Abstract
Milk intake has been associated with risk of neurodegenerative diseases in observational studies. Nevertheless, whether the association is causal remains unknown. We adopted Mendelian randomization design to evaluate the potential causal association between milk intake and common neurodegenerative diseases, including multiple sclerosis (MS), Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and Parkinson’s disease (PD). Genetic associations for neurodegenerative diseases were obtained from the International Multiple Sclerosis Genetics Consortium (n = 80,094), FinnGen consortium (n = 176,899), AD GWAS (n = 63,926), Web-Based Study of Parkinson’s Disease (n = 308,518), PDGene (n = 108,990), and ALS GWAS (n = 80,610). Lactase persistence variant rs4988235 (LCT-13910 C > T) was used as the instrumental variable for milk intake. Genetically predicted higher milk intake was associated with a decreased risk of MS and AD and with an increased risk of PD. For each additional milk intake increasing allele, the odds ratios were 0.94 (95% confidence intervals [CI]: 0.91–0.97; p = 1.51 × 10−4) for MS, 0.97 (0.94–0.99; p = 0.019) for AD and 1.09 (95%CI: 1.06–1.12, p = 9.30 × 10−9) for PD. Genetically predicted milk intake was not associated with ALS (odds ratio: 0.97, 95%CI: 0.94–1.01, p = 0.135). Our results suggest that genetically predicted milk intake is associated with a decreased risk of MS and AD but with an increased risk of PD. Further investigations are needed to clarify the underlying mechanisms.
Keywords: milk, causal effect, neurodegenerative disease
1. Introduction
Neurodegenerative diseases lead to severe social and economic burdens in aging populations [1]. The causes of neurodegenerative diseases are not yet well understood. Dietary factors play a key role in the development of neurodegenerative diseases, among which milk intake has received considerable interest. Milk is a rich source of essential nutrients and a variety of anabolic hormones, which can nourish and facilitate growth [2].
The effects of milk intake on the risk of neurodegenerative diseases have been widely investigated. Observational studies have shown that milk intake is associated with a reduced risk of multiple sclerosis (MS) [3,4] and Alzheimer’s disease (AD) [5,6], but with an increased risk of Parkinson’s disease (PD) [5,7,8,9]. The association between milk intake and amyotrophic lateral sclerosis (ALS) is largely unknown.
Since observational studies are prone to potential biases, especially reverse causality and confounding, the causal effect of milk intake on neurodegenerative diseases risk is unknown. The Mendelian randomization (MR) design adopts genetic variants as the instrumental variable for an exposure to reduce these potential biases, which could enhance the causal inference [10].
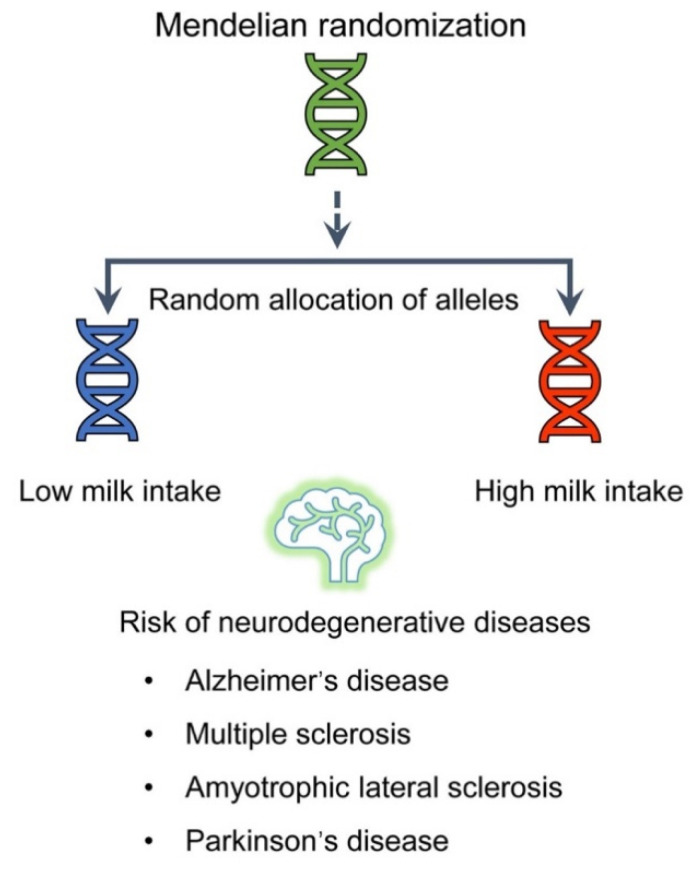
The lactase (LCT) gene encodes lactase, which metabolizes milk sugar. The genetic variant rs4988235, near the LCT gene (LCT-13910 C > T), is related to lactase persistence (LP) and increased milk intake among Europeans [11,12]. Here, we evaluated the causal association of milk intake with neurodegenerative diseases (MS, AD, ALS, and PD) by adopting the genetic variant near LCT as the instrumental variable for milk intake (Figure 1).
