Abstract
Background
Cross-sectional studies have found that individuals with posttraumatic stress disorder (PTSD) exhibit deficits in autonomic functioning. While PTSD rates are twice as high in women compared to men, sex differences in autonomic functioning are relatively unknown among trauma-exposed populations. The current study used a prospective design to examine sex differences in posttraumatic autonomic functioning.
Methods
192 participants were recruited from emergency departments following trauma exposure (Mean age = 35.88, 68.2% female). Skin conductance was measured in the emergency department; fear conditioning was completed two weeks later and included measures of blood pressure (BP), heart rate (HR), and high frequency heart rate variability (HF-HRV). PTSD symptoms were assessed 8 weeks after trauma.
Results
2-week systolic BP was significantly higher in men, while 2-week HR was significantly higher in women, and a sex by PTSD interaction suggested that women who developed PTSD demonstrated the highest HR levels. Two-week HF-HRV was significantly lower in women, and a sex by PTSD interaction suggested that women with PTSD demonstrated the lowest HF-HRV levels. Skin conductance response in the emergency department was associated with 2-week HR and HF-HRV only among women who developed PTSD.
Conclusions
Our results indicate that there are notable sex differences in autonomic functioning among trauma-exposed individuals. Differences in sympathetic biomarkers (BP and HR) may have implications for cardiovascular disease risk given that sympathetic arousal is a mechanism implicated in this risk among PTSD populations. Future research examining differential pathways between PTSD and cardiovascular risk among men versus women is warranted.
Keywords: Trauma, PTSD, Autonomic, Sex, Cardiovascular
1. Introduction
Trauma exposure is very common, with lifetime prevalence rates up to 89% (Kilpatrick et al., 2013). Symptoms of posttraumatic stress disorder (PTSD) affect a substantial proportion of those exposed to trauma and include unwanted re-experiencing of the event, avoidance of trauma reminders, negative changes in thinking and mood, and hyperarousal (APA, 2013). Individuals with PTSD symptoms experience significant functional impairment across multiple domains, and they are at greater risk of having cardiometabolic diseases compared to the general population (Edmondson et al., 2013; Norman et al., 2006; Pacella et al., 2013). One of the proposed mechanisms underlying increased cardiometabolic disease incidence in PTSD is altered functioning of the autonomic nervous system, such as increased heart rate (HR) and blood pressure (BP), and decreased high frequency heart rate variability (HF-HRV; for reviews see Buckley and Kaloupek, 2001 and Michopoulos et al., 2015). While numerous cross-sectional studies have demonstrated that those with PTSD exhibit altered autonomic functioning (reviewed below), there is a paucity of longitudinal studies following individuals in the immediate aftermath of trauma. Further, it is well-established that PTSD rates are twice as high in women compared to men (Kessler et al., 1995), yet sex differences in autonomic functioning are relatively unknown among trauma-exposed populations.
There is a long-standing literature supporting an association between PTSD and autonomic deficits. Compared to healthy and trauma-exposed controls, individuals with PTSD demonstrate increased sympathetic arousal indicated by higher HR and BP both at rest and in response to challenge/stress (e.g., Ehlers et al., 2010; Gerardi et al., 1994; Jovanovic et al., 2009; Peri et al., 2000), coupled with decreased parasympathetic control indicated by lower HF-HRV (e.g., Cohen et al., 1997; Hauschildt et al., 2011; Hopper et al., 2006; Keary et al., 2009; Minassian et al., 2014; Sahar et al., 2001). Another autonomic indicator frequently used in PTSD is skin conductance response, which is a measure of sweat gland activity that is controlled by the sympathetic nervous system and increases with arousal (Hinrichs et al., 2017; Wangelin and Tuerk, 2015). Individuals with PTSD exhibit higher skin conductance responses to conditioned stimuli (e.g., Blechert et al., 2007; Milad et al., 2008; Peri et al., 2000) and are slower to extinguish this response (Milad et al., 2010) compared to healthy and trauma-exposed controls. While these studies have provided insight into the negative autonomic sequalae of trauma, few have utilized prospective designs. Earlier work conducted by Shalev et al. (1998, 2000) indicated that HR in the immediate trauma aftermath was predictive of subsequent PTSD and that autonomic deficits develop along with PTSD. Another study by Minassian et al. (2015) suggested low parasympathetic control prior to deployment was predictive of subsequent PTSD among U.S. Marines. Recently, our group demonstrated that high skin conductance response in the immediate trauma aftermath is a strong prospective indicator of PTSD development (Hinrichs et al., 2019). These findings are promising in terms of their potential to identify PTSD risk, but much more is needed to develop reliable biomarkers of autonomic functioning for use in the immediate trauma aftermath.
Sex differences in PTSD prevalence are well-established, however, little is known about sex differences in autonomic functioning among trauma-exposed samples (for a review, see Seligowski et al., 2020). In a study of assault survivors, women who experienced increased HR during script-driven imagery were more likely to be given a PTSD diagnosis six months later compared to men and compared to women who didn't have such a response (Kleim et al., 2010). Among motor vehicle accident survivors seen in the emergency department, no sex differences were observed in HR, but women were more likely to have PTSD at follow-up (Irish et al., 2011). In contrast, another study found that men with PTSD exhibited higher HRV than trauma-exposed controls, and no effect in women, but it is possible that these differences were due in part to collecting HRV data during a dark-enhanced startle paradigm (Kamkwalala et al., 2012). In terms of skin conductance, women with PTSD appear to demonstrate higher responses during fear conditioning compared to men with PTSD (Inslicht et al., 2013), and high levels of progesterone have been implicated in extinction retention deficits among women with PTSD (Pineles et al., 2016, 2018). Given the apparent link between autonomic deficits and cardiometabolic disease risk in PTSD, as well as the elevated prevalence of PTSD in women, it is necessary to better understand sex differences in autonomic functioning that may confer differential risk following trauma.
The current study used a prospective design to examine several aspects of posttraumatic autonomic functioning as part of the AURORA study (McLean et al., 2020). Men and women were recruited from emergency departments immediately following trauma exposure and their skin conductance response to a trauma interview was gathered at this time. They completed a fear conditioning paradigm two weeks later where HR, HRV, and BP were assessed. We sought to identify potential sex differences in HR, HRV, and BP at this 2-week session, as well as to determine if skin conductance in the immediate post-trauma period was associated with autonomic functioning two weeks later. Given limited sex differences research in PTSD populations, we examined the effects of both sex and 8-week PTSD status on these autonomic indices.
2. Methods
2.1. Participants and procedure
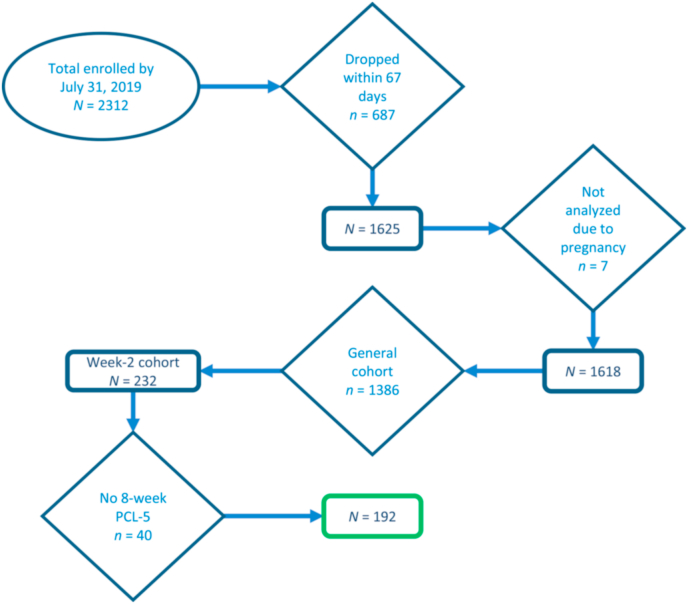
As part of the multi-site AURORA study, participants were recruited from 22 emergency departments (EDs) across the U.S. immediately following a traumatic event (see McLean et al., 2020 for additional detail). Traumatic events included motor vehicle collisions, physical assault, sexual assault, and serious accidents. Exclusion criteria were intracranial injury, long bone fracture or significant extracranial hemorrhage, pregnancy, and admission due to intentional self-injury or suicide attempt. Given the study's aim of examining acute trauma responses, participants were also excluded if they reported ongoing domestic violence. Fear conditioning was completed in a sub-sample of AURORA participants at one of four locations two weeks following the emergency department visit. All participants provided informed consent and the study was approved by each site's Institutional Review Board. See Fig. 1 for a CONSORT diagram outlining participant flow through the study.
