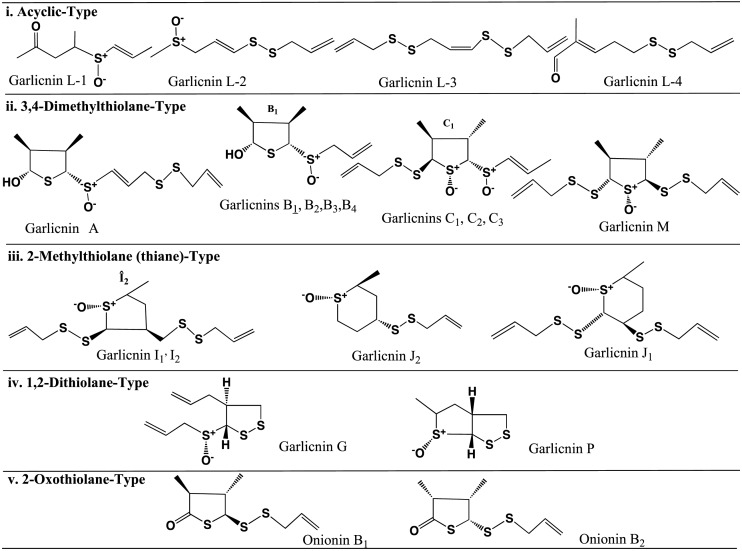
The above garlicnins and onionins were divided into five types: acyclic-type sulfides including garlicnins L-1, L-2, L-3, and L-4; major sulfides, 3,4-dimethylthiolane-type sulfides including garlicnins A, B1, B2, B3, B4, C1, C2, C3, and M, onionins A1, A2, and A3; 2-methylthiolane (and thiane)-type sulfoxides including garlicnins I1, I2, J1 and J2; 1,2-dithiolane-type sulfoxides including garlicnins G and P; and 2-oxothiolane-type sulfides including onionins B1 and B2. The structures of acyclic-type sulfides, that is, garlicnins L-1, L-2, L-3, and L-4, were characterized as E-5-thiaocta-6-ene 4-methyl-2,5-dioxide, E-2,6,7-trithiadeca-4,9-diene 2-oxide, Z-4,5,9,10-tetrathiatrideca-1,7,12-triene, and E-6,7-dithiadeca-2.9-diene 2-methyl-1-oxide, respectively. Regarding the 3,4-dimethylthiolane-type sulfides, we determined the structure of onionin A1, prior to the structure determination of garlicnin B group from garlic, as 3,4-dimethylthiolane S-oxide (1’) in 2010 as shown Fig. 1, based on the 1H-1H COSY analysis that included the correlation between H-5 and H-1’, the proton assignments of H–S+-O− and H-2 at C-2, and determination of the relative configuration by the aromatic solvent-induced NMR shifts [21, 22]. In relation to the structure of onionin A1, we determined the structure of garlicnin B1 (2’) isolated from garlicin in 2012. However, in 2018, Block et al. corrected the structure of garlicnin B1 as 3,4-dimethyl-5-allylsulfinylthiolane- 2-ol (2) [25] as shown in Fig. 1. This correction was made because the proposed continuity of nine carbons was not observed in the 13C-13C NMR incredible natural abundance double quantum transfer experiments (INADEQUATE). In 2019, Kubec et al. corrected onionin A1 as (E)-3,4-dimethyl-5-(1-propenylsulfinyl)thiolane-2-ol (1) as shown in Fig. 1, and he only corrected the part of structure and retained the names onionin A and garlicnin B [26]. Here, we reconfirmed the validity of their claims and we reformed the structures of onionin A1 (1) and garlicnins B1 (2), and determined the absolute configuration [27] of garlicnin B1 as shown in Fig. 2 by the Mosher method [28, 29] and NOESY analysis of 2. Simultaneously, the absolute configurations of onionin A1 and garlicnin A (3) were also deduced because their proton chemical shifts of H-2, H-3, H-4, CH3 at C-3, and CH3 at C-4, and their carbon chemical shifts of C-2, C-3, C-4, CH3 at C-3, and CH3 at C-4 approximated to those of garlicnin B1 (2) as shown in Fig. 2.
