Abstract
The introduction of simian virus 40 large T antigen (SVLT) into human primary cells enables them to proliferate beyond their normal replicative life span. In most cases, this temporary escape from senescence eventually ends in a second proliferative block known as “crisis,” during which the cells cease growing or die. Rare immortalization events in which cells escape crisis are frequently correlated with the presence of telomerase activity. We tested the hypothesis that telomerase activation is the critical step in the immortalization process by studying the effects of telomerase activity in two mortal SVLT-Rasval12-transformed human pancreatic cell lines, TRM-6 and βlox5. The telomerase catalytic subunit, hTRT, was introduced into late-passage cells via retroviral gene transfer. Telomerase activity was successfully induced in infected cells, as demonstrated by a telomerase repeat amplification protocol assay. In each of nine independent infections, telomerase-positive cells formed rapidly dividing cell lines while control cells entered crisis. Telomere lengths initially increased, but telomeres were then maintained at their new lengths for at least 20 population doublings. These results demonstrate that telomerase activity is sufficient to enable transformed cells to escape crisis and that telomere elongation in these cells occurs in a tightly regulated manner.
Primary human and other mammalian cells, when cultured in vitro, generally exhibit a strictly limited proliferative life span, after which they permanently exit the cell cycle but remain viable for long periods in a state termed senescence (12, 53). The predominant theory of senescence is that growth arrest is a response to telomere shortening, which occurs as a result of incomplete replication of the extreme 3′ ends of linear chromosomes (29, 51). Telomerase, a multisubunit riboprotein, repairs and maintains telomeric ends in mammalian germ cells, in simple organisms such as yeast and tetrahymena (4), and in most tumor-derived cell lines (25, 40). The vast majority of normal, cycling somatic cells have no detectable telomerase activity (25). It is hypothesized that senescence occurs when the telomere lengths of one or more chromosomes reach a critical point at which cells are signaled to exit the cell cycle. This model was strengthened by the demonstration that the introduction of telomerase activity into normal, cycling (presenescent) primary cells prolongs the proliferative life span, perhaps indefinitely (6, 45).
The normal limitation on life span can be bypassed by a variety of transforming mechanisms that disable key cell cycle regulators (7, 20, 53–55). Transformation by oncogenes such as simian virus 40 large T antigen (SVLT), human papilloma virus E6 and E7, and adenovirus E1A and E1B triggers entry into the cell cycle and is sufficient to delay senescence but fails to induce immortality in human cells (2, 18, 37, 42). Unchecked proliferation ultimately gives way to slowed growth, altered cell morphology, and high rates of cell death (21). This end point is most commonly referred to as “crisis,” although other terms, such as mortality checkpoint 2 or M2, have been used. Crisis and senescence share the property of growth arrest following a defined number of cell divisions. However, the well-controlled exit from the cell cycle, the preservation of chromosomal integrity, and the gradual loss of viability observed during senescence contrast with the proliferative slowing, chromosomal instability, and ongoing cell death that characterize cells in crisis. It is not known whether crisis and senescence share the same underlying etiology.
Because crisis is characterized by severe chromosomal structural instability (33, 43) and because it has been demonstrated that transformation does not halt telomere shortening during replication (15, 16), it has been speculated that crisis occurs when telomere erosion has progressed beyond a second critical point such that chromosomes become unstable. The best evidence in favor of a relationship between telomere shortening and crisis is the high degree of correlation between immortalization and telomere stabilization. Depending on the cell type, 1 in 105 to 1 in 107 cells will escape crisis and go on to form immortal lines (24, 41). The rare clones that do emerge from crisis invariably exhibit some form of telomere stabilization, usually by telomerase (25) but sometimes by alternate mechanisms (9), implying that telomere stabilization is crucial to continued growth. Conversely, immortal HeLa cells can be forced into crisis when the telomerase holoenzyme is disabled by the expression of antisense hTR (the RNA component of the enzyme) (19).
Specific chromosomal changes observed in crisis are consistent with telomere loss. Mean telomeric TTAGGG lengths reported for cells in crisis are so low (1.5 to 2 kb) (15, 16, 27, 29) as to raise the possibility that at least some chromosome ends may have been eroded into subtelomeric regions (1, 17, 31, 51). Intertelomere associations become common, and dicentric chromosomes increase in frequency (16, 21, 33, 37, 43). These are predicted consequences of telomere loss and are similar to chromosomal defects seen in serially passaged cells from mTR knockout mice (5). Despite the strong correlation between crisis and telomere loss (or immortalization and telomere stabilization), a causal relationship between these events has not been definitively established.
As part of an effort to understand the relationship between pancreatic endocrine cell growth and differentiation, we have been developing cell lines from the human fetal and adult pancreas using a retroviral vector expressing SVLT and H-rasval12 (48, 49). The TRM-6 (49) and βlox5 cell lines were established from human fetal and adult pancreatic islets, respectively. Like other cells transformed or cotransformed with SVLT, these cells exhibit an extended life span but eventually enter a crisis phase characterized by increased cell death, altered morphology, and lack of proliferation. Although many human cell types expressing SVLT have eventually produced rare clones that escaped crisis and went on to form immortal lines, attempts to derive immortal clones from TRM-6 or βlox5 cells have been unsuccessful so far. Because telomerase activation may be the primary means by which cells escape crisis and because introduction of the human telomerase reverse transcriptase (hTRT) subunit (34) has been shown to reconstitute telomerase activity in several human cell types (35, 50), we tested the hypothesis that hTRT expression could rescue TRM-6 and βlox5 cells from crisis.
