Abstract
Plant sterols are compounds with multiple biological functions, mainly cholesterol-reducing. There are no comprehensive databases on plant sterols, which makes it difficult to estimate their intake in the Polish population. This work attempted to use international food databases, additionally supplemented by scientific data from the literature, to create a database of plant sterols, which would cover various kinds of foods and dishes consumed in Poland. The aim was to assess the size and sources of dietary plant sterols in the adult population of Poland. The literature search was conducted using PubMed, Web of Science, Scopus, and Google Scholar to identify possible sources of published food composition data for plant sterols. The study group consisted of 5690 participants of the WOBASZ II survey. We identified 361 dietary sources of plant sterols based on the consumption of foods and dishes reported by participants. Cereals and fats provided 61% of the total plant sterols, and together with vegetables and fruits, this totaled 80%. The median intake of plant sterols in the Polish population was 255.96 mg/day, and for men and women 291.76 and 230.61 mg/day, respectively. Canola oil provided the most plant sterols at 16.92%, followed by white bread at 16.65% and soft margarine at 8.33%. The study found that plant sterol intake in Poland is comparable to other populations, and women’s diets are more dense in plant sterols. Due to the lack of literature sources on plant sterol content in some foods, future studies should expand and complete the databases on plant sterol content in foods.
Keywords: plant sterols, database, Polish population
1. Introduction
Plant sterols are bioactive phytocompounds with a molecular structure similar to cholesterol [1]. The absorption of dietary cholesterol from diets rich in phytosterols is reduced by various mechanisms, mainly associated with the displacement of cholesterol from lipid micelles [2]. To date, more than 250 phytosterols have been identified, which include plant sterols and their saturated forms, stanols [3,4]. In various food sources, β-sitosterol is predominant and accounts for approximately 80% of the phytosterol intake in the diet [5]. Clinical evidence shows that phytosterols have a moderate LDL- and triglyceride-lowering effect [6,7]. Phytosterols are also considered moderately active antioxidants [8] and have immunomodulatory properties [9]. Sitosterol may suppress obesity-related chronic inflammation by reducing circulating interleukin-6 and TNF-α [10]. A growing body of evidence suggests that phytosterols may be an alternative and/or complementary therapy for patients with obesity and diabetes [3]. The consumption of naturally occurring plant sterols has been found to be associated with a lower risk of first myocardial infarction in men [11]. In addition, high doses of plant sterols in the diet, especially β-sitosterol, have been found to prevent the development of cancer [12,13].
Food sources with the highest plant sterol content include vegetable oils, mainly corn oil (746 mg/100 g), and sesame seeds (714 mg/100 g) [14]. A good source of phytosterols is nuts, which provide 30–220 mg/100 g of phytosterols, and cereals that contain phytosterols in the amount of 35–198 mg/100 g [15]. Vegetables contain smaller amounts of phytosterols, with 4–40 mg/100 g, and fruits contain 4–24 mg/100 g [15]. Consumption studies have shown that due to the frequency and volume of consumption, the suppliers of plant sterols are mainly bread, cereals, fats, and vegetables [3,5]. As studies show, population intakes of plant sterols are variable [5,11,16,17,18,19,20,21].
There is a need to develop databases of biologically active compounds to calculate population intakes [22]. Unlike the various databases on food composition, there are no comprehensive databases on plant sterols, which makes it difficult to estimate the intake of plant sterols in populations, as well as their further calculations in epidemiological studies. Earlier population-based studies used different databases prepared for individual studies with different methodologies [5,11,16,17,18,19,20,21]. Some studies used plant sterol databases [16,18,20], but others prepared individual databases based on experimental data [5,11,17,19,21]. There is currently no evaluation of plant sterols at the Polish population level, but an attempt has been made in a pilot study on a sample of students [23].
This work attempted to use international food databases, additionally supplemented by scientific data from the literature, to create a database of plant sterols, which would cover various kinds of foods and dishes consumed in Poland. The aim was to assess the size and sources of dietary plant sterols in the adult population of Poland.
2. Materials and Methods
2.1. Plant Sterol Database and Calculation of Dietary Intake
Since there is no plant sterol database in Poland, its establishment for the purpose of this study was based on international databases, which were published in English and are publicly available [14,24]. A literature review was conducted to search for reliable data sources that would supplement the data taken from international databases. The literature search was conducted using PubMed, Web of Science, Scopus, and Google Scholar to identify possible sources of published food composition data for plant sterols. The search terms included phytosterols, plant sterols, β-sitosterol, campesterol, and stigmasterol combined with food, cereals, vegetables, fruit, berries, nuts, seeds, legumes, beverages, coffee, tea, wine, soda, chocolate, pastry, and cookies.
The plan was to select data sources that were as complete as possible in terms of individual plant sterols (β-sitosterol, campesterol, and stigmasterol). For the total plant sterol content, the full data reported by databases or scientific sources were used or, in the absence of relevant data, the available data for plant sterol content were aggregated. The quality of the data was assessed according to the procedure described by Rand et al. [25], which takes into account the analytical method used, the number of samples, the sample handling procedures, the sampling plan for the selection of foods, and the analytical and quality assurance. The currently available techniques for sterol analysis are gas chromatography (GC), high-pressure liquid chromatography (HPLC), and supercritical fluid chromatography (SFC). GC/FID (flame-ionization detection) or GC/MS (mass spectrometry) can be considered the methods of choice for the determination of phytosterols in foods and diets [26]. For most of the studies, all of the quality criteria were met. For some food products, the number of studies was limited to only one publication; although they did not meet all quality criteria, they were included in the developed database due to lack of other publication sources. Finally, data from 13 data sources were included in the database, with 11 studies meeting the Rand criteria and 2 not meeting these criteria.
In this study, data for fats and oils were extracted from the British database of Food Composition [24], the USDA Database [14], and Normen et al. [27]. Data on plant sterols in cereals were extracted from the British database of Food Composition [24] and Normen et al. [28]. Most of the data for vegetables and potatoes were taken from Normen et al. [29]. Data gaps in the vegetables group were filled in from the publications by Han et al. [30], Piironen et al. [31], Ryan et al. [32], the British database of Food Composition [24], and the USDA Nutrient Database [14]. The plant sterol contents in fruits and berries were compiled from the USDA Database [14], Piironen et al. [31], Normen et al. [29], and Han et al. [30]. The plant sterol contents in nuts and seeds were taken from the USDA Database [14], the British database of Food Composition [24], and Normen et al. [27]. The plant sterols for legumes were compiled from Li et al. [33], Han et al. [30], the USDA Database [14], Ryan et al. [32], and Yamaya et al. [34]. Data for fruit and vegetable juices, sodas, tea, and beer were taken from Decloedt et al. [35]. Data for the plant sterols in wines were taken from Ruggiero et al. [36]. The plant sterol content in the sterolic fraction of coffee was taken from Čížková et al. [37] and recalculated per 100 g of coffee. For pastry and cookies, data were extracted from the British database of Food Composition [24], the USDA Database [14], and Piironen et al. [31]. For chocolate and chocolate candies, data were compiled from Normen et al. [27]. Data on plant sterols in foods are available in Supplementary Table S1.
For the dishes, the individual ingredients were extracted according to recipes of the National Institute of Food and Nutrition of Poland, taking into account the yield factors of the dishes. Data on plant sterols in dishes are available in Supplementary Table S2.
Finally, foods were grouped into 10 categories: cereals (flour, bread, breakfast cereals, bran, groats, and pasta), fruit (processed and non-processed), vegetables (processed and non-processed), potatoes, legumes, fats and oils (oils, margarine, and mayonnaise), coffee (instant and infusion), cookies and cakes, chocolate (chocolate and chocolate candies and bars), and other foods (tea, beer, wine, sodas, mustard, nuts, and seeds). Foods enriched with phytosterols were not included in these calculations because not all manufacturers were willing to disclose their formulations regarding individual phytosterols.
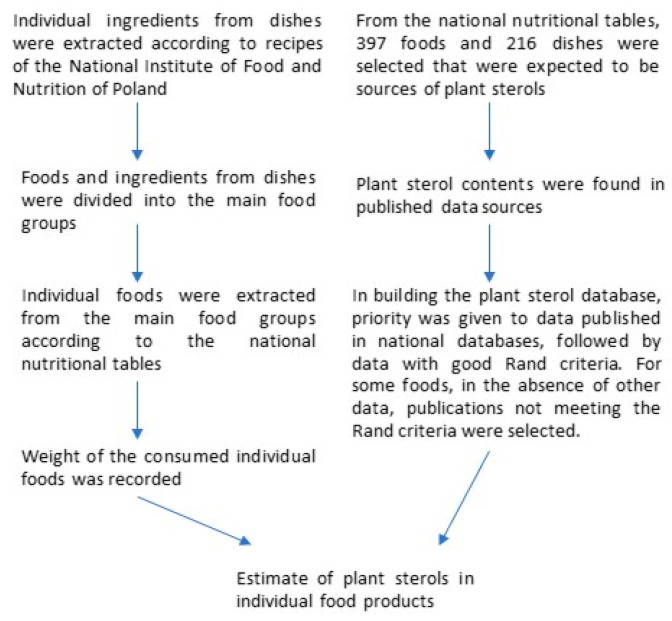
The process used to estimate plant sterols in foods is given in Figure 1.
