Abstract
A standardised, single-centre cross-sectional imaging protocol was utilised to investigate cortical grey matter and cerebral white matter alterations in 36 poliomyelitis survivors in contrast to healthy individuals and patients with amyotrophic lateral sclerosis (ALS) as a ‘disease-control’ group. [1] T1-weighted imaging and 32-direction diffusion tensor imaging data were obtained on a 3 Tesla Philips Achieva MRI system, using an IR-SPGR sequence and SE-EPI sequence respectively. Raw region-of-interest data and percentage change with respect to reference estimated marginal mean values are presented for grey and white matter metrics in key anatomical regions. Poliomyelitis survivors exhibit no frank grey or white matter degeneration. To the contrary, increased partial volumes can be detected in the brainstem, cerebellum and occipital lobes compared to healthy individuals. Higher fractional anisotropy was also noted in the corticospinal tracts, cerebellum, bilateral mesial temporal lobes and inferior frontal brain regions in poliomyelitis survivors in contrast to controls. Anatomical patterns of superior integrity metrics in polio survivors were concordant with anatomical regions of focal degeneration in ALS. Our imaging data indicate cortical and white matter reorganisation in polio survivors, which may be interpreted as compensatory adaptation to severe lower motor neuron injury acquired in infancy.
Keywords: Motor neuron disease, Poliomyelitis, Amyotrophic lateral sclerosis, Neuroimaging, MRI
Specifications Table
| Subject | Neurology, Motor Neuron Disease, Poliomyelitis, Radiology |
| Specific subject area | MRI, Grey matter ROI, White matter ROI, Subcortical volumetry, Poliomyelitis |
| Type of data | Raw volumetric data, Estimated Marginal Means plots, Box plots, Radar graphs |
| How data were acquired | Imaging data were acquired on a Philips Achieva 3T MRI scanner with an 8-channel head coil. |
| Data format | The partial volumetric profiles of cortical grey matter regions are presented as raw data and percentage change with reference to healthy controls is reported adjusted for age, gender, education and total intracranial volumes. Region-of-interest fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) values are reported as raw values and percentage change presented with respect to controls adjusted for age, gender and education. |
| Parameters for data collection | T1-weighted data: 3D Inversion Recovery prepared Spoiled Gradient Recalled echo (IR-SPGR) sequence: spatial resolution: 1 × 1 × 1 mm, Field of view: 256 × 256 × 160 mm, repetition time= 8.5 ms, Echo time = 3.9 ms, Inversion time =1060 ms, flip angle = 8°, SENSE factor = 1.5, sagittal acquisition; 256 slices. Diffusion Tensor Imaging data: spin- echo planar imaging (SE-EPI) sequence, 32-direction SE-EPI sequence. Field of view: 245 × 245 × 150 mm, spatial resolution = 2.5 mm3, 60 slices, repetition time / Echo time = 7639/59 ms. |
| Description of data collection | Data were collected on a 3 Tesla MRI system. Demographic variables were recorded before the MRI scan, and a standardised neurological examination was performed on the day of the MRI. |
| Data source location | Institution: Computational Neuroimaging Group (CNG), Trinity Biomedical Sciences Institute, Trinity College Dublin City/Town/Region: Dublin Country: Ireland |
| Data accessibility | Raw imaging metrics are available online at Mendeley Data; https://data.mendeley.com/datasets/dys423mzjp/1 |