MATERIALS AND METHODS
Plasmids and viruses.
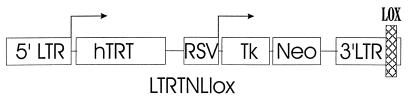
The hTRT-expressing retroviral vector, LTRTNLlox (Fig. 1), was created from the retroviral construct Lluc70AUGNeo (28). The vector was modified by insertion of a loxP site into the XbaI site of the 3′ long terminal repeat (LTR) (13, 38). The luciferase gene from Lluc70AUGNeo was removed by HindIII digestion and replaced with a cassette consisting of a modified Rous sarcoma virus (RSV) LTR promoter (56) and a HincII-HindIII fragment containing the herpes simplex virus thymidine kinase (tk) gene (32). The HincII site is 63 bp upstream of the ATG start codon, and the HindIII site was inserted by PCR, 27 bp downstream of the stop codon. The neomycin phosphotransferase gene is directly 3′ of the tk gene and is expressed by a ribosomal translational scanning mechanism (28). Finally, the hTRT gene (34) was subcloned into a NotI site inserted downstream of the 5′ viral LTR promoter, which drives its expression. The LTPRRTNLlox vector used in the creation of the βlox5 cell line (see below) is identical to LTRTNLlox except that the NotI-flanked SVLT-Po-RasVal12 cassette from LOTPRRNLO (47) replaces the hTRT gene. The green fluorescent protein (GFP)-expressing control construct, LGFPRNL (57a), was created by the insertion of the GFP μ2 gene (14) into the BamHI site of the retroviral vector LZRNL (56). 293GP-derived producer cell lines were created for each construct, and vesicular stomatitis virus G (VSV-G)-pseudotyped retrovirus was harvested as previously described (10, 46).
FIG. 1.
A schematic diagram showing the hTRT-expressing retroviral vector LTRTNLlox. The retroviral LTR has internal promoter activity, which drives the expression of hTRT. A modified RSV promoter drives the expression of the tk and neo genes. The herpes simplex virus thymidine kinase (tk) gene induces gancyclovir sensitivity. The neomycin phosphotransferase gene (neo) imparts G418 resistance. A loxP site (crosshatched box) has been inserted into the 3′ viral LTR. The duplication of the loxP site during retroviral insertion allows the intervening sequences to be eliminated when cre is expressed (13, 38).
Cells and cell culture.
TRM-6 cells are derived from 24-week human fetal pancreatic epithelial cells transformed with SVLT antigen and Rasval12 (49). βlox5 cells are derived from adult human pancreatic islet cells (provided by the Diabetes Research Institute, Miami, Fla.) which were sorted for high flavin adenine dinucleotide autofluorescence in order to enrich for β-cells (44). They were transformed by LTPRRTNLlox. Basinger cells are human primary fibroblast cells (46). All cells were maintained in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum and 400 ng of G418/ml, except for Basinger cells, which were maintained without G418 until after infection with LTRTNLlox or LGFPRNL. Infections were carried out in the presence of 6 μg of Polybrene/ml.
Growth rate analysis.
Cells were seeded into 35-mm wells at a density of 10,000 cells/well. Cells were passaged at 80% confluency (except for controls lacking telomerase activity, which never reached that density) and were counted on days 7, 13, 17, and 22.
TRAP assay.
Cell extracts were prepared, and the telomerase repeat amplification protocol (TRAP) assays were performed with the TRAPEZE enzyme-linked immunosorbent assay (ELISA) telomerase detection kit (Oncor, Gaithersburg, Md.) as directed by the manufacturer. Samples were assayed in duplicate. Heat-treated controls from each sample were included in order to rule out false-positive signals from PCR artifacts.
Telomere length analysis.
Genomic DNA was isolated by proteinase K lysis and phenol-choroform extraction. For telomere length comparisons, 3.0 μg of genomic DNA was digested with RsaI and HinfI restriction enzymes and separated on a 0.6% agarose gel. The DNA was transferred to a positively charged nylon membrane by Southern blotting and was probed with 32P-labeled 5′-(CCCTAA)5. Radioactive signals were either detected on a phosphor screen and scanned with a Storm Scanner (Molecular Dynamics, Sunnyvale, Calif.) or detected on X-ray film and scanned with a Hewlett-Packard ScanJet 6100C. Signal densities were analyzed with Scion Image software (Scion Corp., Frederick, Md.). Mean telomere restriction fragment (TRF) lengths were calculated as previously described (22).
In the comparison of telomere lengths as a function of cell division, population doublings (PD) were determined as follows. Cells were arbitrarily designated PD 0 at the time of infection. The number of cell divisions that occurred in emerging colonies prior to the first passage was estimated at 10 based on the number of cells in a typical colony. Subsequent PD were calculated as a function of passage number and splitting ratio.
RESULTS
Development of an hTRT-expressing retroviral vector.
