Abstract
Preterm infants undergo early separation from parents and are exposed to frequent painful clinical procedures, with resultant short- and long-term effects on their neurodevelopment. We aimed to establish whether the mother’s voice could provide an effective and safe analgesia for preterm infants and whether endogenous oxytocin (OXT) could be linked to pain modulation. Twenty preterm infants were exposed to three conditions—mother’s live voice (speaking or singing) and standard care—in random order during a painful procedure. OXT levels (pg/mL) in saliva and plasma cortisol levels were quantified, and the Premature Infant Pain Profile (PIPP) was blindly coded by trained psychologists. During the mother’s live voice, PIPP scores significantly decreased, with a concomitant increase in OXT levels over baseline. The effect on pain perception was marginally significant for singing. No effects on cortisol levels were found. The mother’s live voice modulated preterm infants’ pain indicators. Endogenous OXT released during vocal contact is a promising protective mechanism during early painful interventions in at-risk populations.
Subject terms: Health care, Paediatrics, Preterm birth, Psychology, Human behaviour
Introduction
Preterm birth rates are continuously increasing in almost all countries, with 15 million premature infants being born every year worldwide1. Despite rapid advances in technology, the number of preterm-born children who show short- and long-term sequelae of prematurity, even before reaching school age, remains high2. Around 40% of low birth weight preterm infants experience a complex spectrum of unfavourable neurodevelopmental outcomes3 when compared with their pairs at term. Thus, prematurity is of great concern for health policies in both low- and high-income countries4,5. The impaired development of preterm infants is not only associated with medical factors, but it is also at least partly a consequence of the atypical early-life environment of these infants3, including exposure to pain and separation from the primary caregivers6–8.
Separation and pain in the Neonatal Intensive Care Unit (NICU)
The effects of separation and pain on premature infant’s development have been at the core of several research studies9. Very preterm infants experience early and prolonged separation from their parents during hospitalisation in the NICU, and this separation has profound impacts on their stress levels8, as well as on their autonomic, neuroendocrine and immune systems10.
This separation can also alter preterm infants’ long-term neurodevelopment, with important effects on emotional and attachment processes for both the infants and their parents11–15. Indeed, in the long term, preterm infants are an at-risk population with higher levels of stress, decreased resilience in coping with difficulties, and less secure attachment than their peers born at term11. A compromised attachment and bonding experience between mothers and their very preterm infants was associated with less intimacy with the infant and with difficulties in regulating socio-emotional stress at 3 months of age16.
Several painful clinical procedures occur for preterm infants in the first days after birth in NICUs in the context of partial or total separation from their parents. Since these procedures occur at a time of heightened sensitivity and rapid neurodevelopment, they might alter, in addition to later pain sensitivity, the brain structure and function of the infants17. Moreover, long-term dysfunctions in the neuroendocrine system and down-regulation of the hypothalamic–pituitary–adrenal axis, with lower cortisol levels, are associated with repeated painful procedures in preterm infants in the NICU18.
Although several epidemiological studies have reported advances in reducing procedural pain and improving the use of analgesic drugs in everyday clinical practice in NICUs19–22, there are consistent disparities in the administration of these analgesics to preterm infants among various countries and among various hospital units within the same country22. Moreover, recent advances in evidence-based studies demonstrate the ambivalence towards the use of drug analgesia techniques in the preterm population and the need to increase research on non-pharmacological forms of analgesia23. Although the role of OXT in the experience of pain has been documented24, its underlying mechanisms are still under debate and the protective impact that increased maternal care could have on OXT—and thus on pain perception in preterm infants—has never been investigated.
Parental modulatory effect on infants’ pain
The positive effects of parental presence on pain modulation are still a matter of discussion, especially very early after birth. Although Piira and colleagues, in a systematic review25, affirmed that parental presence may not have a clear, direct influence on child distress, several research findings support the positive impact of parental contact on infants during noxious stimulation26. In particular, the efficacy of breastfeeding and skin-to-skin contact are accepted as alleviating pain responses during painful procedures27–29. The odour of the mother’s milk also appears to reduce pain from a heel stick in full-term neonates30 and preterm infants31, as well as pre-procedural pain22. Cortical pain responses after venepuncture in preterm infants has recently been investigated and the type of contact that the infant has with the mother has been shown to modulate neonatal brain processing of noxious stimuli32.
Understanding the mechanisms that modulate the effects of parental contact on pain perception in neonates could also provide insight into pain learning and protective actions against repeated pain exposure that can be enhanced in the neonatal period.
Parents in the NICU can modulate their preterm infant’s state and behaviour not only with skin-to-skin contact, but also with the medium of vocalisations. Early vocal contact is an early intervention that actively involves parents in emotional and meaningful vocal contact with their preterm infants during hospitalisation in the NICU33. It sustains the preterm infant’s physiological stability, with a significant decrease in critical events such as bradycardia, apnoea, and hypoxia, and it increases the occurrence of calm awake states34. When preterm infants in the NICU are exposed to more adult voices, they show significantly higher language and cognitive scores at 7 and 18 months35. In contrast, low parental presence and, consequently, decreased interaction and lower language exposure, contribute to the sensory deprivation experienced by infants in neonatal units, with impacts on their brain structure and neurodevelopmental outcomes36.
In two studies, using microanalytic methods, Filippa and colleagues illustrated the impact of live maternal vocalisations, both singing and speaking, on preterm infants’ behaviours37. Similarly, two specific infant pro-social behaviours, eye opening and smiles, are associated with an increase in the emotional content in the mother’s voice38 and with specific acoustical characteristics associated with infant-directed speech and singing39. Father’s vocal contact, like the mother’s voice, also has an impact on the preterm infant’s behavioural organisation and state, with calm awakening effects40.
The key role of OXT
The role of OXT seems to be crucial, both in the paradigm of separation versus reunion of young infants with their mothers and in the inflammatory effects related to pain and stress41,42. OXT is released not only during affiliative interactions43, but also during social vocalisations44. The modulation of this oxytocinergic system is also correlated with positive effects on social behaviours44,45, pain46, stress due to separation47 and anxiety48–50.
