Abstract
Stat5a and Stat5b are rapidly activated by a wide range of cytokines and growth factors, including interleukin-2 (IL-2). We have previously shown that these signal transducers and activators of transcription (STAT proteins) are key regulatory proteins that bind to two tandem gamma interferon-activated site (GAS) motifs within an IL-2 response element (positive regulatory region III [PRRIII]) in the human IL-2Rα promoter. In this study, we demonstrate cooperative binding of Stat5 to PRRIII and explore the molecular basis underlying this cooperativity. We demonstrate that formation of a tetrameric Stat5 complex is essential for the IL-2-inducible activation of PRRIII. Stable tetramer formation of Stat5 is mediated through protein-protein interactions involving a tryptophan residue conserved in all STATs and a lysine residue in the Stat5 N-terminal domain (N domain). The functional importance of tetramer formation is shown by the decreased levels of transcriptional activation associated with mutations in these residues. Moreover, the requirement for STAT protein-protein interactions for gene activation from a promoter with tandemly linked GAS motifs can be relieved by strengthening the avidity of protein-DNA interactions for the individual binding sites. Taken together, these studies demonstrate that a dimeric but tetramerization-deficient Stat5 protein can activate only a subset of target sites. For functional activity on a wider range of potential recognition sites, N-domain-mediated oligomerization is essential.
The interaction of interleukin-2 (IL-2) with high-affinity IL-2 receptors critically regulates the magnitude and duration of the T-cell immune response (17). In peripheral blood lymphocytes (PBL), IL-2 rapidly activates the Janus-family tyrosine kinases Jak1 and Jak3 (1, 2, 14, 22, 26, 33) and the latent STAT (signal transducers and activators of transcription) transcription factors Stat3, Stat5a, and Stat5b (5–7, 10, 18, 19, 25, 32). The STAT proteins then homo- or heterodimerize and translocate to the nucleus, where they bind to and regulate the transcriptional activation of the promoters of target genes.
Dimeric STAT proteins can bind to the palindromic gamma interferon-activated (GAS) sequence TTCNmGAA, where m is 3 for all the STATs except Stat6, which can additionally bind to GAS motifs where m is 4 (reviewed in references 3, 4, 11, and 16). However, there are differences in the fine DNA binding specificities for the various STAT proteins; binding site specificity studies have identified specific nucleotide requirements in the three central nucleotides and in the sequences flanking the core consensus sequence of the different STAT proteins (8, 21, 27, 28, 36).
It has also been shown that, in addition to binding to DNA as dimers, Stat1 and Stat4 can form tetrameric complexes on tandemly linked GAS motifs and that tetramer formation is mediated by the highly conserved N-terminal regions (N domains) of these proteins (30, 35). Such cooperative DNA binding can serve to selectively bind different STAT proteins on a promoter that contains multiple potential STAT binding sites (35). Recently, the crystal structure of the N domain of Stat4 was determined to be a hook-like structure consisting of eight α-helices (31). A tryptophan residue, conserved in all STATs, was shown to be engaged in crucial internal polar interactions between interacting helices in the structure. Mutation of this residue in Stat1 prevented tetramer formation and, consequently, resulted in the loss of gamma interferon-inducible transcriptional activity from a synthetic, model promoter. These results established the importance of this tryptophan residue in the functional activity of Stat1 and suggested that this residue would be important for tetramer formation by other STATs as well.
In this study, we investigated the importance and molecular basis of N-domain-mediated tetramerization of Stat5 in regulating the transcriptional activation of a complex, natural promoter element containing tandem GAS motifs. Stat5 proteins are key signaling molecules that are activated by a variety of different cytokines and growth factors, including IL-2 (16). One important target gene regulated by Stat5 encodes the highly IL-2-inducible component of the IL-2 receptor (IL-2R) complex itself, the IL-2Rα chain (12, 13, 15, 23, 29). An IL-2 response element (positive regulatory region III [PRRIII]) (Fig. 1A) was identified in both murine and human IL-2Rα genes (13, 15, 29). In the human IL-2Rα gene, PRRIII is located 3.7 kb upstream of the transcription initiation sites and is a complex element composed of two GAS motifs bound by Stat5, as well as binding sites for other factors, including the Ets-family protein Elf-1, the high-mobility-group proteins, HMG-I(Y), and a putative GATA-like factor (13, 29). The two GAS motifs are linked in tandem; one is a consensus GAS motif (GASc), while the other is a nonconsensus GAS motif (GASn) (Fig. 1A). Full functional activity of PRRIII is dependent on the simultaneous presence of both of the GAS motifs and the downstream Elf-1 binding site (13). Recently, studies of the murine IL-2Rα IL-2 response element suggested that responsiveness of this element to IL-2 is mediated by cooperative interactions between Stat5 dimers bound to the tandem GAS motifs (20). Based on the information available on the crystal structure of N-terminal dimers of Stat4, we generated putative N-terminal oligomerization mutants of Stat5 and directly demonstrated the importance of N-terminal oligomerization of Stat5 proteins in mediating the IL-2-inducible activation of a naturally occurring promoter element, PRRIII. In so doing, we have also learned more about the requirements for Stat5 tetramerization and Stat5-dependent transcriptional activation.
FIG. 1.
Stat5 binds to tandem GAS motifs in PRRIII. (A) Sequence and organization of PRRIII. Also shown are the sequences of the probes used for the EMSAs depicted in panel B. EBS, Ets binding site. (B) Stat5 binds efficiently only to probes containing both of the GAS motifs. EMSAs were performed with nuclear extracts derived from normal preactivated PBL that were either unstimulated (lanes 1, 3, 5, and 7) or stimulated with 2 nM IL-2 (lanes 2, 4, 6, and 8) for 30 min. The inducible complex, which we have previously shown to contain Stat5 (13), is indicated.