Fig. 1.
CONSORT diagram.
2.2. Measures
PTSD Checklist for DSM-5 (PCL-5; Weathers et al., 2013). The PCL-5 is a 20-item self-report measure of PTSD symptom severity and was administered eight weeks following the ED visit. Responses are on a scale from 0 (not at all) to 4 (extremely). A total score was used to assess overall PTSD severity. A score of 33 or higher was used to indicate probable PTSD diagnosis (Bovin et al., 2016).
2.3. ED skin conductance response measurement
eSense (Mindfield Biosystems, Inc., Berlin, Germany). Using previously validated procedures (Hinrichs et al., 2017), skin conductance levels were recorded with the eSense system during the initial ED visit. eSense software was downloaded to an iPad and two Velcro electrodes, which were connected to the iPad, were attached to the middle and index finger. Participants were asked to describe the traumatic event that brought them to the ED and eSense was used to continuously measure skin conductance levels during the interview. This method has been used previously in the ED among trauma-exposed adults (Hinrichs et al., 2019).
2.4. 2-Week psychophysiological assessment
Approximately two weeks following the ED visit, participants completed a follow-up session that included a seated BP assessment and a fear-potentiated startle (FPS) paradigm, during which HR and HRV were obtained. The FPS paradigm is a Pavlovian fear conditioning paradigm that has been validated in clinical and nonclinical samples (e.g., Glover et al., 2011; Jovanovic et al., 2012; Norrholm et al., 2006; Seligowski et al., 2018). It includes a habituation phase followed by fear acquisition and extinction phases. During habituation, 12 108 dB startle probes were delivered through headphones to assess baseline startle, and eight of these were delivered upon the termination of conditioned stimuli (CS), which were colored shapes presented on a computer screen. During acquisition, one of the shapes (CS+) was paired with an aversive unconditioned stimulus (US), while the other was not (CS-). The US was a 250 ms/140 p.s.i. air blast directed at the larynx. The CS+ and CS- were each presented for 6 s and the startle probe was presented at their termination. During acquisition, the CS+ and startle probe were followed by the US 0.5 s later. Habituation included four trials of each CS (not reinforced with air blasts) and four startle probes alone (noise alone [NA]). Acquisition included three conditioning blocks with four trials of each type (NA, CS+, CS-) in each block. Extinction included four blocks of four trials of each type (NA, CS+ [unreinforced], and CS-) in each block. All trials were on a fixed schedule and the inter-trial interval was 9–22 s.
Psychophysiological data were acquired using Biopac MP150 for Windows (Biopac Systems, Inc., Aero Camino, CA). HR and HF-HRV were continuously measured with Ag/AgCl electrodes in the Lead II placement of electrodes using the electrocardiogram (ECG) module, while eyeblink startle was indexed using the electromyogram (EMG) module, which was measured with two Ag/AgCl electrodes placed below the participant's pupil.
3. Data processing and analysis
3.1. Psychophysiology data processing
Skin conductance data were acquired using eSense on the iPad at a sampling rate of 10 Hz and the data were exported using.csv files. Baseline skin conductance was measured for 2 min and followed by the participant's narrative of their traumatic event. Skin conductance measurements were obtained throughout the narrative.
HR and HF-HRV data were processed using MindWare software, which identifies R-waves and R-R intervals (i.e., the time between heart beats), and detects artifacts, which were visually inspected and corrected (MindWare, Inc.). HR and HF-HRV were derived by spectral analysis of 1-min epochs with a Hamming windowing function and log transformed. Settings for the high frequency band were based on standard recommendations for HF-HRV data (0.12–0.40 Hz; Task Force, 1996). HR and HF-HRV values were averaged over the three acquisition blocks and four extinction blocks. The EMG sampling rate was 1 kHz, which was amplified by a gain of 2000, and filtered using 28 Hz and 500 Hz low and high pass filters; visual inspection was performed using MindWare. Following processing in MindWare, psychophysiological data were exported to SPSS. Consistent with American Heart Association guidelines, hypertension was defined as systolic BP ≥ 130 or diastolic BP ≥ 80 (heart.org).
3.2. Data analysis
Eight-week PCL-5 data were available for 192 participants. Using a cutoff score of 33 (Bovin et al., 2016), a dichotomous PTSD variable was created to indicate probable versus no PTSD. Of those with 8-week PCL-5 data, 158 participants had useable BP from the 2-week session, 151 participants had useable HR and HF-HRV data from 2-week fear conditioning, and 141 had useable eSense data from the ED. Reasons for missingness included data quality (e.g., noisy ECG signals) and incomplete data acquisition (e.g., participant did not complete fear conditioning). A chi-square analysis was used to compare the prevalence of hypertension among men versus women. Six univariate analyses of variance (ANOVAs) were used to test the effects of sex and PTSD on systolic and diastolic BP, average HR during acquisition and extinction, and average HF-HRV during acquisition and extinction. These models controlled for age, race, and body mass index (BMI). Bivariate correlations were used to test associations among eSense and BP, HR, and HF-HRV. All analyses were conducted with SPSS v.24 and significance set to p < .05.
4. Results
Table 1 displays descriptive data among the total sample and by sex, and Table 2 displays bivariate correlations among the total sample. Of the 192 participants with 8-week PCL-5 data (Mage = 35.88, SD = 13.56), 78 (40.6%) met criteria for probable PTSD per the cutoff score of 33. There were no sex differences in putative PTSD diagnosis (χ2 = 0.77, p = .380) or PCL-5 score (F[1190] = 0.74, p = .389). In terms of race, 44.3% (n = 85) identified as Non-Hispanic Black, 33.3% (n = 64) identified as Non-Hispanic White, 17.7% (n = 34) identified as Hispanic, and 4.7% (n = 9) identified as Non-Hispanic “other” racial category.
Table 1.
