Abstract
Arthroplasty implants are comprised of metal alloys designed to function within the human body. Implant-related issues and associated soft-tissue reactions have been well documented for modular revision hip and knee constructs. This case highlights findings of metallosis in the context of polyethylene wear in a failed primary total knee arthroplasty. Fretting of a polyethylene reinforcement pin within the tibial baseplate as a direct result of knee joint instability appears to be the root cause of observed periprosthetic metallosis. Enhanced design principles and improved polyethylene locking mechanisms may be useful to potentially mitigate fretting-related issues in future knee replacement designs. The authors recommend surveillance in patients with this construct especially when prosthetic instability is present.
Keywords: Osteolysis, Metallosis, Primary Total Knee Arthroplasty, Locking Mechanism
Introduction
Total knee arthroplasty (TKA) is one of the most successful interventions for the management of end-stage osteoarthritis. The survivorship of TKA is dependent on surgical technique, implant position, patient factors, and prosthesis design [1]. Modular polyethylene (PE) components enable the versatility to adjust ligamentous tension which is crucial to proper function and longevity of the prosthesis [1,2]. Several PE options are available for each implant system depending on the level of constraint necessary for a particular case.
Today arthroplasty implants are comprised of metal alloys designed to function within the human body. Implant-related issues and associated soft-tissue reactions have been well documented for modular hip replacements and, to a lesser extent, in knee arthroplasty. Most studies focus on metallic debris originating from the modular junctions of revision hip and knee components [3,4]. Some of these issues have been recognized within the primary arthroplasty literature as the result of PE dislodgement or catastrophic failure, thus permitting metal-on-metal articulation [2,5]. Metallic debris has been thought to play a role in propagating the inflammatory cascade within the periprosthetic tissues which can ultimately contribute to failure of the prosthesis [6]. Unfortunately, the interaction of these byproducts with the human immune system is still not completely understood.
We present an interesting case of osteolysis with accompanying metallosis after a primary TKA. Informed consent was obtained for this case study.
Case history
A 46-year-old Caucasian female (body mass index of 30.4 kg/m2) underwent a routine posterior stabilized left total knee replacement (PFC Sigma; Depuy Synthes, Warsaw, IN) using a conventional ultrahigh-molecular-weight fixed-bearing PE component for symptomatic tricompartmental osteoarthritis. The construct used a Stabilized Plus PE component (Depuy Synthes, Warsaw, IN) which has a titanium (Ti) alloy pin with a proximal ribbed portion that reinforces the PE post and a cylindrical distal end protruding from the undersurface of the PE that fits within the corresponding aperture within the center of the tibial baseplate. Initially, the patient had an uneventful postoperative recovery with final postoperative range of motion (0-125°) significantly improved from the preoperative level (15-90°). The prior operative report did not mention difficulties with ligamentous balancing or stability during the index procedure.
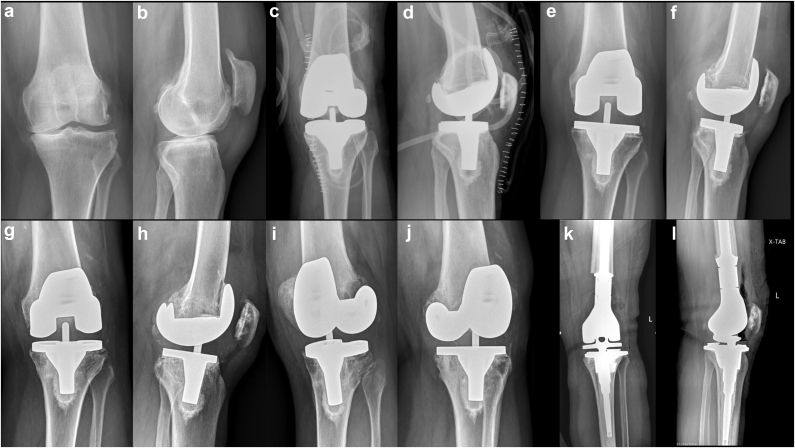
Surveillance radiographs 7 years later revealed progressive osteolysis and worsening signs of knee instability (Fig. 1). Varus collapse of the tibial baseplate and obvious loosening of the knee prosthesis prompted computed tomography (CT) evaluation which denoted large voids of osteolysis within the distal femur and proximal tibia. Thus, the decision was made to proceed with a revision TKA after infection workup was negative.
