Abstract
Acinetobacter baumannii is one of the most successful pathogens causing nosocomial infections and has significantly multidrug-resistant. So far, there are no certain treatments to protect against infection with A. baumannii, therefore an effective A. baumannii vaccine needed. The purpose of this study was to predict antigenic epitopes of CarO protein for designing the A. baumannii vaccine using immunoinformatics analysis. CarO protein is one of the most important factors in the resistance against the antibiotic Carbapenem. In this study, T and B-cell epitopes of CarO protein were predicted and screened based on the antigenicity, toxicity, allergenicity features. The epitopes were linked by suitable linkers. Four different adjuvants were attached to the vaccine constructs which among them, vaccine construct 3 was chosen to predict the secondary and the 3D structure of the vaccine. The refinement process was performed to improve the quality of the 3D model structure; the validation process is performed using the Ramachandran plot and ProSA z-score. The designed vaccine's binding affinity to six various HLA molecules and TLR 2 and TLR4 were evaluated by molecular docking. Finally, in silico gene cloning was performed in the pET28a (+) vector. The findings suggest that the vaccine may be a promising vaccine to prevent A. baumannii infection.
Keywords: Multi-epitope vaccine, Acinetobacter baumannii, CarO, Immunoinformatics
Introduction
Acinetobacter baumannii is an aerobic, pleomorphic, non-motile, typically short, almost round, and rod-shaped Gram-negative bacterium (Perez et al. 2007). It can cause infections in parts of the body such as blood, urinary tract, and lungs (Eliopoulos et al. 2008). As an opportunistic pathogen, A. baumannii has a high incidence among patients in the intensive care unit (ICU) setting and immunocompromised individuals particularly those who have experienced a prolonged hospital stay (Valencia et al. 2009). In addition, the frequency of A. baumannii infections has been enhancing gently (Dijkshoorn et al. 2007). A. baumannii is able to grow at various temperatures and pH conditions. Also, this organism can exploits different types of both carbon and energy sources. These properties cause A. baumannii can persist in either moist or dry conditions in the hospital environment. This hardiness, combined with its resistance to many antimicrobial drugs, contributes to spread it in the hospital setting (Obeidat et al. 2014).
This phenomenon of multidrug-resistant (MDR) pathogens has become a serious concern about nosocomial and community infections (Dijkshoorn et al. 2007; Tohidinia et al. 2020). During 1970s, A. baumannii is thought to have been sensitive to wide range of antibiotics, but today the pathogen emerges immense resistance to most first-line antibiotics (Howard et al. 2012). Pathogenesis in A. baumannii infections is a result of several virulence factors such as porins, capsules, and cell wall lipopolysaccharide, enzymes, biofilm production, motility, and iron-acquisition systems (Eijkelkamp et al. 2014). These virulence factors contribute to the organism to resist in hard environmental conditions and enable development of intense infections (Ayoub Moubareck and Hammoudi Halat 2020). In addition, various resistance mechanisms in this pathogen are identified which resistance with major classes of antibiotics including penicillins, cephalosporins, carbapenems, most aminoglycosides, fluoroquinolones, chloramphenicol, and tetracyclines (Kyriakidis et al. 2021). These mechanism including wide array of antibiotic-hydrolyzing enzymes, efflux pump changes, impermeability, and antibiotic target mutations which gives a unique ability to the organism to maintain a multidrug-resistant phenotype (Asif et al. 2018; Espinal et al. 2019). According to the report of the Centers for Disease Control and Prevention, in 2017, Carbapenem-resistant Acinetobacter caused an estimated 8500 infections in hospitalized patients, and 700 people died as a result. CDC has reported a list of urgent threats bacteria that have the danger of antibiotic resistance. This list includes Carbapenem-resistant Acinetobacter, Candida auris, Clostridioides difficile, Carbapenem-resistant Enterobacterales, Drug-resistant Neisseria gonorrhea. Carbapenem-resistant Acinetobacter has been reported in the first line of this serious danger (Prevention 2019).
Carbapenem is a category of highly effective antibiotic drugs generally used for the treatment of severe or high-risk bacterial infections (Shaker and Shaaban 2017). The growing trend of Carbapenem resistance in A. baumannii recently has become a grave concern around the world because it limits the range of therapeutic alternatives (Higgins et al. 2010). In the context of the increasing worldwide occurrence of Carbapenem-resistant A. baumannii strains, Non-enzymatic molecular mechanisms act in cooperation with Carbapenem-hydrolyzing β-lactamases to provide an intense resistance. However, this process has not been described clearly (Catel-Ferreira et al. 2011). MDR strains showed a porin-mediated mechanism relating to the Carbapenem resistance-associated outer membrane protein, CarO (Catel-Ferreira et al. 2011; Mussi et al. 2007; Siroy et al. 2005). CarO protein generally is an eight‐stranded β‐barrel protein of 29 kDa which plays a key role in selective uptake of the ornithine, other basic amino acids, and uptake of Carbapenem (Catel-Ferreira et al. 2011). Mutations in the CarO gene can alter the structure or decrease the expression of the porin, resulting in a decline of antibiotics entry into the bacteria (Poirel et al. 2006; Zahn et al. 2015). In This study CarO protein was evaluated by available bioinformatics tools for designing an efficient multi-epitope subunit vaccine for the induction of immune responses against A. baumannii infections.
One of the critical issues in immunization is the presence of epitopes including B cells, HTL, and CTL that are recognized by immune system cells and capable of stimulating an immune response (Catel-Ferreira et al. 2011). The epitopes are the parts of a protein that have two shapes, continuous and discontinuous epitopes. Discontinuous epitopes consist of the residues from distant parts of the sequence that collectively made up the epitope and they will usually not recognized individually by antibodies appointed against the discontinuous epitope, therefore these epitopes are not suitable for the multi-epitope vaccine. However, continuous epitopes, also called sequential epitopes, are conformation independent, so they have the ability to be recognized by antibodies individually and independently (Chandra and Singh 2012; Rubinstein et al. 2008). Epitopes can induce a more direct and potent immune response, than the response induced by the whole protein. Therefore, the identification of antigenic epitopes can be more effective and safe in vaccine design (Palatnik-de-Sousa et al. 2018).
Immunoinformatics plays a key role in vaccine design and antibody production (Kazi et al. 2018). In the past, antibody design and vaccine development are expensive and time-consuming. Nowadays, advances in the field of bioinformatics have provided practical tools which can be used to lessen the time and cost for vaccine and antibody design (Anderson et al. 2013; Kazi et al. 2018). The Immunoinformatics approach allows the identification of the immunogenic epitopes from the pathogen genomes. Also, the ideal and immunogenic parts could be developed as potential vaccine candidates to trigger protective immune responses in the hosts (Ali et al. 2017).
Adjuvants are agents or drugs that have few or no antigenic effects or properties but can help enhance the efficacy and immunogenicity of vaccines (Mishra et al. 2020). In many studies, several kinds of adjuvants were used that had significant effects on the immunogenicity of the vaccine (Cayatte et al. 2017; Doener et al. 2019; Gasparini et al. 2001; Suschak et al. 2017; Yang et al. 2020). Adjuvants L7/L12 ribosomal protein (Behbahani 2020; Saadi et al. 2017), beta-defensin (Gupta et al. 2020; Wang et al. 2020), HBHA protein (Kumar et al. 2019; Lei et al. 2020), and HBHA conserved sequence(Rashidian et al. 2020; Solanki and Tiwari 2018) recently been identified as efficient and helpful adjuvants, and we used them in this study.
Infections caused by A. baumannii are becoming extremely difficult to prevent and treat because infection control strategies against the spread of A. baumannii are facing new multidrug-resistant (Rasooli et al. 2020). This study identifies the potential and immunogenic epitopes of CarO protein by bioinformatics tools. Then, linkers and adjuvant were added to the selected epitopes to design final multi-epitope vaccines and was selected the best vaccine construct based on allergenicity, antigenicity, and solubility features. The 3D structure of the protein was predicted, and its affinity to different HLA, TLR2, and TLR4 was investigated by molecular docking. Finally, the vaccine coding gene expression in E. coli was evaluated, and in silico cloning was performed in the pET28a (+) vector. This selected vaccine construct can be developed to cure the infection caused by the A. baumannii.
Method
Sequence Availability and Alignment
The CarO protein sequence was retrieved from NCBI at http://www.ncbi.nlm.nih.gov/protein and saved in FASTA format for further analyses (Pruitt et al. 2005); then Protein BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) against reference sequence on the database was performed using Blosum80 matrix to collect homologous sequences between different bacteria (Tatusova and Madden 1999). Because BLOSUM80 created for highly similar alignments and is used to find related proteins (Wheeler 2003). PRALINE server at https://www.ibi.vu.nl/programs/pralinewww/ was used for generating of alignments. This server based on homology-extended multiple alignments and the integration of predicted secondary structure information with iteration capabilities methods which have many advantages and have made this server stronger (Simossis and Heringa 2005). Alignments can demonstrate the conservancy of the protein residues among various strains (Simossis and Heringa 2003). The suitable vaccine candidate should able effective against all strains of a given pathogen. The conservancy of the amino acids among bacteria other than Acinetobacter implies probable cross-reactivity levels.
Prediction T Cell MHC Class I Epitope
The recognition of MHC-I antigenic peptide which exposed at the target cell surface is crucial for the adaptive immune response (Nguyen van Binh et al. 2006). NetCTL server at http://www.cbs.dtu.dk/services/NetCTL/ was used to predict MHC class I epitopes. In this server, the method integrates prediction of peptide MHC class I binding, proteasomal C terminal cleavage, and TAP transport efficiency (Larsen et al. 2005). Prediction threshold value 0.75 was set for epitope identification. Epitopes were chosen with a combinatorial score > 1 for further analyses (Larsen et al. 2007). Also, IEDB server at http://tools.iedb.org/mhci/ was used to predict peptide binding to MHC class I molecules based on the consensus method. Identified T-cell epitopes with HLA alleles were selected by percentile rank and IC50 values. The lower the percentile rank was shown the higher interaction between the peptide and MHC molecules. In Addition, high binding epitopes have an IC50 value below 50 nM, and weak binding peptides and IC50 value below 500 nM. So, Epitopes with both IC50 value < 50 nM and percentile rank ≤ 0.2 were selected for more analyses (Vita et al. 2019).
Prediction Class I Antigenic, Toxicity and Immunogenicity
A second screening step including their important features such as antigenicity and allergenicity which were checked by AllergenFP server at http://ddg-pharmfac.net/AllergenFP/ and Vaxijen server at http://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html. AllergenFP is generally utilized for the prediction of allergenicity of epitopes for vaccine design (Dimitrov et al. 2014). VaxiJen server used to predict antigenic properties of the peptide at a threshold value of 0.5. This server depends on Auto Cross Covariance (ACC) transformation, and alignment-independent predicted antigenic epitopes by their physicochemical behavior (Doytchinova and Flower 2007). In the following, Epitope/MHC complex should have the ability to evoke an immune response. So, we have used MHC I immunogenicity prediction tool in the IEDB server (Calis et al. 2013). Default parameters were selected and the epitopes which gained the positive value were chosen.