Retroviral gene transfer vectors provide a simple and efficient means of integrating exogenous DNA into the host chromosomes of dividing cells. We designed an hTRT-expressing retroviral vector to test the effects of telomerase activity in populations of SVLT-Rasval12-transformed cells. The hTRT gene (34) was subcloned into a retroviral vector to create pLTRTNLlox. Expression of hTRT is driven by the viral 5′ LTR (Fig. 1). A GFP-expressing retroviral vector, pLGFPRNL, was used as a control. Retroviruses were pseudotyped with the VSV-G envelope protein in order to maximize their ability to infect mammalian cells (10, 46).
To test the ability of our vector to express functional hTRT protein, Basinger human primary fibroblasts (46) were infected with LTRTNLlox or LGFPRNL and selected in G418. A TRAP assay confirmed that only LTRTNLlox-infected cells acquired telomerase activity (data not shown). In agreement with the results of Bodnar et al. (6), Basinger fibroblast cultures expressing hTRT from LTRTNLlox continued to proliferate well beyond the point at which control cells senesced (data not shown).
Expression of hTRT in human pancreatic cell lines confers telomerase activity.
Previous studies found that the hTR RNA and hTLP1 protein subunits of telomerase were ubiquitously expressed (19, 35) and that hTRT was the only missing factor required for telomerase activity (35, 50). However, the number of cell lines tested was small, and the effects of SVLT and H-rasval12 expression on the expression or activity of those telomerase subunits are unknown. Therefore, it was important to determine whether hTRT expression in human pancreatic cell lines is sufficient to reconstitute telomerase activity.
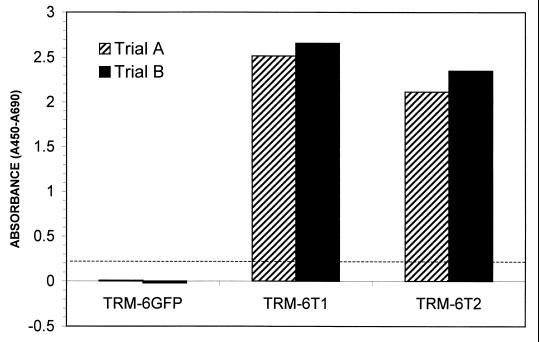
Late-passage TRM-6 cells and βlox5 cells, judged by their slowed growth and the appearance of altered morphology to be approaching crisis, were seeded into 35-mm wells and infected with either LGFPRNL or LTRTNLlox retrovirus at four different multiplicities of infection (MOIs) ranging from 1 to 10. Although selection was precluded by the fact that TRM-6 and βlox5 cells are already G418 resistant, previous studies using GFP as a marker have demonstrated infection efficiencies as high as 40% in TRM-6 cells with LGFPRNL at an MOI of 10 (24a). ELISA-based TRAP assays performed on extracts from LGFPRNL- and LTRTNLlox-infected TRM-6 cells confirmed that the LTRTNLlox-infected cells were strongly positive for telomerase activity for at least 7 weeks after infection, whereas no such activity was detectable in control cells (Fig. 2).
FIG. 2.
A TRAP ELISA was performed on extracts from control (TRM-6GFP) and LTRTNLlox-infected (TRM-6T) cells 48 days postinfection. TRM-6T1 and TRM-6T2 are derived from independent infections of TRM-6 with LTRTNLlox. Samples were tested in duplicate. Absorbance was measured at 450 and 690 nm. The A690 was subtracted to eliminate background absorbance from the plate. The absorbance of controls in which telomerase was inactivated by heat treatment was then subtracted in order to control for PCR artifacts. The final values are shown. The dashed line delineates the threshold above which the assay is considered positive.
TRM-6 and βlox5 cells are consistently rescued from crisis by the introduction of hTRT.
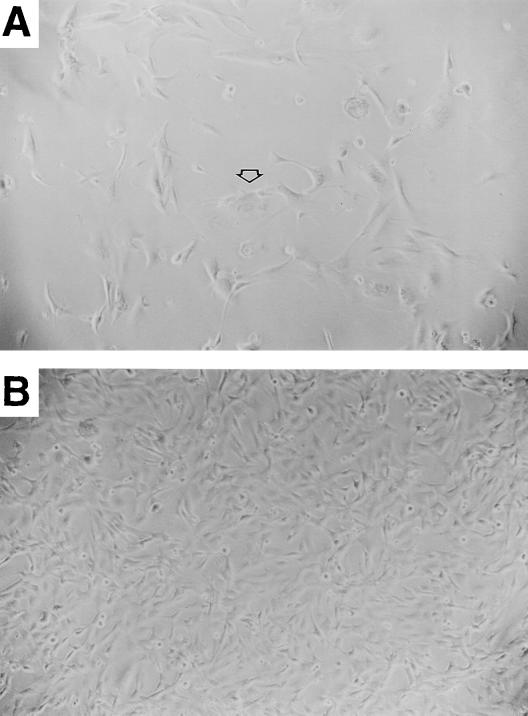
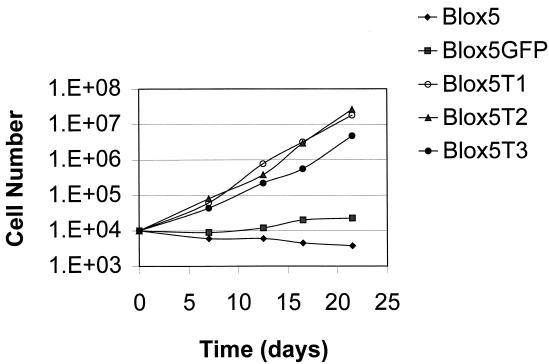
Cells infected with LGFPRNL or LTRTNLlox were passaged continuously. Within 3 weeks (2 to 3 PD), 3 of 3 uninfected cultures and 8 of 8 LGFPRNL-infected control cultures had stopped proliferating (failed to exceed 50% confluency) and comprised mainly large, flattened cells with low nucleus/cytoplasm ratios and highly variable morphology (Fig. 3A). A low level of ongoing cell death was also observed, consistent with crisis. During the same 3-week period, 5 of 5 independent LTRTNLlox-infected TRM-6 cultures and 4 of 4 independent LTRTNLlox-infected βlox5 cultures gave rise to numerous small colonies of rapidly dividing cells (Fig. 3B), generating the polyclonal TRM-6T lines 1 to 5 and βlox5T lines 1 to 4, respectively. These cells were easily distinguishable by their morphology from surrounding cells