Abstract
Estrogenic responses are now known to be mediated by two forms of estrogen receptors (ER), ERα and ERβ, that can function as homodimers or heterodimers. As homodimers the two have been recently shown to exhibit distinct transcriptional responses to estradiol (E2), antiestrogens, and coactivators, suggesting that the ER complexes are not functionally equivalent. However, because the three possible configurations of ER complexes all recognize the same estrogen response element, it has not been possible to evaluate the transcriptional properties of the ER heterodimer complex by transfection assays. Using ER subunits with modified DNA recognition specificity, we were able to measure the transcriptional properties of ERα-ERβ heterodimers in transfected cells without interference from the two ER homodimer complexes. We first demonstrated that the individual activation function 1 (AF-1) domains act in a dominant manner within the ERα-ERβ heterodimer: the mixed agonist-antagonist 4-hydroxytamoxifen acts as an agonist in a promoter- and cell context-dependent manner via the ERα AF-1, while activation of the complex by the mitogen-activated protein kinase (MAPK) pathway requires only the ERα- or ERβ-responsive MAPK site. Using ligand-binding and AF-2-defective mutants, we further demonstrated that while the ERα-ERβ heterodimer can be activated when only one E2-binding competent partner is present per dimer, two functional AF-2 domains are required for transcriptional activity. Taken together, the results of this study of a retinoid X receptor-independent heterodimer complex, the first such study, provide evidence of different stoichiometric requirements for AF-1 and -2 activity and demonstrate that AF-1 receptor-specific properties are maintained within the ERα-ERβ heterodimer.
The estrogen signal is now known to be mediated by two receptors referred to as estrogen receptor α (ERα) and ERβ (13, 14, 19, 26). Both receptors are members of the superfamily of nuclear receptors and have high degrees of identity in their ligand-binding domains (LBDs) and DNA-binding domains (DBDs). ERα and ERβ have similar affinities for estradiol (E2), recognize a consensus estrogen response element (ERE) (19, 26, 35), and are expressed in distinct and overlapping tissues (6) as well as during human breast tumorigenesis (21). Transcriptional regulation by ERα and ERβ involves two activation functions (AFs) that reside on opposite ends of each of the receptors. AF-1 is located in the distinct amino terminus of each receptor, whereas AF-2 is present at the carboxy-terminal end of the well-conserved LBD. Although both AF-1 and AF-2 are required to achieve maximal transcriptional activity, only AF-2 activity is entirely dependent on ligand binding. It has recently been demonstrated that ERα and ERβ have similar properties with respect to their abilities to interact with steroid coactivator 1 (SRC-1), to respond to the mitogen-activated protein kinase (MAPK) pathway, and to be inhibited by antiestrogens (34–36). However, while ERα and ERβ respond to antiestrogens similarly in classical transactivation assays, their responses to antiestrogens have been shown to differ in two different ways. First, 4-hydroxytamoxifen (OHT) acts as an agonist to ERα when assayed on a basal promoter linked to an ERE, but this effect is not observed with ERβ (35, 38). Second, ERα and ERβ signal in opposite directions when assayed with an AP1 element. E2 activates transcription with ERα but inhibits transcription with ERβ (29). In addition, antiestrogens were shown to be potent transcriptional activators of ERβ at an AP1 site. Taken together, these results show that the current characterization of ERβ’s physiological and transcriptional properties is leading to a reevaluation of estrogen and antiestrogen signaling (12).
Nuclear receptors can adopt different configurations when binding their cognate DNA response elements. Steroid receptors usually bind to their response elements as homodimers (2). Some orphan receptors are able to bind DNA as monomers and/or as homodimers. In contrast to steroid receptors, orphan receptor homodimers recognize both palindromic and direct repeat elements (23). Finally, a large number of nuclear receptors, including retinoic acid receptor (RAR), vitamin D3, thyroid receptor (T3R), and peroxisome proliferator-activated receptor (PPAR), form heterodimers with the retinoid X receptor (RXR) (reviewed in reference 23). Two classes of RXR heterodimers have been described: nonpermissive heterodimers, such as RAR-RXR and T3R-RXR, in which RXR acts as a silent partner, and permissive heterodimers, such as PPAR-RXR, that allow RXR activation by natural or synthetic ligands (10, 20). However, under specific conditions, the RXR-RAR heterodimer has been activated by an RXR-specific ligand in the absence of an RAR ligand (31). Intriguingly, the ligand-dependent dissociation of corepressors and subsequent recruitment of coactivators to this complex are mediated by the unliganded RAR subunit of the heterodimer (31). When dimerized with a permissive partner, liganded RXR can contribute to heterodimeric transcriptional activity by acting in synergy with another liganded receptor (17, 18, 32). These observations reveal a complex functional interdependence between partners in RXR heterodimers. This is further evidenced by the recent observation that the association of adjacent AF-2 domains in the RXR-RAR heterodimer may prevent coactivators from binding to the complex until the RAR ligand causes a conformational change in the receptor, releasing the RXR AF-2 domain (39).
It was recently shown that ERα and ERβ could form a heterodimer complex both in vitro and in vivo (7, 28, 30). In contrast to that of RXR heterodimers, the analysis of the transcriptional activity of the ERα-ERβ heterodimer has proved difficult to achieve since there are no specific ligands with which to measure the contributions of each partner in vivo. While it is possible to cotransfect cells under conditions which appear to favor heterodimer formation (7), the proportion of heterodimers contained in these cells and the contribution of residual ERα and ERβ homodimers remain largely undetermined. To address this problem, we have designed a system to measure exclusively the activity of ERα-ERβ heterodimers in transfected cells. By altering the DNA-binding specificity of one ER partner and forcing it to interact with a wild-type ER moiety on a hybrid response element, it is possible to monitor the transcriptional activity of the heterodimer and compare its characteristics with those of both ER homodimers. Our analyses revealed that both partners contribute in an additive fashion to the activity of the dimeric unit. Our results also indicate that the receptor-specific activities of the AF-1 domain from each partner are maintained within the heterodimeric complex and appear to function independently. Furthermore, examination of AF-2 activity indicates that the ER heterodimers, like the RXR heterodimers, adopt a conformation where the AF-2 domain of one dimeric partner will influence the activity of the other. However, both AF-2 domains are required for heterodimer activity. Taken together, our results provide the first insight into the mechanisms of action of AF-1 and AF-2 in the ER heterodimer complex.