Figure 1.
The process used to estimate plant sterol intake.
2.2. Study Group and Data Collection
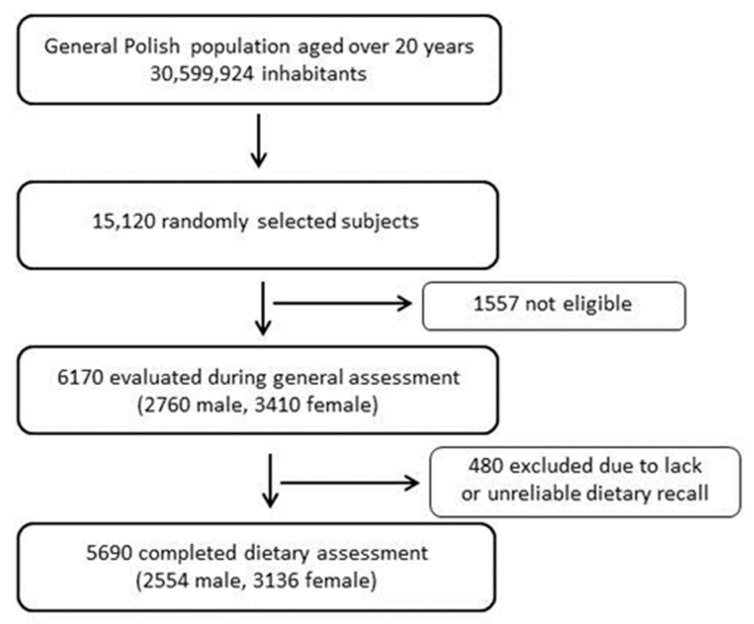
The study group consisted of 5690 participants (2554 men and 3136 women) of the National Multicenter Health Survey II (the Polish acronym is WOBASZ II). WOBASZ II is a cross-sectional study representative of the Polish adult population aged 20 years and over, which was carried out by the National Institute of Cardiology (formerly the Institute of Cardiology), Warsaw, Poland, in the years 2013–2014, in collaboration with five national medical universities. The design and methods of the WOBASZ II survey have been described in detail elsewhere [38]. Daily food consumption data were collected by trained interviewers using a single 24-h dietary recall method. The overall evaluation included a sample of 6170 participants, 480 of whom were excluded due to missing or unreliable dietary recalls. A flowchart of the participants is shown in Figure 2. The WOBASZ II study was approved by the Bioethics Committee of the National Institute of Cardiology (no. 1344), as was the current study (no. 1837). Written informed consent was obtained from all participants.
Figure 2.
Flow chart of the study participants.
Data on the demographic status, diseases, leisure-time physical activity, tobacco use, community size, marital status, and education level of the participants were collected using a standardized questionnaire developed for the WOBASZ II survey. Height and weight measurements were taken by personnel trained in standard procedures. Body mass index (BMI) was calculated from body weight in kilograms divided by the square of the height in meters. Blood pressure (BP) was measured three times on the right arm after 5 min of rest in a sitting position at 1 min intervals, and final BP was reported as the mean of the second and third measurements. The general characteristics of the study group are shown in Table 1.
Table 1.
General description of the studied population.
| Trait | Men and Women N = 5690 |
Men N = 2554 |
Women N = 3136 |
p * |
|---|---|---|---|---|
| Age (year), mean ± SD median (IQR) |
49.58 ± 16.43 50.00 (36.00–62.00) |
48.79 ± 16.27 49.00 (35.00–61.00) |
50.23 ± 16.54 51.00 (37.00–62.00) |
0.0023 |
| BMI (kg/m2), mean ± SD median (IQR) |
27.17 ± 5.19 26.63 (23.54–30.15) |
27.42 ± 4.55 27.07 (24.34–30.02) |
26.96 ± 5.65 26.12 (22.87–30.39) |
<0.0001 |
| Systolic BP (mmHg), mean ± SD median (IQR) |
130.67 ± 19.34 127.5 (117.5–141.0) |
134.44 ± 18.19 131.5 (122.0–144.5) |
127.6 ± 19.71 124.0 (113.5–138.0) |
<0.0001 |
| Diastolic BP (mmHg), mean ± SD median (IQR) |
80.23 ± 10.81 80.0 (72.5–87.0) |
81.51 ± 10.91 81.0 (74.0–88.0) |
79.19 ± 10.62 78.5 (72.0–85.5) |
<0.0001 |
| Fasting glucose (mmol/L), mean ± SD median (IQR) |
5.50 ± 1.46 5.21 (4.84–5.72) |
5.65 ± 1.6 5.35 (4.96–5.84) |
5.38 ± 1.32 5.12 (4.77–5.58) |
<0.0001 |
| Total cholesterol (mmol/L), mean ± SD median (IQR) |
5.20 ± 1.27 5.14 (4.38–5.93) |
5.21 ± 1.33 5.15 (4.36–5.97) |
5.19 ± 1.22 5.14 (4.41–5.90) |
0.7223 |
| LDL-cholesterol (mmol/L), mean ± SD median (IQR) |
3.15 ± 1.03 3.07 (2.42–3.78) |
3.19 ± 1.04 3.15 (2.46–3.86) |
3.11 ± 1.02 3.01 (2.39–3.72) |
0.0002 |
| Diseases (%) | ||||
| Hypertension 1 | 45.22 | 49.56 | 41.69 | <0.0001 |
| Hypercholesterolemia 2 | 67.30 | 68.86 | 66.03 | 0.0262 |
| Diabetes 3 | 10.82 | 11.86 | 9.96 | 0.0249 |
| Age groups (%) | ||||
| 20–40 years | 33.46 | 34.92 | 32.27 | 0.0045 |
| 41–60 years | 38.60 | 38.32 | 38.83 | |
| 61–74 years | 20.42 | 20.52 | 20.34 | |
| >74 years | 7.52 | 6.24 | 8.56 | |
| Commune size (%) | ||||
| <8.000 inhabitants | 35.20 | 33.83 | 36.32 | 0.0849 |
| 8.000–40.000 inhabitants | 30.67 | 30.70 | 30.64 | |
| >40.000 inhabitants | 34.13 | 35.47 | 33.04 | |
| Marital status (%) | ||||
| married | 66.71 | 70.19 | 63.87 | <0.0001 |
| single 4 | 33.29 | 29.81 | 36.13 | |
| Level of education 5 (%) | ||||
| under middle | 17.12 | 14.74 | 19.06 | <0.0001 |
| middle | 38.89 | 36.89 | 40.52 | |
| academic | 19.85 | 17.09 | 22.09 | |
| vocational | 24.14 | 31.28 | 18.33 | |
| Smoking status (%) | ||||
| current smokers | 23.28 | 28.95 | 18.66 | <0.0001 |
| past smokers | 25.46 | 33.62 | 18.82 | |
| never smokers | 51.26 | 37.43 | 62.52 | |
| Leisure-time physical activity 6 (%) | ||||
| low level | 54.25 | 54.98 | 53.67 | 0.2856 |
| middle level | 15.29 | 14.81 | 15.68 | |
| high level | 28.08 | 27.50 | 28.54 | |
| seasonally | 2.38 | 2.71 | 2.11 | |
| BMI (kg/m2) (%) | ||||
| underweight (BMI < 18.5) | 1.61 | 0.90 | 2.20 | <0.0001 |
| normal (BMI 18.5–24.99) | 34.91 | 30.07 | 38.88 | |
| overweight (BMI 25–29.99) | 37.25 | 43.93 | 31.76 | |
| obesity (BMI ≥ 30) | 26.23 | 25.10 | 27.16 | |
| Use of phytosterol-enriched margarines (%) | 1.90 | 1.96 | 1.85 | 0.7660 |
* p calculated for differences between men and women. 1 Hypertension: systolic blood pressure SBP ≥140 mmHg or diastolic blood pressure DBP ≥90 mmHg, or use of antihypertensive drugs. 2 Hypercholesterolemia: TC ≥5 mmol/L or LDL-C ≥3 mmol/L or the participant was taking lipid-lowering medication. 3 Diabetes: blood glucose level was ≥7.0 mmol/L or diabetes was declared in an interview. 4 Singles: widows/widowers, unmarried, divorced, in separation. 5 Education level: under middle—no education, partial or completed education for primary level, partial secondary education; middle—secondary education, partial academic education; academic—tertiary education; vocational—vocational based on primary or on middle school. 6 Physical activity at leisure (for example, jogging, cycling, swimming, gardening for at least 30 min a day): low level—no such physical activity, once a week or less; middle level—every second or third day; high level—everyday, almost every day; seasonally (e.g., skiing in winter or on the plot in summer).
The present study identified 361 dietary sources of plant sterols based on the consumption of foods and dishes reported by participants in the WOBASZ II survey. A small proportion of subjects who consumed phytosterol-enriched products was found (Table 1). Plant sterol daily intake was determined by multiplying the daily consumption of individual food items by the respective total plant sterols, such as the β-sitosterol, campesterol, and stigmasterol contents, in these food items and then summed up.
