Abstract
Eating behavior varies greatly between individuals, but the neurobiological basis of these trait-like differences in feeding remains poorly understood. Central μ-opioid receptors (MOR) and cannabinoid CB1 receptors (CB1R) regulate energy balance via multiple neural pathways, promoting food intake and reward. Because obesity and eating disorders have been associated with alterations in the brain’s opioid and endocannabinoid signaling, the variation in MOR and CB1R system function could potentially underlie distinct eating behavior phenotypes. In this retrospective positron emission tomography (PET) study, we analyzed [11C]carfentanil PET scans of MORs from 92 healthy subjects (70 males and 22 females), and [18F]FMPEP-d2 scans of CB1Rs from 35 subjects (all males, all also included in the [11C]carfentanil sample). Eating styles were measured with the Dutch Eating Behavior Questionnaire (DEBQ). We found that lower cerebral MOR availability was associated with increased external eating—individuals with low MORs reported being more likely to eat in response to environment’s palatable food cues. CB1R availability was associated with multiple eating behavior traits. We conclude that although MORs and CB1Rs overlap anatomically in brain regions regulating food reward, they have distinct roles in mediating individual feeding patterns. Central MOR system might provide a pharmacological target for reducing individual’s excessive cue-reactive eating behavior.
Subject terms: Molecular neuroscience, Physiology
Introduction
Obesity is one of the leading public health issues, resulting from individuals’ long-term excessive energy intake in relation to energy expenditure [1]. Yet, humans vary greatly in their choices and habits related to food intake quantity and quality i.e., eating behavior [2, 3]. Trait-like eating behaviors have been associated with multiple clinical eating disorders in addition to obesity [4–7], but also nonobese individuals vary in how they control their feeding [8]. Interacting with peripheral hormones, central nervous system (CNS) integrates hunger and satiety signals with environmental stimuli to regulate food intake [1]. Large-scale genome-wide association studies have identified limbic system, hippocampus and hypothalamus to be key regions in the CNS contributing to individual’s body mass index (BMI) and eating behavior [9, 10]. Central regulation of feeding is however constantly challenged by the modern environment characterized by abundance of palatable and energy-dense food products, promoting feeding independently of metabolic needs [11, 12]. The prevalence of obesity is increasing in alarming speed, and new targets for anti-obesity pharmacotherapy are acutely needed [13].
Palatability and hedonic properties of food are centrally mediated by μ-opioid receptor (MOR) system [14, 15]. Both endogenous and exogenous opioids stimulate feeding, especially via hedonic hotspots of nucleus accumbens, insula and frontal cortex [16–19]. Conversely, opioid antagonists reduce food intake and related hedonic responses in rodents [19] and humans [20, 21]. Central MORs are also important mediators of homeostatic feeding, even in the absence of subjective pleasure [22]. Human positron emission tomography (PET) studies have revealed that obesity associates with decrease of MORs in appetite regulating brain areas [23, 24], and insular MORs are lowered in patients with bulimia nervosa proportionally to fasting behavior [25]. Central MOR system function varies considerably also in healthy humans [26], and traits linked with feeding control such as impulsivity are associated with MOR availability [27]. Nevertheless, the association between the MOR system and specific patterns of eating behavior remains elusive.
Feeding is also regulated by brain’s endocannabinoid system, which anatomically overlaps with MORs in the central reward circuit [28]. The most abundant central cannabinoid receptors are the CB1 receptors (CB1Rs), which regulate food intake through pathways of ventral striatum, limbic system, and hypothalamus [29, 30]. Functional interplay between MOR and CB1R systems has been established in animal studies, where CB1R-antagonists and MOR-antagonists have synergistic effect on reducing food intake [31], while CB1R-antagonist can be used to block MOR-agonist induced food intake and vice versa [32]. MOR-agonists also directly increase endocannabinoid concentration and CB1R-agonists increase opioid concentration in the brain [33, 34]. In humans with food intake disorders including obesity, anorexia and bulimia nervosa, lowered central CB1R availability in the mesolimbic reward system associates with increased BMI [35]. While CB1R-antagonist rimonabant showed promise as an anti-obesity drug, it had to be withdrawn due to psychiatric side effects [36]. More nuanced understanding of CB1R system and feeding is clearly required to enable further pharmacological advancement.
Variation in central MOR and CB1R function could thus be linked to differences in feeding behavior, but it remains unresolved what specific feeding traits they govern in humans. Individual differences in feeding can be conceptualized based on the psychological mechanisms that contribute to or attenuate development of overweight. In such conceptualization, emotional eating refers to reactive overeating to distress or negative emotions, while external eating refers to tendency to overeat in response to appetitive food-cues. Finally, restrained eating refers to the tendency to eat less than desired [37–39]. Variation in such trait-like feeding patterns contribute to differences in weight gain and maintenance [37, 40], and they can be measured using The Dutch Eating Behavior Questionnaire (DEBQ) [41]. In this retrospective study utilizing PET scans from historical healthy controls, we compiled 92 [11C]carfentanil scans of MOR system and 35 [18F]FMPEP-d2 scans of CB1R system and corresponding DEBQ scores. We tested whether the MOR and CB1R availabilities in food-intake-regulating brain areas associate with individual eating behavior traits measured with DEBQ.
Materials and methods
Subjects
The study subjects were historical controls retrieved from the AIVO neuroinformatics database (http://aivo.utu.fi), a large-scale molecular image database hosted by Turku PET Centre. We identified all the [11C]carfentanil and [18F]FMPEP-d2 baseline PET studies accompanied with completed Finnish version of the DEBQ form [41]. Exclusion criteria were neurologic and psychiatric disorders, current use of medications that could affect CNS or abuse of alcohol or illicit drugs. Subjects were not preselected on the basis of weight or eating habits. Final sample consisted of 92 subjects (70 males and 22 females) scanned with [11C]carfentanil from five distinct projects with three different PET scanners. The [18F]FMPEP-d2 sample consisted of 35 males, all of which were also all included in the [11C]carfentanil male sample. All subjects of the [18F]FMPEP-d2 subsample were nonsmoking males, while in the [11C]carfentanil sample seven females smoked. All [18F]FMPEP-d2 scans were carried out with GE Discovery VCT PET/CT (GE Healthcare). The original data were acquired between 2011 and 2019 in the Turku PET Centre (Turku, Finland). The subjects had completed the DEBQ form on the day of the scanning visit or on the preceding screening day. Characteristics of the study sample are summarized in Table 1, and the information of smoking status and PET scanners are detailed in Supplementary Table 1. The study was conducted in accordance with the Declaration of Helsinki and approved by the Turku University Hospital Clinical Research Services. The subjects had signed written informed consent forms and completed the DEBQ forms as a part of the original study protocols. The references for the original studies are provided in Supplementary Table 2. a priori power analysis based on our prior neuroreceptor PET studies on obesity [24] suggested that a sample size of 32 would be sufficient for establishing the predicted effects of r = 0.5 at power of 0.95.