Fig. 1.
Corrected structures of onionin A1 (1) and garlicnin B1 (2)
Fig. 2.
Structures of onionin A1 (1), garlicnin B1 (2), and garlicnin A (3)
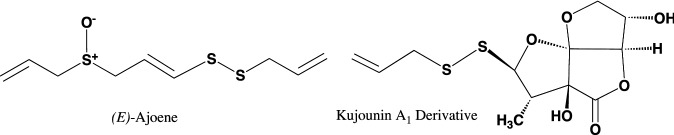
The structures of garlicnins C1, C2, and C3 were determined to be 2-(allyldisulfanyl)-5-(1-propenylsulfinyl)-3,4-dimethylthiolan-S-oxide. Garlicnins C1, C2, and C3 are steric isomers. The structure of garlicnin M was determined to be 2,5-bis(allyldisulfanyl)-3,4-dimethyl-thiolane-S-oxide. Next, the structures of 2-methylthiolane-type sulfoxides, that is, garlicnins I1 and I2 were determined to be 5-methyl-2-(allyldisulfanyl)-3-[(allyldisulfanyl)-methyl]-thiolane-S-oxides, and the structures of 2-methylthiane-type sulfoxides; garlicnins of J1 and J2 were determined to be 6-methyl-2,3-bis(allyldisulfanyl)-thiane-S-oxide and 6-methyl-4-(allyl- disulfanyl)-thiane-S-oxide, respectively. The structures of 1,2-dithiolane-type sulfoxides; garlicnins G and P were determined to be 4-(allyl)-3-(allylsulfinyl)-1,2-dithiolane, and 3-methyl-2,7,8-trithia-bicyclo[3.3.0]octan-2-oxide, respectively. Finally, 2-oxothiolane-type sulfides, onionins B1 and B2 were determined to be 5-(allyldisulfanyl)-3,4-dimethyl-2-oxothiolanes. The structures of the above garlicnins and onionins are summarized in Table 1, together with (E)-ajoene and kujounin A1 derivative as shown in Fig. 3.
Table 1.
Structures of garlicnins and onionins isolated from garlic
Fig. 3.
Structures of (E)-ajoene and kujounin A1 derivative
Hypothetic pathways to respective sulfides
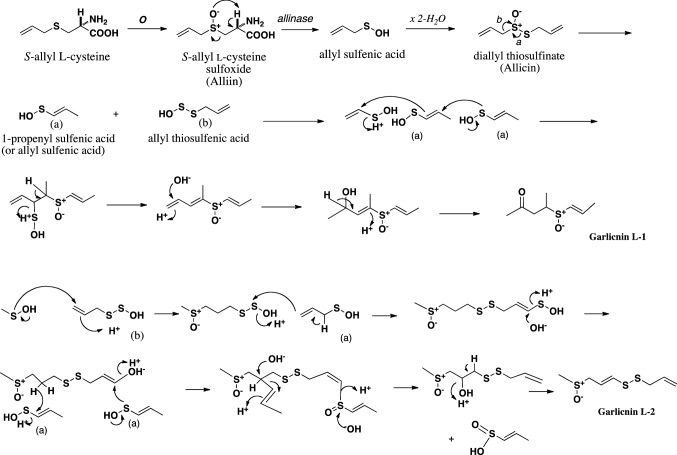
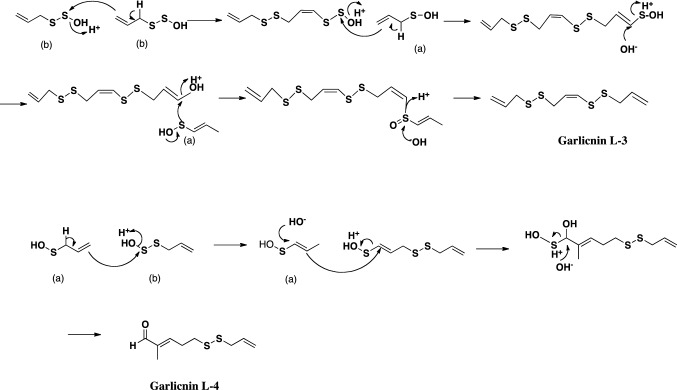
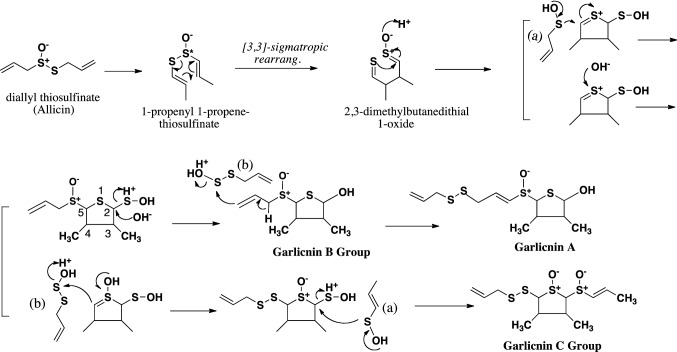
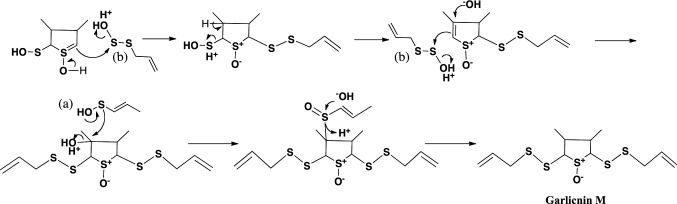