MATERIALS AND METHODS
Plasmids and reagents.
TKLuc, vitellogeninA2-ERE-TKLuc (vERE1TKLuc), pS2Luc, pS2ΔERELuc, pCMXmERβ, and CMXβgal have been previously described (35). Although all experiments were conducted with the shortest form of mouse ERβ, originally cloned by our laboratory (35), the amino acid numbering utilized throughout this paper is based on the longest form of mouse ERβ, currently described with a total length of 549 amino acids (GenBank accession no. AF067422). We have not detected any differences in the ways the short and long forms of ERβ respond to OHT or Ras (data not shown). GRE3TKLuc was constructed by inserting three copies of the consensus glucocorticoid response element (GRE) (2) into TKLuc. The hybrid element reporter plasmid E/GRE2TKLuc (see Fig. 2B for sequence) used in this study was constructed similarly. To replace the thymidine kinase promoter of E/GRE2TKLuc and yield E/GRE2ΔpS2Luc, the ΔpS2 promoter, which contains the inactivated ERE, was PCR amplified from pGL3ΔpS2 (35) and ligated into BamHI/XhoI-digested E/GRE2TKLuc. Human ERα, generously provided by Pierre Chambon (Institut National de la Santé et de la Recherche Médicale, Illkirch, France), was cloned into the EcoRI site of pCMX (37). The GRE-specific mutant of ERα, HE82 (22), was a gift from Sylvie Mader (Université de Montréal, Montréal, Québec, Canada). A GRE-specific mutant of ERβ (22) was constructed by replacing Glu186, Gly187, and Ala190 in the DBD with glycine, serine, and valine, respectively, by PCR mutagenesis with the ExSite kit from Stratagene (La Jolla, Calif.). All other mutants used in this study were constructed in a similar fashion. Wherever possible, the DNA cassettes containing mutated sequences were subcloned back into the original expression vector to rule out the presence of unwanted mutations which may have occurred during the amplification procedure. All mutations were confirmed by sequencing with the T7 sequencing kit from Pharmacia (Piscataway, N.J.). The H-RasV12 expression plasmid was a generous gift from Morag Park (McGill University, Montréal, Québec, Canada). Full-length SRC-1 was a gift from Joe Torchia, University of Western Ontario, London, Ontario, Canada. E2 was obtained from Sigma Chemical Co. (St. Louis, Mo.). [2,4,6,7-3H]-17β-E2 was supplied by Amersham (Arlington Heights, Ill.). EM-652 was synthesized in the medicinal chemistry division of the Laboratory of Molecular Endocrinology, CHUL Research Center, Québec, Québec, Canada. OHT was kindly provided by D. Salin-Drouin, Besins-Iscovesco, Paris, France. Glutathione S-transferase–Sepharose was obtained from Pharmacia.
FIG. 2.
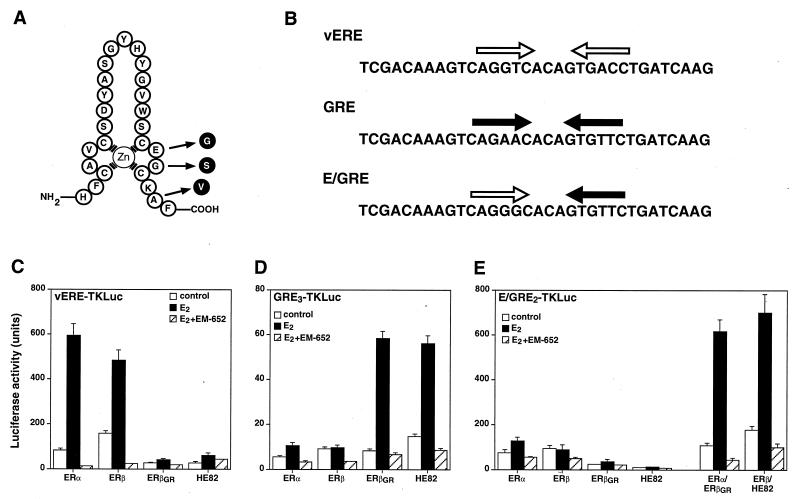
Altered response element specificity of ER DNA-binding mutants. (A) Amino acid sequence of the first zinc finger module of the mouse ERβ DBD. Arrows indicate the positions of the three amino acids that were changed to create the mutant ERβGR, which has the ability to recognize the half-site sequence AGAACA but can no longer bind to the half-site core motif AGGTCA. (B) Sequence of hormone response elements used in this study. White and black arrows illustrate consensus ERE and GRE half-sites, respectively. (C) Cos-1 cells were cotransfected with the vERE1TKLuc reporter construct and either the wild-type ER (ERα and ERβ) or the GRE-specific ER (ERβGR and HE82) expression plasmids. Cells were treated with a control (0.1% ethanol) or 10 nM E2 in the absence or presence of 100 nM of the pure antiestrogen EM-652. (D) Transfection conditions are identical to those for panel C except that the cells were cotransfected with the GRE3TKLuc reporter construct. (E) Cos-1 cells were cotransfected with E/GRE2TKLuc and wild-type or GRE-specific ERs separately or in combination as indicated.
Cell culture and transfection.
All mammalian cell lines were obtained from the American Type Culture Collection. Cos-1, 293T, and HeLa cells were maintained in Dulbecco’s minimal essential medium containing penicillin (25 U/ml), streptomycin (25 U/ml), and 10% fetal calf serum in a humidified atmosphere at 37°C and 5% CO2. Twenty-four hours prior to transfection, the growth medium was changed to phenol red-free Dulbecco’s minimal essential medium containing antibiotics and 10% charcoal dextran-treated fetal calf serum. Cells were seeded in 12-well plates and transfected by the calcium phosphate-DNA precipitation method (11). Typically, 1 to 2 μg of reporter plasmid, 0.5 μg of CMXβgal, 25 to 50 ng of receptor expression vector, and pBluescript KSII (used as carrier DNA) comprised a total of 5 μg per well. After 8 h, the cells were washed and treated with either 10 nM E2 or 100 nM antiestrogen for 16 h. For luciferase assay, the cells were lysed in potassium phosphate buffer containing 1% Triton X-100, and light emission was detected with a luminometer after the addition of luciferin. Values are expressed as arbitrary light units normalized to the β-galactosidase activity of each sample. All results presented in this study are calculated as the means ± standard errors of the means of at least three different experiments conducted in duplicate.