2.3. Data Analysis
Total phytosterol intake, including β-sitosterol, campesterol, and stigmasterol, was calculated by multiplying the daily consumption of individual food items by the respective phytosterol contents in these products. Additionally, the contribution of individual groups of food products and their ingredients to the consumption of different phytosterols was studied. Descriptive statistics were applied to describe the continuous variables (means and standard deviations, as well as median and interquartile range), and the percentages of the respective values were used for categorized variables. The contributions of food categories and individual food items to the intake of particular total and individual phytosterols are presented as percentages. To investigate the differences between men and women, a non-parametric Wilcoxon test or Chi-square test was used, respectively, for quantitative and qualitative variables. The level of significance was considered p < 0.05. Data analyses were processed using Statistical Analysis System (SAS; version 9.4, SAS Institute Inc., Cary, NC, USA).
3. Results
This study identified the top 10 food categories that provided plant sterols for the Polish population, which were cereals, vegetable fats and oils, vegetables, fruits, coffee, cookies and cakes, chocolate products, potatoes, and legumes. The other food products providing lower amounts of plant sterols were classified into the category of “other food products”. Among all of these categories, cereals and fats provided 61% of the total plant sterols, and together with vegetables and fruits, this totaled 80%. Median total plant sterol intake in this study was 255.96 mg/day, and divided by men and women was 291.76 and 230.61 mg/day, respectively (Table 2). Considering individual foods (mg/day), canola oil provided the most plant sterols at 16.92%, followed by white bread at 16.65% and soft margarine at 8.33%. Among vegetables and fruits, there was no single significant source of plant sterols, but raw fruits and vegetables provided the predominant amounts of plant sterols (9.78% and 7.27%, respectively). This pattern of plant sterol sources was reflected in men, while among women, the main contributor was canola oil, followed by white bread, raw fruits, raw vegetables and soft margarine. Gender differences were found for most sources of plant sterol intake.
Table 2.
Contributions of food categories and individual food products to total plant sterol intake (PS), listed according to diminishing order of contribution.
| Food Categories | All N = 5690 |
Men N = 2554 |
Women N = 3136 |
p * | |
|---|---|---|---|---|---|
| Cereals | mg/day (mean ± SD), | 90.65 ± 56.38 | 112.51 ± 63.28 | 72.85 ± 42.42 | <0.0001 |
| median (IQR) | 79.15 (53.94–114.87) | 102.44 (69.27–143.02) | 66.77 (45.90–91.39) | ||
| Contribution to PS (%) | 32.04 | 35.08 | 28.88 | <0.0001 | |
| Major sources (% contribution) ** |
wheat bread (16.65), rolls (6.64), rye bread (5.38) | wheat bread (20.59), rolls (6.85), rye bread (4.82) | wheat bread (12.56), rolls (6.43), rye bread (5.96) | - | |
| Fats | mg/day (mean ± SD), | 81.94 ± 92.30 | 98.34 ± 107.10 | 68.58 ± 75.63 | <0.0001 |
| median (IQR) | 51.65 (19.05–114.67) | 64.75 (24.30–138.68) | 44.47 (15.76–97.22) | ||
| Contribution to PS (%) | 28.95 | 30.66 | 27.20 | 0.0042 | |
| Major sources (% contribution) ** |
oils (19.11) including: canola oil (16.92), sunflower oil (2.06), olive oil (0.04), soft margarines (8.33), mayonnaise (1.05) |
oils (20.02) including: canola oil (18.03), sunflower oil (1.88), olive oil (0.06), soft margarines (9.08), mayonnaise (1.05) |
oils (18.17) including: canola oil (15.77), sunflower oil (2.25), olive oil (0.03), soft margarines (7.56), mayonnaise (1.05) |
- | |
| Fruits | mg/day (mean ± SD), | 27.76 ± 31.23 | 25.69 ± 31.46 | 29.44 ± 30.95 | <0.0001 |
| median (IQR) | 20.19 (0–40.38) | 17.50 (0–39.37) | 21.62 (3.62–42.39) | ||
| Contribution to PS (%) | 9.81 | 8.01 | 11.67 | <0.0001 | |
| Major sources (% contribution) ** |
raw fruits (9.78) including: apples (4.47), bananas (1.04), grapes (0.78), pears (0.52), plums (0.48), strawberries (0.37) |
raw fruits (7.98) including:
apples (4.10), bananas (0.87), grapes (0.57), pears (0.44), plums (0.40), strawberries (0.27) |
raw fruits (11.65) including: apples (5.42), bananas (1.23), grapes (1.00), pears (0.60), plums (0.55), strawberries (0.48) |
- | |
| Vegetables | mg/day (mean ± SD), | 25.37 ± 24.22 | 26.04 ± 24.16 | 24.83± 24.26 | 0.0028 |
| median (IQR) | 20.05 (10.12–33.45) | 21.10 (10.53–34.73) | 19.22 (9.92–32.34) | ||
| Contribution to PS (%) | 8.97 | 8.12 | 9.85 | 0.0224 | |
| Major sources (% contribution) ** |
raw vegetables (7.27), including:
tomatoes (1.11), carrots (0.90), cabbage (0.84), cauliflowers (0.77), peppers (0.45), beetroot (0.49), lettuce (0.47), cucumbers (0.42), vegetable preserves (1.32) |
raw vegetables (6.45) including:
tomatoes (1.02), carrots (0.77), cabbage (0.77), cauliflowers (0.64), beetroot (0.50), peppers (0.39), lettuce (0.38), cucumbers (0.38), vegetable preserves (1.37) |
raw vegetables (8.11) including:
tomatoes (1.21), carrots (1.03), cabbage (0.91), cauliflowers (0.91), peppers (0.52), beetroot (0.49), lettuce (0.47), cucumbers (0.42), vegetable preserves (1.26) |
- | |
| Coffee | mg/day (mean ± SD), | 19.24 ± 20.39 | 17.56 ± 20.84 | 20.61 ± 19.91 | <0.0001 |
| median (IQR) | 21.47 (0–26.84) | 21.47 (0–26.84) | 21.47 (0–26.84) | ||
| Contribution to PS (%) | 6.80 | 5.48 | 8.17 | <0.0001 | |
| Cookies, | mg/day (mean ± SD), | 11.57± 23.84 | 11.36 ± 24.40 | 11.72 ± 23.40 | 0.0055 |
| cakes | median (IQR) | 0 (0–16.50) | 0 (0–15.00) | 0 (0–17.60) | |
| Contribution to PS (%) | 4.08 | 3.54 | 4.65 | 0.0332 | |
| Chocolate | mg/day (mean ± SD), | 6.46 ± 22.86 | 6.78 ± 7.20 | 6.19 ± 21.39 | 0.0477 |
| products | median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | |
| Contribution to PS (%) | 2.28 | 2.11 | 2.46 | 0.3936 | |
| Potatoes | mg/day (mean ± SD), | 6.12 ± 6.34 | 7.30 ± 7.20 | 5.16 ± 5.35 | <0.0001 |
| median (IQR) | 6.05 (0–11.01) | 6.05 (0–12.10) | 4.15 (0–8.07) | ||
| Contribution to PS (%) | 2.16 | 2.27 | 2.05 | 0.5511 | |
| Legumes | mg/day (mean ± SD), | 3.84 ± 18.56 | 4.70 ± 21.97 | 3.13 ± 15.19 | 0.9949 |
| median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | ||
| Contribution to PS (%) | 1.36 | 1.47 | 1.24 | 0.4277 | |
| Other food products | mg/day (mean ± SD), | 10.02 | 10.49 | 9.68 | - |
| Contribution to PS (%) | 3.55 | 3.26 | 3.83 | 0.2434 | |
| Total plant sterol intake | mg/day (mean ± SD), median (IQR) |
282.97 ± 144.50 255.96 (184.98–347.98) |
320.77 ± 160.93 291.76 (209.96–399.07) |
252.19 ± 121.20 230.61 (167.73–308.2) |
<0.0001 |
| Contribution to PS (%) | 100 | 100 | 100 | - | |
* p calculated for differences between men and women. ** In the total and each food category, only individual food products with the strongest impact on the total plant sterol intakes were listed.
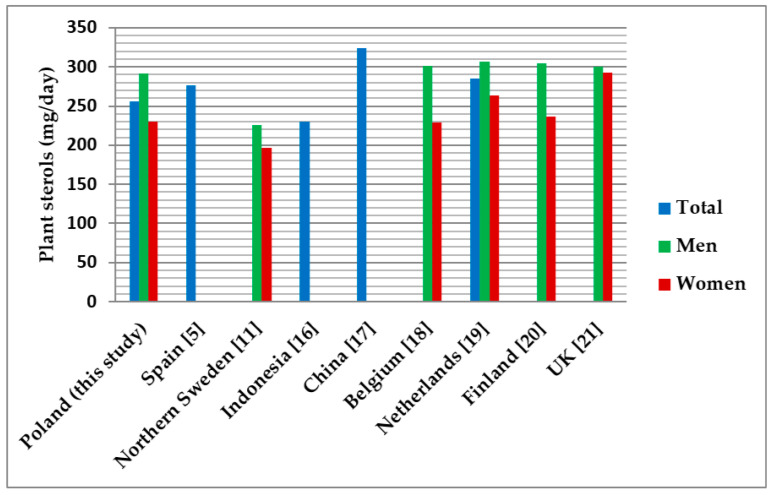
Figure 3 shows the intake of plant sterols in the Polish population (total, men, women) compared to other populations. With a plant sterol intake of 255.96 mg/day, the data for Poles are within the range for other populations.