Table 1.
Characteristics of the studied subjects.
| [11C]carfentanil scans | p value | ||||||
|---|---|---|---|---|---|---|---|
| Males (n = 70) | Females (n = 22) | ||||||
| Mean | SD | Range | Mean | SD | Range | ||
| Age (years) | 27.4 | 7.5 | 19–58 | 47.7 | 10.0 | 20–59 | <0.001 |
| BMI (kg/m2) | 24.5 | 2.8 | 19–31 | 23.7 | 3.1 | 18–31 | 0.27 |
| Total DEBQ score | 67.0 | 12.8 | 40–109 | 73.4 | 12.8 | 46–97 | 0.05 |
| Emotional eating score | 21.0 | 6.8 | 13–40 | 22.1 | 5.7 | 13–32 | 0.44 |
| External eating score | 24.7 | 6.5 | 10–43 | 25.0 | 5.4 | 13–34 | 0.82 |
| Restrained eating score | 21.3 | 5.5 | 11–39 | 26.2 | 5.5 | 10–33 | 0.001 |
| Injected activity (MBq) | 277.0 | 77.9 | 223–508 | 352.3 | 125.5 | 234–519 | 0.01 |
| [18F]FMPEP-d2 scans | |||||||
| Males (n = 35) | |||||||
| Mean | SD | Range | |||||
| Age (years) | 25.9 | 4.3 | 21–35 | ||||
| BMI (kg/m2) | 24.5 | 3.1 | 19–31 | ||||
| Total DEBQ score | 68.6 | 14.5 | 43–109 | ||||
| Emotional eating score | 20.7 | 7.4 | 13–40 | ||||
| External eating score | 27.1 | 6.0 | 14–43 | ||||
| Restrained eating score | 20.8 | 5.6 | 12–32 | ||||
| Injected activity (MBq) | 187.9 | 12.8 | 147–215 | ||||
p value is for two-tailed independent samples t-test between males and females.
Eating behavior assessment with the DEBQ
The DEBQ [41] was used to quantify eating behavior. The DEBQ is a 33-item questionnaire with Likert-type scoring in each item (response options ranging from 1 to 5, from “Never” to “Very often”). It is divided in three dimensions measuring different behavioral traits: Emotional eating, External eating, and Restrained eating [37–39]. The emotional and external overeating are based on psychosomatic and externality theories of eating behavior, while restrained eating dimension centers around food intake self-inhibition [41]. The DEBQ subscales have been designed to measure independent dimensions of feeding behavior [42], and the subscales have good internal consistency, dimensional validity, and test-retest reliability [4, 7, 41, 43].
Image processing and modeling
PET images were preprocessed similarly using automated processing pipeline Magia [44]. [11C]carfentanil data preprocessing has been described previously [26]. Briefly, preprocessing consisted of framewise realignment and coregistration of the PET and magnetic resonance images (MRIs). MOR availability was expressed as [11C]carfentanil binding potential (BPND), which is the ratio of specifically bound radioligand to that of nondisplaceable radioligand in tissue [45]. BPND was estimated with simplified reference tissue model [46]. Occipital cortex served as the reference region, since it contains only negligible number of opioid receptors and yields reliable and reproducible reference estimates [47–49]. Parametric BPND images were calculated and spatially normalized to MNI-space via segmentation of T1-weighted MRIs and smoothed with an 8 mm Gaussian kernel. For [18F]FMPEP-d2, there exists no suitable central reference region (i.e., a region without CB1Rs)—thus, CB1R availability is expressed as the [18F]FMPEP-d2 volume of distribution (VT) [50]. [18F]FMPEP-d2 VT was quantified using graphical analysis by Logan [51]. The frames starting at 36 min and later since injection were used in the model fitting, since Logan plots became linear after 36 min [51]. Plasma activities were corrected for plasma metabolites as described previously [52]. Further details of the scan acquisition and modeling of the [18F]FMPEP-d2 data are described in Supplementary Text 1.
Statistical analysis
The study question was whether the DEBQ subscales (Emotional eating, External eating, Restrained eating) or Total DEBQ scores are associated with [11C]carfentanil BPND or [18F]FMPEP-d2 VT. We used Bayesian hierarchical modeling to analyze these effects in a priori regions of interest (ROIs). We targeted regions with high to moderate density of MORs [26, 53], and with previously proposed roles in obesity [24], food intake disorders [25, 54], and food reward [55–57]. The analyses were harmonized by investigating CB1Rs in the same regions. FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) was used to extract the ten bilateral ROIs: amygdala, caudatus, cerebellum, dorsal anterior cingulate cortex, insula, middle temporal cortex, nucleus accumbens, orbitofrontal cortex, putamen, and thalamus. The Bayesian models were estimated using the R package brms (https://cran.r-project.org/web/packages/brms/index.html) that utilizes the Markov chain Monte Carlo sampling of RStan (https://mc-stan.org/users/interfaces/rstan). Because age influences [11C]carfentanil binding [26, 58] and different PET scanners may yield slightly different BPND estimates [59], both age and PET scanner were controlled for in all [11C]carfentanil models. Age was also controlled for in all [18F]FMPEP-d2 VT models (the scanner-adjustment was not needed since the [18F]FMPEP-d2 data were acquired using a single scanner). For both tracers, we created models separately for the Total DEBQ score as well as its subscales, adjusting for age. [11C]carfentanil BPND and [18F]FMPEP-d2 VT were log-transformed to improve model fit [26]. For both tracers, we estimated varying (random) intercepts for the subjects and ROIs, and varying (random) slopes across ROIs for the predictor of interest (e.g., Total DEBQ score) and age. Compared to a model where the regionally specific effects would be estimated using interaction term for ROI, the hierarchical model produces results that are partially pooled toward each other, thus accounting for the multiple comparisons performed [60]. For both tracers, we also estimated regionally varying (random) residual variances. For [11C]carfentanil data, we also estimated regionally varying (random) intercepts for the scanners. We used the standard normal distribution as a prior distribution for the regression coefficients of the predictors to provide regularization. The standard normal distribution was also used as the prior distribution for the standard deviation of the group-level (random) effects. Otherwise we used the default priors of brms. We used 1000 warmup samples, 1000 post-warmup samples and 10 chains, thus totaling 10,000 post-warmup samples. The sampling parameters were slightly modified to facilitate convergence (adapt_delta = 0.999; max_treedepth = 20). The samplings produced no divergent iterations and the Rhats were all 1.0, suggesting that the chains converged successfully.