Prediction T Cell MHC Class II Epitope and Analysis Antigenic and Toxicity
To identify MHC-II binding epitopes two servers were employed. IEDB server at http://tools.iedb.org/mhci/ were used to compute MHC-II binding epitopes based on the consensus method. The consensus method uses a combination of both stabilization matrix alignment method and average relative binding matrix method. Predicted epitopes were selected relying on percentile rank and IC50 values (percentile rank ≤ 0.2 and IC50 value < 50 nM) (Jensen et al. 2018). NetMHCII 2.3 server at http://www.cbs.dtu.dk/services/NetMHCII/ was used to predict binding of peptides to HLA-DR, HLA-DQ and HLA-DP using artificial neural networks (Kaushik and Therapeutics 2019). Among predicted strong binding epitopes were chosen those had score < 1 for further analyses. Antigenicity, toxicity, and allergenicity were evaluated for predicted epitopes. The antigenicity and allergenicity of the epitopes were calculated by VaxiJen and AllergenFP server respectively (Dimitrov et al. 2014; Doytchinova and Flower 2007). In addition, mutation and toxicity features were checked by ToxinPred server at http://crdd.osdd.net/raghava/toxinpred/. In the silico method, ToxinPred was used to predict the toxicity of peptides and about how to design a peptide or protein with desired toxicity by mutating a minimum number of amino acids (Gupta et al. 2013).
Prediction and Screening of Liner B-Cell Epitopes
The recognition of B-cell epitopes has a key role in vaccine design, immunodiagnostic tests, and antibody production. There are various servers to predict B cell epitopes. Since each server performs based on specific algorithms and exclusive methods in epitope prediction. Therefore, the usage of several tools to predict linear B-cell epitopes in protein sequences are more valid. Ellipro server at http://tools.immuneepitope.org was employed to predict linear antibody epitopes based on a protein antigen's 3D structure (Ponomarenko et al. 2008). Also, liner B cell epitopes were predicted using an artificial neural network by ABCpred server at http://crdd.osdd.net (Saha and Raghava 2006a, b) In addition, SVMTriP (http://sysbio.unl.edu/SVMTriP/) was used in the prediction of the B cell linear epitopes. This server is a Support Vector Machine which used to predict linear antigenic epitopes which combine the Tri-peptide similarity and Propensity scores (SVMTriP) (Yao et al. 2012). For more identification of continuous B-cell epitopes from the CarO protein, Bcpred server at http://ailab.ist.psu.edu/bcpred/predict.html was utilized and the epitopes size of 20 amino acids as well as the specificity threshold of 0.75% were considered. The Bcpred server for predicting employs a subsequence kernel-based SVM classifier (EL‐Manzalawy et al. 2008). Selected epitopes from the servers mentioned above were screened for antigenicity and allergenicity properties (Dimitrov et al. 2014; Doytchinova and Flower 2007).
Construction of Model Vaccine
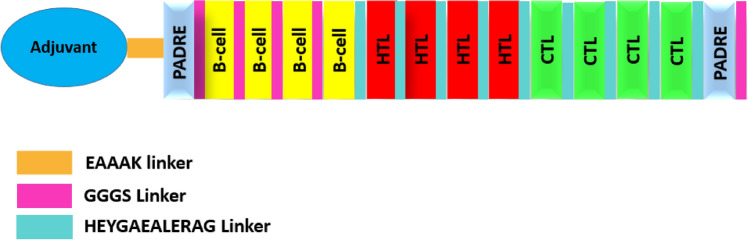
To construct the novel vaccine with low toxicity, allergenicity, and high immunogenicity, we have analyzed the different combinations of sequence constructs. During this novel multi-epitope subunit vaccine construction. Firstly, CTL, HTL, and B cell epitopes were joined with the help of amino acid linkers HEYGAEALERAG and GGGS respectively (Hasan et al. 2019; Nezafat et al. 2014). Secondly, for enhancing the immunogenicity of this sequence constructs were added the four different adjuvants L7/L12 ribosomal protein (Mallick et al. 2007), beta-defensin (Dar et al. 2015), HBHA protein (Nezafat et al. 2014), and HBHA conserved sequence respectively (Rana and Akhter 2016). Adjuvants sequences were added with the help of EAAAK linkers at both N- and C-terminal of Adjuvants (Saadi et al. 2017). PADRE peptide sequences were also incorporated along with the adjuvants by GGGS linker. PADRE peptide induced CD4+ T-cells that improve efficacy and potency of peptide vaccine (Wu et al. 2010).
Allergenicity, Antigenicity and Solubility Evaluation
The Allergenicity of vaccine constructs was predicted by AlgPred server (https://webs.iiitd.edu.in/raghava/algpred/submission.html) which based the SVM module on amino acid composition. A threshold score of prediction has been considered to be − 0.40. Prediction score less than the threshold value point displays the non-allergic feature of the peptide (Saha and Raghava 2006a, b). VaxiJen server and ANTIGENpro program (Kalita et al. 2020) at http://scratch.proteomics.ics.uci.edu were employed for the prediction of vaccine antigenicity behavior. In addition, SOLpro online server at http://scratch.proteomics.ics.uci.edu was utilized to predict the propensity of the protein to be soluble upon overexpression in Escherichia coli. This server uses a two-stage SVM architecture method to evaluate the protein solubility (Magnan et al. 2009).
Secondary Structure Prediction
The PSIPRED at http://bioinf.cs.ucl.ac.uk/psipred/ was used to predict the secondary structure of the vaccine construct. PSIPRED is a simple and accurate secondary structure prediction method, incorporating two feed-forward neural networks that perform an analysis on output obtained from PSI-BLAST (McGuffin et al. 2000). In addition, Phyre2 server at http://www.sbg.bio.ic.ac.uk/~phyre2/html/page.cgi?id=index employed to validate the PSIPRED predictions (Kelley et al. 2015).
Vaccine 3D Modeling, Refinement, and Validation
Homology modeling is the construction of an atomic model of a purpose protein based solely on the target's amino acid sequence and the experimentally determined structures of homologous proteins, referred to as templates (Kelley et al. 2015). While Ab initio prediction is the challenging attempt to predict protein structures based only on sequence information and without using templates (Yang and Zhang 2015). There are many tools and servers that are used for homology modeling and Ab initio prediction. There is no single modeling program or server which is superior in every aspect to others (Dolan et al. 2011). The SWISS-MODEL server at http://swiss-model.expasy.org/ is a fully automated protein structure homology-modeling server (Kiefer et al. 2009). I-TASSER server at https://zhanglab.dcmb.med.umich.edu/I-TASSER/ is based on hierarchical method to protein structure prediction and structure-based function annotation (Zhang 2008).
GMQE (Global Model Quality Estimation) is a quality estimation which combines properties from the target–template alignment and the template search method. QMEAN, which stands for Qualitative Model Energy Analysis, is a composite scoring function describing the major geometrical aspects of protein structures. All 3D models of the protein built by SWISS-MODEL server, were qualitatively estimated by GMQE and QMEAN scores (Schwede et al. 2003). In addition, C-score is a confidence score for estimating the quality of predicted models by I-TASSER. It is calculated based on the significance of threading template alignments and the convergence parameters of the structure assembly simulations (Yang et al. 2015). Finally, Qualitative evaluation of 3D models was done by ProSA at https://prosa.services.came.sbg.ac.at. ProSA specifically faces the needs confronted in the authentication of protein structures acquired from X-ray analysis, NMR spectroscopy, and hypothetical estimations (Wiederstein and Sippl 2007). Rampage at http://mordred.bioc.cam.ac.uk/rapper/rampage.php was also employed for estimation of model quality using Ramachandran plot which is an algorithm for atomic level, high-resolution protein structure improvement (Hooft et al. 1997).
Molecular Docking
The aim of molecular docking is to give a prediction of the binding affinity between a receptor molecule and ligand by computation methods (Ballester and Mitchell 2010). The vaccine construct 3 (V3) was made by I-TASSER server. In addition, PDB IDs of different HLA alleles were downloaded from the RCSB at https://www.rcsb.org/ (Rose et al. 2010). Molecular docking of V3 with 6 various HLA alleles including 1A6A(HLA-DR B1*03:01), 3C5J(HLA-DR B3*02:02), 1H15(HLA-DR B5*01:01), 2FSE(HLA-DRB1*01:01), 2Q6W(HLA-DR B3*01:01), and 2SEB(HLA-DRB1*04:01) was carried out by PatchDock server (Schneidman-Duhovny et al. 2005) at https://bioinfo3d.cs.tau.ac.il/PatchDock/ to represent HLA-peptide interactions. PatchDock predicted a number of 266 solutions that were again subjected to the FireDock server (http://bioinfo3d.cs.tau.ac.il/FireDock/) to refine and re-scoring of molecular docking score (Andrusier et al. 2007). TLRs are the most important membrane-bound PRRs, playing a critical role in the recognition of invading pathogens by the innate immune system. A. baumannii interacts with host cells mainly by engaging TLR2 and TLR4 (Chen 2020). In the same way, the PatchDock server was used to docking of vaccine construct (V3) with TLR2 (PDB ID 6NIG) and TLR4 (PDB ID 4G8A) and then the top 10 models were refined and re-scored by FireDock server. The docking models of TLRs with vaccine construct (V3) were displayed by the HADDOCK 2.4 server at https://wenmr.science.uu.nl/ (Van Zundert et al. 2016). The best cluster was chosen from the docked clusters based on the lowest HADDOCK score. Finally, the interacting residues, hydrogen bonds and hydrogen bonds distances between the vaccine and the TLRs were mapped using PDBsum at http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=index.html (Laskowski 2001).
Prediction of Various Physicochemical Properties
The functionally characterize of vaccine sequence was predicted by Expasy ProtParam server (https://web.expasy.org/protparam/). This server has determined the physicochemical characteristics such as the molecular weight, number of amino acids, PI values, hydropathicity GRAVY values, instability index, aliphatic index, and estimated half-life of the protein. Calculate of these physicochemical properties is on the basis of pK values of different amino acids. The instability index value shows protein is stable or unstable. The instability index value is < 40 for stable protein and > 40 for unstable protein. The aliphatic index determines the volume occupied by the aliphatic side chains of protein (Gasteiger et al. 2005).
Codon Optimization of the Vaccine Construct and In-Silico Cloning
It is important to consider codon optimization when performing expression studies because codon optimization plays a critical role in the success of protein expression (Mauro and Chappell 2018). Java Codon Adaptation Tool (JCAT) at http://www.jcat.de/ was employed to adapt the codon usage of vaccine construct to E. coli host strain 12. Vaccine amino acid sequence was back-translated to DNA sequence and then it was adapted for codon usage to E. coli. The adaptation was based on Codon Adaptation Index values (CAI). The rho-independent transcription terminators, prokaryotic ribosome binding sites, and cleavage sites of some restriction enzymes were avoided during the operation performed by JCAT server (Grote et al. 2005). In addition, to clone the adapted gene sequence of the final vaccine construct (V3) in E. coli pET28a vector (Ali et al. 2021), using Snapgene tool at https://www.snapgene.com/ to ensure the vaccine construct expression (Arumugam 2021) and BglII and ApaI restriction sites were introduced to the N and C-terminals of the sequence, respectively.
Result
The flow chart of experimental process which summarizes an overview of the methodology used in this study is shown in Fig. 1.
Fig. 1.
Flow chart showing an overview of our methodology
Protein Sequencing and Alignments
CarO protein sequence with accession No. AKL79738.1 is available as a query for protein BLAST against Acinetobacter producing a set of sequences containing various species of Acinetobacter. Out of 100 alignments, 20 sequences with the highest similarity (identity ≥ 90%, query coverage: 100% and E value: 0) were selected as T-COFFEE alignment input. BLAST search of Acinetobacter revealed numerous sequences of CarO. These hits showed that the CarO protein is specific for Acinetobacter species. The image of PRALINE alignment results with conservation color scheme is shown in Fig. 2.