that underwent growth arrest, and they were numerous enough to be readily observed at low-power magnification in virtually every field. The presence of colonies on 9 of 9 LTRTNLlox-infected plates and the absence of proliferation on control plates indicate that proliferation and escape from crisis occurred as a direct result of infection by LTRTNLlox. The large number of colonies on LTRTNLlox-infected plates suggests that escape from crisis occurred in a high percentage of infected cells. βlox5T and TRM-6T cells have continued to grow (Fig. 4 and data not shown) and have been maintained in culture for more than 35 and 45 PD, respectively.
FIG. 3.
Differences in growth and morphology of control (TRM-6) and hTRT-expressing (TRM-6T) cells are shown. (A) TRM-6GFP cells have failed to reach confluency after more than 2 weeks in culture. The arrow points to an enlarged cell with a flattened appearance and a decreased nucleus/cytoplasm ratio, typical of those seen in crisis. (B) TRM-6T cells were seeded at the same time as control cells and had been passaged twice at the time that the picture was taken. Magnification, ×10.
FIG. 4.
A growth curve charts the differences in proliferative capacity between control (βlox5 and βlox5GFP) and telomerase-positive cells. Blox5T1, βlox5T2, and βlox5T3 are derived from independent infections of βlox5 with LTRTNLlox.
Induction of telomerase activity lengthens the telomeres of transformed cell lines.
Bodnar et al. showed that hTRT gene transfer resulted in telomere elongation in primary cells (6). In contrast, immortalized, transformed cell lines that have acquired telomerase activity by an as yet uncharacterized genetic or epigenetic event generally retain short, but stable, telomeres (15, 16). The effect of constitutive expression of a transduced hTRT gene on telomere length in transformed cells has not been examined. Therefore, the mean TRF lengths of the hTRT-expressing TRM-6T and βlox5T cells were measured and compared to those of near-crisis and crisis stage control cells. TRFs were generated by cutting genomic DNA with restriction enzymes (RsaI and HinfI) which cut sites in subtelomeric regions but do not cleave telomeric repeats. Genomic DNA for telomere length analysis was harvested as soon as control cells had clearly entered crisis, as determined by growth arrest, and at various time points thereafter up to PD 34 (for βlox5T) and PD 40 (for TRM-6T), calculated as described in Materials and Methods. DNA was also harvested from uninfected βlox5 cells prior to the onset of crisis.
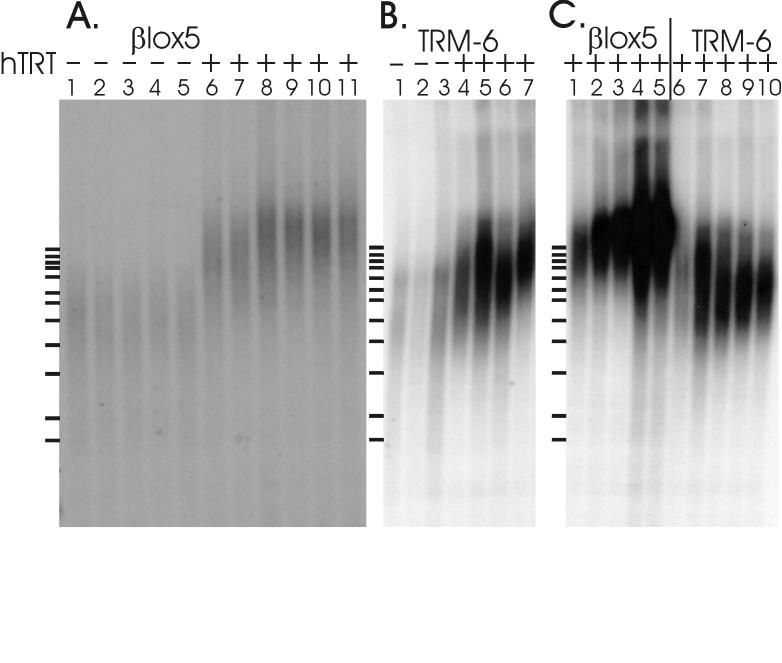
The hTRT-expressing cells have an increase in the average telomere length compared to either crisis phase or precrisis control cells. The βlox5T cells exhibited the most impressive lengthening (Fig. 5A) and the most consistent length regulation between independent lines (Fig. 5A) and over many cell divisions (Fig. 5C, lanes 1 to 5). Marked lengthening of the telomeres was evident at the earliest time point (Fig. 5A, lanes 6 and 7), and they reached a maximum length by PD 15 (Fig. 5A, lanes 8 to 11), after which they appeared to remain stable for at least 20 PD (Fig. 5C). The mean of the TRF lengths of the four independent βlox5T cultures was approximately 15 kb, and they had a narrow distribution. A marked increase in signal intensity at the target length (15 kb) became evident at later PD (Fig. 5C, lanes 4 and 5). This was not due to uneven loading of the DNA, as confirmed by ethidium bromide staining (data not shown). The TRF lengths of TRM-6T cells demonstrated less-dramatic lengthening and more variability between and within independent lines (Fig. 5B). Mean TRF lengths ranged from 7 to 10 kb, with broadly distributed signals, although this distribution narrowed with increasing PD (Fig. 5C, lanes 6 to 10). Interestingly, the gradual convergence of TRM-6T TRF lengths appeared to involve shortening or loss of some long telomere populations as well as elongation of others (Fig. 5C, lanes 6 to 10). The striking difference in the size, distribution, and dynamics of TRF lengths between TRM-6T and βlox5T lines demonstrates cell line-specific regulation of telomere length.