To examine associations in the whole brain, we used nonparametric approach with SnPM13 (http://nisox.org/Software/SnPM13/) to create full-volume linear regression models for BPND and VT values. We used p < 0.01 as the cluster-defining threshold, and only report clusters large enough to be statistically significant at FWE p < 0.05. 5000 permutations were used to estimate the null distribution. We created distinct models for Total DEBQ score and all the subscale scores, adjusting for age and also for PET scanner in [11C]carfentanil models. The PET scanner was entered in the models as a covariate. Based on our earlier large-scale [11C]carfentanil data analysis, BMI in the current study range (18–31) is not associated with MOR availability [26]. However, to rule out the possible effects of sex, smoking and also BMI, we replicated the [11C]carfentanil full volume analysis with these additional covariates. The [18F]FMPEP-d2 models were also replicated with BMI as additional covariate (there were no smokers or females in the [18F]FMPEP-d2 data).
Results
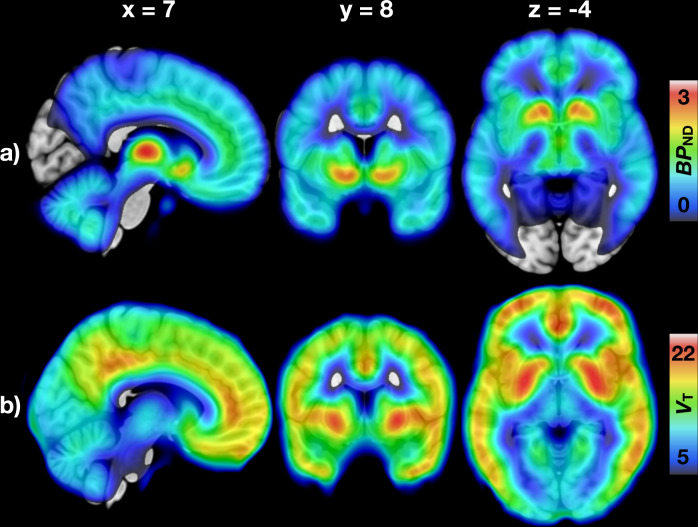
Mean distribution of MORs and CB1Rs (Fig. 1) was consistent with previous studies [26, 50, 53, 61]. Correlations between the DEBQ subscales were positive but only modest, strongest being between Emotional and External eating (r = +0.33). BMI had a significant correlation only with Restrained eating (r = +0.27). Correlations with p values are presented in Supplementary Fig. 1 and Supplementary Table 3.
Fig. 1. Mean distribution of central μ-opioid and CB1 receptors.
a Mean binding potential (BPND) of the 92 subjects (70 males and 22 females) studied with [11C]carfentanil. b Mean volume of distribution (VT) of the 35 males studied with [18F]FMPEP-d2.
Regional analysis of neuroreceptor availability and eating behavior
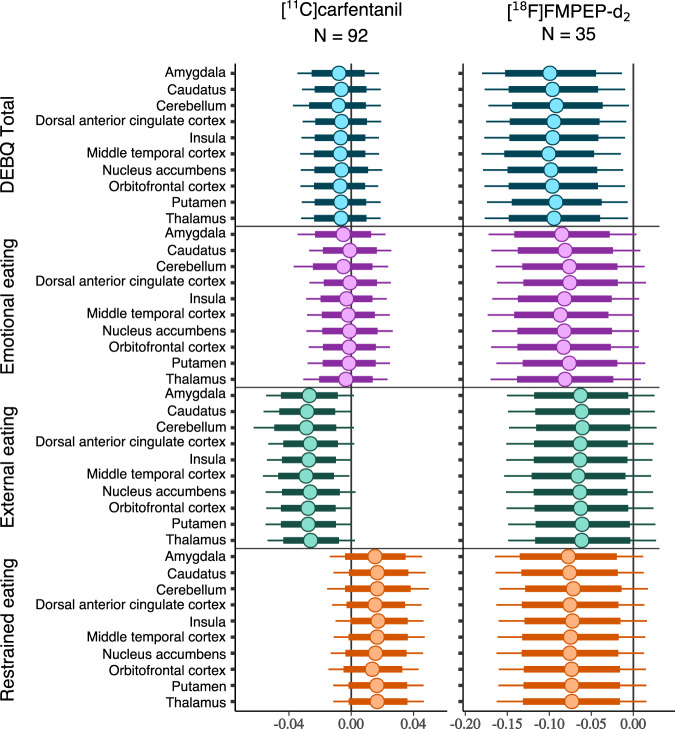
Higher External eating score was associated with lower [11C]carfentanil BPND in all a priori ROIs (Fig. 2). In the [11C]carfentanil models with other DEBQ subscales and Total DEBQ, the 80% confidence intervals overlapped with zero. For [18F]FMPEP-d2, higher Total DEBQ score was associated with lower VT all examined ROIs (Fig. 2). The association directions between VT and all DEBQ subscales were negative, but the 95% confidence intervals overlapped with zero. Complementary visualization of the regional relationships between DEBQ scores and neuroreceptor availability in representative ROIs is presented in Supplementary Fig. 2. In the subsample of 35 males with both [11C]carfentanil and [18F]FMPEP-d2 PET data, there were no significant regional correlations between MOR and CB1R availabilities (Supplementary Fig. 3).
Fig. 2. Regional associations of Total DEBQ and subscale scores with μ-opioid and CB1 receptor availabilities.
