Abstract
Multiple vascular anomalies may be encountered in patients with nutcracker syndrome; further compounding the surgical complexity in managing this condition. A 28-year-old male presented with persistent flank pain and hematuria. Imaging revealed narrowing of the left renal vein at the aortomesenteric junction, and a dilated vein consistent with the left gonadal vein. On surgical exploration, a duplicated IVC was found. The patient underwent a right caval-to-left caval bypass using a cryopreserved femoral vein homograft. The surgery was well tolerated and completely resolved the patient's symptoms.
Keywords: Nutcracker syndrome, IVC Duplication, Hematuria, Congenital anomalies
1. Introduction
Nutcracker syndrome (NCS) is a constellation of symptoms arising from extrinsic compression of the left renal vein (LRV), most commonly between the aorta and the superior mesenteric artery (SMA). The clinical presentation of NCS is variable, ranging from asymptomatic to gross hematuria, flank pain, pelvic congestion, and varicocele formation.1,2 Asymptomatic patients may be managed with surveillance, however, the presence of symptoms necessitates surgical intervention.2
Duplication of the inferior vena cava (IVC) is a rare congenital anomaly that is found in 0.2–3% of the general population. Normal embryogenesis of the IVC occurs through a coordinated series of events involving the development and regression of three paired embryonic veins, with the abnormal persistence of both supracardinal veins resulting in a double IVC.3 Although these patients are often asymptomatic and incidentally diagnosed, their complex anatomy conveys an increased predisposition to surgical complications.
Due to the predominance of genitourinary symptoms associated with NCS, the urologist is often consulted for the clinical and surgical management of this condition. As a result, they should have an adequate understanding of potentially associated vascular abnormalities. Very few cases of a duplicated IVC in NCS have been disclosed, and to the best of our knowledge, this is the first reported case where a left caval-to-right caval anastomosis was performed.
2. Case presentation
A 28-year-old healthy male presented with gross hematuria and left flank pain of 10 months duration. These symptoms were reproducible with exercise and showed progression in frequency and severity over time. Physical exam was unremarkable, showing no signs of costovertebral angle tenderness or palpable varicocele. Laboratory evaluation was insignificant except for microscopic hematuria on urinalysis.
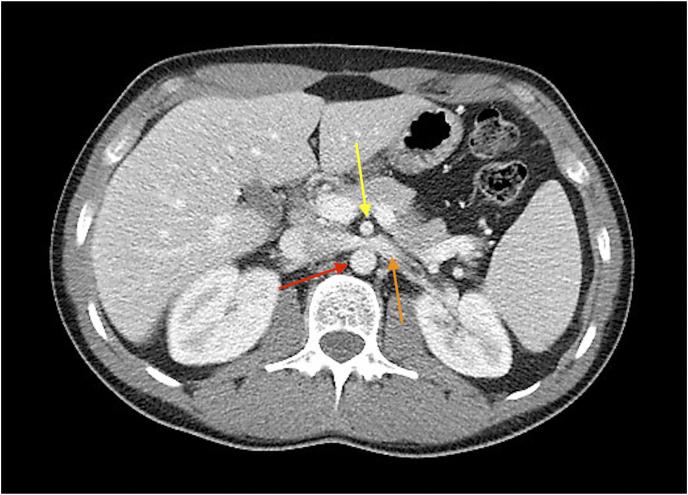
Abdominal computerized tomography (CT) imaging revealed compression of the LRV at the level of the SMA and a dilated left gonadal vein (LGV) measuring 12.8 mm in diameter. The diameter of the LRV was 3.5 mm at the aortomesenteric junction and 8 mm at the portion adjacent to the renal hilum (Fig. 1).
Fig. 1.
Axial computed tomography image showing entrapment of the left renal vein (yellow) between the aorta (red) and the superior mesenteric artery (orange). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
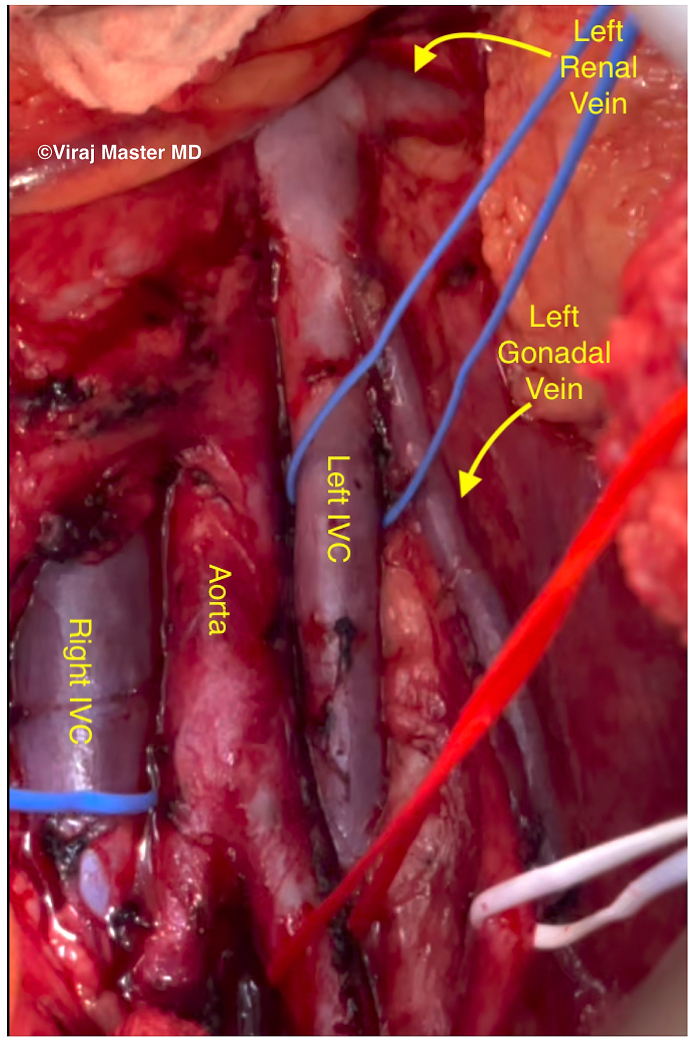
The patient was offered a LGV to IVC transposition via a transperitoneal approach. After mobilization of the splenic flexure and descending colon, the large-caliber vessel believed to be the LGV was visualized. Complete skeletonization revealed a separate LGV entering the lateral aspect of the dilated venous structure. The structure was in fact a duplicated IVC, which continued inferiorly as the left external iliac vein with no communication from the common or left internal iliac veins (Fig. 2). The decision was made to proceed with a left caval-to-right caval bypass using a cryopreserved femoral vein homograft (Fig. 3a).