EMSA.
293T cells were seeded in six-well plates and transfected as described above with 5 μg of expression vector for ERα, expression vector for ERβ, or both. After 24 h, the cells were washed in phosphate-buffered saline and lysed in a buffer containing 20 mM HEPES (pH 7.8), 0.5 M KCl, 20% glycerol, 2 mM dithiothreitol, 0.5 mM EDTA, 0.5 mM EGTA, and protease inhibitors. Ten micrograms of extract was used in each binding reaction and electromobility shift assays (EMSA) were performed as previously described (11) in the presence of 10 nM E2. The antibodies raised against ERα and ERβ were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.).
E2 binding studies.
Estrogen receptors were produced with rabbit reticulocyte lysates (Promega, Madison, Wis.), diluted 30-fold in TEG buffer (10 mM Tris [pH 7.5], 1.5 mM EDTA, 10% glycerol, protease inhibitors), and incubated overnight at 4°C in 5 nM [2,4,6,7-3H]-17β-E2 in a total volume of 150 μl. Unbound steroids were removed with dextran-coated charcoal, and counts per minute were determined by liquid scintillation counting.
RESULTS
Monitoring the transcriptional activity of the ERα-ERβ heterodimeric complex.
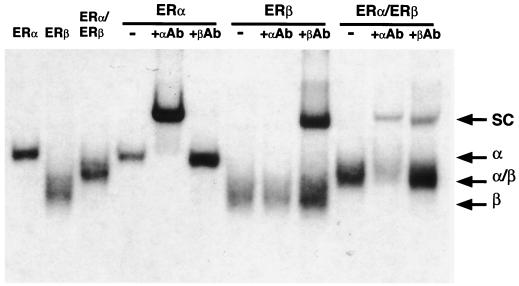
It has been shown recently that ERα and ERβ heterodimerize efficiently when cotranslated in vitro or coexpressed in transfected cells (7, 28, 30). Data presented in Fig. 1 confirm these results and demonstrate that when the human ERα and mouse ERβ used in this study are coexpressed in vivo, ERα and ERβ preferentially configure as heterodimers to bind DNA. Since both ER isoforms bind to the consensus ERE, interpretation of the transcriptional activities of the ERα-ERβ complexes in cotransfection assays is difficult. To avoid this problem, we devised a strategy that takes advantage of the previous observation that the DNA-binding specificity of the ER can be made identical to that of the glucocorticoid receptor by the mutagenesis of three amino acid residues located at the base of the first zinc finger module (22). The altered ERβ is cotransfected with wild-type ERα (or vice versa) and a reporter plasmid containing a hybrid ERE-GRE that allows transcription to occur exclusively in the presence of the ERα-ERβ modified heterodimers. Thus, a mutant ERβ modeled after the ERα mutant HE82 (22) was constructed in which the DNA-binding specificity was changed from that of an ERE (AGGTCA) to that of a GRE (AGAACA). The ERβ mutant E186G/G187S/A190V, referred to as ERβGR (Fig. 2A), displays a complete change in response element specificity, as was observed with the ERα mutant HE82 bearing the same amino acid changes. In the presence of a luciferase reporter gene linked to one copy of the vERE, both ERα and ERβ efficiently induced luciferase activity in the presence of 10 nM E2, whereas receptors containing altered DBDs (ERβGR and HE82) had no activity on this reporter (Fig. 2C). Conversely, both ERβGR and HE82 had considerable transcriptional activity in the presence of E2 when cotransfected with a reporter gene under the control of three copies of a GRE (Fig. 2D). As expected, neither of the wild-type receptors had any transcriptional activity in the presence of this reporter construct. Furthermore, the pure antiestrogen EM-652 (34) was able to inhibit the response to E2 under all conditions tested (Fig. 2C and D). These data demonstrate that the mutations present in ERβGR are sufficient to completely alter the DNA-binding activity of ERβ from ERE specific to GRE specific.
FIG. 1.
ERα and ERβ form heterodimer complexes in vivo. 293T cells were transiently transfected with ER expression vectors as indicated for 24 h, and whole-cell extracts were prepared. A 10-μg sample of each extract was subjected to EMSA in the presence of 50,000 cpm of 32P-labeled ERE. The presence of each receptor in the heterodimeric complexes was identified by incubating the binding reaction mixtures in the presence of ERα- or ERβ-specific antibodies (αAb and βAb), leading to supershifted complexes (SC). Entire binding reaction products were loaded onto a 5% polyacrylamide gel and electrophoresed for 2 to 3 h at 150 V. Dried gels were exposed overnight at −85°C. The positions of the homo- and heterodimeric complexes (α, β, and α/β) are indicated.
To determine whether coexpression of wild-type receptors with their mutant counterparts containing modified DBDs would result in transcriptional activity in the presence of the hybrid element, transfections were conducted to test which response element would be best suited to measure the activity of the heterodimer. All of the hybrid response elements tested contained a variation of an ERE half-site and a GRE half-site separated by three base pairs. Analysis of hybrid elements (ERE-GRE) ranging from those containing ideal consensus sequences to those with severely mutated half-sites allowed us to determine that the best ERE-GRE consisted of a half-site that slightly deviated from the consensus ERE (AGGGCA instead of AGGTCA) paired with a consensus GRE half-site (Fig. 2B and data not shown). Indeed, transfection of this particular reporter construct in the presence of ERα and ERβGR resulted in significant induction by E2 (Fig. 2E). Similar levels of activity were observed when ERβ and HE82 were cotransfected. Again, EM-652 completely abrogated transcriptional activity (Fig. 2E), as did ICI 182,780 and OHT (data not shown). Significantly, neither receptor displayed any transcriptional activity when transfected alone. As shown in Fig. 2E, neither the wild-type nor the GRE-specific ERs were able to significantly stimulate transcription of the reporter gene linked to the ERE-GRE when transfected individually, indicating that the E2-dependent activity observed in the presence of ERα-ERβGR or ERβ-HE82 was due solely to the transactivation by ERα-ERβ heterodimers.