In animal models, the periods of separation between mothers and offspring lead to reduced maternal care and a concomitant alteration in the regulation of OXT and vasopressin51. Early maternal separation interferes with the healthy development of OXT receptors in specific forebrain regions52. Offspring that received less maternal licking and grooming from low sensitive mothers exhibited associated changes in hypothalamic regions implicated in hormonal release and then indirectly related to maternal care53; they also showed reduced OXT receptor protein levels in the medial preoptic area, the lateral septum, the bed nucleus of the stria terminalis, the paraventricular nucleus of the hypothalamus, and the central nucleus of the amygdala54, providing a potential mechanism for the intergenerational transmission of individual differences in maternal behaviour.
From a protective perspective, in contrast, the provision of appropriate maternal care increases OXT levels in infants, and this affects brain organisation early in life in both animals and humans55–58. In particular, it has been shown that positive maternal care behaviours (1) increase OXT receptor binding in brain areas central to parenting and emotional behaviours, such the amygdala and the dorsolateral prefrontal cortex59, and (2) increase the reward that parents derive from their infants60.
Early tactile care behaviours, such as skin-to-skin contact in the newborn period, increase OXT levels in both mothers and infants61,62, decrease salivary cortisol reactivity and improve salivary cortisol concordance between mother and infant12. Interestingly, OXT receptors are present in the peripheral terminal axons of the skin63, and touch-evoked OXT release could also explain the analgesia induced by tactile stimulation64.
As regards the role of OXT in pain experience it has been suggested that in the animal model OXT has precise functions in the physiological responses to pain and stress65. In rodents, OXT has analgesic effects acting on pain matrix structures66. In particular, endogenous oxytocin exerts an analgesic action, for example in newborn pups with a reduction of the depolarizing action of GABA on nociceptive neurons67.
In humans, OXT acts on brain regions involved in pain processing68, but the mechanisms linking OXT and pain perception in humans are not yet fully understood66. Results seem sometimes controversial, but generally promising, suggesting that OXT may decrease pain sensitivity69. OXT reduces visceral pain symptoms in patients with irritable bowel syndrome70, low back pain after intrathecal infusion71, and its intranasal administration diminishes headache72. Additionally, OXT modulates the emotional dimension of pain expression73,74. These observations and others led to the proposal that OXT modulates several dimensions of pain expression, and has strong effects on emotional output, attentional processes, and social interactions75.
Finally, it is known that OXT and cortisol are both implicated in the effects of pain and that OXT can inhibit the function of the hypothalamic–pituitary–adrenal axis at several levels during the production of cortisol76,77. It is also known that basal cortisol secretion is altered in preterm infants78, especially in infants born at low gestational age79, who were exposed to the most invasive procedures during neonatal care in addition to being developmentally the most immature.
In the present study, we aimed to evaluate the effects of reducing separation between the mother and her prematurely-born offspring on pain perception and on OXT release during a routine painful clinical procedure in the NICU. We predicted that early vocal contact would reduce the signs related to preterm infant pain by affecting the oxytocinergic system, generating an increase in endogenous OXT levels correlated with a decrease in infant pain signs.
Methods
Study design and participants
The singlecentre study was conducted in a level II NICU at the Parini Hospital (Aosta, Italy). A total of 68 preterm infants were born in the NICU hospital between January 2018 and April 2019; of these, 47 did not meet the inclusion criteria, their length of stay in the NICU was expected to be less than 48 h, or they were not included because of technical or logistic problems. Infants were assessed for eligibility by a senior clinician; 21 of them met the inclusion criteria, were medically stable and were approached for enrolment and were enrolled. However, one was excluded because of incomplete data. Twenty preterm infants were thus recruited.
The final sample met the following inclusion criteria: (1) age > 29 weeks gestational age, (2) weight > 1000 g and (3) stable medical condition. Preterm infants who needed mechanical ventilation and additional oxygen and who had specific pathological conditions (i.e., genetic malformation, presence of periventricular or intraventricular haemorrhage, periventricular leukomalacia) were excluded from the study, as were mothers with a history of substance abuse or with mental health problems. The data for the mothers and newborns are summarised in Table 1.
Table 1.
Infant demographics.
| Mean (SD, range) | |
|---|---|
| Characteristics at birth | |
| Gestational age at birth (weeks)* | 32.7 (9, 6 days) |
| Birthweight (g) | 2042 (392, 5) |
| Apgar score at 1 min | 7.05 (1, 5) |
| Apgar score at 5 min | 8.1 (1) |
| Mode of delivery | Spontaneous vaginal delivery (40%) |
| Cesarean Sect. (60%) | |
| Sex | Female (45%) |
| Citizenship | Italian (100%) |
| Primiparous | Yes (90%) |
| Mother’s age | 29.2 (4, 62) |
| Characteristics at time of clinical procedure | |
| Gestational age at test (weeks) | 34.8 (10 days) |
| Postnatal age at test (days) | 3 (range 1–8) |
| Weight (g) | 2264 (325) |
| Number of painful procedures before testing | 7 (range 3–15) |
| Phototherapy before testing | Yes (70%) |
*Postmenstrual age should be used for clarity in neonatal practice. In our unit, the infants' age is recorded in the medical and nursing notes as gestational age, and we have used this terminology.
At discharge, all participants had passed the AABR bilateral hearing screening test. The official Hospital Ethical Committee of Aosta reviewed and approved the study (I.C. n. 90.513; date of approval: 10/20/2017), written informed parental consent was obtained and all experiments were performed in accordance with relevant guidelines and regulations.
Procedure
Electronic data capture of heart rate, respiratory rate and oxygen saturation, as well as video recordings, began at least 5 min before the clinical procedure in order to establish a baseline of clinical stability for every infant (baseline duration: 5 min). Each infant was recorded on three test occasions. During the intervention, mothers were asked to speak or to sing to their preterm infants in the incubators for 5 min preceding the heel prick procedure and for the subsequent 5 min.