Figure 3.
Intake of plant sterols in the Polish population and in other countries.
Table 3, Table 4 and Table 5 show the contribution of food categories to the consumption of individual plant sterols such as β-sitosterol, campesterol, and stigmasterol. The median β-sitosterol consumption was 160.85 mg/day, while the intake of campesterol and stigmasterol was 47.45 mg/day and 22.10 mg/day, respectively.
Table 3.
Contributions of food categories and individual food products to β-sitosterol intake (β-SIT), listed according to diminishing order of contribution.
| Food Categories | All N = 5690 |
Men N = 2554 |
Women N = 3136 |
p * | |
|---|---|---|---|---|---|
| Cereals | mg/day (mean ± SD), | 51.37 ± 31.69 | 63.57 ± 35.62 | 41.44 ± 23.84 | <0.0001 |
| median (IQR) | 44.98 (30.27–64.94) | 58.45 (39.20–81.10) | 37.87 (26.28–51.81) | ||
| Contribution to β-SIT (%) | 29.19 | 32.13 | 26.20 | <0.0001 | |
| Major sources (% contribution) ** |
wheat bread (14.88), rolls (6.04), rye bread (4.75) |
wheat bread (18.56), rolls (6.29), rye bread (4.28) |
wheat bread (11.14), rolls (5.79), rye bread (5.22) |
- | |
| Fats | mg/day (mean ± SD), | 50.78 ± 54.93 | 60.81 ± 63.51 | 42.61 ± 45.17 | <0.0001 |
| median (IQR) | 33.86 (12.70–71.60) | 42.15 (16.50–88.00) | 28.40 (11.00–61.14) | ||
| Contribution to β-SIT (%) | 28.86 | 30.73 | 26.95 | 0.0017 | |
| Major sources (% contribution) ** |
oils (18.43) including:
canola oil
(15.88),
sunflower oil (2.40),
olive
oil (0.07), soybean oil (0.07),
soft margarines (9.02), mayonnaise (0.87) |
oils (19.40) including:
canola oil
(17.06),
sunflower oil (2.20),
olive
oil (0.09), soybean oil (0.05),
soft margarines (9.87), mayonnaise (0.88) |
oils (17.44) including:
canola oil
(14.68),
sunflower oil (2.60),
olive
oil (0.05), soybean oil (0.09),
soft margarines (8.16), mayonnaise (0.86) |
- | |
| Fruits | mg/day (mean ± SD), | 25.00 ± 27.70 | 23.21 ± 27.96 | 26.45 ± 27.41 | <0.0001 |
| median (IQR) | 19.50 (0–38.13) | 15.90 (0–36.36) | 19.50 (3.29–39.00) | ||
| Contribution to β-SIT (%) | 14.20 | 11.73 | 16.73 | <0.0001 | |
| Major sources (% contribution) ** |
raw fruits (14.17) including: apples (7.37), bananas (1.29), grapes (1.05), pears (0.81), plums (0.62), strawberries (0.55) |
raw fruits (11.68) including:
apples (6.41), bananas (1.08), grapes (0.77), pears (0.69), plums (0.53), strawberries (0.40) |
raw fruits (16.70) including: apples (8.34), bananas (1.51), grapes (1.33), pears (0.93), plums (0.72), strawberries (0.70), peaches (0,56) |
- | |
| Vegetables | mg/day (mean ± SD), | 15.31 ± 14.51 | 15.68 ± 14.69 | 15.01 ± 14.36 | 0.0037 |
| median (IQR) | 11.96 (5.88–20.15) | 12.60 (6.16–20.84) | 11.56 (5.73–19.60) | ||
| Contribution to β-SIT (%) | 8.70 | 7.92 | 9.49 | 0.0347 | |
| Major sources (% contribution) ** |
raw vegetables (7.11), including:
cabbage (0.98), carrots (0.99), tomatoes (0.91), cauliflowers (0.81), peppers (0.54), beetroot (0.43), onion (0.36), cucumbers (0.33), vegetable preserves (1.23) |
raw vegetables (6.36) including:
cabbage (0.92), carrots (0.86), tomatoes (0.84), cauliflowers (0.68), peppers (0.47), beetroot (0.43), onion (0.38), cucumbers (0.32), vegetable preserves (1.28) |
raw vegetables (7.88) including:
cabbage (1.06), carrots (1.13), tomatoes (0.99), cauliflowers (0.94), peppers (0.61), beetroot (0.42), onion (0.34), cucumbers (0.35), vegetable preserves (1.18) |
- | |
| Coffee | mg/day (mean ± SD), | 9.91 ± 10.50 | 9.05 ± 10.74 | 10.62 ± 10.26 | <0.0001 |
| median (IQR) | 11.06 (0–13.83) | 11.06 (0–13.83) | 11.06 (0–13.83) | ||
| Contribution to β-SIT (%) | 5.64 | 4.57 | 6.72 | 0.0005 | |
| Cookies, | mg/day (mean ± SD), | 7.04 ± 14.04 | 6.97 ± 14.69 | 7.10 ± 13.49 | 0.0058 |
| cakes | median (IQR) | 0 (0–10.20) | 0 (0–10.00) | 0 (0–10.40) | |
| Contribution to β-SIT (%) | 4.00 | 3.52 | 4.49 | 0.0645 | |
| Chocolate | mg/day (mean ± SD), | 3.89 ± 13.77 | 4.10 ± 14.76 | 3.73 ± 12.89 | 0.0485 |
| products | median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | |
| Contribution to β-SIT (%) | 2.22 | 2.07 | 2.36 | 0.4699 | |
| Potatoes | mg/day (mean ± SD), | 4.35 ± 4.50 | 5.18 ± 5.12 | 3.67 ± 3.80 | <0.0001 |
| median (IQR) | 4.30 (0–7.82) | 4.30 (0–8.60) | 2.95 (0–5.73) | ||
| Contribution to β-SIT (%) | 2.47 | 2.62 | 2.32 | 0.4742 | |
| Legumes | mg/day (mean ± SD), | 2.21 ± 11.91 | 2.71 ± 14.03 | 1.80 ± 9.83 | 0.9852 |
| median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | ||
| Contribution to β-SIT (%) | 1.25 | 1.37 | 1.14 | 0.4522 | |
| Other food products | mg/day (mean ± SD), | 6.12 | 6.61 | 5.71 | - |
| Contribution to β-SIT (%) | 3.47 | 3.34 | 3.60 | 0.5732 | |
| Total β-sitosterol intake |
mg/day (mean ± SD), median (IQR) |
175.98 ± 88.00 160.85 (115.80–218.15) |
197.89 ± 98.28 180.84 (131.20–246.86) |
158.14 ± 74.02 146.28 (105.89–196.13) |
<0.0001 |
| Contribution to β-SIT (%) | 100 | 100 | 100 | - | |
* p calculated for differences between men and women. ** In the total and each food category, only individual food products with the strongest impact on the total plant sterol intakes were listed.
Table 4.
Contributions of food categories and individual food products to campesterol intake (CAMP), listed according to diminishing order of contribution.