Fig. 2.
CarO sequence alignment with 20 sequences obtained from protein BLAST against Acinetobacter. The superposition was made with the T-coffee program and adjusted manually. Residues conservancy is depicted by blue to pink colors
Selection of Potent MHC-I Epitopes in Protein and Analysis Eminent Features of Epitopes
On the basis of the high combinatorial score, the best T-cell epitopes were predicted by NetCTL server using the protein sequence. The software identified 36 epitopes in CarO protein. Peptides with combinatorial score > 1 were selected to other analyses (Table 1). In IEDB server, 9 epitopes were elicited with both IC < 200 nM and percentile rank ≤ 0.2 were subjected to afterward analysis (Table 1). After finalizing selected epitopes of both NetCTL and IEDB based above conditions, their important features including allergenicity, antigenicity, and immunogenicity Checked (Table 1). The sequences of IAPYLGFGF, KRIGNGDTL, WQANPYVGL and ALLWQANPY were considered as epitopes of T cells that interact with MHC Class-I and have the most important epitopes due to their features such as Non-allergen, positive scores in immunogenicity, and high scores in antigenicity.
Table 1.
CTL epitopes (MHC I peptides) of CarO protein with their antigenicity, allergenicity and immunogenicity scores
| No | CTL epitope | Position | Vaxijen score | Allergenicity | Immunogenicity | Final decision |
|---|---|---|---|---|---|---|
| 1 | GAAYLDNDY | 121 | − 0.6097 | Allergen | 0.02656 | |
| 2 | RAEVGTTGY | 43 | 1.4273 | Allergen | 0.19614 | |
| 3 | DVSVNGTKY | 79 | 1.7446 | Non allergen | − 0.0798 | |
| 4 | LLWQANPYV | 55 | 0.8355 | Allergen | − 0.02417 | |
| 5 | YIAAGAAYL | 117 | 0.1448 | Allergen | 0.15276 | |
| 6 | YLDNDYDLA | 124 | − 0.0090 | Allergen | 0.03105 | |
| 7 | VLVTTTALL | 6 | − 0.3603 | Allergen | 0.15334 | |
| 8 | GVRGKMSYK | 158 | 2.1964 | Non allergen | − 0.46618 | |
| 9 | YLGFGFAPK | 172 | 1.0001 | Allergen | 0.29854 | |
| 10 | AYLDNDYDL | 123 | − 0.0481 | Allergen | 0.04314 | |
| 11 | SYAFDKNQL | 29 | − 0.0618 | Allergen | − 0.12404 | |
| 12 | IAPYLGFGF | 169 | 1.4363 | Non allergen | 0.13238 | * |
| 13 | GVFGEVGAY | 186 | − 0.2548 | Allergen | 0.25992 | |
| 14 | DVSVNGTKY | 79 | 1.7446 | Non allergen | − 0.0798 | |
| 15 | YIAAGAAYL | 117 | 0.1448 | Allergen | 0.15276 | |
| 16 | SYKNDIAPY | 164 | 0.0616 | Allergen | 0.09691 | |
| 17 | RVLVTTTAL | 5 | − 0.3508 | Allergen | 0.1679 | |
| 18 | IPVGARAEV | 38 | 0.3971 | Allergen | 0.22584 | |
| 19 | NPTSAQDAV | 214 | 1.0699 | Allergen | − 0.18323 | |
| 20 | RVLVTTTAL | 5 | − 0.3508 | Allergen | 0.1679 | |
| 21 | KRIGNGDTL | 133 | 1.1551 | Non allergen | 0.1443 | * |
| 22 | YTGNPKVEL | 195 | 0.8255 | Non allergen | − 0.11597 | |
| 23 | YKNDIAPYL | 165 | − 0.0820 | Allergen | 0.17513 | |
| 24 | YIAAGAAYL | 117 | 0.1448 | Allergen | 0.15276 | |
| 26 | DMDNNNVYL | 90 | 0.3279 | Allergen | 0.02098 | |
| 27 | SYAFDKNQL | 29 | − 0.0618 | Allergen | − 0.12404 | |
| 28 | WQANPYVGL | 57 | 0.8396 | Non allergen | 0.0466 | * |
| 29 | TGYGGALLW | 49 | 0.1673 | Allergen | 0.08689 | |
| 30 | GKVGVNFYW | 241 | 1.6442 | Allergen | 0.17825 | |
| 31 | YLNAEIRPW | 97 | 0.4980 | Allergen | 0.29725 | |
| 32 | FAPKISKNW | 177 | 0.9830 | Allergen | − 0.43251 | |
| 33 | GVFGEVGAY | 186 | − 0.2548 | Allergen | 0.25992 | |
| 34 | ALLWQANPY | 54 | 0.6467 | Non allergen | 0.13138 | * |
| 35 | LYIAAGAAY | 116 | 0.4268 | Allergen | 0.20845 | |
| 36 | AVVHDSYAF | 24 | 0.2032 | Allergen | − 0.06844 | |
| 37 | YLNAEIRPW | 97 | 0.4980 | Allergen | 0.29725 | |
| 38 | YAFDKNQLI | 30 | − 0.8295 | Allergen | − 0.26001 | |
| 39 | DEAVVHDSY | 22 | 0.5088 | Allergen | 0.04695 | |
| 40 | YAFDKNQLI | 30 | − 0.8295 | Allergen | − 0.26001 | |
| 41 | STNPWAQGL | 108 | − 0.0570 | Allergen | 0.16131 | |
| 42 | DMDNNNVYL | 90 | 0.3279 | Allergen | 0.02098 | |
| 43 | AYYTGNPKV | 193 | 0.5286 | Allergen | − 0.07059 | |
| 44 | GVRGKMSYK | 158 | 2.1964 | No allergen | − 0.46618 | |
| 45 | VFGEVGAYY | 187 | − 0.3389 | No allergen | 0.21471 |
Four CTL epitopes were selected (labeled with *) for incorporation in the vaccine construct
Selection of MHC-II Epitopes and Prediction Their Antigenic and Toxicity
In addition to MHC-I epitope prediction, CarO protein was subjected to MHC-II binding prediction, using IEDB and NetMHCII server. The epitopes that elicited higher affinity (IC < 200 nM) and percentile rank ≤ 0.2 were subjected to afterward analysis (Table 2). NetMHCII server predicted more than 200 regions that could have an affinity for MHCII proteins. We selected 17 epitopes because these regions showed strong binding with a score < 1 and promising characteristics. After this process, selected epitopes from both servers were analyzed based on their essential features including mutation, toxicity, allergenicity, and antigenicity (Table 2). Finally, the sequences of GNGDTLSIDGKNYQQ, PWAQGLYIAAGAAYL, WAQGLYIAAGAAYLD, AQGLYIAAGAAYLDN, GLYIAAGAAYLDNDY, QGLYIAAGAAYLDND, ANEIRNDNKYEWMPV, GKMSYKNDIAPYLGF, KMSYKNDIAPYLGFG, RGKMSYKNDIAPYLG, DIAPYLGFGFAPKIS, and YLGFGFAPKISKNWG as the epitopes of T cells that interact with MHC Class-II alleles were considered as the most essential epitopes due to features of Non-allergen, non-toxin and high score in antigenicity.
Table 2.
HTL epitopes (MHC-II peptides) of CarO protein with their antigenicity, allergenicity, toxicity and mutation scores.
| No | HTL epitope | Position | Vaxijen Score | Allergenicity | Toxicity | Mutation | Final decision |
|---|---|---|---|---|---|---|---|
| 1 | KVLRVLVTTTALLAA | 16–2 | − 0.2328 | No allergen | |||
| 2 | LRVLVTTTALLAAGA | 18–4 | − 0.0644 | No allergen | |||
| 3 | MKVLRVLVTTTALLA | 15–1 | − 0.1848 | No allergen | |||
| 4 | VLRVLVTTTALLAAG | 17–3 | − 0.2461 | No allergen | |||
| 5 | GNGDTLSIDGKNYQQ | 150–131 | 1.8936 | No allergen | * | ||
| 6 | LSIDGKNYQQAVPGQ | 155–141 | 1.1512 | Allergen | |||
| 7 | TLSIDGKNYQQAVPG | 154–140 | 1.1523 | Allergen | |||
| 8 | PWAQGLYIAAGAAYL | 125–111 | 0.5259 | No allergen | * | ||
| 9 | WAQGLYIAAGAAYLD | 126–112 | 0.4335 | No allergen | * | ||
| 10 | AQGLYIAAGAAYLDN | 127–113 | 0.4818 | No allergen | * | ||
| 11 | GLYIAAGAAYLDNDY | 129–115 | 0.4596 | No allergen | * | ||
| 12 | QGLYIAAGAAYLDND | 128–114 | 0.4293 | No allergen | * | ||
| 13 | ANEIRNDNKYEWMPV | 241–226 | 1.7000 | No allergen | * | ||
| 14 | GKMSYKNDIAPYLGF | 175–161 | 0.5176 | No allergen | * | ||
| 15 | KMSYKNDIAPYLGFG | 176–162 | 0.4967 | No allergen | * | ||
| 16 | RGKMSYKNDIAPYLG | 174–160 | 0.5852 | No allergen | * | ||
| 17 | VHDSYAFDKNQLIPV | 40–26 | − 0.1666 | No allergen | |||
| 18 | VRGKMSYKNDIAPYL | 173–159 | 0.7463 | Allergen | |||
| 19 | IRPWGASTNPWAQGL | 102–116 | − 0.1476 | No allergen | |||
| 20 | DIAPYLGFGFAPKIS | 168–182 | 1.2750 | No allergen | * | ||
| 21 | TALLAAGAAMADEAV | 11–25 | − 0.0024 | No allergen | |||
| 22 | NPWAQGLYIAAGAAY | 110–124 | 0.1204 | No allergen | |||
| 23 | PWAQGLYIAAGAAYL | 111–125 | 0.0259 | No allergen | |||
| 24 | VHDSYAFDKNQLIPV | 26–40 | − 0.1666 | Allergen | |||
| 25 | VLRVLVTTTALLAAG | 3–17 | − 0.2461 | Allergen | |||
| 26 | KVLRVLVTTTALLAA | 2–16 | − 0.2328 | Allergen | |||
| 27 | IAAGAAYLDNDYDLA | 118–132 | − 0.0341 | No allergen | |||
| 28 | NPWAQGLYIAAGAAY | 110–124 | 0.1204 | No allergen | |||
| 29 | TALLAAGAAMADEAV | 11–25 | − 0.0024 | No allergen | |||
| 30 | VLVTTTALLAAGAAM | 6–20 | − 0.0596 | No allergen | |||
| 31 | AAGAAYLDNDYDLAK | 119–133 | − 0.0505 | No allergen | |||
| 32 | LVTTTALLAAGAAMA | 7–21 | 0.0283 | Allergen | |||
| 33 | YLGFGFAPKISKNWG | 172–186 | 1.7184 | No allergen | * |
Eleven HTL epitopes were selected (labeled with *) for selective step to incorporation in the vaccine construct
Linear B Cell Epitope Prediction
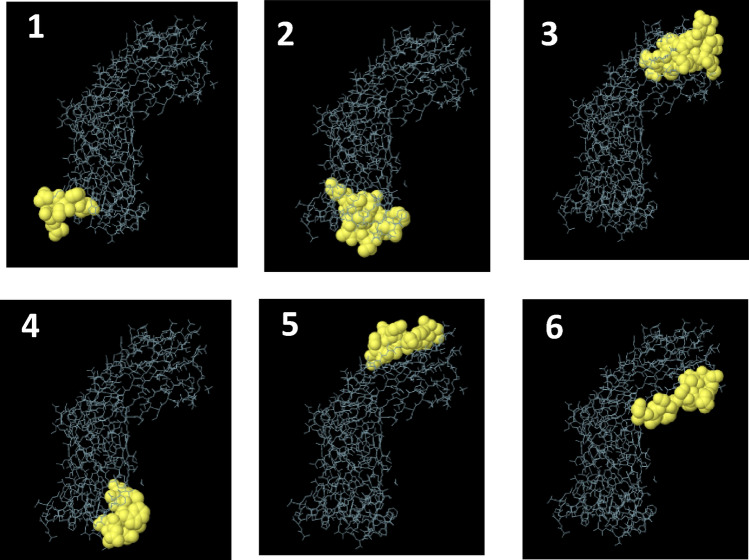
The analysis of the sequences by different bioinformatics programs for T and B cells yielded approximately 100 epitopes. Svmtrip predicted 3 Linear B cell epitopes based on the score. The epitopes recommended by this server are "AMADEAVVHDSYAFDKNQLI", "GAAYLDNDYDLAKRIGNGDT", and "WQANPYVGLA LGYNGGDISW". 6 linear along B cell epitopes were predicted by ElliPro software. The sequences and pictures of 6 linear epitopes with the highest PI (protrusion index) are shown in Fig. 3 and Table 3 (Rows 7–12).