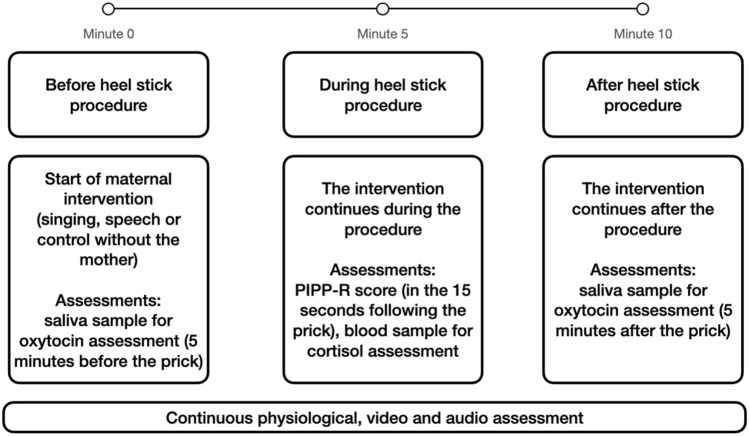
During the control condition (without the mother), the newborn was placed by the nurse in the incubator in the standard care conditions recommended for painful procedures (supine position, wrapped and contained by the nest). During the intervention and control conditions, physiological and video data were continuously captured. See Fig. 1 for procedure diagram of sample collection, assessments and intervention.
Figure 1.
Procedure diagram of the sample collections, assessments and intervention.
In both intervention conditions, speaking and singing, the mothers were asked not to touch the baby but to pay close attention to his/her reactions and to modulate the voice accordingly. A nurse was present during all procedures.
Before each intervention, the background noise levels were acquired via a calibrated sound level meter (Voltcraft Phonometer SL-10; Conrad Electronic, Hirschau, Germany) in the room where the intervention took place, inside the incubator, and 20 cm from the newborn’s head. This measure was assessed at every intervention session to ensure that the mother’s voice was audible to the newborn (i.e., that it exceeded the background noise of 10 dBA)80 in order to support 100% speech and song intelligibility among mothers and preterm newborns. Moreover, mothers were instructed not to exceed the recommended levels for sound pressure81 in order to prevent overstimulation.
Finally, they were instructed to open the incubator window, to speak and to sing through the window, and to keep a distance approximately of 20 cm from the newborn’s head. The correct position was constantly verified by the researcher.
No other specific instructions were given to the mothers, who could use their intuitive parenting behaviours in proximity to the babies. No further analyses were performed on these values.
Testing was performed over three consecutive days in the first days of extra-uterine life (for details on postnatal days, see Table 1). The order of the stimuli (speaking or singing) and the control condition was randomly selected over the three testing days across the infants. The randomisation was performed by using a secure web-based randomisation system, through which the study investigator registered new patients and obtained the treatment arm assignment.
Outcome measures
The co-primary outcome measures were a behavioural pain score calculated after the heel prick with the Premature Infant Pain Profile–Revised (PIPP)82 and salivary OXT levels.
PIPP score
The PIPP score was calculated in the 15-s period after the heel prick procedure by both a trained nurse who was observing the procedure and offline by two blinded independent and trained coders on muted video tracks. The PIPP score is a cluster of physiological and behavioural measures. Physiological assessment was calculated on the heart rate and oxygen saturation levels as collected from the patient monitor by the researcher. Inter-rater reliability was assessed by three independent coders: expert coders 1 and 2 performed blinded ratings from offline muted videos and digitally recorded physiological parameters, whereas coder 3 was a trained nurse and performed a direct online rating of the PIPP scores. The three independent raters achieved a high degree of reliability, as confirmed by Spearman’s correlation analysis, with values of 0.73 for coder 1, 0.78 for coder 2, and 0.70 for coder 3 when compared with the mean of the PIPP scores obtained on all raters.
OXT measure
Saliva samples were collected without stimulants by using an absorbent device83 that was placed in the newborn’s mouth and then centrifuged and stored at − 20 °C until analysis. Saliva samples were collected twice, 5 min before and after the heel prick procedure, in the three conditions. Salivary OXT concentrations (pg/mL) were quantified by radioimmunoassay (RIAgnosis, Munich, Germany). A quantity of 300 μL of saliva was evaporated for each sample (SpeedVac, Thermoscientific Inc, Waltham, MA, USA); 50 μL of assay buffer and 50 μL antibody were then added. After a 60-min preincubation interval, an additional 10 μL of 125 I-labelled tracer (PerkinElmer, Waltham, MA, USA) was used and samples incubated for 3 days at 4 °C. The detection limit was fixed at 0.5 pg/sample range, depending on the age of the tracer, with typical displacements of 20–25% at 2 pg, 60–70% at 8 pg, and 90% at 32 pg of standard neuropeptide. The intra- and inter-assay variabilities were < 10%. When the amount of saliva was insufficient, saliva samples from several infants during the same condition were pooled, and serial dilutions of saliva samples containing high levels of endogenous OXT were run strictly parallel to the standard curve indicating immuno-identity. Single, not pooled, saliva analysis was performed for 73% of the collected samples.
Cortisol measure
Blood samples were collected during the heel prick procedure, centrifuged 10 min at 2000 rpm, and each serum separated from the clot and frozen at − 20 °C until analysis. Cortisol concentrations were measured by using the chemiluminescent immunoassay method on a DiaSorin Liaison XL analyser (Saluggia, Italy) according to the manufacturer’s instructions. Serum samples were analysed in different batches; however, all samples from each newborn were always assayed in the same batch.