The figure shows posterior distributions of the regression coefficients for Total DEBQ and subscale scores on log-transformed [11C]carfentanil binding potential (BPND) and [18F]FMPEP-d2 volume of distribution (VT) in ten regions of interest. Age (and PET scanner for [11C]carfentanil data) are controlled as covariates. The colored circles represent posterior means, the thick horizontal bars 80% posterior intervals, and the thin horizontal bars 95% posterior intervals.
Full volume analysis of central receptor availability and eating behavior
For both tracers, full volume results were consistent with the ROI models. Mean receptor distribution maps and statistically significant DEBQ association maps can be found at NeuroVault (https://neurovault.org/collections/RZFLYXTL/).
μ-opioid receptor availability and DEBQ
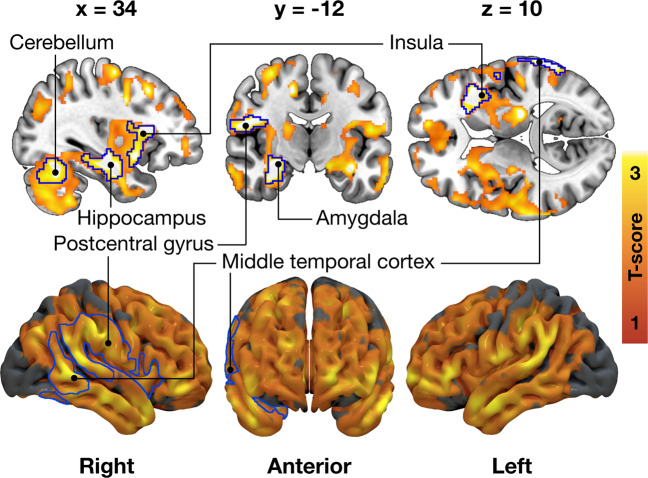
Higher External eating score was associated with lower [11C]carfentanil BPND in multiple brain areas (Fig. 3). Strongest cerebral associations were found in the right frontotemporal cortex and insula (peak voxel coordinates in Supplementary Table 4). Associations with Total DEBQ or other subscale scores were not statistically significant. Complementary analyses of [11C]carfentanil are presented in Supplementary Text 2 and Supplementary Fig. 4. In general, results were similar when additionally controlling for smoking, sex, and BMI. There were no significant associations in the female subsample, likely due to limited statistical power.
Fig. 3. Association between External eating and decreased μ-opioid receptor availability in the 92 subjects (70 males and 22 females) scanned with [11C]carfentanil.
The blue outline marks brain regions where lower [11C]carfentanil binding potential (BPND) associated with higher External eating score, age and PET scanner as nuisance covariates, cluster forming threshold p < 0.01, FWE corrected. In the red–yellow T-score scale shown are also additional bilateral associations significant with more lenient cluster-defining threshold (p < 0.05, FWE corrected) for visualization purposes.
CB1 receptor availability and DEBQ
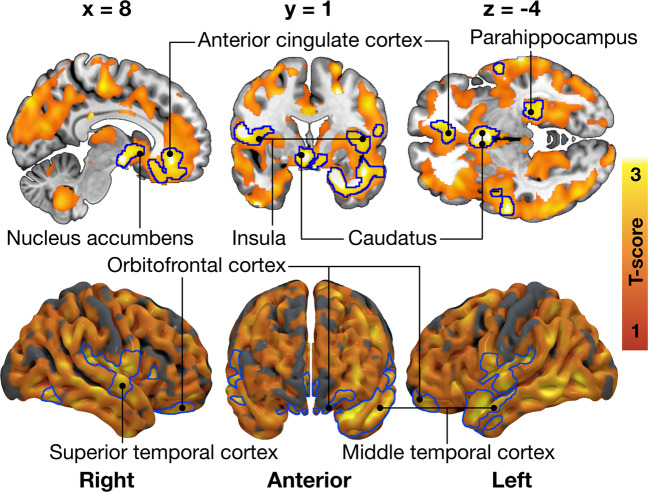
Higher Total DEBQ score was associated with lower [18F]FMPEP-d2 VT bilaterally in multiple brain regions (Fig. 4). Most prominent associations were found in parahippocampus, frontal striatum, insula, anterior cingulate, and frontotemporal cortices (peak voxel coordinates in Supplementary Table 4). Full-volume associations with distinct DEBQ subscales and VT were not statistically significant. Results were essentially the same when controlling for BMI.
Fig. 4. Total Dutch Eating Behavior Questionnaire (DEBQ) score associated with decreased CB1 receptor availability in the 35 males scanned with [18F]FMPEP-d2.
The blue outline marks brain regions where lower [18F]FMPEP-d2 volume of distribution (VT) associated with higher Total DEBQ score, age as a nuisance covariate, cluster forming threshold p < 0.01, FWE corrected. In the red–yellow T-score scale shown are also additional associations significant with more lenient cluster-defining threshold (p < 0.05, FWE corrected) for visualization purposes.
Discussion
Our main finding was that higher DEBQ scores were associated with lower central availability of MORs and CB1Rs in healthy, nonobese humans. MOR and CB1R systems however showed distinct patterns of associations with specific dimensions of self-reported eating: While CB1Rs were associated in general negatively with different DEBQ subscale scores (and most saliently with the Total DEBQ score), MORs were specifically and negatively associated with externally driven eating only. Our results support the view that variation in endogenous opioid and endocannabinoid systems explain interindividual variation in feeding, with distinct effects on eating behavior measured with DEBQ.
Central μ-opioid receptors and external eating behavior
External eating—the tendency to feed when encountering palatable food cues such as advertisements—was associated with lowered MOR availability in multiple brain areas, including insula, cortico-limbic regions and striatum, which are major areas processing environmental food cues and mediating reward [62]. A bulk of studies have shown that these regions are activated by mere perception of food cues or anticipation of feeding [63–65], and our recent work shows that lowered MOR availability is associated with amplified hemodynamic responses to food images in the same regions [15]. Higher score on external eating is associated with increased food craving [66] and cue-induced palatable food intake [38, 67], and may also contribute to short-term weight gain [40]. Altogether these results suggest that central MOR system has an important role in modulating particularly this kind of impulsive feeding that may lead to overweight.
Previous PET studies have established that feeding triggers endogenous opioid release in humans [22, 23]. Binge eating disorder (BED) is accompanied with downregulated central MORs and high External and Emotional eating scores [68]. Morbid obesity is also associated with lowered central MOR availability [23, 24], possibly reflecting receptor downregulation due to repeated overstimulation following feeding. In minipigs, already 12 days of high sucrose intake and following central endogenous neurotransmitter release downregulates MORs in cingulate and prefrontal cortices, nucleus accumbens and elsewhere in striatum [69]. The present findings extend the role of MORs in obesity and eating disorders to different feeding patterns in healthy subjects.