Fig. 3.
Epitope mapping on 3D models. Discovery Studio Visualizer 2.5.5 software was used. From 1 to 6 pictures, 6 linear epitopes with the highest PI score predicted by Ellipro server are shown
Table 3.
B-cell epitopes of CarO protein with their antigenicity and allergenicity scores
| No | Bcell epitope | Position | Vaxijen Score | Allergenicity | Final decision |
|---|---|---|---|---|---|
| 1 | SIDGKNYQQAVPGQEGGVRG | 142–161 | 1.6824 | No allergen | * |
| 2 | LNAEIRPWGASTNPWAQGLY | 98–117 | 0.1589 | Allergen | |
| 3 | KNWGVFGEVGAYYTGNPKVE | 183–202 | 0.0053 | Allergen | |
| 4 | GYNGGDISWTDDVSVNGTKY | 68–87 | 0.9330 | No allergen | * |
| 5 | APVTGNPTSAQDAVDKEANE | 209–228 | 1.1717 | No allergen | * |
| 6 | NQLIPVGARAEVGTTGYGGA | 35–54 | 0.8647 | No allergen | * |
| 7 | KNQLIPV | 34–40 | − 0.7431 | Allergen | |
| 8 | IRPWGASTNRWAQGL | 81–95 | 0.3082 | Allergen | |
| 9 | NVDATRSFRVNNQDFIAGADGV | 113–134 | 0.8684 | No allergen | * |
| 10 | FAPKINKNWGV | 154–164 | 0.7523 | No allergen | * |
| 11 | SGSAVTTGDQSLEEAVNA | 183–200 | 1.2565 | No allergen | * |
| 12 | DISWSDDVKVNGST | 52–65 | 1.2709 | No allergen | * |
| 13 | AMADEAVVHDSYAFDKNQLI | 19–38 | 0.0036 | Allergen | |
| 14 | GAAYLDNDYDLAKRIGNGDT | 121–140 | 0.3189 | Allergen | |
| 15 | WQANPYVGLALGYNGGDISW | 57–76 | 0.4059 | Allergen | |
| 16 | ACINETACTERAMANN | 15–30 | 0.5248 | No allergen | * |
| 17 | EVGAYYTGNPKVELTQ | 232–247 | 0.5643 | No allergen | * |
| 18 | RAEVGTTGYGGALLWQ | 85–100 | 0.9620 | No allergen | * |
| 19 | EAVVHDSYAFDKNQLI | 65–80 | − 0.1973 | Allergen | |
| 20 | GGDISWTDDVSVNGTK | 113–228 | 1.3248 | No allergen | * |
| 21 | DNKYEWMPVGKVGVNF | 274–289 | 0.1697 | Allergen | |
| 22 | PVTGNPTSAQDAVDKE | 252–267 | 1.1236 | No allergen | * |
| 23 | TQYNLAPVTGNPTSAQ | 246–261 | 0.8548 | Allergen | |
| 24 | GQEGGVRGKMSYKNDI | 196–211 | 1.9167 | Allergen |
Thirteen B-cell epitopes were selected (labeled with *) for selective step to incorporation in the vaccine construct
ABCpred result showed 65 hits of 16 metric peptide sequences as B-cell epitopes ranking based on scores. The best epitopes predicted by the server with a score above 0.85 are the sequences of "ACINETACTERAMANN", "EVGAYYTGNPKVELTQ", "RAEVGTTGYGGALLWQ", "EAVVHDSYAFDKNQLI", "GGDISWTDD VSVNGTK", "DNKYEWMPVGKVGVNF", "PVTGNPTSAQDAVDKE", "TQYNLAPVTGNPTSAQ", and "GQEGGVRGKMSYKNDI". BCpred server predicted 6 B-cell epitopes. The scores of epitopes ranging from 0.787 to 0.994 where a high score represents the higher probability of becoming B-cell epitopes and higher affinity for the B-cell receptor. The total promising linear B cell epitopes which were mentioned above were analyzed by the Vaxijen server (≥ 0.4 score) and AllergenFP server in order to participate in the vaccine construct.
Comparison of All the Predicted Epitopes to Select the Final Epitopes
After collecting the immunoinformatics information with the help of T-cell prediction and B-cell prediction, the high scores of HTL, CTL, and B cell epitopes were predicted and analyzed for their essential feature. Finally, to make the final multi-epitope construct, 4 CTL epitopes, 4 HTL epitopes, and 4 B cell epitopes due to features of non-Allergen, non-toxic, and higher score in antigenicity (Table 4).
Table 4.
The final selection of CTL, HTL and B-cell epitopes for multi-epitope vaccine construct
| No | Type of epitope | Sequence of epitope | Antigenicity score |
|---|---|---|---|
| 1 | CTL | IAPYLGFGF | 1.4363 |
| 2 | CTL | KRIGNGDTL | 1.1551 |
| 3 | CTL | WQANPYVGL | 0.8396 |
| 4 | CTL | ALLWQANPY | 0.6467 |
| 5 | HTL | GNGDTLSIDGKNYQQ | 1.8936 |
| 6 | HTL | ANEIRNDNKYEWMPV | 1.7000 |
| 7 | HTL | DIAPYLGFGFAPKIS | 1.2750 |
| 8 | HTL | YLGFGFAPKISKNWG | 1.7184 |
| 9 | B-cell | SIDGKNYQQAVPGQEGGVRG | 1.6824 |
| 10 | B-cell | DISWSDDVKVNGST | 1.2709 |
| 11 | B-cell | GGDISWTDDVSVNGTK | 1.3248 |
| 12 | B-cell | PVTGNPTSAQDAVDKE | 1.1236 |
Construction of Final Vaccine with Different Adjuvants, Linkers, and PADRE Sequence
A total of four vaccine constructs were prepared and further analyzed. All four vaccine constructs were added with the respective adjuvants with the help of EAAAK linker. MHC-I, MHC-II, B-epitopes, and PADRE sequence were joined together by HEYGAEALERAG and GGGS linkers (Fig. 4). Details of the vaccine constructs have been mentioned in Table 5.
Fig. 4.
Schematic diagram of multi-epitope vaccine
Table 5.
Allergenicity prediction of all vaccine construct using Algpred, Vaxijen and scratch protein predictor server
| No | Vaccine construct |
Complete sequence of vaccine construct | Allergenicity (Algpred) (Threshold − 0.4) |
Vaxijen Score |
SOLpro | ANTIGENpro |
|---|---|---|---|---|---|---|
| 1 | V1 | EAAAKMAKLSTDELLDAFKEMTLLELSDFVKKFEETFEVTAAAPVAVAAAGAAPAGAAVEAAEEQSEFDVILEAAGDKKIGVIKVVREIVSGLGLKEAKDLVDGAPKPLLEKVAKEAADEAKAKLEAAGATVTVKEAAAKAKFVAAWTLKAAAGGGSSIDGKNYQQAVPGQEGGVRGGGGSDISWSDDVKVNGSTGGGSGGDISWTDDVSVNGTKGGGSPVTGNPTSAQDAVDKEHEYGAEALERAGGNGDTLSIDGKNYQQHEYGAEALERAGANEIRNDNKYEWMPVHEYGAEALERAGDIAPYLGFGFAPKISHEYGAEALERAGYLGFGFAPKISKNWGHEYGAEALERAGIAPYLGFGFHEYGAEALERAGKRIGNGDTLHEYGAEALERAGWQANPYVGLHEYGAEALERAGALLWQANPYHEYGAEALERAG AKFVAAWTLKAAAGGGS |
0.22954849 Allergen |
0.8945 | 0.954742 | 0.831728 |
| 2 | V2 | EAAAKGIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKKEAAAKAKFVAAWTLKAAAGGGSSIDGKNYQQAVPGQEGGVRGGGGSDISWSDDVKVNGSTGGGSGGDISWTDDVSVNGTKGGGSPVTGNPTSAQDAVDKEHEYGAEALERAGGNGDTLSIDGKNYQQHEYGAEALERAGANEIRNDNKYEWMPVHEYGAEALERAGDIAPYLGFGFAPKISHEYGAEALERAGYLGFGFAPKISKNWGHEYGAEALERAGIAPYLGFGFHEYGAEALERAGKRIGNGDTLHEYGAEALERAGWQANPYVGLHEYGAEALERAGALLWQANPYHEYGAEALERAG AKFVAAWTLKAAAGGGS |
− 0.3428515 Allergen |
1.0459 | 0.972937 | 0.875245 |
| 3 | V3 | EAAAKMAENPNIDDLPAPLLAALGAADLALATVNDLIANLRERAEETRAETRTRVEERRARLTKFQEDLPEQFIELRDKFTTEELRKAAEGYLEAATNRYNELVERGEAALQRLRSQTAFEDASARAEGYVDQAVELTQEALGTVASQTRAVGERAAKLVGIELEAAAKAKFVAAWTLKAAAGGGSSIDGKNYQQAVPGQEGGVRGGGGSDISWSDDVKVNGSTGGGSGGDISWTDDVSVNGTKGGGSPVTGNPTSAQDAVDKEHEYGAEALERAGGNGDTLSIDGKNYQQHEYGAEALERAGANEIRNDNKYEWMPVHEYGAEALERAGDIAPYLGFGFAPKISHEYGAEALERAGYLGFGFAPKISKNWGHEYGAEALERAGIAPYLGFGFHEYGAEALERAGKRIGNGDTLHEYGAEALERAGWQANPYVGLHEYGAEALERAGALLWQANPYHEYGAEALERAGAKFVAAWTLKAAAGGGS |
− 0.40767406 No allergen |
0.9251 | 0.950830 | 0.847375 |
| 4 | V4 | EAAAKMAENSNIDDIKAPLLAALGAADLALATVNELITNLRERAEETRRSRVEESRARLTKLQEDLPEQLTELREKFTAEELRKAAEGYLEAATSELVERGEAALERLRSQQSFEEVSARAEGYVDQAVELTQEALGTVASQVEGRAAKLVGIELEAAAKAKFVAAWTLKAAAGGGSSIDGKNYQQAVPGQEGGVRGGGGSDISWSDDVKVNGSTGGGSGGDISWTDDVSVNGTKGGGSPVTGNPTSAQDAVDKEHEYGAEALERAGGNGDTLSIDGKNYQQHEYGAEALERAGANEIRNDNKYEWMPVHEYGAEALERAGDIAPYLGFGFAPKISHEYGAEALERAGYLGFGFAPKISKNWGHEYGAEALERAGIAPYLGFGFHEYGAEALERAGKRIGNGDTLHEYGAEALERAGWQANPYVGLHEYGAEALERAGALLWQANPYHEYGAEALERAG AKFVAAWTLKAAAGGGS |
− 0.2874046 Allergen |
0.9330 | 0.966516 | 0.819423 |
Prediction Features of Different Vaccine Constructs
Antigenicity of the vaccine proteins was predicted using VaxiJen server and ANTIGENpro. All Constructs were found as potential vaccine candidates with high antigenicity scores and the ability to stimulate an efficient immune response (Table 5). Also all 4 constructs showed the solubility more than 0.7, which indicated that the vaccine construct will be highly soluble during its heterologous expression in the E. coli (Table 5). But, among four constructs, the only vaccine with beta-defensin (V3) was found to be non-allergic in nature (Allergenicity < − 0.4). So, the V3 construct was chosen for further analysis.