Figure 1.
Radiographs: Preoperative anteroposterior (a) and lateral (b) views. Immediate postoperative anteroposterior (c) and lateral (b) views. Five-year postoperative anteroposterior (e) and lateral (f) views. Seven-year postoperative anteroposterior (g), lateral (h), and oblique views (i and j). Note the gradual varus migration of the tibial and femoral components and progressive bone loss around the lateral femoral condyle and lateral tibial plateau. Postoperative anteroposterior (k) and lateral (l) views of the revision surgery to a distal femoral replacement.
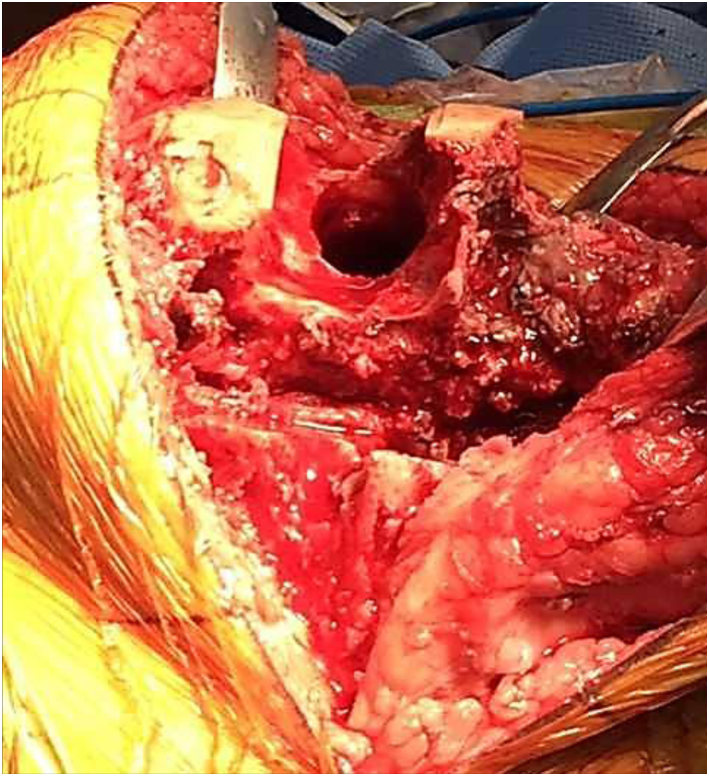
At the time of revision surgery, prominent synovitis was noted throughout the knee joint. The femoral component was found to be grossly loose and therefore removed with minimal effort. There was significant bone loss in the area of the lateral femoral condyle. This region contained unusual gray metallic fibrinous tissue prompting several samples to be sent for histopathologic analysis. In addition, there was complete loss of the lateral stabilizing soft-tissue structures of the knee including the lateral joint capsule, lateral collateral ligament, popliteus tendon, and most of the Iliotibial band (Figure 2, Figure 3). After debriding all nonviable fibrinous tissues, a large uncontained defect (50 mm × 40 mm × 20 mm) remained in the area of the lateral femoral condyle. Extensive bone loss also extended medially into the lateral portion of the intercondylar region of the distal femur. Lesser bone loss was also noted at the posterior medial femoral condyle.
Figure 2.
Intraoperative lateral view depicting gray and white fibrous material in the region of the lateral femoral condyle after removal of femoral implant.
Figure 3.