Healthy humans vary considerably in central MOR availability [26], and it is also possible that lowered MOR availability constitutes a genetically determined [70] risk factor for externally driven eating behavior. In healthy humans, trait impulsivity is associated with central MOR availability [27]. Increased cue-reactivity is prevalent feature of behavioral addictions [71], and patients with BED and pathological gambling have reduced availability of central MORs as measured with in vivo PET [54]. It is thus possible that subjects with lower MOR availability are susceptible for increased external eating in modern environment where they are consistently bombarded with feeding cues in advertisements and food shelves in supermarkets [11]. However, the present data are purely cross-sectional and longitudinal human studies are needed to further disentangle the causes and the effects between the decrease of MORs in relation to external eating.
The association with decreased MORs and high External eating scores was observed in all a priori ROIs. This was partly expected, since it is known that MOR densities exhibit high regional autocorrelation [72], and also in morbidly obese subjects, MOR availability is decreased globally [24]. These data suggest that the mechanism leading to decreased MOR availability affects the brain in a widespread manner. Preclinical research has found that exogenous MOR-agonists stimulate feeding [20], and in obese mice, central concentration of endogenous MOR-agonist (beta-endorphin) is increased manifold compared to controls [73]. Thus, one mechanism potentially leading to excessive external eating and compensatory MOR downregulation [74] could be chronically elevated basal endogenous opioidergic tone. Accordingly, the only available anti-obesity drug directly targeting opioid pathways is a combination of naloxone/bupropion, which blocks central beta-endorphin messaging and leads to stimulation of anorexigenic pathways [75, 76]. Although leading to 5–10% weight loss on average, the drug has major side effects [75]. Thus, it might be important to utilize also other types of pharmacological strategies. For example, in other conditions with chronically elevated opioidergic tone (such as tolerance following opioid abuse), ultra-low dose antagonists have been used successfully to restore MOR-mediated analgesic messaging [77, 78]. Whether these strategies might be applicable also in the treatment of obesity and externally-oriented feeding behavior is to be examined in future studies.
Central CB1 receptors and eating behavior
Higher Total DEBQ score associated with lower availability of central CB1Rs, and ROI-level modeling suggested a consistent negative association with all DEBQ subscales. Compared with the [11C]carfentanil model, wider posterior distributions reflect the uncertainty arising from smaller [18F]FMPEP-d2 sample size. Brain’s endocannabinoid system is a major homeostatic signaling system, with CB1Rs abundant in all known food intake regulating central regions [79]. In previous brain PET studies, similarly lowered CB1R availability has been associated with increased BMI [35, 80], while globally upregulated CB1Rs have been found in anorexia nervosa [81]. These opposite phenotypes on body adiposity spectrum could potentially result from corresponding alterations from CB1R-regulated food intake behaviors. Indeed, stimulation of CB1Rs by pharmacological agonists or endocannabinoids promotes food intake and amplifies the rewarding properties of feeding [82]. In contrast, antagonism of the CB1Rs by rimonabant (withdrawn anti-obesity drug, Acomplia) effectively reduces food intake and increases energy expenditure, but in many patients also induces psychiatric symptoms (e.g., depressive mood, suicidality, anxiety) [79]. Accordingly, the endocannabinoid system function has been connected to several other essential behavioral processes in addition to feeding (e.g., stress-coping, emotion regulation, pain perception) [83, 84]. Being this diverse and complex regulatory system, it may not be possible to pinpoint single distinct aspect of food intake behavior mediated by CB1Rs. Rather, our results add support to central CB1Rs role in regulation of multiple eating behavior traits, with implications on both homeostatic and hedonic feeding [85].
Limitations and methodological considerations
The [11C]carfentanil data were pooled from three PET scanners, which may produce minor variance in outcome measures [59]. However, this was accounted for in the analyses by adding the PET scanner as a nuisance covariate to all full-volume and regional analyses; the chosen outcome metric (BPND) is also robust against such variability. The sample studied with [11C]carfentanil consisted predominantly of males, and our statistical power was compromised for detecting potential sex differences. The male and female samples were not identical with regards of age and Restrained eating score, which is due to the limited availability of database subjects. The sex difference in the [11C]carfentanil dose pertains to the fact that more females compared to males were scanned with HRRT PET scanner, which requires higher tracer doses (Supplementary Table 1)—however, this was accounted for by controlling with the scanner in all analyses as described above. Also all subjects of the [18F]FMPEP-d2 subsample were males, and thus conclusions regarding CB1Rs might not be generalizable to females. Eating behavior was assessed by self-reports, rather than by direct observations. DEBQ has however been found to successfully identify clinically relevant eating styles [4, 5]. Our study had a cross-sectional design, and although we found evidence of eating behavior’s association with MOR and CB1R systems, whether these receptor systems’ alterations directly promote future weight gain is to be examined in longitudinal studies. Our study included subjects with the BMI 18–31, and the findings might not be applicable to severe obesity. However, previous human PET studies have established that morbid obesity and eating disorders characterized by increased BMI are associated with decreased availability of MORs [24, 54] and CB1Rs [35]. Additional studies have found that these clinical conditions are also associated with increased DEBQ scores [5, 68]. Our study shows that MORs and CB1Rs contribute to feeding behavior regulation in a wide BMI range and in both healthy and clinical populations. Finally, in a single PET scan it is not possible to determine the exact proportions for causal factors to the altered receptor availability, which could potentially be affected by changes in receptor density, affinity or endogenous ligand binding [74].
Conclusions
Low cerebral MOR availability is associated with increased externally triggered eating behavior. Modern obesogenic environment may promote food consumption via engaging the opioidergic link of the reward circuit whose tone is linked with cue-reactive eating. Our study suggests that for individuals with aberrant external eating, MOR system might provide a feasible pharmacological target to combat weight gain. Central CB1Rs are in turn associated with multiple eating behavioral traits measured with DEBQ, consistent with endocannabinoid system’s role as a major homeostatic regulatory system at the intersection of feeding, emotion and behavior.