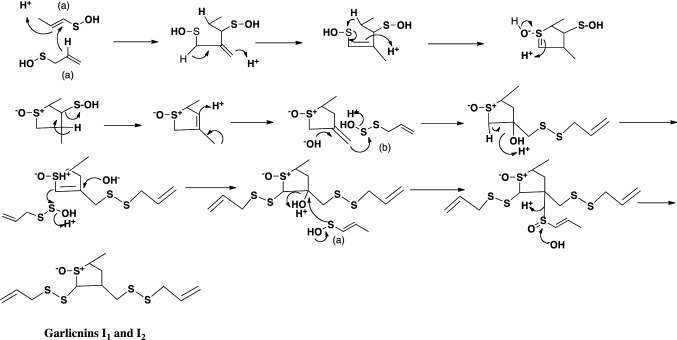
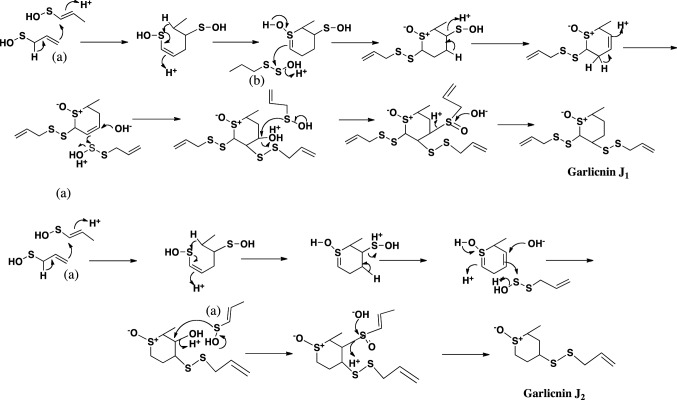
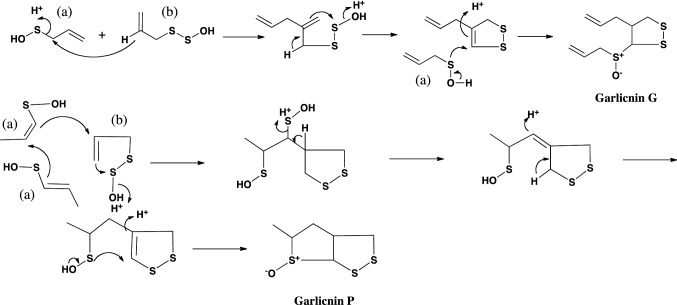
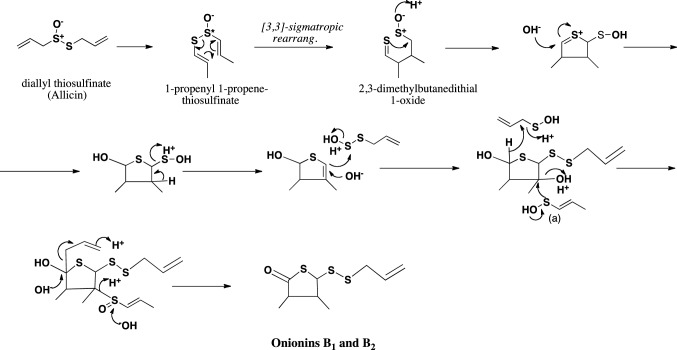
The first acyclic-type sulfides were produced by the arrangement and combination of allyl (or 1-propenyl) sulfenic acid, and allyl thiosulfenic acid derived from allicin (Fig. 4, Fig. 5). In the case of garlicnins L-1 and L-2, vinyl (ethenyl) and methyl sulfenic acid, respectively, were used in the first step of their synthesis. Moreover, on the basis of garlicnin L-2 formation, it was hypothesized that allyl (or 1-propenyl) sulfenic acid would be involved in hydroxylation for oxidative reaction, of which example were observed in the pathway to onionin B group. In the case of garlicnin L-2 formation, 1-propenyl sulfenic acid was likely involved in the dehydroxylation for reductive reaction, for which instances were observed in the pathways to garlicnins L-3, M, I1, I2, J2, and onionin B group. Formation of garlicnin B group was proposed as shown in Fig. 6: allicin was firstly derived from S-allyl L-cysteine, next allicin was transformed into 1-propenyl 1-propene-thiosulfinate via double-bond rearrangement and was then converted to 2,3-dimethylbutanedithial 1-oxide via [3,3]-sigmatropic rearrangement [30]. The generated intermediate was subsequently ring-closed to form a thiolane derivative that reacted with allyl sulfenic acid to finally produce the 3,4-dimethylthiolane-type sulfides, garlicnins B1, B2, B3 and B4. On the other hand, the above thiolane derivative was once hydroxylated on S in the thiolane framework to give thiolane S-oxide, and next reacted with allyl thiosulfenic acid and 1-propenyl sulfenic