Abstract
Context:
Despite the improvement in renal cell carcinoma (RCC) diagnosis and management observed during the last 2 decades, RCC remains one of the most lethal urological malignancies. With the expansion of routine imaging for many disorders, an increasing number of patients who harbour RCC are identified incidentally.
Objective:
To summarise and compare RCC incidence and mortality rates, analyse the magnitude of risk factors, and interpret these epidemiological observations in the context of screening and disease management.
Evidence acquisition:
The primary objective of the current review was to retrieve and describe worldwide RCC incidence/mortality rates. Secondly, a narrative literature review about the magnitude of the known risk factors was performed. Finally, data retrieved from the first two steps were elaborated to define the clinical implications for RCC screening.
Evidence synthesis:
RCC incidence and mortality significantly differ among individual countries and world regions. Potential RCC risk factors include behavioural and environmental factors, comorbidities, and analgesics. Smoking, obesity, hypertension, and chronic kidney disease represent established risk factors. Other factors have been associated with an increased RCC risk, although selection biases may be present and controversial results have been reported.
Conclusions:
Incidence of RCC varies worldwide. Within the several RCC risk factors identified, smoking, obesity, and hypertension are most strongly associated with RCC. In individuals at a higher risk of RCC, the cost effectiveness of a screening programme needs to be assessed on a country-specific level due to geographic heterogeneity in incidence and mortality rates, costs, and management implications. Owing to the low rates of RCC, implementation of accurate biomarkers appears to be mandatory.
Patient summary:
The probability of harbouring kidney cancer is higher in developed countries and among smokers, obese individuals, and individuals with hypertension.
Keywords: Kidney cancer, Renal cancer, Epidemiology, Incidence, Prevalence, Mortality
1. Introduction
Worldwide, renal cell carcinoma (RCC) represents the sixth most frequently diagnosed cancer in men and the 10th in women, accounting for 5% and 3% of all oncological diagnoses, respectively [1]. RCC incidence rates have been increasing, and in higher-income settings, this may partially be due to an increase in the incidental detection of renal masses when abdominal imaging is performed for nonspecific musculoskeletal or gastrointestinal complaints. Although most detected lesions are small tumours, locally advanced disease continues to be diagnosed in a notable proportion of patients, with up to 17% of patients harbouring distant metastases at the time of diagnosis [2].
In Europe and North America, the lifetime risk for developing RCC ranges between 1.3% and 1.8%. According to the most updated data provided by the World Health Organization, there are more than 140 000 RCC-related deaths yearly, with RCC ranking as the 13th most common cause of cancer death worldwide [3].
The aim of this review is to summarise and compare the available evidence on RCC incidence and mortality rates, identify the most strongly associated risk factors, and interpret these epidemiological observations in the context of screening and disease management.
2. Evidence acquisition
The primary objective of the current review was to retrieve and describe worldwide RCC incidence/mortality rates. Secondly, a nonsystematic narrative literature review about the magnitude of the known risk factors was performed. Finally, data retrieved from the first two steps were elaborated to define the clinical implications for RCC screening.
The evidence acquired is presented and discussed according to three constructs.
2.1. Epidemiology
Descriptive analyses of worldwide RCC incidence and mortality rates were retrieved from the most recent version of GLOBOCAN database [3]. Geographic and temporal patterns were examined using age-standardised rates (ASRs) adjusted to the world standard population and expressed per 100 000 alongside cumulative risk—the probability of developing or dying from the disease in a lifetime (defined as over the age range 0–74 yr), in the absence of competing causes of death (see the Supplementary material) [3]. An alphanumeric scoring system describing the availability of incidence and mortality data has been established at the country level and is presented together with the estimates for each country, with the aim of providing a broad indication of the robustness of the estimation (see the Supplementary material) [3]. Acknowledged limitations of the epidemiological sources and a glossary of all terms used throughout the review are available in the Supplementary material.
2.2. Riskfactors
RCC risk factors were derived from English-language original articles, previous systematic/narrative reviews, and meta-analyses published during the last 10 yr (January 2008–January 2018). Genetic and hereditary RCC cases were not the objective of the current review, and only sporadic RCC was considered and discussed.
Between January 2008 and January 2018, a literature Search of the following electronic resources was conducted: Medline (via PubMed) and Scopus. The search strategy included the following search terms: “Kidney Neoplasms” OR “Carcinoma Renal Cell” OR Kidney Neoplasm* OR Renal Neoplasm* OR Renal cell neoplasm* OR Kidney Cancer* OR Renal Cancer* OR Renal Cell Cancer* OR kidney tumor* OR renal tumor* OR renal cell tumor*; and “epidemiology” OR “incidence” OR “prevalence” OR “risk factor”. Only papers reporting duplication, case reports, comments, editorials, and congress abstracts were excluded. Additional relevant articles were selected from manuscript bibliographies. Given the descriptive, retrospective, noncomparative design of the studies identified, evidence synthesis was performed in a descriptive and narrative manner.
2.3. Clinical implications
Finally, data retrieved from the first two steps were elaborated to describe potential clinical implications of these epidemiological findings for RCC screening.
3. Evidence synthesis
3.1. Epidemiology of kidney cancer: demographic factors
The all-age incidence ASRs of RCC for both sexes is 4.4 per 100 000, with a cumulative risk (ages 0–74) of 0.51%. Incidence (Fig. 1), prevalence (Fig. 2) and mortality ( Fig. 3) rates significantly vary worldwide, with Figure 4 depicting the cumulative risk of RCC incidence and mortality stratified according to world region and sex. Worldwide, North America had the highest ASR (11.7 per 100 000) followed by Western Europe (9.8) and Australia/New Zealand (9.2).
Fig. 1 –

World incidence ASRs for both sexes. Numbers are expressed per 100 000 people. Reproduced with permission from the International Agency for Research on Cancer [3]. ASR = age-standardised rate.
Fig. 2 –

World prevalence proportions for both sexes. Numbers are expressed per 100 000 people. Reproduced with permission from the International Agency for Research on Cancer [3].
Fig. 3 –

World mortality ASRs for both sexes. Numbers are expressed per 100 000 people. Reproduced with permission from the International Agency for Research on Cancer [3]. ASR = age-standardised rate.