Supplementary information
Acknowledgements
This work was supported by Academy of Finland grants (#332225, #304385, and #294897 to LN) and Sigrid Juselius Foundation. The work was also funded by Centre of Excellence of Cardiovascular and Metabolic Diseases supported by Academy of Finland (PN). We are grateful to Emil Aaltonen Foundation, Finnish Cultural Foundation (Southwest Finland Fund), and Jenny and Antti Wihuri Foundation for personal grants to TaK. We thank Turunmaa Duodecim Society, Turku University Hospital Foundation for Education and Research, and Jalmari and Rauha Ahokas Foundation for personal grants to LP. We are grateful to Academy of Finland (grant #256836) and Finnish Alcohol Research Foundation for personal grants to VK. Earlier version of the paper has been posted on preprint server bioRxiv (10.1101/2020.12.17.423284).
Author contributions
TaK: Corresponding and first author, study design, study coordination, data acquisition, data modeling, statistical analysis, interpretation of the results, tables and figures, main writer of the paper. ToK: Study design, data modeling, statistical analysis, interpretation of the results, figures, writing of the paper. LP: Data acquisition, interpretation of the results, writing of the paper. JI: Data acquisition, interpretation of the results, writing of the paper. KK: Interpretation of the results, writing of the paper. VK: Interpretation of the results, writing of the paper. JH: Data modeling, interpretation of the results, writing of the paper. PN: Study design, study coordination, interpretation of the results, writing of the paper. LN: Study design, study coordination, statistical analysis, interpretation of the results, figures, writing of the paper, supervision of the study.
Code availability
The code for preprocessing of the PET data (Magia) is available at https://github.com/tkkarjal/magia.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01559-5.
References
- 1.Guyenet SJ, Schwartz MW. Regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab. 2012;97:745–55. doi: 10.1210/jc.2011-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larson N, Story M. A review of environmental influences on food choices. Ann Behav Med. 2009;38:56–73. doi: 10.1007/s12160-009-9120-9. [DOI] [PubMed] [Google Scholar]
- 3.French SA, Epstein LH, Jeffery RW, Blundell JE, Wardle J. Eating behavior dimensions. Associations with energy intake and body weight. A Rev Appetite. 2012;59:541–49. doi: 10.1016/j.appet.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardle J. Eating style: a validation study of the Dutch Eating Behaviour Questionnaire in normal subjects and women with eating disorders. J Psychosom Res. 1987;31:161–9. doi: 10.1016/0022-3999(87)90072-9. [DOI] [PubMed] [Google Scholar]
- 5.Baños RM, Cebolla A, Moragrega I, Van Strien T, Fernández-Aranda F, Agüera Z, et al. Relationship between eating styles and temperament in an Anorexia Nervosa, Healthy Control, and Morbid Obesity female sample. Appetite. 2014;76:76–83. doi: 10.1016/j.appet.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Barrada JR, Van Strien T, Cebolla A. Internal structure and measurement invariance of the Dutch eating behavior questionnaire (DEBQ) in a (nearly) representative Dutch community sample. Eur Eat Disord Rev. 2016;24:503–9. doi: 10.1002/erv.2448. [DOI] [PubMed] [Google Scholar]
- 7.Cebolla A, Barrada JR, van Strien T, Oliver E, Baños R. Validation of the Dutch Eating Behavior Questionnaire (DEBQ) in a sample of Spanish women. Appetite. 2014;73:58–64. doi: 10.1016/j.appet.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Yeomans MR, Leitch M, Mobini S. Impulsivity is associated with the disinhibition but not restraint factor from the Three Factor Eating Questionnaire. Appetite. 2008;50:469–76. doi: 10.1016/j.appet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Silventoinen K, Konttinen H. Obesity and eating behavior from the perspective of twin and genetic research. Neurosci Biobehav Rev. 2020;109:150–65. doi: 10.1016/j.neubiorev.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berthoud H-R. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012;71:478–87. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Sci (N. Y, NY) 1998;280:1371–74. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 13.Malik VS, Willet WC, Hu FB. Nearly a decade on — trends, risk factors and policy implications in global obesity. Nat Rev Endocrinol. 2020;16:615–6. doi: 10.1038/s41574-020-00411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosnell BA, Levine AS. Reward systems and food intake: role of opioids. Int J Obes (Lond) 2009;33:S54–58. doi: 10.1038/ijo.2009.73. [DOI] [PubMed] [Google Scholar]
- 15.Nummenmaa L, Saanijoki T, Tuominen L, Hirvonen J, Tuulari JJ, Nuutila P, et al. opioid receptor system mediates reward processing in humans. Nat Commun. 2018;9:1500. doi: 10.1038/s41467-018-03848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro DC, Berridge KC. Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc Natl Acad Sci. 2017;114:E9125–E9134. doi: 10.1073/pnas.1705753114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by μ-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci. 2011;31:3249–60. doi: 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueiras R, Romero-Picó A, Vazquez MJ, Novelle MG, López M, Diéguez C. The opioid system and food intake: homeostatic and hedonic mechanisms. Obes Facts. 2012;5:196–207. doi: 10.1159/000338163. [DOI] [PubMed] [Google Scholar]
- 20.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26:713–28. doi: 10.1016/S0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 21.Ziauddeen H, Chamberlain SR, Nathan PJ, Koch A, Maltby K, Bush M, et al. Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Mol Psychiatry. 2013;18:1287–93. doi: 10.1038/mp.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuulari JJ, Tuominen L, de Boer FE, Hirvonen J, Helin S, Nuutila P, et al. Feeding Releases Endogenous Opioids in Humans. J Neurosci. 2017;37:8284–91. doi: 10.1523/JNEUROSCI.0976-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burghardt PR, Rothberg AE, Dykhuis KE, Burant CF, Zubieta J-K. Endogenous Opioid Mechanisms Are Implicated in Obesity and Weight Loss in Humans. J Clin Endocrinol Metab. 2015;100:3193–201. doi: 10.1210/jc.2015-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, et al. Obesity is associated with decreased mu-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35:3959–65. doi: 10.1523/JNEUROSCI.4744-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bencherif B, Guarda AS, Colantuoni C, Ravert HT, Dannals RF, Frost JJ. Regional μ-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. J Nucl Med. 2005;46:1349–51. [PubMed] [Google Scholar]