MATERIALS AND METHODS
Cell culture.
293T cells are adenovirus-transformed human kidney epithelial cells expressing the simian virus 40 large T antigen. These cells were cultured in Dulbecco’s modified Eagle medium containing 10% fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM glutamine.
Expression and purification of recombinant Stat5 proteins.
A cDNA fragment encoding human Stat5a (18) was cloned between the SacI and XmaI sites of the baculovirus transfer vector pBacPAK8 (Clontech). The Stat5a W37A mutant was generated by site-directed mutagenesis (MORPH site-specific plasmid DNA mutagenesis kit; 5′-3′ Inc.) and confirmed by DNA sequencing. Human Stat5b (18) was cloned between the EagI and EcoRI sites of the baculovirus transfer vector pVL1392 (PharMingen). Stat5a, Stat5a W37A, and Stat5b constructs were engineered to encode a six-histidine tag at the N terminus, following the third amino acid. Stat5-expressing viruses were generated by transfecting Sf9 cells with the pBacPAK8 or pVL1392 constructs by using the BaculoGold kit. Recombinant proteins were expressed in High Five cells (Invitrogen) that were grown in suspension in Sf900 medium (Gibco BRL). Cells were infected at a multiplicity of infection of 5:1 and harvested at 66 h postinfection. The recombinant proteins were purified by Ni2+ affinity chromatography, essentially as previously described for Stat6 (27). Proteins were eluted with concentrations of imidazole between 70 and 150 mM and quantified by the Bio-Rad protein assay kit. The wild-type Stat5a and Stat5b proteins were expressed at approximately 20 mg/109 cells and purified to approximately 90% homogeneity, as judged by Coomassie blue staining. For unknown reasons, expression of the Stat5a W37A-mutated protein was lower, although similar amounts of Stat5a and Stat5a W37A proteins were added in each experiment. Unexpectedly, Stat5a, Stat5b, and Stat5a W37A were tyrosine phosphorylated, as shown by Western blotting with PY20 and by the proteins’ ability to bind DNA, suggesting that the insect cells contained an endogenous kinase capable of phosphorylating tyrosine 694 of Stat5a and tyrosine 699 of Stat5b. Coinfection with Jak1- or Jak3-encoding baculoviruses did not further increase levels of phosphorylation.
Nuclear extracts and electrophoretic mobility shift assays (EMSAs).
Nuclear extracts were prepared essentially as previously described (19). Protein concentrations were determined with the Bio-Rad protein assay kit. Binding reaction mixtures (20 μl) contained 2 to 5 μg of nuclear extracts from transfected 293T cells or various amounts of recombinant Stat5 proteins, 20,000 cpm of probe (0.1 to 0.2 ng), and 2 μg of poly(dI-dC) in 10 mM Tris HCl (pH 7.5), 10 mM HEPES, 50 mM KCl, 1.25 mM dithiothreitol, 1.1 mM EDTA, and 15% glycerol. Following incubation on ice for 30 min, DNA-protein complexes were analyzed on 6% polyacrylamide gels (59:1 acrylamide-bisacrylamide) run in Tris-borate buffer at 150 V for 2.5 h at room temperature. For DNA off-rate experiments, reaction mixtures were incubated at room temperature for 30 min and then an approximately 1,000-fold molar excess of unlabeled PRRIII oligonucleotide was added for the times indicated in Fig. 2B.
FIG. 2.
Tryptophan 37 is required for the binding of Stat5 to PRRIII as a tetramer. (A) EMSAs were performed with approximately 35 to 40 ng of rStat5a (WT, lanes 1 and 3) or rStat5a W37A (W37A, lanes 2 and 4) proteins and GASc (lanes 1 and 2) or PRRIII (lanes 3 and 4) probes. Threefold more GASc probe than PRRIII probe was used in order to facilitate our detection of the purified proteins binding to the GASc probe. The filled circle indicates the migration of the tetrameric Stat5-PRRIII complex and the open circle indicates the position of the dimeric complex. (B) The tetrameric, but not the dimeric, Stat5 complex is very stable. DNA off-rate experiments were performed by using wild-type PRRIII as a probe and the same amounts of rStat5a (lanes 1 to 6) or rStat5a W37A (lanes 7 to 12) proteins as in panel A. A 1,000-fold molar excess of unlabeled PRRIII oligonucleotide was added (lanes 2 to 6 and 8 to 12), and then samples were incubated with unlabeled PRRIII oligonucleotide for the indicated times. Lanes 1 and 7 show binding in the absence of added cold oligonucleotide. The positions of the tetramer (2X dimer) and dimer are indicated.
Plasmids and mutagenesis.
To construct the expression plasmids pCI-Stat5a and pCI-Stat5b, a SalI fragment containing the full-length Stat5a-coding region was cloned in the SalI site of pCI (Promega) and a SmaI fragment containing the full-length Stat5b cDNA from pSX-Stat5b (18) was cloned in the SmaI site of pCI. The correct orientations of both inserts were confirmed by restriction enzyme digests. PRRIII-E1B-luciferase was constructed by first inserting the PRRIII sequence (13) between the XhoI and SmaI sites of the pGL2-luciferase basic reporter plasmid (Promega Corp.). An oligonucleotide containing the minimal E1B promoter sequence (AGATCTGGGTATATAATAAGCTT) was then inserted downstream of PRRIII and between the BglII and HindIII sites immediately upstream of the luciferase gene. The mutant plasmids pCI-Stat5a W37A, pCI-Stat5b W37A, pCI-Stat5a W37A,K70A, pCI-Stat5b W37A,K70A, pCI-Stat5a K70A, and pCI-Stat5b K70A were generated by performing site-directed mutagenesis on the wild-type Stat5a and Stat5b plasmids with the kit from 5′-3′ Inc. All mutations were verified by sequence analysis. pME18S-IL-2Rβ, pME18S-γc, and pME18S-Jak3 were previously described (24, 26).