acid to generate the garlicnin C group, as shown in Fig. 6. The hypothetical pathway for the production of garlicnin M is shown in Fig. 7. In the production of the 2-methylthiolane(and thiane)-type sulfoxides, the combination of C-2 on allyl sulfenic acid and C-1 on 1-propenyl sulfenic was triggered in the pathways to garlicnins I1 and I2 as shown in Fig. 8, and the combination between the C-1 on 1-propenyl sulfenic acid and C-3 on allyl sulfenic acid occurred for the formation of garlicnin J1 as shown in Fig. 9. In the production of 1,2-dithiolane-type sulfoxides, the first stage was initiated by the combination of C-1 on allyl sulfenic acid and C-2 on allyl thiosulfenic acid in the case of garlicnin G. To produce garlicnin P, the dehydroxylation of allyl thiosulfenic acid resulted in successive rearrangements between allyl thiosulfenic acid and 1-propenyl sulfenic acid to yield garlicnin P as shown in Fig. 10. The 2-oxothiolane-type sulfides, onionins B1 and B2 were produced following hydroxylation to C-2 on the thiolane framework. This method differed from garlicnins C group, in which the hydroxylation to the S atom on the thiolane framework occurred as shown in Fig. 6. Furthermore, allyl sulfenic acid preferred hydroxylation at C-2 and 1-propenyl sulfenic acid may participate in dehydroxylation at C-4 as shown in Fig. 11.
Fig. 4.
Hypothetical pathways to acyclic-type sulfides, garlicnins L-1 and L-2
Fig. 5.
Hypothetical pathways to acyclic-type sulfides, garlicnins L-3 and L-4
Fig. 6.
Hypothetical pathways to 3,4-dimethylthiolane-type sulfides, garlicnins A, B, and C groups
Fig. 7.
Hypothetical pathway to 3,4-dimethylthiolane-type sulfoxide, garlicnins M
Fig. 8.
