Abstract
Background/Purpose:
Heavy alcohol drinking is associated with pancreatitis. Pancreatitis is initiated by the damage to the pancreatic acinar cells. The endoplasmic reticulum (ER) stress has been shown to play an important role in alcohol-induced pancreatic damage. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is an ER stress-inducible protein. The aim of the study was to determine whether MANF can ameliorate alcohol-induced ER stress and cellular damages to pancreatic acinar cells.
Methods:
Alcohol-induced damage to mouse pancreatic 266–6 acinar cells was determined by MTT and flow cytometry. MANF expression was down-regulated by MANF siRNA using a Neon Transfection System. The over-expression of MANF was performed by the infection with the adenoviral vector carrying mouse MANF gene. The expression of ER stress markers was determined by immunoblotting and immunofluorescence.
Results:
Alcohol caused ER stress, oxidative stress and induced apoptosis of 266–6 acinar cells. Recombinant human MANF alleviated alcohol-induced ER stress and cell death by inhibiting IRE1-caspase 12-caspase 3 apoptotic pathway. Overexpression of mouse MANF also protected cells against alcohol-induced apoptosis. In contrast, inhibiting MANF by siRNA exacerbated alcohol-induced cellular damage.
Conclusions:
MANF was protective against alcohol-induced ER stress and cellular injury in pancreatic acinar cells. The findings suggest a potential therapeutic value of MANF for alcoholic pancreatitis.
Keywords: Alcohol abuse, apoptosis, endoplasmic reticulum stress, oxidative stress, pancreatitis, unfolded protein response
INTRODUCTION
Alcohol consumption has long been recognized as a risk factor for pancreatitis, including both acute pancreatitis and chronic pancreatitis[1]. Pancreatitis is an inflammatory disorder characterized by acinar cell injury and death[2]. Epidemiological studies have shown that the risk of developing pancreatitis is positively correlated to the amount of alcohol intake or the duration of alcohol consumption. However, only a small number of heavy alcohol drinkers develop pancreatitis, suggesting either additional risk factors are required for the progression of the disease or alcohol exposure induces adaptive mechanisms which may in turn protect the pancreas from alcohol’s toxic effect. It is therefore important to identify cellular and molecular mechanisms underlying alcoholic pancreatitis.
The acinar cells are the functional unit of exocrine pancreas that synthesize and secrete large amounts of digestive enzymes[3]. Because of the high protein demands, the acinar cells own extensive endoplasmic reticulum (ER) networks that regulate the synthesis, folding, modification and transport of proteins[4]. Upon the insults of physiological and pathological factors, cells may not be able to properly fold and posttranslationally modify protein in the ER, leading to ER stress and trigger a highly-conserved adaptive response termed as unfolded protein response (UPR)[5]. Pancreatic acinar cells are particularly susceptible to ER stress because of their high dependence on ER functionality. The ER stress-induced UPR signaling pathways have been documented in pancreatitis which may function to relieve the stress and restore the ER homeostasis; however, if ER stress is prolonged or sustained, pathological changes in the pancreas occur and damages are induced to the acinar cells[6].
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is an ER stress-inducible protein originally identified as a survival promoting factor for brain dopaminergic neurons[7]. MANF can be induced by a number of ER stress inducers in many cell types in vivo and in vitro and exert a protective role against ER stress-induced damages[8]. The pancreatic-specific MANF deficiency leads to ER stress, apoptosis of pancreatic beta cells and diabetes in mice, whereas recombinant MANF enhances pancreatic beta cell proliferation[9]. In addition, MANF has also been shown to protect human pancreatic beta cells against experimentally stress-induced cell death. However, the role of MANF in pancreatic acinar cells and effects of alcohol is unknown.
In the present study, we used a mouse pancreatic 266–6 acinar cell line to investigate alcohol-induced damage to acinar cells in vitro. We sought to determine whether alcohol exposure causes ER stress and cellular injury. We further investigated the role of MANF in alcohol-induced ER stress and cell death.
MATERIALS AND METHODS
Materials
Anti-GRP78, anti-eIF2a, anti-DNP, and anti-ATF6 antibodies were obtained from Santa Cruz Biotech (Santa Cruz, CA). Anti-cleaved caspase-3, anti-phospho-PERK, and anti-IRE1a antibodies were obtained from Cell Signaling Technology, Inc. (Beverly, MA). Anti-phospho-IRE1a (Ser724), anti-MANF, and anti-caspase-12 antibodies were obtained from Abcam (Cambridge, MA). Anti-X-box binding protein-1s (XBP1s) antibody was from BioLegend (San Diego, CA). 4-PBA and NAC were purchased from Sigma Chemical Co. (St. Louis, MO). 4-Hydroxynonenal (HNE) adduct assay was obtained from Cell Biolabs, Inc. (San Diego, CA). Small interfering (si) RNA for human MANF gene (si MANF) was purchased from invitrogen Co. (Carlsbad, CA). TUNEL Assay Kit was obtained from Abcam (Cambridge, MA). STF-083010 was obtained from Med Chem express (New Jersey, USA). Salubrinal was purchased from Tocris Bioscience (Avonmouth, Bristol, UK). Recombinant human MANF was obtained from Creative BioMart company (Shirley, NY). All other antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Other chemicals/reagents used in this project were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA) unless stated otherwise.