Fig. 4 –
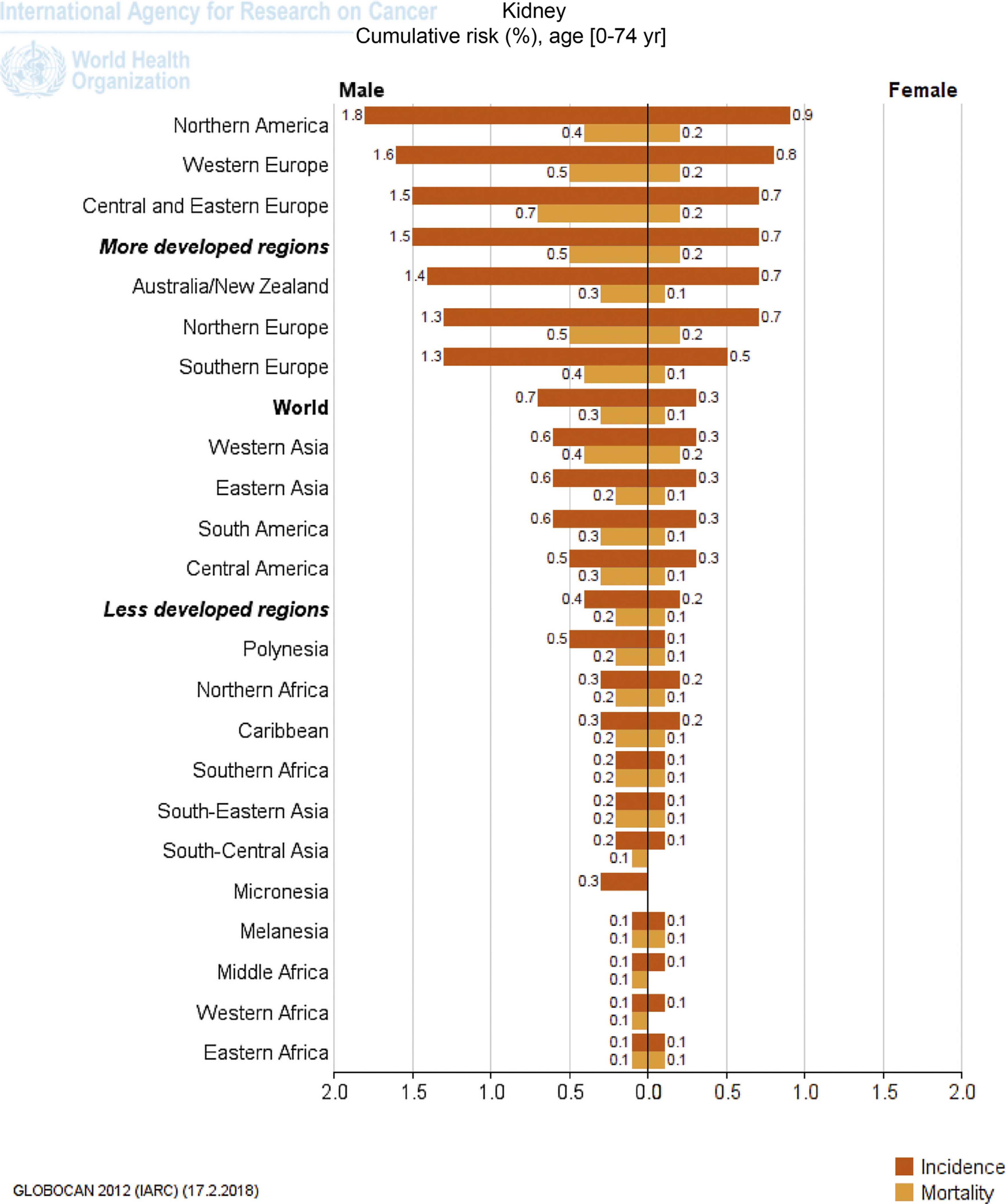
Cumulative risk of incidence and mortality of kidney cancer stratified for gender and world regions. Numbers are expressed per 100 000 people. Reproduced with permission from the International Agency for Research on Cancer [3].
3.1.1. Europe
Incidence rates in both sexes were highest in Western Europe (9.8) but virtually equivalent in all the other European regions (Central and Eastern Europe: 8.7, Northern Europe: 8.3, and Southern Europe) [3]. Table 1 depicts the incidence and cumulative risk of RCC stratified for each country. In terms of survival, the highest estimated mortality rates were observed in Lithuania (4.9), Czech Republic (4.8), Latvia (4.7), and Estonia (4.6), with a cumulative risk of RCC mortality varying from 0.55% to 0.66% [4]. In 2015, 12 547 new cases of RCC were registered in UK, with an incidence ASR of 20.8 (95% confidence interval [CI] 20.5–21.2). Over the last decade in the UK, RCC incidence rates increased by 47% [5]. In terms of mortality, differences across the countries were recorded: although mortality rates have declined in Scandinavian countries, France, Germany, Austria, the Netherlands, and Italy, in some European countries (ie, Ireland, Croatia, Greece, Estonia, and Slovakia), mortality rates are rising [6].
Table 1 –
Incidence and cumulative risk of kidney cancer in Europe
| Population | Qualitya | Numbers | Crude rate | ASR (W) | Cumulative risk |
|---|---|---|---|---|---|
| Czech Republic | A2 | 3313 | 31.4 | 16.7 | 2.01 |
| Lithuania | A1 | 773 | 23.5 | 13.2 | 1.61 |
| Slovakia | A1 | 1063 | 19.4 | 12.5 | 1.49 |
| Estonia | A1 | 284 | 21.2 | 11.7 | 1.39 |
| Belarus | A2 | 1575 | 16.5 | 11.1 | 1.29 |
| Slovenia | A1 | 400 | 19.6 | 11.1 | 1.27 |
| Latvia | A1 | 449 | 20.1 | 10.9 | 1.31 |
| Germany | B2 | 18 615 | 22.7 | 10.6 | 1.27 |
| Croatia | A2 | 821 | 18.7 | 10.0 | 1.16 |
| France (metropolitan) | B2 | 11 023 | 17.4 | 9.7 | 1.14 |
| Norway | A2 | 798 | 16.1 | 9.3 | 1.08 |
| Hungary | G1 | 1554 | 15.6 | 9.1 | 1.03 |
| Russian Federation | D2 | 19 313 | 13.5 | 8.9 | 1.05 |
| Iceland | A1 | 45 | 13.7 | 8.8 | 1.17 |
| The Netherlands | A2 | 2679 | 16.0 | 8.8 | 1.04 |
| Italy | B2 | 11 300 | 18.5 | 8.7 | 1.01 |
| Belgium | A2 | 1763 | 16.3 | 8.7 | 1.03 |
| Ireland | A1 | 571 | 12.5 | 8.4 | 0.99 |
| Luxembourg | D2 | 70 | 13.4 | 8.3 | 1.00 |
| UK | A1 | 9714 | 15.5 | 8.2 | 0.93 |
| Poland | C3 | 5244 | 13.7 | 8.1 | 0.95 |
| Malta | A1 | 57 | 13.6 | 8.0 | 0.91 |
| Austria | A2 | 1322 | 15.7 | 8.0 | 0.95 |
| Finland | A1 | 882 | 16.3 | 7.9 | 0.90 |
| Spain | B2 | 6474 | 13.8 | 7.9 | 0.89 |
| Ukraine | A2 | 5240 | 11.7 | 7.5 | 0.87 |
| Serbia | B2 | 1127 | 11.4 | 7.4 | 0.85 |
| Denmark | A2 | 754 | 13.5 | 7.2 | 0.87 |
| Bulgaria | A2 | 881 | 11.9 | 6.9 | 0.80 |
| Montenegro | G6 | 59 | 9.3 | 6.6 | 0.75 |
| Switzerland | B2 | 948 | 12.3 | 6.5 | 0.74 |
| Sweden | A2 | 1125 | 11.8 | 6.4 | 0.75 |
| Albania | G3 | 228 | 7.1 | 5.8 | 0.71 |
| Romania | E1 | 1940 | 9.1 | 5.7 | 0.66 |
| Bosnia Herzegovina | D5 | 292 | 7.8 | 5.2 | 0.57 |
| Portugal | C3 | 1004 | 9.4 | 5.0 | 0.57 |
| Republic of Moldova | G1 | 230 | 6.5 | 4.6 | 0.55 |
| Greece | G3 | 1094 | 9.6 | 4.5 | 0.48 |
| FYR Macedonia | G3 | 104 | 5.0 | 3.6 | 0.41 |
| Cyprus | A3 | 46 | 4.1 | 3.0 | 0.35 |
ASR = age-standardised rate.