Hypothetical pathway to 2-methylthiolane-type sulfoxides, garlicnins I1 and I2
Fig. 9.
Hypothetical pathway to 2-methylthiane-type sulfoxides, garlicnins J1 and J2
Fig. 10.
Hypothetical Pathway to 1,2-dithiolane-type sulfoxides, garlicnins G and P
Fig. 11.
Hypothetical pathway to 2-oxothiolane-type sulfides, onionins B1 and B2
Effect of 3,4-dimethylthiolane-type sulfide [onionin A1 (1)] on tumor progression and metastasis in tumor injected mice
3,4-Dimethylthiolane-type sulfides, such as onionins A1–A3 from onion and Welsh onion, and garlicnins A, B1–B4, C1–C3, and M from garlic are common compounds among these Allium species and are regarded as major sulfides. Therefore, to examine the antitumor activity, onionin A1 (1) [23], which is representative of the 3,4-dimethylthiolane-type sulfides, was investigated. Onionin A1 is an isomer of garlicnin B1, with an allylsulfinyl group instead of a 1-propenylsulfinyl group at C-5 on the core 3,4-dimethylthiolane 2-ol framework. Therefore, if onionin A1 is active for antitumor effects then garlicnin B1 also expected to be active. We used onionin A1 available at this time for antitumor examination. The effects of onionin A1 on tumor progression and metastasis in mouse osteosarcoma and ovarian cancer-bearing mouse models were investigated. Administration of onionin A1 significantly suppressed both subcutaneous tumor development and lung metastasis in a mouse osteosarcoma (LM-8)-bearing mouse model (A in Fig. 12). Furthermore, onionin A1 significantly suppressed (in promotion stage) tumor progression in a mouse ovarian cancer (iMOC)-bearing mouse model (B in Fig. 12), suggesting that onionin A1 is an orally available small molecule for anti-cancer therapy [31, 32]. The antitumor effects observed in vivo are likely caused by reversal of the antitumor immune system. Activation of the antitumor immune system by onionin A1 might be an effective adjuvant therapy for patients with osteosarcoma, ovarian cancer and other malignant tumors.
Fig. 12.
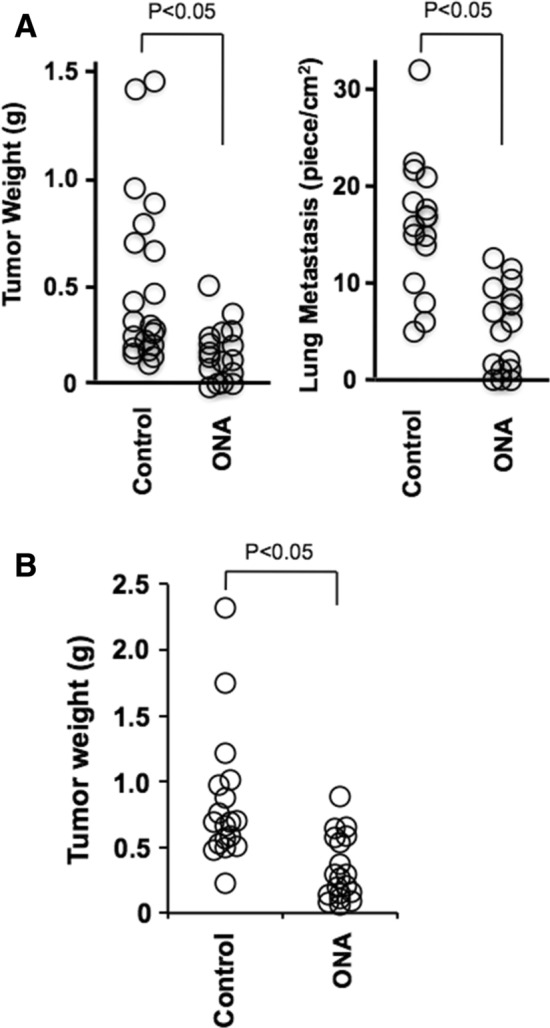
Effect of 3,4-Dimethylthiolane-type sulfide (onionin A1: ONA) on tumor progression and metastasis in tumor injected mice Onionin A1 (ONA) (20 mg/kg) was administered orally before and after the subcutaneous implantation of LM8 cells in the C3H mice (n = 20, each group) for 3 weeks, followed by determination of the subcutaneous tumor weight and presence of lung metastasis (A). As a murine ovarian cancer model, C57B6 mice were injected in the right ovary with iMOC cells and were administered ONA (20 mg/kg) for 3 week, followed by determination of the subcutaneous tumor weight (B)
Conclusion
The identification and characterization of novel sulfides isolated from garlic, onion, and Welsh onion have contributed to the identification of new chemicals and pharmaceutical compounds. Among the 3,4-dimethylthiolane-type of major sulfides, garlicnin B1 (Table 1, Fig. 13) is expected to be developed as a novel anti-cancer agent, as it is readily isolated in high yield, representing approximately 0.05% of Chinese garlic, and is also a synthesizable target because of its structural simplicity. Based on these findings, pharmacological investigations will be conducted to develop healthy foods and anti-cancer agents that can prevent or combat disease.