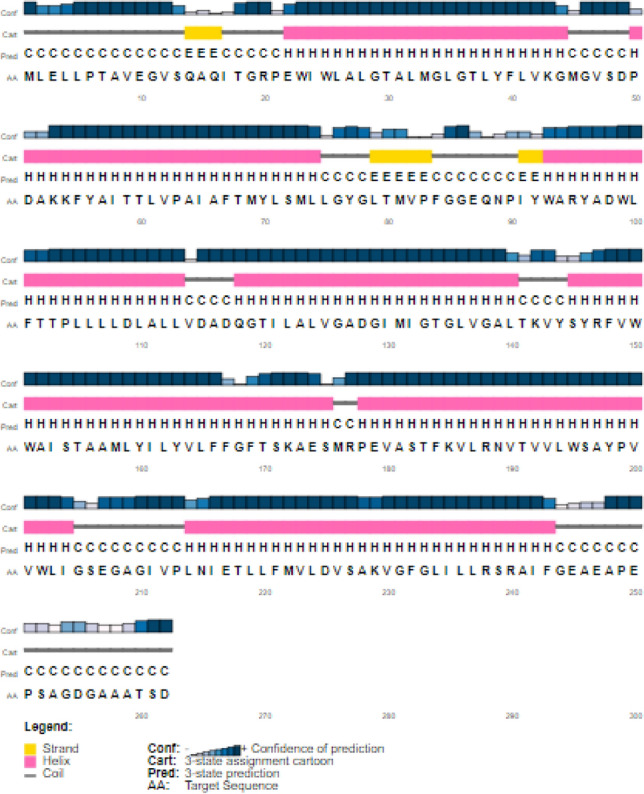
Prediction Secondary Structure
PSIPRED and Phyre2 servers were used to predict the secondary structure of the construct V3. The predicted structure of the protein confirmed to have 68.70% alpha-helix, 27.48% sheet, and 3.82% coil structure (Fig. 5). The results from Phyre2 confirmed the results of the PSIPRED program.
Fig. 5.
Secondary structure prediction of vaccine construct (V3) using PESIPRED server
Vaccine 3D Modeling, Refinement, and Validation
Swiss model and I-TASSER software were recruited for homology modeling and Ab initio prediction. The former prepared 12 different models and the latter introduced 5 models. Among the structures predicted by the Swiss model, the best structures were selected based on the QMEAN and GMQE scores. QMEAN scores about zero show good agreement between the model structure and experimental structures of similar size and scores of − 4.0 or below indicate models with low quality. In addition, the GMQE score is expressed as a number between zero and one, reflecting the expected accuracy of a model built with that alignment and template. Higher numbers illustrate higher reliability. Two 3D models have shown GMQE with scores − 1.41 and − 1.38 and QMEAN with scores of 0.05 and 0.04 respectively. The best structure among five models of I-TASSER were measured on the basis of C-score. The C-score is normally in [− 5, 2] and It should be noted that the higher this score, the more accurate the model quality. Since the C-score of model 2 is higher (= − 0.83) than other models, is expected to have good quality. Finally, three predicted 3D models were selected for further analyses.
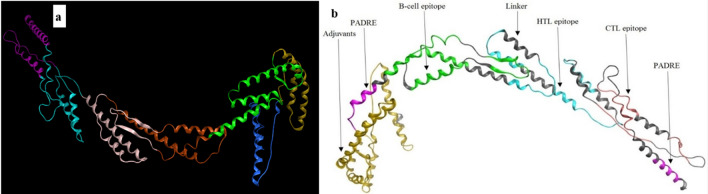
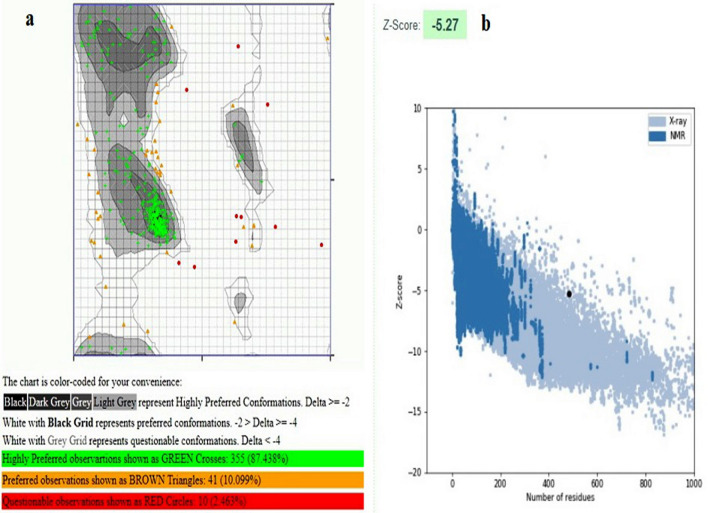
The quality of the 3D models were evaluated by Rampage and Prosa servers. Based on the result of Ramachandran plot and Prosa the best model was chosen from the predicted structure by I-TASSER (Fig. 6a). We showed the Cartoon diagram of a computational structural model of the vaccine in the following figure by using Molecular Operating Environment Software. This figure displays that the sequence of each epitope is properly folded and exposed (Fig. 6b). This structure of vaccine construct show which 87.438% of the residues are in favored regions. Also, Prosa revealed that the predicted model was among other acceptable proteins with z score = − 5.27 (Fig. 7).
Fig. 6.
a The best model of epitope vaccine (V3) predicted by I-TASSER server. b All of the regions of the vaccine for display on the scaffold are labeled and colored in representing adjuvants_yellow, PADRE_purple, B-cell epitopes_green, Linker_gray, HTL_blue, and CTL_pink (Color figure online)
Fig. 7.
Model evaluation. a Ramachandran plot of final epitope vaccine (V3) model. Number of residues in favored region: 355 (87.438%). Number of residues in allowed region: 41(10.099%). Number of residues in outlier region: 10 (2.463%). b Prosa protein structure analysis results. Z score = − 5.27. Overall quality of the ultimate model is acceptable
Molecular Docking
Docking analysis was employed between the vaccine construct 3 and different HLA alleles (Table 6). Construct V3 displayed biologically significant results and HLA-DR B1*01:01 found to be superior among different HLA in terms of free binding energy. Moreover, docking was also used to predict the binding affinity of the designed vaccine construct with human TLR2 and TLR4 receptors using PatchDock and Firedock servers respectively. FireDock output refinement of PatchDock server showed the lowest global energy of − 27.61 and − 50.74 for TLR2 and TLR4 respectively. In order to display docking of protein–ligand complexes was used HADDOCK. The top cluster with the lowest HADDOCK score of − 100.1 ± − 5.4 and − 160.9 ± − 29.4 suggests a good binding affinity between the vaccine and the TLR2 and TLR4. The docked complexes along with hydrogen bonds and distance of hydrogen bonds are shown in Figs. 8 and 9 and Tables 7 and 8.
Table 6.
Prediction binding energy of vaccine construct (V3) with selected HLA molecules by PatchDock, Firedock
| Vaccine constructs |
HLA alleles & their PDB ID’s |
Score | Area | Hydrogen bond energy |
Globel energy |
ACE |
|---|---|---|---|---|---|---|
| V3 | HLA-DRB1*03:01 (1A6A) | 13,384 | 2181.40 | −1.41 | 0.33 | 3.51 |
| HLA-DR B3*02:02 (3C5J) | 14,840 | 2191.30 | − 4.17 | − 10.18 | 12.89 | |
| HLA-DR B5*01:01 (1H15) | 14,816 | 2309.50 | − 1.23 | − 14.97 | 1.11 | |
| HLA-DR B1*01:01 (2FSE) | 13,982 | 1947.70 | − 4.11 | − 21.21 | 8.95 | |
| HLA-DRB3*01:01 (2Q6W) | 15,688 | 2164.50 | − 0.23 | − 3.22 | 2.43 | |
| HLA-DR B1*04:01 (2SEB) | 13,990 | 1956.80 | − 0.44 | − 5.23 | 0.36 |
Fig. 8.
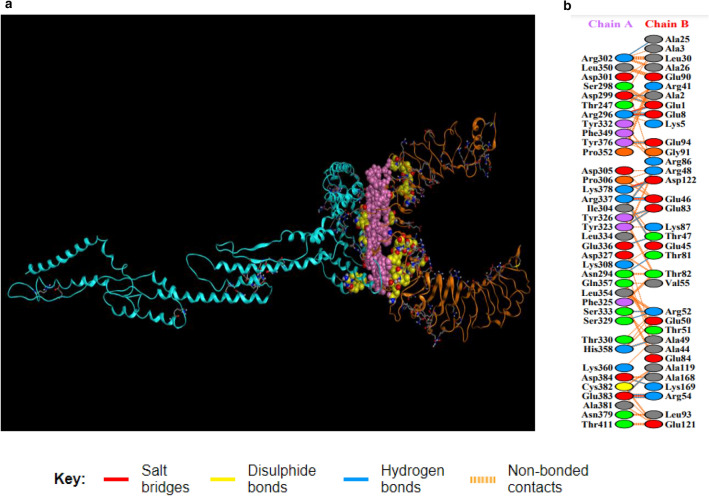
a Showing TLR2/vaccine docked complex. Vaccine construct 3 is shown in blue colour while TLR2 is shown in orange colour. Interacting residues and active pockets between docked TLR2/vaccine are displayed with purple and yellow filling space model respectively. b Few prominent hydrogen bonds within vaccine-TLR2 complex are focused (Color figure online)
Fig. 9.
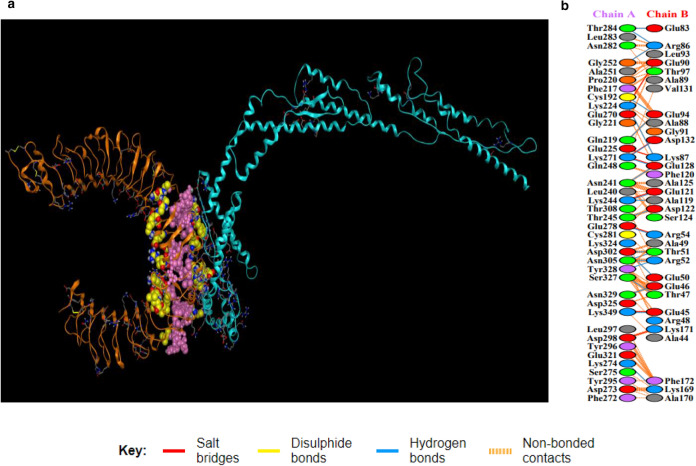
a Showing TLR4/vaccine docked complex. Vaccine construct 3 is shown in blue colour while TLR4 is shown in orange colour. Interacting residues and active pockets between docked TLR4/vaccine are displayed with purple and yellow filling space model respectively. b Few prominent hydrogen bonds within vaccine-TLR4 complex are focused (Color figure online)
Table 7.