Data are sorted by ASRs. Numbers are expressed per 100 000 people. Reproduced with permission from the International Agency for Research on Cancer [3].
For Quality legend, please refer to the Supplementary material (Level of availability of incidence and mortality data).
3.1.2. North and South America
Table 2 depicts the incidence and cumulative risk of RCC stratified for the USA and Canada. North America has the highest worldwide estimated incidence (ASR 12 per 100 000), with cumulative risks of 1.8% and 0.9% for males and females, respectively (Fig. 1). South and Central America have significantly lower RCC incidence (0.6% and 0.5%, respectively, for males and 0.3% for females). In specific countries (ie, Uruguay and Argentina), ASRs and cumulative risk were slightly lower compared with North American ASRs (Table 2).
Table 2 –
Incidence and cumulative risk of kidney cancer in America
| Population | Qualitya | Numbers | Crude rate | ASR (W) | Cumulative risk |
|---|---|---|---|---|---|
| USA | A1 | 58 222 | 18.4 | 12.0 | 1.39 |
| Uruguay | A2 | 465 | 13.7 | 9.4 | 1.12 |
| Canada | A1 | 5579 | 16.1 | 9.3 | 1.06 |
| Argentina | B3 | 4068 | 9.9 | 8.0 | 0.93 |
| Chile | C2 | 1353 | 7.8 | 6.0 | 0.72 |
| Puerto Rico | A2 | 272 | 7.3 | 4.9 | 0.56 |
| Costa Rica | A2 | 179 | 3.7 | 3.7 | 0.42 |
| Peru | E3 | 953 | 3.2 | 3.6 | 0.42 |
| Mexico | E1 | 3851 | 3.3 | 3.5 | 0.40 |
| Bolivia | G6 | 263 | 2.6 | 3.5 | 0.41 |
| Belize | G2 | 8 | 2.5 | 3.2 | 0.27 |
| Cuba | C1 | 517 | 4.6 | 3.1 | 0.34 |
| Brazil | B2 | 6255 | 3.2 | 3.0 | 0.33 |
| Ecuador | C3 | 403 | 2.7 | 2.9 | 0.31 |
| Venezuela | G1 | 747 | 2.5 | 2.7 | 0.30 |
| Colombia | C2 | 1048 | 2.2 | 2.4 | 0.26 |
| Trinidad and Tobago | D2 | 32 | 2.4 | 2.3 | 0.23 |
| Guyana | G2 | 14 | 1.8 | 2.2 | 0.21 |
| France, Guadeloupe | G2 | 14 | 3.0 | 2.1 | 0.31 |
| Panama | G2 | 68 | 1.9 | 2.0 | 0.26 |
| Barbados | G2 | 8 | 2.9 | 1.9 | 0.20 |
| Bahamas | G1 | 7 | 2.0 | 1.8 | 0.24 |
| Suriname | G3 | 9 | 1.7 | 1.6 | 0.19 |
| El Salvador | G2 | 93 | 1.5 | 1.5 | 0.17 |
| Nicaragua | G3 | 63 | 1.1 | 1.5 | 0.16 |
| Guatemala | G2 | 150 | 1.0 | 1.5 | 0.17 |
| Honduras | G6 | 80 | 1.0 | 1.5 | 0.16 |
| Paraguay | G3 | 78 | 1.2 | 1.5 | 0.16 |
| France, Martinique | A2 | 11 | 2.7 | 1.4 | 0.15 |
| Jamaica | C3 | 31 | 1.1 | 1.1 | 0.10 |
| French Guiana | G2 | 2 | 0.8 | 0.7 | 0.06 |
| Haiti | G3 | 58 | 0.6 | 0.7 | 0.08 |
| Dominican Republic | G3 | 52 | 0.5 | 0.6 | 0.06 |
ASR = age-standardised rate.
Data are sorted by ASRs. Numbers are expressed per 100 000 people. Reproduced with permission from the International Agency for Research on Cancer [3].
For Quality legend, please refer to the Supplementary material (Level of availability of incidence and mortality data).
The highest estimated mortality rates were seen in Uruguay (4.4 per 100 000), Argentina (3.6), Chile (3.1), and the USA (2.6), with a cumulative risk of mortality ranging from 0.3% to 0.52%. Data from the US demonstrate an increasing incidence [7]. Specifically, US incidence rate increased from 10.6/100 000 individuals in 2001 to 12.4/ 100 000 individuals in 2010 and increased with age.
3.1.3. Asia
Table 3 depicts the incidence and cumulative risk of RCC stratified for each Asian country. Cumulative risks of incidence were 0.6% and 0.3%, respectively, for males and females in both western and eastern Asia. Israel has the highest estimated incidence in Asia (ASR 10.0 per 100 000), with an overall cumulative risk of 1.2%. The highest estimated mortality rates were observed in Turkey (4.7), the State of Palestine (3.4), the Democratic Republic of Korea (3.4), and Singapore (3.3), with a cumulative risk of mortality between 0.36% and 0.54% per year of age.