FIG. 5.
Southern blots showing TRF lengths in control and telomerase-positive cells. (A) βlox5 derivatives. Lanes: 1, precrisis βlox5 cells; 2, βlox5 cells in crisis; 3 to 5, independent βlox5GFP controls in crisis; 6 to 7, independent βlox5T lines at PD 10; 8 to 11, independent βlox5T lines at PD 14 to 15. (B) TRM-6 derivatives. Lanes: 1, TRM-6 cells in crisis; 2 to 3, TRM-6GFP cells in crisis; 4 to 7, independent TRM-6T lines at PD 17 to 18. (C) Telomere length dynamics. Lanes 1 through 5, βlox5T3 cells at PD 10, 15, 22, 28, and 34, respectively. Lanes 6 through 10, TRM-6T2 cells at PD 10, 17, 25, 34, and 40, respectively.
DISCUSSION
The most important conclusion of this study is that telomerase activity is sufficient to allow transformed cells to escape from crisis. Although hTRT expression is sufficient to prevent senescence in normal, cycling cells, its efficacy in enabling transformed cells to bypass crisis had not been tested previously. Here, we have shown directly that hTRT gene expression is sufficient to initiate telomerase activity and to prevent crisis in late-passage pancreatic epithelial cells transformed with SVLT and Rasval12. Although additional studies will be required to determine whether these results can be generalized to other cell lines and other models of transformation, our findings demonstrate that telomere erosion is a primary factor responsible for the onset of crisis in transformed cell cultures. Previous studies correlating escape from crisis with the appearance of telomerase activity could not rule out the possibility that other events were occurring and playing a role in this process. The consistency and rapidity with which the differences between LTRTNLlox-infected and control cells became evident argues against the need for any additional events.
Another question that our study addresses regards the regulation of telomere length in hTRT-expressing cells. We found that TRM-6 and βlox5 cells at the point of crisis both demonstrated significant telomere elongation upon the introduction of hTRT, with subsequent stabilization. Telomere elongation following the introduction of the hTRT transgene has been demonstrated in other models. Bodnar et al. reported that the telomere lengths of stable, hTRT-expressing clones of primary human fibroblasts and retinal pigment epithelial cells were longer than those of the starting populations at the time of transfection (6). Similar results were reported by Vaziri and Benchimol (45). These experiments, however, were performed in presenescent primary cell cultures, not in transformed cells nearing crisis. In contrast, many previous studies of immortal tumor cell lines have found that they characteristically have stable telomeres that are significantly shorter than those found in young primary cells (17, 39). This suggests that in most spontaneously transformed cells, telomeres are maintained within a constant range but are not elongated. It was presumed that stabilization occurred at the moment at which telomerase became active. Likewise, detailed studies of telomere lengths during spontaneous immortalization of simian virus 40-, adenovirus-, and Epstein-Barr virus-transformed cells found stabilization of telomeres without elongation (15, 16), although telomere elongation was seen in a study of human papillomavirus-transformed cell lines (27).
Any of a number of possible mechanisms could account for the telomere lengthening seen in our system compared to the maintenance of stable, short telomeres seen in most spontaneously immortalized transformed cells. There may be differences in the expression level of hTRT, and consequently in telomerase activity, between spontaneously immortalized cells and cells genetically modified to express hTRT. Indeed, detailed studies of HeLa and 293 cells, both of which have “stable” telomere lengths, demonstrated that telomere stabilization in these cell lines is actually a dynamic process, with subpopulations exhibiting varying levels (or an absence) of telomerase activity at different times (8). Constitutively expressed transgenic hTRT may be less susceptible to repression or inactivation. Another possibility is that telomere length is controlled by other factors that are not reflected in telomerase activity as measured by the TRAP assay. Such factors could be specific to a particular cell type and/or could be influenced by the mechanism by which the cell was transformed.