| Food Categories | All N = 5690 |
Men N = 2554 |
Women N = 3136 |
p * | |
|---|---|---|---|---|---|
| Fats | mg/day (mean ± SD), | 26.55 ± 35.53 | 32.02 ± 41.34 | 22.10 ± 29.23 | <0.0001 |
| median (IQR) | 12.52 (3.75–37.85) | 16.70 (4.65–45.65) | 10.10 (3.00–31.11) | ||
| Contribution to CAMP (%) | 44.95 | 46.30 | 43.47 | 0.0311 | |
| Major sources (% contribution) ** |
oils (34.60) including: canola oil (33.43), sunflower oil (1.07), soybean oil (0.08), olive oil (0.01), soft margarines (8.11), mayonnaise (1.87) |
oils (35.50) including: canola oil (34.48), sunflower oil (0.94), soybean oil (0.06), olive oil (0.01), soft margarines (8.58), mayonnaise (1.81) |
oils (33.61) including: canola oil (32.26), sunflower oil (1.21), soybean oil (0.11), olive oil (0.01), soft margarines (7.60), mayonnaise (1.94) |
- | |
| Cereals | mg/day (mean ± SD), | 18.79 ± 11.57 | 23.34 ± 13.00 | 15.09 ± 8.65 | <0.0001 |
| median (IQR) | 16.50 (11.11–23.84) | 21.46 (14.48–30.02) | 13.79 (9.37–19.06) | ||
| Contribution to CAMP (%) | 31.81 | 33.74 | 29.67 | 0.0009 | |
| Major sources (% contribution) ** |
wheat bread (16.30), rolls (6.91), rye bread (5.49) |
wheat bread (19.52), rolls (6.92), rye bread (4.76) |
wheat bread (12.74), rolls (6.91), rye bread (6.29) |
- | |
| Vegetables | mg/day (mean ± SD), | 3.04 ± 3.81 | 3.06 ± 3.90 | 3.04 ± 3.75 | 0.2297 |
| median (IQR) | 2.00 (0.72–3.99) | 2.09 (0.74–4.08) | 1.95 (0.69–3.91) | ||
| Contribution to CAMP (%) | 5.16 | 4.43 | 5.97 | 0.0098 | |
| Major sources (% contribution) ** |
fresh vegetables (4.29) including:
cauliflowers (1.07), cabbage (0.93), carrots (0.70), peppers (0.51), tomatoes (0.36), vegetable preserves (0.68) |
fresh vegetables (3.62) including:
cabbage (0.75), cauliflowers (0.71), carrots (0.49), peppers (0.36), tomatoes (0.28), vegetable preserves (0.67) |
fresh vegetables (5.04) including:
cauliflowers (1.07), cabbage (0.93), carrots (0.70), peppers (0.51), tomatoes (0.36),
vegetable preserves (0.69) |
- | |
| Cookies, | mg/day (mean ± SD), | 2.99 ± 6.30 | 2.99 ± 6.69 | 3.00 ± 5.95 | 0.0049 |
| cakes | median (IQR) | 0 (0–3.90) | 0 (0–3.60) | 0 (0–4.16) | |
| Contribution to CAMP (%) | 5.07 | 4.32 | 5.90 | 0.0071 | |
| Coffee | mg/day (mean ± SD), | 3.15 ± 3.33 | 2.87 ± 3.41 | 3.37 ± 3.26 | <0.0001 |
| median (IQR) | 3.51 (0–4.39) | 3.51 (0–4.39) | 3.51 (0–4.39) | ||
| Contribution to CAMP (%) | 5.33 | 4.15 | 6.63 | <0.0001 | |
| Fruits | mg/day (mean ± SD), | 1.52 ± 2.47 | 1.38 ± 2.53 | 1.63 ± 2.42 | <0.0001 |
| median (IQR) | 0.60 (0–1.91) | 0.54 (0–1.64) | 0.72 (0.15–2.23) | ||
| Contribution to CAMP (%) | 2.57 | 1.99 | 3.21 | 0.0044 | |
| Major sources (% contribution) ** |
raw fruits (2.56) including: apples (0.61), bananas (0.53), grapes (0.30), mandarins (0.27), plums (0.19), oranges (0.17) |
raw fruits (1.98) including:
apples (0.51), bananas (0.42), grapes (0.22), mandarins (0.21), plums (0.16), oranges (0.11) |
raw fruits (3.20) including: apples (0.72), bananas (0.64), grapes (0.40), mandarins (0.34), oranges (0.24), plums (0.23) |
- | |
| Chocolate | mg/day (mean ± SD), | 0.68 ± 2.41 | 0.71 ± 2.59 | 0.65 ± 2.25 | 0.0462 |
| products | median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | |
| Contribution to CAMP (%) | 1.15 | 1.03 | 1.28 | 0.3669 | |
| Potatoes | mg/day (mean ± SD), | 0.37 ± 0.38 | 0.44 ± 0.44 | 0.31 ± 0.32 | <0.0001 |
| median (IQR) | 0.37 (0–0.67) | 0.37 (0–0.73) | 0.25 (0–0.49) | ||
| Contribution to CAMP (%) | 0.63 | 0.64 | 0.61 | 0.9213 | |
| Legumes | mg/day (mean ± SD), | 0.35 ± 1.75 | 0.42 ± 2.06 | 0.28 ± 1.44 | 0.9950 |
| median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | ||
| Contribution to CAMP (%) | 0.59 | 0.61 | 0.55 | 0.6768 | |
| Other food products | mg/day (mean ± SD), | 1.62 | 1.93 | 1.36 | - |
| Contribution to CAMP (%) | 2.74 | 2.79 | 2.67 | 0.8152 | |
| Total campesterol intake | mg/day (mean ± SD), median (IQR) |
59.06 ± 41.44 47.45 (31.53–74.39) |
69.16 ± 47.68 56.71 (37.17–86.39) |
50.83 ± 33.38 40.88 (27.80–64.90) |
<0.0001 |
| Contribution to CAMP (%) | 100 | 100 | 100 | - | |
* p calculated for differences between men and women. ** In the total and each food category, only individual food products with the strongest impact on the total plant sterol intakes were listed.
Table 5.
Contributions of food categories and individual food products to stigmasterol intake (STIG), listed according to diminishing order of contribution.
| Food Categories | All N = 5690 |
Men N = 2554 |
Women N = 3136 |
p * | |
|---|---|---|---|---|---|
| Coffee | mg/day (mean ± SD), | 6.18 ± 6.55 | 5.64 ± 6.70 | 6.62 ± 6.40 | <0.0001 |
| median (IQR) | 6.90 (0–8.63) | 6.90 (0–8.63) | 6.90 (0–8.63) | ||
| Contribution to STIG (%) | 25.10 | 21.41 | 28.53 | <0.0001 | |
| Vegetables | mg/day (mean ± SD), | 5.72 ± 5.48 | 6.00 ± 5.63 | 5.49 ± 5.34 | 0.0007 |
| median (IQR) | 4.34 (1.90–7.96) | 4.55 (1.95–8.48) | 4.12 (1.85–7.45) | ||
| Contribution to STIG (%) | 23.22 | 22.76 | 23.66 | 0.4179 | |
| Major sources (% contribution) ** |
raw vegetables (17.90) including:
tomatoes (4.63), beets (1.90), cucumbers (1.82), carrots (1.80), parsley (1.88), green beans (1.88), lettuce (1.24), celery (1.08), vegetable preserves (4.10) |
raw vegetables (17.15) including:
tomatoes (4.48), beets (2.02), cucumbers (1.83), carrots (1.64), parsley (1.89), green beans (1.52), lettuce (1.17), celery (1.12),
vegetable preserves (4.62) |
raw vegetables (18.60) including:
tomatoes (4.76), beets (1.79), cucumbers (1.81), carrots (1.96), parsley (1.88), green beans (2.21), lettuce (1.31), celery (1.04), vegetable preserves (3.62) |
- | |
| Fats | mg/day (mean ± SD), | 4.15 ± 4.93 | 5.02 ± 5.79 | 3.44 ± 3.97 | <0.0001 |
| median (IQR) | 2.59 (0.81–5.77) | 3.23 (1.05–7.10) | 2.28 (0.66–4.97) | ||
| Contribution to STIG (%) | 16.85 | 19.05 | 14.84 | <0.0001 | |
| Major sources (% contribution) ** |
soft margarines (11.82), oils (3.75), mixed fats (0.65), mayonnaise (0.60) | soft margarines (13.90), oils (3.69), mixed fats (0.79), mayonnaise (0.63) | soft margarines (9.91), oils (3.80), mixed fats (0.52), mayonnaise (0.56) | - | |
| Cereals | mg/day (mean ± SD), | 3.19 ± 2.26 | 3.91 ± 2.43 | 2.60 ± 1.93 | <0.0001 |
| median (IQR) | 2.72 (1.82–3.98) | 3.53 (2.36–5.02) | 2.33 (1.57–3.19) | ||
| Contribution to STIG (%) | 12.93 | 14.83 | 11.18 | <0.0001 | |
| Major sources (% contribution) ** |
wheat bread (6.48), rolls (2.57), rye bread (2.48), cereals (0,66) | wheat bread (8.49), rolls (2.69), rye bread (2.44), cereals (0,47) | wheat bread (4.62), rolls (2.29), rye bread (2.69), cereals (0,84) | - | |
| Chocolate | mg/day (mean ± SD), | 1.58 ± 5.59 | 1.66 ± 6.00 | 1.51 ± 5.22 | 0.0474 |
| products | median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | |
| Contribution to STIG (%) | 6.40 | 6.29 | 6.52 | 0.7579 | |
| Legumes | mg/day (mean ± SD), | 1.21 ± 6.04 | 1.47 ± 7.23 | 0.99 ± 4.86 | 0.9904 |
| median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | ||
| Contribution to STIG (%) | 4.91 | 5.58 | 4.28 | 0.0208 | |
| Fruits | mg/day (mean ± SD), | 0.92 ± 1.81 | 0.82 ± 1.71 | 1.00 ± 1.88 | <0.0001 |
| median (IQR) | 0.18 (0–0.88) | 0.15 (0–0.60) | 0.22 (0.02–1.13) | ||
| Contribution to STIG (%) | 3.73 | 3.13 | 4.29 | 0.0211 | |
| Major sources (% contribution) ** |
raw fruits (3.72) including: bananas (1.68), apples (0.40), nectarines (0.30), plums (0.29), peaches (0.40) |
raw fruits (3.12) including: bananas (1.33), apples (0.37), nectarines (0.27), plums (0.26), peaches (0.25) |
raw fruits (4.28) including: bananas (1.68), apples (0.44), nectarines (0.33), plums (0.32), peaches (0.53) |
- | |
| Potatoes | mg/day (mean ± SD), | 0.61 ± 0.63 | 0.73 ± 0.72 | 0.52 ± 0.53 | <0.0001 |
| median (IQR) | 0.60 (0–1.10) | 0.60 (0–1.21) | 0.41 (0–0.81) | ||
| Contribution to STIG (%) | 2.48 | 2.77 | 2.22 | 0.1861 | |
| Cookies, | mg/day (mean ± SD), | 0.59 ± 1.52 | 0.57 ± 1.62 | 0.61 ± 1.44 | 0.0004 |
| cakes | median (IQR) | 0 (0–0.40) | 0 (0–0.25) | 0 (0–0.50) | |
| Contribution to STIG (%) | 2.40 | 2.17 | 2.61 | 0.2589 | |
| Other food products | mg/day (mean ± SD), | 0.48 | 0.54 | 0.44 | - |
| Contribution to STIG (%) | 1.98 | 2.01 | 1.88 | 0.7530 | |
| Total stigmasterol intake | mg/day (mean ± SD), median (IQR) |
24.63 ± 14.49 22.10 (14.53–30.92) |
26.36 ± 16.02 23.49 (15.14–32.91) |
23.22 ± 12.94 21.11 (14.16–29.19) |
<0.0001 |
| Contribution to STIG (%) | 100 | 100 | 100 | - | |
* p calculated for differences between men and women. ** In the total and each food category, only individual food products with the strongest impact on the total plant sterol intakes were listed.