Figure 1.
Overview of this MR study.
2. Methods
2.1. Study Design
This study is based on the MR design, which relies on three main assumptions. First, the instrumental variable should affect the exposure. Second, the instrumental variable should not be related to confounders. Third, the instrumental variable should affect the outcome only through the exposure.
2.2. Outcome Data Sources
Summary-level data for MS were obtained from the International Multiple Sclerosis Genetics Consortium (IMSGC, MS genome-wide association study (GWAS): 14,802 cases and 26,703 controls [13]; MS Immunochip: 14,498 cases and 24,091 controls, Table 1) [14]. Genetic results for AD were obtained from the the International Genomics of Alzheimer’s Project (IGAP, 21,982 cases and 41,944 controls) [15] and FinnGen consortium R4 release (3,060 cases and 173,839 controls). Genetic association estimates for ALS were retrieved from a recent large GWAS of ALS (20,806 cases and 59,804 controls) [16]. Summary-level data for PD were obtained from the Web-Based Study of Parkinson’s Disease (PDWBS, 6,476 cases 302,042 controls) [17] and PDGene (13,708 cases, 95,282 controls) [18]. In our study, the analysis was restricted to European subjects to minimize population stratification bias. Details of case ascertainment and genotyping for these neurodegenerative diseases have been described in the original studies.
Table 1.
Studies and datasets adopted in the present analyses.
| Outcome | Consortium | Population | Cases | Controls |
|---|---|---|---|---|
| Multiple sclerosis | International Multiple Sclerosis Genetics Consortium-MS GWAS | European | 14,802 | 26,703 |
| International Multiple Sclerosis Genetics Consortium-MS Immunochip | European | 14,498 | 24,091 | |
| Alzheimer’s disease | International Genomics of Alzheimer’s Project | European | 21,982 | 41,944 |
| FinnGen consortium | European | 3,060 | 173,839 | |
| Parkinson’s disease | Web-Based Study of Parkinson’s Disease | European | 6,476 | 302,042 |
| PDGene | European | 13,708 | 95,282 | |
| Amyotrophic lateral sclerosis | Project MinE, the database of Genotypes and Phenotypes, the HYPERGENES Project, the Wellcome Trust Case Control Consortium, National Institute on Aging | European | 20,806 | 59,804 |
2.3. Genetic Instrument
We adopted rs4988235 as the genetic instrument for milk intake, which is located 13,910 bp upstream from the LCT gene (Figure 2). This genetic variant has been shown to be strongly associated with milk intake in Europeans [11,12], thereby fulfilling the first MR assumption. In the EPIC-InterAct study, the median milk intake was 162 g/day, and each additional lactase persistence allele (T) of rs4988235 was associated with 17.1 g/day (95% confidence interval [CI]: 10.6–23.6, p = 2 × 10−7) higher milk intake [12]. In a Danish cohort comprising 73,715 participants, milk intake increased by 0.58 (95% CI: 0.49–0.68; p = 9 × 10−36) glasses/week per an additional T-allele of rs4988235 [11]. The genetic variant associated with milk intake (rs4988235) explained 2% of the variance in milk intake and had an F statistic of 515 [11].
