LIPOSOMES
ETHOSOMES
|
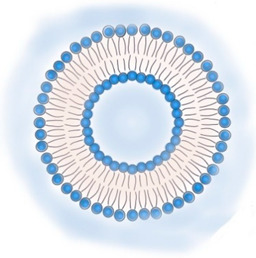
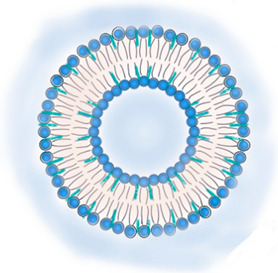
Aqueous phases in the core and surroundings of synthetic vesicles formed by self-assembly of lipid bilayers. Unilamellar (1 bilayer), oligolamellar (2–4 bilayers), and multilamellar (>4 bilayers) classifications are based on the number of lipid bilayers. Small (100 nm), large (100–500 nm), and giant (>500nm) are the classifications based on their size. Ethosomes are phospholipid-based vesicles with high ethanol content (20–45%).
|
Biocompatible and biodegradable. Administration routes are limited (mainly intranasal and intravenous). Production processes are difficult to scale. Liposomes in their natural state are quickly absorbed by the reticuloendothelial system and cleared from circulation. This property has been used to deliver ARVs to macrophages. The transdermal delivery of ARV is achieved by the incorporation of edge activators (e.g., surfactants, monoolein forming transferosomes) or ethanol (forming ethosomes) in the lipid bilayer. The protection of sensitive therapeutics can be achieved by using antioxidant agents in their composition (e.g., α-tocopherol, forming tocosome). Can encapsulate hydrophilic, hydrophobic, or amphiphilic drugs. Limited hydrophilic drug-loading capacity. Low long-term physical and biological stability, which hinders their use for long-term drug delivery.
|
[19,53,57,58] |
CUBOSOMES
|
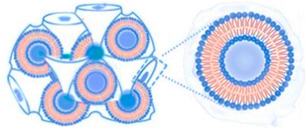
Highly stable structures organized in curved bicontinuous lipid bilayers forming soft 3D honeycomb-like structures. Composed by a continuous periodic bilayer and two non-connected water channels. Main components: glyceryl monooleate/monoolein (GMO) and phytantriol.
|
Incapability to modulate inner pore and channel sizes. Difficult loading of large molecules and difficult scale-up processes. Biocompatible and bioadhesive. Increase drug solubility and bioavailability through a variety of routes, including intranasal delivery to the brain and transdermal delivery. More stable than liposomes. High degree of encapsulation efficiency.
|
[59,60,61,62,63] |
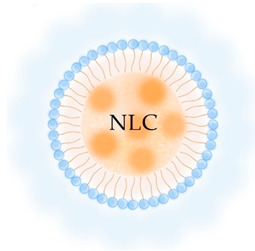
LIPID NANOPARTICLES
|
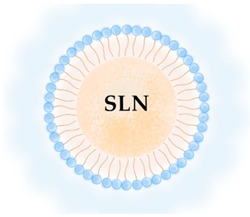
Colloidal self-assembled dispersions with a hydrophobic matrix and a surfactant layer that facilitates dispersion in water. At body and room temperatures, lipid nanoparticles are solid. Solid lipid nanoparticles (SLN) are lipid nanoparticles with hydrophobic matrices made up of solid lipids. Nanostructured lipid carriers (NLC) have lipid matrices with solid lipids and liquid lipids (oils).
|
Ease manufacturing and scale-up Low-cost and recognized as safe (GRAS) excipients, and biocompatibility. Greater drug stability and better control over drug-release kinetics than liposomes, cubosomes, and nanoemulsions. In comparison to other nanocarriers, they can entrap a greater amount of lipophilic drugs, but are inadequate for encapsulating hydrophilic and amphiphilic drugs. Good blood stability. Receptor-mediated transcytosis allows lipid nanoparticles to cross the BBB (targeting low-density lipoproteins receptors).
|
[7,64,65] |
LIPID EMULSIONS
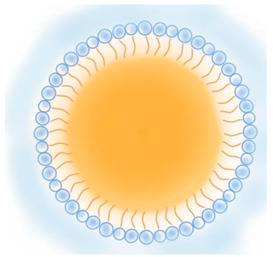
Nanoemulsion O/W
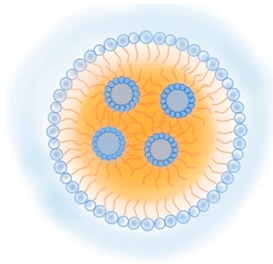
SNEDDS W/O/W
|
Colloidal systems made of immiscible liquid phases, categorized in water-in-oil (w/o) or oil-in-water (o/w), according to the phase dispersed in the other phase (continuous phase) and stabilized by surfactants. Microemulsions are thermo-dynamically stable dispersions that can be generated with low external energy. The droplet sizes of the dispersed phase are < 1000 nm, typically range between 10 and 200 nm, resulting in optically clear dispersion. Nanoemulsions are thermo-dynamically unstable and require high external energy to be produced. The dispersed phase droplets are < 500 nm typically 100 nm. Over time, nanoemulsions are more prone to instability. Self-emulsifying drug delivery systems (SEDDS) are emulsions that, when gently agitated, form fine oil-in-water droplets without the need for a dissolution process. These include self-micro emulsifying delivery systems (SMEDDS) with droplet sizes < 50 nm; self-nanoemulsifying drug delivery systems (SNEDDS) with droplet sizes of 20 to 200 nm; and solid self-nanoemulsifying oily formulations (S-SNEOF) where the drug is precipitated as a result of the evaporation of the co-solvent.
|
Increase drug oral bioavailability as their droplets preserve the drug from gastrointestinal degradation and can be dispersed quickly in blood and lymph (thereby avoiding the first-pass metabolism), but are also administrated by other routes: topical, and intravenous. Composed by GRAS lipids. However, to stabilize the droplets, high concentrations of surfactants are used, and thus their toxicity and biocompatibility may be compromised. Easy to manufacture and scale up, although the production methods can be expensive. In comparison to liposomes are more stable and provide higher encapsulation efficiency than lipophilic drugs. SNEDDS have higher physicochemical stability than classical nanoemulsions. SNEOFs promote lymphatic absorption by inhibiting first-pass metabolism and P-glycoprotein (P-gp) efflux, resulting in the complete eradication of HIV in lymphatic reservoirs.
|
[29,66] |