- 26.Kantonen T, Karjalainen T, Isojärvi J, Nuutila P, Tuisku J, Rinne J, et al. Interindividual variability and lateralization of μ-opioid receptors in the human brain. NeuroImage. 2020;217:116922. doi: 10.1016/j.neuroimage.2020.116922. [DOI] [PubMed] [Google Scholar]
- 27.Love TM, Stohler CS, Zubieta J-K. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009;66:1124–34. doi: 10.1001/archgenpsychiatry.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cota D, Tschöp MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Bermudez-Silva FJ, Cardinal P, Cota D. The role of the endocannabinoid system in the neuroendocrine regulation of energy balance. J Psychopharmacol. 2012;26:114–24. doi: 10.1177/0269881111408458. [DOI] [PubMed] [Google Scholar]
- 30.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 31.Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology. 2001;159:111–6. doi: 10.1007/s002130100910. [DOI] [PubMed] [Google Scholar]
- 32.Solinas M, Goldberg SR. Motivational Effects of Cannabinoids and Opioids on Food Reinforcement Depend on Simultaneous Activation of Cannabinoid and Opioid Systems. Neuropsychopharmacology. 2005;30:2035–45. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- 33.Solinas M, Zangen A, Thiriet N, Goldberg SR. Beta-endorphin elevations in the ventral tegmental area regulate the discriminative effects of Delta-9-tetrahydrocannabinol. Eur J Neurosci. 2004;19:3183–92. doi: 10.1111/j.0953-816X.2004.03420.x. [DOI] [PubMed] [Google Scholar]
- 34.Viganò D, Valenti M, Cascio MG, Di Marzo V, Parolaro D, Rubino T. Changes in endocannabinoid levels in a rat model of behavioural sensitization to morphine. Eur J Neurosci. 2004;20:1849–57. doi: 10.1111/j.1460-9568.2004.03645.x. [DOI] [PubMed] [Google Scholar]
- 35.Ceccarini J, Weltens N, Ly HG, Tack J, Van Oudenhove L, Van Laere K. Association between cerebral cannabinoid 1 receptor availability and body mass index in patients with food intake disorders and healthy subjects: a [18 F] MK-9470 PET study. Transl Psychiatry. 2016;6:e853–e853. doi: 10.1038/tp.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Marzo V, Després J-P. CB1 antagonists for obesity—what lessons have we learned from rimonabant? Nat Rev Endocrinol. 2009;5:633–8. doi: 10.1038/nrendo.2009.197. [DOI] [PubMed] [Google Scholar]
- 37.Van Strien T, Herman CP, Verheijden MW. Eating style, overeating, and overweight in a representative Dutch sample. Does external eating play a role? Appetite. 2009;52:380–7. doi: 10.1016/j.appet.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Van Strien T, Peter Herman C, Anschutz D. The predictive validity of the DEBQ‐external eating scale for eating in response to food commercials while watching television. Int J Eat Disord. 2012;45:257–62. doi: 10.1002/eat.20940. [DOI] [PubMed] [Google Scholar]
- 39.Van Strien T, Frijters JE, Van Staveren WA, Defares PB, Deurenberg P. The predictive validity of the Dutch restrained eating scale. Int J Eat Disord. 1986;5:747–55. doi: 10.1002/1098-108X(198605)5:4<747::AID-EAT2260050413>3.0.CO;2-6. [DOI] [Google Scholar]
- 40.Van Strien T, Peter Herman C, Verheijden MW. Eating style, overeating and weight gain. A prospective 2-year follow-up study in a representative Dutch sample. Appetite. 2012;59:782–9. doi: 10.1016/j.appet.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5:295–315. doi: 10.1002/1098-108X(198602)5:2<295::AID-EAT2260050209>3.0.CO;2-T. [DOI] [Google Scholar]
- 42.Van Strien T, Schippers GM, Cox WM. On the relationship between emotional and external eating behavior. Addict Behav. 1995;20:585–94. doi: 10.1016/0306-4603(95)00018-8. [DOI] [PubMed] [Google Scholar]
- 43.Malesza M, Kaczmarek MC. One year reliability of the Dutch eating behavior questionnaire: an extension into clinical population. J Public Health. 2019;29:463–69. doi: 10.1007/s10389-019-01147-4. [DOI] [Google Scholar]
- 44.Karjalainen T, Tuisku J, Santavirta S, Kantonen T, Bucci M, Tuominen L, et al. Magia: Robust Automated Image Processing and Kinetic Modeling Toolbox for PET Neuroinformatics. Front Neuroinform. 2020;14:3. doi: 10.3389/fninf.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2007;27:1533–39. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 46.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–8. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 47.Frost JJ, Douglass KH, Mayberg HS, Dannals RF, Links JM, Wilson AA, et al. Multicompartmental Analysis of [11C]-Carfentanil Binding to Opiate Receptors in Humans Measured by Positron Emission Tomography. J Cereb Blood Flow Metab. 1989;9:398–409. doi: 10.1038/jcbfm.1989.59. [DOI] [PubMed] [Google Scholar]
- 48.Hirvonen J, Aalto S, Hagelberg N, Maksimow A, Ingman K, Oikonen V, et al. Measurement of central µ-opioid receptor binding in vivo with PET and [11 C] carfentanil: a test–retest study in healthy subjects. Eur J Nucl Med Mol Imaging. 2009;36:275–86. doi: 10.1007/s00259-008-0935-6. [DOI] [PubMed] [Google Scholar]
- 49.Pfeiffer A, Pasi A, Mehraein P, Herz A. Opiate receptor binding sites in human brain. Brain Res. 1982;248:87–96. doi: 10.1016/0006-8993(82)91150-7. [DOI] [PubMed] [Google Scholar]
- 50.Terry GE, Hirvonen J, Liow JS, Zoghbi SS, Gladding R, Tauscher JT, et al. Imaging and quantitation of cannabinoid CB1 receptors in human and monkey brains using 18F-labeled inverse agonist radioligands. J Nucl Med. 2010;51:112–20. doi: 10.2967/jnumed.109.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol. 2000;27:661–70. doi: 10.1016/S0969-8051(00)00137-2. [DOI] [PubMed] [Google Scholar]
- 52.Lahesmaa M, Eriksson O, Gnad T, Oikonen V, Bucci M, Hirvonen J, et al. Cannabinoid type 1 receptors are upregulated during acute activation of brown adipose tissue. Diabetes. 2018;67:1226–36. doi: 10.2337/db17-1366. [DOI] [PubMed] [Google Scholar]