We consider it unlikely that the apparent telomere lengthening observed in our experiments represents telomere stabilization in a few rare cells having unusually long telomeres at the time at which the culture was infected with LTRTNLlox. This is most clear in the βlox5 experiment, in which the vast majority of the parental cells clearly had very short TRFs. Since the independent βlox5T lines represent polyclonal populations, as indicated by the large number of colonies observed, stabilization without elongation should have generated a majority of very short TRFs. Instead, all four lines had a narrow distribution of TRF lengths, with a mean of 15 kb. It is highly unlikely that any cells in a population on the verge of crisis, as these were, would have TRFs in the 15-kb range. Previous studies have consistently shown that crisis occurs when TRF lengths average less than 4 kb (15, 16, 27, 29). Furthermore, in two cases we were able to demonstrate mean TRF lengths in samples taken very early after LTRTNLlox infection that were intermediate between those of control cells and the length at which the same lines ultimately stabilized (Fig. 5A, lanes 6 to 7, and 5C, lane 1). Finally, as discussed further below, independent βlox5T lines had very similar mean TRF lengths and TRF length distributions. More variability between individual cell lines might have been expected if atypical populations were being selected by telomere stabilization.
Once the telomeres are elongated, it is clear that the ultimate length is tightly regulated in a cell line-specific manner. Multiple independent (but concurrent) infections of each of the two cell lines studied here demonstrated that the telomeres in independent lines originating from the same parent strain were consistently lengthened to similar extents but that TRM-6T lines maintained much shorter TRF lengths than βlox5T lines. Elongation occurred early after infection and remained stable for at least 20 cell divisions. βlox5T cells demonstrated much more stringently regulated telomere lengths than TRM-6T cells, with little deviation occurring either with increasing PD or between independent lines. Interestingly, the probe signal increased in intensity at later PDs in βlox5T cells, although the mean TRF length remained essentially unchanged. While the significance of this is unclear at present, one possible explanation is that telomere lengthening continues to occur over many generations, with an increasing proportion of cells in the population and/or an increasing proportion of chromosomes in each cell having undergone telomere elongation at each time point. TRM-6T cells also demonstrated an increasingly focused signal, although TRF length modification in these lines seemed to involve loss or shortening of longer telomere subpopulations over time. The increasing homogeneity of TRF lengths in the cultures could simply reflect selective expansion of one or a few clones within the population. This does not change the observation that telomere length is closely regulated. It is not known what determines the ultimate length at which telomeres will be maintained in a given cell type, although mean TRF lengths within species (germ lines) or cell lines are stringently maintained (3, 15, 26, 58). Mean TRF lengths in the βlox5T lines are similar to those reported in human fetal tissues (11, 57), although the TRM-6T lines failed to reach this length. The stabilization of telomere length over time in the hTRT-expressing lines demonstrates that regulation of telomere length persists despite constitutive overexpression of hTRT mRNA. This is consistent with previous evidence suggesting that telomere lengths are regulated by other means, such as factors that bind directly to telomeric DNA (23, 30, 36, 52, 59). Alternatively, telomere stabilization may simply reflect an equilibrium between telomerase activity and telomere loss, either of which could be positively or negatively affected by as yet uncharacterized regulatory factors. The fact that telomere length increased in our cells (and in those of others using an hTRT transgene) could be due to a partial or temporary overwhelming of inhibitory regulators by overexpression of hTRT. Future studies will be needed to look more specifically at the relationship between hTRT expression, telomerase activity, and telomere length regulatory mechanisms.
ACKNOWLEDGMENTS
This work was supported by grants from the Stern Foundation and the Juvenile Diabetes Foundation International, and by a pilot grant from the Howard Hughes Medical Institute. T. Halvorsen is a recipient of a Medical Scientist Training Program Award. F. Levine is a member of the UCSD Center for Molecular Genetics, UCSD Cancer Center, and Whittier Institute for Diabetes.
We thank Camillo Recordi for providing adult human islets, L. Yu for providing reagents, and G. Beattie, A. Hayek, and members of the Levine laboratory for helpful discussions.