Descriptive statistics for total sample and by sex.
| Male (n = 61) |
Female (n = 131) |
Total (N = 192) |
Missing values |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Race | ||||||||
| Non-Hispanic Black | 20 | 32.8 | 65 | 49.6 | 85 | 44.3 | – | – |
| Non-Hispanic White | 26 | 42.6 | 38 | 29.0 | 64 | 33.3 | – | – |
| Hispanic | 13 | 21.3 | 21 | 16.0 | 34 | 17.7 | – | – |
| Non-Hispanic other/not listed | 2 | 3.3 | 7 | 5.3 | 9 | 4.7 | – | – |
| Hypertension (2-weeks) | 26 | 42.6 | 21 | 16.0 | 21 | 16.0 | – | – |
| PTSD (8-weeks) | 22 | 36.1 | 56 | 42.7 | 78 | 40.6 | – | – |
| M | SD | M | SD | M | SD | n | % | |
| Age | 37.61 | 14.83 | 35.07 | 12.91 | 35.88 | 13.56 | 0 | – |
| BMI | 27.13 | 5.16 | 29.82 | 7.43 | 28.91 | 6.85 | 20 | – |
| eSense Baseline (ED) | 3.78 | 2.90 | 2.71 | 1.86 | 3.09 | 2.33 | 51 | – |
| eSense Start (ED) | 4.33 | 4.21 | 2.93 | 2.07 | 3.43 | 3.06 | 53 | – |
| eSense Max (ED) | 6.18 | 6.08 | 5.02 | 3.49 | 5.43 | 4.59 | 51 | – |
| eSense End (ED) | 4.71 | 5.22 | 3.29 | 2.14 | 3.81 | 3.62 | 69 | – |
| BP – Systolic (2-weeks) | 133.98 | 20.42 | 120.65 | 15.33 | 125.46 | 18.43 | 34 | – |
| BP – Diastolic (2-weeks) | 83.30 | 13.17 | 81.80 | 11.38 | 82.34 | 12.03 | 35 | – |
| HR – Acquisition (2-weeks) | 68.44 | 10.12 | 74.31 | 10.15 | 72.44 | 10.47 | 41 | – |
| HRV – Acquisition (2-weeks) | 6.16 | 1.38 | 6.02 | 1.33 | 6.06 | 1.34 | 41 | – |
| HR – Extinction (2-weeks) | 70.29 | 10.93 | 75.69 | 10.38 | 73.96 | 10.82 | 51 | – |
| HRV – Extinction (2-weeks) | 6.01 | 1.46 | 5.89 | 1.40 | 5.93 | 1.42 | 52 | – |
| PTSD symptoms (8-weeks) | 27.10 | 17.67 | 29.58 | 18.96 | 28.79 | 18.55 | 0 | – |
Note. BMI = body mass index; BP = blood pressure; HR = heart rate; HRV = heart rate variability.
Table 2.
Descriptives and bivariate correlations among study variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. eSense Baseline (ED) | – | ||||||||||
| 2. eSense Start (ED) | .947** | – | |||||||||
| 3. eSense Max (ED) | . 782** | .830** | – | ||||||||
| 4. eSense End (ED) | .823** | .891** | .947** | – | |||||||
| 5. BP – Systolic (2-weeks) | -.088 | -.155 | -.134 | -.135 | – | ||||||
| 6. BP – Diastolic (2-weeks) | -.048 | -.068 | -.052 | -.083 | .728** | – | |||||
| 7. HR – Acquisition (2-weeks) | .029 | .111 | .095 | .133 | -.133 | .056 | – | ||||
| 8. HRV – Acquisition (2-weeks) | .081 | .094 | .141 | .062 | -.092 | -.205 | -.539** | – | |||
| 9. HR – Extinction (2-weeks) | .039 | .093 | .099 | .137 | -.088 | .071 | .967** | -.473** | – | ||
| 10. HRV – Extinction (2-weeks) | .029 | .083 | .090 | .049 | -.192 | -.240* | -.506** | .924** | -.503** | – | |
| 11. PTSD symptoms (8-weeks) | -.030 | -.023 | -.067 | -.100 | -.021 | .041 | -.096 | -.043 | -.103 | .043 | – |
| Mean | 2.96 | 3.15 | 4.99 | 3.45 | 126.14 | 82.38 | 71.57 | 6.12 | 72.80 | 6.03 | 28.41 |
| SD | 2.14 | 2.27 | 3.43 | 2.22 | 19.52 | 12.58 | 10.69 | 1.28 | 10.95 | 1.38 | 18.50 |
| Minimum | .43 | .44 | .45 | .43 | 96.00 | 54.00 | 48.47 | 3.15 | 49.75 | 2.69 | 0 |
| Maximum | 9.02 | 9.26 | 14.90 | 10.12 | 207.00 | 120.00 | 94.96 | 10.17 | 92.81 | 10.36 | 75 |
Note. *p < .05; **p < .01; BP = blood pressure; HR = heart rate; HRV = heart rate variability.
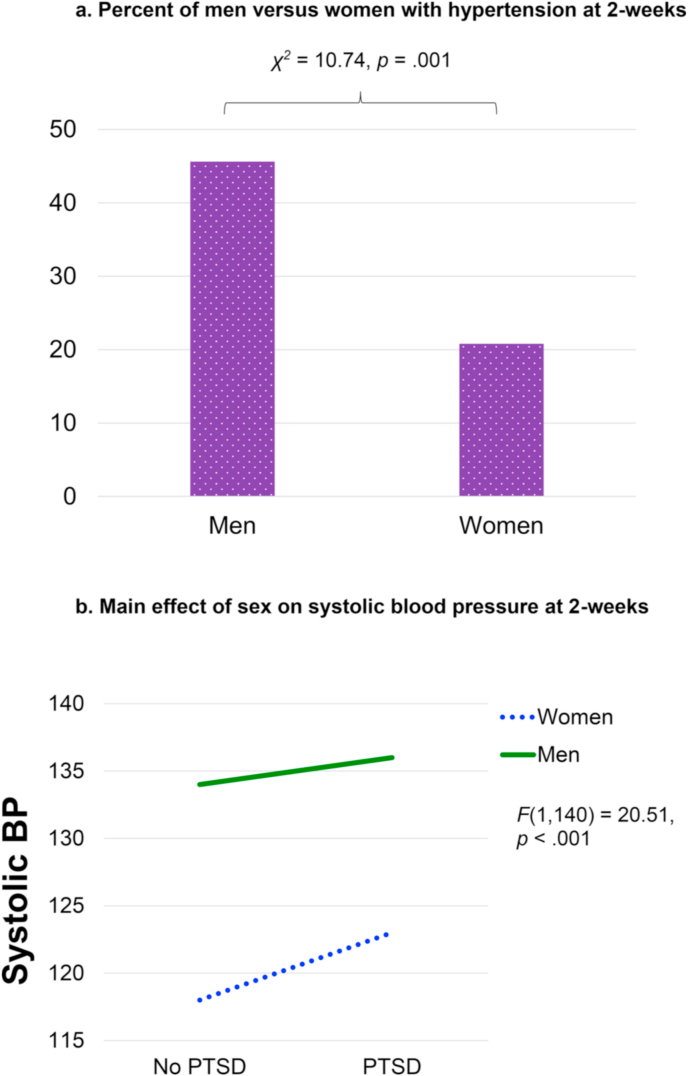
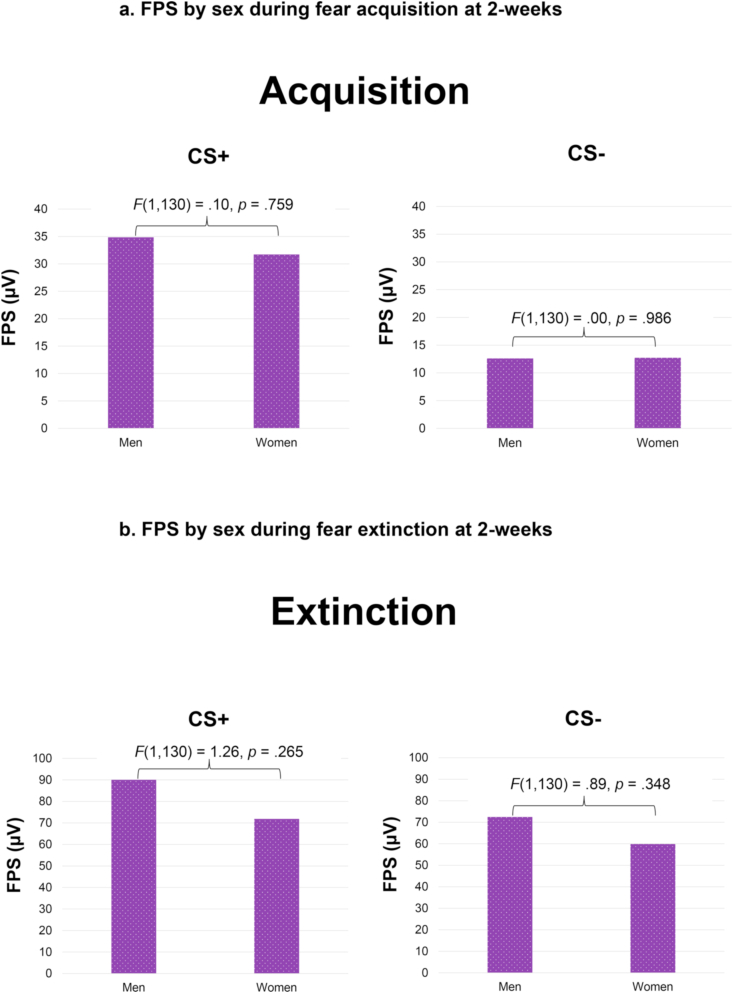
Men were significantly more likely to meet criteria for hypertension than women, χ2 = 10.74, p = .001. Results of the first univariate ANOVA indicated a significant main effect of sex on systolic BP, F(1,140) = 20.51, p < .001, such that it was higher in men compared to women (Fig. 2). The PTSD by sex interaction was not significant, F(1,140) = 0.34, p = .564, and there were no significant findings for diastolic BP. There was not a significant sex difference in baseline HF-HRV, F(1,173) = 0.41, p = .523. Further, there were no significant sex differences in eyeblink startle to the CS + or CS- during acquisition or extinction (see Fig. 3), nor any significant PTSD by sex interactions when controlling for age, race, and BMI (p's > 0.05).