Cell culture and treatment
The mouse-derived pancreatic acinar cell line (266–6) was obtained from American Type Tissue Collection (Rockville, MD). 266–6 cells were maintained in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% streptomycin/penicillin. For alcohol exposure, a method utilizing sealed containers was used to maintain ethanol concentrations in the culture medium. To choose appropriate concentration and time, acinar cells were incubated with complete medium containing 0.2%, 0.4% or 0.8% (volume/volume) ethanol for 24, 48, 72 hours. Recombinant human MANF was added into the medium at a working concentration of 10 ng/ml two hours before adding alcohol. 4-PBA (5 mM), STF083010 (10 μM), salubrinal (10 μM) and NAC (1 mM) were similarly administered.
MANF adenoviruses (AD-MANF) infection
MANF adenoviruses (AD-MANF) were obtained from Applied Biological Materials (ABM). A DNA sequence encoding mouse MANF was inserted into the pAdenoG-HA vector and then packaged into adenoviruses (Cat No. 210179A). An empty vector virus, AD-vector (Cat. No. 000047A, ABM) was used as the control virus. Both adenoviruses were prepared at titer more than 1×106pfu/ml and amplified by transducing HEK293 cells. To obtain the overexpression of MANF in 266–6 cells, we infected cells with AD-MANF or AD-vector for 48 hours according to the protocol of the ABM. In brief, acinar cells were seeded in 6-well plates at 50% confluency one day before transduction. In transduction, acinar cells were incubated with culture media containing AD-MANF or AD-vector for 2 hours and then replaced with fresh media. After transduction for 48 hours, acinar cells were collected for the subsequent analysis of cell viability, immunoblotting and flow cytometry.
MANF siRNA transfection
Transient transfection of MANF siRNA, control siRNA was performed using a Neon Transfection System (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer’s protocol. Briefly, 266–6 cells were electroporated and incubated with indicated siRNAs. Experiments were initiated forty-eight hours after the transfection.
Cell viability
The viability of 266–6 acinar cells was determined by a 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described in detail previously.
TUNEL assay
The apoptosis of 266–6 acinar cells was detected by the TUNEL assay kit. After the treatment, acinar cells were centrifugated, fixed, washed, and added appropriate reagents according to the protocol provided by the manufacturer. Cells were analyzed within 3 hours of staining. The proportion of apoptosis was analyzed by flow cytometry (Ex/Em = 488/520 nm for FITC, and 488/623 nm for PI).
Immunofluorescence microscopy
The procedure for immunofluorescent microscopy has been previously described. Briefly, 266–6 cells were seeded on coverslips pre-coated with fibronectin (10 μg/ml). Cells were fixed with 3.7% paraformaldehyde for 10 min, washed 3 times in PBS, and permeabilized with 0.5% Triton X-100 for 5 min. Cells were blocked with 5% BSA, incubated with anti-MANF antibody (1:100), anti-caspase12 (1:200) primary antibodies at 4°C overnight followed by incubation with the corresponding Alexa Fluorlabeled secondary antibodies. After rinsing with PBS, coverslips were mounted with Prolong Gold anti-fade reagent, and images were photographed using an Olympus inverted fluorescent microscope (IX81) with the same exposure time and amplifier offset.
Immunoblotting
The procedure for immunoblotting has been previously described. 266–6 cells were lysed in modified RIPA buffer (150 mM NaCl, 50 mM Tris, 1% NP-40, 0.25% sodium deoxycholate) containing 1 mM sodium orthovanadate, 1 mM phenylmethanesulfonyl fluoride (PMSF), 5 μg/ml of aprotinin, and 2 μg/ml of leupeptin. 30–50 ug of extracted protein was used for immunoblotting to examine the levels of ER stress markers -(GRP78, p-PERK, IRE1α, ATF6, p-eIF2α, XBP1s), MANF, oxidative stress markers (DNP and 4-HNE) and apoptotic markers (cleaved caspase-3 and caspase-12). The final dilutions for primary antibodies were: GRP78, 1:1,000; p-PERK, 1:1,000; IRE1α, 1:1,000; ATF6, 1:1,000; XBP-1, 1:1,000; p-eIF2α, 1:1,000; cleaved caspase-3, 1:1,000; caspase-12,1:1,000; HNE, 1:1,000; DNP, 1:1,000; and actin, 1:5,000. The nitrocellulose membranes were first probed with specific primary antibodies overnight at 4°C. After washing with phosphate-buffered saline (PBS) containing 0.05% Tween-20 three times, the membranes were incubated with secondary antibodies for 1 hour at room temperature. Protein-specific signals were then detected with enhanced chemiluminescence substrate (GE Healthcare, Chalfont, Buckinghamshire, UK) using a Chemi™Doc imaging system (Bio-Rad 215 Laboratories, Hercules, CA) and then quantified with the software of Image lab 5.2 (Bio-Rad Laboratories, Hercules, CA).