Transfections and reporter assays.
293T cells were transfected by the calcium phosphate method. In reporter assays, cells were transfected with 1 μg of the reporter plasmid, PRRIII-E1B-luciferase, 25 or 50 ng of each Stat5 expression plasmid (pCI-Stat5a and pCI-Stat5b), 2 μg of pME18S-IL-2Rβ, 500 ng of pME18S-γc, 250 ng of pME18S-Jak3, and 0.5 ng of the transfection control reporter plasmid, pRL-TKLuciferase (Promega Corp.). Twenty-four hours after transfection, cell cultures were split into two sets, and one set of cells was treated with 2 to 4 nM IL-2 for 14 to 16 h while the other set was left untreated. Dual luciferase assays were performed according to the manufacturer’s protocol (Promega Corp.). All transfection experiments were performed in triplicate, and the data are presented as the means ± standard deviations. In transfections for EMSAs, 0.5 μg of each Stat5 expression plasmid was used to enhance the signal, and stimulation with 2 nM IL-2 was performed for 30 min. Nuclear extracts and EMSAs were then performed as described above.
RESULTS
PRRIII exhibits much higher Stat5 DNA binding activity than either of the individual GAS motifs within PRRIII.
We previously demonstrated that neither GASc nor GASn motifs derived from PRRIII (Fig. 1A) can activate transcription when cloned upstream of a heterologous promoter (13). To determine if Stat5 proteins could bind to these GAS motifs, we performed EMSAs with oligonucleotides comprising GASc, GASn, or GASc+n or full-length PRRIII and nuclear extracts prepared from PBL that were preactivated with phytohemagglutinin, rested, and then either stimulated with IL-2 or left untreated (Fig. 1B). Preactivation of cells ensured that they were primed to be maximally responsive to IL-2 treatment. Consistent with the inability of these isolated GAS motifs to activate transcription, inducible Stat5 binding to GASc or GASn probes was not discernible (Fig. 1, lanes 1 to 4) indicating that neither site alone is of sufficiently high affinity to efficiently bind Stat5 proteins present in these nuclear extracts. With long exposure times, weak binding of Stat5 was detectable with the GASc probe (data not shown). However, IL-2-induced Stat5 DNA binding activity was readily detected with a probe spanning both GAS motifs (Fig. 1, lane 6), suggesting that activation of PRRIII may depend on the ability of Stat5 proteins to bind synergistically to the tandem GAS motifs. Interestingly, Stat5 binding to full-length PRRIII was greater than that observed with the GASc+n probe (Fig. 1, lane 8 versus 6), suggesting that other proteins that bind to PRRIII may additionally stabilize Stat5 binding to PRRIII.
Stat5 binds to PRRIII as a tetramer.
To further analyze the requirements for Stat5 tetramerization and thus for binding to PRRIII, we constructed putative N-terminal oligomerization mutants of Stat5, based on the crystal structure of the N domain of Stat4 (31). Tryptophan 37 (W37) is the key residue at the heart of the polar interaction interface in N-terminally oligomerized Stat4 and is important for gamma interferon-stimulated gene activation in vivo (31). Because this residue is conserved in all STAT proteins, we substituted an alanine in that location in both Stat5a and Stat5b. Additionally, glutamic acid 66 (E66) in Stat4 was shown to make direct and water-mediated contacts with W37 (31). Therefore, we sought to evaluate the importance of the correspondingly positioned lysine 70 residue in Stat5a and Stat5b. Thus, both W37 and K70 were replaced with alanine residues individually and in combination in both Stat5a and Stat5b.
To verify that Stat5 bound as a tetramer to PRRIII and that mutations of W37 could abolish tetramer formation, we generated wild-type (rStat5a) and W37A mutant Stat5a (rStat5a W37A) proteins using a baculovirus expression system. EMSAs were first performed with these purified recombinant proteins and a 32P-labeled GASc probe (Fig. 2A, lanes 1 and 2). Although little if any binding of Stat5 proteins to GASc was detected from nuclear extracts (Fig. 1, lane 2), purified Stat5 protein bound to this probe (Fig. 2A, lane 1), indicating that this site functions as a consensus GAS motif when sufficient amounts of Stat5 protein are added. Purified rStat5a W37A protein formed a dimeric complex that comigrated with and was similar in intensity to the major complex obtained with wild-type rStat5a (Fig. 2A, lane 2 versus 1); thus, as expected, the W37A mutation did not diminish the rates of Stat5a binding as a dimer. We next analyzed the complexes that these purified proteins formed with a 32P-labeled PRRIII probe containing two tandem GAS motifs (Fig. 2A, lanes 3 and 4). Binding of purified rStat5a produced a major low-mobility band (Fig. 2A, lane 3), which presumably corresponds to two Stat5 dimers bound in a tetrameric complex, as well as a weaker, faster-migrating band, which, after normalization for the fact that the PRRIII probe is longer than the GASc probe, presumably represents a single dimer bound to DNA, similar to that seen in lanes 1 and 2 of Fig. 2A. In contrast to rStat5a, the purified rStat5a W37A protein primarily formed the dimer-DNA complex with greater mobility (Fig. 2A, lane 4). Taken together, these results suggest that Stat5a binds to PRRIII predominantly as a tetramer and that the formation of this complex is mediated through protein-protein interactions involving W37 in the N domain of Stat5a.