Fig. 2a.
Percent of men versus women with hypertension at 2-weeks
Figure 2b. Main effect of sex on systolic blood pressure at 2-weeks.
Fig. 3a.
FPS by sex during fear acquisition at 2-weeks
Figure 3b. FPS by sex during fear extinction at 2-weeks.
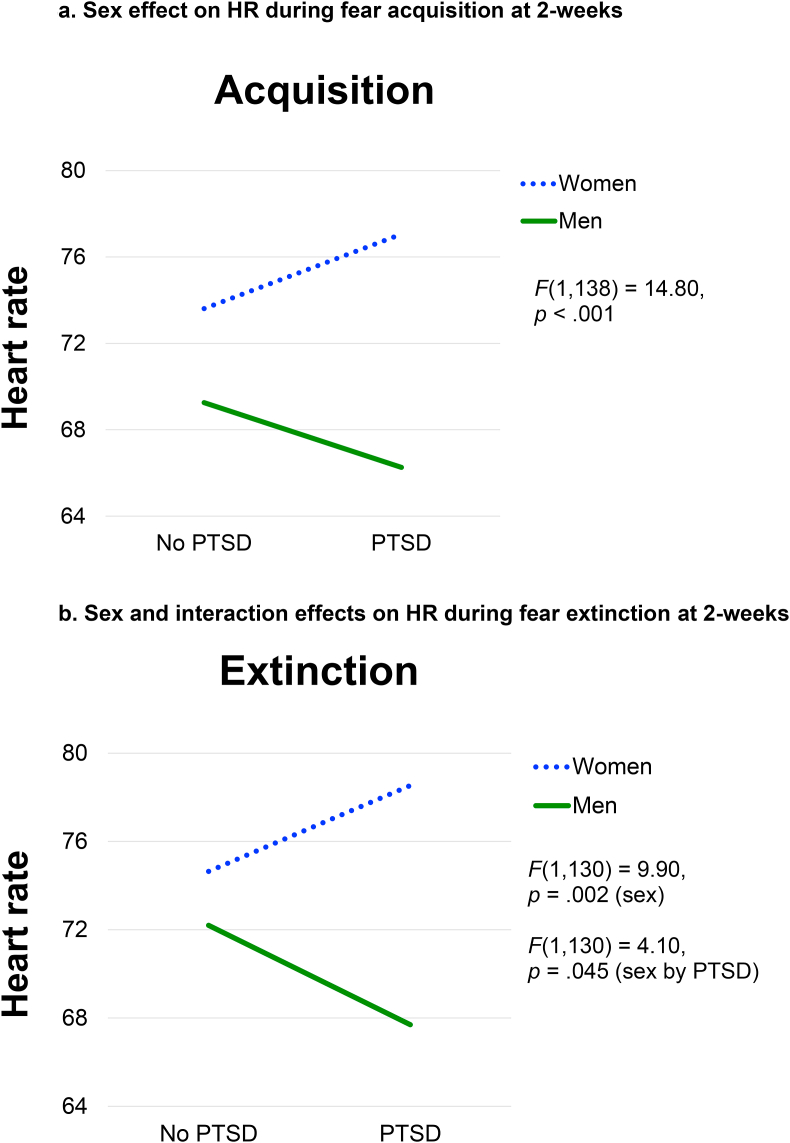
There was a significant main effect of sex on HR during fear acquisition, F(1,138) = 14.80, p < .001. The PTSD by sex interaction was not significant. During fear extinction, there was a significant main effect of sex on HR, F(1,130) = 9.90, p = .002, as well as a significant PTSD by sex interaction, F(1,130) = 4.10, p = .045. Post-hoc tests for this interaction indicated that HR was significantly different for men versus women only in the PTSD group, F(1,123) = 10.94, p = .001, whereas there was no significant effect of PTSD within either sex. As depicted in Fig. 4, women demonstrated higher HR compared to men during both fear acquisition and extinction, and this effect was stronger in the PTSD group during extinction, where HR was significantly higher in women (M = 78.53) compared to men (M = 67.70).
Fig. 4a.
Sex effect on HR during fear acquisition at 2-weeks
Figure 4b. Sex and interaction effects on HR during fear extinction at 2-weeks.