Fig. 13.
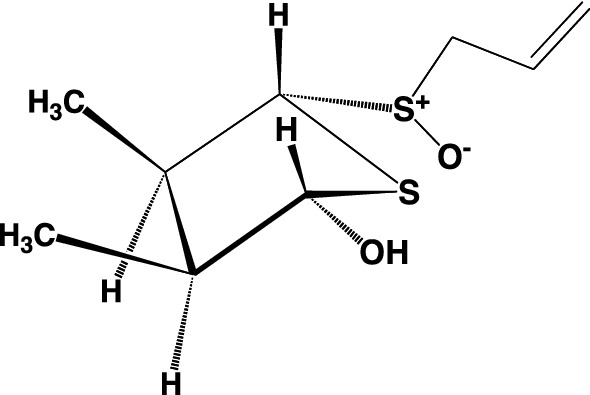
Garlicnin B1 (2)
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Theisen C. What ever happened to…? Looking back 10 years. J Nat Cancer Inst. 2001;93(14):1049–1050. doi: 10.1093/jnci/93.14.1049. [DOI] [PubMed] [Google Scholar]
- 2.Dewick PM. Medicinal natural products a biosynthetic approach. UK: John Willey & Sons; 2002. [Google Scholar]
- 3.Rose P, Whiteman M, Moore PK, Zhu YZ. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: the chemistry of potential therapeutic agents. Nat Prod Rep. 2005;22:351–368. doi: 10.1039/b417639c. [DOI] [PubMed] [Google Scholar]
- 4.Amagase H. Clarifying the real bioactivie constituents of garlic. J Nut. 2006;136:716–725. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 5.Corzo-Martinerz M, Corzo N, Villamiel M. Diological properties of onion and garlic. Trends Food Sci Technol. 2007;18:609–625. doi: 10.1016/j.tifs.2007.07.011. [DOI] [Google Scholar]
- 6.Smith S. Chemistry in a salad bowl: Allium chemistry and biochemistry. In: Block E, editor. Garlic and other alliums. The lore and science: Royal Society of Chemistry; RSC Publishing; 2010. pp. 100–223. [Google Scholar]
- 7.Cavallito CJ, Bailey JH. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J Am Chem Soc. 1944;66:1950–1951. doi: 10.1021/ja01239a048. [DOI] [PubMed] [Google Scholar]
- 8.Stoll A, Seebeck F. Die synthese des natuerlichen alliins und seiner drei optisch aktiven isomeren. 5. Mitteilung ueber substanzen Helv Chim Acta. 1951;34:481–487. doi: 10.1002/hlca.19510340212. [DOI] [Google Scholar]
- 9.Brodnitz M, Pascale JV, van Derslice IJ. Flavor components of garlic extract. J Agric Food Chem. 1971;19:273–275. doi: 10.1021/jf60174a007. [DOI] [Google Scholar]
- 10.Block E, Ahmad S. (E, Z)-Ajoene: a potent antithromboic agent from garlic. J Am Chem Soc. 1984;106:8295–8296. doi: 10.1021/ja00338a049. [DOI] [Google Scholar]
- 11.Fenwick GR, Hanley AB (1985) Critical reviews in food science and nutrition. In: Furia TE (ed) The genus Allium, part 2, vol 22. CRC Press, Boca Raton, FL, pp 273–377 [DOI] [PubMed]
- 12.El-Aasr M, Fujiwara Y, Takeya M, Ono M, Nakano D, Okawa M, Kinjo J, Ikeda T, Miyashita H, Yoshimitsu H, Nohara T. Garlicnin A from the fraction regulating macrophage activation of Allium sativum. Chem Pharm Bull. 2011;59:1340–1343. doi: 10.1248/cpb.59.1340. [DOI] [PubMed] [Google Scholar]
- 13.Nohara T, Kiyota Y, Sakamoto T, Manabe H, Ono M, Ikeda T, Fujiwara Y, Nakano D, Kinjo J. Garlicnins B1, C1, and D, from the fraction regulating macrophage activation of Allium sativum. Chem Pharm Bull. 2012;60:747–751. doi: 10.1248/cpb.60.747. [DOI] [PubMed] [Google Scholar]