| Related research article | Authors: Stacey Li Hi Shing, Jasmin Lope, Mary Clare McKenna, Rangariroyashe H. Chipika, Orla Hardiman, Peter Bede Title: Increased cerebral integrity metrics in poliomyelitis survivors: putative adaptation to longstanding lower motor neuron degeneration. Journal: Journal of Neurological Sciences https://doi.org/10.1016/j.jns.2021.117361. |
Value of the data
-
•
Quantitative radiological data from poliomyelitis survivors allow the interpretation of imaging data in other lower motor neuron conditions
-
•
White matter integrity metrics in polio survivors permits the juxtaposition of white matter measures in upper motor neuron conditions
-
•
This dataset can be utilised for meta-analyses across multiple data sources
-
•
Radiological data from polio survivors helps the validation of cerebral compensation studies in spinal conditions
-
•
Grey and white matter data from heathy controls may be utilised for the interpretation of findings in other neurological conditions
1. Data Description
Region of interest quantitative imaging data is presented in a cohort of poliomyelitis survivors [2]. We present both grey and white matter imaging metrics in 36 poliomyelitis survivors who underwent magnetic resonance imaging using a standardised imaging protocol. To aid data interpretation, imaging data from healthy controls (HC, n = 117) and patients with amyotrophic lateral sclerosis (ALS, n = 88) were also acquired. Imaging metrics from poliomyelitis survivors and HC cohorts are available online at Mendeley Data; http://dx.doi.org/10.17632/dys423mzjp.1 White matter integrity was evaluated using multiple diffusivity metrics; fractional anisotropy (FA), axial diffusivity (AD) and radial diffusivity (RD). In sharp contrast to other motor neuron diseases (MND), there is a paucity of imaging studies undertaken in polio survivors [3], [4], [5]. Existing imaging studies in motor neuron disease primarily focus on amyotrophic lateral sclerosis (ALS) [6] and primary lateral sclerosis (PLS) [7] and lower motor neuron predominant conditions are under evaluated [8], [9], [10]. Existing studies of lower motor neuron conditions typically solely evaluate spinal cord changes and cerebral changes are less well characterised [11, 12]. MNDs are also primarily associated with neurodegenerative change[5], decreased grey matter volume [13], cortical thinning [14], thalamus degeneration [15], fractional anisotropy reductions [16], and network dysfunction [17] despite emerging evidence of compensatory changes [18,19]. In this dataset, raw grey and white matter profiles of the study groups are presented in Fig. 1 and Fig. 2 as box plots. Additionally, demographically adjusted estimated marginal means are presented for key grey and white matter variables in Figs. 3 and 4. The comparative profile of study groups with reference to healthy individuals are illustrated by radar plots to further highlight regional grey (Fig. 5) and white matter alterations. (Fig. 6) Percentage change in grey matter partial volumes are calculated based on the estimated marginal means corrected for age, gender, education and total intracranial volumes. Percentage change in white matter diffusivity metrics are based on the estimated marginal means corrected for age, gender and education.
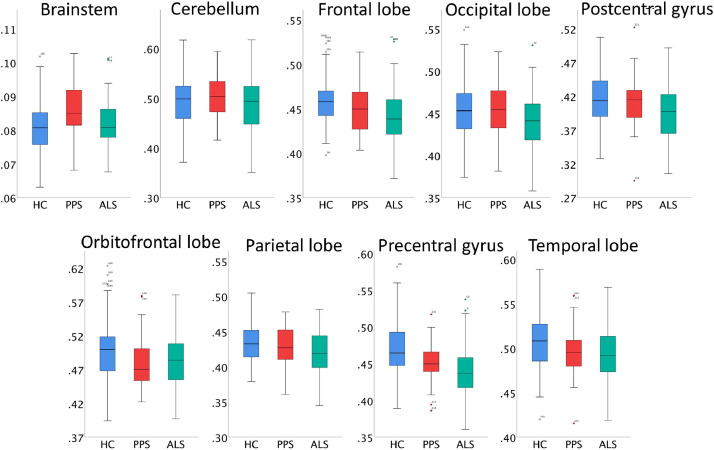
Fig. 1.
The comparative regional partial volume profile of poliomyelitis survivors (PPS), amyotrophic lateral sclerosis (ALS) and healthy controls (HC).
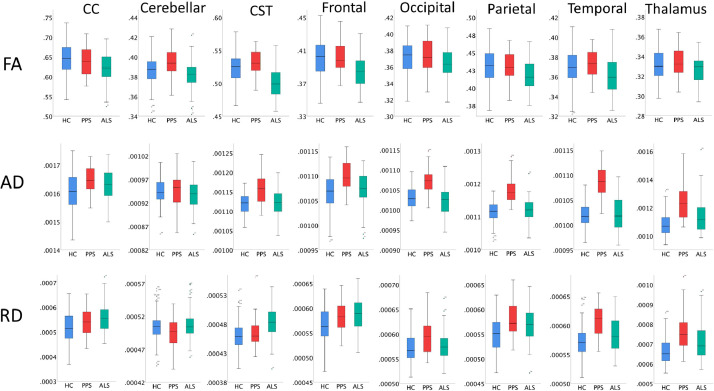
Fig. 2.