Transcriptional properties of the ERα-ERβ heterodimer AF-1 domain.
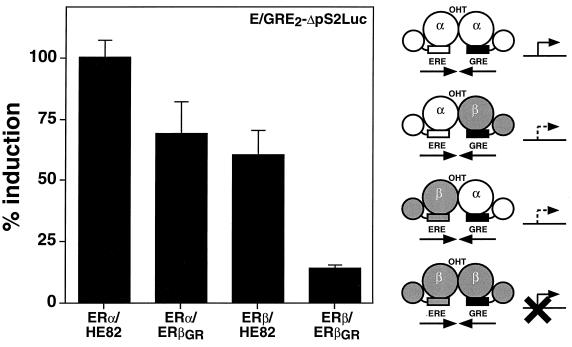
The establishment of a system that can reliably monitor the measurement of ERα-ERβ heterodimer activity in cells allowed us to define the transcriptional properties of the heterodimeric complex and compare its properties to that of both ER homodimers. We first analyzed the characteristics of the amino-terminal regions that contain distinct AF-1 domains. It had been previously established that OHT acts as a partial agonist on ERα but not on ERβ in an AF-1-dependent manner (3, 35, 38). The activity of ERα-ERβ AF-1 was evaluated by determining if OHT could have any agonistic activity on the heterodimer complex. For this assay, we studied a reporter construct (E/GRE2ΔpS2Luc) that contains two copies of the hybrid element preceding the ΔpS2 promoter (4) in which the natural ERE has been inactivated by a point mutation. This reporter gene could be efficiently activated when HeLa cells were cotransfected with ERα and HE82 in the presence of OHT (Fig. 3). As observed previously with the wild-type receptor, the activity of the ERβ homodimer (ERβ-ERβGR) cotransfected with E/GRE2ΔpS2Luc was virtually unaffected by OHT. However, both configurations of the ERα-ERβ heterodimer (namely, ERα-ERβGR and ERβ-HE82) were activated to approximately 50% of the level observed with ERα-HE82 in the presence of OHT (Fig. 3). As was observed in previous experiments, all dimers were activated by E2 to similar degrees and none of the receptors could be activated when transfected alone in the presence of E/GRE2ΔpS2Luc (data not shown).
FIG. 3.
The ERα-ERβ heterodimer is activated by the mixed agonist-antagonist OHT. HeLa cells were cotransfected with the reporter construct E/GRE2ΔpS2Luc and ERα or ERβ expression vectors as indicated. The cells were treated with 100 nM OHT. Results are expressed as the percentage of the ERα-HE82 homodimer response for OHT-dependent activation. A schematic representation of the effect of OHT on the transcriptional activity of each heterodimer complex is displayed on the right of the graph. ERα and ERβ are represented as white and shaded models, respectively. Modified DBDs are depicted as black boxes.
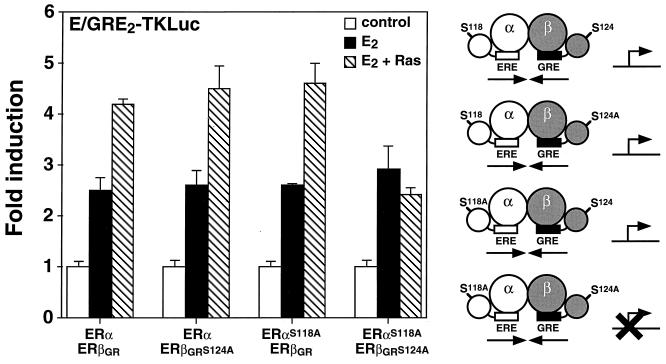
We next wanted to determine whether the ERα-ERβ heterodimer would be sensitive to the action of the MAPK pathway. As shown in Fig. 4, the transcriptional activity of the heterodimer was enhanced when the heterodimer was cotransfected in the presence of E2 with H-RasV12, a dominant active form of H-Ras, and the hybrid E/GRE2TKLuc reporter in Cos-1 cells. The presence of Ser118 has been shown to be necessary for maximal activity of AF-1 in human ERα and for mediating the effect of the MAPK pathway on the transcriptional activity of the ER (1, 5, 16). In addition, we have previously shown that Ser124 (formerly Ser60) of murine ERβ is necessary for Ras activation in the presence of E2 (35). In an attempt to investigate the role of these serine residues within the context of the heterodimer, we mutated Ser118 and Ser124 to alanine in human ERα and mouse ERβGR, respectively. Interestingly, mutation of either Ser118 in ERα or Ser124 in ERβGR did not affect the ability of Ras to activate the ERα-ERβ heterodimer (Fig. 4). In contrast, a heterodimer complex in which mutations had inactivated both AF-1 domains was unable to respond to Ras in the presence of E2 (Fig. 4). Furthermore, both serine mutants were tested as homodimers and found to be nonresponsive to transfected Ras (data not shown). These results indicate that the ER heterodimer can be activated by Ras in a manner similar to that of the ER homodimers and that a single responsive MAPK phosphorylation site within the ER heterodimer complex is necessary for this activation to occur. Taken together, the analyses of two properties inherent to the AF-1 domain of ERs—activation by Ras and OHT agonism—suggest that a single AF-1 domain is required to confer signal-specific responsiveness to the heterodimer.
FIG. 4.
Enhancement of the transcriptional activity of the ERα-ERβ heterodimer by cotransfection of H-RasV12. Cos-1 cells were transfected with 50 ng each of ERα and ERβGR or the serine-to-alanine mutants as indicated. The cells were treated with a control (0.1% ethanol) or 10 nM E2 or also cotransfected with 100 ng of a dominant active form of Ras, H-RasV12, in the presence of E2. Results are expressed as the response over basal levels in the absence of a ligand. A schematic representation of the effect of Ras on the transcriptional activity of each heterodimer complex is displayed on the right of the graph. Symbols are the same as in Fig. 3.
Transcriptional activity of the ERα-ERβ heterodimer LBD.