Figure 2.
Rs4988235 on chromosome 2q21 and linkage disequilibrium pattern of single-nucleotide polymorphisms based on 1000 genomes (phase_3, CEU).
2.4. Statistical Analysis
The ratio estimate for rs4988235 was calculated by dividing the beta-coefficient of outcome by the beta-coefficient of milk intake. Odds ratios (ORs) and corresponding 95% CIs were computed per additional T-allele of rs4988235. For outcomes with two independent samples, the MR estimates were combined with a fixed-effects meta-analysis. We evaluated the heterogeneity between studies with the I2 statistics. The analyses were performed using Stata 14.0.
3. Results
3.1. Basic Characteristics
Studies and datasets adopted in the present MR analyses were shown in Table 1. The outcome data sources included four neurodegenerative diseases (MS, AD, ALS, and PD). All analyses in the present MR study were based on individuals of European ancestry (Table 1).
The associations of the T allele of rs4988235 with neurodegenerative diseases are shown in Table 2. Summary-level data (regression coefficient and standard error) for rs4988235 were available in all outcome databases (Table 2).
Table 2.
Association between rs4988235 variant T allele and neurodegenerative diseases. Beta, regression coefficient; SE, standard error.
| Disease | Database | Beta | SE | p |
|---|---|---|---|---|
| Multiple sclerosis | MS GWAS | −0.054 | 0.025 | 2.93 × 10−2 |
| MS Immunochip | −0.063 | 0.020 | 1.97 × 10−3 | |
| Alzheimer’s disease | IGAP | −0.028 | 0.016 | 7.88 × 10−2 |
| FinnGen consortium | −0.048 | 0.029 | 9.76 × 10−2 | |
| Parkinson’s disease | PDWBS | 0.048 | 0.021 | 2.37 × 10−2 |
| PDGene | 0.117 | 0.020 | 7.41 × 10−9 | |
| Amyotrophic lateral sclerosis | ALS GWAS | −0.028 | 0.019 | 1.35 × 10−1 |
3.2. Genetically Predicted Milk Intake and Risk of Neurodegenerative Diseases
Genetically predicted milk intake was associated with a decreased risk of MS in both the MS GWAS (OR: 0.95, 95%CI: 0.90–0.99) and MS Immunochip (OR: 0.94, 95%CI: 0.90–0.98). In the combined analysis, the OR and 95% CI for each additional milk consumption increasing allele (T) was 0.94 (0.91–0.97; p = 1.51 × 10−4, Figure 3) without heterogeneity between studies (p = 0.788, I2 = 0%).
Figure 3.
Association of genetically predicted higher milk intake with neurodegenerative diseases. CI: confidence interval, OR: odds ratio, IGAP: International Genomics of Alzheimer’s Project, PDWBS: Web-Based Study of Parkinson’s Disease.
Genetically predicted milk intake was also inversely associated with AD risk (Figure 3). In the combined analysis of IGAP and FinnGen consortium, the OR for each additional milk intake-increasing allele (T) was 0.97 (0.94–0.99, p = 0.019, Figure 3) without heterogeneity between studies (p = 0.545, I2 = 0%). Genetically predicted milk intake was not associated with ALS (OR: 0.97; 95% CI: 0.94–1.01; p = 0.135, Figure 3).
Genetically predicted milk intake was associated with an increased risk of PD in both PDWBS (OR: 1.05, 95%CI: 1.01–1.09) and PDGene (OR: 1.12, 95%CI: 1.08–1.17). In the combined analysis of PDWBS and PDGene, the OR and 95% CI for each additional milk intake-increasing allele (T) was 1.09 (1.06–1.12; p = 9.3 × 10−9, Figure 3).