Data Analysis
All values were reported as mean ± SEM, and analyzed using unpaired t-test or one-way analysis of variance (ANOVA) with the software GraphPad Prism 6 (GraphPad Software; La Jolla, CA). Differences in which p was<0.05 were considered statistically significant.
RESULTS
Alcohol causes ER stress and inhibits cell viability in pancreatic 266–6 acinar cells
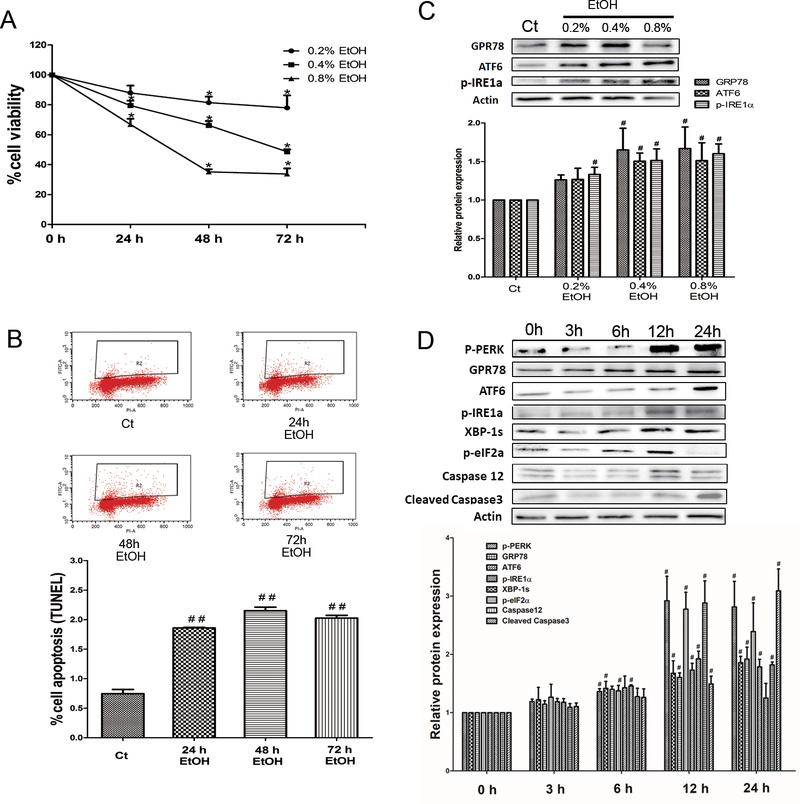
Pancreatic 266–6 acinar cells were exposed to ethanol at 0.2%, 0.4% and 0.8% for 24 h, 48 h, or 72 h. At the concentration of 0.4% and 0.8%, ethanol significantly reduced cell viability after 24 h of exposure; ethanol at the concentration of 0.2% decreased cell viability after 48 h of exposure (Fig. 1A). The ethanol-induced cell death was confirmed by flow cytometry. Ethanol at the concentration of 0.4% induced apotosis of pancreatic acinar cells after 24 h of exposure (Fig. 1B). To investigate the effect of ethanol on ER stress, we treated pancreatic 266–6 acinar cells with ethanol at the concentration of 0.2%, 0.4%, or 0.8% for 12 h. Ethanol at 0.4% and 0.8% induced the expression of ER stress markers (Fig. 1C). We next performed a time-course study of ethanol’s effect on ER stress. As shown in Fig. 1D, ethanol at the concentration of 0.4% upregulated the expression of various ER stress markers, such as GRP78, p-perk, ATF6, p-IRE1α, xbp-1s, p-eIF2a, and caspase12, as early as 6 h of exposure. Ethanol also increased the expression of an apoptotic marker, cleaved-caspase 3. Based on the finding that ethanol at the concentration of 0.4% was effective causing apoptosis and ER stress in 266–6 cells, we therefore used 0.4% ethanol for the rest of our study.
Fig 1. Effects of alcohol on the viability and ER stress in pancreatic acinar cells.
(A) Pancreatic 266–6 acinar cells were exposed to ethanol (EtOH: 0.2%, 0.4%, or 0.8%) for different times and cell viability was determined by MTT assay. Each data point was mean ± SEM; n=6; *P<0.05, **P<0.01 compared to 0 h. (B) The effect of ethanol (0.4%) on apoptosis was determined by flow cytometry. n=3; ##P<0.01 versus control (Ct). (C) The effect of ethanol (EtOH: 0.2%, 0.4%, or 0.8%) on the expression of ER stress markers was determined by immunoblotting. n=3; #P<0.05 versus control (Ct). (D) A time-course study of the effect of ethanol (0.4%) on ER stress markers was determined by immunoblotting. n=3; #P<0.05 versus 0 h.
Inhibition of oxidative stress and ER stress offers protection against alcohol-induced cellular injury.
There is considerable evidence of interaction between ER stress and oxidative stress. Alcohol-induced ER stress may be associated with oxidative stress which could also play a role in alcohol-induced pancreatic damage. Therefore we investigated the effect of alcohol exposure on oxidative stress in the pancreatic 266–6 acinar cells. 4-hydroxynonenal (4-HNE) and 2,4-dinitrophenol (DNP) have been commonly used as sensitive and reliable biomarkers for oxidative stress. In the time course study, alcohol was shown to increase the expression of 4-HNE and DNP in 266–6 acinar cells in a time-dependent manner (Fig. 2A).