List of residues involved in hydrogen bonds and hydrogen bonds distance in TLR2-vaccine interactions
| No. | Res name | Res no. | Chain | Res name | Res no. | Chain | Distance hydrogen bonds |
|---|---|---|---|---|---|---|---|
| 1 | ASN | 294 | A | THR | 81 | B | 3.03 |
| 2 | ARG | 296 | A | GLU | 1 | B | 2.67 |
| 3 | ARG | 296 | A | GLU | 8 | B | 2.75 |
| 4 | ARG | 296 | A | GLU | 8 | B | 2.6 |
| 5 | ASP | 299 | A | GLU | 1 | B | 2.75 |
| 6 | ASP | 299 | A | ALA | 2 | B | 2.84 |
| 7 | ARG | 302 | A | ALA | 25 | B | 2.79 |
| 8 | ILE | 304 | A | ARG | 48 | B | 2.69 |
| 9 | TYR | 323 | A | GLU | 83 | B | 2.63 |
| 10 | TYR | 326 | A | GLU | 83 | B | 3.11 |
| 11 | SER | 329 | A | ARG | 52 | B | 3.04 |
| 12 | SER | 333 | A | ARG | 52 | B | 2.77 |
| 13 | GLU | 336 | A | THR | 47 | B | 2.65 |
| 14 | ARG | 337 | A | GLU | 45 | B | 2.97 |
| 15 | ARG | 337 | A | GLU | 46 | B | 3 |
| 16 | ARG | 337 | A | GLU | 46 | B | 2.78 |
| 17 | HIS | 358 | A | ALA | 49 | B | 2.66 |
| 18 | TYR | 376 | A | GLU | 94 | B | 2.61 |
| 19 | LYS | 378 | A | ASP | 122 | B | 2.62 |
| 20 | GLU | 383 | A | ALA | 119 | B | 3.1 |
| 21 | GLU | 383 | A | ARG | 54 | B | 2.66 |
| 22 | GLU | 383 | A | ARG | 54 | B | 3.06 |
| 23 | ASP | 384 | A | LYS | 169 | B | 2.67 |
Table 8.
List of residues involved in hydrogen bonds and hydrogen bonds distance in TLR4-vaccine interactions
| No. | Res name | Res no. | Chain | Res name | Res no. | Chain | Distance hydrogen bonds |
|---|---|---|---|---|---|---|---|
| 1 | CYS | 192 | A | GLU | 94 | B | 2.98 |
| 2 | GLN | 219 | A | GLU | 94 | B | 2.65 |
| 3 | LYS | 224 | A | LYS | 87 | B | 2.67 |
| 4 | ASN | 241 | A | GLU | 128 | B | 3.1 |
| 5 | LYS | 244 | A | ALA | 119 | B | 2.79 |
| 6 | LYS | 244 | A | GLU | 121 | B | 2.65 |
| 7 | THR | 245 | A | ASP | 122 | B | 2.64 |
| 8 | GLY | 252 | A | ARG | 86 | B | 2.86 |
| 9 | LYS | 271 | A | GLU | 128 | B | 2.54 |
| 10 | LYS | 271 | A | ASP | 132 | B | 2.71 |
| 11 | LYS | 274 | A | LYS | 169 | B | 3.31 |
| 12 | GLU | 278 | A | ARG | 54 | B | 2.57 |
| 13 | THR | 284 | A | GLU | 83 | B | 3.05 |
| 14 | THR | 284 | A | ARG | 86 | B | 2.84 |
| 15 | ASP | 302 | A | GLU | 50 | B | 3 |
| 16 | ASP | 302 | A | THR | 51 | B | 2.67 |
| 17 | ASN | 305 | A | ARG | 52 | B | 2.7 |
| 18 | ASN | 305 | A | THR | 51 | B | 2.78 |
| 19 | SER | 327 | A | THR | 47 | B | 2.62 |
| 20 | TYR | 328 | A | ARG | 52 | B | 3.04 |
| 21 | ASN | 329 | A | GLU | 46 | B | 2.67 |
| 22 | LYS | 349 | A | GLU | 45 | B | 2.65 |
Physicochemical Analysis of Vaccine Construct (V3)
The vaccine construct (V3) was characterized on the basis of various physicochemical properties using ProtParam tool. The molecular weight of the vaccine construct was found 51.57 kDa which shows its good antigenic potential. GRAVY (a hydropathic index) was − 0.527 (a negative value), which represents the hydrophilic nature of constructs. The calculated instability index of the protein was 23.43 which classified it as a stable one. The estimated half-life of the constructed vaccine was expected to be 1 h in mammalian reticulocytes in vitro while more than 10 h in E. coli in vivo. Aliphatic value 71.90 also showed that the vaccine construct has a good characteristic to initiate an immunogenic reaction.
In-Silico Cloning of Chimeric Vaccine Construct (V3) for Its Heterologous Expression
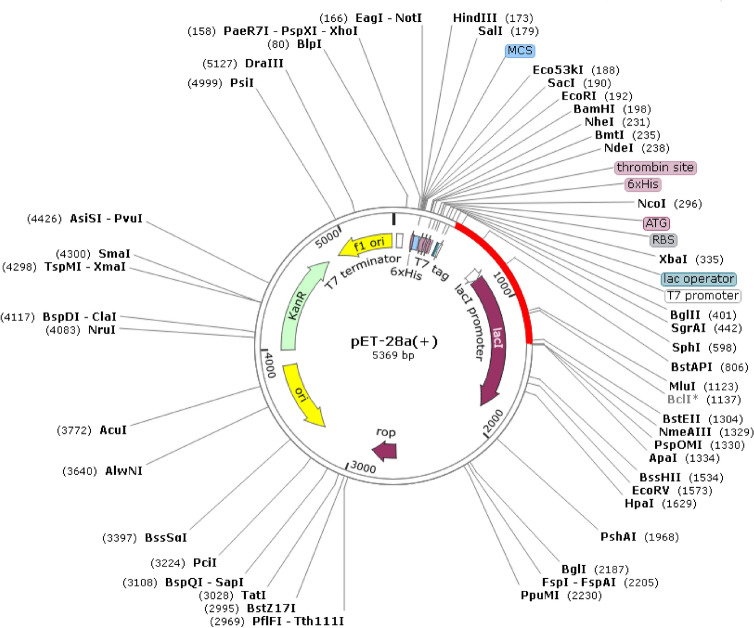
Reverse translation and codon optimization were performed by considering the expression system of the host in order to express the multi-epitope vaccine. The optimum range of GC content was considered to be between 30 and 70%. The GC content of the optimized codons (53.95%) was significant. Also, the high value of the CAI score shows a high expression of the gene. Our construct has a codon adaptation index (CAI) of 1. Recognition sites for BglII and ApaI restriction enzymes were added to the 5′ and 3′ end of the optimized gene and then the final vaccine construct (V3) with a total length of 1465 bp was cloned into pET28a vector for its heterologous cloning and expression in E. coli (Fig. 10). Polyhistidine-tag is an amino acid motif in proteins that typically consists of at least six histidines (His) residues which is often at the N- or C-terminus with spaced or attached to inserted protein. His-tag will be used to purify protein in further experiments. In addition, the His-tag is part of the pET28a vector structure.
Fig. 10.
In silico cloning of the gene sequence of the final vaccine construct V3 into pET28a (+) expression vector. The target sequence was inserted between BglII (401) and ApaI (1334)
Discussion
The emergence of multidrug resistance (MDR) A. baumannii is recognized as a serious health concern in hospitals across the world (Savov et al. 2019). CarO from A. baumannii is a membrane porin that uptake small molecules such as l-ornithine, Carbapenem, and other basic amino acids (Labrador-Herrera et al. 2020; Tohidinia et al. 2020). In a study, researchers were demonstrated outer membrane vesicles (OMVs) of A. baumannii had able to induce protective immunity against A. baumannii. Among OMV proteins, CarO was identified as a highly immunogenic protein (McConnell et al. 2011). Currently, there is no definite cure to protect against the A. baumannii due to increase MDR. Therefore, it is essential to take preventive measures against it (Solanki and Tiwari 2018). The employment of bioinformatics tools in the field of vaccinology can be aiding the selection of potential vaccine candidates and simplify the optimization of the chosen immunogen (Gupta and Kumar 2020; Tohidinia and Sefid 2020).
The entire proteome of the A. baumannii ATCC 19606 strain was retrieved from NCBI and the result of BLAST from various sequences of CarO showed it is specific to Acinetobacter species. Alignment of CarO sequences from various strains indicates that such vaccines are able to trigger antibodies with complete specificity for A. baumannii and CarO is a conserved protein among Acinetobacter species. Therefore, it can one of the best choices for vaccine design. A multi-epitope vaccine consists of several B and T epitopes with sensible linkers in order to avoid the generation of junctional epitopes and increase the antigen presentation process (Forouharmehr 2021; Shey et al. 2019). A multi-epitope vaccine can trigger both humoral and cell-mediated immunity (Ali et al. 2017). The cell-mediated immune response is mediated by T-cells whereas the humoral immune response is mediated by produced B-cells (Channappanavar et al. 2014; Kozakiewicz et al. 2013). Therefore, the correct and accurate selection of TCL, HTL, and B-cell epitopes to design a multi-epitope vaccine has a direct relationship with the immune system response.
Approximately 300 epitopes of CarO protein were predicted by IEDB with recommended methods and NetCTL server to be MHC I peptides that can bind to a large number of HLA-A and HLA-B alleles with a high binding affinity. 350 epitopes of CarO protein were also predicted by using the MHC-II binding prediction tool of IEDB and NetMHCII server as MHC II peptides. But the most important epitopes were selected among the identified epitopes with certain scores mentioned in the method section and then their important characteristics were examined. One of the most important features considered for selecting CTL epitopes was the Vaxijen score. The higher Vaxijen score indicating the higher antigenicity of an amino acid sequence and is able to further stimulate the immune system (Saha et al. 2017). In this study, only CTL epitopes with non-allergenic and positive immunogenicity behavior were selected for further analysis. In addition, the characteristics of the allergenicity, antigenicity, toxicity, and mutation were examined for the selection of HTL epitopes. Finally, according to all these characteristics, sequences IAPYLGFGF, KRIGNGDTL, WQANPYVGL, and ALLWQANPY as CTL epitopes and GNGDTLSIDGKNYQQ, ANEIRNDNKYEWMPV, DIAPYLGFGFAPKIS, and YLGFGFAPKISKNWG as HTL epitopes were selected to participate in the structure of the vaccine. Amino acid sequences that are linear in shape are called continuous or linear epitopes (Abboud et al. 2009) which were predicted by ElliPro, ABCpred, Svmtrip, and BCpred servers. Through the epitope prediction servers carried out in this project we were able to compile 82 potential linear B cell epitopes in CarO protein. Among promising linear B cell epitopes, four B cell epitopes were selected based on their features such as antigenicity and allergenicity.