REFERENCES
- 1.Allshire R C, Dempster M, Hastie N D. Human telomeres contain at least three types of G-rich repeat distributed nonrandomly. Nucleic Acids Res. 1989;17:4611–4627. doi: 10.1093/nar/17.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin T, Vogt P K. Cell transformation by viruses. In: Fields B N, et al., editors. Virology. 2nd ed. New York, N.Y: Raven Press, Ltd.; 1990. pp. 317–368. [Google Scholar]
- 3.Blackburn E H. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn E H. Telomeres. Trends Biochem Sci. 1991;16:378–381. doi: 10.1016/0968-0004(91)90155-o. [DOI] [PubMed] [Google Scholar]
- 5.Blasco M A, Lee H W, Hande M P, Samper E, Lansdorp P M, DePinho R A, Greider C W. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA [see comments] Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 6.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Extension of life-span by introduction of telomerase into normal human cells [see comments] Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 7.Brown J P, Wei W, Sedivy J M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 8.Bryan T M, Englezou A, Dunham M A, Reddel R R. Telomere length dynamics in telomerase-positive immortal human cell populations. Exp Cell Res. 1998;239:370–378. doi: 10.1006/excr.1997.3907. [DOI] [PubMed] [Google Scholar]
- 9.Bryan T M, Englezou A, Gupta J, Bacchetti S, Reddel R R. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J-K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler M G, Tilburt J, DeVries A, Muralidhar B, Aue G, Hedges L, Atkinson J, Schwartz H. Comparison of chromosome telomere integrity in multiple tissues from subjects at different ages. Cancer Genet Cytogenet. 1998;105:138–144. doi: 10.1016/s0165-4608(98)00029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campisi J. The biology of replicative senescence. Eur J Cancer. 1997;33:703–709. doi: 10.1016/S0959-8049(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 13.Choulika A, Guyot V, Nicolas J-F. Transfer of single gene-containing long terminal repeats into the genome of mammalian cells by a retroviral vector carrying the cre gene and the loxP site. J Virol. 1996;70:1792–1798. doi: 10.1128/jvi.70.3.1792-1798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 15.Counter C M, Avilion A A, LeFeuvre C E, Stewart N G, Greider C W, Harley C B, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Counter C M, Botelho F M, Wang P, Harley C B, Bacchetti S. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein-Barr virus-transformed human B lymphocytes. J Virol. 1994;68:3410–3414. doi: 10.1128/jvi.68.5.3410-3414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lange T, Shiue L, Myers R M, Cox D R, Naylor S L, Killery A M, Varmus H E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990;10:518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Silva R, Whitaker N J, Rogan E M, Reddel R R. HPV-16 E6 and E7 genes, like SV40 early region genes, are insufficient for immortalization of human mesothelial and bronchial epithelial cells. Exp Cell Res. 1994;213:418–427. doi: 10.1006/excr.1994.1218. [DOI] [PubMed] [Google Scholar]
- 19.Feng J, Funk W D, Wang S S, Weinrich S L, Avilion A A, Chiu C P, Adams R R, Chang E, Allsopp R C, Yu J, Le S, West M D, Harley C B, Andrews W H, Greider C W, Villeponteau B. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 20.Garkavtsev I, Hull C, Riabowol K. Molecular aspects of the relationship between cancer and aging: tumor suppressor activity during cellular senescence. Exp Gerontol. 1998;33:81–94. doi: 10.1016/s0531-5565(97)00086-7. [DOI] [PubMed] [Google Scholar]
- 21.Girardi A J, Jensen F C, Koprowski H. SV40-induced transformation of human diploid cells: crisis and recovery. J Cell Comp Physiol. 1965;65:69–84. doi: 10.1002/jcp.1030650110. [DOI] [PubMed] [Google Scholar]
- 22.Harley C B, Futcher A B, Greider C W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 23.Holt S E, Wright W E, Shay J W. Multiple pathways for the regulation of telomerase activity. Eur J Cancer. 1997;33:761–766. doi: 10.1016/S0959-8049(97)00066-X. [DOI] [PubMed] [Google Scholar]
- 24.Huschtscha L I, Holliday R. Limited and unlimited growth of SV40-transformed cells from human diploid MRC-5 fibroblasts. J Cell Sci. 1983;63:77–99. doi: 10.1242/jcs.63.1.77. [DOI] [PubMed] [Google Scholar]
- 24a.Itkin-Ansari, P. R. Unpublished data.
- 25.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Specific association of human telomerase activity with immortal cells and cancer [see comments] Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 26.Kipling D, Cooke H J. Hypervariable ultra-long telomeres in mice. Nature. 1990;347:400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- 27.Klingelhutz A J, Barber S A, Smith P P, Dyer K, McDougall J K. Restoration of telomeres in human papillomavirus-immortalized human anogenital epithelial cells. Mol Cell Biol. 1994;14:961–969. doi: 10.1128/mcb.14.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine F, Yee J-K, Friedmann T. Efficient gene expression in mammalian cells from a dicistronic transcriptional unit in an improved retroviral vector. Gene. 1991;108:167–174. doi: 10.1016/0378-1119(91)90431-a. [DOI] [PubMed] [Google Scholar]
- 29.Levy M Z, Allsopp R C, Futcher A B, Greider C W, Harley C B. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225:951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- 30.McEachern M J, Blackburn E H. Runaway telomere elongation caused by telomerase RNA gene mutations. Nature. 1995;376:403–409. doi: 10.1038/376403a0. [DOI] [PubMed] [Google Scholar]