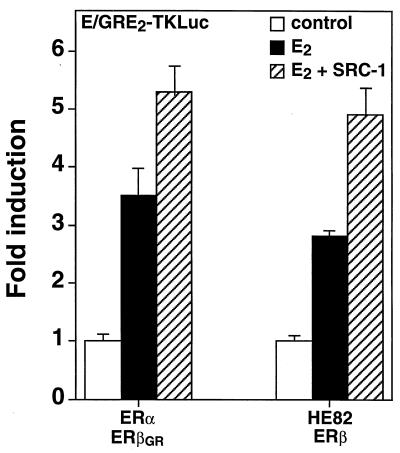
The results obtained from the analysis of AF-1 heterodimer function suggest that neither of the dimer partners was predominant over the other and that the functions of the partners within a dimer might actually be partially independent from one another. We wanted to determine if the AF-2 functions of the ERα-ERβ heterodimer would also involve such independent activation from both ER partners. As SRC-1 has been previously shown to interact with ERα-ERβ heterodimers bound to DNA (7), we first tested whether the ER heterodimeric complex would respond to SRC-1 in vivo. Cos-1 cells were transfected with ERα, ERβGR, and the E/GRE2-TKLuc reporter in the presence or absence of an expression vector encoding full-length SRC-1. As depicted in Fig. 5, the transcriptional activity of the ERα-ERβGR heterodimer could be efficiently stimulated by SRC-1 in the presence of 10 nM E2. Similarly, a heterodimer which formed between HE82 and ERβ was also stimulated by SRC-1 (Fig. 5). These results indicate that the coactivator-interacting surface of the ERα-ERβ heterodimer closely resembles that of the native ERα and ERβ homodimers.
FIG. 5.
Induction of E2-dependent transcriptional activity of the ERα-ERβ heterodimer by SRC-1. Cos-1 cells were cotransfected with E/GRE2TKLuc and 50 ng of either ERα-ERβGR or HE82-ERβ and 100 ng of SRC-1 expression vector. The cells were treated with a control (0.1% ethanol) or 10 nM E2. The effect of SRC-1 on response to E2 is also indicated. Results are expressed as the response over basal levels in the absence of a ligand.
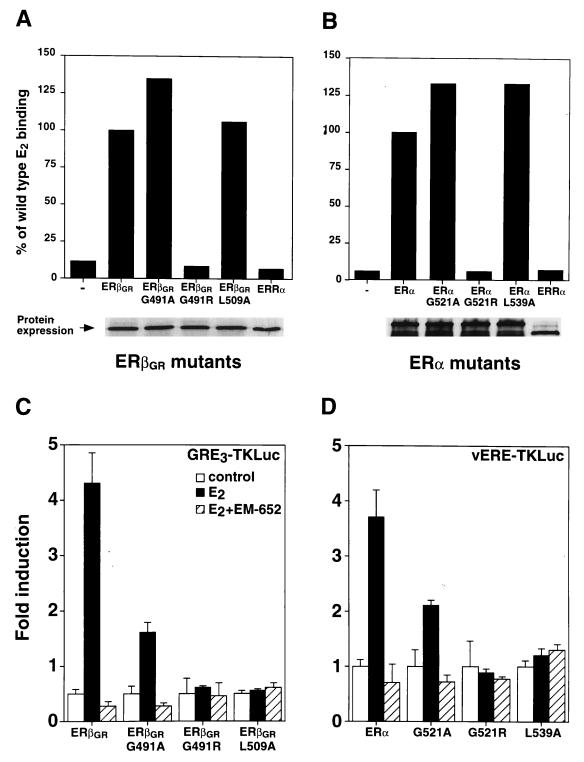
To investigate the contribution of each partner’s LBD to the heterodimer, mutations were introduced within the LBDs of ERα and ERβ to study the dependence of heterodimer activity on an intact AF-2 motif and on E2 binding. In order to create AF-2-defective mutants, the first leucine residue in the AF-2 core motif was replaced with an alanine, a mutation which has previously been shown to abolish ERα activity (8). These mutations correspond to position 509 in ERβGR (L509A) and 539 in human ERα (L539A). In addition, ligand-binding mutants were generated by replacing glycine residues with arginine at position 491 in ERβGR (G491R) and at position 521 in ERα (G521R) (9). The receptors were synthesized in vitro with rabbit reticulocyte lysates and tested for their abilities to bind radiolabeled E2. As shown in Fig. 6A and B, both ERβGRG491R and ERαG521R are unable to bind E2. In controls, replacement of the arginine residue for an alanine did not impede ligand binding, indicating that modification of this glycine did not cause an overall disruption of the LBD structure. Conversely, AF-2-defective mutants of both ERs (ERβGRL509A and ERαL539A) bound ligands similarly to the wild-type receptor (Fig. 6A and B). The human orphan receptor estrogen-related receptor α (ERRα) (33) was used as a control and did not bind E2. Finally, [35S]methionine incorporation showed that all proteins were produced in equal amounts in each sample (Fig. 6A and B, bottom panels). We next assessed the transcriptional activities of these mutants in Cos-1 cells. As shown in Fig. 6C, both the E2-binding mutant, ERβGRG491R, and AF-2-defective mutant, ERβGRL509A, were inactive when cotransfected with GRE3TKLuc in the presence of 10−8 M E2. Similarly, the corresponding mutants generated for ERα were transcriptionally inactive when cotransfected with vERE1TKLuc (Fig. 6D). Not surprisingly, the glycine-to-alanine mutants of both receptors could be stimulated by E2, although the levels of induction were decreased compared to that of the wild type (Fig. 6C and D).
FIG. 6.
Functional characterization of ERβGR and ERα LBD mutants. (A) E2 binding analysis of in vitro-translated ERβGR mutants. Controls were conducted by using either unprogrammed reticulocyte lysates (−) or human ERRα, both of which are unable to bind E2. Results are expressed as the percentage of ERβGR ligand binding, which was arbitrarily set at 100%. The bottom panel shows that all receptors were expressed in equal amounts by 35S-labeling in parallel reactions. (B) Conditions were identical to those for panel A except that an analysis of the corresponding ERα mutants was conducted. (C) Cos-1 cells were cotransfected with the GRE3TKLuc reporter construct and wild-type or mutated ERβGR. The cells were treated with a control (0.1% ethanol) or 10 nM E2 in the absence or presence of 100 nM EM-652. (D) Conditions were identical to those for panel C except that Cos-1 cells were cotransfected with ERα receptors and the vERE1TKLuc reporter construct.