Intraoperative anterior view of the distal femur demonstrating complete loss of lateral femoral condyle after debridement of nonviable fibrous tissue.
After removing the PE component from the tibial tray, gross examination noted signs of macroscopic wear and delamination; however, there were no obvious signs of catastrophic failure. Interestingly, the PE reinforcement pin also displayed signs of wear.
After careful removal of the tibial baseplate, a contained defect (25 mm × 20 mm × 20 mm) was noted in the area of the lateral tibial plateau. Again metallic-stained fibrinous tissues were debrided from the defect and sent for analysis. The decision was made to proceed with a cemented distal femoral replacement given the extensive loss of lateral femoral condyle and supporting capsuloligamentous structures (Fig. 1). The patient had an uneventful recovery process, and she continues her rehabilitation program focusing on functional balance and endurance exercises 18 months after revision surgery.
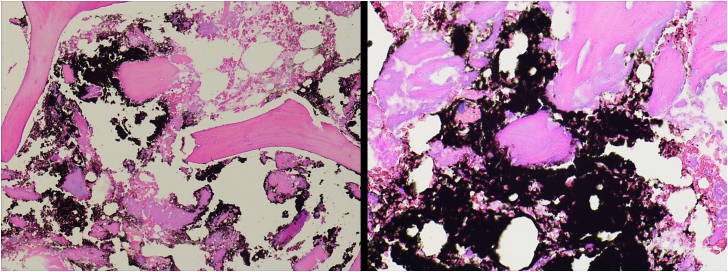
Intraoperative cultures failed to isolate bacteria. Histopathologic analysis confirmed tissue fibrosis with histiocytic proliferation with black-pigmented debris consistent with metallosis (Fig. 4). Laboratory analysis of the explanted components was performed to determine the root cause of the metallosis present in the periprosthetic tissues.
Figure 4.
Representative histology samples from the fibrous tissue collected from the lateral femoral condyle displaying benign bone fragments with areas of remodeling and fibrosis among black pigmented debris material. (left 100x; right 200x).
Retrieval analysis
First, the PE component was visually inspected using optical microscopy (Keyence VHX-6000; Osaka, Japan). The component was then imaged using micro-CT (Scanco μCT-80; Switzerland) to document the as-retrieved condition of the bearing surface through the coronal plane. The middle portion of the PE was excluded to avoid imaging artifact because of the presence of the reinforcement pin. The PE insert was then sectioned along the mid-coronal line to liberate the pin. Imaging of the pin was performed using a scanning electron microscope (Tescan VEGA3; Brno, Czech Republic) and an electron dispersive spectroscope (EDAX LLC, Mahwah, NJ) to determine the nature of damage on the surface of the pin.
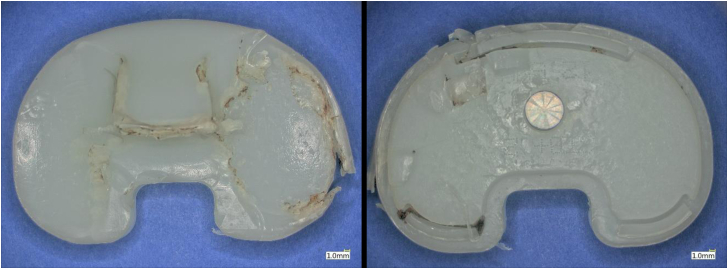
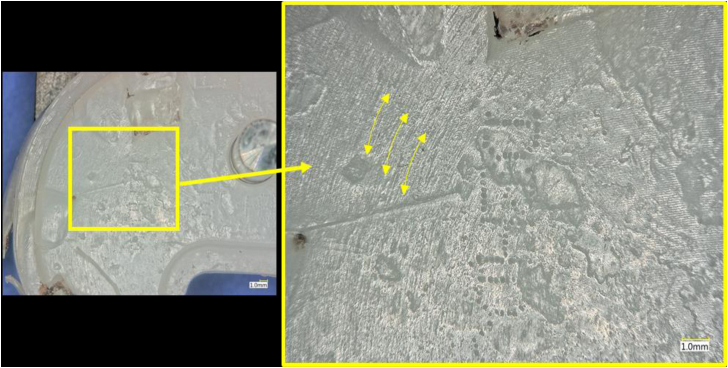
Visual assessment of the PE component revealed signs of wear, burnishing, and local delamination (Fig. 5). The damage of the bearing surface of the PE was more extensive on the medial aspect than the lateral. There was evidence of impingement between the PE component and the femoral box given the extent of damage noted along the anteromedial aspect of the PE post. Inspection of the backside of the PE component also exhibited signs of wear (Fig. 6). These wear findings are most consistent with rotational instability.