Fig 2. Effects of inhibition of oxidative stress and ER stress on alcohol-induced cellular death.
(A) The effect of EtOH on oxidative stress was determined by immunoblotting analysis of DNP and 4-HNE. n=3; #P<0.05 versus 0 h. (B) 266–6 cells were treated with ER stress inhibitors 4-PBA (5 mM), STF083010 (10 μM), salubrinal (10 μM) and antioxidant NAC (1 mM) for 24 hours. The effect of ER stress inhibitors and antioxidant NAC on cell viability was determined by MTT assay. n=6; ##P<0.01 versus Ct, *P<0.05 versus EtOH. (C) 266–6 cells were treated with NAC (1 mM) for 12 hours. The effect of NAC on ER stress markers was determined by immunoblotting. n=3; #P<0.05 versus Ct, *P<0.05 versus EtOH. (D) Cells were treated with EtOH with/without NAC (1 mM) and ER stress inhibitors 4-PBA (5 mM), STF083010 (10 μM), salubrinal (10 μM) for 24 hours, and cell viability was determined by MTT assay. n=6; ##P<0.01 versus Ct, *P<0.05 versus EtOH, ΔP<0.05 versus without NAC.
To determine whether ER stress and oxidative stress contributed to alcohol-induced cellular injury, we inhibited alcohol-induced ER stress and oxidative stress using specific inhibitors and antioxidants. 4-PBA, STF083010, and salubrinal have been used to experimentally alleviate ER stress. 4-PBA is often considered an “ER stress inhibitor” primarily due to its properties as a chemical chaperone to improve protein folding and reduce ER stress in vivo and in vitro. STF083010 is a novel small-molecule inhibitor of IRE1. Salubrinal selectively inhibits eIF2α dephosphorylation and has been shown to protect cells against ER stress-mediated apoptosis. NAC is a thiol-containing antioxidant and potent free radical scavenger. 4-PBA (5 mM), STF083010 (10 μM), salubrinal (10 μM) and NAC (1 mM) showed little effect on acinar cells viability (Fig. 2B). NAC inhibited the expression of alcohol-induced p-perk, ATF6, p-IRE1α and 4-HNE (Fig. 2C), suggesting that there was an interaction between alcohol-induced oxidative stress and ER stress. Pretreatment with 4-PBA (5 mM), STF083010 (10 μM), salubrinal (10 μM) and NAC (1 mM) significantly protected alcohol-induced cellular injury to acinar cells (Fig. 2D). At the same time, combined 4-PBA (5 mM) or STF083010 (10 μM) or salubrinal (10 μM) with NAC (1mM) seemed to be more effective than without NAC (ER stress inhibitors alone). Notably, the combination of salubrinal with NAC had a stronger protective effect than salubrinal alone. There is no significant difference between the effects of NAC+ER stress inhibitors (4-PBA or STF) and ER stress inhibitors alone. (Fig. 2D).
Exogenous MANF alleviates alcohol-induced apoptosis by inhibiting IRE1-caspase 12-caspase 3 apoptotic pathway in 266–6 acinar cells.
MANF is an ER stress-inducible protein. To determine the role of MANF in alcohol-induced cellular injury to acinar cells, we firstly examined the effect of alcohol on the expression of MANF. The expression of MANF gradually increased following alcohol treatment; the increase became significantly after 12 h of alcohol exposure (Fig. 3A). To determine whether MANF could protect acinar cells against alcohol-induced cellular injury, we treated cells with exogenous recombinant human MANF (10 ng/ml). MANF significantly relieved alcohol-induced inhibition on the cell viability as determined by MTT assay (Fig. 3B). Consistently, exogenous recombinant human MANF also significantly alleviated alcohol-induced apoptosis as determined by flow cytometry (Fig. 3C). To determine the effects of exogenous MANF on ER stress, we examined the effect of alcohol and MANF on the expression ER stress markers. As shown in Fig. 3D, MANF significantly inhibited alcohol-activated p-IRE1α-caspase12-caspase3 pathway. Together these results suggested that MANF may alleviate the cytotoxicity of alcohol by inhibiting p-IRE1/caspase 12/caspase 3 apoptotic pathway.
Fig 3. Effects of exogenous MANF on alcohol-induced cellular injury and ER stress.
(A) The effect of EtOH on the expression of MANF was determined by immunoblotting. n=3; #P<0.05, ##P<0.01 versus 0 h. (B) The effect of exogenous MANF (10 ng/ml) on ethanol-induced reduction of cell viability was determined by MTT assay at 24 h and 48 h following EtOH exposure. n=6; ##P<0.01 versus Ct, *P<0.05 versus EtOH. (C) Cells were treated with EtOH with/without MANF (10 ng/ml) for 24 hours. The effect of exogenous MANF on apoptosis was determined by flow cytometry. n=3; ##P<0.01 versus Ct, *P<0.05 versus EtOH. (D) Cells were treated with EtOH with/without MANF (10 ng/ml) for 12 hours. The effect of EtOH and exogenous MANF on the expression of ER stress markers was determined by immunoblotting. n=3; #P<0.05 versus Ct, *P<0.05 versus EtOH.