The comparative raw white matter region-of-interest profiles of poliomyelitis survivors (PPS), amyotrophic lateral sclerosis (ALS) and healthy controls (HC). CC: Corpus callosum; CST: Corticospinal tract; FA: fractional anisotropy; AD: axial diffusivity; RD: Radial diffusivity.
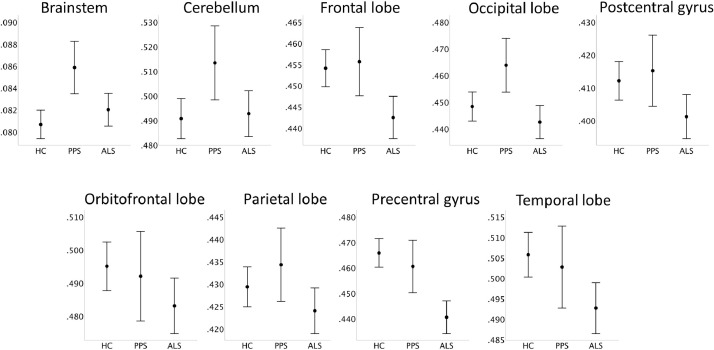
Fig. 3.
The volumetric profile of healthy controls (HC), poliomyelitis survivors (PPS) and patients with ALS based on estimated marginal means adjusted for the following values: Age = 59.84, Gender = 1.47, Education = 13.78, TIV = 1,424,021.23. Error bars represent 95% confidence interval.
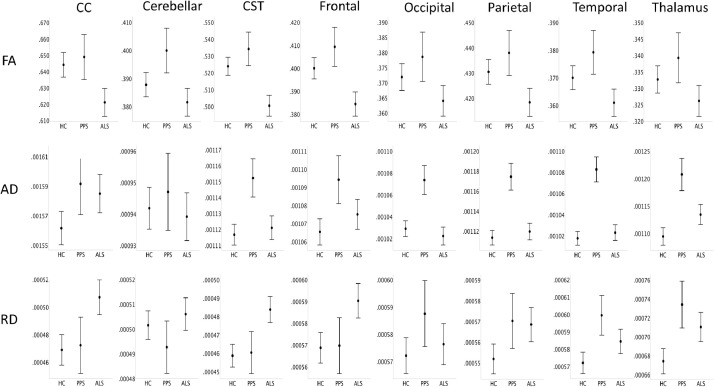
Fig. 4.
The diffusivity profile of healthy controls (HC), poliomyelitis survivors (PPS) and patients with ALS based on estimated marginal means adjusted for the following values: Age=59.84, Gender=1.47, Education=13.78. Error bars represent 95% confidence interval.
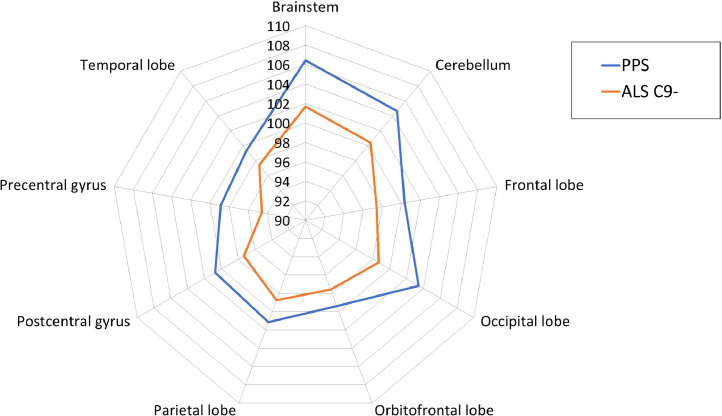
Fig. 5.
The grey matter ROIs profile of poliomyelitis survivors (PPS) and patients with ALS with reference to healthy controls. 100% represents the estimated marginal means of healthy controls for each grey matter ROI. Estimated marginal means of volumes were calculated with the following values: Age = 59.84, Gender = 1.47, Education = 13.78, TIV = 1,424,021.23.