- 14.Nohara T, Fuiiwara Y, Ikeda T, Murakami K, Ono M, Nakano D, Kinjo J. Cyclic sulfoxides garlicnins B2, B3, B4, C2, and C3 from Allium sativum. Chem Pharm Bull. 2013;61:695–699. doi: 10.1248/cpb.c13-00082. [DOI] [PubMed] [Google Scholar]
- 15.Ono M, Fujiwara Y, Ikeda T, Pan C, El-Aasr M, Lee JH, Nakano D, Kinjo J, Nohara T. Atypical cyclic sulfides, garlicnins G, I, and J, extracted from Allium satuvum. Chem Pharm Bull. 2017;54:102–106. doi: 10.1248/cpb.c16-00648. [DOI] [PubMed] [Google Scholar]
- 16.Nohara T, Ono M, Nishioka N, Masuda F, Fujiwara Y, Ikeda T, Nakano D, Kinjo J. New cyclic sulfides I2, M, N, and O, from Allium sativum. J Nat Med. 2018;72:326–331. doi: 10.1007/s11418-017-1133-2. [DOI] [PubMed] [Google Scholar]
- 17.Nohara T, Ono M, Nishioka N, Masuda F, Fujiwara Y, Ikeda T, Nakano D, Kinjo J. New cyclic sulfides extracted from Allium sativum: garlicnins P, J2 and Q. J Nat Med. 2018;72:335–341. doi: 10.1007/s11418-017-1151-0. [DOI] [PubMed] [Google Scholar]
- 18.Nohara T, Fuiiwara Y, Ikeda T, Yamaguchi K, Manabe H, Murakami K, Ono M, Nakano D, Kinjo J. Acyclic sulfides, garlicnins L-1-L-4, E, and F, from Allium sativum. Chem Pharm Bull. 2014;62:477–482. doi: 10.1248/cpb.c14-00003. [DOI] [PubMed] [Google Scholar]
- 19.Nohara T, Ono M, Ikeda T, Fujiwara Y, Nakano D, Kinjo J. Two new cyclic sulfides, onionin B1 and B2, from onion. Curr Top Phytochem. 2018;14:71–75. [Google Scholar]
- 20.Fukaya M, Nakamura S, Nakagawa R, Nakashima S, Yamashita M, Masuda H. Rare sulfur-containing compounds, kujounins A1 and A2 and Allium sulfoxide A1, from Allium fistulosum”Kujou”. Org Lett. 2018;20:28–31. doi: 10.1021/acs.orglett.7b03234. [DOI] [PubMed] [Google Scholar]
- 21.Juaristi E, Cruz-Sanchez JS, Petson A, Glass RS. Conformational preference of the S=O group. 3. Continued evidence for a very strong S-S=O anomeric interaction from The NMR spectroscopic study of 4,4,5,5-tetramethyl-1,2-dithiane-1-oxide. Tetrahedron. 1988;44:5653–5660. doi: 10.1016/S0040-4020(01)81426-5. [DOI] [Google Scholar]
- 22.Ronayne J, Williams DH. The mechanism of solvent shifts of proton resonances induced by benzene. J Chem Soc B. 1967;1967:540–546. doi: 10.1039/j29670000540. [DOI] [Google Scholar]
- 23.El-Aasr M, Fujiwara Y, Takeya M, Ikeda T, Tsukamoto S, Ono M, Nakano D, Okawa M, Kinjo J, Yoshimitsu H, Nohara T. Onionin A from Allium cepa inhibits macrophage activation. J Nat Prod. 2010;73:1306–1308. doi: 10.1021/np100105u. [DOI] [PubMed] [Google Scholar]