Fig. 2.
Schematic drawing detailing the vasculature anatomy of the presented patient.
Fig. 3a.
(Left): Intraoperative photograph demonstrating a duplicated IVC inserting into the left renal vein.
Circumferential dissection of both IVCs were performed. The venous graft was then sewn into the left IVC, which was proximally and distally clamped during this time. The anastomosis between the venous graft and the right IVC was executed in a similar fashion (Fig. 3b). After the anastomosis was completed, a Doppler ultrasound was performed showing brisk and open flow in the bypass graft.
Fig. 3b.
(Right): Intraoperative photograph of a left caval-to-right caval bypass using a cryopreserved femoral vein homograft.
The estimated blood loss was 100 mL. The patient had an uncomplicated hospital stay and was discharged on post-operative day 2. At the patient's follow-up appointment, his hematuria and flank pain were completely resolved.
3. Discussion
We report a unique case of NCS in the presence of a duplicated IVC. Due to interrelated mechanisms of embryologic formation, vascular anomalies of the IVC and renal veins often coexist, providing potential reasoning for our patient's anatomic and clinical presentation.3
Patients with either NCS or a duplicated IVC are often asymptomatic and thus incidentally diagnosed during radiographic evaluation. The diameters of the IVCs in the setting of duplication are frequently asymmetrical, which may contribute to diagnostic errors.3 Dilatation of the LGV is often seen in NCS, and due to its similar location and course, may obscure the presence of a duplicated IVC on imaging studies. However, IVC duplication and dilatation of the LGV may be distinguished anatomically. Distally, the duplicated IVC will either bifurcate or continue inferiorly as the left external iliac vein, whereas the LGV will emerge from the inguinal canal.3,4
The presentation of NCS varies with the degree of LRV entrapment, with insufficient venous outflow commonly manifesting as flank pain and gross hematuria. Prolonged venous reflux may result in gonadal varices, pelvic congestion, and the formation of collateral veins to facilitate hilar decompression.1,2 As a potential source of intraoperative hemorrhage, identification of these anomalous tributary vessels is of key importance during surgical intervention.
Our patient's worsening of symptoms with exercise may partially be attributed to the physiologic increase in venous return during physical activity. In a non-exertional state, the duplicated IVC assisted in draining the left kidney, transmitting blood from the LRV to the left common iliac vein, essentially bypassing the site of compression. When exercising, the increase in venous pressure in the left IVC exceeded that of the LRV, reversing the direction of flow. Renal capillary rupture and capsular distension secondary to elevated intrarenal pressure caused the patient's hematuria and flank pain, respectively.
Definitive surgical correction and endovascular stenting are the predominant interventions used in the management of NCS. Reports vary on the efficacy of intra-vascular approaches as stent migration and re-stenosis may result in symptom recurrence.5
4. Conclusion
Additional vascular anomalies frequently coexist in patients with NCS. Duplication of the IVC is extremely rare and complicates surgical correction of LRV entrapment. A right caval-to-left caval bypass is a unique option for the management of NCS in the setting of a double IVC, and to our knowledge, has yet to be reported in the literature.
Declaration of competing interest
None of the contributing authors have any conflict of interest, including specific financial interests or relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
References
- 1.Kurklinsky A.K., Rooke T.W. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. 2010;85(6):552–559. doi: 10.4065/mcp.2009.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ananthan K., Onida S., Davies A.H. Nutcracker syndrome: an update on current diagnostic criteria and management guidelines. Eur J Vasc Endovasc Surg. 2017;53(6):886–894. doi: 10.1016/j.ejvs.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Bass J.E., Redwine M.D., Kramer L.A., Huynh P.T., Harris J.H., Jr. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 2000;20(3):639–652. doi: 10.1148/radiographics.20.3.g00ma09639. [DOI] [PubMed] [Google Scholar]
- 4.Morita S., Higuchi M., Saito N., Mitsuhashi N. Pelvic venous variations in patients with congenital inferior vena cava anomalies: classification with computed tomography. Acta Radiol. 2007;48(9):974–979. doi: 10.1080/02841850701499409. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z., Zheng X., He Y. Stent migration after endovascular stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. 2016;4(2):193–199. doi: 10.1016/j.jvsv.2015.10.005. [DOI] [PubMed] [Google Scholar]