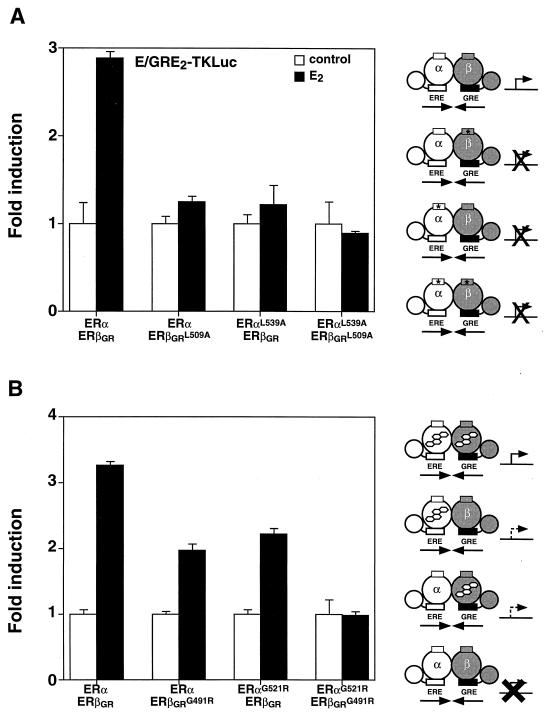
The function of the ERα-ERβ heterodimer was further investigated by determining the effect of limiting the dimer complex to one active AF-2 domain. This experiment was carried out in Cos-1 cells by cotransfecting either wild-type ERα with ERβGRL509A or ERαL539A with wild-type ERβGR in the presence of E/GRE2TKLuc. In both cases, the heterodimer could not be stimulated by E2 (Fig. 7A), suggesting that two functional AF-2 motifs are required for transcriptional activity. As was the case for the homodimers (Fig. 6), inactivation of AF-2 in both partners (ERαL539A and ERβGRL509A) (Fig. 7A) resulted in an inactive heterodimer. Next, heterodimer complexes in which only one molecule of ligand could bind to each dimer unit were monitored for E2 responsiveness. As shown in Fig. 7B, cotransfection of ERα with ERβGRG491R resulted in a heterodimer that was approximately half as active as the wild-type complex. In a similar manner, the reversed heterodimer (ERαG521R-ERβGR) also showed reduced transcriptional activity. Again, forming a heterodimer containing the mutation in both partners resulted in an inactive receptor complex (Fig. 7B).
FIG. 7.
The ERα-ERβ heterodimer requires two functional LBDs for maximal transcriptional activity. (A) Heterodimers with one or two AF-2 defective partners were analyzed by cotransfecting Cos-1 cells with the E/GRE2TKLuc reporter construct and wild-type or AF-2-defective mutants of ERβGR or ERα. The cells were treated with a control (0.1% ethanol) or 10 nM E2. (B) Transcriptional activity of heterodimers bound to only one molecule of E2 per dimer is shown. Conditions for treatment were identical to those used for panel A. A schematic representation of the effects on transcriptional activity of inactivating the AF-2 domain and of the E2-binding capacity of each heterodimer complex is displayed on the right of the graph. Symbols are the same as in Fig. 3.
DISCUSSION
Heterodimerization provides a mechanism by which nuclear receptors can expand their repertoire of physiological actions by combining the transcriptional properties of two distinct partners. For the well-studied RXR heterodimers, this mechanism allows for activation by two distinct ligands and synergistic interactions between partners (reviewed in reference 41). However, the recent observation that ERα and ERβ preferentially form heterodimers illustrates an unusual occurrence in steroid receptor signaling. While heterodimerization of ERα and ERβ has been shown to occur in vitro and in vivo (7, 28, 30), it was unclear how such a heterodimer would function in cells at the transcriptional level. Using ERα and ERβ with modified DNA-binding specificity together with hybrid response elements, we were able to specifically monitor the transcriptional activities of heterodimeric complexes in response to different signaling pathways. The main conclusions of our study of the ERα-ERβ heterodimer are that (i) the specific activities of distinct AF-1 domains are conserved within the heterodimer complex; (ii) both AF-2 domains are required for transcriptional activation in vivo; and (iii) despite the requirement for two AF-2 domains, a single liganded ER subunit is sufficient to activate transcription. These results support a model of the ERα-ERβ heterodimer with different stoichiometric requirements for AF-1 and -2. Furthermore, since the pathways leading to AF-1 activation of ERα and ERβ are likely to differ in vivo, a convergence of these activation pathways may occur when estrogenic signals are transduced by the ERα-ERβ heterodimeric complex.
AF-1 activities in an ERα-ERβ heterodimer complex.
The first question we wished to address was whether the AF-1 domains of ERα and ERβ retain their distinct transcriptional properties within the context of the heterodimer. The AF-1 domain has been shown to transduce the MAPK signal to both ERs (5, 16, 35), while it specifically confers OHT inducibility on ERα (24, 35, 38). The results presented in this study first show that the ERα-ERβ heterodimer remains sensitive to the action of the MAPK pathway. More importantly, our data indicate that each AF-1 domain within the ERα-ERβ heterodimer could be activated independently from the other. The observation that mutation of either Ser118 in ERα or Ser124 in ERβ within the heterodimer did not affect Ras activation (Fig. 4) demonstrates that stimulation of ER activity by Ras requires the presence of only one responsive MAPK site per dimer. Similarly, our observation that OHT stimulates the activity of the ERα-ERβ complex demonstrates that ERα AF-1 can function independently in the heterodimer. However, OHT produced only an intermediate agonistic response in the presence of the heterodimer, suggesting a localized contribution from the ERα partner. This result further demonstrates that the presence of ERβ does not hinder the OHT-induced conformational change in ERα required to transmit the signal from the LBD to the AF-1 domain and that concomitant OHT binding to the ERβ moiety does not prevent ERα activation. This is in sharp contrast to the RAR-RXR heterodimeric complex in which the binding of an RXR homodimer antagonist induces conformational changes in RAR, leading to transcriptional activation by RAR AF-2 (31).