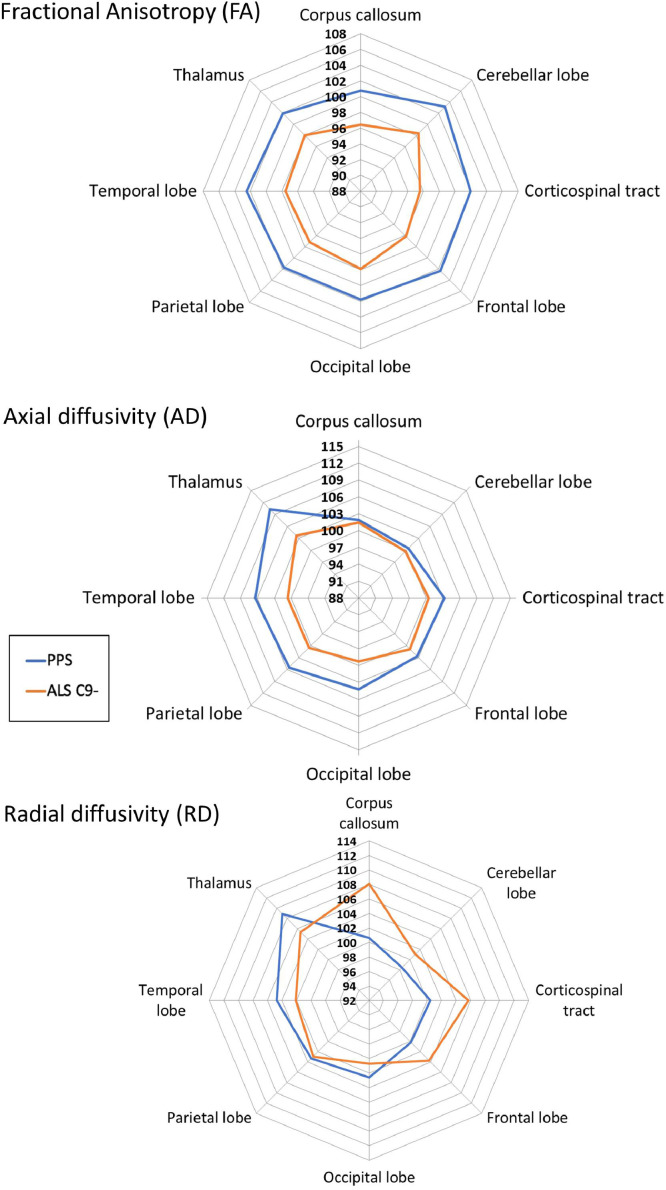
Fig. 6.
The diffusivity profile of poliomyelitis survivors (PPS) and patients with (ALS) with reference to healthy controls. 100% represents estimated marginal means of healthy controls for each structure. Estimated marginal means of the relevant metrics were calculated with the following values: Age=59.84, Gender=1.47, Education=13.78.
2. Experimental Design, Materials and Methods
This prospective, single-centre neuroimaging study was approved by the Ethics Committee of Beaumont Hospital, Dublin and each participant provided informed consent prior to participation. Participating patients with amyotrophic lateral sclerosis (ALS) according to the El Escorial research criteria. The study was specifically designed to characterise the cortical grey matter and cerebral white matter alterations in poliomyelitis survivors in contrast to ALS, a mixed upper- and lower-motor neuron disease (UMN/LMN), and healthy controls (HC). Patients underwent standardised clinical assessments and imaging with a uniform MR protocol on the same day. The imaging protocol implemented included T1-weighted, diffusion tensor imaging (DTI) and Fluid-attenuated inversion recovery (FLAIR) pulse sequences. T1-weighted images were acquired using a 3D Inversion Recovery prepared Spoiled Gradient Recalled echo (IR-SPGR) sequence with a spatial resolution of 1 × 1 × 1 mm and field of view of 256 × 256 × 160 mm; repetition time (TR) = 8.5 ms, echo time (TE) = 3.9 ms, Inversion time (TI) =1060 ms, flip angle = 8°, SENSE factor = 1.5. DTI were acquired using a 32-direction SE-EPI sequence with field of view: 245 × 245 × 150 mm, spatial resolution of 2.5 mm3, 60 slices, repetition time / Echo time of 7639/59 ms. FLAIR images were acquired to assess for vascular white matter lesion load and images were individually reviewed by the same experienced neurologist. Total intracranial volumes (TIV) were estimated for each participant to be used as covariates for volumetric statistics. An atlas-based approach was implemented to define regions-of-interest (ROIs) and retrieve MR metrics from pre-defined anatomical regions [20,21]. FMRIB's software library (FSL) v6.0 was used to process diffusion data. A tensor model was fitted to raw DTI data to generate maps of fractional anisotropy (FA), axial diffusivity (AD) and radial diffusivity (RD). Multivariate analyses of covariance (MANCOVA) were performed to explore intergroup differences adjusting for age, gender and education for white matter metrics and also using TIV to compare partial volume profiles Table 1.