Figure 5.
Representative images of the bearing surface and backside of the retrieved tibial insert in this case.
Figure 6.
Optical micrographs of the PE component backside, showing evidence of wear that is consistent with rotational motion of the PE component against the CoCr baseplate. Yellow arrows indicate direction of rotational wear.
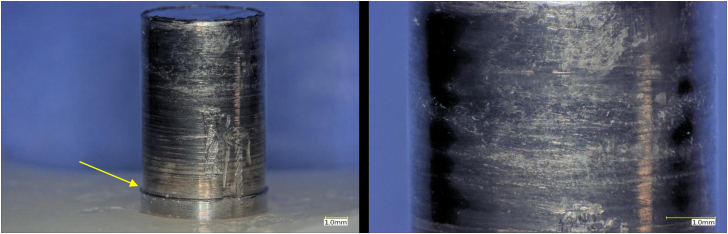
Optical microscopy of the pin contained within the PE component showed that there was extensive abrasion and material loss due to wear while assembled within the tibial component. (Fig. 7). It was unclear if some of the features and overall appearance of the pin were consistent with corrosion after visual inspection.
Figure 7.
Optical micrographs of the Ti post within the tibial insert. There is a clear region of material loss (yellow arrow).
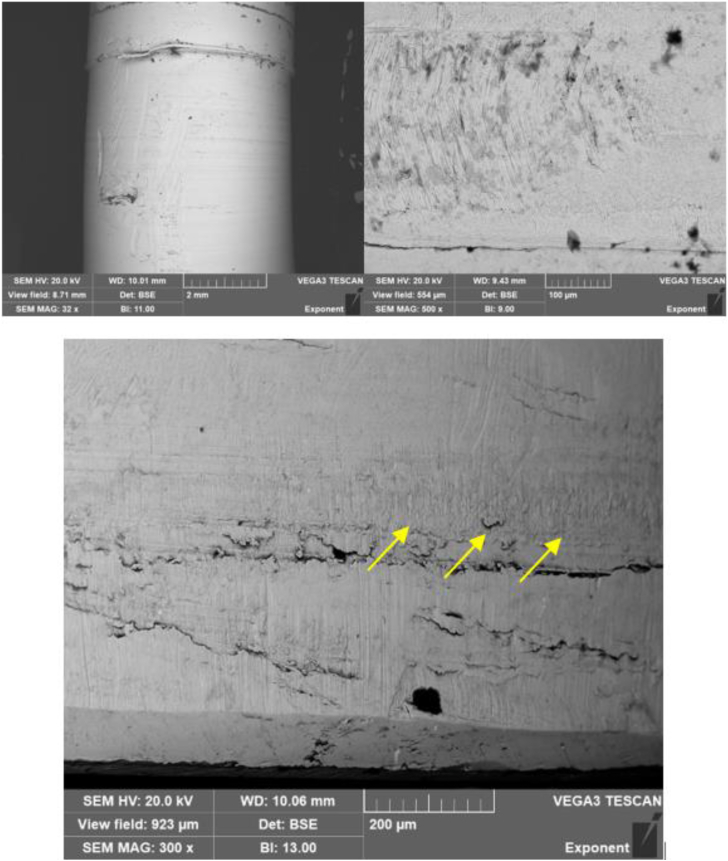
Scanning electron microscopy (SEM) and energy dispersive x-ray spectroscopy (EDS) verified if the nature of the damage to the pin was primarily mechanical (Fig. 8). The surface of the pin, within the section of material loss, had localized regions consistent with fretting. In addition, some larger scale mechanical damage was noted, which may be consistent with either insertion or removal of the PE component during the index procedure or at the time of revision surgery, respectively. Importantly, there was no apparent evidence of material transfer between the pin and the accompanying receiving feature on the tibial baseplate. There was localized evidence of mechanically assisted corrosion damage on the distal region of the pin. This, however, appeared to be outweighed by the magnitude of the material loss that could be attributed to larger scale relative motion between the pin and baseplate. The damage mechanisms observed in the backside surface of the PE indicate that there was likely both axial and torsional components to the micromotion, both from the observed fretting and marks on the reinforcement pin and from the wear patterns observed on the condylar bearing surfaces of the PE insert (Fig. 8, top right).