Statistical analysis
General linear mixed models in R software (version 2.15.0) were used. R Studio (version 0.97.551) was applied instead of classic analysis of variance in order to include random factors (e.g., pooled samples, identity; Team, 2013). To test the significance of the different experimental conditions, we systematically used chi square tests for the comparison of alternative models (e.g., a model with main effects compared with a model that also included the interaction). The following fixed effects factors were specified: the three Conditions (during mother’s singing [Singing], during mother’s speech [Speaking], and without mothers [Control]) and the Time factor (Pre and Post conditions for OXT levels). The random factors included in our models were dyad ID, and pooled or not pooled samples. Our dependent variables were cortisol levels, OXT levels and PIPP scores.
For cortisol analysis, we identified the outliers for values > 22.15, i.e., higher than double the standard deviation of the mean of the total conditions. In the analysis, we substituted the missing values (5%) with the mean of the same conditions (6.4339 for control; 6.6927 for singing; 7.6883 for speaking).
Results
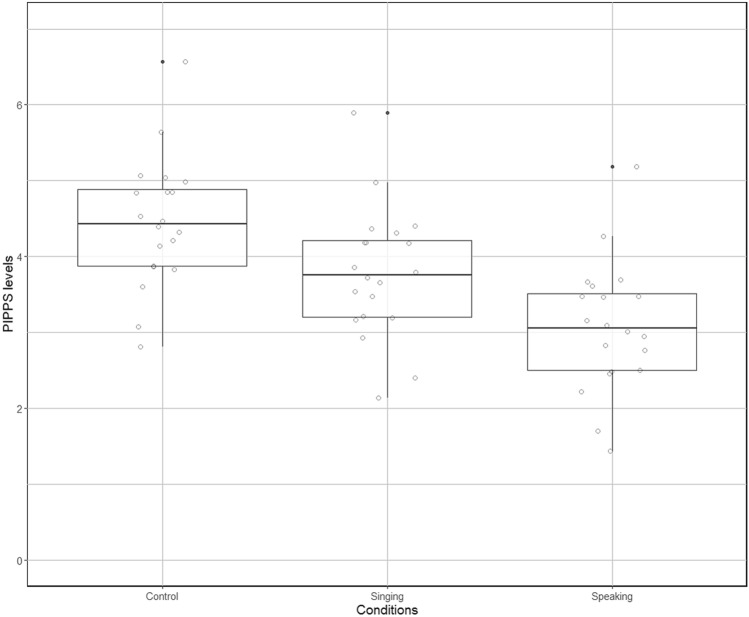
The statistical model that included Condition (Singing, Speaking, and Control modalities) explained significantly more variance of the PIPP score than did a simpler model with only random effects (IDs and Pooled/NoPooled; χ2 (2) = 7.16, p = 0.028). Planned contrasts revealed that preterm infants’ PIPP scores were significantly lower in the Speaking condition than in the Control condition in the absence of the mother’s voice (χ2 (1) = 7.45, p = 0.006). No significant differences were found for the Singing condition for the same comparison (χ2 (1) = 1.76, p = 0.18; see Fig. 2).
Figure 2.
Preterm infants Pain Profile scores presentation in the Control, Singing and Speaking conditions during painful procedures.
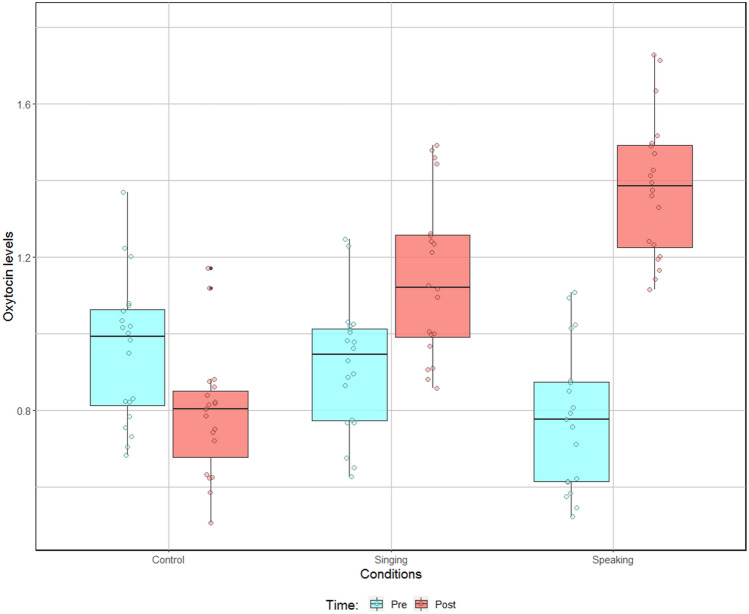
OXT analysis revealed that the statistical model that included the planned interaction of Condition (Singing, Speaking, and Control modalities) with Time (Pre and Post modalities) explained significantly more variance of OXT levels than did the model with main effects of both Condition and Time (χ2 (2) = 6.99, p = 0.03). Note that the comparisons of models for each main effect of Condition and Time were not significant (χ2 (2) = 1.79, p = 0.41, and χ2 (1) = 2.87, p = 0.09, respectively). Planned contrasts revealed (1) no significant differences for the Pre modality of the Time factor between the three Conditions (Control vs. Singing: χ2 (1) = 0.06, p = 0.80; Control vs. Speaking: χ2 (1) = 0.91, p = 0.34; Singing vs. Speaking: χ2 (1) = 0.49, p = 0.48); (2) significant differences for the Post modality of the Time factor between Speaking and Control modalities of the Condition factor (χ2 (1) = 7.72, p = 0.005); (3) marginally significant differences between Control and Singing (χ2 (1) = 2.91, p = 0.088); and (4) no significant differences between Singing and Speaking (χ2 (1) = 1.15, p = 0.28; Fig. 3). Note that the Pre vs. Post comparisons confirmed the significant increase of OXT levels for the Speaking modality (χ2 (1) = 7.99, p = 0.0047), whereas the same contrast did not reach significance for both the Control and the Singing modalities (χ2 (1) = 0.82, p = 0.37, and χ2 (1) = 1.11, p = 0.29, respectively). This analysis was not significantly affected by the infant’s sex (χ2 (1) = 0.13, p = 0.71).