- 24.Nohara T, Fujiwara Y, Kudo R, Yamaguchi K, Ikeda T, Murakami K, Ono M, Kajimoto T, Takeya M. Isolation and characterization of new onionins A2 and A3 from Allium cepa, and of onionins A1, A2, and A3 from Allium fistulosum. Chem Pharm Bull. 2014;62:1141–1145. doi: 10.1248/cpb.c14-00461. [DOI] [PubMed] [Google Scholar]
- 25.Block E, Dethier B, Bechand B, Cotelesage JJH, George GN, Goto K, Pickering IJ, Rengifo EM, Sheridan R, Sneeden EY, Vogt L. Ajothiolanes: 3,4-dimethylthiolane natural products from garlic (Allium sativum) J Agric Food Chem. 2018;66:10193–10204. doi: 10.1021/acs.jafc.8b03638. [DOI] [PubMed] [Google Scholar]
- 26.Štefanová I, Zápal J, Moos M, Kuzma M, Kubec R. Isoalliin-derived thiolanes formed in homogenized onion. J Agric Food Chem. 2019;67:9895–9906. doi: 10.1021/acs.jafc.9b01384. [DOI] [PubMed] [Google Scholar]
- 27.Nohara T, El-Aasr M, Ikeda T, Gao S, Yokomizo K, Determination of absolute configuration of most abundant garlic sulfide, garlicnin B1. Curr Top Phytochem (in press)
- 28.Dale JA, Mosher HS. Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and α-methoxy-α-trifluoromethyl-phenylacetate (MTPA) esters. J Am Chem Soc. 1973;95:512–519. doi: 10.1021/ja00783a034. [DOI] [Google Scholar]
- 29.Sullivan GR, Dale JA, Mosher HS. Correlation of configuration and fluorine-19 chemical shifts of α-methoxy-α-trifluoromethylphenyl acetate derivatives. J Org Chem. 1973;38:2143–2147. doi: 10.1021/jo00952a006. [DOI] [Google Scholar]
- 30.Bayer T, Wagner H, Block E, Grisoni S, Zhao SH, Neszmeiyi A. Zwiebelanes: novel biologically active 2,3-dimethyl-5,6-dithiabicyclo-[2.1.1]hexane 5-oxides from onion. J Am Chem Soc. 1989;111:3085–3086. doi: 10.1021/ja00190a064. [DOI] [Google Scholar]
- 31.Fujiwara Y, Horlad H, Shiraishi D, Tsuboki J, Kudo R, Ikeda T, Nohara T, Takeya M, Komohara Y. Onionin A, a sulfer-containing compound isolated from onion, impairs tumor development and lung metastasis by inhibiting the protumoral and immune-suppressive functions of myeloid cells. Mol Nut & Food Res. 2016;60:2467–2480. doi: 10.1002/mnfr.201500995. [DOI] [PubMed] [Google Scholar]
- 32.Tsuboki J, Fujiwara Y, Horlad H, Shiraishi D, Nohara T, Tayama S, Motohara T, Saito Y, Ikeda T, Takaishi K, Tashiro H, Yonemoto Y, Katabuchi H, Takeya M, Komohara, Onionin A inhibits ovarian cancer progression by suppressing cancer cell proliferation and the protumour function of macrophages. Sci Rep. 2016;6:29588. doi: 10.1038/srep29588. [DOI] [PMC free article] [PubMed] [Google Scholar]