The main food categories providing β-sitosterol were cereals (29.19%), fats (28.86%), fruits (14.20%), and vegetables (8.70%), with a total share of 80.95% of the β-sitosterol supply (Table 3). Among the food products, β-sitosterol was supplied by canola oil (15.88%), followed by wheat bread (14.88%) and soft margarine (9.02%). Women had a lower β-sitosterol intake compared to men at 146.28 mg/day vs. 180.84 mg/day, respectively.
The main sources of campesterol were fats (44.95%) and cereal products (31.81%), which together accounted for 76.76% of the campesterol intake (Table 4). For individual products, campesterol was supplied by canola oil (33.43%), white bread (16.30%), and soft margarines (8.11%). Men consumed more campesterol compared to women (56.71 vs. 40.88 mg/day, respectively).
As for stigmasterol, its main sources were the following product groups: coffee (25.10%), vegetables (23.22%), fats (16.85%), and cereal products (12.93%). The foods supplying the highest amounts of stigmasterol included coffee (as a food product; 25.10%), soft margarine (11.82%), and white bread (6.48%). The median intake of stigmasterol was higher in men at 23.49 mg/day compared to women at 21.11 mg/day.
On a per milligram basis, men consumed more total and individual plant sterols (Table 6). However, per 1000 kcal, significantly more plant sterols as total and individual sterols were consumed by women (p < 0.0001), except for campesterol, for which the difference was not statistically significant.
Table 6.
Comparison of total and individual sterol intakes (in mg and in mg/1000 kcal) by men and women.
| Plant Sterols (mg) | Men | Women | p-Value |
|---|---|---|---|
| Total plant sterols | 320.8 | 252.2 | <0.0001 |
| Total plant sterols/1000 kcal | 141.0 | 154.2 | <0.0001 |
| β-sitosterol | 197.8 | 158.1 | <0.0001 |
| β-sitosterol/1000 kcal | 87.1 | 96.8 | <0.0001 |
| Campesterol | 69.2 | 50.8 | <0.0001 |
| Campesterol/1000 kcal | 29.8 | 30.4 | 0.2279 |
| Stigmasterol | 26.4 | 23.2 | <0.0001 |
| Stigmasterol/1000 kcal | 12.0 | 14.8 | <0.0001 |
4. Discussion
This is the first report on dietary plant sterol intake and its dietary sources in the Polish population. Due to the lack of plant sterols in Polish food composition tables, the database used for this study included international databases available in English supplemented with data from research papers on plant sterol contents in food products. In our study, the consumption of plant sterols from enriched food products was not taken into consideration, since the percentage of consumers of phytosterol-enriched products was low (2%). In comparison, it has been estimated that regular consumers of products with added plant sterols represent approximately 10–15% of the EU population [39].
Typical contemporary Western diets provide much lower amounts of phytosterols [40] than estimated for distant human ancestors, whose diet provided 1 g/day of phytosterols [41]. The dietary phytosterol intake in population studies is usually between 200 and 400 mg/day [21,42], even in those populations with more beneficial dietary habits [43], and this amount is too low to show significant LDL cholesterol-lowering effects demonstrated for 1 g of phytosterols [44]. Contrary to this, the PREDIMED study found that even small amounts of plant sterols from natural foods may exert a cholesterol-lowering effect [45]. A recent meta-analysis of 124 clinical studies demonstrated that a phytosterol intake between 0.6 and 3.3 g/day is associated with a gradual decrease in the concentration of LDL-cholesterol from 6% to 12% [46]. Scientific evidence indicates that even moderate doses of phytosterols delivered via a normal diet can provide a protective effect on the lipid profile by reducing cholesterol absorption [47,48], but a lipid-lowering effect may depend on the inter-individual variation in response to phytosterols [49].
The daily intake of total plant sterols in our study (255.96 mg/day) is similar to that of the Spanish population, where it was estimated to be 276 mg, with the largest contribution of beta-sitosterol (79.7%) [5]. In different populations, plant sterol intake ranged from 230 to 324 mg/day. Among other things, these differences may be due to the dietary habits of different populations or the availability of different food products on the market. Some differences may also be due to the food intake methodology. Some studies were based on a 24-h interview or dietary records, and others on a frequency of intake. Our results confirm earlier findings that β-sitosterol is the most important contributor (67.8%) to the intake of total dietary plant sterols. Regarding gender differences in plant sterol intake, in our study, the intake was 291.76 mg/day for men and 230.61 mg/day for women. These results are similar to most other populations where gender differences in plant sterol intake were observed among men and women [20]. These differences may be due to differences in food intake between the two sexes. Women tend to consume smaller portions of foods, which translates into fewer ingredients including plant sterols.
As per our study, the consumption pattern of total plant sterols from major food groups such as cereal products, vegetable oils and fats, vegetables, and fruits is similar to the intervention group in the PREDIMED study and to the U.K. population [21,45]. Of these, cereal products and oils provided nearly 61% of plant sterols, and when combined with vegetables and fruits, nearly 80%. However, unlike the PREDIMED study, where legumes were the fifth contributor to total plant sterols, in our study, the additional sources of plant sterols included coffee, cookies and cakes, chocolate products, and potatoes, while legumes were only ninth in providing plant sterols. Together, these minor sources of plant sterols accounted for 16.68% of plant sterol intake. The other sources of plant sterols accounted for 3.55%; these included, among others, nuts and seeds, which are normally a good source of plant sterols, but because of their low intake [50], they were not a significant source of plant sterols for the Polish population. The PREDIMED intervention study indicated an important role for the Mediterranean diet, in combination with nuts, in providing plant sterols in the diet and providing a cholesterol-lowering effect [45]. Considering this, Poles should be encouraged to increase their nut consumption and improve their dietary habits, which are far from the recommended for the prevention of cardiovascular diseases [51,52]. Regarding individual dietary sources of total plant sterols, canola oil and white bread predominated, followed by soft margarine. Similar to a Chinese study, canola oil was the main provider of plant sterols among vegetable fats and oils [53].
As in the study of EPIC-Norfolk population [21], women in the WOBASZ survey had a higher plant sterol density than men. Interestingly, when converted per 1000 kcal, the total plant sterol content did not differ from the values obtained in the EPIC-Norfolk study. For men and women in our study, the amount of plant sterols was 141.0 mg and 154.2 mg, respectively, and in the EPIC-Norfolk study, for men it was 137.33 mg and for women it was 152.4 mg/day.
Limitations
Some plant sterol values in this study may have been underestimated because only three major sterols (sitosterol, campesterol, and stigmasterol) are typically included in the totals, despite the contribution of other sterols. Although the compiled database facilitated the calculation of plant sterols, there are some shortcomings due to the lack of data for individual plant sterols. This is mainly due to the fact that the literature data do not provide information on the content of plant sterols in certain food products. For some foods, the values of plant sterols (total and individual) were not found, e.g., no studies were found for chard. No data were found for campesterol or stigmasterol in radishes, wines, and mushrooms. For foods such as chives, blueberries, cherries, pears, raspberries, blackcurrants, walnuts, and pumpkin seeds, no value was found for stigmasterol. Therefore, the values obtained for the sum of individual plant sterols could be lower than the total plant sterol content. Moreover, there are no specific data on the composition of plant sterols in enriched margarine, which is related, among other things, to proprietary manufacturing technologies. In addition, since a small percentage of study participants consumed phytosterol-enriched margarine, they were not included in the calculation of dietary plant sterols.
Furthermore, a limitation of the study is the inclusion in the plant sterol database of results from several less rigorous literature sources than those given in the Rand criteria.