- 53.Nummenmaa L, Tuominen L. Opioid system and human emotions. Br J Pharmacol. 2018;175:2737–49. doi: 10.1111/bph.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Majuri J, Joutsa J, Johansson J, Voon V, Alakurtti K, Parkkola R, et al. Dopamine and Opioid Neurotransmission in Behavioral Addictions: a Comparative PET Study in Pathological Gambling and Binge Eating. Neuropsychopharmacology. 2017;42:1169–77. doi: 10.1038/npp.2016.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 56.Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Science (New York, NY), 2019;363:1–10. [DOI] [PMC free article] [PubMed]
- 57.Owens MM, Gray JC, Amlung MT, Oshri A, Sweet LH, MacKillop J. Neuroanatomical foundations of delayed reward discounting decision making. NeuroImage. 2017;161:261–70. doi: 10.1016/j.neuroimage.2017.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156:842–8. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 59.Nummenmaa L, et al. Lowered endogenous mu-opioid receptor availability in subclinical depression and anxiety. Neuropsychopharmacology. 2020;45:1953–59. doi: 10.1038/s41386-020-0725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gelman A, Hill J, Yajima M. Why we (usually) don’t have to worry about multiple comparisons. J Res Educ Effectiveness. 2012;5:189–211. doi: 10.1080/19345747.2011.618213. [DOI] [Google Scholar]
- 61.Laurikainen H, Tuominen L, Tikka M, Merisaari H, Armio RL, Sormunen E, et al. Sex difference in brain CB1 receptor availability in man. Neuroimage. 2019;184:834–42. doi: 10.1016/j.neuroimage.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 62.Berthoud H-R, Münzberg H, Morrison CD. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology. 2017;152:1728–38. doi: 10.1053/j.gastro.2016.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PloS ONE. 2012;7:e31089. doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stice E, Spoor S, Bohon C, Small D. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Sci (N. Y, NY) 2008;322:449–52. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–35. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burton P, Smit HJ, Lightowler HJ. The influence of restrained and external eating patterns on overeating. Appetite. 2007;49:191–7. doi: 10.1016/j.appet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 67.Anschutz DJ, Van Strien T, Van De Ven MOM, Engels RCME. Eating styles and energy intake in young women. Appetite. 2009;53:119–22. doi: 10.1016/j.appet.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 68.Joutsa J, Karlsson HK, Majuri J, Nuutila P, Helin S, Kaasinen V, et al. Binge eating disorder and morbid obesity are associated with lowered mu-opioid receptor availability in the brain. Psychiatry Res: Neuroimaging. 2018;276:41–5. doi: 10.1016/j.pscychresns.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Winterdahl M, Noer O, Orlowski D, Schacht AC, Jakobsen S, Alstrup A, et al. Sucrose intake lowers μ-opioid and dopamine D2/3 receptor availability in porcine brain. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-53430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weerts EM, McCaul ME, Kuwabara H, Yang X, Xu X, Dannals RF, et al. Influence of OPRM1 Asn40Asp variant (A118G) on [11C]carfentanil binding potential: preliminary findings in human subjects. Int J Neuropsychopharmacol. 2013;16:47–53. doi: 10.1017/S146114571200017X. [DOI] [PubMed] [Google Scholar]
- 71.Antons S, Brand M, Potenza MN. Neurobiology of cue-reactivity, craving, and inhibitory control in non-substance addictive behaviors. J Neurological Sci. 2020;415:116952. doi: 10.1016/j.jns.2020.116952. [DOI] [PubMed] [Google Scholar]
- 72.Tuominen L, Nummenmaa L, Keltikangas‐Järvinen L, Raitakari O, Hietala J. Mapping neurotransmitter networks with PET: an example on serotonin and opioid systems. Hum brain Mapp. 2014;35:1875–84. doi: 10.1002/hbm.22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khawaja X, Bailey C, Green I. Central mu, delta, and kappa opioid binding sites, and brain and pituitary beta-endorphin and met-enkephalin in genetically obese (ob/ob) and lean mice. Life Sci. 1989;44:1097–1105. doi: 10.1016/0024-3205(89)90337-8. [DOI] [PubMed] [Google Scholar]
- 74.Henriksen G, Willoch F. Imaging of opioid receptors in the central nervous system. Brain. 2008;131:1171–96. doi: 10.1093/brain/awm255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14:12–24. doi: 10.1038/nrendo.2017.122. [DOI] [PubMed] [Google Scholar]
- 76.Ornellas T, Chavez B. Naltrexone SR/Bupropion SR (Contrave): a new approach to weight loss in obese adults. Pharm Ther. 2011;36:255–62. [PMC free article] [PubMed] [Google Scholar]
- 77.Barnett V, Twycross R, Mihalyo M, Wilcock A. Opioid antagonists. J Pain Symptom Manag. 2014;47:341–352. doi: 10.1016/j.jpainsymman.2013.12.223. [DOI] [PubMed] [Google Scholar]
- 78.Mattioli T-AM, Milne B, Cahill CM. Ultra-low dose naltrexone attenuates chronic morphine-induced gliosis in rats. Mol Pain. 2010;6:1744-8069-1746-1722. doi: 10.1186/1744-8069-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bermudez-Silva FJ, Viveros MP, McPartland JM, Rodriguez, de Fonseca F. The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol Biochem Behav. 2010;95:375–82. doi: 10.1016/j.pbb.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 80.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–9. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gérard N, Pieters G, Goffin K, Bormans G, Van Laere K. Brain Type 1 Cannabinoid Receptor Availability in Patients with Anorexia and Bulimia Nervosa. Biol Psychiatry. 2011;70:777–84. doi: 10.1016/j.biopsych.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 82.Richard D, Guesdon B, Timofeeva E. The brain endocannabinoid system in the regulation of energy balance. Best Pract Res Clin Endocrinol Metab. 2009;23:17–32. doi: 10.1016/j.beem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:438–55. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 84.Lutz B, Marsicano G, Maldonado R, Hillard CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci. 2015;16:705–18. doi: 10.1038/nrn4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quarta C, Mazza R, Obici S, Pasquali R, Pagotto U. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends Mol Med. 2011;17:518–26. doi: 10.1016/j.molmed.2011.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code for preprocessing of the PET data (Magia) is available at https://github.com/tkkarjal/magia.