In the construction of vaccines, the selected epitopes were connected by HEYGAEALERAG and GGGS linkers. Each of these constructions was connected to 4 different adjuvants with the EAAAK linker. Finally, after examining the allergenicity, antigenicity, and solubility behaviors vaccine construct 3 which had HBHA adjuvant was selected due to non-allergic in nature and high allergenicity and antigenicity score to prediction the second and third structures.
To ensure effective binding between HLA molecules and vaccine construct 3, a docking study was performed. The vaccine construct 3 was subjected to the PatchDock server as a receptor and 6 different HLA alleles including 1A6A(HLA-DR B1*03:01), 3C5J(HLA-DR B3*02:02), 1H15(HLA-DR B5*01:01), 2FSE(HLA-DRB1*01:01), 2Q6W(HLA-DR B3*01:01), and 2SEB(HLA-DRB1*04:01) as a ligand were selected for docking analysis. All the selected HLA showed low binding energy with V3 which was biologically significant. HLA-DR B1*01:01 was found to be best considering the free binding energy. Moreover, to display interactions among human TLRs (TLR2 and TLR4) and the vaccine structure 3, HADDOCK server were applied for molecular docking. Docking results demonstrated that the multi-epitope vaccine we designed has the potential to bind the mention receptors. Also, the PDBsum server showed 22 and 23 hydrogen bonds between the vaccine and TLR2 and TLR4 respectively. Hydrogen bonds can increase the strength and stability of a vaccine-receptor interaction substantially (Fox et al. 2013). Physicochemical properties of the V3 sequence were analyzed using ProtParam server which showed nature of V3 is appropriate for vaccine construct. Finally, the vaccine construct V3 was reverse transcribed and adapted for E. coli strain K12 former to insertion within pET28a (+) vector for its cloning and expression. In our study, our in silico results were based on various databases. We suggest using model animals for further analysis and experimental validation of this designed vaccine.
Acknowledgements
We thank Yazd University for granting us a permission to use its web server.
Funding
This study was supported by an unrestricted free access to Yazd University web site for data collection.
Declarations
Conflict of interest
The authors have received no financial support for the elaboration of this manuscript. Yazd University did not play any decision-making role in the study analysis or writing of the manuscript. All authors declare no potential conflicts of interest.
Ethical Approval
As the present study involved no experimental with animals or human, hence there was no need for approval by the Ethics Committee of Yazd University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maryam Touhidinia, Email: Maryamtohidi90@yahoo.com.
Fatemeh Sefid, Email: sefid.fateme@yahoo.com.
Mozhgan Bidakhavidi, Email: M_bidakhavidi@yahoo.com.
References
- Abboud N, et al. Identification of linear epitopes in Bacillus anthracis protective antigen bound by neutralizing antibodies. J Biol Chem. 2009;284:25077–25086. doi: 10.1074/jbc.M109.022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, Pandey RK, Khatoon N, Narula A, Mishra A, Prajapati VK. Exploring dengue genome to construct a multi-epitope based subunit vaccine by utilizing immunoinformatics approach to battle against dengue infection. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-09199-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, et al. Proteome wide vaccine targets prioritization and designing of antigenic vaccine candidate to trigger the host immune response against the Mycoplasma genitalium infection. Microb Pathog. 2021;152:104771. doi: 10.1016/j.micpath.2021.104771. [DOI] [PubMed] [Google Scholar]
- Anderson L, Dormitzer P, Nokes D, Rappuoli R, Roca A, Graham B. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31:B209–B215. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrusier N, Nussinov R, Wolfson HJ. FireDock: fast interaction refinement in molecular docking. Proteins. 2007;69:139–159. doi: 10.1002/prot.21495. [DOI] [PubMed] [Google Scholar]
- Arumugam S. In-silico design of envelope based multi-epitope vaccine candidate against Kyasanur forest disease virus. Sci Rep. 2021 doi: 10.21203/rs.3.rs-155690/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif M, Alvi IA, Rehman SU. Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect Drug Resist. 2018;11:1249. doi: 10.2147/IDR.S166750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub Moubareck C, Hammoudi Halat D. Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics. 2020;9:119. doi: 10.3390/antibiotics9030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester PJ, Mitchell JB. A machine learning approach to predicting protein–ligand binding affinity with applications to molecular docking. Bioinformatics. 2010;26:1169–1175. doi: 10.1093/bioinformatics/btq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbahani M. In silico design of novel multi-epitope recombinant vaccine based on coronavirus surface glycoprotein. Bioinformatics. 2020;17:122. [Google Scholar]
- Calis JJ, et al. Properties of MHC class I presented peptides that enhance immunogenicity. PLoS Comput Biol. 2013;9:e1003266. doi: 10.1371/journal.pcbi.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catel-Ferreira M, et al. Structure–function relationships of CarO, the carbapenem resistance-associated outer membrane protein of Acinetobacter baumannii. J Antimicrob Chemother. 2011;66:2053–2056. doi: 10.1093/jac/dkr267. [DOI] [PubMed] [Google Scholar]
- Cayatte C, Marin A, Rajani GM, Schneider-Ohrum K, Snell Bennett A, Marshall JD, Andrianov AK. PCPP-adjuvanted respiratory syncytial virus (RSV) sF subunit vaccine: self-assembled supramolecular complexes enable enhanced immunogenicity and protection. Mol Pharm. 2017;14:2285–2293. doi: 10.1021/acs.molpharmaceut.7b00118. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2019) 2019 AR threats report. https://www.cdc.gov/drugresistance/biggest-threats.html#acine
- Chandra S, Singh TR. Linear B cell epitope prediction for epitope vaccine design against meningococcal disease and their computational validations through physicochemical properties. Netw Model Anal Health Inf Bioinform. 2012;1:153–159. doi: 10.1007/s13721-012-0019-1. [DOI] [Google Scholar]
- Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. Host innate immune responses to Acinetobacter baumannii infection. Front Cell Infect Microbiol. 2020;10:486. doi: 10.3389/fcimb.2020.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar A, Tipu M, Townsend H, Potter A, Gerdts V, Tikoo S. Administration of poly [di (sodium carboxylatoethylphenoxy) phosphazene](PCEP) and avian beta defensin as adjuvants in inactivated inclusion body hepatitis virus and its hexon protein-based experimental vaccine formulations in chickens. Avian Dis. 2015;59:518–524. doi: 10.1637/11202-052815-Reg.1. [DOI] [PubMed] [Google Scholar]
- Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- Dimitrov I, Naneva L, Doytchinova I, Bangov I. AllergenFP: allergenicity prediction by descriptor fingerprints. Bioinformatics. 2014;30:846–851. doi: 10.1093/bioinformatics/btt619. [DOI] [PubMed] [Google Scholar]
- Doener F, et al. RNA-based adjuvant CV8102 enhances the immunogenicity of a licensed rabies vaccine in a first-in-human trial. Vaccine. 2019;37:1819–1826. doi: 10.1016/j.vaccine.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Dolan MA, Noah JW, Hurt D. Homology modeling. Totowa: Humana Press; 2011. Comparison of common homology modeling algorithms: application of user-defined alignments; pp. 399–414. [DOI] [PubMed] [Google Scholar]
- Doytchinova IA, Flower DR. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007;8:4. doi: 10.1186/1471-2105-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp BA, Stroeher UH, Hassan KA, Paulsen IT, Brown MH. Comparative analysis of surface-exposed virulence factors of Acinetobacter baumannii. BMC Genomics. 2014;15:1–12. doi: 10.1186/1471-2164-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos GM, Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- EL‐Manzalawy Y, Dobbs D, Honavar V Predicting linear B-cell epitopes using string kernels. J Mol Recognit. 2008;21:243–255. doi: 10.1002/jmr.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinal P, et al. Relationship between different resistance mechanisms and virulence in Acinetobacter baumannii. Microb Drug Resist. 2019;25:752–760. doi: 10.1089/mdr.2018.0182. [DOI] [PubMed] [Google Scholar]
- Forouharmehr A. Engineering an efficient poly-epitope vaccine against Toxoplasma gondii infection: a computational vaccinology study. Microb Pathog. 2021;152:104646. doi: 10.1016/j.micpath.2020.104646. [DOI] [PubMed] [Google Scholar]
- Fox CB, Kramer RM, Barnes VL, Dowling QM, Vedvick TS. Working together: interactions between vaccine antigens and adjuvants. Ther Adv Vaccines. 2013;1:7–20. doi: 10.1177/2051013613480144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini R, Montomoli E, Fragapane E, Senatore F, Minutello M, Podda A. Increased immunogenicity of the MF59-adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur J Epidemiol. 2001;17:135–140. doi: 10.1023/A:1017919305501. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, Wilkins MR, Appel RD, Bairoch A. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. The proteomics protocols handbook. Totowa: Humana Press; 2005. pp. 571–607. [Google Scholar]
- Grote A, Hiller K, Scheer M, Münch R, Nörtemann B, Hempel DC, Jahn D. JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005;33:W526–W531. doi: 10.1093/nar/gki376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Kumar A. Designing an efficient multi-epitope vaccine against Campylobacter jejuni using immunoinformatics and reverse vaccinology approach. Microb Pathog. 2020;147:104398. doi: 10.1016/j.micpath.2020.104398. [DOI] [PubMed] [Google Scholar]
- Gupta S, Kapoor P, Chaudhary K, Gautam A, Kumar R, Raghava GP, Open Source Drug Discovery Consortium In silico approach for predicting toxicity of peptides and proteins. PLoS ONE. 2013;8:e73957. doi: 10.1371/journal.pone.0073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Regar H, Verma VK, Prusty D, Mishra A, Prajapati VK. Receptor-ligand based molecular interaction to discover adjuvant for immune cell TLRs to develop next-generation vaccine. Int J Biol Macromol. 2020;152:535–545. doi: 10.1016/j.ijbiomac.2020.02.297. [DOI] [PubMed] [Google Scholar]
- Hasan M, Ghosh PP, Azim KF, Mukta S, Abir RA, Nahar J, Khan MMH. Reverse vaccinology approach to design a novel multi-epitope subunit vaccine against avian influenza A (H7N9) virus. Microb Pathog. 2019;130:19–37. doi: 10.1016/j.micpath.2019.02.023. [DOI] [PubMed] [Google Scholar]
- Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65:233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- Hooft RW, Sander C, Vriend G. Objectively judging the quality of a protein structure from a Ramachandran plot. Bioinformatics. 1997;13:425–430. doi: 10.1093/bioinformatics/13.4.425. [DOI] [PubMed] [Google Scholar]
- Howard A, O’Donoghue M, Feeney A, Sleator R. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KK, et al. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology. 2018;154:394–406. doi: 10.1111/imm.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalita P, Padhi AK, Zhang KY, Tripathi T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb Pathog. 2020;145:104236. doi: 10.1016/j.micpath.2020.104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik V, Therapeutics In silico identification of epitope-based peptide vaccine for Nipah virus. Int J Peptide Res Ther. 2019 doi: 10.1007/s10989-019-09917-0. [DOI] [Google Scholar]