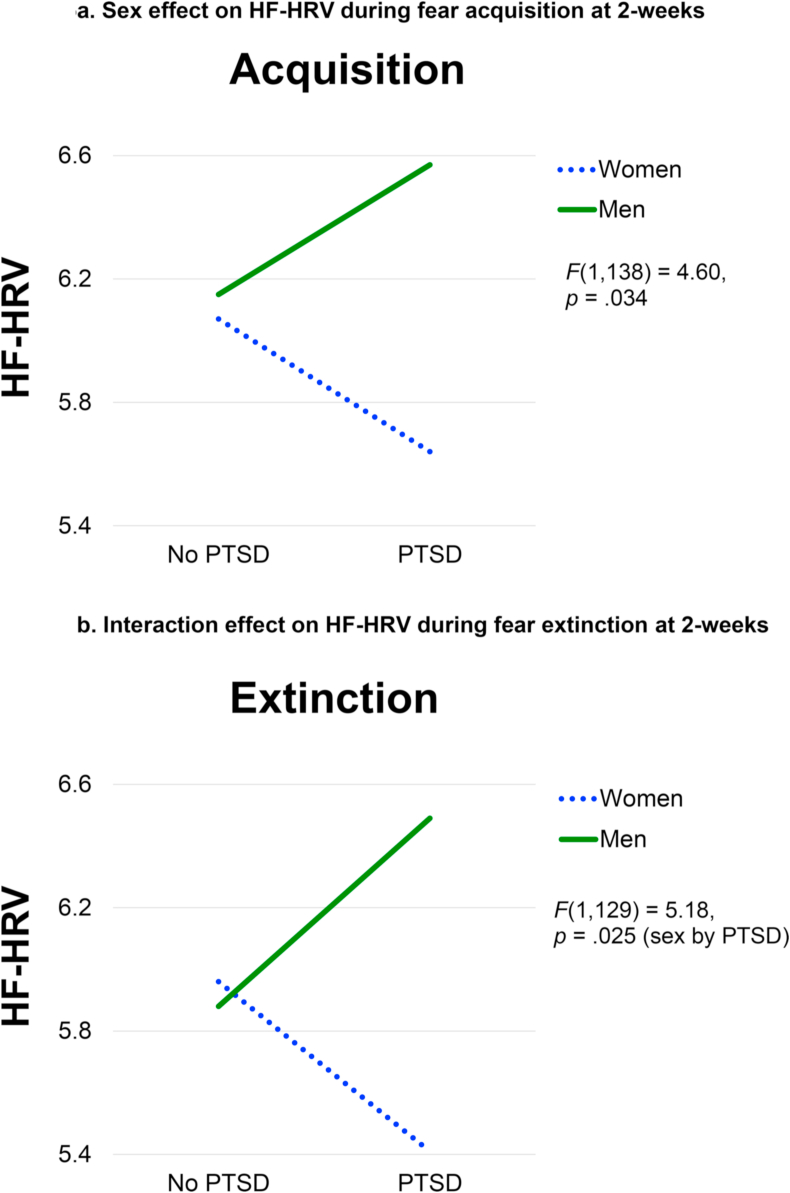
There was a significant main effect of sex on HF-HRV during fear acquisition, F(1,138) = 4.60, p = .034. The PTSD by sex interaction was not significant, F(1,138) = 3.41, p = .067. During fear extinction, there was a significant PTSD by sex interaction, F(1,129) = 5.18, p = .025. Post-hoc tests for this interaction indicated that HF-HRV was significantly different for men versus women only in the PTSD group, F(1,122) = 7.20, p = .008, whereas there was no significant effect of PTSD within either sex. As depicted in Fig. 5, women demonstrated lower HF-HRV compared to men during fear acquisition, and this effect was stronger in the PTSD group during extinction, where HF-HRV was significantly lower in women (M = 5.41) compared to men (M = 6.49).
Fig. 5a.
Sex effect on HF-HRV during fear acquisition at 2-weeks
Figure 5b. Interaction effect on HF-HRV during fear extinction at 2-weeks.
Next, we examined associations among eSense skin conductance in the ED and 2-week BP, HR, and HF-HRV. Skin conductance values were significantly and positively associated with HR and negatively associated with HF-HRV only among women who developed PTSD (p's < .05; see Table 3), indicative of increased sympathetic arousal and decreased parasympathetic control during fear conditioning. When controlling for age, race, and BMI using linear regression models, only the association between baseline eSense and extinction HF-HRV in women with PTSD remained significant (β = −0.36, p = .006). This suggests that increased sympathetic arousal in the immediate aftermath of trauma was associated with worse parasympathetic responses to fear extinction in women but not men with PTSD. No significant findings were observed in men or in women without PTSD.
Table 3.
Descriptives and bivariate correlations of study variables among women with PTSD.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. eSense Baseline (ED) | – | ||||||||||
| 2. eSense Start (ED) | .918** | – | |||||||||
| 3. eSense Max (ED) | . 698** | .825** | – | ||||||||
| 4. eSense End (ED) | .672** | .776** | .863** | – | |||||||
| 5. BP – Systolic (2-weeks) | .067 | .025 | .056 | .139 | – | ||||||
| 6. BP – Diastolic (2-weeks) | -.182 | -.169 | .021 | -.029 | .851** | – | |||||
| 7. HR – Acquisition (2-weeks) | .287 | .390* | .339 | .386 | -.237 | -.256 | – | ||||
| 8. HRV – Acquisition (2-weeks) | -.259 | -.232 | -.157 | -.368 | .268 | .327 | -.566** | – | |||
| 9. HR – Extinction (2-weeks) | .369 | .411* | .417* | .536* | -.123 | -.207 | .967** | -.460* | – | ||
| 10. HRV – Extinction (2-weeks) | -.491* | -.341 | -.301 | -.530* | -.029 | .093 | -.401* | .878** | -.339 | – | |
| 11. PTSD symptoms (8-weeks) | -.118 | -.178 | -.309 | -.235 | -.262 | -.232 | -.331 | .174 | -.387 | .321 | – |
| Mean | 2.69 | 3.03 | 5.36 | 3.27 | 122.58 | 83.08 | 73.04 | 6.16 | 74.45 | 6.23 | 46.98 |
| SD | 1.87 | 2.10 | 3.34 | 1.75 | 18.95 | 13.95 | 11.60 | 1.05 | 11.20 | 1.13 | 10.50 |
| Minimum | .43 | .50 | .62 | .43 | 96.00 | 57.00 | 53.41 | 3.32 | 54.02 | 3.30 | 33 |
| Maximum | 8.80 | 8.95 | 14.90 | 6.58 | 171.00 | 118.00 | 94.96 | 7.49 | 92.81 | 7.80 | 75 |
Note. *p < .05; **p < .01; BP = blood pressure; HR = heart rate; HRV = heart rate variability.
5. Discussion
This study used a prospective design to examine sex differences in autonomic functioning among a sample of recently traumatized men and women. Sex differences were observed and varied by biomarker. While men demonstrated significantly higher BP and rates of hypertension, women demonstrated significantly higher HR and lower HF-HRV, and these effects were strongest among women who subsequently developed PTSD. Further, acute sympathetic arousal (indexed via skin conductance response) associated with HR and HF-HRV during fear conditioning but only among women who developed PTSD.
Our findings regarding BP and hypertension are consistent with what is commonly observed in the general population, such that men are more likely than women to experience hypertension (American Heart Association, heart.org). This sex difference is known to decrease among older age groups and it is thought that decreasing estradiol levels as a result of menopause in women play a role (i.e., estradiol is cardioprotective and may explain lower rates of hypertension in pre-menopausal women; for reviews, see Colafella and Denton, 2018; Regitz-Zagrosek et al., 2016). It is important to note that the average age in our sample was 35 and thus most women were pre-menopausal. While prior studies have found that individuals with PTSD demonstrate higher BP than those without PTSD (for a review, see Buckley and Kaloupek, 2001), we did not observe an effect of PTSD status. One potential explanation is that our sample is not as highly traumatized as comparisons in prior work (e.g., most participants were in motor vehicle collisions). Similarly, BP was assessed with only one measurement and this occurred two weeks following trauma exposure. It is therefore possible that the higher levels of BP observed in prior PTSD studies were a result of more chronic PTSD symptoms and sympathetic hyperarousal, which our study did not capture.
In terms of HR and HF-HRV, our findings indicate that women experienced worse autonomic functioning during fear conditioning compared to men, and this was particularly seen in those women who subsequently developed PTSD. Specifically, trauma-exposed women demonstrated stronger sympathetic arousal and worse parasympathetic control than men during fear learning, where main effects of sex were observed, and those with PTSD demonstrated particularly worse functioning during extinction. This is consistent with prior literature implicating extinction deficits as a biomarker specific to PTSD (Jovanovic et al., 2012) and further suggests that women may be more likely than men to experience these deficits. Given that HF-HRV has been shown to be higher in healthy women compared to men (for a review, see Koenig and Thayer, 2016), our findings also highlight the importance of trauma and PTSD status in sex differences in autonomic function. The lack of sex differences in eyeblink startle (a brainstem-mediated reflex and not an autonomic indicator) suggests that there may be specificity of our sex-based findings to peripheral autonomic and cardiovascular physiology (i.e., HR and HF-HRV). Additionally, low levels of estradiol have been implicated as a contributing factor to fear inhibition and extinction deficits in women with PTSD (indexed via eyeblink startle; Glover et al., 2012, 2013) as well as healthy controls (indexed via skin conductance; Milad et al., 2010). Thus, future research is needed to determine if an interaction between PTSD status and high versus low estradiol confers greater risk for autonomic and inhibition/extinction deficits (indexed via startle) in trauma-exposed women.
eSense has previously demonstrated utility in predicting PTSD status and symptom trajectory when used to measure skin conductance in recently traumatized individuals in emergency departments (Hinrichs et al., 2019). Our findings suggest it may have additional, and perhaps more specific utility among women, such that eSense skin conductance levels were significantly associated with future HR and HF-HRV only in women who developed PTSD. Given that autonomic deficits have been implicated in the increased risk of cardiovascular disease in PTSD, future research testing eSense as a predictor of autonomic functioning and subsequent cardiac events could be extremely useful in determining which trauma-exposed individuals are at highest risk for developing cardiovascular disease. Findings from the current study indicate that this may be a particularly useful tool among women, though replication is needed.