Two functional AF-2 domains are required for ER activation.
Analysis of the transcriptional properties of the AF-2 domain of the heterodimer revealed important differences between the interaction of ERα with ERβ moieties and the results obtained for AF-1. First, both AF-2 domains were required to generate a transcriptionally active ER dimer (Fig. 7A). Comparable results were obtained when either AF-2 domain was inactivated in RXR-RAR heterodimers in P19 cells (25). In contrast, experiments conducted with the permissive RXR heterodimers RXR-LXR and RXR-PPAR demonstrated that the AF-2 domain of RXR was dispensable for transcriptional activity (32, 40, 42). Insight into the possible mechanisms of allosteric interactions between adjacent AF-2 domains is provided by recent studies showing that the AF-2 domain of RXR can physically interact with the RAR partner (39). The binding of a ligand to RAR promotes the recruitment of an LXXLL motif of SRC-1 which displaces the RXR AF-2 domain. This allows the RXR ligand to bind and attract a second LXXLL motif from the same SRC-1 molecule. While our studies do not provide direct evidence that ERα-ERβ heterodimers function by the same mechanism, the requirement for both AF-2 domains suggests that similar allosteric interactions between ER dimeric partners are possible. The study of allosteric interactions in nonpermissive RXR dimers, such as RXR-RAR and RXR-T3R, indicated that these heterodimers could be activated by a single ligand (10, 20, 31). We observed similar effects when only one partner of the ERα-ERβ heterodimer was bound to E2 (Fig. 7B). However, while RXR heterodimers generally react synergistically when both ligands are bound, the effect of dual ligand binding on ER heterodimers is additive.
Analyses of the properties of ER heterodimers’ AF-2 and ligand-binding requirements also permitted us to address another important question pertaining to the stoichiometry of receptor-coactivator interactions. Data from Westin and coworkers (39) and the recently elucidated cocrystal structure of the ligand-bound PPARγ and an SRC-1 peptide (27) suggest that two LXXLL motifs from the same SRC-1 molecule will interact with each nuclear receptor heterodimer. This conclusion agrees with the recent finding that single molecules of SRC-1 appear to bind to ERα homodimers in vitro (15). Further support for this hypothesis is provided by our observations that an ER heterodimer complex containing one E2-binding-deficient partner (ERαG521R or ERβGRG491R) (Fig. 7B) which is unable to interact with SRC-1 in vitro (data not shown) is still able to activate transcription. Since the presence of one E2 molecule per dimer allows only one LXXLL motif to interact, these observations suggest a stoichiometry of one SRC-1 molecule per ERα-ERβ heterodimer.
Physiological implications.
In this paper we describe the first detailed analysis of the transcriptional properties of the ERα-ERβ heterodimer complex. Although to date virtually all studies have focused on RXR-dependent heterodimers, our results provide preliminary insights into the function and physiological role of a novel heterodimer within the steroid receptor subfamily. Our findings have several implications for the interpretation of how estrogenic stimuli are transmitted within cells containing both ERs. More specifically, our data indicate that the transcriptional activity of neither ERα nor ERβ is able to predominate within an ERα-ERβ heterodimer. For example, an agonist or antagonist which may preferentially bind to or regulate one ER over the other will be unable to discriminate between homo- or heterodimers in cells where both ERα and ERβ are expressed. In addition, our studies show that OHT is able to act as an agonist of the ERα-ERβ heterodimer under the same conditions necessary for agonism of ERα, suggesting that OHT could act as an agonist in tissues preferentially expressing heterodimers. Although the presence of the ERα-ERβ heterodimer in specific tissues ultimately depends on the coexpression of ERα and ERβ, our results indicate that the heterodimeric ER complex possesses the attributes necessary to transduce the estrogenic signal in response to a wide spectrum of physiological cues.
ACKNOWLEDGMENTS
We thank P. Chambon for the gift of human ERα, J. Torchia for the human SRC-1 cDNA, S. Mader for HE82, and M. Park for the gift of the H-RasV12 expression vector.
Financial support was provided by the Medical Research Council of Canada, the National Cancer Institute of Canada, and the Cancer Research Society Inc. to V. Giguère. G. B. Tremblay is a postdoctoral fellow, and V. Giguère is a scientist of the Medical Research Council of Canada.