Table 3 –
Incidence and cumulative risk of kidney cancer in Asia
| Population | Qualitya | Numbers | Crude rate | ASR (W) | Cumulative risk |
|---|---|---|---|---|---|
| Israel | A2 | 1002 | 13.0 | 10.0 | 1.17 |
| Korea, Republic of | A2 | 5651 | 11.6 | 8.0 | 0.91 |
| Turkey | C6 | 3992 | 5.4 | 5.6 | 0.62 |
| Japan | B1 | 16 830 | 13.3 | 5.3 | 0.59 |
| Singapore | A1 | 401 | 7.6 | 5.2 | 0.61 |
| Korea, Democratic Republic of | G6 | 1318 | 5.4 | 4.3 | 0.53 |
| China | C4 | 66 466 | 4.9 | 3.8 | 0.43 |
| Qatar | A3 | 33 | 1.7 | 3.5 | 0.45 |
| Jordan | D5 | 129 | 2.0 | 3.2 | 0.37 |
| Lebanon | D6 | 142 | 3.3 | 3.2 | 0.35 |
| Mongolia | D5 | 66 | 2.3 | 3.1 | 0.38 |
| Syrian Arab Republic | G6 | 467 | 2.2 | 3.1 | 0.34 |
| State of Palestine | F6 | 71 | 1.7 | 3.1 | 0.36 |
| Kazakhstan | G2 | 491 | 3.0 | 2.9 | 0.33 |
| Iraq | F6 | 581 | 1.7 | 2.9 | 0.32 |
| Georgia | G2 | 167 | 3.9 | 2.7 | 0.29 |
| Bahrain | A3 | 23 | 1.7 | 2.6 | 0.25 |
| Iran, Islamic Republic of | C6 | 1641 | 2.2 | 2.6 | 0.28 |
| Malaysia | C2 | 611 | 2.1 | 2.4 | 0.28 |
| Saudi Arabia | D6 | 454 | 1.6 | 2.3 | 0.26 |
| United Arab Emirates | D6 | 64 | 0.8 | 2.3 | 0.29 |
| Kyrgyzstan | G2 | 91 | 1.7 | 2.2 | 0.26 |
| Kuwait | A2 | 34 | 1.2 | 2.2 | 0.26 |
| Timor-Leste | G6 | 14 | 1.2 | 2.1 | 0.26 |
| Oman | A3 | 36 | 1.2 | 2.1 | 0.25 |
| Armenia | G3 | 78 | 2.5 | 1.9 | 0.22 |
| Turkmenistan | G2 | 75 | 1.5 | 1.8 | 0.21 |
| Brunei | F5 | 6 | 1.5 | 1.8 | 0.16 |
| Indonesia | F6 | 3225 | 1.3 | 1.5 | 0.17 |
| Philippines | B2 | 1008 | 1.0 | 1.4 | 0.16 |
| Tajikistan | G3 | 63 | 0.9 | 1.4 | 0.16 |
| Afghanistan | G6 | 237 | 0.7 | 1.3 | 0.16 |
| Azerbaijan | G2 | 135 | 1.4 | 1.3 | 0.15 |
| Pakistan | E6 | 1646 | 0.9 | 1.3 | 0.14 |
| Uzbekistan | G2 | 283 | 1.0 | 1.2 | 0.14 |
| Thailand | B3 | 1017 | 1.5 | 1.2 | 0.13 |
| Myanmar | G6 | 476 | 1.0 | 1.1 | 0.12 |
| Lao PDR | G6 | 52 | 0.8 | 1.1 | 0.12 |
| Nepal | G6 | 218 | 0.7 | 1.0 | 0.12 |
| India | C5 | 9658 | 0.8 | 0.9 | 0.11 |
| Cambodia | G6 | 101 | 0.7 | 0.9 | 0.10 |
| Viet Nam | E4 | 810 | 0.9 | 0.9 | 0.09 |
| Sri Lanka | D6 | 221 | 1.0 | 0.9 | 0.09 |
| Bangladesh | F6 | 900 | 0.6 | 0.8 | 0.08 |
| Yemen | E6 | 112 | 0.4 | 0.6 | 0.07 |
| Bhutan | D6 | 3 | 0.4 | 0.6 | 0.06 |
ASR = age-standardised rate.
Data are sorted by ASRs. Numbers are expressed per 100 000 people. Reproduced with permission from the International Agency for Research on Cancer [3].
For Quality legend, please refer to the Supplementary material (Level of availability of incidence and mortality data).
3.1.4. Africa
Table 4 depicts the incidence and cumulative risk of RCC stratified for each African country. Overall, Africa has the lowest cumulative risk of incidence and mortality, below 0.2% for both sexes. Mauritius has the highest estimated incidence in Africa (4.2), with a cumulative risk of 0.37%. All other African countries showed significantly lower ASRs. The highest estimated mortality rates were seen in Egypt (2.4), Libya (2.3), Mali (1.8), and Tunisia (1.7), with a cumulative mortality risk between 0.17% and 0.27%.
Table 4 –
Incidence and cumulative risk of kidney cancer in Africa
| Population | Qualitya | Numbers | Crude rate | ASR (W) | Cumulative risk |
|---|---|---|---|---|---|
| Mauritius | D2 | 53 | 4.0 | 4.2 | 0.37 |
| Libya | C6 | 133 | 2.1 | 2.7 | 0.30 |
| Egypt | C3 | 1740 | 2.1 | 2.4 | 0.26 |
| Tunisia | C6 | 237 | 2.2 | 2.2 | 0.25 |
| France, La Reunion | D2 | 21 | 2.4 | 2.1 | 0.19 |
| Ethiopia | E6 | 1412 | 1.6 | 2.1 | 0.20 |
| Mali | E6 | 307 | 1.9 | 1.9 | 0.15 |
| Eritrea | G6 | 77 | 1.4 | 1.9 | 0.18 |
| Somalia | G6 | 131 | 1.3 | 1.8 | 0.17 |
| Algeria | C6 | 454 | 1.2 | 1.5 | 0.16 |
| Sudan | F6 | 462 | 1.2 | 1.5 | 0.15 |
| Morocco | E6 | 451 | 1.4 | 1.5 | 0.15 |
| Djibouti | G6 | 11 | 1.2 | 1.5 | 0.14 |
| South Sudan | G6 | 117 | 1.1 | 1.4 | 0.13 |
| Kenya | E6 | 380 | 0.9 | 1.4 | 0.15 |
| Zimbabwe | C6 | 137 | 1.1 | 1.3 | 0.11 |
| Uganda | C6 | 305 | 0.9 | 1.2 | 0.09 |
| Namibia | D6 | 19 | 0.8 | 1.2 | 0.14 |
| South African Republic | D3 | 506 | 1.0 | 1.2 | 0.13 |
| Senegal | G6 | 134 | 1.0 | 1.1 | 0.09 |
| Niger | E6 | 92 | 0.6 | 1.0 | 0.12 |
| Benin | F6 | 77 | 0.8 | 1.0 | 0.09 |
| Chad | G6 | 89 | 0.8 | 0.9 | 0.09 |
| Western Sahara | G6 | 5 | 0.9 | 0.9 | 0.06 |
| Angola | G6 | 109 | 0.5 | 0.8 | 0.08 |
| Central African Republic | G6 | 34 | 0.7 | 0.8 | 0.07 |
| Madagascar | G6 | 139 | 0.6 | 0.8 | 0.07 |
| Botswana | D6 | 12 | 0.6 | 0.8 | 0.10 |
| Gabon | F6 | 12 | 0.8 | 0.8 | 0.05 |
| Zambia | E6 | 80 | 0.6 | 0.7 | 0.07 |
| Burundi | G6 | 44 | 0.5 | 0.7 | 0.05 |
| Rwanda | F6 | 60 | 0.5 | 0.7 | 0.05 |
| Malawi | C6 | 132 | 0.8 | 0.7 | 0.04 |
| Ghana | F6 | 173 | 0.7 | 0.7 | 0.05 |
| Mauritania | G6 | 23 | 0.6 | 0.7 | 0.05 |
| Cote d’Ivoire | F6 | 127 | 0.6 | 0.7 | 0.06 |
| Nigeria | E6 | 936 | 0.6 | 0.6 | 0.05 |
| Togo | F6 | 36 | 0.6 | 0.6 | 0.06 |
| Congo, Democratic | G6 | 337 | 0.5 | 0.6 | 0.04 |
| Republic of Cameroon | E6 | 116 | 0.6 | 0.5 | 0.04 |
| Burkina Faso | F6 | 70 | 0.4 | 0.5 | 0.05 |
| Congo, Republic of | E6 | 21 | 0.5 | 0.5 | 0.03 |
| Sierra Leone | G6 | 24 | 0.4 | 0.4 | 0.04 |
| Lesotho | G6 | 6 | 0.3 | 0.4 | 0.03 |
| Liberia | G6 | 14 | 0.3 | 0.3 | 0.03 |
| Equatorial Guinea | G6 | 3 | 0.4 | 0.3 | 0.02 |
| Guinea | E6 | 27 | 0.3 | 0.3 | 0.03 |
| Guinea-Bissau | G6 | 5 | 0.3 | 0.3 | 0.02 |
| Tanzania | E6 | 106 | 0.2 | 0.2 | 0.01 |
| Swaziland | D6 | 2 | 0.2 | 0.2 | 0.02 |
| Mozambique | E6 | 34 | 0.1 | 0.1 | 0.00 |
| Comoros | G6 | 1 | 0.1 | 0.1 | 0.00 |
| The Gambia | D6 | 0 | 0.0 | 0.0 | 0.00 |
| Cape Verde | G6 | 0 | 0.0 | 0.0 | 0.00 |
ASR = age-standardised rate.