4. Discussion
In the present study, we investigated the causal relationship between genetically predicted milk intake and the risk of four common neurodegenerative diseases using the MR design. To the best of our knowledge, this is the first study using the MR method to explore the causal association between milk intake and risk of neurodegenerative diseases.
Milk is widely consumed worldwide and is a crucial modifiable risk factor for neurodegenerative diseases. In recent years, increasing attention has been paid to the relationship between milk intake and risk of neurodegenerative diseases. Milk is rich in nutrients such as protein, fat, vitamins, and calcium [19]. Our MR study showed that genetically predicted higher milk intake was associated with a decreased risk of MS and AD but with an increased risk of PD, consistent with results of observational studies [3,4,5,6].
A previous case-control study (660 patients and 421 controls) showed that milk intake was associated with decreased risk of MS [3]. Another case-control study (536 patients and 399 controls) indicated that dairy product consumption was significantly lower in MS patients (56.6%) than in control participants (67.5%, p = 0.01) [4]. For AD, results of a meta-analysis indicated that elevated milk intake was associated with a lower AD risk (OR = 0.63, 95%CI: 0.44–0.90) [6].
The protective effect of milk intake on MS and AD might be partially mediated through vitamin D and calcium. Vitamin D regulates calcium and phosphate metabolism. An association between low vitamin D levels and increased risk of MS was observed [20]. In a prospective nested case-control study (148 MS cases and 296 controls), the risk of MS was reduced by 41% for a 50-nmol/L increase in 25-hydroxyvitamin D [21]. MR studies have also indicated that circulating vitamin D levels are inversely associated with MS risk [22,23]. Furthermore, in the prospective Rotterdam study, lower serum vitamin D levels were associated with an increased risk of AD (hazard ratio: 1.13; 95% CI: 1.03–1.24) [24].
Calcium is essential for maintaining physiological processes in the cells [25]. Dysregulation of calcium homeostasis has been associated with MS and AD development [25,26]. A case-control study showed that the frequency of calcium supplementation among the control population (30.4%) was significantly higher than in MS patients (14.9%, p < 0.001), and calcium supplementation was associated with decreased risk of MS (OR = 0.44, 95%CI: 0.27–0.71) [3]. Furthermore, higher genetically predicted serum calcium has been shown to be associated with a lower risk of AD (OR = 0.57, 95% CI: 0.35–0.95) [27]. Thus, milk could have a protective effect against MS and AD through vitamin D and calcium.
Our results indicate that higher milk intake is associated with an increased risk of PD. This finding is consistent with results of observational studies [5,7,8,9]. A previous study indicated that milk could increase risk of PD by decreasing the serum uric acid level [28]. Many studies have demonstrated that urate has a protective effect against PD, and increasing plasma urate might decrease PD risk and postpone PD progression [29,30].
A limitation is that potential non-linear relationships between milk intake and risk of neurodegenerative diseases could not be evaluated in the present study due to the summary-level data used in our MR analyses. Secondly, we could not assess potential mechanisms behind the observed associations between milk intake and neurodegenerative diseases. Thirdly, whether our results could be applied to other forms of dairy products needs to be investigated in future. Finally, we cannot exclude that the used genetic instrument for milk intake affects the risk of neurodegenerative diseases through other pathways than via milk intake, thereby violating the second and third MR assumptions. For example, an increased intake of milk is likely to lead to reduced intake of other foods (to maintain energy balance) that might be associated with risk of neurodegenerative diseases. However, to date, no other food item has been established to affect the risk of the studied neurodegenerative diseases.
5. Conclusions
This MR study found that genetically predicted milk intake might be associated with a decreased risk of MS and AD but with an increased risk of PD. Further investigations are needed to clarify the potential underlying mechanisms.