We next investigated the relative stability of the binding of tetrameric versus dimeric complexes by comparing the lengths of time required to compete the faster and more slowly migrating complexes formed by rStat5a and rStat5a W37A (Fig. 2B). The lesser-mobility tetrameric complex was fully competed only after 60 min of incubation with a 1,000-fold molar excess of unlabeled PRRIII oligonucleotide, while the greater-mobility dimeric complex (observed with both rStat5a and rStat5a W37A proteins) was efficiently competed within 5 min. These results demonstrate that the tetrameric Stat5 complex formed on PRRIII is much more stable than the dimeric complex and that the increased stability of this complex is dependent on W37 in the N domain of Stat5.
Stat5 tetramer formation is important for transcriptional activation of PRRIII.
We next assessed the in vivo importance of Stat5 tetramer formation in IL-2-induced PRRIII activation using an IL-2R reconstitution system in 293T cells (37). 293T cells were transfected with expression vectors encoding IL-2Rβ, γc, Jak3, and either wild-type Stat5a or Stat5b or mutant versions of these proteins. Nuclear extracts were prepared from these transfections, and the relative expressions of the wild-type or mutant Stat5a and Stat5b proteins are shown in Fig. 3A. EMSAs were performed using these nuclear extracts and a 32P-labeled PRRIII probe (Fig. 3B). We observed a low level of constitutive Stat5 binding in the absence of IL-2 treatment in transfected 293T cells. This is consistent with our finding that there is a low level of constitutive tyrosine phosphorylation of overexpressed Stat5 proteins in these cells (possibly due to the high levels of activated Jak1 kinase in these cells [unpublished observations]). Nevertheless, IL-2 greatly enhanced the formation of an inducible complex when wild-type Stat5a or Stat5b was transfected either alone or in combination (Fig. 3B, lanes 2, 10, and 18); the complex formed in each case was a single, slowly migrating band indicative of tetramer formation. Interestingly, Stat5a reproducibly exhibited higher DNA binding activity to PRRIII than did Stat5b (Fig. 3B, lane 2 versus 10). As expected, the W37A mutants of both Stat5a and Stat5b exhibited only very low levels of tetrameric complex formation (Fig. 3B, lanes 4 and 12 versus 2 and 10). The K70A mutant also showed a decreased level of complex formation (Fig. 3B, lanes 6 and 14), while the simultaneous mutation of W37 and K70 virtually abrogated the binding of Stat5a and Stat5b proteins to PRRIII (Fig. 3B, lanes 8 and 16). Additionally, coexpression of the W37A-K70A doubly mutated versions of Stat5a and Stat5b resulted in no DNA binding activity (Fig. 3B, lane 20). We hypothesize that the presence of tetrameric, rather than dimeric, mutant protein complexes could be due to heterodimerization of the mutant Stat5 proteins with endogenous wild-type Stat5 proteins.
FIG. 3.
DNA binding properties of wild-type (WT) and N-terminal oligomerization mutants of Stat5 in an IL-2R reconstituted system. (A) Nuclear extracts were prepared from 293T cells that were transfected with IL-2Rβ, γc, Jak3, and expression plasmids encoding wild-type or mutant Stat5a and Stat5b proteins. Transfected cells were either not stimulated or induced with 2 nM IL-2 for 30 min prior to preparation of nuclear extracts. The relative expression of the Stat5 proteins used in the EMSAs depicted in panel B are shown. Ten micrograms of the IL-2-induced nuclear extracts used in panel A were run on an 8% Tris-glycine gel and Western blotted with a pan-Stat5 antibody (Ab) (Transduction Labs). (B) W37 and K70 of Stat5a and Stat5b are important for stable tetramer formation on PRRIII. EMSAs were performed with 5 μg of nuclear extracts as in panel A and the wild-type PRRIII probe.
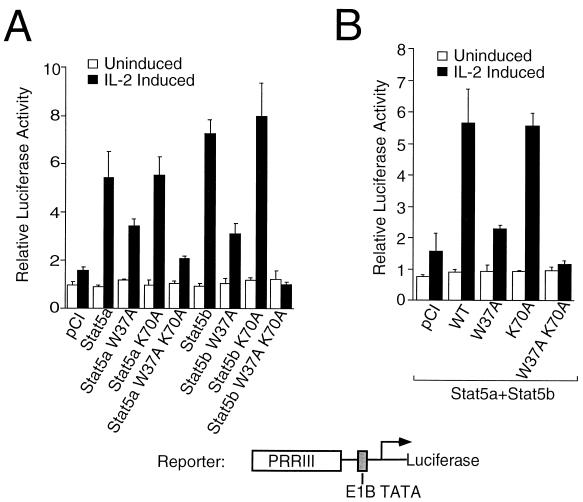
We next investigated the ability of the N-domain oligomerization mutants of Stat5 to mediate IL-2-induced transcriptional activation of PRRIII. 293T cells were transfected with IL-2R components and Jak3 as described above, along with a PRRIII-E1B-luciferase reporter construct (Fig. 4). PRRIII-E1B-luciferase exhibited a low level of IL-2 inducibility in the absence of transfected Stat5 proteins (Fig. 4A and B), consistent with the presence of low levels of endogenous Stat5 proteins in 293T cells. Overexpression of wild-type Stat5a or Stat5b potently increased the IL-2-induced activity of the reporter gene (Fig. 4A). The W37A mutants of Stat5a and Stat5b showed decreased IL-2 inducibility of PRRIII, but surprisingly, the K70A mutation did not significantly affect the functional activity of Stat5a or Stat5b. Importantly, however, the double mutation of Stat5a or Stat5b profoundly diminished IL-2-induced activation of PRRIII (Fig. 4A). Similar results were found when the W37A or W37A K70A mutants of Stat5a and Stat5b were coexpressed (Fig. 4B).