Table 1.
Data categories and measures ALS-amyotrophic lateral sclerosis; FA-Fractional Anisotropy; AD-Axial Diffusivity; RD-Radial Diffusivity.
| Data categories | Specific measures |
|---|---|
| Grey matter region-of-interest | Brainstem |
| Cerebellum | |
| Frontal lobe | |
| Occipital lobe | |
| Orbitofrontal cortex | |
| Parietal lobe | |
| Precentral gyrus | |
| Temporal lobe | |
| White matter region-of-interest | Corpus callosum |
| Cerebellum | |
| Corticospinal tract | |
| Frontal lobe | |
| Occipital lobe | |
| Parietal lobe | |
| Temporal lobe | |
| Thalamus | |
| Diffusivity metrics evaluated | Fractional anisotropy (FA) |
| Axial diffusivity (AD) | |
| Radial diffusivity (RD) | |
| Percentage change with respect to healthy controls |
Grey matter ROIs: Brainstem Cerebellum Frontal lobe Occipital lobe Orbitofrontal cortex Parietal lobe Precentral gyrus Temporal lobe |
|
White matter ROIs (FA, AD, RD): Corpus callosum Cerebellum Corticospinal tract Frontal lobe Occipital lobe Parietal lobe Temporal lobe Thalamus |
Ethics Statement
This study was approved by the Ethics Committee of Beaumont Hospital, Dublin and each participant provided informed consent prior to participation.
CRediT Author Statement
Stacey Li Hi Shing and Peter Bede: Drafting the manuscript; Stacey Li Hi Shing, Jasmin Lope, Rangariroyashe H. Chipika and Peter Bede Neuroimaging analyses; Stacey Li Hi Shing, Rangariroyashe H. Chipika and Orla Hardiman: Clinical assessments; Stacey Li Hi Shing, Jasmin Lope, Rangariroyashe H. Chipika, Orla Hardiman and Peter Bede: Review of the manuscript for intellectual content.
Declaration of Competing Interest
The authors have no competing interests to declare.
Acknowledgments
We acknowledge all poliomyelitis survivors, patients with amyotrophic lateral sclerosis and healthy controls for agreeing to participate in this research study. Without their contribution, this study would not have been possible. This study was supported by the Health Research Board (HRB EIA-2017–019), the Spastic Paraplegia Foundation (SPF), the EU Joint Programme – Neurodegenerative Disease Research (JPND), the Andrew Lydon scholarship, and the Irish Institute of Clinical Neuroscience (IICN), the Iris O'Brien Foundation.