Our findings regarding sex differences in autonomic functioning may have clinical implications. Specifically, men and women differed in their sympathetic arousal, with men demonstrating higher BP and women demonstrating higher HR. Further, women demonstrated lower parasympathetic function than men. As mentioned above, autonomic deficits have been implicated in the increased risk of cardiovascular disease in PTSD. Our findings suggest that the specific autonomic mechanisms through which cardiovascular disease develops could differ for men versus women with PTSD. For example, there is preliminary evidence that blockade of the renin-angiotensin system (responsible for BP regulation) via ace-inhibitors and angiotensin receptor blockers is associated with decreased likelihood of PTSD diagnosis (Khoury et al., 2012; Seligowski et al., 2021). We recently observed a sex effect such that the protective effects of these medications may be greater among men versus women (Seligowski et al., 2021). Thus, medications targeting BP may be more effective in men versus women with PTSD because men are more likely to experience hypertension and therefore see an effect of such medications. Prospective trials of antihypertensive medications for PTSD are needed to further explore sex differences in their effects. Another possible avenue for future trials is to determine if the autonomic deficits we observed during extinction in women with PTSD translate to clinical outcomes (e.g., do women with PTSD experience less symptom reduction from exposure treatments than men?). Thus far, sex differences in exposure-based treatments have not been reported, but we are not aware of any trials that examined sex differences in autonomic functioning during these treatments.
While capturing acute trauma reactions with a prospective design is a strength of this study, an important limitation is that our sample is not as highly symptomatic as comparisons from the literature. For example, we did not see main effects of PTSD status on BP, HR, of HF-HRV and this may be due to the recency of trauma exposure and the absence of severe PTSD symptoms in this cohort. Another limitation relates to trauma type, such that the index trauma for most participants was a motor vehicle collision and the incidence of PTSD in that population is lower than that of other trauma types, such as interpersonal violence and combat exposure (Kessler et al., 2017). Additionally, while we used a recommended cutoff for provisional PTSD diagnosis (Bovin et al., 2016) at 8-weeks, the current study relied on self-reported symptoms and did not include a structured clinical interview of PTSD. Future studies with more robust PTSD assessment among individuals with a broader range of trauma exposure will be needed to replicate and extend our findings. Despite these limitations, this study adds to a very scant literature regarding both 1) prospective assessments of posttraumatic autonomic functioning and 2) sex differences in posttraumatic autonomic functioning.
The current study identified sex differences in multiple domains of autonomic functioning among a recently traumatized sample. Our findings suggest that men and women demonstrate different patterns of sympathetic arousal, with men exhibiting higher BP and women exhibiting higher HR. Women also exhibited worse parasympathetic function as indicated by lower HF-HRV during fear conditioning, as was particularly seen in women who developed PTSD. Acute sympathetic arousal indexed by skin conductance in the emergency department was associated with HR and HF-HRV among women who developed PTSD, suggesting it may be a useful biomarker of subsequent autonomic functioning in this population. Additional studies examining subsequent sex differences in cardiovascular risk as a result of differential autonomic mechanisms are warranted.
CRediT authorship contribution statement
Antonia V. Seligowski: Data processing, Formal analysis, Data interpretation, Writing – original draft. Elizabeth R. Steuber: Data processing, Formal analysis, Data interpretation, Writing – original draft. Rebecca Hinrichs: Data processing, Formal analysis. Mariam H. Reda: Data processing, Formal analysis. Charis N. Wiltshire: Data processing, Formal analysis. Cassandra P. Wanna: Data processing, Formal analysis. Sterling J. Winters: Data processing, Formal analysis. Karlye A. Phillips: Data processing, Formal analysis. Stacey L. House: Funding acquisition, recruitment, logistics. Francesca L. Beaudoin: Funding acquisition, recruitment, logistics. Xinming An: Funding acquisition, recruitment, logistics. Jennifer S. Stevens: Funding acquisition, recruitment, logistics. Donglin Zeng: Funding acquisition, recruitment, logistics. Thomas C. Neylan: Funding acquisition, recruitment, logistics. Gari D. Clifford: Funding acquisition, recruitment, logistics. Sarah D. Linnstaedt: Funding acquisition, recruitment, logistics. Laura T. Germine: Funding acquisition, recruitment, logistics. Kenneth A. Bollen: Funding acquisition, recruitment, logistics. Guia Guffanti: Funding acquisition, recruitment, logistics. Scott L. Rauch: Funding acquisition, recruitment, logistics. John P. Haran: Funding acquisition, recruitment, logistics. Alan B. Storrow: Funding acquisition, recruitment, logistics. Christopher Lewandowski: Funding acquisition, recruitment, logistics. Paul I. Musey: Funding acquisition, recruitment, logistics. Phyllis L. Hendry: Funding acquisition, recruitment, logistics. Sophia Sheikh: Funding acquisition, recruitment, logistics. Christopher W. Jones: Funding acquisition, recruitment, logistics. Brittany E. Punches: Funding acquisition, recruitment, logistics. Michael C. Kurz: Funding acquisition, recruitment, logistics. Vishnu P. Murty: Funding acquisition, recruitment, logistics. Meghan E. McGrath: Funding acquisition, recruitment, logistics. Lauren A. Hudak: Funding acquisition, recruitment, logistics. Jose L. Pascual: Funding acquisition, recruitment, logistics. Mark J. Seamon: Funding acquisition, recruitment, logistics. Elizabeth M. Datner: Funding acquisition, recruitment, logistics. Anna M. Chang: Funding acquisition, recruitment, logistics. Claire Pearson: Funding acquisition, recruitment, logistics. David A. Peak: Funding acquisition, recruitment, logistics. Roland C. Merchant: Funding acquisition, recruitment, logistics. Robert M. Domeier: Funding acquisition, recruitment, logistics. Niels K. Rathlev: Funding acquisition, recruitment, logistics. Brian J. O'Neil: Funding acquisition, recruitment, logistics. Leon D. Sanchez: Funding acquisition, recruitment, logistics. Steven E. Bruce: Funding acquisition, recruitment, logistics. Mark W. Miller: Funding acquisition, recruitment, logistics. Robert H. Pietrzak: Funding acquisition, recruitment, logistics. Jutta Joormann: Funding acquisition, recruitment, logistics. Deanna M. Barch: Funding acquisition, recruitment, logistics. Diego A. Pizzagalli: Funding acquisition, recruitment, logistics. John F. Sheridan: Funding acquisition, recruitment, logistics. Beatriz Luna: Funding acquisition, recruitment, logistics. Steven E. Harte: Funding acquisition, recruitment, logistics. James M. Elliott: Funding acquisition, recruitment, logistics. Karestan C. Koenen: Methodology, Conceptualization. Ronald C. Kessler: Methodology, Conceptualization. Samuel A. McLean: Methodology, Conceptualization. Kerry J. Ressler: Methodology, Conceptualization. Tanja Jovanovic: Data processing, Formal analysis, Data interpretation, Writing – original draft.