- 31.McElligott R, Wellinger R J. The terminal DNA structure of mammalian chromosomes. EMBO J. 1997;16:3705–3714. doi: 10.1093/emboj/16.12.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKnight S L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980;8:5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moorhead P S, Saksela E. The sequence of chromosome aberrations during SV40 transformation of a human diploid cell strain. Hereditas. 1965;52:271–284. doi: 10.1111/j.1601-5223.1965.tb01960.x. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T M, Morin G B, Chapman K B, Weinrich S L, Andrews W H, Lingner J, Harley C B, Cech T R. Telomerase catalytic subunit homologs from fission yeast and human [see comments] Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama J, Tahara H, Tahara E, Saito M, Ito K, Nakamura H, Nakanishi T, Ide T, Ishikawa F. Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat Genet. 1998;18:65–68. doi: 10.1038/ng0198-65. [DOI] [PubMed] [Google Scholar]
- 36.Runge K W, Zakian V A. Introduction of extra telomeric DNA sequences into Saccharomyces cerevisiae results in telomere elongation. Mol Cell Biol. 1989;9:1488–1497. doi: 10.1128/mcb.9.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sack G H., Jr Human cell transformation by simian virus 40—a review. In Vitro. 1981;17:1–19. doi: 10.1007/BF02618025. [DOI] [PubMed] [Google Scholar]
- 38.Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci USA. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt H, Blin N, Zankl H, Scherthan H. Telomere length variation in normal and malignant human tissues. Genes Chromosomes Cancer. 1994;11:171–177. doi: 10.1002/gcc.2870110306. [DOI] [PubMed] [Google Scholar]
- 40.Shay J W, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 41.Shay J W, Van Der Haegen B A, Ying Y, Wright W E. The frequency of immortalization of human fibroblasts and mammary epithelial cells transfected with SV40 large T-antigen. Exp Cell Res. 1993;209:45–52. doi: 10.1006/excr.1993.1283. [DOI] [PubMed] [Google Scholar]
- 42.Shay J W, Wright W E, Werbin H. Defining the molecular mechanisms of human cell immortalization. Biochim Biophys Acta. 1991;1072:1–7. doi: 10.1016/0304-419x(91)90003-4. [DOI] [PubMed] [Google Scholar]
- 43.Stewart N, Bacchetti S. Expression of SV40 large T antigen, but not small T antigen, is required for the induction of chromosomal aberrations in transformed human cells. Virology. 1991;180:49–57. doi: 10.1016/0042-6822(91)90008-y. [DOI] [PubMed] [Google Scholar]
- 44.Van de Winkle M, Maes E, Pipeleers D. Islet cell analysis and purification by light scatter and autofluorescence. Biochem Biophys Res Commun. 1982;107:525–532. doi: 10.1016/0006-291x(82)91523-6. [DOI] [PubMed] [Google Scholar]
- 45.Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Beattie G, Hayek A, Levine F. Transformation of human primary fibroblasts by pseudotyped retroviral vectors expressing multiple dominant oncogenes from an inducible promoter. Gene. 1996;182:145–150. doi: 10.1016/s0378-1119(96)00536-7. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Beattie G M, Mally M I, Cirulli V, Itkin-Ansari P, Lopez A D, Hayek A, Levine F. Isolation and characterization of a cell line from the epithelial cells of the human fetal pancreas. Cell Transplant. 1997;6:59–67. doi: 10.1177/096368979700600110. [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Beattie G M, Mally M I, Cirulli V, Lopez A D, Hayek A, Levine F. Development and characterization of cell line from human pancreatic beta-cells and beta-cell precursors using retroviral vectors expressing SV40 T-antigen and H-rasval12. Diabetes. 1996;45(Suppl. 2):285A. [Google Scholar]
- 49.Wang S, Beattie G M, Mally M I, Lopez A D, Hayek A, Levine F. Analysis of a human fetal pancreatic islet cell line. Transplant Proc. 1997;29:2219. doi: 10.1016/s0041-1345(97)00306-0. [DOI] [PubMed] [Google Scholar]
- 50.Weinrich S L, Pruzan R, Ma L, Ouellette M, Tesmer V M, Holt S E, Bodnar A G, Lichtsteiner S, Kim N W, Trager J B, Taylor R D, Carlos R, Andrews W H, Wright W E, Shay J W, Harley C B, Morin G B. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- 51.Wellinger R J, Sen D. The DNA structures at the ends of eukaryotic chromosomes. Eur J Cancer. 1997;33:735–749. doi: 10.1016/S0959-8049(97)00067-1. [DOI] [PubMed] [Google Scholar]
- 52.Wright W E, Brašiškyte̊ D, Piatyszek M A, Shay J W. Experimental elongation of telomeres extends the lifespan of immortal × normal cell hybrids. EMBO J. 1996;15:1734–1741. [PMC free article] [PubMed] [Google Scholar]
- 53.Wynford-Thomas D. Proliferative lifespan checkpoints: cell-type specificity and influence on tumour biology. Eur J Cancer. 1997;33:716–726. doi: 10.1016/S0959-8049(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 54.Wynford-Thomas D. Telomeres, p53 and cellular senescence. Oncol Res. 1996;8:387–398. [PubMed] [Google Scholar]
- 55.Wynford-Thomas D, Jones C J, Wyllie F S. The tumour suppressor gene p53 as a regulator of proliferative life-span and tumour progression. Biol Signals. 1996;5:139–153. doi: 10.1159/000109184. [DOI] [PubMed] [Google Scholar]
- 56.Xu L, Yee J-K, Wolff J, Friedmann T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989;171:331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]
- 57.Youngren K, Jeanclos E, Aviv H, Kimura M, Stock J, Hanna M, Skurnick J, Bardeguez A, Aviv A. Synchrony in telomere length of the human fetus. Hum Genet. 1998;102:640–643. doi: 10.1007/s004390050755. [DOI] [PubMed] [Google Scholar]
- 57a.Yu, L. Unpublished data.
- 58.Zakian V A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]
- 59.Zhong Z, Shiue L, Kaplan S, de Lange T. A mammalian factor that binds telomeric TTAGGG repeats in vitro. Mol Cell Biol. 1992;12:4834–4843. doi: 10.1128/mcb.12.11.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]