FIG. 4.
Transactivation properties of the W37 and K70 mutant forms of Stat5a and Stat5b. (A) Mutations of W37 alone and of both W37 and K70 in Stat5a and Stat5b significantly reduce their transactivation functions. 293T cells were transfected with IL-2Rβ, γc, Jak3, and wild-type or mutant Stat5a and Stat5b proteins as well as with 1 μg of a PRRIII-luciferase reporter construct and 0.5 ng of a control luciferase plasmid, pRL-TKLuciferase. Twenty-four hours after transfection, cells were split into two groups, and one-half was stimulated with 2 nM IL-2 for 11 to 14 h. The relative luciferase activity is the activity of the reporter plasmid after normalization for the activity of the control reporter plasmid. (B) Experiments were performed as for panel A, but both Stat5a and Stat5b expression plasmids were simultaneously expressed. WT, wild type.
Stat5 dimers can activate a PRRIII mutant containing high-affinity GAS motifs.
The above results demonstrate the importance of N-domain-mediated tetramer formation for Stat5 binding and consequently for IL-2-induced PRRIII activation. We therefore hypothesized that augmenting the affinity of the GAS motifs in PRRIII might eliminate the requirement for Stat5 tetramer formation for maximal transcriptional activation. We initially mutated the GASn motif into a consensus site (Fig. 5A, mutant M9) so that both GAS motifs in this mutant PRRIII could potentially bind dimers of Stat5 independently. 293T cells were then transfected with the IL-2 receptor components and Jak3, together with an M9-luciferase reporter construct. As expected, IL-2-induced activation of this reporter by wild-type Stat5 proteins was modestly higher than that obtained with the wild-type PRRIII reporter plasmid (Fig. 4B versus 5B; also data not shown). Interestingly, the Stat5 W37A mutants did not maximally activate this reporter (Fig. 5B), suggesting that N-domain-mediated tetramer formation of Stat5 proteins is required for binding to the two GAS motifs in this mutant PRRIII sequence. Consistent with this finding, in EMSAs, Stat5 protein from transfected 293T nuclear extracts bound to M9 only as a tetramer and similarly produced Stat5 W37A mutant protein bound much less efficiently to this probe (data not shown).
FIG. 5.
A mutant PRRIII that contains tandem strong GAS motifs can be activated by a tetramerization-defective mutant of Stat5. (A) The sequences of wild-type (WT) PRRIII and mutants M9 and M10 are shown. EBS, Ets binding site. (B) The Stat5 W37A protein exhibits diminished activation of the M9-luciferase reporter construct. 293T cells were reconstituted as described before, except that 1 μg of the mutant reporter M9-luciferase was transfected in these experiments. (C) Stat5a and Stat5b can bind to the mutant M10 PRRIII probe equally well as dimers and 2× dimers. EMSAs were performed with 5 or 10 ng of rStat5a (lanes 1 to 4) or rStat5b (lanes 5 to 8) proteins and wild-type (lanes 1, 2, 5, and 6) or M10 (lanes 3, 4, 7, and 8) probes. The greater-mobility (dimeric) Stat5-PRRIII complex is stably formed with the M10 probe but not with the wild-type probe. The positions of the dimer and 2× dimer Stat5 complexes are indicated. (D) rStat5a W37A protein can also bind as dimers or 2× dimers to the high-affinity M10 probe. EMSAs were performed with a labeled M10 probe and approximately 40 ng of rStat5a or rStat5a W37A proteins. (E) The Stat5 W37A proteins can activate transcription of an M10-luciferase reporter construct with similar potency to that of WT Stat5 proteins. 293T cells were reconstituted as described before, except that 1 μg of the mutant reporter, M10-luciferase, was transfected in these experiments.
Based on a DNA binding site selection analysis performed for Stat5 (28a), we prepared another mutant of PRRIII (M10) that contains additional mutations (including some outside the TTCN3GAA core GAS motif) and transforms the GASc and GASn sequences into strong binding sites (Fig. 5A). EMSAs were performed with 32P-labeled wild-type and mutant M10 PRRIII probes and purified rStat5a or rStat5b proteins (Fig. 5C). As expected, purified rStat5a (Fig. 5C, lanes 1 to 4) and rStat5b (Fig. 5C, lanes 5 to 8) efficiently bound to wild-type and M10 PRRIII probes as tetramers. Moreover, the M10 (but not wild-type) PRRIII probes also efficiently formed the faster-migrating dimeric complex (Fig. 5C, lanes 3, 4, 7, and 8), reflecting the presence in M10 of two strongly binding GAS motifs. This also explains why overall binding to the M10 probe was somewhat greater than to the wild-type probe.