- Kazi A, Chuah C, Majeed ABA, Leow CH, Lim BH, Leow CY. Current progress of immunoinformatics approach harnessed for cellular-and antibody-dependent vaccine design. Pathog Glob Health. 2018;112:123–131. doi: 10.1080/20477724.2018.1446773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozakiewicz L, Phuah J, Flynn J, Chan J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. In: Divangahi M, editor. The new paradigm of immunity to tuberculosis. Advances in experimental medicine and biology. New York: Springer; 2013. pp. 225–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sunagar R, Gosselin E. Bacterial protein toll-like-receptor agonists: a novel perspective on vaccine adjuvants. Front Immunol. 2019;10:1144. doi: 10.3389/fimmu.2019.01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakidis I, Vasileiou E, Pana ZD, Tragiannidis A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens. 2021;10:373. doi: 10.3390/pathogens10030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrador-Herrera G, et al. Virulence role of the outer membrane protein CarO in carbapenem-resistant Acinetobacter baumannii. Virulence. 2020;11:1727–1737. doi: 10.1080/21505594.2020.1855912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen MV, Lundegaard C, Lamberth K, Buus S, Brunak S, Lund O, Nielsen M. An integrative approach to CTL epitope prediction: a combined algorithm integrating MHC class I binding, TAP transport efficiency, and proteasomal cleavage predictions. BMC Bioinform. 2005;35:2295–2303. doi: 10.1002/eji.200425811. [DOI] [PubMed] [Google Scholar]
- Larsen MV, Lundegaard C, Lamberth K, Buus S, Lund O, Nielsen M. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinform. 2007;8:424. doi: 10.1186/1471-2105-8-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski RA. PDBsum: summaries and analyses of PDB structures. Nucleic Acids Res. 2001;29:221–222. doi: 10.1093/nar/29.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Shao J, Ma F, Lei C, Chang H, Zhang Y. Enhanced efficacy of a multi-epitope vaccine for type A and O foot-and-mouth disease virus by fusing multiple epitopes with Mycobacterium tuberculosis heparin-binding hemagglutinin (HBHA), a novel TLR4 agonist. Mol Immunol. 2020;121:118–126. doi: 10.1016/j.molimm.2020.02.018. [DOI] [PubMed] [Google Scholar]
- Magnan CN, Randall A, Baldi P. SOLpro: accurate sequence-based prediction of protein solubility. Bioinformatics. 2009;25:2200–2207. doi: 10.1093/bioinformatics/btp386. [DOI] [PubMed] [Google Scholar]
- Mallick A, et al. Escheriosome-mediated delivery of recombinant ribosomal L7/L12 protein confers protection against murine brucellosis. Vaccine. 2007;25:7873–7884. doi: 10.1016/j.vaccine.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Mauro VP, Chappell SA. Recombinant protein expression in mammalian cells. New York: Springer; 2018. Considerations in the use of codon optimization for recombinant protein expression; pp. 275–288. [DOI] [PubMed] [Google Scholar]
- McConnell MJ, Domínguez-Herrera J, Smani Y, López-Rojas R, Docobo-Pérez F, Pachón J. Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant Acinetobacter baumannii. Infect Immun. 2011;79:518–526. doi: 10.1128/IAI.00741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- Mishra R, Krishan S, Siddiqui AN, Kapur P, Khayyam KU, Sharma M. Potential role of adjuvant drugs on efficacy of first line oral antitubercular therapy: drug repurposing. Tuberculosis. 2020;120:101902. doi: 10.1016/j.tube.2020.101902. [DOI] [PubMed] [Google Scholar]
- Mussi MA, Relling VM, Limansky AS, Viale AM. CarO, an Acinetobacter baumannii outer membrane protein involved in carbapenem resistance, is essential for l-ornithine uptake. FEBS Lett. 2007;581:5573–5578. doi: 10.1016/j.febslet.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Nezafat N, Ghasemi Y, Javadi G, Khoshnoud MJ, Omidinia E. A novel multi-epitope peptide vaccine against cancer: an in silico approach. J Theor Biol. 2014;349:121–134. doi: 10.1016/j.jtbi.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Nguyen van Binh P, Duc HT. Epitopes of the class I major histocompatibility complex (MHC-I) recognized in the syngeneic or allogeneic context predominantly linked to antigenic peptide loading to its binding groove. Clin Exp Immunol. 2006;145:372–379. doi: 10.1111/j.1365-2249.2006.03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeidat N, Jawdat F, Al-Bakri AG, Shehabi AA. Major biologic characteristics of Acinetobacter baumannii isolates from hospital environmental and patients' respiratory tract sources. Am J Infect Control. 2014;42:401–404. doi: 10.1016/j.ajic.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Palatnik-de-Sousa C, Soares I, Rosa D. Editorial: epitope discovery and synthetic vaccine design. Front Immunol. 2018;9:826. doi: 10.3389/fimmu.2018.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect. 2006;12:826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- Ponomarenko J, Bui H-H, Li W, Fusseder N, Bourne PE, Sette A, Peters B. ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinform. 2008;9:514. doi: 10.1186/1471-2105-9-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt KD, Tatusova T, Maglott DR. NCBI Reference Sequence (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2005;33:D501–D504. doi: 10.1093/nar/gki025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A, Akhter Y. A multi-subunit based, thermodynamically stable model vaccine using combined immunoinformatics and protein structure based approach. Immunobiology. 2016;221:544–557. doi: 10.1016/j.imbio.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Rashidian E, Forouharmehr A, Nazifi N, Jaydari A, Shams N. Computer-aided design of a novel poly-epitope protein in fusion with an adjuvant as a vaccine candidate against leptospirosis. Curr Proteomics. 2020;17:1–11. [Google Scholar]
- Rasooli I, Abdolhamidi R, Jahangiri A, Astaneh SDA. Outer membrane protein, Oma87 prevents Acinetobacter baumannii infection. Int J Pept Res Ther. 2020;26(4):2653–2660. doi: 10.1007/s10989-020-10056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose PW, et al. The RCSB Protein Data Bank: redesigned web site and web services. Nucleic Acids Res. 2010;39:D392–D401. doi: 10.1093/nar/gkq1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ND, Mayrose I, Halperin D, Yekutieli D, Gershoni JM, Pupko T. Computational characterization of B-cell epitopes. Mol Immunol. 2008;45:3477–3489. doi: 10.1016/j.molimm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Saadi M, Karkhah A, Nouri HR. Development of a multi-epitope peptide vaccine inducing robust T cell responses against brucellosis using immunoinformatics based approaches. Infect Genet Evol. 2017;51:227–234. doi: 10.1016/j.meegid.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Saha S, Raghava GPS. AlgPred: prediction of allergenic proteins and mapping of IgE epitopes. Nucleic Acids Res. 2006;34:W202–W209. doi: 10.1093/nar/gkl343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Raghava GPS. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins. 2006;65:40–48. doi: 10.1002/prot.21078. [DOI] [PubMed] [Google Scholar]
- Saha CK, Hasan MM, Hossain MS, Jahan MA, Azad AK. In silico identification and characterization of common epitope-based peptide vaccine for Nipah and Hendra viruses. Asian Pac J Trop Med. 2017;10:529–538. doi: 10.1016/j.apjtm.2017.06.016. [DOI] [PubMed] [Google Scholar]
- Savov E, Trifonova A, Kovachka K, Kjosseva E, Strateva T. Antimicrobial in vitro activities of ceftazidime-avibactam, meropenem-vaborbactam and plazomicin against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa—a pilot Bulgarian study. Infect Dis. 2019;51:870–873. doi: 10.1080/23744235.2019.1653491. [DOI] [PubMed] [Google Scholar]
- Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 2005;33:W363–W367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker MA, Shaaban MI. Formulation of carbapenems loaded gold nanoparticles to combat multi-antibiotic bacterial resistance: in vitro antibacterial study. Int J Pharm. 2017;525:71–84. doi: 10.1016/j.ijpharm.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Shey RA, et al. In-silico design of a multi-epitope vaccine candidate against onchocerciasis and related filarial diseases. Sci Rep. 2019;9:1–18. doi: 10.1038/s41598-019-40833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simossis VA, Heringa J. The PRALINE online server: optimising progressive multiple alignment on the web. Amsterdam: Elsevier; 2003. [DOI] [PubMed] [Google Scholar]
- Simossis VA, Heringa J. PRALINE: a multiple sequence alignment toolbox that integrates homology-extended and secondary structure information. Comput Biol Chem. 2005;33:W289–W294. doi: 10.1093/nar/gki390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siroy A, et al. Channel formation by CarO, the carbapenem resistance-associated outer membrane protein of Acinetobacter baumannii. Antimicrob Agents Chemother. 2005;49:4876–4883. doi: 10.1128/AAC.49.12.4876-4883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki V, Tiwari V. Subtractive proteomics to identify novel drug targets and reverse vaccinology for the development of chimeric vaccine against Acinetobacter baumannii. Sci Rep. 2018;8:1–19. doi: 10.1038/s41598-018-26689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suschak JJ, Williams JA, Schmaljohn CS. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum Vaccines Immunother. 2017;13:2837–2848. doi: 10.1080/21645515.2017.1330236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusova TA, Madden TL. BLAST 2 sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 1999;174:247–250. doi: 10.1111/j.1574-6968.1999.tb13575.x. [DOI] [PubMed] [Google Scholar]
- Tohidinia M, Sefid F. Identification B and T-cell epitopes and functional exposed amino acids of S protein as a potential vaccine candidate against SARS-CoV-2/COVID-19. Microb Pathog. 2020;148:104459. doi: 10.1016/j.micpath.2020.104459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohidinia M, Moshtaghioun SM, Sefid F, Falahati A. Functional exposed amino acids of CarO analysis as a potential vaccine candidate in Acinetobacter Baumannii. Int J Pept Res Ther. 2020;26:1185–1197. doi: 10.1007/s10989-019-09923-2. [DOI] [Google Scholar]
- Valencia R, et al. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect Control Hosp Epidemiol. 2009;30:257. doi: 10.1086/595977. [DOI] [PubMed] [Google Scholar]
- Van Zundert G, et al. The HADDOCK2. 2 web server: user-friendly integrative modeling of biomolecular complexes. J Mol Biol. 2016;428:720–725. doi: 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Vita R, et al. The immune epitope database (IEDB): 2018 update. Nucleic Acids Res. 2019;47:D339–D343. doi: 10.1093/nar/gky1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, et al. The improvement of immune effect of recombinant human beta-defensin 2 on hepatitis B vaccine in mice. Viral Immunol. 2020;34:96–111. doi: 10.1089/vim.2020.0052. [DOI] [PubMed] [Google Scholar]
- Wheeler D. Selecting the right protein-scoring matrix. Curr Protoc Bioinform. 2003 doi: 10.1002/0471250953.bi0305s00. [DOI] [PubMed] [Google Scholar]
- Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35:W407–W410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-Y, Monie A, Pang X, Hung C-F, Wu TC. Improving therapeutic HPV peptide-based vaccine potency by enhancing CD4+ T help and dendritic cell activation. J Biomed Sci. 2010;17:1–10. doi: 10.1186/1423-0127-17-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang Y. Protein structure and function prediction using I-TASSER. Curr Protoc Bioinform. 2015 doi: 10.1002/0471250953.bi0508s52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, et al. Effectiveness, immunogenicity, and safety of influenza vaccines with MF59 adjuvant in healthy people of different age groups: a systematic review and meta-analysis. Medicine. 2020;99:e19095. doi: 10.1097/MD.0000000000019095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B, Zhang L, Liang S, Zhang C. SVMTriP: a method to predict antigenic epitopes using support vector machine to integrate tri-peptide similarity and propensity. PLoS ONE. 2012;7:e45152. doi: 10.1371/journal.pone.0045152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn M, D'agostino T, Eren E, Baslé A, Ceccarelli M, Van Den Berg B Small-molecule transport by CarO, an abundant eight-stranded β-barrel outer membrane protein from Acinetobacter baumannii. J Mol Biol. 2015;427:2329–2339. doi: 10.1016/j.jmb.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008;9:1–8. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]