Figure 3.
Preterm infants’ oxytocin levels (pg/mL) before and after painful procedures in the Control, Singing and Speaking conditions.
The cortisol levels showed no significant differences when we included the Condition factor compared with a model with only the random factors (χ2 (2) = 2.17, p = 0.34). Spearman correlation analysis was performed and no significant correlation between cortisol levels and OXT levels in preterm infants was found for the Pre condition (rs = − 0.06, p = 0.65, N = 60) and the Post condition (rs = 0.07, p = 0.59, N = 60). Similarly, no significant correlation between cortisol levels and PIPP scores was found (rs = − 0.01, p = 0.93, N = 60).
Discussion
The main aims of this study were to investigate how mothers’ interventions through vocalisations (speaking and singing), compared with a control condition without the mother, could modulate (1) the signs of pain in the preterm infants, (2) the endogenous amount of OXT in the preterm infants’ saliva samples, and (3) the level of cortisol in their plasma samples after acutely painful clinical procedures. The results showed that maternal infant-directed speech has a beneficial effect on preterm infants’ pain, as evidenced by a significant decrease in PIPP scores. Furthermore, OXT levels significantly increased during the mother’s speech, but only marginally for singing, when compared with the absence of the mother. No effects of the three conditions—speaking, singing or standard care—on infants’ plasma cortisol levels were found.
To our knowledge, this is the first study to not only provide the preliminary data on the potential analgesic properties of the live maternal voice during painful procedures in preterm infants, but also to show that endogenous OXT regulation could act as a potential protective mechanism for early pain perception. Further data based on preclinical animal models are needed to demonstrate the causality link between maternal vocalizations, OXT levels and pain perception. The administration of an OXT receptor antagonist to offspring during a painful procedure, in the presence of maternal vocalizations, could show the existence of a causal link. These preliminary results extend previous studies of infants conducted in an animal model, which showed that early relational experiences can persistently affect social behaviours by modifying the OXT system55. In particular, it is known that experiences of early contact have long-term effects—even intergenerational effects84—through the modulation of the OXT system. Although singing was associated with an increase in OXT, only in the speaking condition were significant correlations demonstrated. The OXT distributions are often heterogeneous and this may explain the marginal effects for the singing condition in a small population.
Early and repeated painful experiences (here especially in preterm infants) induce long-term over-sensitisation to pain and stress, and have significant consequences for infant social and emotional competencies7. Concerning maternal separation, the OXT system might also play a crucial role in repairing and reconstructing the infant’s resilience in response to painful stimuli.
One of the main aims of pain management in neonates is to maximise the their capacity to cope with and recover from painful experiences85. If the present results are confirmed through studies that investigate pain responses in preterm infants at the brain level and through robust animal models in which OXT antagonists are manipulated during painful procedures, new early protective interventions can be designed for preemies in the NICU. Parental vocalizations should thus be encouraged during all phases of painful procedures in the NICU, including the preparation phase, the acute pain phase and the consolatory phase following the procedure.
The active involvement of parents in the early care of preterm infants is one of the primary goals of infant- and family-centred developmental care86. Positive social and emotional interactions between parents and infants in the NICU have long-term effects not only on infants, but also on parent mental health outcomes87, reducing parental depression and anxiety, and reinforcing the parental role.
The present preliminary results, if supported by further studies and in line with early family-centred interventions, could suggest that maternal direct vocalizations should be integrated in standard care in the NICU during infants’ painful and stressful experiences.
Study limitations
One limitation of the present study is the small sample size, although this vulnerable population needs individualized studies in order to design future preventive health interventions. Several difficulties led to our study having a small sample size, including the vulnerability of the preterm population and the active involvement of parents during painful procedures, which is not part of standard care in an NICU. Future research is needed to enlarge the sample size, both in number and types of involved patients. Fragile very preterm infants, born at less than 32 weeks of gestational age, and newborn infants requiring painful procedures during hospitalization should be included in future studies. In the present study, only maternal vocalizations were manipulated, but the introduction of a control condition with a silent maternal presence is recommended for future studies, in the absence of painful procedures88. Another limitation of the study is the blood sample for cortisol analysis. In order to avoid an additional painful procedure for research purposes, we took additional blood during the same procedure. Thus, we had no possibility of analysing the cortisol changes in blood samples at different time points, i.e., after 5 and 10 min following the procedure. Finally, it is known that OXT distribution is heterogeneous, especially in very small saliva samples such as those for preterm infants. However, the existing correlations between OXT levels and PIPP scores are preliminary promising results. Future studies, robust preclinical research and investigations into preterm infant pain through neuroimaging techniques are needed to replicate the present results.
Conclusion
The universal right to pain relief, especially in vulnerable patient populations, is undeniable89. The non-noxious perspective in protective medicine should be at the core of future research, and the search for alternative, safe and effective pain management must be a primary concern of researchers and of science.
We believe that our study is a starting point for further investigations into the role of maternal vocalizations as a protective factor for preterm infants against the effects of pain and separation during hospitalisation in the NICU. The specific role of endogenous OXT is a promising mechanism of action for early protective intervention in at-risk populations, who are too often exposed to pain, stress and separation from their caregivers during hospitalisation.
Acknowledgements
We wish to thank the preterm infants and parents for their participation; the medical staff, in particular the nursing staff of Parini Hospital; and the Aosta Analysis Lab. We also thank the Zonta and the Lions Club Aosta for their economic support. Special thanks to NCCR Evolving language (51NF40_180888), financed by the Swiss National Science Foundation, for indirectly supporting this project.