We also investigated which types of complexes were formed by the rStat5a W37A protein and the M10 probe (Fig. 5D). In contrast to the results obtained with the binding of rStat5a W37A protein to wild-type PRRIII, where the occupancy of only a single GAS site could be detected, the tetramerization-deficient Stat5 protein also formed the slower complex, indicative of the simultaneous occupancy of each GAS site in M10 by a Stat5 dimer (Fig. 5D, lane 2). Corresponding to the ability of Stat5 dimers to bind to both of the GAS motifs in M10 without the need for N-domain-mediated interactions, wild-type and W37A-mutant Stat5a and Stat5b proteins were capable of activating transcription from the M10 mutant reporter construct to similar levels (Fig. 5E). As expected, presumably because of its stronger binding sites for Stat5, M10-E1B-luciferase showed higher levels of IL-2-inducible activity with endogenous or transfected Stat5 proteins than did the wild-type PRRIII-E1B-luciferase reporter construct (Fig. 4 versus 5; also data not shown). These results not only demonstrate that there is no impairment of the intrinsic transactivation potential of Stat5 W37A proteins but also underscore the ability of Stat5 proteins to efficiently extend the repertoire of their recognition sites to nonconsensus tandem binding sites by virtue of N-domain-mediated oligomerization.
DISCUSSION
In this study, we have directly demonstrated the importance of the tetramerization of Stat5 for IL-2-induced regulation of the IL-2 response element (PRRIII) in the human IL-2Rα gene. This extends the work of Meyer et al., who provided evidence that Stat5 could form oligomeric complexes on the murine IL-2Rα gene but did not directly establish the importance of N-domain-mediated tetramer formation for IL-2-induced transcriptional activation (20). Our study is based on the crystal structure of the Stat4 N domain and the description of the interface between N domains (31). We describe the first testing of the predictions from this structure concerning STAT oligomerization in the context of the transcriptional regulation of a “natural” promoter.
PRRIII is a composite element whose full functional activity requires two tandemly linked GAS motifs and a juxtaposed Ets binding site (13, 15, 29). Based on our ability to detect physiological levels of Stat5 binding only when both GAS motifs from PRRIII were present (13) (Fig. 1), we speculated that binding to these sites was dependent on synergistic interactions between two Stat5 dimers. In their study of the IL-2 response element in the murine IL-2Rα promoter, Meyer et al. demonstrated the formation of a lesser-mobility complex that corresponds to a Stat5 tetramer. Surprisingly, however, the higher-mobility, dimeric Stat5 complex was often detected as the favored form, and the lesser-mobility complex was detected even when only a single GAS motif was present in the probe (20). In contrast, we show that in the human IL-2Rα promoter, cooperative Stat5 binding uniformly results in the formation of a stable tetramer (Fig. 2B). Moreover, we demonstrate that mutation of W37 in Stat5 (which is required for N-domain-mediated tetramerization of Stat1) (31) substantially decreased tetrameric Stat5 complex formation and concomitantly decreased IL-2-induced transcriptional activation of PRRIII, thus directly linking tetramerization to IL-2 responsiveness.
In addition to W37, we studied the importance of another residue, K70. K70 in Stat5 spatially corresponds to E66 of Stat4, which makes important water-mediated and direct contacts with W37 (31). We therefore speculated that K70 might make important contacts with W37 in Stat5. Although K70 is opposite in charge to E66, both residues can form charge-stabilized hydrogen bonds which contribute to the polar hydrophilic interface described in the Stat4 structure. Thus, these two residues are functional homologues within different STATs. Interestingly, although mutation of K70 substantially diminished Stat5 DNA binding activity, the K70A mutants of Stat5a and Stat5b retained somewhat more binding activity than the W37A mutants (Fig. 3B and data not shown). It is well established that individual residues in a protein-protein interface contribute to varying extents to the overall binding energy (34); therefore, it is not surprising that two different point mutations (such as K70A and W37A) in a complex interface of presumably approximately a dozen residues (31) differ in the severity of their effects on binding.
Although the K70A mutation had a significant effect on binding (Fig. 3), it had no obvious effect on transcriptional activation in the 293T overexpression-reconstitution system (Fig. 4). This presumably indicates that sufficient tetramer formation can occur in these cells to allow this effect. This may result in part from dimerization with endogenous wild-type Stat5 in 293T cells and could reflect a greater level of homo- or heterodimerization of Stat5 K70 mutants in vivo than could be detected under the in vitro experimental conditions of EMSAs. The fact that the mutation of both W37 and K70 most dramatically diminished both Stat5 DNA binding and transactivation strongly suggests that both residues are required for efficient tetramerization of Stat5.
This study demonstrates that dimeric Stat5 is not competent to be the transcriptionally active molecule for all Stat5-responsive promoters and that further oligomerization (e.g., tetramerization in the case of the IL-2Rα promoter) is absolutely required for the activation of certain genes. This pivotal role of higher-order aggregates in the activation of promoters with multiple, weak STAT binding sites is demonstrated in Fig. 5. This experiment shows that attenuated protein-protein interactions can be compensated for by increased protein-DNA binding strength. Conversely, N-domain-mediated oligomerization enables Stat5 to extend its range of target sites considerably by virtue of additionally utilizing nonconsensus sites for binding. Thus, dimeric, but tetramerization-deficient, STATs have a more limited set of binding sites than do tetramerization-competent STATs.
At present it is unclear how the ability of STATs to form higher-order oligomers is linked to promoter selectivity. Xu et al. (35) have shown differential binding site occupancy by oligomerized STATs and suggest that this phenomenon reflects binding site preferences. In this regard, we were unable to achieve stable binding of dimeric Stat5 to the M9 mutant PRRIII sequence that converts the GASn to a consensus GAS by a point mutation to change TTCTGATAA to TTCTGAGAA. Instead, to achieve high-affinity binding, we made additional changes in the nucleotides in the central GASn core and in the sequences immediately flanking both the GAS motifs (mutant M10) in accordance with a binding site selection analysis performed with recombinant Stat5 proteins (28a). This result suggests that the affinity of binding of Stat5 proteins to a given GAS element can be influenced not only by the central three nucleotides within the core GASc motif but also by the surrounding nucleotides.