Data are sorted by ASRs. Numbers are expressed per 100 000 people. Reproduced with permission from the International Agency for Research on Cancer [3].
For Quality legend, please refer to the Supplementary material (Level of availability of incidence and mortality data).
3.1.5. Oceania
Table 5 depicts the incidence and cumulative risk of RCC stratified by country. Cumulative risks of incidence were 1.4% and 0.7% for males and females, respectively. Australia and New Zealand observed the highest estimated incidence (ASR 9.5 and 8.2 per 100 000, respectively), with an overall cumulative risk of 1%. The highest estimated mortality rates were observed in Australia (3.5 per 100 000) and New Zealand (3.0), with a cumulative risk of mortality between 0.31% and 0.41%.
Table 5 –
Incidence and cumulative risk of kidney cancer in Australia
| Population | Qualitya | Numbers | Crude rate | ASR (W) | Cumulative risk |
|---|---|---|---|---|---|
| Australia | A1 | 3501 | 15.3 | 9.5 | 1.08 |
| New Zealand | A1 | 586 | 13.1 | 8.2 | 0.97 |
| New Caledonia | D5 | 15 | 5.8 | 4.9 | 0.57 |
| French Polynesia | D5 | 12 | 4.3 | 4.3 | 0.53 |
| Guam | D6 | 4 | 2.2 | 1.8 | 0.11 |
| Papua New Guinea | G6 | 37 | 0.5 | 0.8 | 0.08 |
| Fiji | D3 | 4 | 0.5 | 0.4 | 0.04 |
| Samoa | D6 | 0 | 0.0 | 0.0 | 0.00 |
| Solomon Islands | G6 | 0 | 0.0 | 0.0 | 0.00 |
| Vanuatu | D6 | 0 | 0.0 | 0.0 | 0.00 |
ASR = age-standardised rate.
Data are sorted by ASRs. Numbers are expressed per 100 000 people. Reproduced with permission from the International Agency for Research on Cancer [3].
For Quality legend, please refer to the Supplementary material (Level of availability of incidence and mortality data).
3.2. Riskfactors
Age and gender are strongly related with the risk of RCC: the estimated incidence increases in the older population. Indeed, the ASR per 10 000 is 0.5 below the age of 40 yr and progressively increases to 35.0 over 75 yr of age (40–44 yr: 2.9, 45–49 yr: 5.3, 50–54 yr: 8.8, 56–59 yr: 13.0, 60–64 yr: 17.9, 65–69 yr: 22.6, 70–74 yr: 26.9). Peak incidence is between 60 and 70 yr. In the UK in 2013–2015, more than a third (36%) of new cases occurred in people aged ≥75 yr. Incidence ASRs rise steadily from around age 40 to 44 yr and more steeply from around age 65–69 yr [5]. The highest rates are in those over 85 yr old. There is a 1.5:1 male predominance, and incidence rates are significantly higher in males than in females across all age groups. The gap is widest in older patients [5].
The evaluation of RCC risk factors is biased by the fact that incidental cancer detection by ultrasounds and computed tomography (CT)/magnetic resonance imaging performed for other reasons might artificially have increased the association between RCC and specific cofactors [2]. Potential risk factors include lifestyle factors, comorbidities, drugs, and environmental causes.
3.2.1. Lifestyle factors
3.2.1.1. Body mass index.
Obesity is commonly linked to several cancers, including RCC [8]. Translational studies demonstrated that RCC could be induced using prolonged intake of a high-fat diet, which are confirmed with pathological changes observed through histological sectioning [9]. Macleod et al. [10] relied on a prospective cohort of 77 260 residents of Washington, aged 50–76 yr, who completed a questionnaire on demographic, lifestyle, and health data to validate established and putative risk factors for incident RCC [10]. The study confirmed that obesity is significantly associated with RCC (body mass index ≥35 vs <25 kg/m2: hazard ratio [HR] 1.71, 95% CI 1.06–2.79) [10]. The relative risk estimate corresponding to roughly 5 kg of body weight increases the risk of RCC by 25% in men and 35% in women [10–12]. The association between obesity and increased risk of RCC was confirmed in both the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial and the National Lung Screening Trial [13]. The biological mechanisms behind this association are unclear, although recent evidence suggests that the effects of circulating hormones such as insulin-like growth factors and adipokines might play a role [11]. Other possible biological mechanisms involved are sex steroids and chronic inflammation [12]. The use of statins among patients with RCC is associated with significantly improved cancer-specific and overall survival [14,15]. However, a recent meta-analysis demonstrated no association between the use of statins and risk of harbouring RCC [14].
3.2.1.2. Physical activity.
Physical activity may decrease RCC risk by reducing obesity, blood pressure, insulin resistance, and lipid peroxidation. A recent meta-analysis reported an inverse association between physical activity and RCC risk (relative risk [RR] 0.88, 95% CI 0.79–0.97) [15]. Combining risk estimates from high-quality studies, this association was even stronger (RR 0.78, 95% CI 0.66–0.92) [15].
3.2.1.3. Fruit or vegetable intake.
Fruit and vegetable consumption (in particular, cruciferous vegetables) has been associated with a reduction in the risk of RCC in some reports [16,17]. However, in the European Prospective Investigation into Cancer and Nutrition (EPIC) study, Weikert et al. [18] examined the association between fruits and vegetables and the risk of RCC. Dietary intake data and complete follow-up information on cancer incidence were available for 375 851 participants. During an average follow-up of 6 yr, 306 RCC cases were identified (0.1%). No significant associations between fruit and vegetable consumption and RCC risk were observed despite a wide range of intake (HR 0.97, 95% CI 0.85–1.11) [18]. Similarly, in the Vitamin and Lifestyle (VITAL) study, no association between fruit or vegetable intake and RCC was recorded [10]. When pooling the data from all available studies, a significantly decreased risk of RCC was observed in those eating cruciferous vegetables (RR 0.73, 95% CI 0.63–0.83) and in a subgroup of case-control studies (RR 0.69, 95% CI 0.60–0.78), but not in cohort studies (RR 0.96, 95% CI 0.71– 1.21) [17].