Author contributions
M.F., D.G. and M.G.M. designed the study; M.F. and C.S. collected the data; D.G. and R.D. performed the data analysis; and all the authors contributed to the interpretation of the results. M.F. wrote the first draft and all the authors substantively revised it.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blencowe H, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. The Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Jarjour IT. Neurodevelopmental outcome after extreme prematurity: A review of the literature. Pediatr. Neurol. 2015;52(2):143–152. doi: 10.1016/j.pediatrneurol.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Adams-Chapman I, et al. Neurodevelopmental impairment among extremely preterm infants in the neonatal research network. Pediatrics. 2018;141(5):e20173091. doi: 10.1542/peds.2017-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick MC, et al. Prematurity: An overview and public health implications. Annu. Rev. Public Health. 2011;32:367–379. doi: 10.1146/annurev-publhealth-090810-182459. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Born Too Soon: The Global Action Report on Preterm Birth. (2012).
- 6.Anand K, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Neonatology. 2000;77(2):69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 7.Grunau, R.E., L. Holsti, and J.W. Peters. Long-term consequences of pain in human neonates. In Seminars in Fetal and Neonatal Medicine. (Elsevier, 2006). [DOI] [PubMed]
- 8.Flacking R, et al. Closeness and separation in neonatal intensive care. Acta Paediatr. 2012;101(10):1032–1037. doi: 10.1111/j.1651-2227.2012.02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filippa M, et al. Pain, parental involvement, and oxytocin in the neonatal intensive care unit. Front. Psychol. 2019;10:715. doi: 10.3389/fpsyg.2019.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev. Psychopathol. 2001;13(3):419–449. doi: 10.1017/S0954579401003029. [DOI] [PubMed] [Google Scholar]
- 11.Korja R, Latva R, Lehtonen L. The effects of preterm birth on mother–infant interaction and attachment during the infant's first two years. Acta Obstet. Gynecol. Scand. 2012;91(2):164–173. doi: 10.1111/j.1600-0412.2011.01304.x. [DOI] [PubMed] [Google Scholar]
- 12.Morelius E, et al. A randomised trial of continuous skin-to-skin contact after preterm birth and the effects on salivary cortisol, parental stress, depression, and breastfeeding. Early Hum. Dev. 2015;91(1):63–70. doi: 10.1016/j.earlhumdev.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Montirosso R, et al. Measuring maternal stress and perceived support in 25 Italian NICUs. Acta Paediatr. 2012;101(2):136–142. doi: 10.1111/j.1651-2227.2011.02440.x. [DOI] [PubMed] [Google Scholar]
- 14.Montirosso R, et al. Maternal stress and depressive symptoms associated with quality of developmental care in 25 Italian Neonatal Intensive Care Units: A cross sectional observational study. Int. J. Nurs. Stud. 2014;51(7):994–1002. doi: 10.1016/j.ijnurstu.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Kommers D, et al. Suboptimal bonding impairs hormonal, epigenetic and neuronal development in preterm infants, but these impairments can be reversed. Acta Paediatr. 2016;105(7):738–751. doi: 10.1111/apa.13254. [DOI] [PubMed] [Google Scholar]
- 16.Provenzi L, et al. Maternal sensitivity buffers the association between SLC6A4 methylation and socio-emotional stress response in 3-month-old full term, but not very preterm infants. Front. Psych. 2017;8:171. doi: 10.3389/fpsyt.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranger M, Grunau RE. Early repetitive pain in preterm infants in relation to the developing brain. Pain management. 2014;4(1):57–67. doi: 10.2217/pmt.13.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunau RE, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113(3):293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roofthooft DW, et al. Eight years later, are we still hurting newborn infants? Neonatology. 2014;105(3):218–226. doi: 10.1159/000357207. [DOI] [PubMed] [Google Scholar]
- 20.Johnston C, et al. Pain in Canadian NICUs: Have we improved over the past 12 years? Clin. J. Pain. 2011;27(3):225–232. doi: 10.1097/AJP.0b013e3181fe14cf. [DOI] [PubMed] [Google Scholar]
- 21.Allegaert K, Van Den Anker JN. Neonatal pain management: Still in search of the Holy Grail. Int. J. Clin. Pharmacol. Ther. 2016;54(7):514. doi: 10.5414/CP202561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carbajal R, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300(1):60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 23.Hartley C, et al. Analgesic efficacy and safety of morphine in the Procedural Pain in Premature Infants (Poppi) study: Randomised placebo-controlled trial. The Lancet. 2018;392(10164):2595–2605. doi: 10.1016/S0140-6736(18)31813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poisbeau P, Grinevich V, Charlet A. Behavioral Pharmacology of Neuropeptides: Oxytocin. Springer; 2017. Oxytocin signaling in pain: Cellular, circuit, system, and behavioral levels; pp. 193–211. [DOI] [PubMed] [Google Scholar]
- 25.Piira T, et al. The role of parental presence in the context of children's medical procedures: A systematic review. Child Care Health Dev. 2005;31(2):233–243. doi: 10.1111/j.1365-2214.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 26.Pillai Riddell RR, et al. Cochrane review: Non-pharmacological management of infant and young child procedural pain. Evidence Based Child Health A Cochrane Rev. J. 2012;7(6):1905–2121. doi: 10.1002/ebch.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cignacco E, et al. The efficacy of non-pharmacological interventions in the management of procedural pain in preterm and term neonates: A systematic literature review. Eur. J. Pain. 2007;11(2):139–152. doi: 10.1016/j.ejpain.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Mooncey S, et al. The effect of mother-infant skin-to-skin contact on plasma cortisol and β-endorphin concentrations in preterm newborns. Infant Behav. Dev. 1997;20(4):553–557. doi: 10.1016/S0163-6383(97)90045-X. [DOI] [Google Scholar]
- 29.Johnston, C. et al. Skin‐to‐skin care for procedural pain in neonates. Cochrane Database Syst. Rev. (2), CD008435. 10.1002/14651858.CD008435.pub3 (2017). [DOI] [PMC free article] [PubMed]
- 30.Nishitani S, et al. The calming effect of a maternal breast milk odor on the human newborn infant. Neurosci. Res. 2009;63(1):66–71. doi: 10.1016/j.neures.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Baudesson de Chanville A, et al. Analgesic effect of maternal human milk odor on premature neonates: A randomized controlled trial. J. Hum. Lact. 2017;33(2):300–308. doi: 10.1177/0890334417693225. [DOI] [PubMed] [Google Scholar]
- 32.Jones L, et al. The impact of parental contact upon cortical noxious‐related activity in human neonates. Eur. J. Pain. 2020;25(1):149–159. doi: 10.1002/ejp.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filippa M, Kuhn P, Westrup B. Early Vocal Contact and Preterm Infant Brain Development. New York: Springer; 2017. [Google Scholar]