Footnotes
Potential conflicts of interest: None declared
References
- 1.Li Hi Shing S. Increased cerebral integrity metrics in poliomyelitis survivors: putative adaptation to longstanding lower motor neuron degeneration. J. Neurol. Sci. 2021 doi: 10.1016/j.jns.2021.117361. [DOI] [PubMed] [Google Scholar]
- 2.Li Hi Shing S. Post-polio syndrome: more than just a lower motor neuron disease. Front. Neurol. 2019;10:773. doi: 10.3389/fneur.2019.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christidi F. Clinical and radiological markers of extra-motor deficits in amyotrophic lateral sclerosis. Front. Neurol. 2018;9:1005. doi: 10.3389/fneur.2018.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasseroleslami B. Characteristic increases in EEG connectivity correlate with changes of structural MRI in amyotrophic lateral sclerosis. Cereb. Cortex. 2019;29(1):27–41. doi: 10.1093/cercor/bhx301. [DOI] [PubMed] [Google Scholar]
- 5.Hardiman O. 2016 ed. Springer Cham Heidelberg New York Dordrecht London© Springer International Publishing Switzerland 2016: Springer International Publishing; 2016. Neurodegenerative Disorders: A Clinical Guide; pp. 1–336. [Google Scholar]
- 6.Grollemund V. Machine learning in amyotrophic lateral sclerosis: achievements, pitfalls, and future directions. Front. Neurosci. 2019;13:135. doi: 10.3389/fnins.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finegan E. Primary lateral sclerosis: a distinct entity or part of the ALS spectrum? Amyotroph. Lateral Scler. Frontotemporal Degener. 2019;20(3–4):133–145. doi: 10.1080/21678421.2018.1550518. [DOI] [PubMed] [Google Scholar]
- 8.Querin G. The spinal and cerebral profile of adult spinal-muscular atrophy: a multimodal imaging study. Neuroimage Clin. 2019;21 doi: 10.1016/j.nicl.2018.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Querin G. Biomarkers of Spinal and Bulbar Muscle Atrophy (SBMA): a comprehensive review. Front. Neurol. 2018;9:844. doi: 10.3389/fneur.2018.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebouteux M.V. Revisiting the spectrum of lower motor neuron diseases with snake eyes appearance on magnetic resonance imaging. Eur. J. Neurol. 2014;21(9):1233–1241. doi: 10.1111/ene.12465. [DOI] [PubMed] [Google Scholar]
- 11.El Mendili M.M. Spinal cord imaging in amyotrophic lateral sclerosis: historical concepts-novel techniques. Front. Neurol. 2019;10:350. doi: 10.3389/fneur.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Querin G. Multimodal spinal cord MRI offers accurate diagnostic classification in ALS. J. Neurol. Neurosurg. Psychiatry. 2018;89(11):1220–1221. doi: 10.1136/jnnp-2017-317214. [DOI] [PubMed] [Google Scholar]
- 13.Bede P. Brainstem pathology in amyotrophic lateral sclerosis and primary lateral sclerosis: a longitudinal neuroimaging study. Neuroimage Clin. 2019;24 doi: 10.1016/j.nicl.2019.102054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chipika R.H. Tracking a fast-moving disease: longitudinal markers, monitoring, and clinical trial endpoints in ALS. Front. Neurol. 2019;10:229. doi: 10.3389/fneur.2019.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chipika R.H. Switchboard" malfunction in motor neuron diseases: selective pathology of thalamic nuclei in amyotrophic lateral sclerosis and primary lateral sclerosis. Neuroimage Clin. 2020;27 doi: 10.1016/j.nicl.2020.102300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster C. The segmental diffusivity profile of amyotrophic lateral sclerosis associated white matter degeneration. Eur. J. Neurol. 2016;23(8):1361–1371. doi: 10.1111/ene.13038. [DOI] [PubMed] [Google Scholar]
- 17.Dukic S. Patterned functional network disruption in amyotrophic lateral sclerosis. Hum. Brain Mapp. 2019;40(16):4827–4842. doi: 10.1002/hbm.24740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abidi M. Adaptive functional reorganization in amyotrophic lateral sclerosis: coexisting degenerative and compensatory changes. Eur. J. Neurol. 2020;27(1):121–128. doi: 10.1111/ene.14042. [DOI] [PubMed] [Google Scholar]
- 19.Bede P. Degenerative and regenerative processes in amyotrophic lateral sclerosis: motor reserve, adaptation and putative compensatory changes. Neural. Regen. Res. 2021;16(6):1208–1209. doi: 10.4103/1673-5374.300440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bede P. Virtual brain biopsies in amyotrophic lateral sclerosis: diagnostic classification based on in vivo pathological patterns. Neuroimage Clin. 2017;15:653–658. doi: 10.1016/j.nicl.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finegan E. The clinical and radiological profile of primary lateral sclerosis: a population-based study. J. Neurol. 2019;266(11):2718–2733. doi: 10.1007/s00415-019-09473-z. [DOI] [PubMed] [Google Scholar]