Acknowledgments
We thank the IMSGC, IGAP, FinnGen consortium, ALS consortium, Web-Based Study of Parkinson’s Disease, and PDGene for providing summary statistics data for the analyses.
Author Contributions
Z.Z. and X.L. designed the research, handled funding, performed the statistical analysis, and drafted the manuscript. M.W., S.Y. and S.C.L. interpreted the data and made critical revisions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (U20A20357 and 81771285).
Institutional Review Board Statement
The MR study only uses publicly available data. Ethical approval for each of the studies included in the investigation can be found in the original publications.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to conduct the analyses in present study were obtained from public GWASs summary statistics (please see Table 1 and Table 2).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hou Y., Dan X., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L., Bohr V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 2.Willett W.C., Ludwig D.S. Milk and Health. N. Engl. J. Med. 2020;382:644–654. doi: 10.1056/NEJMra1903547. [DOI] [PubMed] [Google Scholar]
- 3.Abbasi M., Nabavi S.M., Fereshtehnejad S.M., Jou N.Z., Ansari I., Shayegannejad V., Mohammadianinejad S.E., Farhoudi M., Noorian A., Razazian N., et al. Multiple sclerosis and environmental risk factors: A case-control study in Iran. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2017;38:1941–1951. doi: 10.1007/s10072-017-3080-9. [DOI] [PubMed] [Google Scholar]
- 4.Shaygannejad V., Rezaie N., Paknahad Z., Ashtari F., Maghzi H. The environmental risk factors in multiple sclerosis susceptibility: A case-control study. Adv. Biomed. Res. 2016;5:98. doi: 10.4103/2277-9175.183665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X., Chen X., Xu Y., Yang J., Du L., Li K., Zhou Y. Milk consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses in humans. Nutr. Metab. 2021;18:7. doi: 10.1186/s12986-020-00527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu L., Sun D. Meta-Analysis of Milk Consumption and the Risk of Cognitive Disorders. Nutrients. 2016;8:824. doi: 10.3390/nu8120824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes K.C., Gao X., Kim I.Y., Wang M., Weisskopf M.G., Schwarzschild M.A., Ascherio A. Intake of dairy foods and risk of Parkinson disease. Neurology. 2017;89:46–52. doi: 10.1212/WNL.0000000000004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang W., Ju C., Jiang H., Zhang D. Dairy foods intake and risk of Parkinson’s disease: A dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2014;29:613–619. doi: 10.1007/s10654-014-9921-4. [DOI] [PubMed] [Google Scholar]
- 9.Olsson E., Byberg L., Hoijer J., Kilander L., Larsson S.C. Milk and Fermented Milk Intake and Parkinson’s Disease: Cohort Study. Nutrients. 2020;12:2763. doi: 10.3390/nu12092763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith G.D., Ebrahim S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 11.Bergholdt H.K.M., Larsen M.K., Varbo A., Nordestgaard B.G., Ellervik C. Lactase persistence, milk intake, hip fracture and bone mineral density: A study of 97 811 Danish individuals and a meta-analysis. J. Intern. Med. 2018;284:254–269. doi: 10.1111/joim.12753. [DOI] [PubMed] [Google Scholar]
- 12.Vissers L.E.T., Sluijs I., Van der Schouw Y.T., Forouhi N.G., Imamura F., Burgess S., Barricarte A., Boeing H., Bonet C., Chirlaque M.D., et al. Dairy Product Intake and Risk of Type 2 Diabetes in EPIC-InterAct: A Mendelian Randomization Study. Diabetes Care. 2019;42:568–575. doi: 10.2337/dc18-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Multiple Sclerosis Genetics Consortium Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365 doi: 10.1126/science.aav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Multiple Sclerosis Genetics Consortium. Beecham A.H., Patsopoulos N.A., Xifara D.K., Davis M.F., Kemppinen A., Cotsapas C., Shah T.S., Spencer C., Booth D., et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat. Genet. 