Overexpression of MANF protects pancreatic acinar cells aganist alcohol-induced apoptosis
To confirm the protective effect of MANF against alcohol-induced cellular injury, we transfected pancreatic 266–6 acinar cells with AD-vector (control group) and AD-MANF (overexpression of mouse MANF). As determined by MTT assay, overexpression of MANF ameliorated alcohol-induced inhibition of cell viability (Fig. 4A). Consistently, the alcohol-induced apoptosis was also significantly reduced by overexpression of MANF (Fig. 4B). We further explored the effect of overexpressing MANF on alcohol-activated p-IRE1a-caspase12-caspase3 pathway. As shown in Fig. 4C, the overexpression of MANF inhibited the alcohol-activated p-IRE1a/caspase12/caspase3 pathway.
Fig 4. Effects of overexpression of MANF on alcohol-induced cellular injury and ER stress.
(A) Pancreatic 266–6 acinar cells were infected with AD-vector (control group) or AD-MANF (overexpression of MANF) and then exposed to EtOH for 24 hours. Cell viability was determined by MTT assay. n=6; ##P<0.01 versus no ethanol treatment group, *P<0.05 versus AD-vector + EtOH. (B) Cells were infected with AD-vector or AD-MANF vector, and then exposed to EtOH for 24 hours. Cell apoptosis was determined by flow cytometry. n=3; ##P<0.01 versus no ethanol treatment group, *P<0.05 versus AD-vector + EtOH. (C) Cells were infected with AD-vector or AD-MANF vector, and then exposed to EtOH for 12 hours. The expression of IRE1a, caspase12, and cleaved caspase3 was determined by immunoblotting. The expression of HA tag and MANF was indicative of successfully MANF infection. n=3; #P<0.05 versus not ethanol treatment group.
Silencing MANF by siRNA renders IRE1a/caspase12/caspase3 pathway more susceptible to alcohol
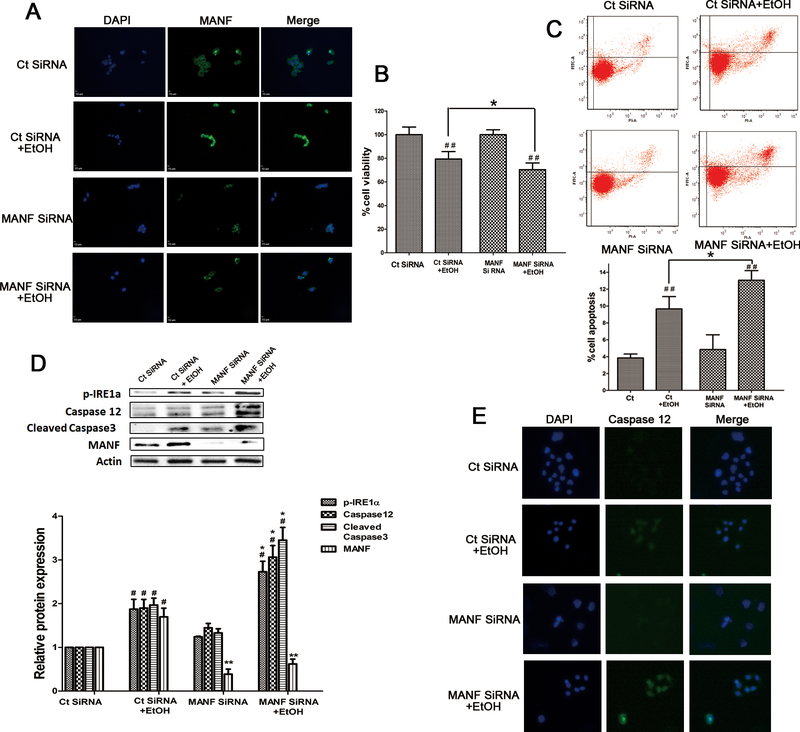
To further determine the role of MANF in alcohol-induced cellular injury, we transfected 266–6 acinar cells with control siRNA and MANF siRNA. MANF siRNA decreased the basal level and alcohol-induced MANF expression (Fig. 5A and 5D). MANF siRNA significantly increased alcohol-induced cell death which was determined by MTT assay and flow cytometry (Fig. 5B and 5C). We further explored the effect of MANF siRNA on alcohol-induced activation of IRE1a/caspase12/caspase3 pathway. As shown in Fig. 5D, MANF siRNA significantly enhanced alcohol-induced activation of p-IRE1α/caspase12/cleaved-caspase3. In addition, MANF siRNA inceased the alcohol-induced expression of caspase-12 confirmed by immunofluorescence study (Fig. 5E). The results indicated that silencing MANF by siRNA rendered IRE1a/caspase12/caspase3 more susceptible to alcohol exposure.
Fig 5. Effects of MANF siRNA on alcohol-induced cellular injury and ER stress.