Declaration of competing interest
In the last three years GDS has received research funding from the NSF, NIH and LifeBell AI, and unrestricted donations from AliveCor, Amazon Research, the Center for Discovery, the Gordon and Betty Moore Foundation, MathWorks, Microsoft Research, the Gates Foundation, Google, One Mind Foundation, and Samsung Research. GDS has financial interest in AliveCor and receives unrestricted funding from the company. He is also the CTO of MindChild Medical and CSO of LifeBell AI and has ownership in both companies. These relationships are unconnected to the current work. In the past three years, LTG has served on the Scientific Advisory Board of Sage Bionetworks, for which she received a small honorarium. SLR reports grants from NIH during the conduct of the study; personal fees from SOBP (Society of Biological Psychiatry) paid role as secretary, other from Oxford University Press royalties, other from APP (American Psychiatric Publishing Inc.) royalties, other from VA (Veterans Administration) per diem for oversight committee, and other from Community Psychiatry paid board service, including equity outside the submitted work; and Leadership roles on Board or Council for SOBP, ADAA (Anxiety and Depression Association of America), and NNDC (National Network of Depression Centers). SS has received funding from the Florida Medical Malpractice Joint Underwriter's Association Dr. Alvin E. Smith Safety of Healthcare Services Grant; Allergan Foundation; the NIH/NIA-funded Jacksonville Aging Studies Center (JAX-ASCENT; R33AG05654); and the Substance Abuse and Mental Health Services Administration (1H79TI083101-01); and the Florida Blue Foundation. CWJ reports no direct conflicts related to this paper. He has been an investigator on studies funded by Hologic Inc, Janssen, AstraZeneca, and Vapotherm, for which his department has received research funding. JJ receives consulting payments from Janssen Pharmaceuticals. Over the past three years, DAP has received consulting fees from Albright Stonebridge Group, BlackThorn Therapeutics, Boehringer Ingelheim, Compass Pathway, Concert Pharmaceuticals, Engrail Therapeutics, Neurocrine Biosciences, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals as well as one honorarium from Alkermes. In addition, he has received stock options from BlackThorn Therapeutics, and research support from National Institute of Mental Health, Dana Foundation, Brain and Behavior Research Foundation, and Millennium Pharmaceuticals. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. JME reports support from the National Institutes of Health (NIH) through Grant Numbers R01HD079076 & R03HD094577, Eunice Kennedy Shriver National Institute of Child Health & Human Development, and National Center for Medical Rehabilitation Research. He also reports funding from the New South Wales Health Spinal Cord Injury Research Grants Program and consulting fees (<$15,000 per annum) from Orofacial Therapeutics, LLC. In the past 3 years, RCK was a consultant for Datastat, Inc., Holmusk, RallyPoint Networks, Inc., and Sage Pharmaceuticals. He has stock options in Mirah, PYM, and Roga Sciences. KJR has received consulting income from Alkermes, research support from NIH, Genomind and Brainsway, and he is on scientific advisory boards for Janssen and Verily, all of which is unrelated to the present work. All other authors have no conflicts of interest to disclose.
Acknowledgements
We would like to thank the many research assistants involved in the AURORA study for their assistance with participant recruitment and data acquisition. Data and/or research tools used in the preparation of this manuscript were obtained from the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in mental health. Dataset identifier: NIMH Data Archive Digital Object Identifier 10.15154/1521155. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or of the Submitters submitting original data to NDA. Support for title page creation and format was provided by AuthorArranger, a tool developed at the National Cancer Institute.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100384.
Funding
This research was supported by NIH U01 MH110925, the US Army Medical Research and Material Command, The One Mind Foundation, and The Mayday Fund.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- American Psychiatric Association . 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [DOI] [PubMed] [Google Scholar]
- Blechert J., Michael T., Vriends N., Margraf J., Wilhelm F.H. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav. Res. Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bovin M.J., Marx B.P., Weathers F.W., Gallagher M.W., Rodriguez P., Schnurr P.P., Keane T.M. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders–fifth edition (PCL-5) in veterans. Psychol. Assess. 2016;28:1379. doi: 10.1037/pas0000254. [DOI] [PubMed] [Google Scholar]
- Buckley T.C., Kaloupek D.G. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom. Med. 2001;63:585–594. doi: 10.1097/00006842-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Cohen H., Kotler M., Matar M.A., Kaplan Z., Miodownik H., Cassuto Y. Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biol. Psychiatr. 1997;41:627–629. doi: 10.1016/s0006-3223(96)00525-2. [DOI] [PubMed] [Google Scholar]
- Colafella K.M.M., Denton K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Rev. Nephrol. 2018;14:185. doi: 10.1038/nrneph.2017.189. [DOI] [PubMed] [Google Scholar]
- Edmondson D., Kronish I.M., Shaffer J.A., Falzon L., Burg M.M. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am. Heart J. 2013;166:806–814. doi: 10.1016/j.ahj.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A., Suendermann O., Boellinghaus I., Vossbeck-Elsebusch A., Gamer M., Briddon E., Martin M.W., Glucksman E. Heart rate responses to standardized trauma-related pictures in acute posttraumatic stress disorder. Int. J. Psychophysiol. 2010;78:27–34. doi: 10.1016/j.ijpsycho.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardi R.J., Keane T.M., Cahoon B.J., Klauminzer G.W. An in vivo assessment of physiological arousal in posttraumatic stress disorder. J. Abnorm. Psychol. 1994;103:825–827. doi: 10.1037//0021-843x.103.4.825. [DOI] [PubMed] [Google Scholar]
- Glover E.M., Jovanovic T., Mercer K.B., Kerley K., Bradley B., Ressler K.J., Norrholm S.D. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol. Psychiatr. 2012;72:19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover E.M., Mercer K.B., Norrholm S.D., Davis M., Duncan E., Bradley B., Ressler K.J., Jovanovic T. Inhibition of fear is differentially associated with cycling estrogen levels in women. J. Psychiatry Neurosci. 2013;38:341. doi: 10.1503/jpn.120129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover E.M., Phifer J.E., Crain D.F., Norrholm S.D., Davis M., Bradley B., Ressler K.J., Jovanovic T. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depress. Anxiety. 2011;28:1058–1066. doi: 10.1002/da.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschildt M., Peters M.J.V., Moritz S., Jelinek L. Heart rate variability in response to affective scenes in posttraumatic stress disorder. Biol. Psychol. 2011;88:215–222. doi: 10.1016/j.biopsycho.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Hinrichs R., Michopoulos V., Winters S., Rothbaum A.O., Rothbaum B.O., Ressler K.J., Jovanovic T. Mobile assessment of heightened skin conductance in posttraumatic stress disorder. Depress. Anxiety. 2017;34:502–507. doi: 10.1002/da.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs R., van Rooij S.J., Michopoulos V., Schultebraucks K., Winters S., Maples-Keller J., Rothbaum A.O., Stevens J.S., Galatzer-Levy I., Rothbaum B.O., Ressler K.J. Increased skin conductance response in the immediate aftermath of trauma predicts PTSD risk. Chronic Stress. 2019;3 doi: 10.1177/2470547019844441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J.W., Spinazzola J., Simpson W.B., van der Kolk B.A. Preliminary evidence of parasympathetic influence on basal heart rate in posttraumatic stress disorder. J. Psychosom. Res. 2006;60:83–90. doi: 10.1016/j.jpsychores.