2013;45:1353–1360. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunkle B.W., Grenier-Boley B., Sims R., Bis J.C., Damotte V., Naj A.C., Boland A., Vronskaya M., Van der Lee S.J., Amlie-Wolf A., et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat. Genet. 2019;51:414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolas A., Kenna K.P., Renton A.E., Ticozzi N., Faghri F., Chia R., Dominov J.A., Kenna B.J., Nalls M.A., Keagle P., et al. Genome-wide Analyses Identify KIF5A as a Novel ALS Gene. Neuron. 2018;97:1268–1283. doi: 10.1016/j.neuron.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang D., Nalls M.A., Hallgrimsdottir I.B., Hunkapiller J., Van der Brug M., Cai F., International Parkinson’s Disease Genomics Consortium. 23andMe Research Team. Kerchner G.A., Ayalon G., et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 2017;49:1511–1516. doi: 10.1038/ng.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nalls M.A., Pankratz N., Lill C.M., Do C.B., Hernandez D.G., Saad M., DeStefano A.L., Kara E., Bras J., Sharma M., et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weaver C.M. How sound is the science behind the dietary recommendations for dairy? Am. J. Clin. Nutr. 2014;99:1217S–1222S. doi: 10.3945/ajcn.113.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charoenngam N., Holick M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients. 2020;12:2097. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munger K.L., Levin L.I., Hollis B.W., Howard N.S., Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA J. Am. Med. Assoc. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 22.Yuan S., Xiong Y., Larsson S.C. An atlas on risk factors for multiple sclerosis: A Mendelian randomization study. J. Neurol. 2020;268:10119. doi: 10.1007/s00415-020-10119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs B.M., Noyce A.J., Giovannoni G., Dobson R. BMI and low vitamin D are causal factors for multiple sclerosis: A Mendelian Randomization study. Neurol. Neuroimmunol. Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Licher S., De Bruijn R., Wolters F.J., Zillikens M.C., Ikram M.A., Ikram M.K. Vitamin D and the Risk of Dementia: The Rotterdam Study. J. Alzheimers Dis. JAD. 2017;60:989–997. doi: 10.3233/JAD-170407. [DOI] [PubMed] [Google Scholar]
- 25.Enders M., Heider T., Ludwig A., Kuerten S. Strategies for Neuroprotection in Multiple Sclerosis and the Role of Calcium. Int. J. Mol. Sci. 2020;21:1663. doi: 10.3390/ijms21051663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaFerla F.M. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat. Rev. Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 27.He Y., Zhang H., Wang T., Han Z., Ni Q.B., Wang K., Wang L., Zhang Y., Hu Y., Jin S., et al. Impact of Serum Calcium Levels on Alzheimer’s Disease: A Mendelian Randomization Study. J. Alzheimers Dis. JAD. 2020;76:713–724. doi: 10.3233/JAD-191249. [DOI] [PubMed] [Google Scholar]
- 28.Dalbeth N., Wong S., Gamble G.D., Horne A., Mason B., Pool B., Fairbanks L., McQueen F.M., Cornish J., Reid I.R., et al. Acute effect of milk on serum urate concentrations: A randomised controlled crossover trial. Ann. Rheum. Dis. 2010;69:1677–1682. doi: 10.1136/ard.2009.124230. [DOI] [PubMed] [Google Scholar]
- 29.Gao X., O’Reilly E.J., Schwarzschild M.A., Ascherio A. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology. 2016;86:520–526. doi: 10.1212/WNL.0000000000002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisskopf M.G., O’Reilly E., Chen H., Schwarzschild M.A., Ascherio A. Plasma urate and risk of Parkinson’s disease. Am. J. Epidemiol. 2007;166:561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to conduct the analyses in present study were obtained from public GWASs summary statistics (please see Table 1 and Table 2).