(A) Pancreatic 266–6 acinar cells were transfected with control siRNA or MANF siRNA, and then exposed to EtOH for 24 hours. The expression of MANF was detected by immunofluorescence. (B) Cells were transfected with control siRNA or MANF siRNA, and then exposed to EtOH for 24 hours. Cell viability was determined by MTT assay. n=6; ##P<0.01 versus no ethanol treatment group, *P<0.05 versus control siRNA group + EtOH. (C) Cells were transfected with control siRNA and MANF siRNA, and then exposed to EtOH for 24 hours. Cell apoptosis was determined by flow cytometry. n=3; ##P<0.01 versus no ethanol treatment group, *P<0.05 versus control siRNA + EtOH. (D) Cells were transfected with control siRNA or MANF siRNA, and then exposed to EtOH for 12 hours. The expression of IRE1a, caspase12, cleaved caspase3, and MANF was determined by immunoblotting. #P<0.05 versus no ethanol treatment group. *P<0.05, **P<0.01 versus control siRNA + EtOH. (E) The effect of MANF siRNA and ethanol on the expression of caspase 12 was examined by immunofluorescence.
DISCUSSION
Heavy alcohol drinking is a risk factor for pancreatitis, including acute pancreatitis and chronic pancreatitis[10], and it is believed that pancreatitis is initiated in the pancreatic acinar cells and characterized by acinar cell injury and death[11]. The apoptosis and necrosis of acinar cells have been observed in both clinical and experimental pancreatitis[12]. In these studies, mild AP and pancreatic atrophy were found to be associated with acinar cell apoptosis, whereas severe AP were found to be linked to acinar cell necrosis. However, the mechanisms regulating cell death remain to be investigated.
The pancreatic acinar cells are secretory cells with a role as biosynthetic factories producing and secreting a large number of digestive enzymes. They heavily depend on their extensive ER networks to ensure high rates of enzyme production[4]. Under ER stress conditions, acinar cells activate UPR adaptive system to either restore ER homeostasis or induce cell death if homeostasis cannot be restored. The ER stress and UPR system have been documented to play a role in the disease progression of several disorders, including diabetes, cardiovascular diseases, neurodegenerative disorders and intestinal inflammation[13]. In human and experimental pancreatitis, the UPR has been shown to act as a binary switch regulating the survival or death of acinar cells affected by ER stress[14].
In this study, we used an in vitro model, pancreatic 266–6 acinar cells, to investigate alcohol-induced cellular injury and the role of MANF. We demonstrated that alcohol caused concentration-dependent cellular damage. We thereafter focused on the effect of 0.4% of ethanol; this concentration has been widely used for in vitro alcohol exposure and is relevant to human heavy alcohol drinking[15]. We showed that alcohol caused apoptotic cell death which was demonstrated by MTT assay, TUNEL and the activation of caspase 3. Alcohol also induced ER stress which was evident by the increased expression of a number of ER stress-associated proteins including GRP78, p-PERK, ATF6, caspase 12, and IRE1α in a dose- and time-dependent manner. Caspase 12 is an ER-specific pro-apoptotic protein[16]. Alcohol activated caspase 12, suggesting that the cell death may be mediated by ER stress-associated apoptosis. Alcohol exposure has been shown to induce ER stress and cell death in numerous cell types in vitro and in vivo[17].
Oxidative stress is defined as an imbalance between reactive oxygen species (ROS) production and the capability of the cell to detoxify oxidants[18]. Oxidative stress is considered as a potential mechanism for alcohol-induced pancreatic damage[19]. There is considerable cross-talk between oxidative stress and ER stress and the interaction has been associated with the pathogenesis of a variety of diseases[20]. Oxidative stress has been proposed to cause pancreatic β-cell dysfunction and apoptosis via ER stress-triggered signaling pathways[21]. In this study, we found that alcohol caused oxidative stress which was demonstrated by the activation of DNP and 4-HNE, the two biomarkers for oxidative stress. The treatment of antioxidant NAC alone partially rescued alcohol-induced cell death and deceased alcohol-induced expression of ER stress markers including IRE1a, PERK and ATF6, suggesting a role of oxidative stress in alcohol-induced ER stress and cytotoxicity. It is noted that the treatment of NAC combined with salubrinal, the inhibitor of eIF2α dephosphorylation, appeared to have a stronger protective effect than salubrinal alone (Fig. 2D), indicative of the involvement of cooperative interaction of ER stress and oxidative stress. A previous study showed that eIF2a was involved in oxidative stress-induced death of neuronal cells[22].
Although heavy alcohol drinking is a risk factor for pancreatitis, only a small number of alcohol abusers acquire pancreatic diseases. Many believe that it is because additional risk factors, either environmental or genetic, are required to develop the disease. The other possibility is that acinar cells may activate adaptive systems such as UPR that can protect against alcohol cytotoxicity[23]. In this study, we showed that alcohol exposure induced the expression of MANF which is an important factor of the UPR system. MANF has been shown to be protective in many pathological conditions, including Parkinson disease[24], cerebral ischemia[25] and cardiac diseases[26]. In addition, MANF has been found to be essential for the proliferation and survival of pancreatic beta cells in mice and humans[9]. In the present study, exogenous MANF (Fig. 3B and 3C) or overexpression of MANF using adenovirus (Fig. 4A and 4B) offered protection against alcohol-induced cell death. In contrast, knockdown of MANF by siRNA rendered 266–6 acinar cells more susceptible to alcohol cytotoxicity (Fig. 5B and 5C), which demonstrates the protective role of MANF in acinar cells.