REFERENCES
- 1.Ali S, Metzger D, Bornert J-M, Chambon P. Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO J. 1993;12:1153–1160. doi: 10.1002/j.1460-2075.1993.tb05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 3.Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry M, Nunez A-M, Chambon P. Estrogen-responsive element of the human pS2 gene is an imperfectly palindromic sequence. Proc Natl Acad Sci USA. 1989;86:1218–1222. doi: 10.1073/pnas.86.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunone G, Briand P-A, Miksicek R J, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 6.Couse J F, Lindzey J, Grandien K, Gustafsson J A, Korach K S. Tissue distribution and quantitative analysis of estrogen receptor-α (ER-α) and estrogen receptor-β (ER-β) messenger ribonucleic acid in the wild-type and ER-α-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 7.Cowley S M, Hoare S, Mosselman S, Parker M G. Estrogen receptors α and β form heterodimers on DNA. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 8.Danielian P S, White R, Lees J A, Parker M G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992;11:1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fawell S E, Lees J A, White R, Parker M G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990;60:953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- 10.Forman B M, Umesono K, Chen J, Evans R M. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 11.Giguère V, Shago M, Zirngibl R, Tate P, Rossant J, Varmuza S. Identification of a novel isoform of the retinoic acid receptor γ expressed in the mouse embryo. Mol Cell Biol. 1990;10:2335–2340. doi: 10.1128/mcb.10.5.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giguère V, Tremblay A, Tremblay G B. Estrogen receptor β: reevaluation of estrogen and antiestrogen signaling. Steroids. 1998;63:335–339. doi: 10.1016/s0039-128x(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 13.Green S, Walter P, Kumar V, Krust A, Bornet J M, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erbA. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 14.Greene G L, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- 15.Kalkhoven E, Valentine J E, Heery D M, Parker M G. Isoforms of steroid receptor co-activator 1 differ in their ability to potentiate transcription by the oestrogen receptor. EMBO J. 1998;17:232–243. doi: 10.1093/emboj/17.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 17.Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci USA. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kliewer S A, Umesono K, Noonan D J, Heyman R A, Evans R M. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuiper G G J M, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurokawa R, DiRenzo J, Boehm M, Sugarman J, Gloss B, Rosenfeld G M, Heyman R A, Glass C K. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature. 1994;371:528–531. doi: 10.1038/371528a0. [DOI] [PubMed] [Google Scholar]
- 21.Leygue E, Dotzlaw H, Watson P H, Murphy L C. Altered estrogen receptor α and β messenger RNA expression during human breast tumorigenesis. Cancer Res. 1998;58:3197–3201. [PubMed] [Google Scholar]
- 22.Mader S, Kumar V, de Verneuil H, Chambon P. Three amino acids of the oestrogen receptor are essential to its ability to distinguish an oestrogen from a glucocorticoid-responsive element. Nature. 1989;338:271–274. doi: 10.1038/338271a0. [DOI] [PubMed] [Google Scholar]
- 23.Mangelsdorf D J, Evans R M. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 24.McInerney E M, Weis K E, Sun J, Mosselman S, Katzenellenbogen B S. Transcription activation by the human estrogen receptor subtype β (ERβ) studied with ERβ and ERα receptor chimeras. Endocrinology. 1998;139:4513–4522. doi: 10.1210/endo.139.11.6298. [DOI] [PubMed] [Google Scholar]
- 25.Minucci S, Leid M, Toyama R, Saint-Jeannet J-P, Peterson V J, Horn V, Ishmael J E, Bhattacharyya N, Dey A, Dawid I B, Ozato K. Retinoid X receptor (RXR) within the RXR-retinoic acid receptor heterodimer binds its ligand and enhances retinoid-dependent gene expression. Mol Cell Biol. 1997;17:644–655. doi: 10.1128/mcb.17.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosselman S, Polman J, Dijkema R. ERβ: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 27.Nolte R T, Wisely G B, Westin S, Cobb J E, Lambert M H, Kurokawa R, Rosenfeld M G, Willson T M, Glass C K, Milburn M V. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ. Nature. 1998;395:137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 28.Pace P, Taylor J, Suntharalingam S, Coombes R C, Ali S. Human estrogen receptor β binds DNA in a manner similar to and dimerizes with estrogen receptor α. J Biol Chem. 1997;272:25832–25838. doi: 10.1074/jbc.272.41.25832. [DOI] [PubMed] [Google Scholar]
- 29.Paech K, Webb P, Kuiper G G J M, Nilsson S, Gustafsson J-Å, Kushner P J, Scanlan T S. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 30.Pettersson K, Grandien K, Kuiper G G J M, Gustafsson J-Å. Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 31.Schulman I G, Li C, Schwabe J W R, Evans R M. The phantom ligand effect: allosteric control of transcription by the retinoid X receptor. Genes Dev. 1997;11:299–308. doi: 10.1101/gad.11.3.299. [DOI] [PubMed] [Google Scholar]
- 32.Schulman I G, Shao G, Heyman R A. Transactivation by retinoid X receptor–peroxisome proliferator-activated receptor γ (PPARγ) heterodimers: intermolecular synergy requires only the PPARγ hormone-dependent activation function. Mol Cell Biol. 1998;18:3483–3494. doi: 10.1128/mcb.18.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sladek R, Bader J-A, Giguère V. The orphan nuclear receptor estrogen-related receptor α is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol. 1997;17:5400–5409. doi: 10.1128/mcb.17.9.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tremblay A, Tremblay G B, Labrie C, Labrie F, Giguère V. EM-800, a novel antiestrogen, acts as a pure antagonist of the transcriptional functions of estrogen receptors α and β. Endocrinology. 1998;139:111–118. doi: 10.1210/endo.139.1.5702. [DOI] [PubMed] [Google Scholar]
- 35.Tremblay G B, Tremblay A, Copeland N G, Gilbert D J, Jenkins N A, Labrie F, Giguère V. Cloning, chromosomal localization and functional analysis of the murine estrogen receptor β. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay G B, Tremblay A, Labrie F, Giguère V. Ligand-independent activation of the estrogen receptors α and β by mutations of a conserved tyrosine can be abolished by antiestrogens. Cancer Res. 1998;58:877–881. [PubMed] [Google Scholar]
- 37.Umesono K, Murakami K K, Thompson C C, Evans R M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe T, Inoue S, Ogawa S, Ishii Y, Hiroi H, Ikeda K, Orimo A, Muramatsu M. Agonistic effect of tamoxifen is dependent on cell type, ERE-promoter context, and estrogen receptor subtype: functional difference between estrogen receptors α and β. Biochem Biophys Res Commun. 1997;236:140–145. doi: 10.1006/bbrc.1997.6915. [DOI] [PubMed] [Google Scholar]
- 39.Westin S, Kurokawa R, Nolte R T, Wisely G B, McInerney E M, Rose D W, Milburn M V, Rosenfeld M G, Glass C K. Interactions controlling the assembly of nuclear-receptor heterodimers and co-activators. Nature. 1998;395:199–202. doi: 10.1038/26040. [DOI] [PubMed] [Google Scholar]
- 40.Wiebel F F, Gustafsson J-Å. Heterodimeric interaction between retinoid X receptor α and orphan nuclear receptor OR1 reveals dimerization-induced activation as a novel mechanism of nuclear receptor activation. Mol Cell Biol. 1997;17:3977–3986. doi: 10.1128/mcb.17.7.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willy P J, Mangelsdorf D J. Nuclear orphan receptors: the search for novel ligands and signaling pathways. In: O’Malley B W, editor. Hormones and signaling. Vol. 1. San Diego, Calif: Academic Press; 1998. pp. 307–358. [Google Scholar]
- 42.Willy P J, Mangelsdorf D J. Unique requirements for retinoid-dependent transcriptional activation by the orphan receptor LXR. Genes Dev. 1997;11:289–298. doi: 10.1101/gad.11.3.289. [DOI] [PubMed] [Google Scholar]