3.2.1.4. Alcohol.
Moderate alcohol intake showed a protective effect on RCC incidence relative to abstinence [19,20]. Lew et al. [20] analysed the participants included in the NIH-AARP Diet and Health Study (n = 492 187 and 1814 RCC cases), and found an inverse association between alcohol intake and the risk of RCC. When the association was examined after excluding RCC cases diagnosed within the first 2 yr of follow-up, the results did not change appreciably. In male patients, the inverse effect was observed with beer consumption; in women, the inverse effect was observed with wine and liquor consumption, but not beer. The multivariate RR for an increment of one drink per day of alcohol drinks among drinkers was 0.96 (95% CI 0.94–0.99) in men and 0.73 (95% CI 0.60–0.88) in women [20]. Similarly, Pelucchi et al. [21] evaluated data from two Italian multicentre case-control studies, including 1115 incidental, histologically confirmed RCC cases and 2582 controls hospitalised with acute, non-neoplastic conditions. Compared with nondrinkers, the multivariate odds ratios of RCC were 0.87 (95% CI 0.73–1.04) for four or fewer drinks per day, 0.76 (95% CI 0.59–0.99) for more than four to eight or fewer drinks per day, and 0.70 (95% CI 0.50–0.97) for more than eight alcoholic drinks per day, with a significant inverse trend in risk (p = 0.01) [21]. Within the PLCO Trial, increasing alcohol consumption was associated with a reduced RCC risk compared with nondrinkers (>9.75 g/d: HR 0.67, 95% CI 0.50–0.89) [22]. Conversely, in the VITAL study, no association between alcohol intake and RCC was recorded [10]. A recent meta-analysis confirmed that alcohol intake from wine, beer, and liquor is associated with a reduced risk of RCC [23]. However, when these associations were examined separately by sex, statistically significant inverse associations were restricted to wine among females (RR 0.82, 95% CI 0.73–0.91) and to beer and liquor among males (RR 0.87, 95% CI 0.83–0.91 and RR 0.95, 95% CI 0.92–0.99, respectively) [23].
3.2.1.5. Smoking.
Smoking has been linked to a number of common cancers, including RCC. Tobacco smoke includes a mix of carcinogens implicated in the aetiology of renal cell cancer. In the VITAL study, smoking was independently associated with RCC (>37.5 pack-years vs never: HR 1.58, 95% CI 1.09–2.29) [10]. Similarly, in the PLCO trial, the intensity of smoking was confirmed to be significantly associated with a higher risk of developing RCC and with a higher risk of high-grade RCC [13]. Moreover, relative risk is directly associated with smoking duration and decreases over time after cessation. A recent meta-analysis of >24 papers demonstrated a pooled RR of RCC incidence of 1.31 (1.22–1.40) for all smokers, 1.36 (1.19–1.56) for current smokers, and 1.16 (1.08–1.25) for former smokers. The corresponding RCC cancer-specific mortality risks were 1.23 (1.08–1.40), 1.37 (1.19–1.59), and 1.02 (0.90–1.15) [24].
3.2.2. Comorbidities
3.2.2.1. Hypertension.
There is evidence that hypertension is an independent risk factor for RCC [10,25,26]. A number of prospective studies investigated the association between blood pressure and the risk of RCC, using either recorded BP levels or reported hypertension as the principal exposure variable [10,27]. In the VITAL study, hypertension was independently associated with RCC risk (HR 1.70, 95% CI 1.30–2.22) [10]. A recent meta-analysis of 18 prospective studies further supports a positive association between hypertension and RCC risk. A history of hypertension was associated with 67% increased risk of RCC, and each 10-mmHg increase in blood pressure was associated with 10– 22% increased risk of RCC, after accounting for heterogeneity and publication bias [27]. The biological mechanisms behind this relationship remain unclear, but some authors have hypothesised the involvement of chronic renal hypoxia and lipid peroxidation with formation of reactive oxygen species. Hypertensive individuals may suffer chronic renal hypoxia caused by the transcription of hypoxia-inducible factors that promote tumour cell proliferation and angiogenesis [28,29]. Importantly, hypertensive patients may also be more likely to get cross-sectional renal imaging and therefore identify incidental renal tumours.
3.2.2.2. Urinary stones.
A recent meta-analysis evaluated the association between a history of kidney stones and RCC [30]. The pooled RR of RCC in patients with kidney stoneswas 1.76 (95% CI 1.24–2.49). Subgroup analysis demonstrated that the history of kidney stones was significantly associated with increased RCC risk only in males (RR 1.41, 95% CI 1.11–1.80), but not in females (RR 1.13 [95% CI 0.86–1.49]) [30].
3.2.2.3. Diabetes.
Type 2 diabetes is associated with an increased risk of several types of cancer [31,32]. However, its relationship with RCC remains unclear. In the VITAL study, there was no observed relationship between diabetes and RCC after accounting for multiple confounders [10]. Conversely, in the Nurses’ Health study [33], relying on 330 cases of pathologically confirmed incident RCC among roughly 120 000 women, type 2 diabetes was significantly associated with an increased risk of RCC (HR 1.60 [95% CI 1.19–2.17]). These associations were consistent across different strata of BMI, smoking, and hypertension. Moreover, RCC risk increased with an increasing number of comorbidities, including obesity, hypertension, and type 2 diabetes. Specifically, women who had all these three conditions had four-fold higher probability of RCC development compared with women without comorbidities [33].
3.2.2.4. Liver and chronic kidney diseases.
Cystic degenerative changes (acquired cystic kidney disease) and a higher incidence of RCC are typical features of end-stage kidney disease. RCC of native end-stage kidneys are found in about 4% of patients. Their lifetime risk of developing RCC is at least 10 times higher than in the general population [34,35].
In the VITAL study, the presence of a kidney disease (HR 2.58, 95% CI 1.21–5.50) or viral hepatitis (HR 1.80, 95% CI 1.03–3.14) was independently associated with RCC [10]. Hepatitis C virus (HCV) infection causes cirrhosis and hepatocellular carcinoma, but is also aetiologically linked to several extrahepatic medical conditions including renal disorders and malignancies. In RCC patients, the rate of HCAB positivity has been reported to be higher (8%) than that in colon cancer patients (1%; p < 0.01), and RCC patients with HCV RNA positivity were significantly younger than RCC patients who were HCV RNA negative (p = 0.01) [36].
3.2.3. Reproductive and hormonal factors
An increased risk of RCC has been associated with parity among women in several cohort studies, although the association is not conclusive [37–40]. Associations with other reproductive factors, including the use of oral contraceptives and hormone replacement therapy, are not consistently observed [41].
3.2.4. Analgesics
Analgesics are the most commonly used over-the-counter drugs worldwide, with epidemiological data suggesting that analgesic use increases the risk of RCC. Cho and colleagues [42] examined the relationship between analgesic use and RCC risk in the Nurses’ Health Study and the Health Professionals Follow-up Study. Aspirin and acetaminophen use were not associated with RCC risk. However, regular use of nonaspirin nonsteriodal anti-inflammatory drugs (NSAIDs) was associated with an increased risk of RCC (pooled multivariate relative risk 1.51 [95% CI 1.12–2.04)]. A meta-analysis by Choueiri et al. [43] evaluated the association between analgesic use and RCC risk. Study-specific effect estimates were pooled to compute the overall relative risk using a random-effects model for each analgesic category. The use of acetaminophen and nonaspirin NSAIDs was associated with an increased risk of RCC (pooled RR 1.28, 95% CI 1.15–1.44, and 1.25, 95% CI 1.06–1.46, respectively). For aspirin use, no association was found except for non-US studies (five studies, pooled RR 1.17, 95% CI 1.04–1.33). Similar increases in risks were seen with higher analgesic intake [43].
3.2.5. Environmental factors
In terms of occupational exposure, RCC generally is not considered an occupational disease; but elevated risk has been linked to specific occupations and specific industrial agents. Trichloroethylene is by far the most extensively examined chemical in relation to RCC risk [37,38]. Despite the evidence being limited, exposure to x radiation and gamma-radiation industrial agents, including arsenic, inorganic arsenic compounds, cadmium, perfluorooctanoic acid, welding fumes, nitrate, and radon in drinking water, are not regarded as potential RCC risk factors [44–47]. However, more robust studies are needed to confirm these findings.