Figure 8.
Representative SEM images of the anterior aspect of the metallic post using backscattered imaging. Top left: There is a visible transition between the as-manufactured region and region of apparent material loss on the pin (32x). Top right, bottom: higher magnification images (500x, 300x) of the region of material loss. In the bottom image, the yellow arrows indicate a localized region of mechanically assisted corrosion damage.
EDS mapping of the pin did not reveal evidence of oxygen in the areas investigated, which would be expected if there had been any accumulation of oxide due to corrosion. Rather, the majority of the elemental composition of the interrogated areas were Ti, aluminum (Al), and vanadium (V), as would be expected from a Ti alloy consistent with the manufacturer specifications [7]. This further supports that mechanically assisted corrosion, although it may have occurred locally, was not the primary mechanism responsible for the magnitude of material loss from the pin. EDS analysis again demonstrated that there was no evidence of transfer of cobalt-chromium (CoCr) material from the tibial baseplate to the Ti pin. One area exhibited material transfer on the surface of the pin. However, a small amount of iron (2.15 weight %) and cobalt (0.9 weight %) was present. Because iron would not be expected to be present due to abrasion with the tibial baseplate, its origin was most likely a surgical tool at the time of the index procedure or revision surgery (Fig. 9).
Figure 9.
Image of one region of interest of possible material transfer and corresponding maps of elemental composition for titanium and iron. The material transferred to the surface was comprised primarily of iron (bottom right).
Discussion
We have described a peculiar case of osteolysis with accompanying metallosis in a failed primary TKA. There has only been one prior report of a reinforcement pin leading to metallosis. Bal and Greenberg reported failure of a PE post which permitted the proximal portion of the reinforcement pin to contact the cam of the femoral component resulting in metallosis and catastrophic instability of a TKA [8]. They concluded the PE post can fail even when reinforced with a metal pin citing patient, implant, and technical factors [8]. Our case examined the distal aspect of the Ti alloy reinforcement pin contained within the CoCr baseplate.
Findings at the time of the revision surgery included osteolysis and metallosis in the context of clearly observable PE wear coupled with obvious material loss of the reinforcing pin. Overall, the condition of the PE component and reinforcement pin was consistent with instability, which had resulted in wear and material loss at the junction between the pin and tibial baseplate. The findings from visual inspection, SEM, and EDS analysis do not suggest that corrosion was the predominant damage mechanism at this interface. Although there was localized evidence of mechanically assisted corrosion on the distal surface of the reinforcement pin, this appeared to be outweighed significantly by the apparent magnitude of overall material loss. Had corrosion of the pin been the primary mechanism of the observed material loss, it would be expected that inspection via SEM and EDS would have revealed evidence of Ti oxide accumulation on the surface of the pin in conjunction with pitting or other mode of corrosion. However, the morphology of the damage did not indicate that electrochemical processes had dominated the material loss behavior at the interface between the Ti pin and the CoCr baseplate. Therefore, the more plausible explanation for the metallic staining of the periprosthetic tissues is the mechanical abrasion resulting from fretting of the reinforcement pin within the baseplate as a direct result of knee joint instability from osteolysis. To our knowledge, this is the first report of metallosis resulting from fretting between the PE reinforcement pin and the tibial baseplate.