The protection of MANF may depend on its ability to alleviate ER stress. There are three major ER stress sensors, that is ATF6, IRE1, and PERK; they are involved in ER stress-associated diseases[27]. Whereas ATF6 and PERK are dispensible for apoptosis; IRE1α is necessary and sufficient to trigger apoptosis[28]. In our study, all three ER stress sensors were activated by alcohol exposure (Fig. 1D), but only IRE1a was decreased by exogenous recombinant human MANF (Fig. 3D). The alcohol activation of caspase-12 and caspase-3 was also decreased by exogenous MANF. Similarly, alcohol-induced activation of IRE1α/caspase12/caspase3 cascade was inhibited by the overexpression of MANF using an adenovirus expression system (Fig. 4C). In contrast, siRNA knockdown of MANF made cells more susceptible to alcohol-induced cellular injury and the activation of IRE1α/caspase12/caspase3 cascade (Fig. 5). Taken together, our findings suggest MANF is protective against alcohol-induced cellular damage by inhibiting IRE1α/caspase12/caspase3 pathway.
The effects of alcohol abuse on the promotion of pancreatitis are complex; multiple pathways are involved the pathogenesis of pancreatitis including trypsinogen activation, NF-κB amplification, ER stress, oxidative stress, and autophagy impairment. MANF may offer protection by affecting multiple pathways. For example, MANF may inhibit inflammation and mediate the cross-talk between the NF-κB pathway and ER stress[29]. MANF alleviates cell damage and protects dopamine neurons by regulating autophagy pathways[30]. In the present study, we focused on the effect of MANF on ER stress and oxidative stress which are considered major pathways associated with alcoholic pancreatitis. We will further evaluate the effect of MANF on other pathways in future.
In summary, our results demonstrate that MANF is an important component of UPR system and protective against alcohol-induced cellular damage to acinar cells. The protection of MANF is likely mediated by inhibiting the activation of IRE1a/caspase12/caspase3 pathway. The finding has clinical implications for the treatment of alcoholic pancreatitis. Futher studies using animal models of over-expression of MANF through either intra-pancreatic injection or genetic manipulation will further establish MANF as a valuable therapeutic target.
Acknowledgments
This research is supported by grants from the National Institutes of Health (NIH) (AA017226 and AA015407). This research is supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development [Biomedical Laboratory Research and Development: Merit Review (BX001721)] to Jia Luo. It is also supported in part by grants Key Project of Anhui province university outstanding youth talent fund (gxyqZD2018023) to Huaxun Wu.
Abbreviation
- ATF6
Activating transcription factor 6
- DAPI
4’, 6-diamidino-2-phenylindole
- DNP
2,4-dinitrophenylhydrazine
- eIF2α
Eukaryotic initiation factor 2α
- ER
Endoplasmic reticulum
- HNE
4-Hydroxynonenal
- IRE1α
Inositol-requiring kinase
- MANF
Mesencephalic astrocyte-derived neurotrophic factor
- PERK
Protein kinase RNA-like endoplasmic reticulum kinase
- 4-PBA
Sodium phenylbutyrate
- TUNEL
TdT mediated dUTP Nick End Labeling
- XBPIs
X-box binding protein-ls
Footnotes
Disclosures
The authors declare no commercial or financial conflict of interest.