3.3. Epidemiology in kidney cancer: what are the clinical implications for screening?
Screening is a strategy used to detect disease within an asymptomatic population. The rationale for screening is that the detection of asymptomatic disease might lead to an earlier staged disease (stage migration) [48] and better outcomes from treatment [49,50]. However, the cost effectiveness of a screening programme depends on many other aspects: (1) incidence and prevalence of the disease, (2) sensitivity and specificity of the detection method, (3) impact of an early diagnosis on the natural history of the disease, (4) impact of overtreatment, and (5) healthcare expenditures. The increased use of CT in the past—especially in the USA and Europe—resulted in a simulated form of screening, which led to potential overtreatment [51]. Moreover, exaggerate cross-sectional imaging (as has been suggested for the USA [51]) may introduce “contamination” of screening studies. In other words, introducing cross-sectional imaging theoretically in countries without this opportunity may result in a higher yield of screen-detected tumours than, for example, in the USAwhere a proportion of the population is being imaged for various reasons anyway.
Focusing on RCC, the highest incidence of RCC reaches 10–15 per 100 000 with an estimated cumulative risk of incident cases of roughly 0.5% per year of age. Important variations exist according to the geographic area. Obesity, hypertension, and smoking independently double the adjusted RR of developing RCC.
Different screening modalities have been analysed in the setting of sporadic RCC. Some authors proposed the use of urine dipstick, but the low diagnostic yield and the low accuracy in detecting RCC preclude any role for this option [52]. Several serum and urine biomarkers have been suggested (eg, aquaporin 1, perilipin 2, KIM1, and others [53]), although none have achieved clinical importance. Screening computed tomography was demonstrated to be non–cost effective, with a high proportion of false-positive cases. Conversely, abdominal ultrasound arises as a potential tool due to its characteristics (noninvasive, well-established, and widely utilised), although no robust data are available to determine whether ultrasound-based screening may be able to affect the natural history of the diseases due to high intervariability and low sensitivity for the detection of small renal masses. Finally, several authors have proposed targeted screening programmes for RCC in patients at a higher risk according to their clinical characteristics and comorbidities [52].
Assessing the epidemiological and risk factor data in the setting of RCC, some considerations regarding the role of screening are worth mentioning. Firstly, the cost effectiveness of a screening programme needs to be assessed on a country-specific level since key differences in RCC incidence, risk factor prevalence, and healthcare context exist among the different countries. Secondly, if risk factors are not present, RCC incidence is very low and–unless new biomarkers will become available—a screening programme relying on the available imaging technology is difficult to be cost effective. Thirdly, given the RCC incidence data we demonstrate, a screening programme would subject participants to a significant risk of overdiagnosis, overtreatment, and emotional impact-related consequences.
4. Conclusions
RCC incidence and mortality rates vary significantly around the globe. Potential risk factors include behavioural and environmental factors, comorbidities, and analgesics. To date, the consistent risk factors for RCC are smoking, obesity, hypertension, and chronic kidney disease. Many other factors have been associated with an increased RCC risk, although these associations may be confounded by selection biases and inconsistent results in the available studies. In individuals at a higher risk of RCC, the cost effectiveness of a screening programme needs to be assessed on a country-specific level. Owing to the low incidence of RCC, there is an unmet need for accurate biomarkers.
Supplementary Material
Financial disclosures:
Umberto Capitanio certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eururo.2018.08.036.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Capitanio U, Montorsi F. Renal cancer. Lancet 2016;387:894–906. [DOI] [PubMed] [Google Scholar]
- [3].Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. [Google Scholar]
- [4].Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–403. [DOI] [PubMed] [Google Scholar]
- [5]. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer/incidence-heading-Zero.
- [6].Levi F, Ferlay J, Galeone C, et al. The changing pattern of kidney cancer incidence and mortality in Europe. BJU Int 2008;101:949–58. [DOI] [PubMed] [Google Scholar]
- [7].King SC, Pollack LA, Li J, King JB, Master VA. Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United States 2001 to 2010. J Urol 2014;191:1665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bergström A, Hsieh CC, Lindblad P, Lu CM, Cook NR, Wolk A. Obesity and renal cell cancer—a quantitative review. Br J Cancer 2001;85:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang X-F, Ma G, Feng N-H, Yu D-S, Wu Y, Li C. Twist2 and CD24 expression alters renal microenvironment in obesity associated kidney cancer. Eur Rev Med Pharmacol Sci 2018;22:358–64. [DOI] [PubMed] [Google Scholar]
- [10].Macleod LC, Hotaling JM, Wright JL, et al. Risk factors for renal cell carcinoma in the VITAL study. J Urol 2013;190:1657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liao LM, Hofmann JN, Cho E, Pollak MN, Chow W-H, Purdue MP. Circulating levels of obesity-related markers and risk of renal cell carcinoma in the PLCO cancer screening trial. Cancer Causes Control 2017;28:801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gild P, Ehdaie B, Kluth LA. Effect of obesity on bladder cancer and renal cell carcinoma incidence and survival. Curr Opin Urol 2017;27:409–14. [DOI] [PubMed] [Google Scholar]
- [13].Lotan Y, Karam JA, Shariat SF, et al. Renal-cell carcinoma risk estimates based on participants in the prostate, lung, colorectal, and ovarian cancer screening trial and national lung screening trial. Urol Oncol 2016;34, 167.e9–16. [DOI] [PubMed] [Google Scholar]
- [14].Zhang X-L, Liu M, Qian J, et al. Statin use and risk of kidney cancer: a meta-analysis of observational studies and randomized trials. Br J Clin Pharmacol 2014;77:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Behrens G, Leitzmann MF. The association between physical activity and renal cancer: systematic review and meta-analysis. Br J Cancer 2013;108:798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao J, Zhao L. Cruciferous vegetables intake is associated with lower risk of renal cell carcinoma: evidence from a meta-analysis of observational studies. PLoS One 2013;8:e75732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu B, Mao Q, Wang X, et al. Cruciferous vegetables consumption and risk of renal cell carcinoma: a meta-analysis. Nutr Cancer 2013;65:668–76. [DOI] [PubMed] [Google Scholar]
- [18].Weikert S, Boeing H, Pischon T, et al. Fruits and vegetables and renal cell carcinoma: findings from the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 2006;118:3133–9. [DOI] [PubMed] [Google Scholar]