In conclusion, we have demonstrated that tetramerization of Stat5 is essential for the potent transcriptional regulation of PRRIII. This finding is presumably relevant for other Stat5-dependent genes whose promoters contain tandem GAS motifs. Indeed, based on the absolute conservation of W37 in all known STAT proteins (31), it seems likely that N-domain-mediated tetramerization is a mechanism commonly used by these proteins. Our data demonstrate that Stat5 W37A can bind as a dimer and mediate transcriptional activation of tandem high-affinity sites (e.g., the PRRIII M10 mutant [Fig. 5E]), but it is incapable of potently activating the wild-type PRRIII sequence. The ability of wild-type STAT proteins to form tetramers enables these proteins to achieve stable binding to tandem weak sites, such as those found in PRRIII, thus extending the repertoire of putative binding sites. This underscores the role of tetramerization in selective activation of tandem GAS motifs to mediate gene-specific activation. Finally, the fact that STAT proteins have been reported to interact with other transcription factors (reviewed in references 9 and 16) whose binding sites are juxtaposed may be another mechanism that allows a gene to achieve maximal transcriptional activation in response to specific external stimuli.
ACKNOWLEDGMENTS
We thank Jian-Xin Lin and Ming-hua Zhu for critical comments.
REFERENCES
- 1.Beadling C, Guschin D, Witthuhn B A, Ziemiecki A, Ihle J N, Kerr I M, Cantrell D A. Activation of JAK kinases and STAT proteins by interleukin-2 and interferon α, but not the T cell antigen receptor, in human T lymphocytes. EMBO J. 1994;13:5606–5615. doi: 10.1002/j.1460-2075.1994.tb06898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boussiotis V A, Barber D L, Nakarai T, Freeman G J, Gribben J G, Bernstein G M, d’Andrea A D, Ritz J, Nadler L M. Prevention of T cell anergy by signaling through the γc chain of the IL-2 receptor. Science. 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 3.Darnell J E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 4.Decker T, Kovarik P, Meinke A. GAS elements: a few nucleotides with a major impact on cytokine-induced gene expression. J Interferon Cytokine Res. 1997;17:121–134. doi: 10.1089/jir.1997.17.121. [DOI] [PubMed] [Google Scholar]
- 5.Fujii H, Nakagawa Y, Schindler U, Kawahara A, Mori H, Gouilleux F, Groner B, Ihle J N, Minami Y, Miyazaki T, Taniguchi T. Activation of Stat5 by interleukin 2 requires a carboxyl-terminal region of the interleukin 2 receptor β chain but is not essential for the proliferative signal transmission. Proc Natl Acad Sci USA. 1995;92:5482–5486. doi: 10.1073/pnas.92.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaffen S L, Lai S Y, Xu W, Gouilleux F, Groner B, Goldsmith M A, Greene W C. Signaling through the interleukin 2 receptor β chain activates a STAT-5-like DNA-binding activity. Proc Natl Acad Sci USA. 1995;92:7192–7196. doi: 10.1073/pnas.92.16.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilmour K, Pine R, Reich N C. Interleukin 2 activates STAT5 transcription factor (mammary gland factor) and specific gene expression in T lymphocytes. Proc Natl Acad Sci USA. 1995;92:10772–10776. doi: 10.1073/pnas.92.23.10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horvath C M, Wen Z, Darnell J E., Jr A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 1995;9:984–994. doi: 10.1101/gad.9.8.984. [DOI] [PubMed] [Google Scholar]
- 9.Horvath C M, Darnell J E., Jr The state of the STATs: recent developments in the study of signal transduction to the nucleus. Curr Opin Cell Biol. 1997;9:233–239. doi: 10.1016/s0955-0674(97)80067-1. [DOI] [PubMed] [Google Scholar]
- 10.Hou J, Schindler U, Henzel W J, Wong S C, McKnight S L. Identification and purification of human Stat proteins activated in response to interleukin-2. Immunity. 1995;2:321–329. doi: 10.1016/1074-7613(95)90140-x. [DOI] [PubMed] [Google Scholar]
- 11.Ihle J N. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 12.Imada K, Bloom E T, Nakajima H, Horvath-Arcidiacono J A, Udy G B, Davey H, Leonard W J. Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998;188:2067–2074. doi: 10.1084/jem.188.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John S, Robbins C M, Leonard W J. An IL-2 response element in the human IL-2 receptor α chain promoter is a composite element that binds Stat5, Elf-1, HMG-I(Y) and a GATA family protein. EMBO J. 1996;15:5627–5635. [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston J A, Kawamura M, Kirken R A, Chen Y Q, Blake T B, Shibuya K, Ortaldo J R, McVicar D W, O’Shea J J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 15.Lécine P, Algarté M, Rameil P, Beadling C, Bucher P, Nabholz M, Imbert J. Elf-1 and Stat5 bind to a critical element in a new enhancer of the human interleukin-2 receptor α gene. Mol Cell Biol. 1996;16:6829–6840. doi: 10.1128/mcb.16.12.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard W J, O’Shea J J. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 17.Lin J-X, Leonard W J. Signaling from the IL-2 receptor to the nucleus. Cytokine Growth Factor Rev. 1997;8:313–332. doi: 10.1016/s1359-6101(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 18.Lin J X, Mietz J, Modi W S, John S, Leonard W J. Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J Biol Chem. 1996;271:10738–10744. [PubMed] [Google Scholar]