This study used single 24-h recall as a tool to measure food intake, which is an appropriate method for large-scale studies. However, 24-h recall does not account for variability in food intake and may not describe a typical diet.
5. Conclusions
This is the first study to evaluate the intake of plant sterols in the Polish population. Since no plant sterols are listed in Polish food composition tables, a database was developed using published data sources. The study found that plant sterol intake in Poland is 255.96 mg/day, which is comparable to other populations, despite different methodologies of nutritional assessment and slightly different databases. The main dietary sources of plant sterols in this study were cereals, fats, vegetables, and fruits, which is consistent with data for other populations. This study found that women’s diets are more dense in plant sterols, which is in agreement with other studies. Due to the lack of literature sources on plant sterol content in some foods, future studies should expand and complete the databases on plant sterol content in foods.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13082722/s1, Table S1: Content of selected plant sterols (stigmasterol, campesterol, beta-sitosterol) and total plant sterols in food products (mg/100 g of product), Table S2: Content of selected plant sterols (stigmasterol, campesterol, beta-sitosterol) and total plant sterols in dishes (mg/100 g of dish).
Author Contributions
Conceptualization, A.W. and A.M.W.; methodology, A.M.W., A.W. and M.E.Z.; software, A.W., A.M.W. and A.C.-M.; validation, A.W., A.M.W. and I.M.-C.; formal analysis, A.C.-M. and A.W.; investigation, A.W. and A.M.W.; resources, A.M.W., A.W., M.E.Z., I.M.-C. and W.D.; data curation, A.W. and A.M.W.; writing—original draft preparation, A.M.W.; writing—review and editing, A.W., M.E.Z., I.M.-C., A.C.-M. and W.D.; visualization, A.W. and A.M.W.; supervision, A.W., A.M.W. and W.D.; project administration, A.M.W. and A.W.; funding acquisition, A.W. and A.M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Cardiology (Grant no. 2.20/I/20) and Medical University of Bialystok (Grant no. SUB/3/DN/21/001/3317).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Bioethics Committee of the National Institute of Cardiology (protocol code 1344, date of approval 5 November 2012, and protocol code 1837, date of approval 14 January 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data in this study are made available upon request to the authors at the following e-mail address: anna.witkowska@umb.edu.pl or awaskiewicz@ikard.pl.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dufourc E.J. Sterols and membrane dynamics. J. Chem. Biol. 2008;1:63–77. doi: 10.1007/s12154-008-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Smet E., Mensink R.P., Plat J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present. Mol. Nutr. Food Res. 2012;56:1058–1072. doi: 10.1002/mnfr.201100722. [DOI] [PubMed] [Google Scholar]
- 3.Vezza T., Canet F., de Marañón A.M., Bañuls C., Rocha M., Víctor V.M. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants. 2020;9:1266. doi: 10.3390/antiox9121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng S., Belwal T., Li L., Limwachiranon J., Liu X., Luo Z. Phytosterols and their derivatives: Potential health-promoting uses against lipid metabolism and associated diseases, mechanism, and safety issues. Compr. Rev. Food Sci. Food Saf. 2020;19:1243–1267. doi: 10.1111/1541-4337.12560. [DOI] [PubMed] [Google Scholar]
- 5.Jiménez-Escrig A., Santos-Hidalgo A.B., Saura-Calixto F. Common sources and estimated intake of plant sterols in the Spanish diet. J. Agric. Food Chem. 2006;54:3462–3471. doi: 10.1021/jf053188k. [DOI] [PubMed] [Google Scholar]
- 6.Shaghaghi M.A., Harding S.V., Jones P.J.H. Water dispersible plant sterol formulation shows improved effect on lipid profile compared to plant sterol esters. J. Funct. Foods. 2014;6:280–289. doi: 10.1016/j.jff.2013.10.017. [DOI] [Google Scholar]
- 7.Plat J., Brufau G., Dallinga-Thie G.M., Dasselaar M., Mensink R.P. A plant stanol yogurt drink alone or combined with a low-dose statin lowers serum triacylglycerol and non-HDL cholesterol in metabolic syndrome patients. J. Nutr. 2009;139:1143–1149. doi: 10.3945/jn.108.103481. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida Y., Niki E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003;49:277–280. doi: 10.3177/jnsv.49.277. [DOI] [PubMed] [Google Scholar]
- 9.Brüll F., De Smet E., Mensink R.P., Vreugdenhil A., Kerksiek A., Lütjohann D., Wesseling G., Plat J. Dietary plant stanol ester consumption improves immune function in asthma patients: Results of a randomized, double-blind clinical trial. Am. J. Clin. Nutr. 2016;103:444–453. doi: 10.3945/ajcn.115.117531. [DOI] [PubMed] [Google Scholar]
- 10.Kurano M., Hasegawa K., Kunimi M., Hara M., Yatomi Y., Teramoto T., Tsukamoto K. Sitosterol prevents obesity-related chronic inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1863:191–198. doi: 10.1016/j.bbalip.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Klingberg S., Ellegård L., Johansson I., Jansson J.H., Hallmans G., Winkvist A. Dietary intake of naturally occurring plant sterols is related to a lower risk of a first myocardial infarction in men but not in women in northern Sweden. J. Nutr. 2013;143:1630–1635. doi: 10.3945/jn.113.178707. [DOI] [PubMed] [Google Scholar]
- 12.Ramprasath V.R., Awad A.B. Role of phytosterols in cancer prevention and treatment. J. AOAC Int. 2015;98:735–738. doi: 10.5740/jaoacint.SGERamprasath. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L., Zhao X., Xu J., Li C., Yu Y., Wang W., Zhu L. The Protective Effect of Dietary Phytosterols on Cancer Risk: A Systematic Meta-Analysis. J. Oncol. 2019;2019:7479518. doi: 10.1155/2019/7479518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Composition of Foods, Raw, Processed, Prepared. National Nutrient Database for Standard Reference Release 27. USDA, 2015. Modified in 2019. [(accessed on 25 May 2019)]; Available online: https://data.nal.usda.gov/dataset/composition-foods-raw-processed-prepared-usda-national-nutrient-database-standard-reference-release-27.
- 15.Tolve R., Cela N., Condelli N., Di Cairano M., Caruso M.C., Galgano F. Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study. Foods. 2020;9:470. doi: 10.3390/foods9040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martianto D., Bararah A., Andarwulan N., Średnicka-Tober D. Cross-Sectional Study of Plant Sterols Intake as a Basis for Designing Appropriate Plant Sterol-Enriched Food in Indonesia. Nutrients. 2021;13:452. doi: 10.3390/nu13020452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M., Huang W., Hu Y., Zhang L., Shao Y., Wang M., Zhang F., Zhao Z., Mei X., Li T., et al. Phytosterol profiles of common foods and estimated natural intake of different structures and forms in China. J. Agric. Food Chem. 2018;66:2669–2676. doi: 10.1021/acs.jafc.7b05009. [DOI] [PubMed] [Google Scholar]
- 18.Sioen I., Matthys C., Huybrechts I., Van Camp J., De Henauw S. Consumption of plant sterols in Belgium: Estimated intakes and sources of naturally occurring plant sterols and β-carotene. Br. J. Nutr. 2011;105:960–966. doi: 10.1017/S0007114510004587. [DOI] [PubMed] [Google Scholar]
- 19.Normen A.L., Brants H.A.M., Voorrips L.E., Anderson H.A., van den Brandt P.A., Goldbohm R.A. Plant sterol intakes and colorectal cancer risk in The Netherlands Cohort Study on diet and cancer. Am. J. Clin. Nutr. 2001;74:141–148. doi: 10.1093/ajcn/74.1.141. [DOI] [PubMed] [Google Scholar]
- 20.Valsta L.M., Lemström A., Ovaskainen M.L., Lampi A.M., Toivo J., Korhonen T., Piironen V. Estimation of plant sterol and cholesterol intake in Finland: Quality of new values and their effect on intake. Br. J. Nutr. 2004;92:671–678. doi: 10.1079/BJN20041234. [DOI] [PubMed] [Google Scholar]
- 21.Klingberg S., Andersson H., Mulligan A., Bhaniani A., Welch A., Bingham S., Khaw K.T., Andersson S., Ellegård L. Food sources of plant sterols in the EPIC Norfolk population. Eur. J. Clin. Nutr. 2008;62:695–703. doi: 10.1038/sj.ejcn.1602765. [DOI] [PubMed] [Google Scholar]
- 22.Amirabdollahian F., Ash R. An estimate of phytate intake and molar ratio of phytate to zinc in the diet of the people in the United Kingdom. Public Health Nutr. 2010;13:1380–1388. doi: 10.1017/S1368980010000704. [DOI] [PubMed] [Google Scholar]
- 23.Przysławski J., Stelmach M., Grygiel-Górniak M., Dubec A. An assessment of dietary habits in the group of studying adolescents especially taking into consideration plant sterols intake—Pilot study. Nowiny Lek. 2009;77:299–304. [Google Scholar]
- 24.Food Standard Agency, Public Health England McCance and Widdowson’s the Composition of Foods: 2019. [(accessed on 25 May 2019)]; Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid.