- [19].Jay R, Brennan P, Brenner DR, et al. Alcohol consumption and the risk of renal cancers in the European Prospective Investigation into Cancer and Nutrition (EPIC). Wozniak MB, Brennan P, Brenner DR, Overvad K, Olsen A, Tjønneland A, Boutron-Ruault MC, Clavel-Chapelon F, Fagherazzi G, Katzke V, Kühn T, Boeing H, Bergmann MM, Steffen A, Naska A, Trichopoulou A, Trichopoulos D, Saieva C, Grioni S, Panico S, Tumino R, Vineis P, Bueno-de-Mesquita HB, Peeters PH, Hjartåker A, Weiderpass E, Arriola L, Molina-Montes E, Duell EJ, Santiuste C, Alonso de la Torre R, Barricarte Gurrea A, Stocks T, Johansson M, Ljungberg B, Wareham N, Khaw KT, Travis RC, Cross AJ, Murphy N, Riboli E, Scelo G. Int J Cancer. 2015October15;137 (8):1953–66. [Epub 2015 Apr 28]. doi: 10.1002/ijc.29559. Urol Oncol 2017;35(3):117. [DOI] [PubMed] [Google Scholar]
- [20].Lew JQ, Chow W-H, Hollenbeck AR, Schatzkin A, Park Y. Alcohol consumption and risk of renal cell cancer: the NIH-AARP diet and health study. Br J Cancer 2011;104:537–41. 10.1038/sj.bjc.6606089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pelucchi C, Galeone C, Montella M, et al. Alcohol consumption and renal cell cancer risk in two Italian case-control studies. Ann Oncol 2008;19:1003–8. [DOI] [PubMed] [Google Scholar]
- [22].Karami S, Daugherty SE, Purdue MP. A prospective study of alcohol consumption and renal cell carcinoma risk. Int J Cancer 2015;137:238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xu X, Zhu Y, Zheng X, Xie L. Does beer, wine or liquor consumption correlate with the risk of renal cell carcinoma? A dose-response meta-analysis of prospective cohort studies. Oncotarget 2015;6:13347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cumberbatch MG, Rota M, Catto JWF, La Vecchia C. The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol 2016;70:458–66. [DOI] [PubMed] [Google Scholar]
- [25].Chien C-C, Han M-M, Chiu Y-H, et al. Epidemiology of cancer in end-stage renal disease dialysis patients: a national cohort study in Taiwan. J Cancer 2017;8:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mazzucotelli V, Piselli P, Verdirosi D, et al. De novo cancer in patients on dialysis and after renal transplantation: north-western Italy, 1997–2012. J Nephrol 2017;30:851–7. [DOI] [PubMed] [Google Scholar]
- [27].Hidayat K, Du X, Zou S-Y, Shi B-M. Blood pressure and kidney cancer risk. J Hypertens 2017;35:1333–44. [DOI] [PubMed] [Google Scholar]
- [28].Sharifi N, Farrar WL. Perturbations in hypoxia detection: a shared link between hereditary and sporadic tumor formation? Med Hypotheses 2006;66:732–5. 10.1016/j.mehy.11.2005.003. [DOI] [PubMed] [Google Scholar]
- [29].Gago-Dominguez M, Castelao JE, Yuan J-M, Ross RK, Yu MC. Lipid peroxidation: a novel and unifying concept of the etiology of renal cell carcinoma (United States). Cancer Causes Control 2002;13:287–93. [DOI] [PubMed] [Google Scholar]
- [30].Cheungpasitporn W, Thongprayoon C, O’Corragain OA, et al. The risk of kidney cancer in patients with kidney stones: a systematic review and meta-analysis. QJM 2015;108:205–12. [DOI] [PubMed] [Google Scholar]
- [31].Hendriks SH, Schrijnders D, van Hateren KJ, et al. Association between body mass index and obesity-related cancer risk in men and women with type 2 diabetes in primary care in the Netherlands: a cohort study (ZODIAC-56). BMJ Open 2018;8:e018859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol 2018;6:e6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Joh H-K, Willett WC, Cho E. Type 2 diabetes and the risk of renal cell cancer in women. Diabetes Care 2011;34:1552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Christensson A, Savage C, Sjoberg DD, et al. Association of cancer with moderately impaired renal function at baseline in a large, representative, population-based cohort followed for up to 30 years. Int J Cancer 2013;133:1452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lowrance WT, Ordoñez J, Udaltsova N, Russo P, Go AS. CKD and the risk of incident cancer. J Am Soc Nephrol 2014;25:2327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gonzalez HC, Lamerato L, Rogers CG, Gordon SC. Chronic hepatitis C infection as a risk factor for renal cell carcinoma. Dig Dis Sci 2015;60:1820–4. 10.1007/s10620-015-3521-3. [DOI] [PubMed] [Google Scholar]
- [37].Lambe M, Lindblad P, Wuu J, Remler R, Hsieh CC. Pregnancy and risk of renal cell cancer: a population-based study in Sweden. Br J Cancer 2002;86:1425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kabat GC, Silvera SAN, Miller AB, Rohan TE. A cohort study of reproductive and hormonal factors and renal cell cancer risk in women. Br J Cancer 2007;96:845–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Molokwu JC, Prizment AE, Folsom AR. Reproductive characteristics and risk of kidney cancer: Iowa Women's Health Study. Maturitas 2007;58:156–63. [DOI] [PubMed] [Google Scholar]
- [40].Lee JE, Hankinson SE, Cho E. Reproductive factors and risk of renal cell cancer: the Nurses’ Health Study. Am J Epidemiol 2009;169:1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chow W-H, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010;7:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cho E, Curhan G, Hankinson SE, et al. Prospective evaluation of analgesic use and risk of renal cell cancer. Arch Intern Med 2011;171:1487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Choueiri TK, Je Y, Cho E. Analgesic use and the risk of kidney cancer: a meta-analysis of epidemiologic studies. Int J Cancer 2014;134:384–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Desimone MC, Rathmell WK, Threadgill DW. Pleiotropic effects of the trichloroethylene-associated P81S VHL mutation on metabolism, apoptosis, and ATM-mediated DNA damage response. J Natl Cancer Inst 2013;105:1355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Moore LE, Boffetta P, Karami S, et al. Occupational trichloroethylene exposure and renal carcinoma risk: evidence of genetic susceptibility by reductive metabolism gene variants. Cancer Res 2010;70:6527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Abdul KSM, Jayasinghe SS, Chandana EPS, Jayasumana C, De Silva PMCS. Arsenic and human health effects: a review. Environ Toxicol Pharmacol 2015;40:828–46. [DOI] [PubMed] [Google Scholar]
- [47].Song JK, Luo H, Yin XH, et al. Association between cadmium exposure and renal cancer risk: a meta-analysis of observational studies. Sci Rep 2015;5:17976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Di Trapani E, Dell’Oglio P, Larcher A, et al. Pathological high-risk renal cell carcinoma: trends in clinical characteristics over 25 years. Anticancer Res 2018;38:4123–30. [DOI] [PubMed] [Google Scholar]
- [49].Rouprêt M. Smoking status is not sufficient to accurately target patients who would benefit from screening for bladder and kidney cancer. Eur Urol Focus 2015;1:52–3. [DOI] [PubMed] [Google Scholar]
- [50].Pinsky PF, Dunn B, Gierada D, et al. Incidental renal tumours on low-dose CT lung cancer screening exams. J Med Screen 2017;24:104–9. [DOI] [PubMed] [Google Scholar]
- [51].Welch HG, Skinner JS, Schroeck FR, Zhou W, Black WC. Regional variation of computed tomographic imaging in the United States and the risk of nephrectomy. JAMA Intern Med 2018;178:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rossi SH, Klatte T, Usher-Smith J, Stewart GD. Epidemiology and screening for renal cancer. World J Urol 2018;36:1341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Morrissey JJ, Mobley J, Figenshau RS, Vetter J, Bhayani S, Kharasch ED. Urine aquaporin 1 and perilipin 2 differentiate renal carcinomas from other imaged renal masses and bladder and prostate cancer. Mayo Clin Proc 2015;90:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