- 34.Filippa M, et al. Live maternal speech and singing have beneficial effects on hospitalized preterm infants. Acta Paediatr. 2013;102(10):1017–1020. doi: 10.1111/apa.12356. [DOI] [PubMed] [Google Scholar]
- 35.Caskey M, et al. Adult talk in the NICU with preterm infants and developmental outcomes. Pediatrics. 2014;133(3):e578–e584. doi: 10.1542/peds.2013-0104. [DOI] [PubMed] [Google Scholar]
- 36.Pineda RG, et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J. Pediatrics. 2014;164(1):52–60. doi: 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filippa M, et al. Live maternal speech and singing increase self-touch and eye-opening in preterm newborns: A preliminary study. J. Nonverbal Behav. 2020;44:453–473. doi: 10.1007/s10919-020-00336-0. [DOI] [Google Scholar]
- 38.Filippa M, Monaci MG, Grandjean D. Emotion attribution in nonverbal vocal communication directed to preterm infants. J. Nonverbal Behav. 2019;43(1):91–104. doi: 10.1007/s10919-018-0288-1. [DOI] [Google Scholar]
- 39.Filippa M, et al. Changes in infant-directed speech and song are related to preterm infant facial expression in the neonatal intensive care unit. Interact. Stud. 2018;19(3):427–444. doi: 10.1075/is.16019.fil. [DOI] [Google Scholar]
- 40.Saliba S, et al. Fathers’ and mothers’ infant directed speech influences preterm infant behavioral state in the NICU. J. Nonverbal Behav. 2020;44(4):437–451. doi: 10.1007/s10919-020-00335-1. [DOI] [Google Scholar]
- 41.Eliava M, et al. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron. 2016;89(6):1291–1304. doi: 10.1016/j.neuron.2016.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zinni M, et al. Modulating the oxytocin system during the perinatal period: A new strategy for neuroprotection of the immature brain? Front. Neurol. 2018;9:229. doi: 10.3389/fneur.2018.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker SC, et al. C-tactile afferents: Cutaneous mediators of oxytocin release during affiliative tactile interactions? Neuropeptides. 2017;64:27–38. doi: 10.1016/j.npep.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Theofanopoulou C, Boeckx C, Jarvis ED. A hypothesis on a role of oxytocin in the social mechanisms of speech and vocal learning. Proc. R. Soc. B Biol. Sci. 1861;2017(284):20170988. doi: 10.1098/rspb.2017.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tops M, et al. Oxytocin receptor gene associated with the efficiency of social auditory processing. Front. Psych. 2011;2:60. doi: 10.3389/fpsyt.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burkett JP, et al. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351(6271):375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziabreva I, et al. Separation-induced receptor changes in the hippocampus and amygdala of Octodon degus: Influence of maternal vocalizations. J. Neurosci. 2003;23(12):5329–5336. doi: 10.1523/JNEUROSCI.23-12-05329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braun S, Scheich H. Influence of experience on the representation of the “mothering call” in frontoparietal and auditory cortex of pups of the rodent Octodon degus: FDG mapping. J. Comp. Physiol. A. 1997;181(6):697–709. doi: 10.1007/s003590050151. [DOI] [PubMed] [Google Scholar]
- 49.Poeggel G, Braun K. Early auditory filial learning in degus (Octodon degus): Behavioral and autoradiographic studies. Brain Res. 1996;743(1–2):162–170. doi: 10.1016/S0006-8993(96)01039-6. [DOI] [PubMed] [Google Scholar]
- 50.Braun K, et al. Influence of parental deprivation on the behavioral development in Octodon degus: Modulation by maternal vocalizations. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2003;42(3):237–245. doi: 10.1002/dev.10096. [DOI] [PubMed] [Google Scholar]
- 51.Veenema AH. Toward understanding how early-life social experiences alter oxytocin-and vasopressin-regulated social behaviors. Horm. Behav. 2012;61(3):304–312. doi: 10.1016/j.yhbeh.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Lukas M, et al. Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology. 2010;58(1):78–87. doi: 10.1016/j.neuropharm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 53.Champagne F, et al. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav. Neurosci. 2007;121(6):1353. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- 55.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24(1):1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 56.Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 2000;12(12):1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 57.Feldman R, et al. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010;35(8):1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Chisholm, J. S. et al.Early Stress: Perspectives from Developmental Evolutionary Ecology. 2005.
- 59.Antonucci, L. A. et al.The Interaction Between OXTR rs2268493 and Perceived Maternal Care is Associated With Amygdala–Dorsolateral Prefrontal Effective Connectivity During Explicit Emotion Processing 1–13 (European Archives of Psychiatry and Clinical Neuroscience, 2019). [DOI] [PubMed]
- 60.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cong X, et al. Parental oxytocin responses during skin-to-skin contact in pre-term infants. Early Hum. Dev. 2015;91(7):401–406. doi: 10.1016/j.earlhumdev.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 62.Vittner D, et al. Increase in oxytocin from skin-to-skin contact enhances development of parent–infant relationship. Biol. Res. Nurs. 2018;20(1):54–62. doi: 10.1177/1099800417735633. [DOI] [PubMed] [Google Scholar]
- 63.González-Hernández A, et al. Peripheral oxytocin receptors inhibit the nociceptive input signal to spinal dorsal horn wide-dynamic-range neurons. Pain. 2017;158(11):2117–2128. doi: 10.1097/j.pain.0000000000001024. [DOI] [PubMed] [Google Scholar]
- 64.Matthiesen AS, et al. Postpartum maternal oxytocin release by newborns: Effects of infant hand massage and sucking. Birth. 2001;28(1):13–19. doi: 10.1046/j.1523-536x.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 65.Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary adrenal axis in male and female rats: Partial action within the paraventricular nucleus. J. Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 66.Mazzuca M, Minlebaev M, Shakirzyanova A, Tyzio R, Taccola G, Janackova S, Khazipov R. Newborn analgesia mediated by oxytocin during delivery. Front. Cell. Neurosci. 2011;5:3. doi: 10.3389/fncel.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boll S, De Minas AA, Raftogianni A, Herpertz SC, Grinevich V. Oxytocin and pain perception: From animal models to human research. Neuroscience. 2018;387:149–161. doi: 10.1016/j.neuroscience.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 68.Bushnell, M. C., & Apkarian, A. V. Representation of pain in the brain. In Wall and Melzack’s Textbook of Pain, 5th edition 107–124 (Elsevier, 2006).