REFERENCES
- [1].Irving HM, Samokhvalov AV, Rehm J. Alcohol as a risk factor for pancreatitis. A systematic review and meta-analysis. JOP. 2009;10(4):387–392. [PMC free article] [PubMed] [Google Scholar]
- [2].Lugea A, Waldron RT, Mareninova OA, Shalbueva N, Deng N, Su HY, et al. Human Pancreatic Acinar Cells:Proteomic Characterization, Physiologic Responses, and Organellar Disorders in exVivo Pancreatitis. Am J Pathol. 2017; 187(12): 2726–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Logsdon CD, Ji B. The role of protein synthesis and digestive enzymes in acinar cell injury. Nat Rev Gastroenterol Hepatol. 2013;10(6):362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pandol SJ. The Exocrine Pancreas. San Rafael (CA): Morgan & Claypool Life Sciences; 2010.49(2):271–275. [PubMed] [Google Scholar]
- [5].Karagöz GE, Acosta-Alvear D, Walter P. The Unfolded Protein Response: Detecting and Responding to Fluctuations in the Protein-Folding Capacity of the Endoplasmic Reticulum. Cold Spring Harb Perspect Biol. 2019;11(9):a033886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lugea A, Gerloff A, Su HY, Xu Z, Go A, Hu C, et al. The Combination of Alcohol and Cigarette Smoke Induces Endoplasmic Reticulum Stress and Cell Death in Pancreatic Acinar Cells. Gastroenterology. 2017;153(6):1674–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, et al. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci. 2003;20(2):173–188. [DOI] [PubMed] [Google Scholar]
- [8].Sousa-Victor P, Jasper H, Neves J. Trophic Factors in Inflammation and Regeneration: The Role of MANF and CDNF. Front Physiol. 2018;9:1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Danilova T, Belevich I, Li H, Palm E, Jokitalo E, Otonkoski T, Lindahl M. MANF Is Required for the Postnatal Expansion and Maintenance of Pancreatic β-Cell Mass in Mice. Diabetes. 2019;68(1):66–80. [DOI] [PubMed] [Google Scholar]
- [10].Lankisch PG, Apte M, Banks PA. Acute pancreatitis [published correction appears in Lancet. 2015 Nov 21;386(10008):2058]. Lancet. 2015;386(9988):85–96. [DOI] [PubMed] [Google Scholar]
- [11].Lugea A, Waldron RT, Pandol SJ. Pancreatic adaptive responses in alcohol abuse: Role of the unfolded protein response. Pancreatology. 2015;15(4 Suppl):S1–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bhatia M Apoptosis of pancreatic acinar cells in acute pancreatitis: is it good or bad?. J Cell Mol Med. 2004;8(3):402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang G, Wang X, Gillette TG, Deng Y, Wang ZV. Unfolded Protein Response as a Therapeutic Target in Cardiovascular Disease. Curr Top Med Chem. 2019;19(21):1902–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen Y, Zhang J, Zhao Q, Chen Q, Sun Y, Jin Y, Wu J. Melatonin Induces Anti-Inflammatory Effects to Play a Protective Role via Endoplasmic Reticulum Stress in Acute Pancreatitis. Cell Physiol Biochem. 2016;40(5):1094–1104. [DOI] [PubMed] [Google Scholar]
- [15].Jones AW, Harding P. Driving under the influence with blood alcohol concentrations over 0.4 g%. Forensic Sci Int. 2013;231(1–3):349–353. [DOI] [PubMed] [Google Scholar]
- [16].Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186–194. [DOI] [PubMed] [Google Scholar]
- [17].Wang S, Pacher P, De Lisle RC, Huang H, Ding WX. A Mechanistic Review of Cell Death in Alcohol-Induced Liver Injury. Alcohol Clin Exp Res. 2016;40(6):1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mittler R Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410. [DOI] [PubMed] [Google Scholar]
- [19].Whitcomb DC. Genetics and alcohol: a lethal combination in pancreatic disease?. Alcohol Clin Exp Res. 2011;35(5):838–842. [DOI] [PubMed] [Google Scholar]
- [20].Dandekar A, Mendez R, Zhang K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol Biol. 2015;1292:205–214. [DOI] [PubMed] [Google Scholar]
- [21].Wang J, Yang X, Zhang J. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic β cells. Cell Signal. 2016;28(8):1099–1104. [DOI] [PubMed] [Google Scholar]
- [22].Tan S, Somia N, Maher P, Schubert D. Regulation of antioxidant metabolism by translation initiation factor 2alpha. J Cell Biol. 2001;152(5):997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lugea A, Tischler D, Nguyen J, Gong J, Gukovsky I, French SW, et al. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology. 2011;140(3):987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang Z, Shen Y, Luo H, Zhang F, Peng D, Jing L, et al. MANF protects dopamine neurons and locomotion defects from a human α-synuclein induced Parkinson’s disease model in C. elegans by regulating ER stress and autophagy pathways. Exp Neurol. 2018;308:59–71. [DOI] [PubMed] [Google Scholar]
- [25].Yang W, Shen Y, Chen Y, Chen L, Wang L, Wang H, et al. Mesencephalic astrocyte-derived neurotrophic factor prevents neuron loss via inhibiting ischemia-induced apoptosis. J Neurol Sci. 2014;344(1–2):129–138. [DOI] [PubMed] [Google Scholar]
- [26].Glembotski CC. Functions for the cardiomyokine, MANF, in cardioprotection, hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51(4):512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kadowaki H, Nishitoh H. Signaling pathways from the endoplasmic reticulum and their roles in disease. Genes (Basel). 2013;4(3):306–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Banerjee A, Ahmed H, Yang P, Czinn SJ, Blanchard TG. Endoplasmic reticulum stress and IRE-1 signaling cause apoptosis in colon cancer cells in response to andrographolide treatment. Oncotarget. 2016;7(27):41432–41444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hakonen E, Chandra V, Fogarty CL, Yu NY, Ustinov J, Katayama S, et al.Mesencephalic astrocyte-derived neurotrophic factor is involved in inflammation by negatively regulating the NF-κB pathway.Sci Rep.2015;5:8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Voutilainen MH, Arumäe U, Airavaara M, Saarma M.MANF protects dopamine neurons and locomotion defects from a human α-synuclein induced Parkinson’s disease model in C. elegans by regulating ER stress and autophagy pathways.Exp Neurol.2018;308:59–71. [DOI] [PubMed] [Google Scholar]