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Inslicht S.S., Metzler T.J., Garcia N.M., Pineles S.L., Milad M.R., Orr S.P. Sex differences in fear conditioning in posttraumatic stress disorder. J. Psychiatr. Res. 2013;47:64–71. doi: 10.1016/j.jpsychires.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish L.A., Fischer B., Fallon W., Spoonster E., Sledjeski E.M., Delahanty D.L. Gender differences in PTSD symptoms: an exploration of peritraumatic mechanisms. J. Anxiety Disord. 2011;25:209–216. doi: 10.1016/j.janxdis.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T., Norrholm S.D., Sakoman A.J., Esterajher S., Kozarić-Kovacić D. Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. Int. J. Psychophysiol. 2009;71:264–268. doi: 10.1016/j.ijpsycho.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T., Kazama A., Bachevalier J., Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamkwalala A., Norrholm S.D., Poole J.M., Brown A., Donley S., Duncan E. Dark-enhanced startle responses and heart rate variability in a traumatized civilian sample: putative sex-specific correlates of posttraumatic stress disorder. Psychosom. Med. 2012;74:153–159. doi: 10.1097/PSY.0b013e318240803a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keary T.A., Hughes J.W., Palmieri P.A. Women with posttraumatic stress disorder have larger decreases in heart rate variability during stress tasks. Int. J. Psychophysiol. 2009;73:257–264. doi: 10.1016/j.ijpsycho.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatr. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Aguilar-Gaxiola S., Alonso J., Benjet C., Bromet E.J., Cardoso G., Degenhardt L., de Girolamo G., Dinolova R.V., Ferry F., Florescu S. Trauma and PTSD in the WHO world mental health surveys. Eur. J. Psychotraumatol. 2017;8:1353383. doi: 10.1080/20008198.2017.1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury N.M., Marvar P.J., Gillespie C.F., Wingo A., Schwartz A., Bradley B., Kramer M., Ressler K.J. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J. Clin. Psychiatr. 2012;73:849–855. doi: 10.4088/JCP.11m07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D.G., Resnick H.S., Milanak M.E., Miller M.W., Keyes K.M., Friedman M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM‐IV and DSM‐5 criteria. J. Trauma Stress. 2013;26:537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim B., Wilhelm F.H., Glucksman E., Ehlers A. Sex differences in heart rate responses to script-driven imagery soon after trauma and risk of posttraumatic stress disorder. Psychosom. Med. 2010;72:917–924. doi: 10.1097/PSY.0b013e3181f8894b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J., Thayer J.F. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci. Biobehav. Rev. 2016;64:288–310. doi: 10.1016/j.neubiorev.2016.03.007. [DOI] [PubMed] [Google Scholar]
- McLean S.A., Ressler K., Koenen K.C., Neylan T., Germine L., Jovanovic T., Kessler R.C. The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol. Psychiatr. 2020;25:283–296. doi: 10.1038/s41380-019-0581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V., Norrholm S.D., Jovanovic T. Diagnostic biomarkers for posttraumatic stress disorder: promising horizons from translational neuroscience research. Biol. Psychiatr. 2015;78:344–353. doi: 10.1016/j.biopsych.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Orr S.P., Lasko N.B., Chang Y., Rauch S.L., Pitman R.K. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J. Psychiatr. Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Zeidan M.A., Contero A., Pitman R.K., Klibanski A., Rauch S.L., Goldstein J.M. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A., Geyer M.A., Baker D.G., Nievergelt C.M., O'Connor D.T., Risbrough V.B. Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosom. Med. 2014;76:292–301. doi: 10.1097/PSY.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A., Maihofer A.X., Baker D.G., Nievergelt C.M., Geyer M.A., Risbrough V.B. Association of predeployment heart rate variability with risk of postdeployment posttraumatic stress disorder in active-duty marines. JAMA Psychiatry. 2015;72:979–986. doi: 10.1001/jamapsychiatry.2015.0922. [DOI] [PubMed] [Google Scholar]
- Norman S.B., Means-Christensen A.J., Craske M.G., Sherbourne C.D., Roy-Byrne P.P., Stein M.B. Associations between psychological trauma and physical illness in primary care. J. Trauma Stress. 2006;19:461–470. doi: 10.1002/jts.20129. [DOI] [PubMed] [Google Scholar]
- Norrholm S.D., Jovanovic T., Vervliet B., Myers K.M., Davis M., Rothbaum B.O., Duncan E.J. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn. Mem. 2006;13:681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacella M.L., Hruska B., Delahanty D.L. The physical health consequences of PTSD and PTSD symptoms: a meta-analytic review. J. Anxiety Disord. 2013;27:33–46. doi: 10.1016/j.janxdis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Peri T., Ben-Shakhar G., Orr S.P., Shalev A.Y. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol. Psychiatr. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Pineles S.L., Nillni Y.I., King M.W., Patton S.C., Bauer M.R., Mostoufi S.M. Extinction retention and the menstrual cycle: different associations for women with posttraumatic stress disorder. J. Abnorm. Psychol. 2016;125:349–355. doi: 10.1037/abn0000138. [DOI] [PubMed] [Google Scholar]
- Pineles S.L., Nillni Y.I., Pinna G., Irvine J., Webb A., Arditte Hall K.A. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology. 2018;93:133–141. doi: 10.1016/j.psyneuen.2018.04.024. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V., Oertelt-Prigione S., Prescott E., Franconi F., Gerdts E., Foryst-Ludwig A., Maas A.H., Kautzky-Willer A., Knappe-Wegner D. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur. Heart J. 2016;37:24–34. doi: 10.1093/eurheartj/ehv598. [DOI] [PubMed] [Google Scholar]
- Sahar T., Shalev A.Y., Porges S.W. Vagal modulation of responses to mental challenge in posttraumatic stress disorder. Biol. Psychiatr. 2001;49:637–643. doi: 10.1016/s0006-3223(00)01045-3. [DOI] [PubMed] [Google Scholar]
- Seligowski A.V., Bondy E., Singleton P., Orcutt H.K., Ressler K.J., Auerbach R.P. Testing neurophysiological markers related to fear-potentiated startle. Psychiatr. Res. 2018;267:195–200. doi: 10.1016/j.psychres.2018.06.023. [DOI] [PubMed] [Google Scholar]
- Seligowski A.V., Duffy L.A., Merker J.B., Michopoulos V., Gillespie C.F., Marvar P.J., Stein M.B., Ressler K.J. The renin–angiotensin system in PTSD: a replication and extension. Neuropsychopharmacology. 2021;46:1–6. doi: 10.1038/s41386-020-00923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligowski A.V., Harnett N.G., Merker J.B., Ressler K.J. Nervous and endocrine system dysfunction in posttraumatic stress disorder: an overview and consideration of sex as a biological variable. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:381–391. doi: 10.1016/j.bpsc.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A.Y., Peri T., Brandes D., Freedman S., Orr S.P., Pitman R.K. Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. Am. J. Psychiatr. 2000;157:255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- Shalev A.Y., Sahar T., Freedman S., Peri T., Glick N., Brandes D. A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Arch. Gen. Psychiatr. 1998;55:553–559. doi: 10.1001/archpsyc.55.6.553. [DOI] [PubMed] [Google Scholar]
- Wangelin B.C., Tuerk P.W. Taking the pulse of prolonged exposure therapy: physiological reactivity to trauma imagery as an objective measure of treatment response. Depress. Anxiety. 2015;32:927–934. doi: 10.1002/da.22449. [DOI] [PubMed] [Google Scholar]
- Weathers F., Litz B., Keane T., Palmieri T., Marx B.P., Schnurr P. The PTSD checklist for DSM-5 (PCL-5). Scale available from the national center for PTSD at. 2013. www.ptsd.va.gov
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.