- 25.Rand W.M., Pennington J.A.T., Murphy S.P., Klensin J.C. Compiling Data for Food Composition Data Bases. United Nations University Press; Tokyo, Japan: 1991. [(accessed on 10 January 2019)]. Available online: http://www.unu.edu/unupress/unupbooks/80772e/80772E00.htm. [Google Scholar]
- 26.Lagarda M.J., García-Llatas G., Farré R. Analysis of phytosterols in foods. J. Pharm. Biomed. Anal. 2006;41:1486–1496. doi: 10.1016/j.jpba.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 27.Normen L., Ellegard L., Brants H., Dutta P., Andersson H. A phytosterol database: Fatty foods consumed in Sweden and the Netherlands. J. Food Compos. Anal. 2007;20:193–201. doi: 10.1016/j.jfca.2006.06.002. [DOI] [Google Scholar]
- 28.Normén L., Bryngelsson S., Johnsson M., Evheden P., Ellegård L., Brants H., Andersson H., Dutta P. The Phytosterol Content of Some Cereal Foods Commonly Consumed in Sweden and in the Netherlands. J. Food Compos. Anal. 2002;15:693–704. doi: 10.1006/jfca.2002.1098. [DOI] [Google Scholar]
- 29.Normén L., Johnsson M., Andersson H., van Gameren Y., Dutta P. Plant sterols in vegetables and fruits commonly consumed in Sweden. Eur. J. Nutr. 1999;38:84–89. doi: 10.1007/s003940050048. [DOI] [PubMed] [Google Scholar]
- 30.Han J.H., Yang Y.X., Feng M.Y. Contents of phytosterols in vegetables and fruits commonly consumed in China. Biomed. Environ. Sci. 2008;21:449–453. doi: 10.1016/S0895-3988(09)60001-5. [DOI] [PubMed] [Google Scholar]
- 31.Piironen V., Toivo J., Puupponen-Pimia R., Lampi A.M. Plant sterols in vegetables, fruits and berries. J. Sci. Food Agric. 2003;83:330–337. doi: 10.1002/jsfa.1316. [DOI] [Google Scholar]
- 32.Ryan E., Galvin K., O’Connor T.P., Maguire A.R., O’Brien N.M. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum. Nutr. 2007;62:85–91. doi: 10.1007/s11130-007-0046-8. [DOI] [PubMed] [Google Scholar]
- 33.Li Y.C., Li C.L., Li R., Chen Y., Zhang M., Guo P.P., Shi D., Ji X.N., Feng R.N., Sun C.H. Associations of dietary phytosterols with blood lipid profiles and prevalence of obesity in Chinese adults, a cross-sectional study. Lipids Health Dis. 2018;17:54. doi: 10.1186/s12944-018-0703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaya A., Endo Y., Fujimoto K., Kitamura K. Effects of genetic variability and planting location on the phytosterol content and composition in soybean seeds. Food Chem. 2007;102:1071–1075. doi: 10.1016/j.foodchem.2006.07.001. [DOI] [Google Scholar]
- 35.Decloedt A.I., Van Landschoot A., Watson H., Vanderputten D., Vanhaecke L. Plant-Based Beverages as Good Sources of Free and Glycosidic Plant Sterols. Nutrients. 2017;10:21. doi: 10.3390/nu10010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruggiero A., Vitalini S., Burlini N., Bernasconi S., Iriti M. Phytosterols in grapes and wine, and effects of agrochemicals on their levels. Food Chem. 2013;141:3473–3479. doi: 10.1016/j.foodchem.2013.05.153. [DOI] [PubMed] [Google Scholar]
- 37.Čížková H., Soukupová V., Voldřich M., Ševčík R. Differentiation of coffee varieties according to their sterolic profile. J. Food Nutr. Res. 2007;46:28–34. [Google Scholar]
- 38.Drygas W., Niklas A.A., Piwońska A., Piotrowski W., Flotyńska A., Kwaśniewska M., Nadrowski P., Puch-Walczak A., Szafraniec K., Bielecki W., et al. Multi-center National Population Health Examination Survey (WOBASZ II study): Assumptions, methods and implementation. Kardiol. Pol. 2016;74:681–690. doi: 10.5603/KP.a2015.0235. [DOI] [PubMed] [Google Scholar]
- 39.European Food Safety Authority (EFSA) Consumption of Food and Beverages with Added Plant Sterols. EFSA J. 2008;133:1–21. doi: 10.2903/j.efsa.2008.133r. [DOI] [Google Scholar]
- 40.Ras R.T., van der Schouw Y.T., Trautwein E.A., Sioen I., Dalmeijer G.W., Zock P.L., Beulens J.W.J. Intake of phytosterols from natural sources and risk of cardiovascular disease in the European Prospective Investigation into Cancer and Nutrition-the Netherlands (EPIC-NL) population. Eur. J. Prev. Cardiol. 2015;22:1067–1075. doi: 10.1177/2047487314554864. [DOI] [PubMed] [Google Scholar]
- 41.Jew S., AbuMweis S.S., Jones P.J.H. Evolution of the human diet: Linking our ancestral diet to modern functional foods as a means of chronic disease prevention. J. Med. Food. 2009;12:925–934. doi: 10.1089/jmf.2008.0268. [DOI] [PubMed] [Google Scholar]
- 42.Sirirat R., Heskey C., Haddad E., Tantamango-Bartley Y., Fraser G., Mashchak A., Jaceldo-Siegl K. Comparison of phytosterol intake from FFQ with repeated 24-h dietary recalls of the Adventist Health Study-2 calibration sub-study. Br. J. Nutr. 2019;121:1424–1430. doi: 10.1017/S0007114519000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaceldo-Siegl K., Lütjohann D., Sirirat R., Mashchak A., Fraser G.E., Haddad E. Variations in dietary intake and plasma concentrations of plant sterols across plant-based diets among North American adults. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreau R.A. Composition of Plant Sterols and Stanols in Supplemented Food Products. J. AOAC Int. 2015;98:685–690. doi: 10.5740/jaoacint.SGEMoreau. [DOI] [PubMed] [Google Scholar]
- 45.Escurriol V., Cofán M., Serra M., Bulló M., Basora J., Salas-Salvadó J., Corella D., Zazpe I., Martínez-González M.A., Ruiz-Gutiérrez V., et al. Serum sterol responses to increasing plant sterol intake from natural foods in the Mediterranean diet. Eur. J. Nutr. 2009;48:373–382. doi: 10.1007/s00394-009-0024-z. [DOI] [PubMed] [Google Scholar]
- 46.Ras R.T., Geleijnse J.M., Trautwein E.A. LDL cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014;112:214–219. doi: 10.1017/S0007114514000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanclemente T., Marques-Lopes I., Fajo-Pascual M., Cofán M., Jarauta E., Ros E., Puzo J., García-Otín A.L. Naturally-occurring phytosterols in the usual diet influence cholesterol metabolism in healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2012;22:849–855. doi: 10.1016/j.numecd.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Racette S.B., Lin X., Lefevre M., Spearie C.A., Most M.M., Ma L., Ostlund R.E., Jr. Dose effects of dietary phytosterols on cholesterol metabolism: A controlled feeding study. Am. J. Clin. Nutr. 2010;91:32–38. doi: 10.3945/ajcn.2009.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trautwein E.A., Vermeer M.A., Hiemstra H., Ras R.T. LDL-Cholesterol Lowering of Plant Sterols and Stanols-Which Factors Influence Their Efficacy? Nutrients. 2018;10:1262. doi: 10.3390/nu10091262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witkowska A.M., Waśkiewicz A., Zujko M.E., Szcześniewska D., Śmigielski W., Stepaniak U., Pająk A., Drygas W. The Consumption of Nuts is Associated with Better Dietary and Lifestyle Patterns in Polish Adults: Results of WOBASZ and WOBASZ II Surveys. Nutrients. 2019;11:1410. doi: 10.3390/nu11061410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waśkiewicz A., Szcześniewska D., Szostak-Węgierek D., Kwaśniewska M., Pająk A., Stepaniak U., Kozakiewicz K., Tykarski A., Zdrojewski T., Zujko M.E., et al. Are dietary habits of the Polish population consistent with the recommendations for prevention of cardiovascular disease?—WOBASZ II project. Kardiol. Pol. 2016;74:969–977. doi: 10.5603/KP.a2016.0003. [DOI] [PubMed] [Google Scholar]
- 52.Drywień M.E., Hamulka J., Jezewska-Zychowicz M. Perceived Nutrition and Health Concerns: Do They Protect against Unhealthy Dietary Patterns in Polish Adults? Nutrients. 2021;13:170. doi: 10.3390/nu13010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang R., Xue L., Zhang L., Wang X., Qi X., Jiang J., Yu L., Wang X., Zhang W., Zhang Q., et al. Phytosterol Contents of Edible Oils and Their Contributions to Estimated Phytosterol Intake in the Chinese Diet. Foods. 2019;8:334. doi: 10.3390/foods8080334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in this study are made available upon request to the authors at the following e-mail address: anna.witkowska@umb.edu.pl or awaskiewicz@ikard.pl.