Contact between 2 dissimilar metals in an aqueous environment occurs commonly in orthopedics, but this does not always imply galvanic corrosion ensues. One case study concluded a Ti alloy interference screw in direct contact with a CoCr baseplate led to TKA failure, which they attributed to possible galvanic corrosion because an electrochemical gradient may have been present [9]. However, it is generally accepted that galvanic corrosion is not likely to occur between Ti and CoCr alloys [10]. Studies have shown galvanic corrosion is more common when pairing stainless steel with another dissimilar metal [11]. Furthermore, as we have demonstrated, fretting can occur without significant evidence of corrosion, which can result in the release of metallic debris without observable corrosion damage on the surface of interest.
The backside burnishing and wear of the PE indicates movement of the component relative to the baseplate and corroborates the observed surface damage of the reinforcing pin. PE wear can occur on both the articular surface (in contact with the femoral component) and the backside (in contact with the tibial baseplate) [2]. The wear at the articular surface of PE occurs primarily through abrasion and adhesion mechanisms during loading and articulation of the knee joint, while the undersurface of the PE wears due to micromotion between the PE and the tibial baseplate [2,12].
PE wear is contingent upon numerous factors. In this case, the use of a conventional non-highly cross-linked PE component is a material factor that may have influenced the amount of wear produced from the articular surface, all else being equal [13]. A relevant clinical factor responsible for wear includes the abnormal biomechanical loading of the articular surface as a result of varus collapse secondary to osteolysis. In this respect, the occurrence of osteolysis, which may have been, in part, a result of PE or metallic wear debris, created biomechanical conditions in the patient that produced more PE wear debris and likely exacerbated the biomechanical conditions that caused abrasion of the Ti reinforcing pin [1,14].
The observed backside wear, while lesser in apparent magnitude than that of the articular surface, exhibited signs of rotational motion which supports the hypothesis of biomechanical instability [2,12]. Backside wear occurs as a result of micromotion between the PE component and tibial baseplate and is contingent upon the design of the locking mechanism to mitigate micromotion and, to a lesser extent, the surface roughness of the tibial baseplate [12]. In this case, the relative contributions to micromotion of the abnormal biomechanics vs the locking mechanism’s inability to resist motion because of those biomechanical forces cannot be completely deconvolved. Therefore, future retrieval studies are required to ascertain the extent to which the locking mechanism in this TKA design adequately resists micromotion and, therefore, backside wear, in patients without such biomechanical complications secondary to osteolysis.
It has been well established that PE wear causes particle-induced osteolysis which is responsible for aseptic loosening of TKA implants [2]. Interestingly, the magnitude of response to PE debris now appears to be a function of genetic expression according to recent studies [6,15,16]. There is a notion that PE debris in the presence of metallic byproducts amplifies the local immune response within the periprosthetic tissues of arthroplasty implants [6,14]. Kaufman et al investigated the human macrophage response to PE, Ti alloy, CoCr, and alumina debris particles [14]. They demonstrated metallic debris, particularly Ti, aluminum, and vanadium alloy, can activate proinflammatory cytokines (interleukin 1 alpha, tumor necrosis factor alpha, interleukin 1 beta, and monocyte chemoattractant protein-1) which are known proponents of the osteolysis cascade [14]. Research is ongoing to further understand the potential link between osteolysis and metallic debris.