- 19.Lin J-X, Migone T-S, Tsang M, Friedmann M, Weatherbee J A, Zhou L, Yamauchi A, Bloom E T, Mietz J, John S, Leonard W J. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 20.Meyer W K, Reichenbach P, Schindler U, Soldaini E, Nabholz M. Interaction of STAT5 dimers on two low affinity binding sites mediates interleukin 2 (IL-2) stimulation of IL-2 receptor alpha gene transcription. J Biol Chem. 1997;272:31821–31828. doi: 10.1074/jbc.272.50.31821. [DOI] [PubMed] [Google Scholar]
- 21.Mikita T, Campbell D, Wu P, Williamson K, Schindler U. Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol Cell Biol. 1996;16:5811–5820. doi: 10.1128/mcb.16.10.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu Z-J, Oishi I, Silvennoinen O, Witthuhn B A, Ihle J N, Taniguchi T. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima H, Liu X, Wynshaw-Boris A, Rosenthal L A, Imada K, Finbloom D S, Hennighausen L, Leonard W J. An indirect effect of Stat5a in IL-2-induced proliferation: a critical role for Stat5a in IL-2-mediated IL-2 receptor α chain induction. Immunity. 1997;7:691–701. doi: 10.1016/s1074-7613(00)80389-1. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura Y, Russell S M, Mess S A, Friedmann M, Erdos M, Francois C, Jacques Y, Adelstein S, Leonard W J. Heterodimerization of the IL-2 receptor β- and γ-chain cytoplasmic domains is required for signalling. Nature. 1994;396:330–333. doi: 10.1038/369330a0. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen M, Svejgaard A, Skov S, Odum N. Interleukin-2 induces tyrosine phosphorylation and nuclear translocation of Stat3 in human T lymphocytes. Eur J Immunol. 1994;24:3082–3086. doi: 10.1002/eji.1830241225. [DOI] [PubMed] [Google Scholar]
- 26.Russell S M, Johnston J A, Noguchi M, Kawamura M, Bacon C M, Friedmann M, Berg M, McVicar D W, Witthuhn B A, Silvennoinen O, Goldman A S, Schmalstieg F C, Ihle J N, O’Shea J J, Leonard W J. Interaction of IL-2Rβ and γc chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 27.Schindler U, Wu P, Rothe M, Brasseur M, McKnight S L. Components of a Stat recognition code: evidence for two layers of molecular selectivity. Immunity. 1995;2:689–697. doi: 10.1016/1074-7613(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 28.Seidel H M, Lawrence M H, Lamb P, Darnell J E, Jr, Stein R B, Rosen J. Spacing of palindromic half sites as a determinant of selective STAT (signal transducers and activators of transcription) DNA binding and transcriptional activity. Proc Natl Acad Sci USA. 1995;92:3041–3045. doi: 10.1073/pnas.92.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Soldaini, E. Unpublished data.
- 29.Sperisen P, Wang S M, Soldaini E, Pla M, Rusterholz C, Bucher P, Corthesy P, Reichenbach P, Nabholz M. Mouse interleukin-2 receptor α gene expression. Interleukin-1 and interleukin-2 control transcription via distinct cis-acting elements. J Biol Chem. 1995;270:10743–10753. doi: 10.1074/jbc.270.18.10743. [DOI] [PubMed] [Google Scholar]
- 30.Vinkemeier U, Cohen S L, Moarefi I, Chait B T, Kuriyan J, Darnell J E., Jr DNA binding of in vitro activated Stat1α, Stat1β and truncated Stat1: interaction between NH2-terminal domains stabilizes binding of two dimers to tandem DNA sites. EMBO J. 1996;15:5616–5626. [PMC free article] [PubMed] [Google Scholar]
- 31.Vinkemeier U, Moarefi I, Darnell J E, Jr, Kuriyan J. Structure of the amino-terminal protein interaction domain of Stat4. Science. 1998;279:1048–1052. doi: 10.1126/science.279.5353.1048. [DOI] [PubMed] [Google Scholar]
- 32.Wakao H, Harada N, Kitamura T, Mui A L, Miyajima A. Interleukin 2 and erythropoietin activate STAT5/MGF via distinct pathways. EMBO J. 1995;14:2527–2535. doi: 10.1002/j.1460-2075.1995.tb07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witthuhn B A, Silvennoinen O, Miura O, Lai K S, Cwik C, Liu E T, Ihle J N. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 34.Xu D, Lin S L, Nussinov R. Protein binding versus protein folding: the role of hydrophilic bridges in protein associations. J Mol Biol. 1997;265:68–84. doi: 10.1006/jmbi.1996.0712. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Sun Y-L, Hoey T. Cooperative DNA binding and sequence-selective recognition conferred by the STAT amino-terminal domain. Science. 1996;273:794–797. doi: 10.1126/science.273.5276.794. [DOI] [PubMed] [Google Scholar]
- 36.Yan R, Small S, Desplan C, Dearolf C R, Darnell J E., Jr Identification of a Stat gene that functions in Drosophila development. Cell. 1996;84:421–430. doi: 10.1016/s0092-8674(00)81287-8. [DOI] [PubMed] [Google Scholar]
- 37.Zhu M-H, Berry J A, Russell S M, Leonard W J. Delineation of the regions of interleukin-2 (IL-2) receptor β chain important for association of Jak1 and Jak3. J Biol Chem. 1998;273:10719–10725. doi: 10.1074/jbc.273.17.10719. [DOI] [PubMed] [Google Scholar]