- 69.Rash JA, Aguirre-Camacho A, Campbell TS. Oxytocin and pain: A systematic review and synthesis of findings. Clin. J. Pain. 2014;30(5):453–462. doi: 10.1097/AJP.0b013e31829f57df. [DOI] [PubMed] [Google Scholar]
- 70.Yang J. Intrathecal administration of oxytocin induces analgesia in low back pain involving the endogenous opiate peptide system. Spine. 1994;19(8):867–871. doi: 10.1097/00007632-199404150-00001. [DOI] [PubMed] [Google Scholar]
- 71.Louvel D, Delvaux M, Felez A, Fioramonti J, Bueno L, Lazorthes Y, et al. Oxytocin increases thresholds of colonic visceral perception in patients with irritable bowel syndrome. Gut. 1996;39:741–747. doi: 10.1136/gut.39.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang YL, Yuan Y, Yang J, Wang CH, Pan YJ, Lu L, et al. The interaction between the oxytocin and pain modulation in headache patients. Neuropeptides. 2013;47:93–97. doi: 10.1016/j.npep.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 73.Herpertz SC, Bertsch K. A new perspective on the pathophysiology of borderline personality disorder: A model of the role of oxytocin. Am. J. Psychiatry. 2015;172:840–851. doi: 10.1176/appi.ajp.2015.15020216. [DOI] [PubMed] [Google Scholar]
- 74.Wigton R, Radua J, Allen P, Averbeck B, Meyer-Lindenberg A, McGuire P, et al. Neurophysiological effects of acute oxytocin administration: Systematic review and meta-analysis of placebo-controlled imaging studies. J. Psychiatry Neurosci. 2015;40:E1–E22. doi: 10.1503/jpn.130289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tracy LM, Georgiou-Karistianis N, Gibson SJ, Giummarra MJ. Oxytocin and the modulation of pain experience: Implications for chronic pain management. Neurosci. Biobehav. Rev. 2015;55:53–67. doi: 10.1016/j.neubiorev.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 76.Neumann ID. Involvement of the brain oxytocin system in stress coping: Interactions with the hypothalamo-pituitary-adrenal axis. Prog. Brain Res. 2002;139:147–162. doi: 10.1016/S0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- 77.Moberg KU, Prime DK. Oxytocin effects in mothers and infants during breastfeeding. Infant. 2013;9(6):201–206. [Google Scholar]
- 78.Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114(1):e77–e84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grunau RE, et al. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J. Pediatr. 2007;150(2):151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuhn P, et al. Infants born very preterm react to variations of the acoustic environment in their incubator from a minimum signal-to-noise ratio threshold of 5 to 10 dBA. Pediatr. Res. 2012;71(1):386–392. doi: 10.1038/pr.2011.76. [DOI] [PubMed] [Google Scholar]
- 81.White RD, Smith JA, Shepley MM. Recommended standards for newborn ICU design. J. Perinatol. 2013;33(1):S2–S16. doi: 10.1038/jp.2013.10. [DOI] [PubMed] [Google Scholar]
- 82.Stevens B, et al. Premature infant pain profile: Development and initial validation. Clin. J. Pain. 1996;12(1):13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 83.Team, R. C. R: A Language and Environment for Statistical Computing (2013).
- 84.Maestripieri D, Lindell SG, Higley JD. Intergenerational transmission of maternal behavior in rhesus macaques and its underlying mechanisms. Dev. Psychobiol. 2007;49(2):165–171. doi: 10.1002/dev.20200. [DOI] [PubMed] [Google Scholar]
- 85.Carbajal R, Gall O, Annequin D. Pain management in neonates. Expert Rev. Neurother. 2004;4(3):491–505. doi: 10.1586/14737175.4.3.491. [DOI] [PubMed] [Google Scholar]
- 86.Westrup B, Sizun J, Lagercrantz H. Family-centered developmental supportive care: A holistic and humane approach to reduce stress and pain in neonates. J. Perinatol. 2007;27(1):S12–S18. doi: 10.1038/sj.jp.7211724. [DOI] [Google Scholar]
- 87.Melnyk BM, et al. Reducing premature infants' length of stay and improving parents' mental health outcomes with the Creating Opportunities for Parent Empowerment (COPE) neonatal intensive care unit program: A randomized, controlled trial. Pediatrics. 2006;118(5):e1414–e1427. doi: 10.1542/peds.2005-2580. [DOI] [PubMed] [Google Scholar]
- 88.Filippa M, et al. Effects of early vocal contact in the neonatal intensive care unit: Study protocol for a multi-centre, randomised clinical trial. Int. J. Environ. Res. Public Health. 2021;18(8):3915. doi: 10.3390/ijerph18083915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moultrie F, et al. A universal right to pain relief: Balancing the risks in a vulnerable patient population. Lancet Child Adolescent Health. 2019;3(2):62–64. doi: 10.1016/S2352-4642(18)30269-4. [DOI] [PubMed] [Google Scholar]