Our case used a Ti alloy pin mating with CoCr baseplate, but interestingly, this was not always the intended couple. According to the original patent from Hurlburt, the constrained PE component was intended to have a Ti alloy pin mate with a Ti alloy baseplate [7]. Interestingly in 2004, owing to a peak in osteolysis-related TKA failures, the manufacturer converted to a polished CoCr baseplate in an effort to reduce backside PE wear [17]. Today, there are at least 2 device manufacturers that use a CoCr pin to reinforce a constrained PE post within a CoCr baseplate. Although a CoCr couple may mitigate debris formation at this interface, the potential for ionic debris due to micromotion still exists [12].
The authors believe enhanced design principles and improved PE locking mechanisms may be useful in promoting a rigid interface between the PE component and tibial baseplate to potentially mitigate fretting related to the reinforcing pin in future TKA designs. Although monolithic tibial components and all-PE tibial components can avoid backside wear altogether, their use is not always practical. The findings of this case report may justify a larger retrieval study to further investigate this particular reinforced PE design. The authors believe limiting additional metallic interfaces will help to prevent future complications as the multifactorial nature of the body’s response to metal and ionic debris as a result of wear and corrosion is still not fully understood.
Summary
We have described a rare cause of metallosis observed in an unstable primary TKA with a specific reinforced PE articulation. Overall, the findings of significant material loss from the reinforcing pin most likely explain the metallosis observed at revision and, in the context of the analyses performed, appears to have been a result of mechanical abrasion through fretting as well as larger scale relative motion between the pin and baseplate. Surgeons should recognize this particular PE design may affect late performance of a TKA. The authors recommend surveillance in patients with this construct especially when prosthetic instability is present. Future research is needed by way of larger retrieval studies aimed at elucidating the relative contributions of joint instability, the biomechanical forces on reinforced PE components, stability of the locking mechanism, and the resultant production of PE and wear debris on the evolution of osteolysis in metal-reinforced PE components.
Conflicts of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: E. Ouellette is a paid employee of Exponent, Inc. S. Kurtz is an officer and shareholder of Exponent, Inc, which, a scientific and engineering consulting firm, received fees from companies and suppliers for paid presentations; received research support as a principal investigator from Ferring Pharmaceuticals, Smith & Nephew, Stryker, Zimmer Biomet, Depuy Synthes, Medtronic, Invibio, Stelkast, Formae, Kyocera Medical, Wright Medical Technology, Ceramtec, DJO, Celanese, Aesculap, Simplify Medical, Active Implants; and received royalties or financial and material support from Elsevier. M. Bullock is in the speakers' bureau of or gave paid presenations for Smith & Nephew; is a paid consultant for Smith & Nephew; is an unpaid consultant for Osso VR; has stock or stock options in Stryker; received educational support from Stryker, Smith & Nephew, Zimmer Biomet, and DePuy; is in the editorial board of Arthroplasty Today; and is a board member of AAHKS Patient Education Society and West Virginia Orthopaedic Society Education Committee.
Informed patient consent
The author(s) confirm that informed consent has been obtained from the involved patient(s) or if appropriate from the parent, guardian, power of attorney of the involved patient(s); and, they have given approval for this information to be published in this case report (series).
Acknowledgments
The authors would like to thank Avalon L. Bullock and Harbor R. Bullock for their proofreading contributions to this manuscript.
Appendix A. Supplementary data
Conflict of Interest Statement for Denning.
References
- 1.Kretzer J.P., Jakubowitz E., Sonntag R., Hofmann K., Heisel C., Thomsen M. Effect of joint laxity on polyethylene wear in total knee replacement. J Biomech. 2010;43:1092. doi: 10.1016/j.jbiomech.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Chakrabarty G., Vashishtha M., Leeder D. Polyethylene in knee arthroplasty: a review. J Clin Orthop Trauma. 2015;6:108. doi: 10.1016/j.jcot.2015.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spece H., Underwood R.J., Baykal D. Is there material loss at the conical junctions of modular components for total knee arthroplasty? J Arthroplasty. 2019;34:2479. doi: 10.1016/j.arth.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Arnholt C.M., MacDonald D.W., Tohfafarosh M. Mechanically assisted taper corrosion in modular TKA. J Arthroplasty. 2014;29:205. doi: 10.1016/j.arth.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salem K.H., Lindner N., Tingart M., Elmoghazy A.D. Severe metallosis-related osteolysis as a cause of failure after total knee replacement. J Clin Orthop Trauma. 2020;11:165. doi: 10.1016/j.jcot.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samelko L., Caicedo M., McAllister K., Jacobs J., Hallab N.J. Metal-induced delayed type hypersensitivity responses potentiate particle induced osteolysis in a sex and age dependent manner. PLoS One. 2021;16:e0251885. doi: 10.1371/journal.pone.0251885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurlburt R. Tibial insert reinforcement pin. (United States patent No. 5658344), United States patent and trademark office. 1995. https://patents.google.com/patent/US5658344 [accessed 12.04.20]
- 8.Bal B.S., Greenberg D. Failure of a metal-reinforced tibial post in total knee arthroplasty. J Arthroplasty. 2007;22:464. doi: 10.1016/j.arth.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Zee M.J.M., van Bemmel B.C., van Raay J.J.A.M. Massive osteolysis due to galvanic corrosion after total knee arthroplasty: a rare cause for early revision? J Surg Case Rep. 2020;2020:rjaa002. doi: 10.1093/jscr/rjaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert J., Mali S., Sivan S. Modularity and tapers in total joint replacement devices. ASTM International; West Conshohocken, Pennsylvania: 2015. Corrosion of modular tapers in total joint replacements: a critical assessment of design, materials, surface structure, mechanics, electrochemistry, and biology; p. 192. [Google Scholar]
- 11.Hansen D. Metal corrosion in the human body: the ultimate bio-corrosion scenario. Electrochem Soc. 2008;17:31. [Google Scholar]
- 12.Sisko Z.W., Teeter M.G., Lanting B.A. Current total knee designs: does baseplate roughness or locking mechanism design affect polyethylene backside wear? Clin Orthop Relat Res. 2017;475:2970. doi: 10.1007/s11999-017-5494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partridge T.C.J., Baker P.N., Jameson S.S., Mason J., Reed M.R., Deehan D.J. Conventional versus highly cross-linked polyethylene in primary total knee replacement: a comparison of revision rates using data from the national joint registry for England, Wales, and Northern Ireland. J Bone Joint Surg Am. 2020;102:119. doi: 10.2106/JBJS.19.00031. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman A.M., Alabre C.I., Rubash H.E., Shanbhag A.S. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: analysis of multiple cytokines using protein arrays. J Biomed Mater Res A. 2008;84:464. doi: 10.1002/jbm.a.31467. [DOI] [PubMed] [Google Scholar]
- 15.Beck R.T., Illingworth K.D., Saleh K.J. Review of periprosthetic osteolysis in total joint arthroplasty: an emphasis on host factors and future directions. J Orthop Res. 2012;30:541. doi: 10.1002/jor.21554. [DOI] [PubMed] [Google Scholar]
- 16.Gordon A., Greenfield E.M., Eastell R., Kiss-Toth E., Wilkinson J.M. Individual susceptibility to periprosthetic osteolysis is associated with altered patterns of innate immune gene expression in response to pro-inflammatory stimuli. J Orthop Res. 2010;28:1127. doi: 10.1002/jor.21135. [DOI] [PubMed] [Google Scholar]
- 17.Hug K., Henderson R., Hansen B., Wellman S., Vail T., Bolognesi M. Polished cobalt-chrome vs titanium tibial trays in total knee replacement (a comparison using the PFC Sigma system) Duke Orthop J. 2012;2:5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.